Nanogold Particles Suppresses 5-Flurouracil-Induced Renal Injury: An Insight into the Modulation of Nrf-2 and Its Downstream Targets, HO-1 and γ-GCS
Abstract
1. Introduction
2. Results
2.1. AuNPs Treatment Attenuated 5-FU-Induced Biochemical and Histopathological Renal Injury
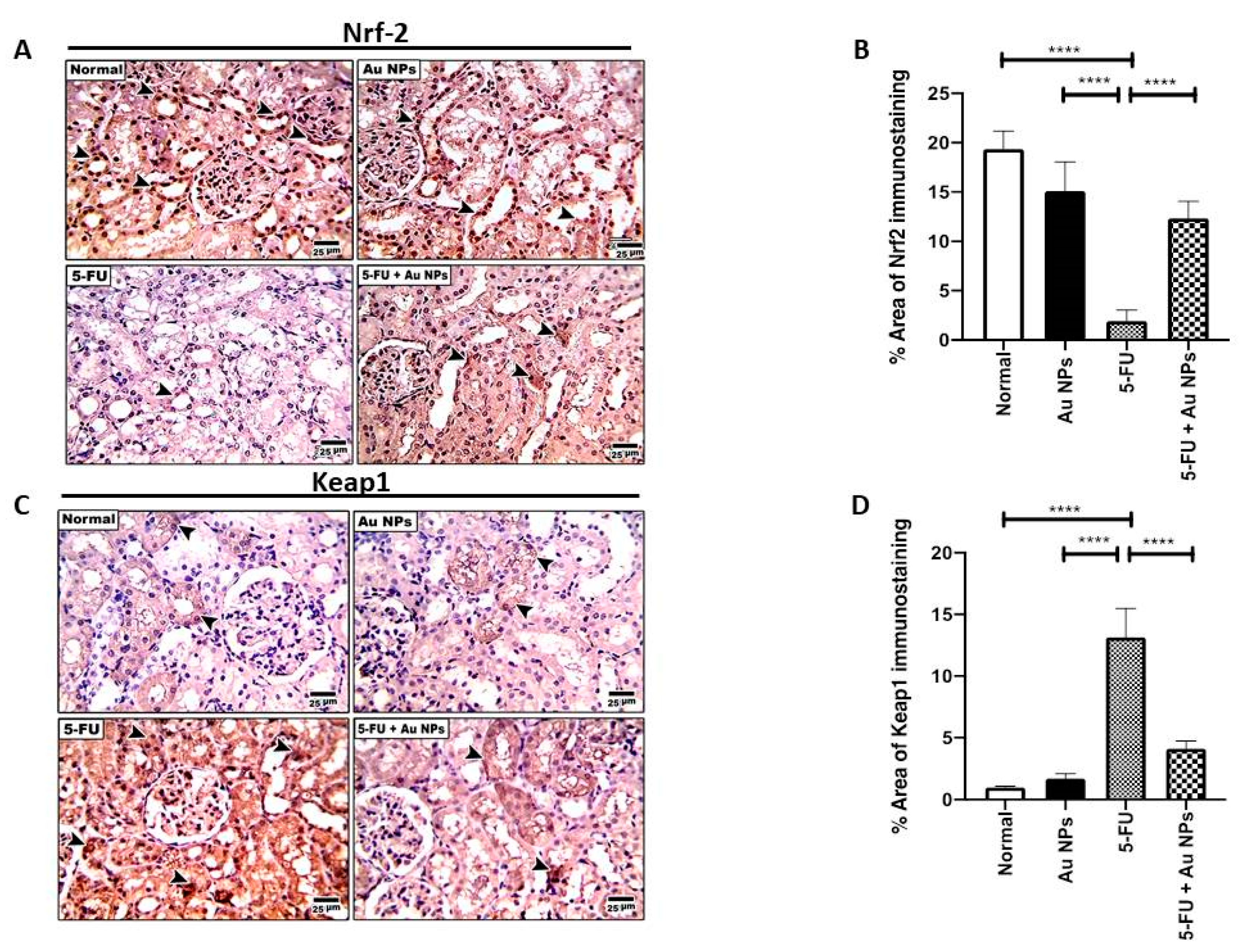
2.2. AuNPs Treatment Corrected 5-FU-Induced Imbalance of Nrf-2/Keap-1 Axis in Renal Tissues
2.3. AuNPs Treatment Restored 5-FU-Induced Suppression of Nrf-2 Downstream Targets, HO-1 and γ-GCS in Renal Tissues
2.4. AuNPs Treatment Potentiated 5-FU-Induced Cytotoxic Effect on MCF-7 Cells
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
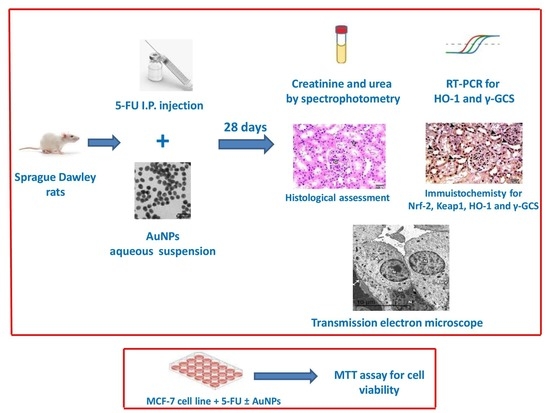
4.2. Animals and Experimental Design
4.3. Assessment of Serum Markers of Renal Injury; Creatinine and Urea
4.4. Real Time Polymerase Chain Reaction (RT-PCR)
4.5. Histological Assessment
4.6. Immunohistochemical Analysis of Renal Expression of Nuclear Factor Erythroid 2–Related Factor 2 (Nrf-2), Gamma-Glutamylcysteine Synthetase (γ-GCSc), Kelch-like ECH-Associated Protein 1 (Keap1) and Heme Oxygenase-1 (HO-1)
4.7. Electron Microscopy Investigation
4.8. MTT Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mawalizadeh, F.; Mohammadzadeh, G.; Khedri, A.; Rashidi, M. Quercetin potentiates the chemosensitivity of MCF-7 breast cancer cells to 5-fluorouracil. Mol. Biol. Rep. 2021, 48, 7733–7742. [Google Scholar] [CrossRef] [PubMed]
- Na, D.; Chae, J.; Cho, S.Y.; Kang, W.; Lee, A.; Min, S.; Kang, J.; Kim, M.J.; Choi, J.; Lee, W.; et al. Predictive biomarkers for 5-fluorouracil and oxaliplatin-based chemotherapy in gastric cancers via profiling of patient-derived xenografts. Nat. Commun. 2021, 12, 4840. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, C.; Patel, G. Thermosensitive nanohydrogel of 5-fluorouracil for head and neck cancer: Preparation, characterization and cytotoxicity assay. Int. J. Nanomed. 2018, 13, 31–33. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Peng, C.; Yan, J.; Chen, P.; Jiang, C.; Sang, S.; Yuan, Y.; Hong, Y.; Yao, M. Sanguisorba officinalis L. suppresses 5-fluorouracil-sensitive and-resistant colorectal cancer growth and metastasis via inhibition of the Wnt/β-catenin pathway. Phytomedicine 2021, 94, 153844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Xiang, D.; Yang, J.; Liu, D.; Ren, X.; Zhang, C. Assessment of dose-response relationship of 5-fluorouracil to murine intestinal injury. Biomed. Pharm. 2018, 106, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Negrei, C.; Hudita, A.; Ginghina, O.; Galateanu, B.; Voicu, S.N.; Stan, M.; Costache, M.; Fenga, C.; Drakoulis, N.; Tsatsakis, A.M. Colon Cancer Cells Gene Expression Signature As Response to 5- Fluorouracil, Oxaliplatin, and Folinic Acid Treatment. Front. Pharmacol. 2016, 7, 172. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Al-Zahrani, A.M.; Khan, A.Q.; Al-Shahrani, H.M.; Ali Al Amri, M. Taurine ameliorates 5-flourouracil-induced intestinal mucositis, hepatorenal and reproductive organ damage in Wistar rats: A biochemical and histological study. Hum. Exp. Toxicol. 2016, 35, 10–20. [Google Scholar] [CrossRef]
- Ishibashi, M.; Ishii, M.; Yamamoto, S.; Mori, Y.; Shimizu, S. Possible involvement of TRPM2 activation in 5-fluorouracil-induced myelosuppression in mice. Eur. J. Pharmacol. 2021, 891, 173671. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Khan, A.Q.; Al-Qasim, A.M.; Al-Yousef, Y. Ascorbic acid attenuates antineoplastic drug 5-fluorouracil induced gastrointestinal toxicity in rats by modulating the expression of inflammatory mediators. Toxicol. Rep. 2015, 2, 908–916. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, F.R.; Shang, J.Y.; Liu, Y.Y.; Lv, X.F.; Yuan, J.N.; Zhang, T.T.; Li, K.; Lin, X.C.; Liu, X.; et al. Renal inhibition of miR-181a ameliorates 5-fluorouracil-induced mesangial cell apoptosis and nephrotoxicity. Cell Death Dis. 2018, 9, 610. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef]
- Rashid, S.; Ali, N.; Nafees, S.; Hasan, S.K.; Sultana, S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem. Toxicol. 2014, 66, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gelen, V.; Şengül, E.; Yıldırım, S.; Senturk, E.; Tekin, S.; Kükürt, A. The protective effects of hesperidin and curcumin on 5-fluorouracil-induced nephrotoxicity in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 47046–47055. [Google Scholar] [CrossRef] [PubMed]
- Elghareeb, M.M.; Elshopakey, G.E.; Hendam, B.M.; Rezk, S.; Lashen, S. Synergistic effects of Ficus Carica extract and extra virgin olive oil against oxidative injury, cytokine liberation, and inflammation mediated by 5-Fluorouracil in cardiac and renal tissues of male albino rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 4558–4572. [Google Scholar] [CrossRef]
- Yousef, H.N.; Aboelwafa, H.R. The potential protective role of taurine against 5-fluorouracil-induced nephrotoxicity in adult male rats. Exp. Toxicol. Pathol. 2017, 69, 265–274. [Google Scholar] [CrossRef]
- Puelles, V.G.; Hoy, W.E.; Hughson, M.D.; Diouf, B.; Douglas-Denton, R.N.; Bertram, J.F. Glomerular number and size variability and risk for kidney disease. Curr. Opin. Nephrol. Hypertens 2011, 20, 7–15. [Google Scholar] [CrossRef]
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, O.C. Nanomedicines for renal disease: Current status and future applications. Nat. Rev. Nephrol. 2016, 12, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Wiraja, C.; Mori, Y.; Ichimura, T.; Hwang, J.; Xu, C.; Bonventre, J.V. Nephrotoxicity Assessment with Human Kidney Tubuloids using Spherical Nucleic Acid-Based mRNA Nanoflares. Nano Lett. 2021, 21, 5850–5858. [Google Scholar]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Eladl, M.A.; Al-Gayyar, M.M.H. Renal protective effects of arjunolic acid in a cisplatin-induced nephrotoxicity model. Cytokine 2016, 77, 26–34. [Google Scholar] [CrossRef]
- Badawoud, M.H.; Elshal, E.B.; Zaki, A.I.; Amin, H.A. The possible protective effect of L-arginine against 5-fluorouracil-induced nephrotoxicity in male albino rats. Folia Morphol. 2017, 76, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F. Gold-based therapy: From past to present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef] [PubMed]
- Barathmanikanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.; Youn, H.S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnology 2010, 8, 16. [Google Scholar] [CrossRef]
- Kondel, R.; Shafiq, N.; Kaur, I.P.; Singh, M.P.; Pandey, A.K.; Ratho, R.K.; Malhotra, S. Effect of Acyclovir Solid Lipid Nanoparticles for the Treatment of Herpes Simplex Virus (HSV) Infection in an Animal Model of HSV-1 Infection. Pharm. Nanotechnol. 2019, 7, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Javan, F.; Vatanara, A.; Azadmanesh, K.; Nabi-Meibodi, M.; Shakouri, M. Encapsulation of ritonavir in solid lipid nanoparticles: In-vitro anti-HIV-1 activity using lentiviral particles. J. Pharm. Pharmacol. 2017, 69, 1002–1009. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, E1979. [Google Scholar] [CrossRef]
- Fahmy, E.K.; El-Sherbiny, M.; Said, E.; Elkattawy, H.A.; Qushawy, M.; Elsherbiny, N. Tranilast ameliorated subchronic silver nanoparticles-induced cerebral toxicity in rats: Effect on TLR4/NLRP3 and Nrf-2. Neurotoxicology 2021, 82, 167–176. [Google Scholar] [CrossRef]
- Vilar, C.; Ribeiro, S.B.; de Araújo, A.A.; Guerra, G.; de Araújo Júnior, R.F.; Brito, G.; Leitão, R.; Pontes, D.L.; Gasparotto, L.; Oliveira, M.; et al. Effect of Gold Nanoparticle on 5-Fluorouracil-Induced Experimental Oral Mucositis in Hamsters. Pharmaceutics 2020, 12, E304. [Google Scholar] [CrossRef]
- Kajita, M.; Hikosaka, K.; Iitsuka, M.; Kanayama, A.; Toshima, N.; Miyamoto, Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic. Res. 2007, 41, 615–626. [Google Scholar] [CrossRef]
- Alomari, G.; Al-Trad, B.; Hamdan, S.; Aljabali, A.; Al-Zoubi, M.; Bataineh, N.; Qar, J.; Tambuwala, M.M. Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy. Drug Deliv. Transl. Res. 2020, 10, 216–226. [Google Scholar] [CrossRef]
- Kirdaite, G.; Leonaviciene, L.; Bradunaite, R.; Vasiliauskas, A.; Rudys, R.; Ramanaviciene, A.; Mackiewicz, Z. Antioxidant effects of gold nanoparticles on early stage of collagen-induced arthritis in rats. Res. Vet. Sci. 2019, 124, 32–37. [Google Scholar] [CrossRef]
- Sul, O.J.; Kim, J.C.; Kyung, T.W.; Kim, H.J.; Kim, Y.Y.; Kim, S.H.; Kim, J.S.; Choi, H.S. Gold nanoparticles inhibited the receptor activator of nuclear factor-κb ligand (RANKL)-induced osteoclast formation by acting as an antioxidant. Biosci. Biotechnol. Biochem. 2010, 74, 2209–2213. [Google Scholar] [CrossRef]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Wang, Y.; Chen, Y.; Li, D.; Li, G.; Xiao, H.; Hou, J.; Wang, Z.; Hu, L.; Wang, L.; et al. Angelica Polysaccharide Antagonizes 5-FU-Induced Oxidative Stress Injury to Reduce Apoptosis in the Liver Through Nrf2 Pathway. Front. Oncol. 2021, 11, 720620. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huo, X.; Gao, L.; Sun, G.; Li, C. Hepatoprotective Effect of Carboxymethyl Pachyman in Fluorouracil-Treated CT26-Bearing Mice. Molecules 2017, 22, E756. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, H.; Wen, Y.; Li, B.; Zhao, Y.; Xing, J.; Zhang, M.; Chen, Y. Nrf2 Inhibits Periodontal Ligament Stem Cell Apoptosis under Excessive Oxidative Stress. Int. J. Mol. Sci. 2017, 18, E1076. [Google Scholar] [CrossRef]
- Armitage, M.; Wilkin, T.; Scott-Morgan, L.; Casey, C.; Betts, P. On the relationship between islet cell antibodies and insulin autoantibodies in patients at risk from insulin dependent diabetes. Autoimmunity 1988, 1, 275–283. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; El-Sherbiny, M. Thymoquinone attenuates Doxorubicin-induced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chem. Biol. Interact. 2014, 223, 102–108. [Google Scholar] [CrossRef]
- Gelen, V.; Şengül, E.; Yıldırım, S.; Atila, G. The protective effects of naringin against 5-fluorouracil-induced hepatotoxicity and nephrotoxicity in rats. Iran. J. Basic Med. Sci. 2018, 21, 404–410. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Alsheblak, M.M.; Elsherbiny, N.M.; El-Karef, A.; El-Shishtawy, M.M. Protective effects of L-carnosine on CCl4 -induced hepatic injury in rats. Eur. Cytokine Netw. 2016, 27, 6–15. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 7, 1304–1309. [Google Scholar] [CrossRef]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, B.K. The Nrf2-Keap1 defence pathway: Role in protection against drug-induced toxicity. Toxicology 2008, 246, 24–33. [Google Scholar] [CrossRef]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1-Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-H.; Shieh, J.-M.; Tsou, C.-J.; Wu, W.-B. Gold nanoparticles induce heme oxygenase-1 expression through Nrf2 activation and Bach1 export in human vascular endothelial cells. Int. J. Nanomed. 2015, 10, 5925–5939. [Google Scholar]
- He, J.; Zhang, X.; Lian, C.; Wu, J.; Fang, Y.; Ye, X. KEAP1/NRF2 axis regulates H2O2-induced apoptosis of pancreatic β-cells. Gene 2019, 691, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Eisa, N.H.; El-Sherbiny, M.; Said, E. Chemo-preventive effect of crocin against experimentally-induced hepatocarcinogenesis via regulation of apoptotic and Nrf2 signaling pathways. Environ. Toxicol. Pharmacol. 2020, 80, 103494. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.Y.; Bach, F.H. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef]
- Ko, W.-C.; Shieh, J.-M.; Wu, W.-B. P38 MAPK and Nrf2 Activation Mediated Naked Gold Nanoparticle Induced Heme Oxygenase-1 Expression in Rat Aortic Vascular Smooth Muscle Cells. Arch. Med. Res. 2020, 51, 388–396. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino. Acids. 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.D.C.; Azevedo, Í.M.; Lima, M.L.; Araújo Filho, I.; Moreira, M.D. Effects of simvastatin on 5-fluorouracil-induced gastrointestinal mucositis in rats. Rev. Col. Bras. Cir. 2018, 45, e1968. [Google Scholar] [CrossRef]
- Ferreira, G.K.; Cardoso, E.; Vuolo, F.S.; Michels, M.; Zanoni, E.T.; Carvalho-Silva, M.; Gomes, L.M.; Dal-Pizzol, F.; Rezin, G.T.; Streck, E.L.; et al. Gold nanoparticles alter parameters of oxidative stress and energy metabolism in organs of adult rats. Biochem. Cell Biol. 2015, 93, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O.; Al-Mutabagani, L.A.; Sabry, D.; Elsherbiny, N.M. Antineoplastic Activity of Chrysin against Human Hepatocellular Carcinoma: New Insight on GPC3/SULF2 Axis and lncRNA-AF085935 Expression. Int. J. Mol. Sci. 2020, 21, E7642. [Google Scholar] [CrossRef]




| Gene Symbol | Forward | Reverse | Gene Bank |
|---|---|---|---|
| HO-1 | 5′-GAGCGCCCACAGCTCGACAG-3′ | 5′-GTGGGCCACCAGCAGCTCAG-3′ | XM_032887931.1 |
| γ-GCS | 5′-AGACACGGCATCCTCCAGTT-3′ | 5′-CTGACACGTAGCCTCGGTAA-3′ | NM_012815.2 |
| GAPDH | 5′-ATGGTGAAGGTCGGTGTGAACG-3′ | 5′-TGGTGAAGACGCCAGTAGACTC-3′ | XM_017592435.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sherbiny, M.; Fahmy, E.K.; Eisa, N.H.; Said, E.; Elkattawy, H.A.; Ebrahim, H.A.; Elsherbiny, N.M.; Ghoneim, F.M. Nanogold Particles Suppresses 5-Flurouracil-Induced Renal Injury: An Insight into the Modulation of Nrf-2 and Its Downstream Targets, HO-1 and γ-GCS. Molecules 2021, 26, 7684. https://doi.org/10.3390/molecules26247684
El-Sherbiny M, Fahmy EK, Eisa NH, Said E, Elkattawy HA, Ebrahim HA, Elsherbiny NM, Ghoneim FM. Nanogold Particles Suppresses 5-Flurouracil-Induced Renal Injury: An Insight into the Modulation of Nrf-2 and Its Downstream Targets, HO-1 and γ-GCS. Molecules. 2021; 26(24):7684. https://doi.org/10.3390/molecules26247684
Chicago/Turabian StyleEl-Sherbiny, Mohamed, Eslam K. Fahmy, Nada H. Eisa, Eman Said, Hany A. Elkattawy, Hasnaa Ali Ebrahim, Nehal M. Elsherbiny, and Fatma M. Ghoneim. 2021. "Nanogold Particles Suppresses 5-Flurouracil-Induced Renal Injury: An Insight into the Modulation of Nrf-2 and Its Downstream Targets, HO-1 and γ-GCS" Molecules 26, no. 24: 7684. https://doi.org/10.3390/molecules26247684
APA StyleEl-Sherbiny, M., Fahmy, E. K., Eisa, N. H., Said, E., Elkattawy, H. A., Ebrahim, H. A., Elsherbiny, N. M., & Ghoneim, F. M. (2021). Nanogold Particles Suppresses 5-Flurouracil-Induced Renal Injury: An Insight into the Modulation of Nrf-2 and Its Downstream Targets, HO-1 and γ-GCS. Molecules, 26(24), 7684. https://doi.org/10.3390/molecules26247684