Determination of 60 Migrant Substances in Plastic Food Contact Materials by Vortex-Assisted Liquid-Liquid Extraction and GC-Q-Orbitrap HRMS
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of the Target Analytes
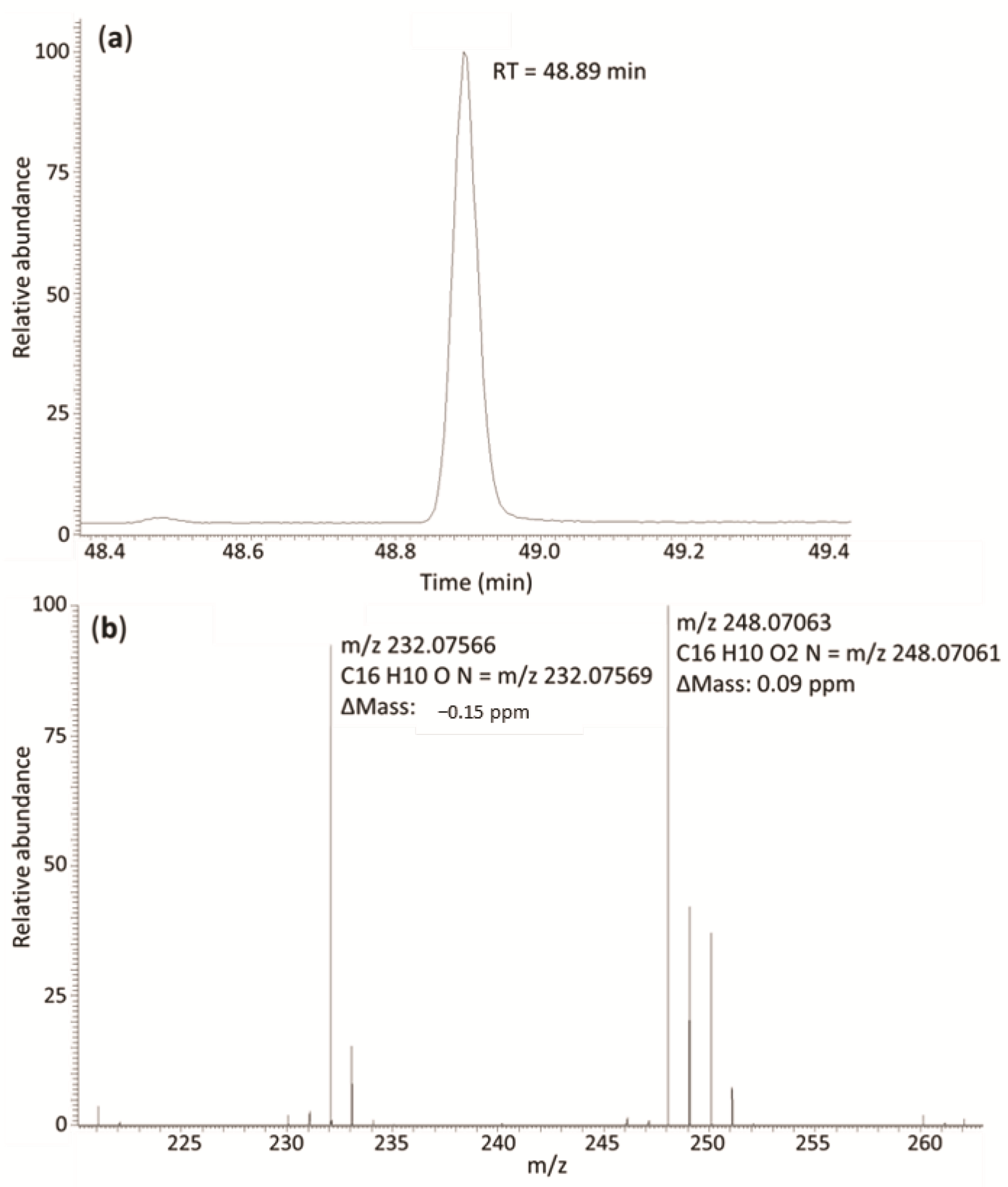
2.2. Selection of the Quantification and Confirmation Ions
2.3. Study of the Experimental Variables Involved in the VA-LLE Procedure
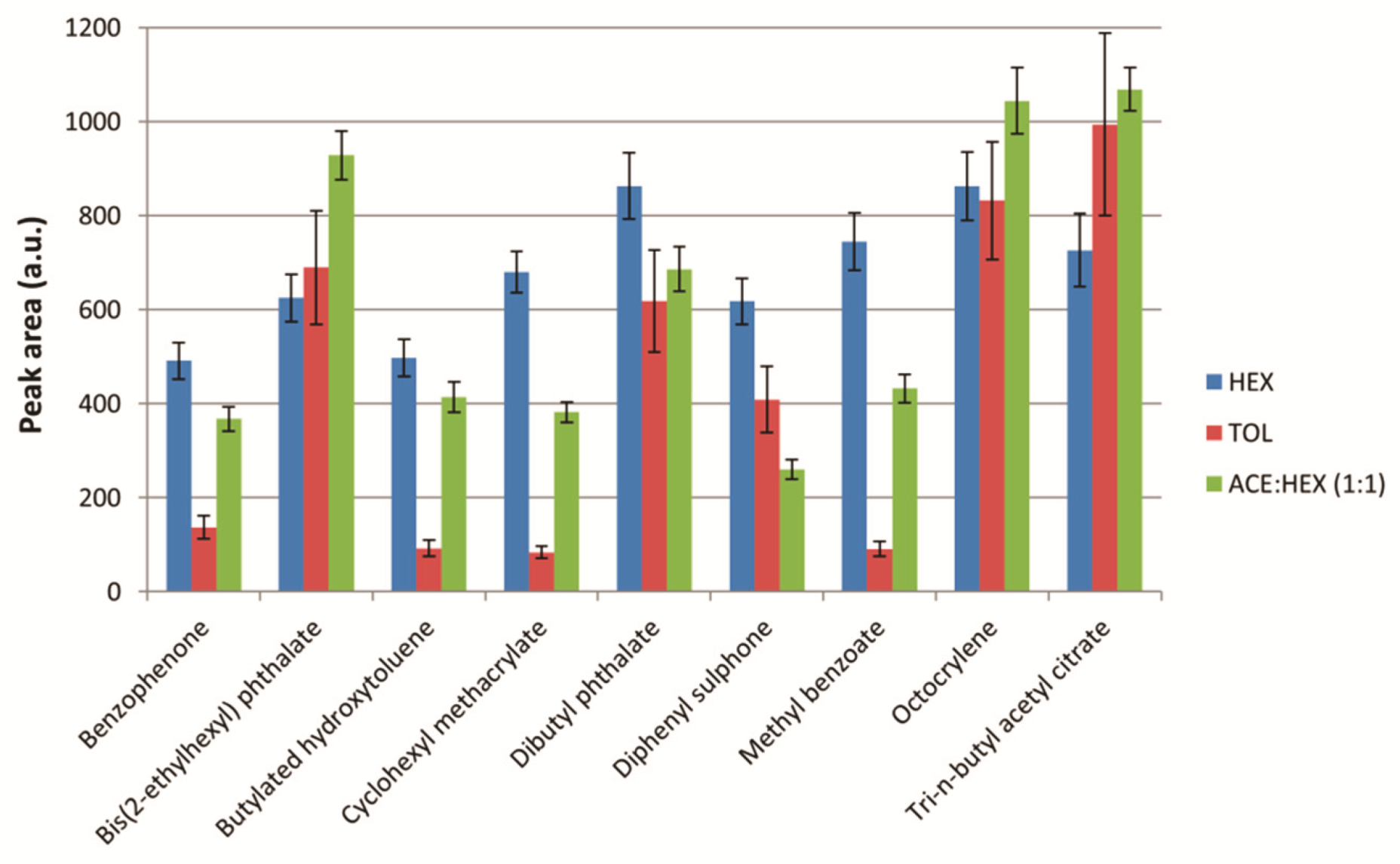
2.3.1. Extraction Solvent Composition
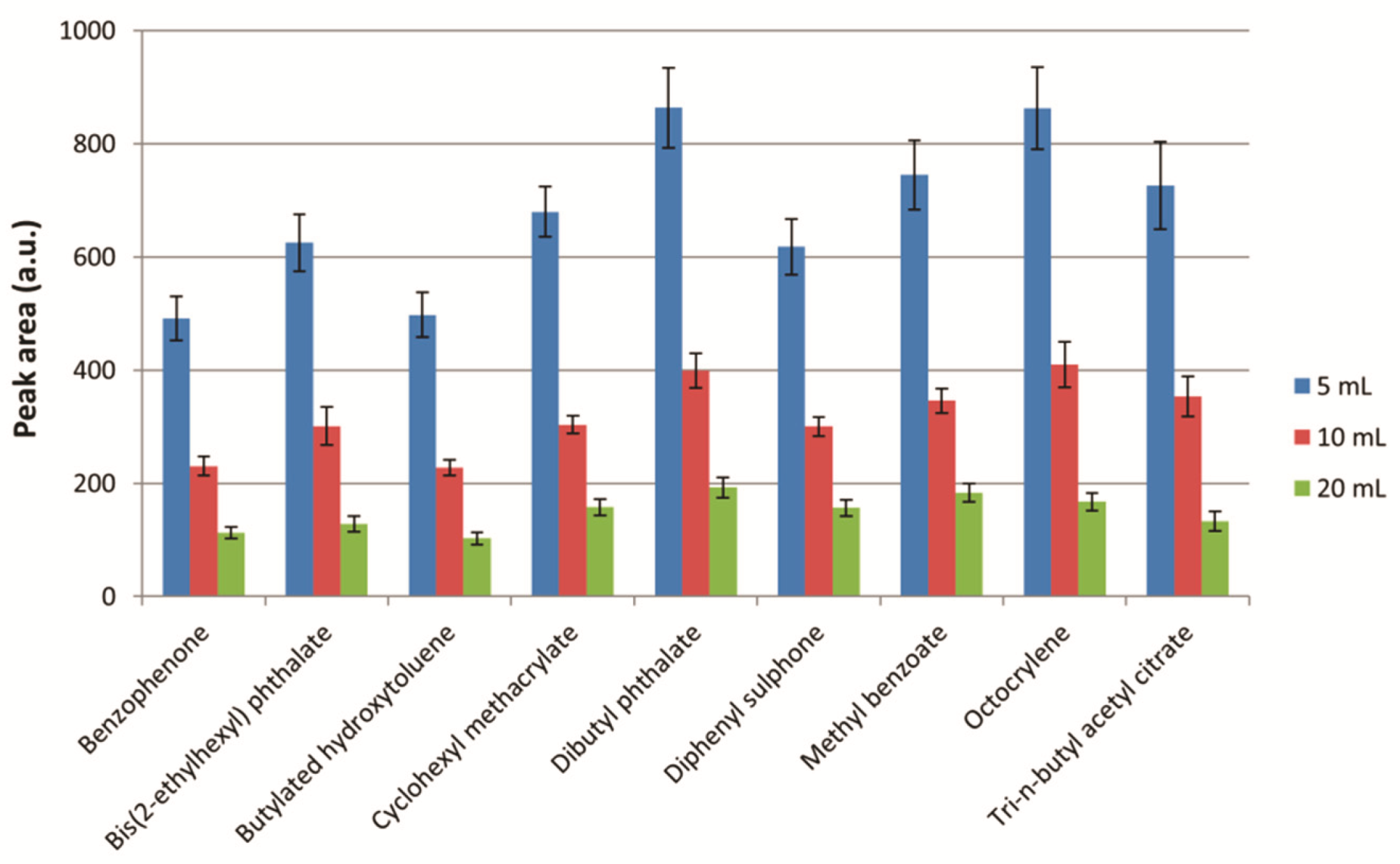
2.3.2. Extraction Solvent Volume
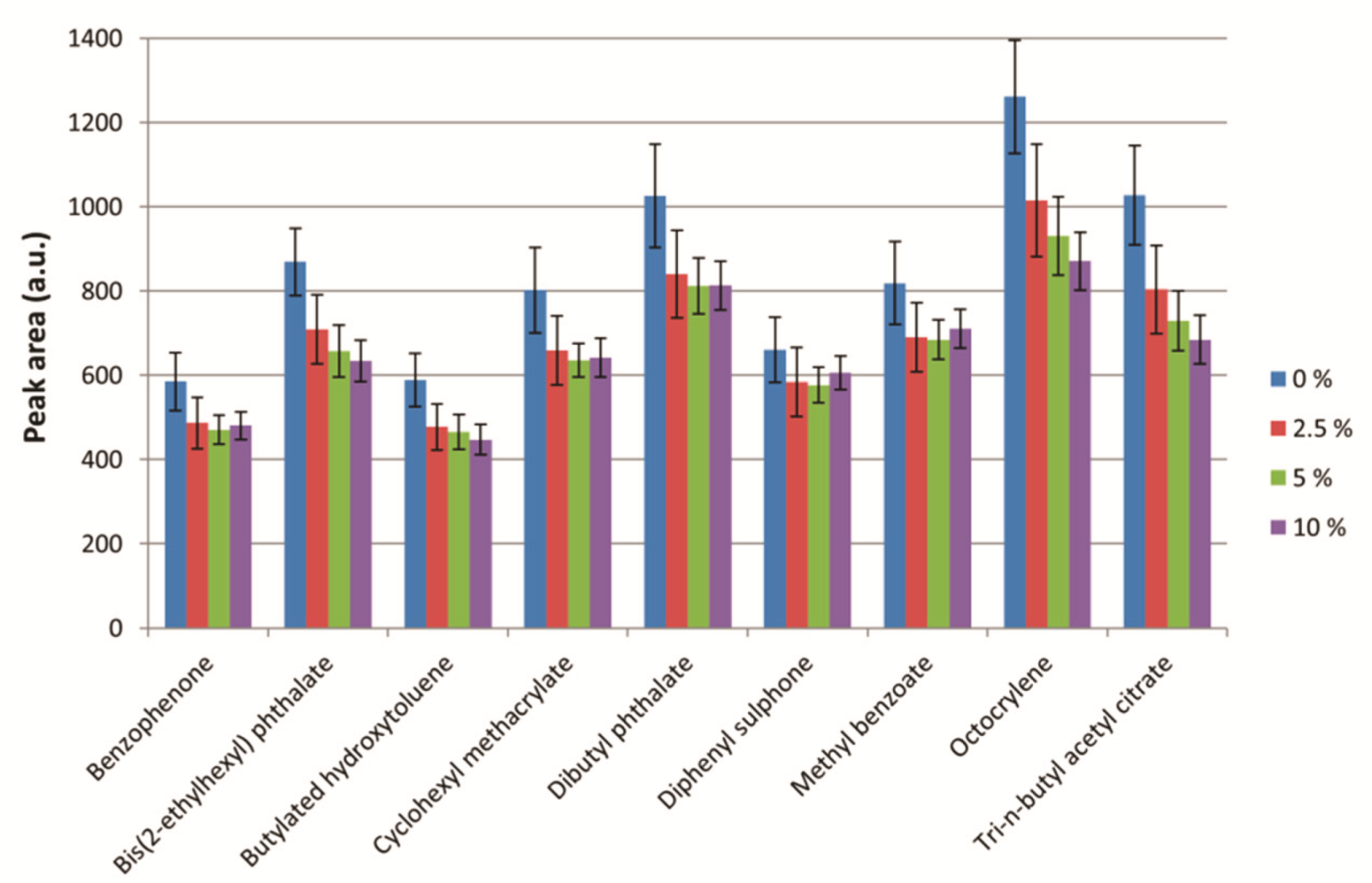
2.3.3. Ionic Strength
2.3.4. Extraction Time
2.4. Study of the Extraction Efficiency
2.5. Study of Stability of the Target Analytes
2.6. Method Validation
2.6.1. Study of Matrix Effects
2.7. Analysis of Field Samples
3. Materials and Methods
3.1. Reagents
3.2. Samples
3.3. Instruments
3.4. Proposed Method
3.4.1. Preparation of Samples. Migration Tests
3.4.2. Preparation of Standards
3.4.3. Vortex-Assisted Liquid–Liquid Extraction (VA-LLE)
3.4.4. GC-HRMS Analysis
3.4.5. Quantification and Confirmation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: http://data.europa.eu/eli/reg/2011/10/2020-09-23 (accessed on 2 December 2021).
- International Life Science Institute (ILSI) Europe. Guidance on Best Practices on the Risk Assessment of Non-Intentionally Added Substances (NIAS) in Food Contact Materials and Articles; ILSI Europe: Brussels, Belgium, 2015. [Google Scholar]
- Sanchís, Y.; Yusà, V.; Coscollà, C. Analytical strategies for organic food packaging contaminants. J. Chromatogr. A 2017, 1490, 22–46. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Gómez Ramos, M.J.; Bauer, A.; Fernández-Alba, A.R. An overview of non-targeted screening strategies based on high resolution accurate mass spectrometry for the identification of migrants coming from plastic food packaging materials. TrAC Trends Anal. Chem. 2019, 110, 191–203. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C. Analytical approaches for analysis of safety of modern food packaging: A review. Molecules 2020, 25, 752. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Lu, Z.; Hu, Y.; Xie, Z.; Li, G. Research progress on sample pretreatment methods for migrating substances from food contact materials. J. Sep. Sci. 2021, 44, 879–894. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. UPLC-ESI-Q-TOF-MSE and GC-MS identification and quantification of non-intentionally added substances coming from biodegradable food packaging. Anal. Bioanal. Chem. 2015, 407, 6781–6790. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Cimmino, S.; Silvestre, C.; Tadeo, J.L.; García-Valcárcel, A.I.; Fernández-Alba, A.R.; Hernando, M.D. Characterization of non-intentionally added substances (NIAS) and zinc oxide nanoparticle release from evaluation of new antimicrobial food contact materials by both LC-QTOF-MS, GC-QTOF-MS and ICP-MS. Anal. Methods 2016, 8, 7209–7216. [Google Scholar] [CrossRef]
- Carrero-Carralero, C.; Escobar-Arnanz, J.; Ros, M.; Jiménez-Falcao, S.; Sanz, M.L.; Ramos, L. An untargeted evaluation of the volatile and semi-volatile compounds migrating into food simulants from polypropylene food containers by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Talanta 2019, 195, 800–806. [Google Scholar] [CrossRef]
- Yusà, V.; López, A.; Dualde, P.; Pardo, O.; Fochi, I.; Pineda, A.; Coscollà, C. Analysis of unknowns in recycled LDPE plastic by LC-Orbitrap Tribrid HRMS using MS3 with an intelligent data acquisition mode. Microchem. J. 2020, 158, 105256. [Google Scholar] [CrossRef]
- Horodytska, O.; Cabanes, A.; Fullana, A. Non-intentionally added substances (NIAS) in recycled plastics. Chemosphere 2020, 251, 126373. [Google Scholar] [CrossRef]
- Yusà, V.; López, A.; Dualde, P.; Pardo, O.; Fochi, I.; Miralles, P.; Coscollà, C. Identification of 24 unknown substances (IAS/NIAS) from food contact polycarbonate by LC-Orbitrap Tribrid HRMS-DDMS3: Safety assessment. Int. J. Anal. Chem. 2021, 2021, 6654611. [Google Scholar] [CrossRef]
- Miralles, P.; López, A.; Dualde, P.; Coscollà, C.; Yusà, V. LC-Orbitrap Tribrid high-resolution MS using data dependent-tandem mass spectrometry with triple stage fragmentation as a screening tool to perform identification and risk assessment of unknown substances in food contact epoxy resin. J. Sep. Sci. 2021, 44, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Yusà, V.; Pineda, A.; Coscollà, C. A fast and automated strategy for the identification and risk assessment of unknown substances (IAS/NIAS) in plastic food contact materials by GC-Q-Orbitrap HRMS: Recycled LDPE as a proof-of-concept. Toxics 2021, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Bo, H.; Duan, W.; Jia, H.; Chen, R.; Ma, Y.; Ai, L. Determination of 46 plasticizers in food contact polyvinyl chloride packaging materials and their migration into food simulants by gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2011, 29, 42–48. [Google Scholar] [CrossRef]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal. Bioanal. Chem. 2018, 410, 3789–3803. [Google Scholar] [CrossRef] [PubMed]
- García Ibarra, V.; Sendón, R.; García-Fonte, X.X.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Migration studies of butylated hydroxytoluene, tributyl acetylcitrate and dibutyl phthalate into food simulants. J. Sci. Food Agric. 2019, 99, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, T.; Bogdanovic, D.; Kostic, I.; Kocic, G.; Nikolic, G.; Pavlovic, R. Phthalates leaching from plastic food and pharmaceutical contact materials by FTIR and GC-MS. Environ. Sci. Pollut. Res. 2021, 28, 31380–31390. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Chen, Z.; Liang, F.; Chen, X.; Wu, S.; Li, Y.; Sun, X. Simultaneous determination of migration amounts of antioxidants and ultraviolet absorbents by high performance liquid chromatography in food contact materials. Chin. J. Chromatogr. 2014, 32, 616–622. [Google Scholar] [CrossRef][Green Version]
- Qiu, Y.; Li, G.; Long, M.; Li, Y.; Xia, Z. Rapid determination of 13 ultraviolet absorbents in plastic food contact materials by ultra-performance convergence chromatography. Chin. J. Chromatogr. 2019, 37, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Zhao, X.; Chi, H.; Gao, X.; Zhou, M.; Liu, W. Simultaneous determination of ten benzotriazole ultraviolet stabilizers in food contact plastic materials by solid phase extraction and ultra performance liquid chromatography with tandem mass spectrometry. Chin. J. Chromatogr. 2015, 33, 571–576. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Lin, J.; Xie, T.; Chu, N. Simulatenous determination of the specific migration amounts of light stabilizers and antioxidants in plastic materials in contact with foodstuffs by ultra-high performance liquid chromatography/orbitrap high resolution mass spectrometry. Chin. J. Chromatogr. 2017, 35, 509–519. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Rose, M.; Charlton, C. 4-Nonylphenol (NP) in food contact materials: Analytical methodology and occurrence. Food Addit. Contam. 2008, 25, 364–372. [Google Scholar] [CrossRef]
- Oca, M.L.; Ortiz, M.C.; Herrero, A.; Sarabia, L.A. Optimization of a GC/MS procedure that uses parallel factor analysis for the determination of bisphenols and their diglycidyl ethers after migration from polycarbonate tableware. Talanta 2013, 106, 266–280. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, G.; Long, M.; Ruan, Y.; Tan, C.; Zhang, L.; Xia, Z. Determination of 11 kinds of bisphenols in plastic food contact materials by ultra-performance convergence chromatography. Chin. J. Chromatogr. 2020, 48, 255–261. [Google Scholar]
- Bignardi, C.; Cavazza, A.; Lagana, C.; Salvadeo, P.; Corradini, C. UHPLC-high-resolution mass spectrometry determination of bisphenol A and plastic additives released by polycarbonate tableware: Influence of ageing and surface damage. Anal. Bioanal. Chem. 2015, 407, 7917–7924. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Xiao, D.; Liang, F.; Chen, Z.; Chen, X.; Sun, X.; Li, Y. Determination of migration of 25 primary aromatic amines from food contact plastic materials by gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2013, 31, 46–52. [Google Scholar] [CrossRef][Green Version]
- Mortensen, S.K.; Trier, X.T.; Foverskov, A.; Petersen, J.H. Specific determination of 20 primary aromatic amines in aqueous food simulants by liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. A 2005, 1091, 40–50. [Google Scholar] [CrossRef]
- Sanchís, Y.; Coscollà, C.; Roca, M.; Yusà, V. Target analysis of primary aromatic amines combined with a comprehensive screening of migrating substances in kitchen utensils by liquid chromatography-high resolution mass spectrometry. Talanta 2015, 138, 290–297. [Google Scholar] [CrossRef]
- Sanches Silva, A.; Sendón García, R.; Cooper, I.; Franz, R.; Paseiro Losada, P. Compilation of analytical methods and guidelines for the determination of selected model migrants from plastic packaging. Trends Food Sci. Technol. 2006, 17, 535–546. [Google Scholar] [CrossRef]
- Dopico-García, M.S.; Noguerol-Cal, R.; Castro-López, M.M.; Cela-Pérez, M.C.; Pinon-Giz, E.; López-Vilarino, J.M.; González-Rodríguez, M.V. Determination of polyolefin additives by reversed phase-liquid chromatography. Cent. Eur. J. Chem. 2012, 10, 585–610. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Nunez, O.; Lucci, P. Recent advances in LC-MS analysis of food-packaging contaminants. TrAC Trends Anal. Chem. 2013, 42, 99–124. [Google Scholar] [CrossRef]
- Hoppe, M.; de Voogt, P.; Franz, R. Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates—A review. Trends Food Sci. Technol. 2016, 50, 118–130. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Shi, C.; Hua, H.; Rong, Y.; Lu, J.; Yang, F. Rapid determination of 48 contaminant residues in food contact plastic products by ultra performance liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometry coupled with microwave-assisted extraction. Chin. J. Chromatogr. 2018, 36, 634–642. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Lopes, J.A.; Hoekstra, E.; Emons, H. Development and validation of a multi-analyte GC-MS method for the determination of 84 substances from plastic food contact materials. Anal. Bioanal. Chem. 2020, 412, 5419–5434. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.D.; Lopes, J.A.; Gika, H.; Dalsgaard, T.K.; Theodoridis, G. A fast SALLE GC-MS/MS multi-analyte method for the determination of 75 food packaging substances in food simulants. Food Chem. 2021, 361, 129998. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Recommendation (EU) 2019/794 of 15 May 2019 on a Coordinated Control Plan with a View to Establishing the Prevalence of Certain Substances Migrating from Materials and Articles Intended to Come into Contact with Food. Available online: http://data.europa.eu/eli/reco/2019/794/oj (accessed on 2 December 2021).
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 2 December 2021).

| Analyte | CAS No. | FCM No. a | SML (mg kg−1) b |
|---|---|---|---|
| 1,4-Butanediol dimethacrylate | 2082-81-7 | 434 | 0.05 |
| 1,4-Dichlorobenzene | 106-46-7 | 217 | 12 |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 6846-50-0 | 497 | 5 |
| 2-Ethyl-1-hexanol | 104-76-7 | 209 | 30 |
| 2-Ethylhexyl acrylate | 103-11-7 | 206 | 0.05 |
| 3-(4-Isopropylphenyl)-2-methylpropionaldehyde | 103-95-7 | - | - |
| 3-(Trimethoxysilyl)propyl methacrylate | 2530-85-0 | 788 | 0.05 |
| 4,4′-Difluorobenzophenone | 345-92-6 | 337 | 0.05 |
| 4-Methylphenol | 106-44-5 | 216 | - |
| 4-tert-Butylphenol | 98-54-4 | 186 | 0.05 |
| Allyl methacrylate | 96-05-9 | 175 | 0.05 |
| α-Methylstyrene | 98-83-9 | 187 | 0.05 |
| α-Pinene | 80-56-8 | 155 | - |
| Benzaldehyde | 100-52-7 | 195 | - |
| Benzophenone | 119-61-9 | 286 | 0.6 |
| Benzyl butyl phthalate | 85-68-7 | 159 | 30(1) |
| Benzyl methacrylate | 2495-37-6 | 447 | 6(2) |
| β-Pinene | 127-91-3 | 314 | - |
| Bis(2-ethylhexyl) adipate | 103-23-1 | 207 | 18(1) |
| Bis(2-ethylhexyl) phthalate | 117-81-7 | 283 | 1.5(1) |
| Bis(4-chlorophenyl) sulphone | 80-07-9 | 152 | 0.05 |
| Butyl acrylate | 141-32-2 | 325 | 6(3) |
| Butyl benzoate | 136-60-7 | 320 | - |
| Butyl lactate | 138-22-7 | 322 | - |
| Butyl methacrylate | 97-88-1 | 184 | 6(2) |
| Butylated hydroxytoluene | 128-37-0 | 315 | 3 |
| Camphor | 76-22-2 | 136 | - |
| Cyclohexyl methacrylate | 101-43-9 | 197 | 0.05 |
| Diallyl phthalate | 131-17-9 | 316 | ND |
| Dibutyl adipate | 105-99-7 | - | - |
| Dibutyl maleate | 105-76-0 | - | - |
| Dibutyl phthalate | 84-74-2 | 157 | 0.3(1) |
| Diethyl phthalate | 84-66-2 | - | - |
| Diisobutyl phthalate | 84-69-5 | - | - |
| Dimethyl isophthalate | 1459-93-4 | 420 | 0.05 |
| Dimethyl terephthalate | 120-61-6 | 288 | - |
| Diphenyl phthalate | 84-62-8 | - | - |
| Diphenyl sulfone | 127-63-9 | 313 | 3 |
| Divinyl benzene | 1321-74-0 | 405 | ND |
| Ethyl benzoate | 93-89-0 | 172 | - |
| Ethylene glycol dimethacrylate | 97-90-5 | 185 | 0.05 |
| Etocrylene | 5232-99-5 | 487 | 0.05 |
| Isobutyl acrylate | 106-63-8 | 218 | 6(3) |
| Lauryl acrylate | 2156-97-0 | 437 | 0.05 |
| Methyl benzoate | 93-58-3 | 171 | - |
| Methyl dihydrojasmonate | 24851-98-7 | - | - |
| Methyl salicilate | 119-36-8 | 284 | 30 |
| Octocrylene | 6197-30-4 | 492 | 0.05 |
| Phenol | 108-95-2 | 241 | 3 |
| Phenyl methacrylate | 2177-70-0 | 439 | 6(2) |
| Propyl benzoate | 2315-68-6 | 441 | - |
| Styrene | 100-42-5 | 193 | - |
| tert-Butyl methacrylate | 585-07-9 | 355 | 6(2) |
| Triethoxyvinylsilane | 78-08-0 | 142 | 0.05 |
| Triethyl citrate | 77-93-0 | 140 | 60(1) |
| Triethyl phosphite | 122-52-1 | 293 | ND |
| Trimethylolpropane trimethacrylate | 3290-92-4 | 463 | 0.05 |
| Tri-n-butyl acetyl citrate | 77-90-7 | 138 | 60(1) |
| Vinyl laurate | 2146-71-6 | 436 | - |
| Vinyltrimethoxysilane | 2768-02-7 | 453 | 0.05 |
| Analyte | Coefficient of Determination (R2) a | Relative Recovery (%) b | Precision (RSD, %) c | ||||
|---|---|---|---|---|---|---|---|
| 40 µg L−1 | 160 µg L−1 | 400 µg L−1 | 40 µg L−1 | 160 µg L−1 | 400 µg L−1 | ||
| 1,4-Butanediol dimethacrylate | 0.998 | 95 ± 6 | 106 ± 3 | 101 ± 1 | 5.8 | 2.9 | 1.3 |
| 1,4-Dichlorobenzene | 0.998 | 105 ± 9 | 101 ± 6 | 99 ± 3 | 8.7 | 5.7 | 3.5 |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 0.999 | 106 ± 3 | 97 ± 2 | 95 ± 2 | 3.2 | 2.0 | 1.7 |
| 2-Ethyl-1-hexanol | 0.997 | 92 ± 8 | 101 ± 4 | 100 ± 4 | 8.3 | 4.1 | 4.1 |
| 2-Ethylhexyl acrylate | 0.999 | 101 ± 8 | 113 ± 4 | 99 ± 2 | 7.5 | 3.8 | 1.6 |
| 3-(4-Isopropylphenyl)-2-methylpropionaldehyde | 0.994 | 104 ± 3 | 96 ± 3 | 101 ± 2 | 2.5 | 3.5 | 2.1 |
| 3-(Trimethoxysilyl)propyl methacrylate | 0.993 | 108 ± 5 | 81 ± 3 | 83 ± 1 | 4.8 | 4.2 | 1.2 |
| 4,4′-Difluorobenzophenone | 0.997 | 89 ± 5 | 106 ± 3 | 97 ± 2 | 6.0 | 3.3 | 2.1 |
| 4-Methylphenol | 0.997 | 87 ± 5 | 101 ± 7 | 103 ± 5 | 6.0 | 6.8 | 5.1 |
| 4-tert-Butylphenol | 0.997 | 94 ± 5 | 103 ± 7 | 105 ± 5 | 5.0 | 6.4 | 4.7 |
| Allyl methacrylate | 0.996 | 105 ± 10 | 99 ± 2 | 101 ± 4 | 9.5 | 2.4 | 4.1 |
| α-Methylstyrene | 0.994 | 103 ± 9 | 102 ± 3 | 108 ± 4 | 8.6 | 3.0 | 3.8 |
| α-Pinene | 0.993 | 100 ± 4 | 97 ± 2 | 100 ± 3 | 4.4 | 2.5 | 2.7 |
| Benzaldehyde | 0.996 | 90 ± 7 | 99 ± 4 | 102 ± 4 | 8.3 | 3.7 | 3.6 |
| Benzophenone | 0.997 | 92 ± 7 | 106 ± 4 | 102 ± 7 | 8.0 | 4.1 | 7.1 |
| Benzyl butyl phthalate | 0.994 | 97 ± 4 | 104 ± 5 | 102 ± 1 | 4.1 | 5.3 | 0.9 |
| Benzyl methacrylate | 0.999 | 94 ± 7 | 106 ± 5 | 102 ± 4 | 7.6 | 5.2 | 4.4 |
| β-Pinene | 0.996 | 104 ± 6 | 101 ± 3 | 100 ± 4 | 6.0 | 2.7 | 3.6 |
| Bis(2-ethylhexyl) adipate | 0.988 | 82 ± 4 | 103 ± 8 | 111 ± 11 | 4.6 | 7.8 | 10.0 |
| Bis(2-ethylhexyl) phthalate | 0.992 | 99 ± 4 | 103 ± 5 | 113 ± 8 | 4.4 | 4.8 | 7.2 |
| Bis(4-chlorophenyl) sulphone | 0.997 | 90 ± 7 | 95 ± 8 | 95 ± 2 | 8.1 | 8.6 | 2.1 |
| Butyl acrylate | 0.998 | 106 ± 9 | 94 ± 2 | 104 ± 2 | 8.7 | 2.3 | 2.2 |
| Butyl benzoate | 0.999 | 92 ± 8 | 107 ± 5 | 100 ± 1 | 8.3 | 4.4 | 0.8 |
| Butyl lactate | 0.996 | 96 ± 3 | 101 ± 1 | 100 ± 6 | 3.6 | 0.7 | 6.0 |
| Butyl methacrylate | 0.991 | 98 ± 10 | 99 ± 3 | 103 ± 4 | 10.0 | 3.2 | 4.1 |
| Butylated hydroxytoluene | 0.997 | 94 ± 5 | 106 ± 5 | 100 ± 1 | 5.1 | 4.7 | 1.3 |
| Camphor | 0.997 | 93 ± 7 | 112 ± 3 | 106 ± 2 | 7.6 | 2.8 | 2.2 |
| Cyclohexyl methacrylate | 0.997 | 100 ± 9 | 113 ± 7 | 103 ± 1 | 9.3 | 6.2 | 1.3 |
| Diallyl phthalate | 0.998 | 97 ± 7 | 98 ± 3 | 100 ± 1 | 6.8 | 3.5 | 0.6 |
| Dibutyl adipate | 0.998 | 90 ± 5 | 100 ± 5 | 100 ± 6 | 6.0 | 5.1 | 6.0 |
| Dibutyl maleate | 0.993 | 100 ± 5 | 92 ± 5 | 101 ± 1 | 5.1 | 5.7 | 1.0 |
| Dibutyl phthalate | 0.997 | 99 ± 6 | 105 ± 5 | 100 ± 1 | 6.1 | 4.7 | 0.8 |
| Diethyl phthalate | 0.999 | 98 ± 2 | 104 ± 4 | 96 ± 2 | 2.5 | 3.7 | 1.9 |
| Diisobutyl phthalate | 0.997 | 92 ± 7 | 105 ± 5 | 96 ± 1 | 7.6 | 4.8 | 0.9 |
| Dimethyl isophthalate | 0.994 | 92 ± 9 | 101 ± 2 | 99 ± 1 | 9.4 | 1.8 | 1.3 |
| Dimethyl terephthalate | 0.994 | 88 ± 6 | 99 ± 4 | 98 ± 1 | 7.0 | 3.7 | 1.1 |
| Diphenyl phthalate | 0.998 | 91 ± 4 | 96 ± 6 | 102 ± 1 | 4.6 | 6.5 | 0.8 |
| Diphenyl sulphone | 0.994 | 88 ± 9 | 105 ± 4 | 97 ± 8 | 10.2 | 3.8 | 8.1 |
| Divinyl benzene | 0.997 | 104 ± 7 | 107 ± 5 | 108 ± 3 | 6.8 | 4.9 | 2.5 |
| Ethyl benzoate | 0.995 | 96 ± 8 | 106 ± 1 | 106 ± 7 | 8.4 | 1.4 | 6.4 |
| Ethylene glycol dimethacrylate | 0.999 | 96 ± 6 | 103 ± 5 | 101 ± 1 | 6.6 | 4.4 | 1.4 |
| Etocrylene | 0.998 | 93 ± 6 | 97 ± 6 | 104 ± 9 | 6.5 | 6.0 | 8.5 |
| Isobutyl acrylate | 0.997 | 93 ± 4 | 99 ± 3 | 98 ± 2 | 4.5 | 3.2 | 2.1 |
| Lauryl acrylate | 0.989 | 94 ± 8 | 113 ± 7 | 99 ± 3 | 8.6 | 6.2 | 3.4 |
| Methyl benzoate | 0.990 | 93 ± 8 | 103 ± 3 | 102 ± 6 | 8.5 | 3.3 | 6.0 |
| Methyl dihydrojasmonate | 0.993 | 99 ± 6 | 97 ± 5 | 102 ± 1 | 6.1 | 5.6 | 1.0 |
| Methyl salicylate | 0.996 | 95 ± 6 | 102 ± 4 | 107 ± 6 | 6.3 | 3.5 | 5.9 |
| Octocrylene | 0.994 | 102 ± 3 | 91 ± 7 | 109 ± 5 | 2.6 | 7.6 | 4.8 |
| Phenol | 0.990 | 93 ± 5 | 97 ± 5 | 106 ± 4 | 5.1 | 4.8 | 4.0 |
| Phenyl methacrylate | 0.999 | 96 ± 7 | 103 ± 2 | 105 ± 3 | 7.0 | 1.9 | 3.1 |
| Propyl benzoate | 0.998 | 99 ± 8 | 108 ± 2 | 101 ± 1 | 8.3 | 2.0 | 1.3 |
| Styrene | 0.995 | 100 ± 3 | 96 ± 3 | 100 ± 2 | 2.6 | 3.0 | 2.1 |
| tert-Butyl methacrylate | 0.992 | 103 ± 5 | 100 ± 2 | 96 ± 2 | 4.4 | 2.5 | 1.8 |
| Triethoxyvinylsilane | 0.995 | 98 ± 8 | 96 ± 6 | 85 ± 3 | 8.7 | 6.5 | 3.8 |
| Triethyl citrate | 0.999 | 99 ± 4 | 91 ± 5 | 98 ± 9 | 4.4 | 5.7 | 8.9 |
| Triethyl phosphite | 0.992 | 81 ± 1 | 99 ± 4 | 82 ± 4 | 1.1 | 3.6 | 4.6 |
| Trimethylolpropane trimethacrylate | 0.994 | 106 ± 4 | 93 ± 4 | 104 ± 1 | 3.4 | 4.9 | 0.9 |
| Tri-n-butyl acetyl citrate | 0.995 | 101 ± 4 | 88 ± 7 | 103 ± 10 | 4.2 | 7.5 | 9.3 |
| Vinyl laurate | 0.999 | 90 ± 6 | 110 ± 5 | 100 ± 2 | 6.9 | 4.4 | 1.8 |
| Vinyltrimethoxysilane | 0.999 | 101 ± 11 | 100 ± 9 | 98 ± 10 | 11.1 | 8.8 | 10.6 |
| Sample | Food Category of Intended Use | Worst Foreseeable Food Contact Time (t) and Temperature (T) | Selected Conditions for Migration Test |
|---|---|---|---|
| 1 | Preserved vegetables or preserved animal products (fish, meat) in an oily medium | Above 30 days, 20 °C < T ≤ 40 °C | Food simulant A, 10 days at 60 °C |
| 2 | Clear drinks: Water, ciders, fruit or vegetable juices, infusions, coffee, tea, energy drinks, etc. | 6 hours < t ≤ 24 h, 20 °C < T ≤ 40 °C | Food simulant B, 24 h at 40 °C |
| 3 | Alcoholic beverages of an alcoholic strength <20% (wine, beer, etc.) or clear drinks | 1 hour < t ≤ 2 h, 20 °C < T ≤ 40 °C | Food simulant C, 2 h at 40 °C |
| 4 | Milk and milk-based drinks whole, partly dried and skimmed, or partly skimmed | 6 hours < t ≤ 24 h, 20 °C < T ≤ 40 °C, including a hot filling up to 70 °C | Food simulant D1, 2 hours at 70 °C and 24 h at 40 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles, P.; Yusà, V.; Sanchís, Y.; Coscollà, C. Determination of 60 Migrant Substances in Plastic Food Contact Materials by Vortex-Assisted Liquid-Liquid Extraction and GC-Q-Orbitrap HRMS. Molecules 2021, 26, 7640. https://doi.org/10.3390/molecules26247640
Miralles P, Yusà V, Sanchís Y, Coscollà C. Determination of 60 Migrant Substances in Plastic Food Contact Materials by Vortex-Assisted Liquid-Liquid Extraction and GC-Q-Orbitrap HRMS. Molecules. 2021; 26(24):7640. https://doi.org/10.3390/molecules26247640
Chicago/Turabian StyleMiralles, Pablo, Vicent Yusà, Yovana Sanchís, and Clara Coscollà. 2021. "Determination of 60 Migrant Substances in Plastic Food Contact Materials by Vortex-Assisted Liquid-Liquid Extraction and GC-Q-Orbitrap HRMS" Molecules 26, no. 24: 7640. https://doi.org/10.3390/molecules26247640
APA StyleMiralles, P., Yusà, V., Sanchís, Y., & Coscollà, C. (2021). Determination of 60 Migrant Substances in Plastic Food Contact Materials by Vortex-Assisted Liquid-Liquid Extraction and GC-Q-Orbitrap HRMS. Molecules, 26(24), 7640. https://doi.org/10.3390/molecules26247640








