Secondary Metabolites Found among the Species Trattinnickia rhoifolia Willd
Abstract
1. Introduction
2. Taxonomy of the Burseraceae Family
3. Chemical Characteristics of Burseraceae
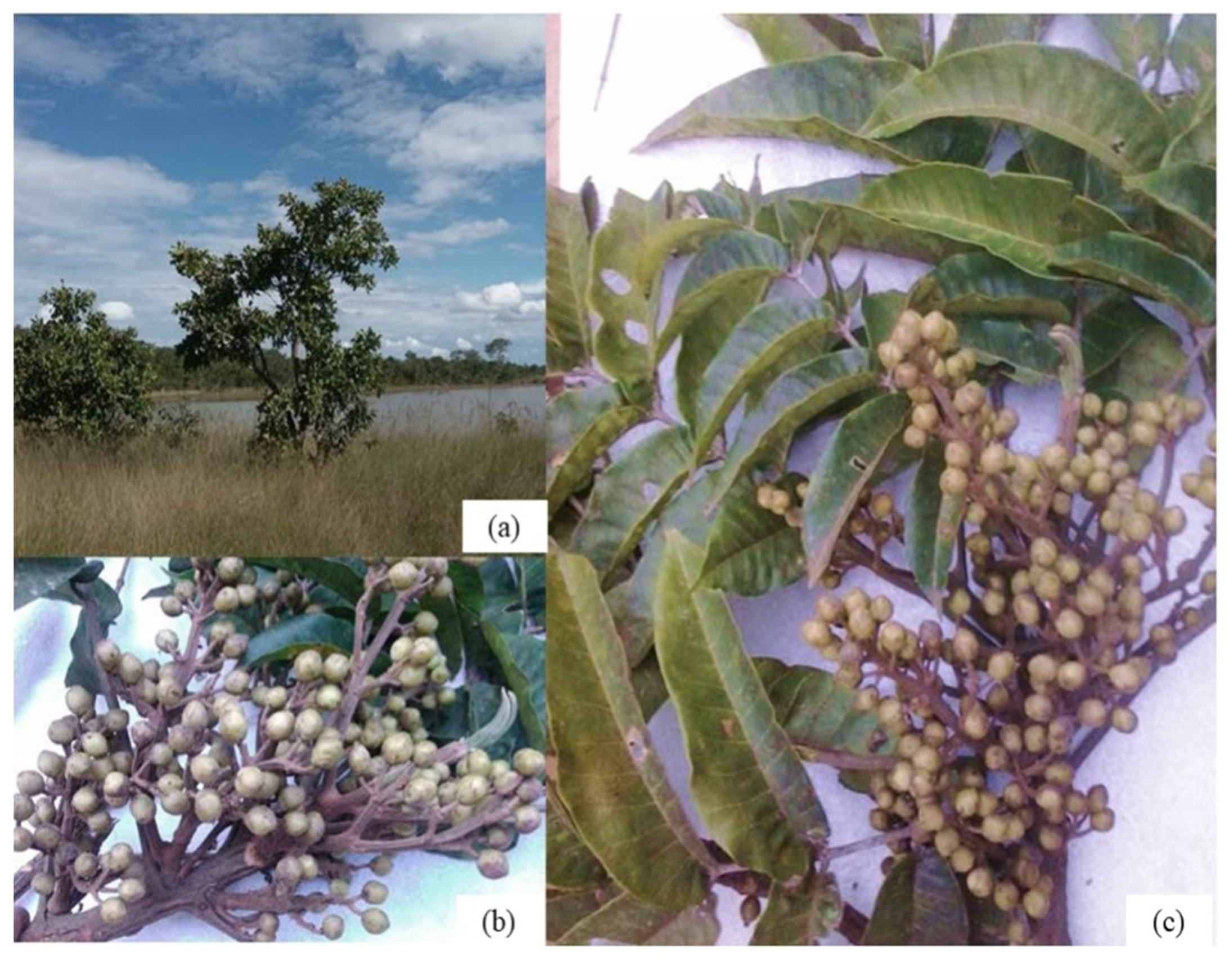
Characteristics of the Species Trattinnickia Rhoifolia Willd
4. Potential Use of the Species Trattinnickia rhoifolia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organização Mundial da Saúde (OMS). Doenças Tropicais Negligenciadas: Tratamento de Mais de um Bilhão de Pessoas em 2017. [atualizado em 2017 em 08 de setembro; citou 2017 em 9 de outubro]. Available online: http://www.who.int/neglected_diseases/news/treating-morethan-one-billion-people-2017/en/ (accessed on 24 November 2021).
- Bolzani, V.S.; Barreiro, E.J.; Viegas, J.R.C. Os produtos naturais e a química medicinal moderna. Q. Nova 2009, 29, 326–337. [Google Scholar]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifúngis, propriedades fitostoxixixixixicas e inseticidas de óleo essencial isolados dos acutidens origanum turcos e seus três componentes, carvacrol, timol e p-cymene. Tecnol. Bioresour. 2008, 99, 89–94. [Google Scholar]
- Morel, C.M. Inovação em saúde e doenças negligenciadas. CSP 2006, 22, 1522–1523. [Google Scholar] [CrossRef]
- Reis, L.C.; Brito, M.E.F.; Souza, M.A.; Pereira, V.R.A. Mecanismos imunológicos na resposta celular e humoral na leishmaniose tegumentar americana. J. Trop. Pathol. 2006, 35, 103–115. [Google Scholar] [CrossRef][Green Version]
- Gil, E.S.; Paula, J.R.; Nascimento, F.R.F.; Bezerra, J.C.B. Produtos naturais com potencial leishmanicida. Rev. Ciênc. Farm. Básica Apl. 2008, 29, 223–230. [Google Scholar]
- Thulin, M.; Beier, B.A.; Razafimandimbison, S.G.; Banks, H.I. Ambilobea, um novo gênero de Madagascar, a posição de Aucoumea, e comenta sobre a classificação tribal da família incenso e mirra (Burseraceae). J. Nórdico Botânica. 2008, 26, 218–229. [Google Scholar]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática; Instituto Plantarum de Estudos da Flora Ltd.: Nova Odessa, Bazil, 2005. [Google Scholar]
- Noguera, B.; Díaz, E.; García, M.V.; SanFeliciano, A.; López-Perezc, J.L.; Israelb, A. Anti-inflammatory activity of leaf extract and fractions of Bursera simaruba (L.) Sarg (Burseraceae). J. Ethnopharmacol. 2004, 92, 129–133. [Google Scholar] [CrossRef]
- Giraldo, M.C.R.; Susunaga, C.M.S. Atividade Antimicrobiana Presente em Partes Aéreas da Espécie Birsera Simaoruba e Graveolens de Birsera (Burseraceas) Contra Microrganismos Como: Tumefaciens Agrobacterium, Erwinia CAROTOVORA, Fusarium Oxysporum, Trichoderma Viride e Botry Cinetisrea. Doctoral Thesis, Universidade de Santa-fé de Bogotá, Bogotá, Colombia, 2000. [Google Scholar]
- Rüdiger, A.L. Estudo Fitoquímico e Citotóxico de Oleorresinas de Burseraceae. Doctoral Thesis, Universidade Federal do Alagoas, Maceio, Brazil, 2012. [Google Scholar]
- Langenheim, J.H. Resinas Vegetais: Química, Evolução, Ecologia e Etnobotânica; Timber Press: Cambridge, OR, USA, 2003. [Google Scholar]
- Pereira, I.F.; Costa APFd Srbek-Araujo, A.C.; Guimarães, L.J.; Merencio, A.F.; Silva, A.G. A dispersão dos diásporos do protium icicariba (Burseraceae) um sistema em rede ou multifatorial? J. Chem. Ecol. 2020, 46, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Passero, L.F.; Laurenti, M.D.; Santos-Gomes, G.; Soares Campos, B.L.; Sartorelli, P.; Lago, J.H. Plants used in traditional medicine: Extracts and secondary metabolites exhibiting antileishmanial activity. Curr. Clin. Pharmacol. 2014, 9, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Duwiejua, M. Isolamento da picopoligramaina da resina de Bursera simaruba. Planta Med. 1993, 59, 10–35. [Google Scholar]
- Mesa, M.E.S.; Peñaranda, L.M.O. Avaliação da Atividade Antimicrobiana dos Extratos de Partes Aéreas da Espécie Bursera Simaruba e Bursera Graveolens, Contra Alguns Microrganismos Patogênicos. Ph.D. Thesis, Universidade de Bogotá, Bogotá, Colombia, 2003. [Google Scholar]
- Khalid, S.A.; do Burseraceae, Q. (Eds.) Química e Taxonomia Química dos Rutales; Acadêmico: London, UK, 2003. [Google Scholar]
- Rüdigera, A.L.S.; Siani, A.C.; Veiga, V. Plant revise a química e a farmacologia do gênero américa do Sul protium. f. (Burseraceae). Comentários Farmacogn. 2007, 1, 93–104. [Google Scholar]
- Khalid, S.A. Química do Burseraceae. In Química e Taxonomia QuíMica dos Rutales; Waterman, P.G., do Grundon, M.F., Eds.; Acadêmico: London, UK, 1983; pp. 281–297. [Google Scholar]
- Ortega, E.A.P. Contribuição Para o Estudo Fitoquímico de Bursera Simaruba (L.) Sarg. Ph.D. Thesis, Universidade de Bogotá, Bogotá, Colombia, 2002. [Google Scholar]
- Braz Filho, R. Contribuição Fitoquímica para o Desenvolvimento de um País Emergente, Quim. Nova. 2020, 33, 229–239. [Google Scholar]
- Laffitte, E.; Revuz, J. Talidomida: Uma droga antiga com novas aplicações clínicas. Opinião Espec. Sobre Segurança Drog. 2000, 127, 603–613. [Google Scholar]
- Aragão, G.F.; Carneiro, L.M.V.; Junior, A.P.F.; Vieira LCBandeira, P.N.; Lemos, T.L.G. Um possível mecanismo para efeitos antidepressivos de alfa e beta-amilina de Protium heptaphyllum (aubl.). Farmacol. Bioquímica E Comport. 2006, 85, 27–35. [Google Scholar]
- Melnikova, N.; Malygina, D.; Balakireva, A.; Peretyagin, P.; Revin, V.; Devyataeva, A.; Malafeeva, K.; Revin, V.O. Efeito de Difosfato de Betulin em Curativos de Feridas de NPs de Celulose Bacteriana-ZnO na Agregação de Plaquetas e a Atividade de Oxidoreductases Reguladas por NAD(P)+/NAD(P)H-Balance in Burns on Rats. Moléculas 2021, 26, 5478. [Google Scholar]
- Camargo, J.R. Isolamento de Ingredientes Biologicamente Ativos Presentes em Espécies Colombianas da Família Burseraceae. Ph.D. Thesis, Pontifícia Universidade Javeriana, Bogotá, Colombia, 2000. [Google Scholar]
- Alvarenga, R.F.; Silva, D.R.C.; Maria, E. Síntese de Indicuminas: Análise Retrossintética Para a Obtenção de Cumarinolignoides. In Anais do Congresso Fluminense de Inovação Tecnológica; UENF: Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Otuki, M.F.; Vieira-Lima, F.; Malheiros, A.; Yunes, R.A.; Calixto, J.B. Topical anti-inflammatory effects of the ether extract from Protium kleinii and α-amyrin pentacyclic triterpene. Eur. J. Pharmacol. 2005, 507, 253–259. [Google Scholar] [CrossRef]
- Siani, A.C.; Nakamura, M.J.; Tappin, M.R.R.; Monteiro, S.S.; Guimarães, A.C.; Ramos, M.F.S. Composição química da Burseraceae sul-americana não volátil oleoresins e avaliação preliminar de solubilidade de sua mistura comercial. Análises Fitoquímicas 2012, 23, 529–539. [Google Scholar]
- Oliveira, L.M.; Queiroz, D.P.K.; Melo, L.E.S.; Marques, M.O.M.; Facanali, R.; Lima, M.P. Constituintes voláteis dos galhos de quatro espécies de Protium Ocorrentes na Flora da Reserva Ducke, Sci. Amazon. 2018, 7, 68–73. [Google Scholar]
- Do Brasil, F. Burseraceae in flora do Brasil 2020 em Construção; Jardim Botânico: Rio de Janeiro, Bazil, 2012. [Google Scholar]
- Di Stasi, L.C.; Hiruma-Lima, C.A. Plantas Medicinais na Amazônia e na Mata Atlântica; Editora Universidade Estadual Paulista: São Paulo, Bazil, 2002. [Google Scholar]
- Daly, D.C.; Melo, M.F. Quatro novas espécies de Trattinnickia da América do Sul. Estudos em Neotropical. Burseraceae XXII. Brittonia. 2017, 69, 1–11. [Google Scholar]
- David, K.; Shirley, C. Phytosterols benefícios para a saúde e preocupações potenciais: Uma revisão. Pesqui. Nutr. 2005, 413–428. [Google Scholar]
- Júnior, F.P.; Araújo, V.F. Plantas da Amazônia para produção cosmética: Uma abordagem química 60 espécies do extrativismo florestal não-madeireiro da Amazônia/Floriano. Projeto Non-Wood II PD 31/99. Rev. Unb. Brasília 2005, 2, 12–244. [Google Scholar]
- Zoghbi, M.G.B.; Cunha, E.V.L.; Filho, W.W. Óleo essencial de Protium unifoliolatum (Burseraceae). Acta Amaz. 1993, 23, 15–16. [Google Scholar] [CrossRef]
- Jing, Y.K.; Xia, L.J.; Chen, D.; Han, R.; Fan, Q.C.; Waxman, S. Boswellic acid acetate induz apoptose através de vias mediadas por caspase em células de leucemia mielóide. Ter. Do Câncer Molecular. 2005, 4, 381–388. [Google Scholar]
- Salazar, U.J.; Porcar, R.C. Componentes químicos das folhas de Trattinickia rhoifolia, Av. En Química 2010, 5, 63–65. [Google Scholar]
- Oliveira, F.A.; Chaves, M.H.; Almeida, F.R.C.; Lima, R.C.P., Jr.; Silva, R.M.; Maia, J.L.; Brito, G.A.C.C.; Santos, F.A.; Rao, V.S. Protective effect of α-and β-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J. Etnopharmacolology 2005, 98, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, D.E.; Onofre, S.B. Atividade antimicrobiana de resina de óleo produzida pela Copaiba Copaifera multijuga Hayne (Leguminosae), Rev Brasileira de Farmacognosia Braz. J. Pharmacogn. 2009, 19, 577–581. [Google Scholar]
- Lorenzi, H.; Matos, A.F.J. Plantas Medicinas no Brasil: Nativas e Exóticas; Instituto Plantarum: Nova Odessa, Bazil, 2002. [Google Scholar]
- Cervantes-García, E.; García-González, R.; Salazar-Schettino, P.M. Características gerais de Staphylococcus aureus. Rev. Lat. Patol. Clin. Med. Lab. 2014, 61, 28–40. [Google Scholar]
- Ziech, R.E.; Farias, L.D.; Balzan, C.; Ziech, M.F.; Heinzmann, B.M.; Lameira, O.A. Atividade antimicrobiana do oleorresina de copaíba (Copaifera reticulata) frente a Staphylococcus coagulase positiva isolados de casos de otite em cães. Pesq. Vet. Bras 2013, 33, 909–913. [Google Scholar] [CrossRef]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G.; Olaniru, O.B. A review on dietary phytosterols: Their occurrence, metabolism and health benefit. Asian. J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Gutmann, H.; Bruggisser, R.; Schaffner, W.; Bugman, K.; Botomino, A.; Drewe, J. Transporte de amentoflavone através da barreira do cérebro de sangue in vitro. Planta Med. 2002, 68, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Saponara, R.; Bosisio, E. Inibição de adipocyte de rato cAMP phosphodiesterase por biflavonas de Ginkgo biloba L. J. Nat. Prod. 1998, 2, 98–100. [Google Scholar]
- Banerjee, T.; Valacchi, G.; Ziboh, Z. Inibição da expressão ciclooxygenase-2 induzida pela TNFa por amentoflavone através da supressão da ativação NF-kB em células A549. Mol. Cell. Bioquímica 2002, 238, 5–10. [Google Scholar]
- Zhu, L.; Ning, N.; Li, Y.; Zhang, Q.-F.; Xie, Y.-C.; Irshad, M.; Feng, X.; Tao, X.-J. Biatractylolide Modula PI3K-Akt-GSK3?-Caminhos dependentes para proteger contra danos celulares induzidos por glutamato nas células PC12 e SH-SY5Y. Med. Complementar Altern. Baseada Evid. 2017, 2007, 1291458. [Google Scholar]
- Bandeira, P.N.; Lemos, T.L.C.; Sônia, M.O.C.; Santos, H.S. Obtenção de derivados da mistura triterpenoídica amirina. Braz. J. Pharmacogn. 2007, 17, 204–208. [Google Scholar] [CrossRef]
- Mahato, S.B.; Nandy, A.K.; Roy, G. Triterpenóides. Fitoquímica 1992, 31, 2199–2249. [Google Scholar]
| Parts Used | Isolated or Characterized Constituents | Pharmacological Activities | ||
|---|---|---|---|---|
| Aerial | Sesquiterpenes: α-selinene (1), β-selinene (2), β-bisabolene (3) Monocyclic sesquiterpene: α-humulene (4) Bicyclic sesquiterpene: β-caryophyllene (5) | Anti-inflammatory, analgesic, anti-edemic, bactericide and insecticide [20,35,37,38,39] | ||
| Aerial oil-resin | Sesquiterpenes: β-bisabolene (3) | Fungicidal and antimicrobial tests show efficacy against Staphylococcus aureus [40,41] | ||
| Aerial leaves | Linear alkane: Eicosane (6); nonacosane (7) Alkane: octadecane (8), octacosane (9), nonacosane (7), tetrapentacontane (10) | Antiparasitic against strays of Leishmania maior, L. donovani, Trypanossoma brucei, cytotoxic activity against various types of tumor cells [33], healing [38,42], anti-inflammatory and analgesic, anti-edemic, anti-inflammatory, bactericide and insecticide, fungicide [38,39] and antimicrobial [39,43] | ||
| Leaf | Biflavonoid: amentoflavane (11), Oxyphytosterols: 5α, 6α epoxy-β-sitosterol (12), 5β, 6 β-epoxy-beta-sitosterol (13) | Indicative of inhibition of the adenosine receptors GABA-A and B, 5-hydroxytryptamide, central benzodiazepine, forskolin and inositol triphosphate, as well as MAO A and B enzymes [42]. Inhibition of these enzymes results in an antidepressant response [39] | ||
| Leaf | Biflavonoid: amentoflavone (11) | Inhibitory activity of cAMP phosphodiesterase, [39,41,44] antioxidant, antifungal and anti-inflammatory activities [45,46], as well as indicative anti-HIV activities [39,47] | ||
| Resin | Monoterpenes: α-pinene (14), camphene (15), sabinene (16), β-pinene (17), α- phellandrene (18), α-terpinene (19), p-cymene (18) (20), β-phellandrene (21), γ-terpinene (22), α-terpinolene (23), trans-α-di- dihydroterpineol (24), terpinen-4-ol (25), p-cymen- 8-ol (26), α-terpineol (27) | Anti-inflammatory, inhibition of nitric oxide production, in vitro antitumor [48,49] | ||
| Leaf | Fatty acids: pentadecanoic acid (28); nonadecanoic acid (29); methyl-11,14,17-eicosatrienoate ester (30) | Antioxidant, antifungal and anti-inflammatory [48] | ||
| Leaf | Sesquiterpenoid: biatractylolide (31) | Anti-tumor and antioxidant, reduction of AChE activity, improvement of brain and memory capacities [47] | ||
| Resin | Triterpenes: ursane (32), α-amrinone (33),α-amyrin (34), 3-epi-α-amyrin (35), 3α,16β-dihydroxyurs-12-ene (36), β-amrinone oleananes (37), β-amyrin (38), 3-epi-β-amyrin (39), 3α,16β-dihydroxyolean-12-ene (40), 3α-hydroxytyrucal-8,24-dien-21-oic tirucallan acid (41), 3α-hydroxytirucal-7,24-dien-21-oic (42) 3-oxotyrucal-8,24-dien-21-oic (43), dammarane dammarenediol-II (44), 3α,20(S)-dihydroxydamar-24-ene (45), 3β-phenylacetoxyurs-12-ene (46), 3β-phenylacetoxyolean-12-ene (47) and 3β,16β,11α-trihydroxyurs-12-ene (48) Monoterpene: 2(S*)-phenylacetoxy-4(R*)-p-mentha-1(7),5-diene (49) | anticancer, anti-inflammatory, antileprotic, antiviral, antibacterial, antifungal, antidiuretic, giardicide and inhibition of acetylcholinesterase enzymes [48,49] | ||
α-selinene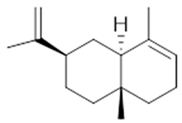 (1) | β-selinene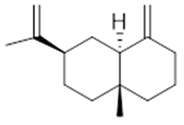 (2) | β-bisabolene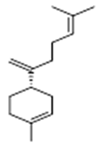 (3) | α-humulene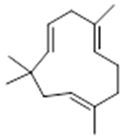 (4) | β-caryophyllene (5) |
Eicosane (6) | Nonacosane (7) | Octadecane (8) | ||
Octacosane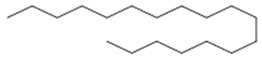 (9) | tetrapentacontane (10) | |||
amentoflavone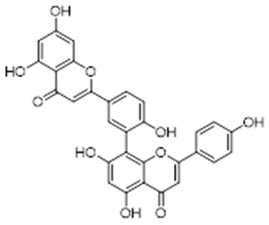 (11) | 5a, 5α, 6α epoxy-β-sitosterol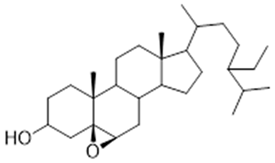 (12) | 5β, 6 β-epoxy-beta-sitosterol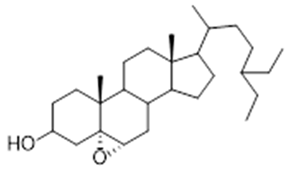 (13) | ||
α-pinene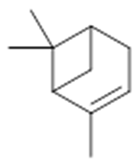 (14) | camphene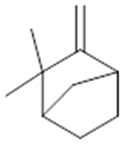 (15) | Sabinene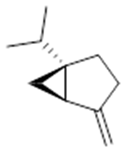 (16) | β-pinene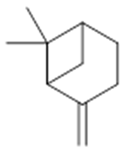 (17) | a-phellandrene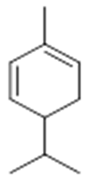 (18) |
α-terpinene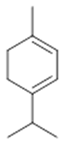 (19) | p-cymene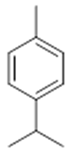 (20) | β-phellandrene (21) | γ-terpinene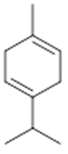 (22) | α-terpinolene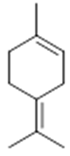 (23) |
trans-α-di-dihydroterpineol (24) | terpinen-4-ol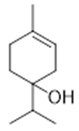 (25) | p-cymen-8-ol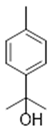 (26) | α-terpineol (27) | |
pentadecanoic acid (28) | Nonadecanoic acid (29) | |||
methyl-11,14,17-eicosatrienoate ester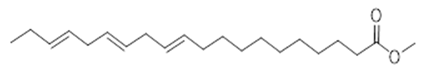 (30) | biatractylolide (31) | |||
ursane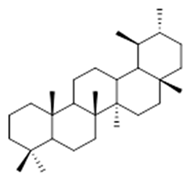 (32) | α-amrinone (33) | α-amyrin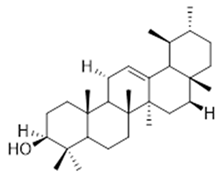 (34) | ||
3-epi-α-amyrin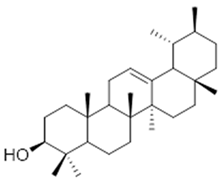 (35) | 3α,16β-dihydroxyurs-12-ene (36) | β-amrinone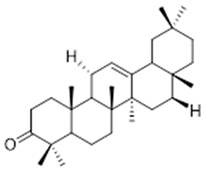 (37) | ||
β-amyrin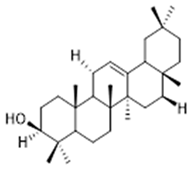 (38) | 3-epi-β-amyrin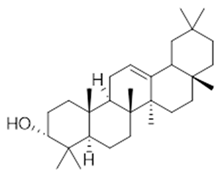 (39) | 3α-hydroxytyrucal-8,24-dien-21-oic (40) | ||
3α-hydroxytyrucal-7,24-dien-21-oic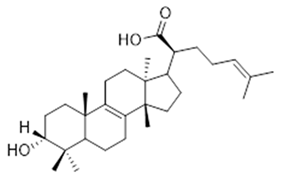 (41) | 3-oxotyrucal-7,24-dien-21-oic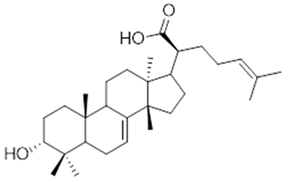 (42) | 3-oxotyrucal-8,24-dien-21-oic (43) | ||
dammarane dammarenediol-II (44) | 3α,20(S)-dihydroxydamar-24-ene (45) | 3β-phenylacetoxyurs-12-ene (46) | ||
3β-phenylacetoxyolean-12-ene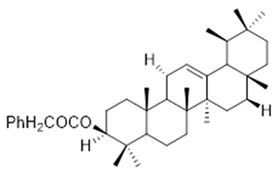 (47) | 3β,16β,11α-trihydroxyurs-12-ene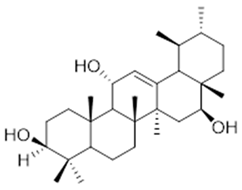 (48) | 2(S*)-phenylacetoxy-4(R*)-p-mentha-1(7),5-diene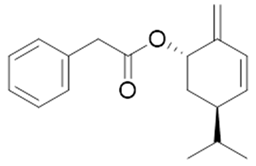 (49) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.A.; Ortíz, B.L.S.; de Carvalho Rocha Koga, R.; Sales, P.F.; da Cunha, D.B.; Guerra, A.L.M.; de Souza, G.C.; Carvalho, J.C.T. Secondary Metabolites Found among the Species Trattinnickia rhoifolia Willd. Molecules 2021, 26, 7661. https://doi.org/10.3390/molecules26247661
de Souza AA, Ortíz BLS, de Carvalho Rocha Koga R, Sales PF, da Cunha DB, Guerra ALM, de Souza GC, Carvalho JCT. Secondary Metabolites Found among the Species Trattinnickia rhoifolia Willd. Molecules. 2021; 26(24):7661. https://doi.org/10.3390/molecules26247661
Chicago/Turabian Stylede Souza, Agerdânio Andrade, Brenda Lorena Sánchez Ortíz, Rosemary de Carvalho Rocha Koga, Priscila Faimann Sales, Divino Bruno da Cunha, Ana Luiza Mantovaneli Guerra, Gisele Custódio de Souza, and José Carlos Tavares Carvalho. 2021. "Secondary Metabolites Found among the Species Trattinnickia rhoifolia Willd" Molecules 26, no. 24: 7661. https://doi.org/10.3390/molecules26247661
APA Stylede Souza, A. A., Ortíz, B. L. S., de Carvalho Rocha Koga, R., Sales, P. F., da Cunha, D. B., Guerra, A. L. M., de Souza, G. C., & Carvalho, J. C. T. (2021). Secondary Metabolites Found among the Species Trattinnickia rhoifolia Willd. Molecules, 26(24), 7661. https://doi.org/10.3390/molecules26247661








