HPLC/DAD, Antibacterial and Antioxidant Activities of Plectranthus Species (Lamiaceae) Combined with the Chemometric Calculations
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization
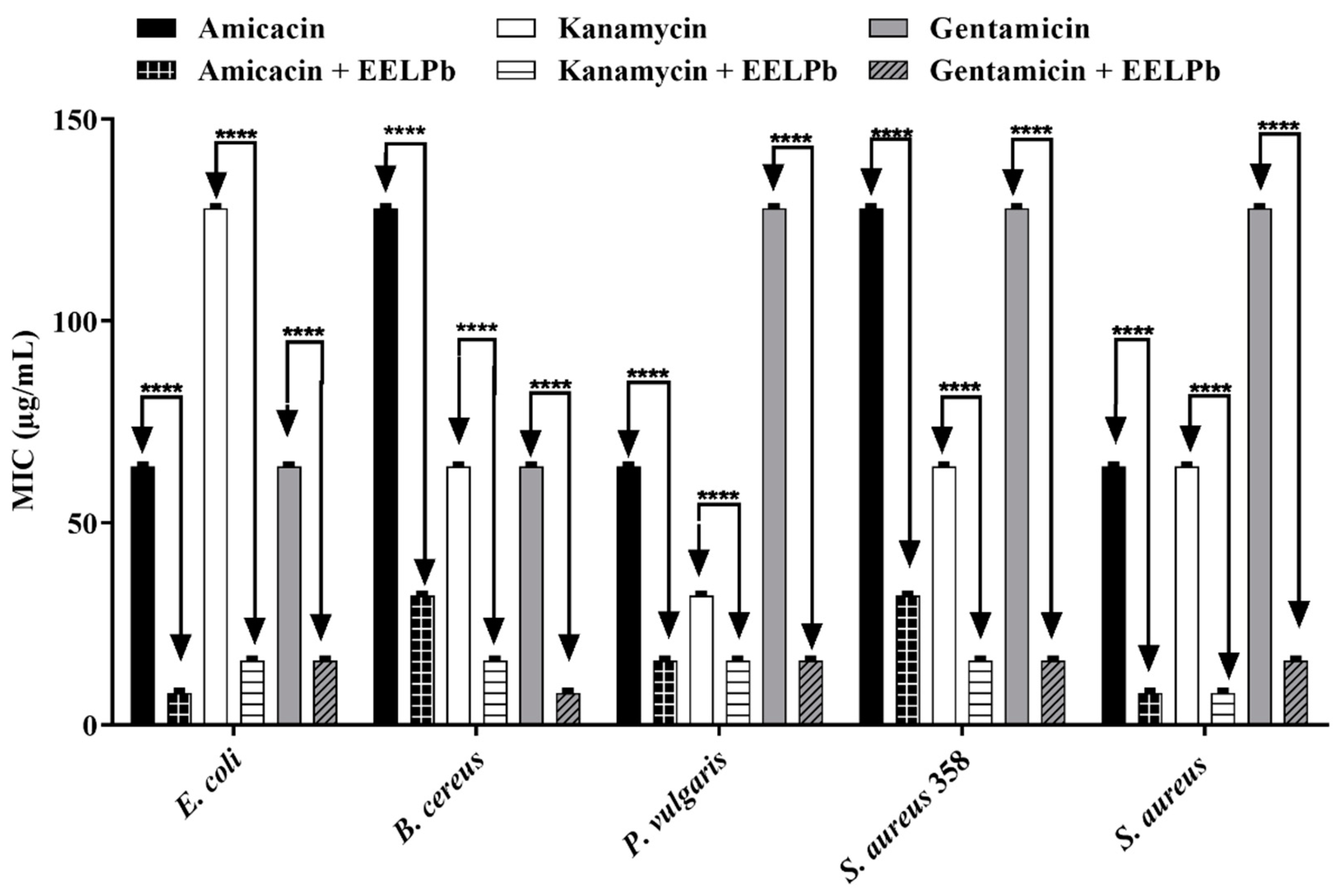
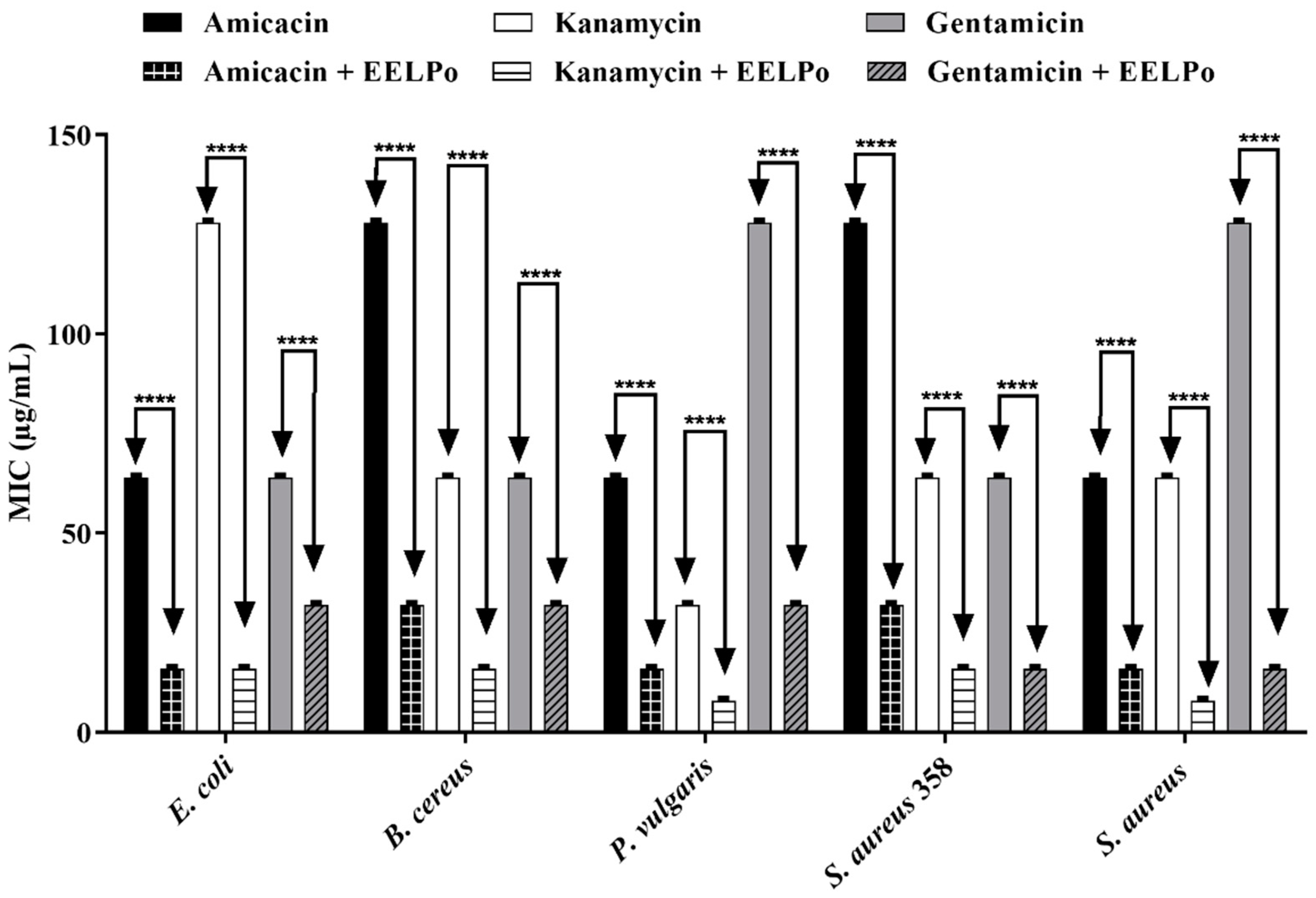
2.2. Antimicrobial Activity
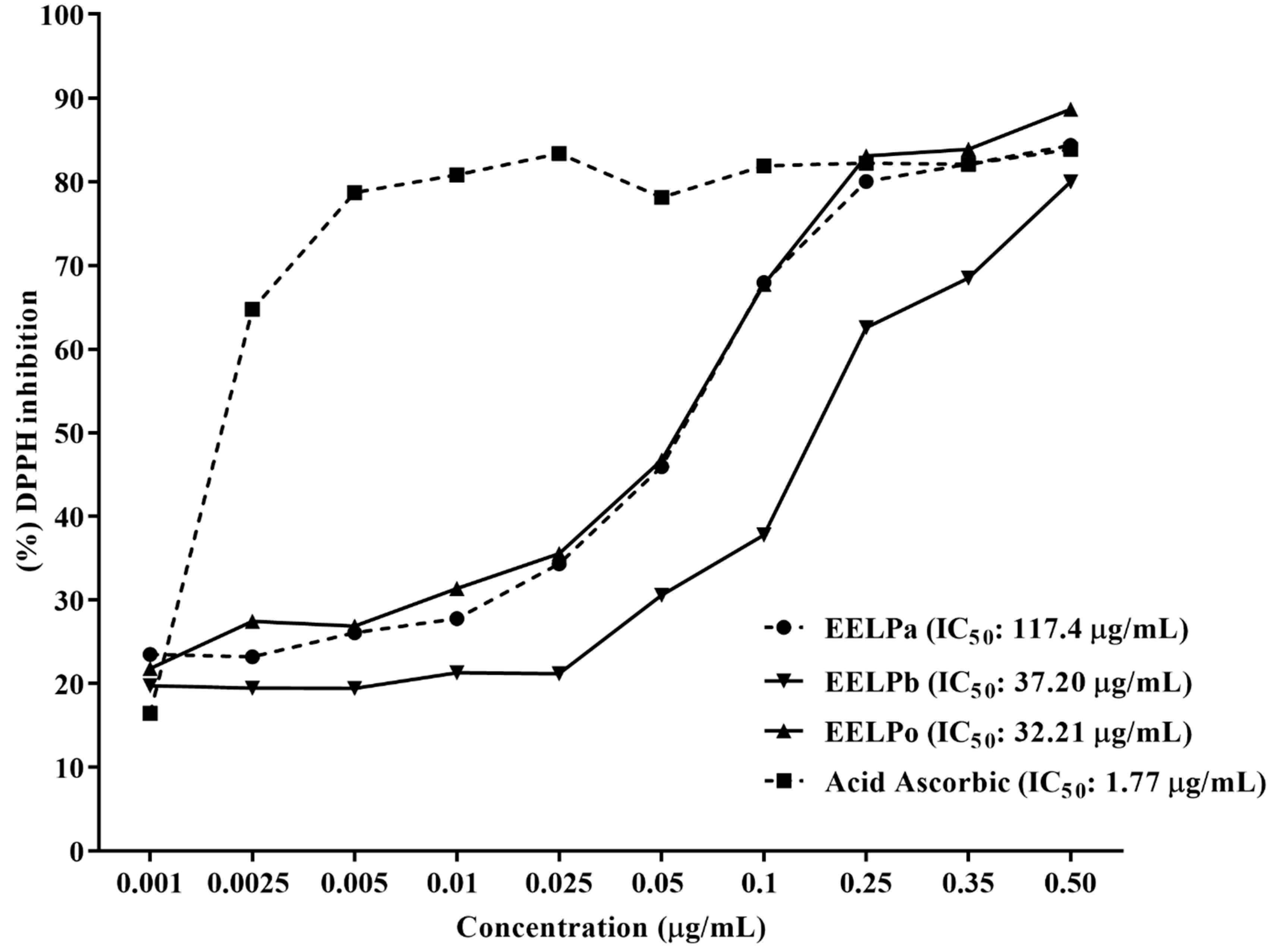
2.3. Antioxidant Activity
2.4. Chemometric Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Preparation of Extracts
3.3. Chemical Characterization
3.3.1. Phytochemical Screening
3.3.2. Phenolics and Flavonoids Compounds by HPLC/DAD
Chemical, Apparatus, and General Procedures
HPLC/DAD Analysis
3.4. Antibacterial Activity
3.4.1. Minimal Inhibitory Concentration Test (MIC)
3.4.2. Modulating the Action of Antibiotics
3.5. DPPH Free Radical Scavenging
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abadi, A.T.B.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization report: Current crisis of antibiotic resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Amakye, W.K.; Hou, C.; Xie, L.; Lin, X.; Gou, N.; Yuan, E.; Ren, J. Bioactive anti-aging agents and the identification of new anti-oxidant soybean peptides. Food Biosci. 2021, 42, 101194. [Google Scholar] [CrossRef]
- Marinho, T.A.; Oliveira, M.G.; Menezes-Filho, A.C.P.; Castro, C.F.S.; Oliveira, I.M.M.; Borges, L.L.; Melo-Reis, P.R.; Silva, N.J., Jr. Phytochemical characterization, and antioxidant and antibacterial activities of the hydroethanolic extract of Anadenanthera peregrina stem bark. Braz. J. Biol. 2022, 82, e234476. [Google Scholar] [CrossRef]
- Lambrechts, I.A.; Lall, N. Traditional usage and biological activity of Plectranthus madagascariensis and its varieties: A review. J. Ethnopharmacol. 2021, 269, 113663. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais do Brasil: Nativas e Exóticas, 1st ed; Instituto Plantarum: Nova Odessa, BR, USA, 2002. [Google Scholar]
- Musila, F.M.; Lukhoba, C.W.; Nguta, J.M.; Dossaji, S.F. Phylogeny of Ten Kenyan Plectranthus Species in the Coleus Clade Inferred from Leaf Micromorphology, Rbcl and MatK Genes. J. Bot. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Tomchinsky, B.; Ming, L.C.; Kinupp, V.F.; Hidalgo, A.D.F.; Chaves, F.C.M. Ethnobotanical study of antimalarial plants in the middle region of the Negro River, Amazonas, Brazil. Acta Amaz. 2017, 47, 203–212. [Google Scholar] [CrossRef]
- Zhang, B.; Wijesundara, N.M.; Abbey, L.; Rupasinghe, H.V. Growing medium amendments effect on growth, secondary metabolites and anti-streptococcal activity of two species of Plectranthus. J. Appl. Res. Med. Aromat. Plants 2017, 5, 53–59. [Google Scholar] [CrossRef]
- Cordeiro, M.F.; Nunes, T.R.S.; Bezerra, F.G.; Damasco, P.K.M.; Silva, W.A.V.; Ferreira, M.R.A.; Magalhães, O.M.C.; Soares, L.A.L.; Cavalcanti, I.M.F.; Pitta, M.G.R.; et al. Phytochemical characterization and biological activities of Plectranthus barbatus Andrews. Braz. J. Biol. 2021, 82, e23629. [Google Scholar] [CrossRef]
- Galbiatti, M.I.; Cassola, F.; Mesquita, A.T.; Pinheiro, G.P.; Mayer, J.L.S.; Sawaya, A.C.H.F. Plectranthus neochilus Schltr.: Anatomic and cytogenetic analyses and chemical characterization of its essential oil. S. Afr. J. Bot. 2021, 143, 97–106. [Google Scholar] [CrossRef]
- Grayer, R.J.; Eckert, M.R.; Lever, A.; Veitch, N.C.; Kite, G.C.; Paton, A.J. Distribution of exudate flavonoids in the genus Plectranthus. Biochem. Syst. Ecol. 2010, 38, 335–341. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Garros, L.; Drouet, S.; Renouard, S.; Lainé, E.; Hano, C. Green ultrasound assisted extraction of trans rosmarinic acid from Plectranthus scutellarioides (L.) R. Br. leaves. Plants 2019, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Kisangau, D.P.; Hosea, K.M.; Joseph, C.C.; Lyaruu, H.V. In vitro antimicrobial assay of plants used in traditional medicine in Bukoba rural district, Tanzania. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, phytochemical, pharmacological and nutritional significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef] [PubMed]
- Magesh, R.; Poorani, R.M.; Karthikeyan, V.; Sivakumar, K.; Mohanapriya, C. Proportionate phytochemical screening and assessment of antioxidant potency on selected species of Lamiaceae family. Int. J. Pharm. Phytochem. Res. 2015, 7, 1066–1072. [Google Scholar]
- Patel, R.; Mahobia, N.; Waseem, N.; Upwar, N.; Singh, S. Phyto-physicochemical investigation of leaves of Plectranthus amboinicus (Lour) Spreng. Pharm. J. 2010, 2, 536–542. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Wang, X.; Wang, L.; Zhang, M.; Ren, M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop. Pasture Sci. 2018, 69, 1208–1214. [Google Scholar] [CrossRef]
- Hiba, H.; Janeeshma, E.; Puthur, J.T. Dynamic alterations of metabolites in Plectranthus amboinicus (Lour.) Spreng. to encounter drought and Zn toxicity. Braz. J. Bot. 2021, 44, 587–599. [Google Scholar] [CrossRef]
- Matias, D.; Nicolai, M.; Fernandes, A.S.; Saraiva, N.; Almeida, J.; Saraiva, L.; Faustino, C.; Diaz-Lanza, A.M.; Reis, C.P.; Rijo, P. Comparison study of different extracts of Plectranthus madagascariensis, P. neochilus and the rare P. porcatus (Lamiaceae): Chemical characterization, antioxidant, antimicrobial and cytotoxic activities. Biomolecules 2019, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Ntungwe, E.; Domínguez-Martín, E.M.; Teodósio, C.; Teixidó-Trujillo, S.; Capote, N.A.; Saraiva, L.; Diaz-Lanza, A.M.; Duarte, N.; Rijo, P. Preliminary Biological Activity Screening of Plectranthus spp. Extracts for the search of anticancer lead molecules. Pharmaceuticals 2021, 14, 402. [Google Scholar] [CrossRef]
- Lemma, B.; Grehl, C.; Zech, M.; Mekonnen, B.; Zech, W.; Nemomissa, S.; Bekele, T.; Glaser, B. Phenolic compounds as unambiguous chemical markers for the identification of keystone plant species in the bale mountains, Ethiopia. Plants 2019, 8, 228. [Google Scholar] [CrossRef]
- Guendouze-Bouchefa, N.; Madani, K.; Chibane, M.; Boulekbache-Makhlouf, L.; Hauchard, D.; Kiendrebeogo, M.; Stévigny, C.; Okusa, P.N.; Duez, P. Phenolic compounds, antioxidant and antibacterial activities of three Ericaceae from Algeria. Ind. Crops Prod. 2015, 70, 459–466. [Google Scholar] [CrossRef]
- Chaowuttikul, C.; Palanuvej, C.; Ruangrungsi, N. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz. J. Pharm. Sci. 2020, 70, 459–466. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, A.E.; Azadniya, E.; Ramírez Moreno, E.; Morlock, G.E. Bioprofiling of Mexican Plectranthus amboinicus (Lour.) essential oil via planar chromatography–effect-directed analysis combined with direct analysis in real time high-resolution mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 344–350. [Google Scholar] [CrossRef]
- Nicolai, M.; Mota, J.; Fernandes, A.S.; Pereira, F.; Pereira, P.; Reis, C.P.; Velasco, M.V.R.; Baby, A.R.; Rosado, C.; Rijo, P. Assessment of the Potential Skin Application of Plectranthus ecklonii Benth. Pharmaceuticals 2020, 13, 120. [Google Scholar] [CrossRef]
- Ramborger, B.P.; Paz, M.E.G.; Denardin, E.L.G.; Soares, J.J.; Roehrs, R. A review of anatomical, physiological, biological characteristics and uses of Plectranthus neochilus. Ciênc. Nat. 2020, 42, 12. [Google Scholar] [CrossRef]
- Rojas, E.R.; Billings, G.; Odermatt, P.D.; Auer, G.K.; Zhu, L.; Miguel, A.; Chang, F.; Weibel, D.B.; Theriot, J.A.; Huang, K.C. The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature 2018, 559, 617–621. [Google Scholar] [CrossRef]
- Almeida, C.F.C.B.R.; Cabral, D.L.V.; Almeida, C.C.B.R.; Amorim, E.L.C.; Araújo, J.M.; Albuquerque, U.P. Comparative study of the antimicrobial activity of native and exotic plants from the Caatinga and Atlantic Forest selected through an ethnobotanical survey. Pharm. Biol. 2012, 50, 201–207. [Google Scholar]
- Oliveira, F.F.M.; Torres, A.F.; Gonçalves, T.B.; Santiago, G.M.P.; Carvalho, C.B.M.; Aguiar, M.B.; Camara, L.M.C.; Rabenhorst, S.H.; Martins, A.M.C.; Valença-Junior, J.T.; et al. Efficacy of Plectranthus amboinicus (Lour.) Spreng in a murine model of methicillin-resistant Staphylococcus aureus skin abscesses. Evid. Based Complement. Altern. Med. 2013, 2013, 291592. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.; Khaled, J.M.; El-Gamal, A.A.; Noman, O.M.; Kumar, A.; Alajmi, M.F.; Al-Rehaily, A.J.; Al-Said, M.S. Comparative evaluation of cytotoxic, antimicrobial and antioxidant activities of the crude extracts of three Plectranthus species grown in Saudi Arabia. Saudi Pharm. J. 2019, 27, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Costa, P.A.; Ribon, A.O.; Purgato, G.A.; Gaspar, D.M.; Diaz, M.A. Plant extracts display synergism with different classes of antibiotics. An. Acad. Bras. Ciênc. 2019, 91, e20180117. [Google Scholar] [CrossRef]
- Nascimento, F.R.; Albuquerque, K.R.; Oliveira, M.R.; Pizziolo, V.R.; Brasileiro, B.G.; Diaz, G.; Diaz, M.A. Antibiotic activity of Plectranthus ornatus Codd., a Traditional Medicinal Plant. An. Acad. Bras. Ciênc. 2017, 89, 2461–2469. [Google Scholar] [CrossRef]
- Araújo, S.G.; Alves, L.F.; Pinto, M.E.A.; Oliveira, G.T.; Siqueira, E.P.; Ribeiro, R.I.; Ferreira, J.M.S.; Lima, L.A. Volatile compounds of Lamiaceae exhibit a synergistic antibacterial activity with streptomycin. Braz. J. Microbiol. 2014, 45, 1341–1347. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Alves, E.F.; Santos, B.S.; Sobral, C.E.S.; Ferreira, J.V.D.A.; Lavor, A.K.L.S.; Figueredo, F.G.; Lima, L.F.; Santos, F.A.V.; Peixoto, F.S.N.; et al. Biological activities and chemical characterization of Cordia verbenacea DC. as tool to validate the ethnobiological usage. Evid. Based Complement. Altern. Med. 2013, 2013, 164215. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- Gomes, J.M.; Terto, M.V.C.; Santos, S.G.; Silva, M.S.; Tavares, J.F. Seasonal variations of polyphenols content, sun protection factor and antioxidant activity of two Lamiaceae species. Pharmaceutics 2021, 13, 110. [Google Scholar] [CrossRef]
- Rai, V.; Pai, V.R.; Kedilaya, P. A preliminary evaluation of anticancer and antioxidant potential of two traditional medicinal plants from Lamiaceae-Pogostemon heyneanus and Plectranthus amboinicus. J. Appl. Pharm. Sci. 2016, 6, 73–78. [Google Scholar] [CrossRef]
- Alves, F.A.R.; Morais, S.M.D.; Sobrinho, A.C.N.; Silva, I.N.G.; Martins, C.G.; Silva, A.A.S.; Fontenelle, R.O.S. Chemical composition, antioxidant and antifungal activities of essential oils and extracts from Plectranthus spp. against dermatophytes fungi. Rev. Bras. Saúde Prod. Anim. 2018, 19, 105–115. [Google Scholar] [CrossRef][Green Version]
- Medrado, H.H.; Santos, E.O.D.; Ribeiro, E.M.; David, J.M.; David, J.P.; Araújo, J.F.; Vale, A.E.; Bellintani, M.C.; Brandão, H.N.; Meira, P.R. Rosmarinic and cinnamic acid derivatives of in vitro tissue culture of Plectranthus ornatus: Overproduction and correlation with antioxidant activities. J. Braz. Chem. Soc. 2017, 28, 505–511. [Google Scholar] [CrossRef]
- Hasibuan, P.A.Z.; Sitorus, P.; Satria, D.; Sibuea, R.D. Antioxidant properties and cytotoxic activity of ethyl acetate fraction of Plectranthus amboinicus (Lour.) Spreng. leaves on HeLa and T47D cell lines. IJCC 2019, 10, 37–45. [Google Scholar] [CrossRef][Green Version]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar]
- Santos, J.F.S.; Tintino, S.R.; Freitas, T.S.; Campina, F.F.; Menezes, I.R.A.; Siqueira-Júnior, J.P.; Coutinho, H.D.M.; Cunha, F.A. In vitro e in silico evaluation of the inhibition of Staphylococcus aureus efflux pumps by caffeic and gallic acid. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 22–28. [Google Scholar] [CrossRef]
- Jhanji, R.; Singh, A.; Kumar, A. Antibacterial potential of selected phytomolecules: An experimental study. Microbiol. Immunol. 2021, 65, 325–332. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of phenolic acid antimicrobial and antioxidant structure–property relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Lal, A.F.; Singh, S.; Franco, F.C.; Bhatia, S. Potential of polyphenols in curbing quorum sensing and biofilm formation in Gram-negative pathogens. As. Pac. J. Trop. Biomed. 2021, 11, 231. [Google Scholar]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Matos, F.J.A. Introdução A Fitoquímica Experimenta, 2nd ed; Edições UFC: Fortaleza, Brazil, 1997. [Google Scholar]
- Simões, C.M.O.; Schenkel, E.P.; Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Farmacognosia: Da Planta ao Medicamento, 6th ed.; Editora da UFRGS: Porto Alegre, Brazil, 2007; 1104p. [Google Scholar]
- IHC. Validation of Analytical Procedures: Text and Methodology; Blackwell Publishing Ltd.: Oxford, UK, 2005. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Coutinho, H.D.M.; Costa, J.G.M.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, H.J.; Kang, S.S. Alatemin, cassiaside and rubrofusarin gentiobioside, radical scavenging principles from the seeds of Cassia tora on 1,1-diphenyl-2-picrylhydrazyl(DPPH) radical. Arch. Pharm. Res. 1994, 17, 462–466. [Google Scholar] [CrossRef] [PubMed]



| Samples | Secondary Metabolites | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | PT | TT | LA | AC | F | FV | FVN | XT | CH | AR | CQ | AL | SP | |
| EELPa | + | + | + | - | + | + | - | + | + | - | + | - | - | + |
| EELPb | + | + | + | - | - | + | - | + | + | + | + | - | - | + |
| EELPo | + | + | + | - | - | + | - | + | + | + | + | - | - | + |
| Compounds | EELPa | EELPb | EELPo | |||
|---|---|---|---|---|---|---|
| mg/g | % | mg/g | % | mg/g | % | |
| Gallic Acid | 1.13 ± 0.04 a | 0.11 | 0.74 ± 0.03 a | 0.07 | 1.85 ± 0.02 a | 0.18 |
| Catechin | 1.89 ± 0.02 b | 0.18 | 1.91 ± 0.01 b | 0.19 | 3.04 ± 0.01 b | 0.30 |
| Chlorogenic Acid | 4.06 ± 0.03 c | 0.40 | 3.87 ± 0.02 c | 0.38 | 4.51 ± 0.03 c | 0.45 |
| Caffeic Acid | 2.15 ± 0.01 d | 0.21 | 5.01 ± 0.01 d | 0.50 | 9.76 ± 0.05 d | 0.97 |
| Rutin | 1.09 ± 0.03 a | 0.10 | 0.26 ± 0.01 e | 0.02 | 3.12 ± 0.01 b | 0.31 |
| Quercitrin | 1.83 ± 0.02 b | 0.18 | 1.83 ± 0.03 bf | 0.18 | 4.93 ± 0.03 e | 0.49 |
| Isoquercitrin | - | - | 0.91 ± 0.04 g | 0.09 | 4.27 ±0.02 c | 0.42 |
| Quercetin | 1.65 ± 0.01 e | 0.16 | 2.34 ± 0.01 h | 0.23 | 2.63 ± 0.01 f | 0.26 |
| Kaempferol | 0.28 ± 0.01 f | 0.02 | 1.76 ± 0.05 f | 0.17 | 2.45 ± 0.04 f | 0.24 |
| Kaempferol glycoside | 1.81 ± 0.02 b | 0.18 | 0.85 ± 0.02 g | 0.08 | 1.36 ± 0.03 g | 0.13 |
| Total | 15.89 ± 0.14 a | 1.37 | 19.48 ± 0.22 b | 1.91 | 37.92 ± 0.25 c | 3.75 |
| Microorganism | MIC (µg/mL) | ||
|---|---|---|---|
| EELPa | EELPb | EELPo | |
| E. coli ATCC 25922 | 64 | 128 | 128 |
| P. vulgaris ATCC 13315 | 128 | 128 | 128 |
| B. cereus ATCC 33018 | 256 | 256 | 256 |
| P. aeruginosa ATCC 15442 | 256 | 128 | 256 |
| S. aureus ATCC 12692 | 16 | 16 | 32 |
| S. aureus SA 358 | 16 | 128 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, F.F.G.; Boligon, A.A.; Menezes, I.R.A.; Galvão-Rodrigues, F.F.; Salazas, G.J.T.; Nonato, C.F.A.; Braga, N.T.T.M.; Correia, F.M.A.; Caldas, G.F.R.; Coutinho, H.D.M.; et al. HPLC/DAD, Antibacterial and Antioxidant Activities of Plectranthus Species (Lamiaceae) Combined with the Chemometric Calculations. Molecules 2021, 26, 7665. https://doi.org/10.3390/molecules26247665
Rodrigues FFG, Boligon AA, Menezes IRA, Galvão-Rodrigues FF, Salazas GJT, Nonato CFA, Braga NTTM, Correia FMA, Caldas GFR, Coutinho HDM, et al. HPLC/DAD, Antibacterial and Antioxidant Activities of Plectranthus Species (Lamiaceae) Combined with the Chemometric Calculations. Molecules. 2021; 26(24):7665. https://doi.org/10.3390/molecules26247665
Chicago/Turabian StyleRodrigues, Fabíola F. G., Aline A. Boligon, Irwin R. A. Menezes, Fábio F. Galvão-Rodrigues, Gerson J. T. Salazas, Carla F. A. Nonato, Nara T. T. M. Braga, Fabrina M. A. Correia, Germana F. R. Caldas, Henrique D. M. Coutinho, and et al. 2021. "HPLC/DAD, Antibacterial and Antioxidant Activities of Plectranthus Species (Lamiaceae) Combined with the Chemometric Calculations" Molecules 26, no. 24: 7665. https://doi.org/10.3390/molecules26247665
APA StyleRodrigues, F. F. G., Boligon, A. A., Menezes, I. R. A., Galvão-Rodrigues, F. F., Salazas, G. J. T., Nonato, C. F. A., Braga, N. T. T. M., Correia, F. M. A., Caldas, G. F. R., Coutinho, H. D. M., Siyadatpanah, A., Kim, B., Costa, J. G. M., & Barros, A. R. C. (2021). HPLC/DAD, Antibacterial and Antioxidant Activities of Plectranthus Species (Lamiaceae) Combined with the Chemometric Calculations. Molecules, 26(24), 7665. https://doi.org/10.3390/molecules26247665








