Recent Advancements in the Development of Anti-Breast Cancer Synthetic Small Molecules
Abstract
:1. Introduction
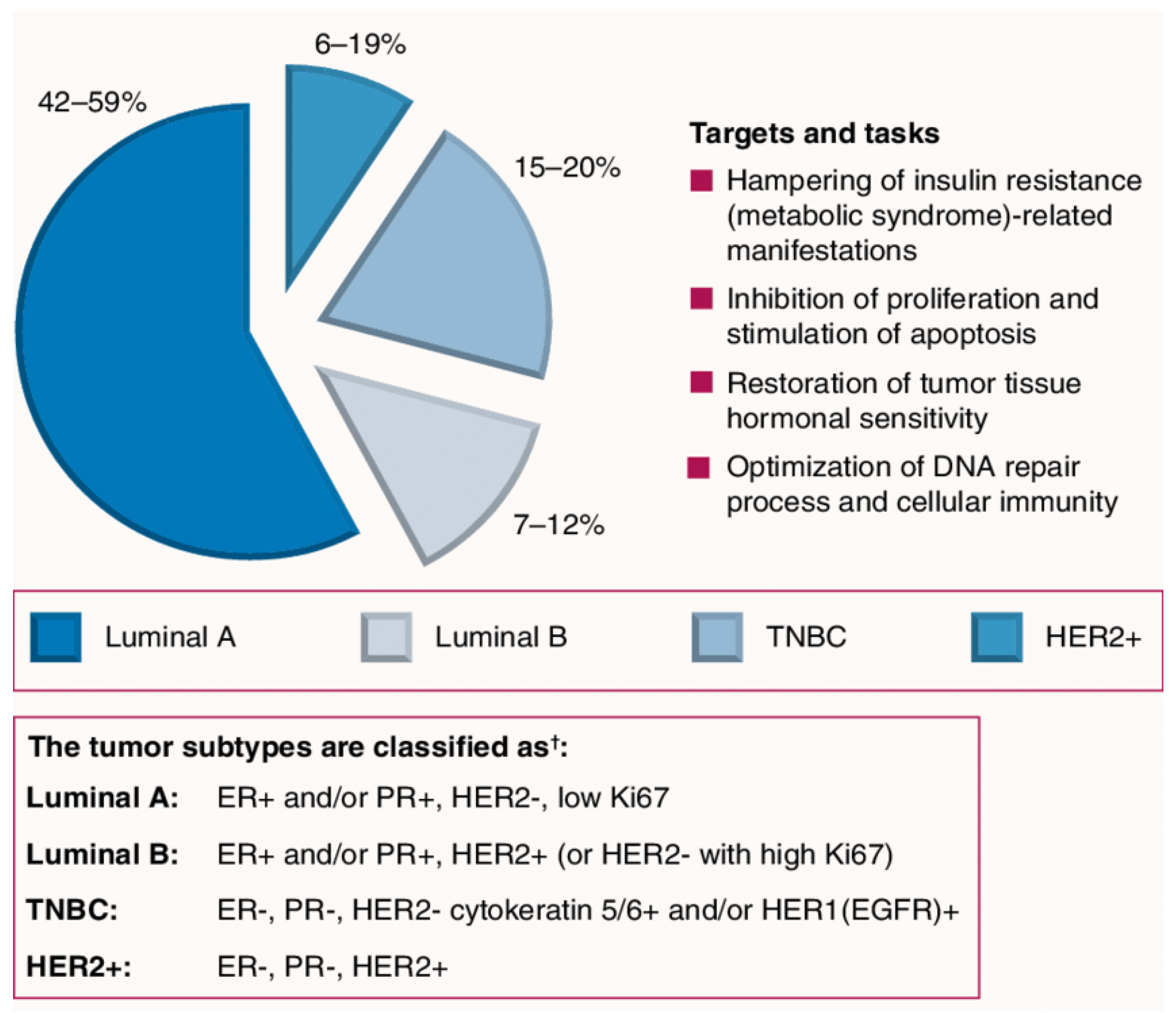
Different Subtypes of Breast Cancer
- Luminal A: This group has tumors that are estrogen-receptor and/or progesterone-receptor positive (+ve ER and/or +ve PR), but it is human epidermal growth factor receptor negative (−ve HER2). In addition, this type possesses protein Ki-67 in low levels, which is beneficial to control the growth of cancer cells. This type generally has the best prognosis with low grade and grows slowly.
- Luminal B: This group has tumors that are positive for ER and HER2 but negative for PR and possesses protein Ki-67 in high levels. Luminal B BCs mainly grow faster than luminal A BCs, and their prognosis is slightly worse.HER2-enriched (HR−/HER2+): This group has tumors that are negative ER, PR and positive for HER2. It is treated with targeted therapy to target the HER2 protein although it grows faster than luminal cancers with a worse prognosis. Examples of targeted therapies include Pertuzumab, Trastuzumab Deruxtecan, Trastuzumab Emtansine, Lapatinib, Trastuzumab, and Neratinib.Basal-like: This group is also named triple-negative breast cancer; it is negative for ER, PR and HER2. Unfortunately, it is the most common type with women with the gene mutation of BRCA1 among young and African-American women, and it is characterized by the missing signature of three biomarkers (PR, ER, HER2 proteins). Accordingly, it is the most aggressive type of BC.
2. Conventional Treatment and Its Drawbacks
3. Recent Drugs Approved for Breast Cancer Management
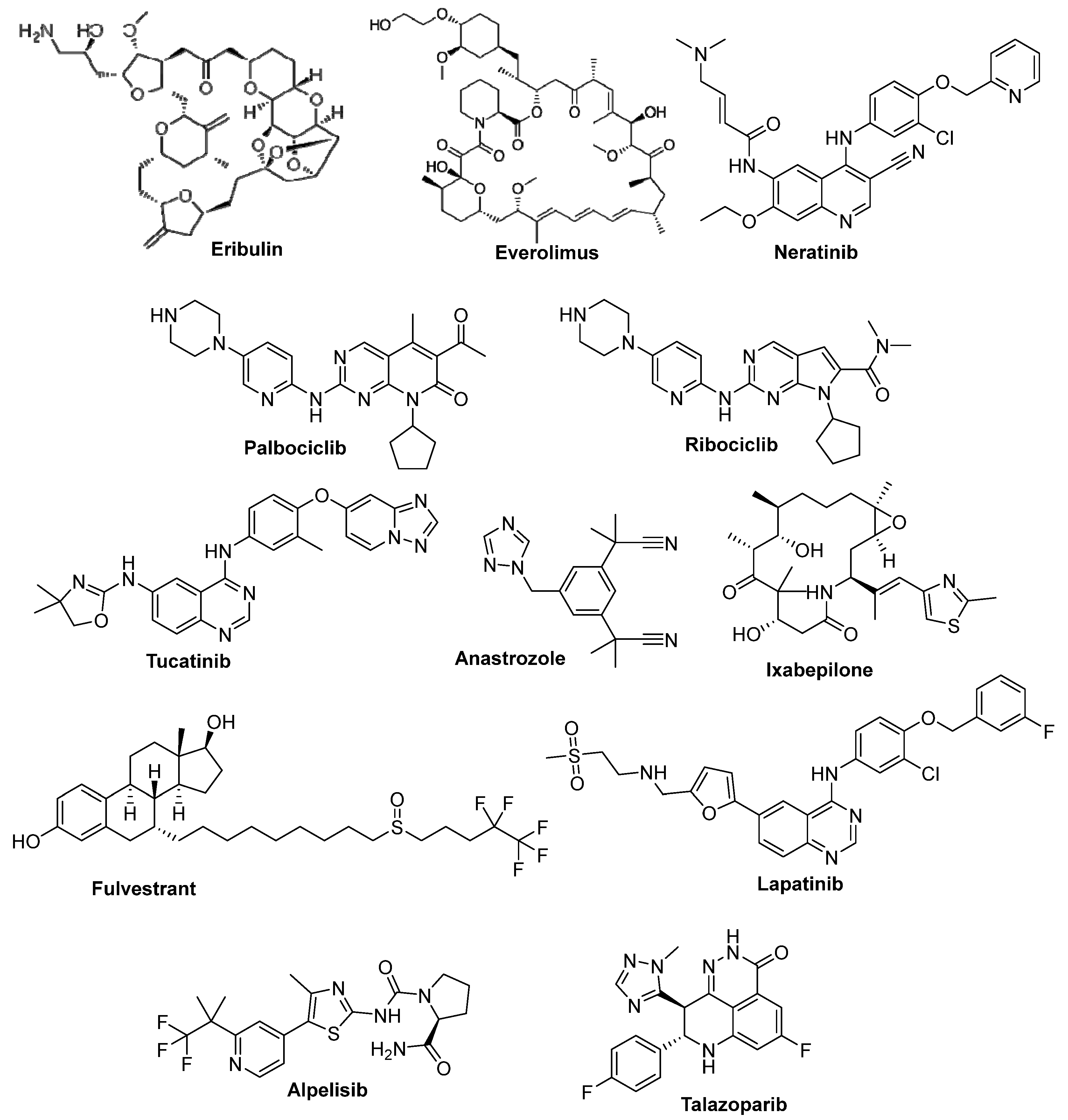
3.1. Eribulin
3.2. Everolimus
3.3. Neratinib
3.4. Palbociclib
3.5. Ribociclib
3.6. Tucatinib
3.7. Anastrozole
3.8. Ixabepilone
3.9. Fulvestrant
3.10. Lapatinib Oral-Active
3.11. Pertuzumab
3.12. Alpelisib
3.13. Talazoparib
4. Recently Developed Synthetic Anti-Breast Cancer Small Molecules
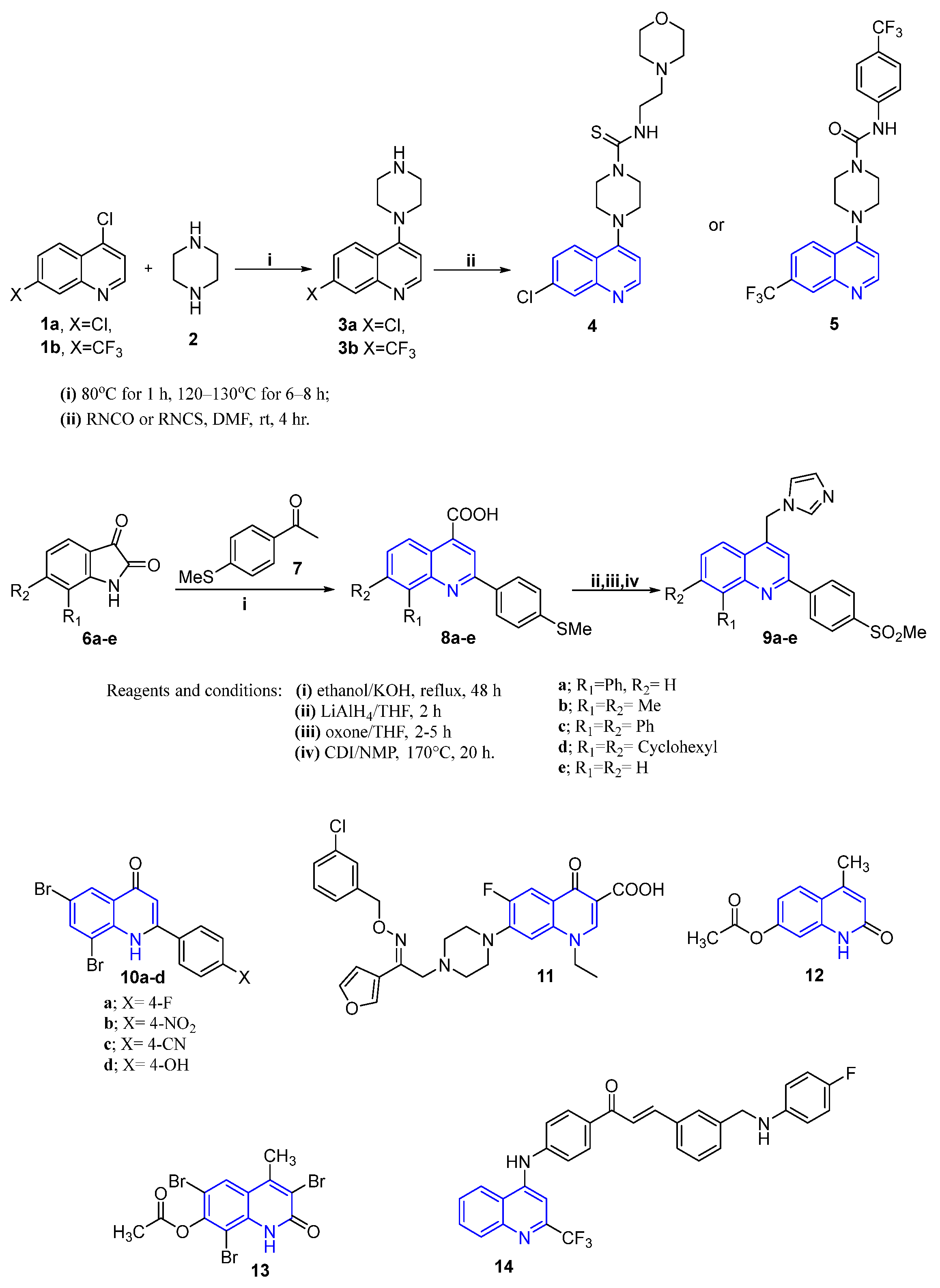
4.1. Quinoline-Based Small Molecules
4.2. Quinazoline- and Quinazolinone-Based Small Molecules
4.3. Pyridine and Fused Pyridine Derivatives
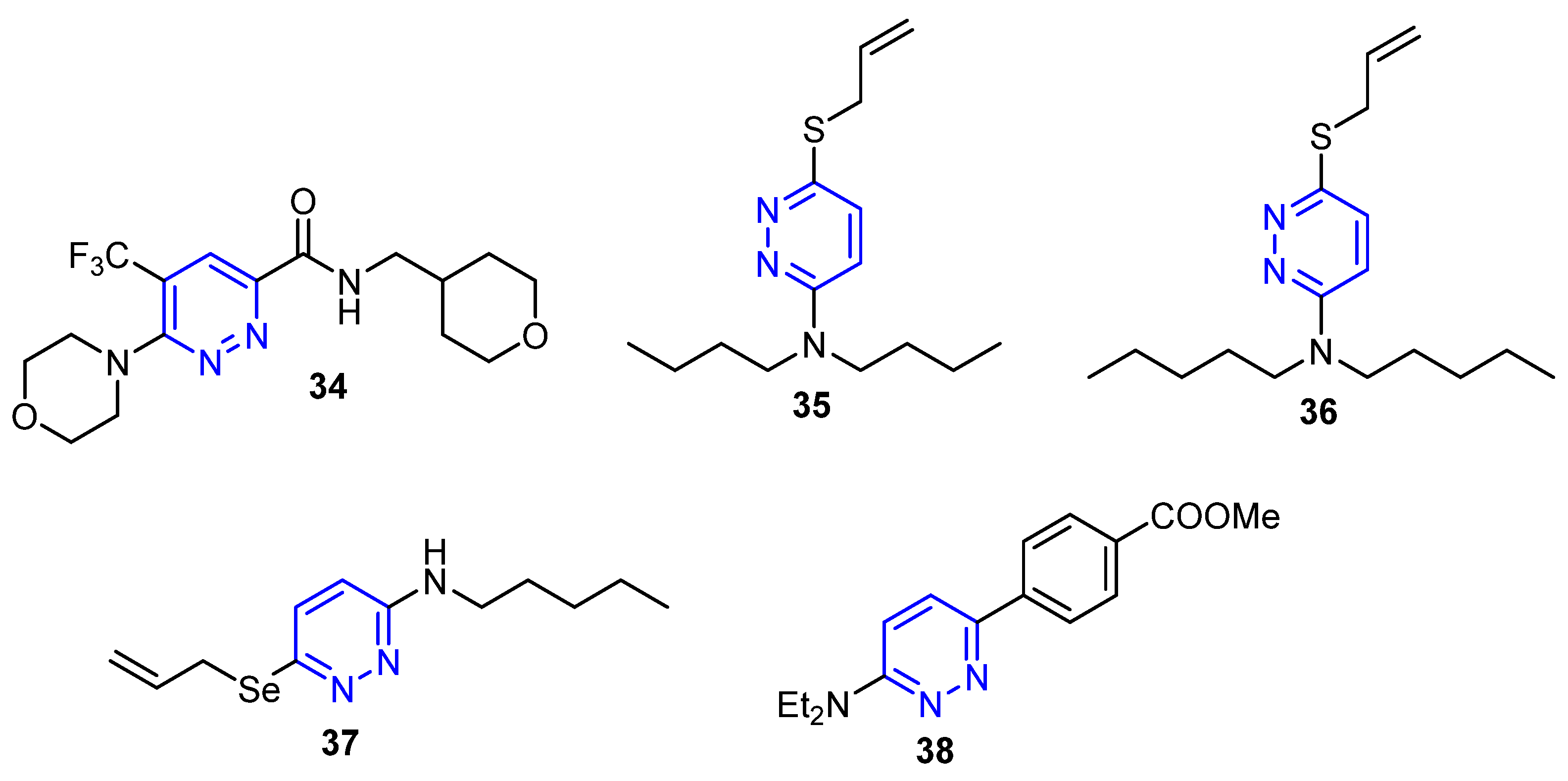
4.4. Pyridazine-Based Small Molecules
4.5. Non-Fused and Fused Pyrimidines Moiety
4.6. Imidazole and Benzimidazole Derivatives
4.7. Coumarin Derivatives
4.8. Tetrazole-Bearing Derivatives
4.9. Indole- and Oxindole-Based Anti-Breast Cancer Agents
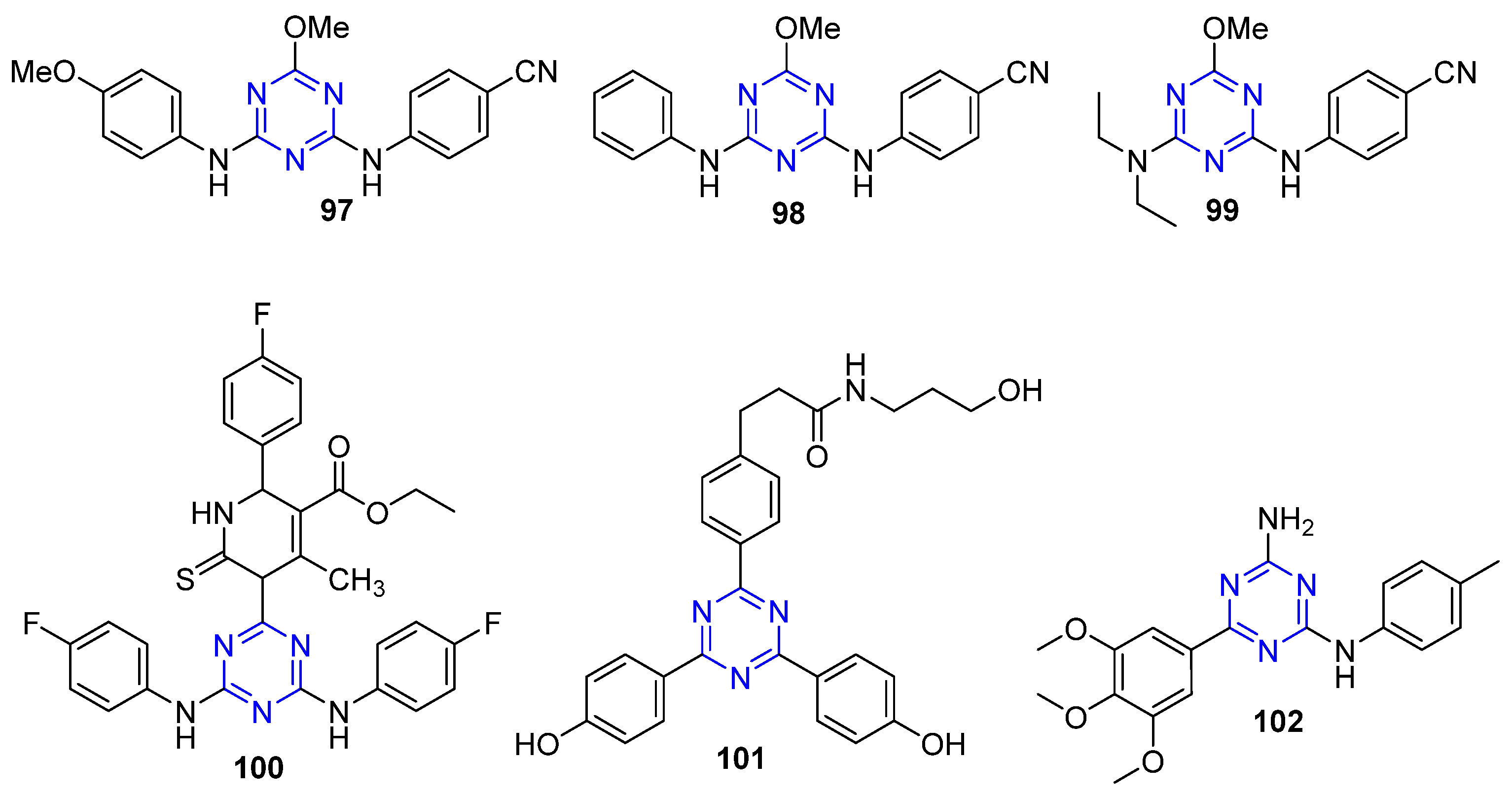
4.10. Triazine-Based Derivatives as Anti-Breast Cancer Agents
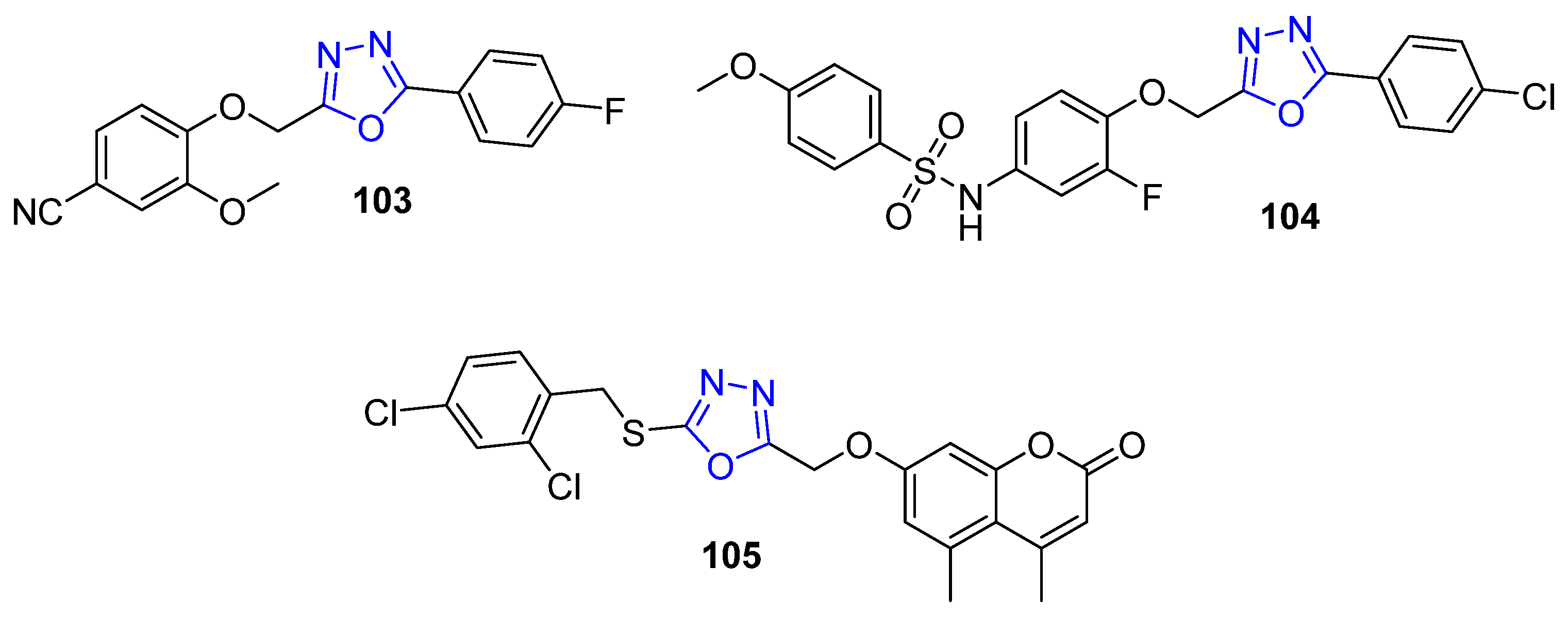
4.11. Oxadiazole-Bearing Small Molecules as Anti-Breast Cancer Agents
4.12. Thiazolidine Derivatives
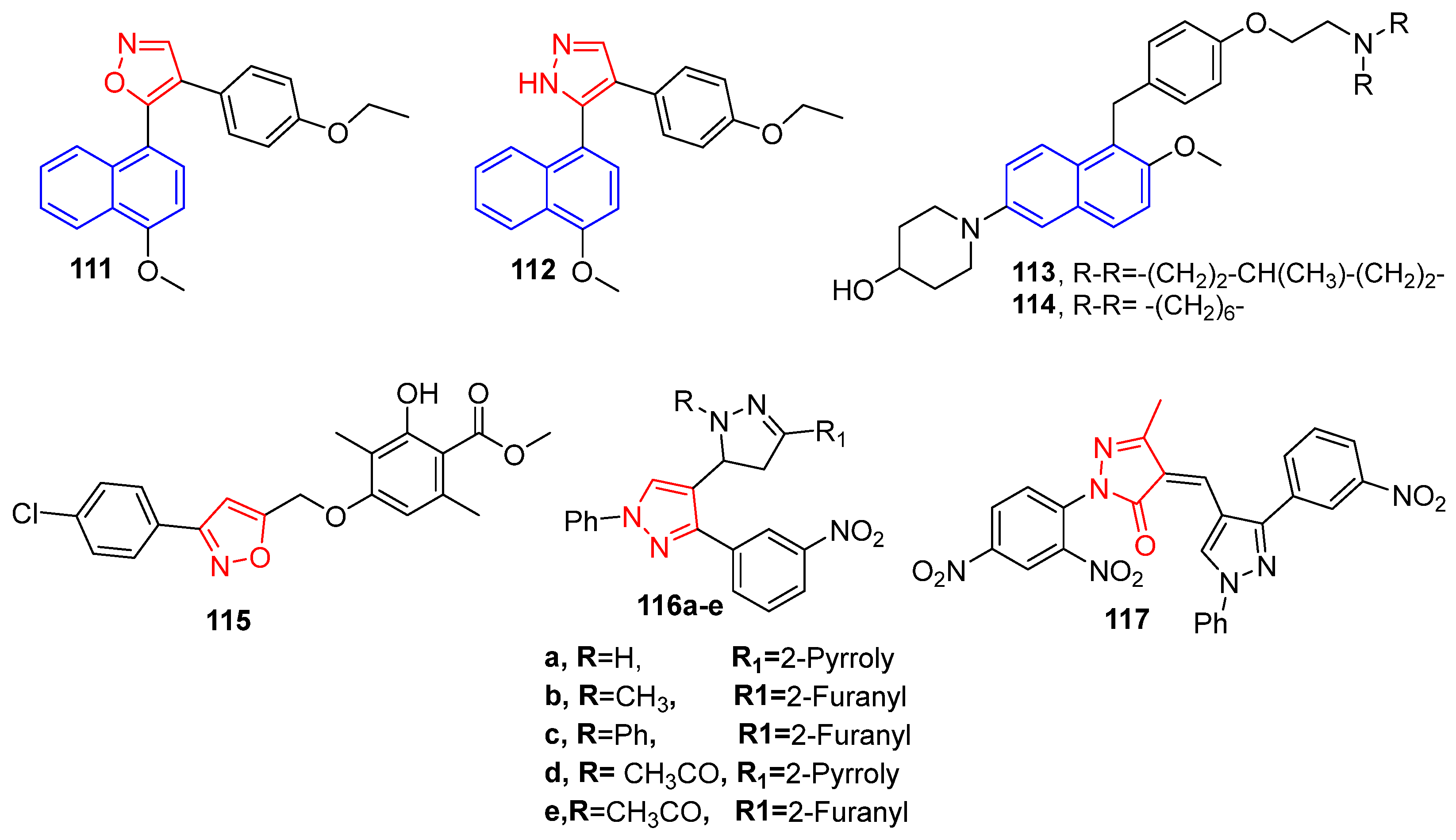
4.13. Anti-Breast Cancer Agents Incorporating Naphthalene, Isoxazole and Pyrazole Moieties
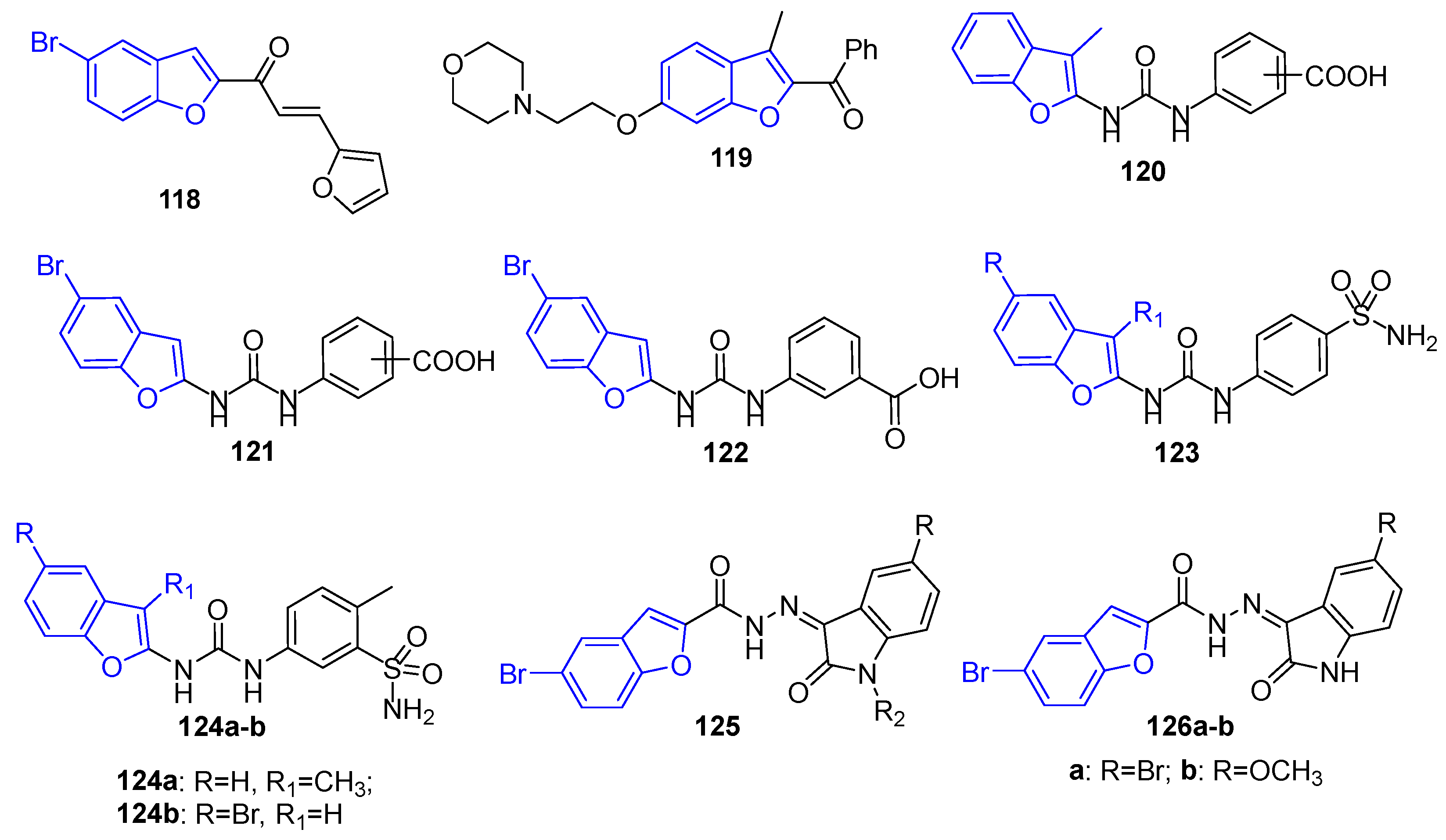
4.14. Benzofuran Derivatives
5. Miscellaneous Anti-Breast Cancer Agents
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Prev. Biomark. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ACS: American Cancer Society. Key Breast Cancer Statistics. Available online: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Breast/ (accessed on 24 August 2021).
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Lukong, K.E. Understanding breast cancer—The long and winding road. BBA Clin. 2017, 7, 64–77. [Google Scholar] [CrossRef]
- Fedele, M.; Cerchia, L.; Chiappetta, G. The epithelial-to-mesenchymal transition in breast cancer: Focus on basal-like carcinomas. Cancers 2017, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.Y.; Shanmugam, M.K.; Sethi, G.; Bishayee, A. Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anticancer Drugs 2016, 27, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kar, S.; Lai, X.; Cai, W.; Arfuso, F.; Sethi, G.; Lobie, P.E.; Goh, B.C.; Lim, L.H.; Hartman, M.J. Triple negative breast cancer in Asia: An insider’s view. Cancer Treat. Rev. 2018, 62, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Valero, V.; Hortobagyi, G.N. Emerging targeted therapies for breast cancer. J. Clin. Oncol. 2010, 28, 3366–3379. [Google Scholar] [CrossRef] [Green Version]
- Perez, E.A.; Spano, J.P. Current and emerging targeted therapies for metastatic breast cancer. Cancer 2012, 118, 3014–3025. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S.; Narasimhan, B. Estrogen alpha receptor antagonists for the treatment of breast cancer: A review. Chem. Cent. J. 2018, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ming, B.; Gong, G.H.; Wang, D.; Bao, G.L.; Yu, L.J. Current research on anti-breast cancer synthetic compounds. RSC Adv. 2018, 8, 4386–4416. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, A.S.; Gomaa, R.M.; Elmorsy, M.A. Design and synthesis of 2-phenyl benzimidazole derivatives as VEGFR-2 inhibitors with anti-breast cancer activity. Chem. Biol. Drug. Des. 2019, 93, 454–463. [Google Scholar] [CrossRef]
- Branowska, D.; Ławecka, J.; Sobiczewski, M.; Karczmarzyk, Z.; Wysocki, W.; Wolińska, E.; Olender, E.; Mirosław, B.; Perzyna, A.; Bielawska, A.; et al. Synthesis of unsymmetrical disulfanes bearing 1,2,4-triazine scaffold and their in vitro screening towards anti-breast cancer activity. Mon. Chem.—Chem. Mon. 2018, 149, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef] [PubMed]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S. MicroRNA therapeutics in triple negative breast cancer. Arch. Pathol. Clin. Res. 2017, 1, 9–17. [Google Scholar]
- Moo, T.A.; Sanford, R.; Dang, C.; Morrow, M. Overview of breast cancer therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P.S. Treatment of breast cancer. Chemotherapy 2010, 81, 1339–1346. [Google Scholar]
- Nicholls, L.; Gorayski, P.; Harvey, J. Osteoradionecrosis of the ribs following breast radiotherapy. Case Rep. Oncol. 2015, 8, 332–338. [Google Scholar] [CrossRef]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updates 2020, 50, 100682. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yu, A. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Rivera, E. Implications of anthracycline-resistant and taxane resistant metastatic breast cancer and new therapeutic options. Breast J. 2010, 16, 252–263. [Google Scholar] [CrossRef]
- Perez, I.E.; Alam, S.T.; Hernandez, G.A. Cancer therapy-related cardiac dysfunction: An overview for the clinician. Clin Med. Insights Cardiol. 2019, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Yang, Y.; Li, L.; Yue, Z.; Lan, L.; Pan, Z. Capecitabine monotherapy in advanced breast cancer resistant to anthracycline and taxane: A meta-analysis. J. Cancer Res. Ther. 2018, 14, 957–963. [Google Scholar] [CrossRef]
- Bryer, E.; Henry, D. Chemotherapy-induced anemia: Etiology, pathophysiology, and implications for contemporary practice. Int. J. Clin. Transfus. Med. 2018, 6, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Pedersini, R.A.; Vassalli, L.A.; Claps, M.B.; Tulla, A.B.; Rodella, F.B.; Grisanti, S.B.; Amoroso, V.B.; Roca, E.B.; Simoncini, E.L.C.; Berruti, A.B. Eribulin in heavily pretreated metastatic breast cancer patients in the real world: A retrospective study. Oncology 2018, 94 (Suppl. S1), 10–15. [Google Scholar] [CrossRef]
- Houghton, P.J. Everolimus. Clin. Cancer Res. 2010, 16, 1368–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zureick, A.H.; McFadden, K.A.; Mody, R.; Koschmann, C. Successful treatment of a TSC2-mutant glioblastoma with everolimus. BMJ Case Rep. CP 2019, 31, e227734. [Google Scholar] [CrossRef] [PubMed]
- Peri, M.; Fazio, N. Clinical evaluation of everolimus in the treatment of neuroendocrine tumors of the lung: Patient selection and special considerations. A systematic and critical review of the literature. Lung Cancer Targets Ther. 2020, 11, 41–52. [Google Scholar] [CrossRef]
- Nasrazadani, A.; Brufsky, A. Neratinib: The emergence of a new player in the management of HER2+ breast cancer brain metastasis. Future Oncol. 2020, 16, 247–254. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Chen, J. Mechanisms of the CDK4/6 inhibitor palbociclib (PD 0332991) and its future application in cancer treatment (Review). Oncol. Rep. 2018, 39, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Hortobagyi, G.N. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: A review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res. 2018, 20, 123. [Google Scholar] [PubMed] [Green Version]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; de Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbi, S.; Rampa, A.; Belluti, F.; Bisi, A. Nonsteroidal aromatase inhibitors for the treatment of breast cancer: An update. Anticancer Agents Med. Chem. 2014, 14, 54–65. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A.; Lee, F.F.Y. Ixabepilone: Targeting βIII-tubulin expression in taxane-resistant malignancies. Mol. Cancer Ther. 2009, 8, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Bergh, J.; Jonsson, P.E.; Lidbrink, E.K.; Trudeau, M.; Eiermann, W.; Brattstrom, D.; Lindeman, J.P.O.; Wiklund, F.; Henriksson, R. FACT: An open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J. Clin. Oncol. 2012, 30, 1919–1925. [Google Scholar]
- Sardesai, S.D.; Storniolo, A.M. Lapatinib: An oral dual tyrosine kinase inhibitor for HER-2-positive breast cancer. Womens Health 2015, 11, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.B.; Reardon, J.; Olson, E.M. Pertuzumab for the treatment of patients with previously untreated her2-positive metastatic breast cancer. Drugs Today 2012, 48, 713–722. [Google Scholar]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufamn, B.; et al. For the SOLAR-1 study group. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Exman, P.; Barroso-Sousa, R.; Tolaney, S.M. Evidence to date: Talazoparib in the treatment of breast cancer. OncoTargets Ther. 2019, 12, 5177–5187. [Google Scholar] [CrossRef] [Green Version]
- Viswas, R.S.; Pundir, S.; Lee, H. Design and synthesis of 4-piperazinyl quinoline derived urea/thioureas for anti-breast cancer activity by a hybrid pharmacophore approach. J. Enzyme Inhib. Med. Chem. 2019, 34, 620–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarghi, A.; Ghodsi, R.; Azizi, E. Design, synthesis and biological evaluation of 4-(imidazolylmethyl)-2-(4-methylsulfonyl Phenyl)-quinoline derivatives as selective COX-2 inhibitors and in-vitro anti-breast cancer agents. Iran. J. Pharm. Res. 2016, 15, 169–177. [Google Scholar]
- Bheemanapalli, L.N.; Kaur, A.; Arora, R.; Akkinepally, R.R.; Javali, N.M. Synthesis, evaluation of 6,8-dibromo-2-aryl-2,3-dihydroquinolin-4(1H)-ones in MCF-7 (breast cancer) cell lines and their docking studies. Med. Chem. Res. 2012, 21, 1741–1750. [Google Scholar] [CrossRef]
- Mohammadhosseini, N.; Pordeli, M.; Safavi, M.; Firoozpour, L.; Amin, F.; Ardestani, S.K.; Edraki, N.; Shafee, A.; Foroumadi, A. Novel N-2-(furyl)-2-(chlorobenzyloxyimino) ethyl piperazinyl quinolones: Synthesis, cytotoxic evaluation and structure–activity relationship. Iran. J. Pharm. Res. 2015, 14, 1095–1103. [Google Scholar]
- Moustafa, A.M.Y.; Bakare, S.B. Synthesis of some hybrid 7-hydroxy quinolinone derivatives as anti breast cancer drugs. Res. Chem. Intermed. 2019, 45, 3895–3912. [Google Scholar] [CrossRef]
- Patel, K.S.; Rathi, J.C.; Dhiman, N. Design, synthesis and molecular modeling of new quinoline analogues as potential anti-cancer agents. Mater. Today Proc. 2020, 28, 77–84. [Google Scholar] [CrossRef]
- Yin, S.; Tang, C.; Wang, B.; Zhang, Y.; Zhou, L.; Xue, L.; Zhang, C. Design, synthesis and biological evaluation of novel EGFR/HER2 dual inhibitors bearing a oxazolo[4,5-g] quinazolin-2(1H)-one scaffold. Eur. J. Med. Chem. 2016, 120, 26–36. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Belal, A.; Youns, M. Design, synthesis, molecular modeling and anti-breast cancer activity of novel quinazolin-4-one derivatives linked to thiazolidinone, oxadiazole or pyrazole moieties. Med. Chem. Res. 2015, 24, 2993–3007. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Hashim, A.A. Design, synthesis of novel quinazolin-4-one derivatives and biological evaluation against human MCF-7 breast cancer cell line. Res. Chem. Intermed. 2016, 42, 1777–1789. [Google Scholar] [CrossRef]
- Faraj, F.L.; Zahedifard, M.; Paydar, M.; Looi, C.Y.; Majid, N.A.; Ali, H.M.; Ahmad, N.; Gwaram, N.S.; Abdulla, M.A. Synthesis, characterization, and anticancer activity of new quinazoline derivatives against MCF-7 cells. J. Sci. World 2014, 2014, 212096. [Google Scholar]
- Wang, S.; Zhang, Y.; Ren, T.; Wu, Q.; Lu, H.; Qin, X.; Liu, Y.; Ding, H.; Zhao, Q. A novel 4-aminoquinazoline derivative, DHW-208, suppresses the growth of human breast cancer cells by targeting the PI3K/AKT/mTOR pathway. Cell Death Dis. 2020, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Khalili, F.; Akrami, S.; Safavi, M.; Mohammadi-Khanaposhtani, M.; Saeedi, M.; Ardestani, S.K.; Larijani, B.; Zonouzi, A.; Tehrani, M.B.; Mahdavi, M. Design, synthesis, in vitro cytotoxic activity evaluation, and study of apoptosis inducing effect of new styrylimidazo[1,2-a]pyridines as potent anti-breast cancer agents. Anti-Cancer Agents Med. Chem. 2019, 19, 265–275. [Google Scholar] [CrossRef]
- Pang, C.; Sun, C.; Wang, J.; Xiao, D.; Ding, L.; Bu, H. Novel 2H-pyrazolo[4,3-c]hexahydropyridine derivatives: Synthesis, crystal structure, fluorescence properties and cytotoxicity evaluation against human breast cancer cells. Sci. China Chem. 2013, 56, 702–715. [Google Scholar]
- Prasad, S.S.; Kumar, K.S.; Jayaprakash, S.H.; Krishna, B.S.; Sundar, C.S.; Rao, P.V.; Babu, T.M.; Rajendra, W.; Reddy, S.C. Design, synthesis, antioxidant, and anti-breast cancer Activities of novel diethyl (alkyl/aryl/ heteroarylamino)(4-(pyridine-2-yl)phenyl)methylphosphonates. Arch. Pharm. 2013, 346, 380–391. [Google Scholar]
- Rahnamay, M.; Mahdavi, M.; Shekarchi, A.A.; Zare, P.; Feizi, M.A.H. Cytotoxic and apoptosis inducing effect of some pyrano [3, 2-c] pyridine derivatives against MCF-7 breast cancer cells. Acta Biochim. Pol. 2018, 65, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Sabt, A.; Eldehna, W.M.; Al-Warhi, T.; Alotaibi, O.J.; Elaasser, M.M.; Suliman, H.; Abdel-Aziz, H.A. Discovery of 3,6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: Synthesis, biological evaluation and in silico insights. J. Enzyme Inhib. Med. Chem. 2020, 35, 1616–1630. [Google Scholar] [CrossRef]
- Kim, C.; Park, E.H.; Park, M.S. Novel alkylaminopyridazine derivatives: Synthesis and their anti-proliferative effects against MCF-7 cells. Bull. Korean Chem. Soc. 2013, 34, 3317–3321. [Google Scholar]
- Kim, C.; Kim, S.B.; Park, M.-S. Synthesis of novel 3-allylseleno-6 alkylthiopyridazines:their anticancer activity against MCF-7 cells. Arch. Pharm. Res. 2014, 37, 452–458. [Google Scholar]
- Sengmany, S.; Sitter, M.; Léonel, E.; Gall, E.L.; Loirand, G.; Martens, T.; Dubreuil, D.; Dilasser, F.; Rousselle, M.; Sauzeau, V.; et al. Synthesis and biological evaluation of 3-amino-, 3-alkoxy- and 3-aryloxy-6-(hetero)arylpyridazines as potent antitumor agents. Bioorg. Med. Chem. Lett. 2019, 29, 755–760. [Google Scholar] [CrossRef]
- Kolawole, O.A.; Banjo, S. In vitro biological estimation of 1,2,3-triazolo[4,5-d]pyrimidine derivatives as anti-breast cancer agent: DFT, QSAR and docking studies. Curr. Pharm. Biotechnol. 2020, 21, 70–78. [Google Scholar] [CrossRef]
- Amr, A.E.E.; Ibrahimd, A.A.; El-Shehry, M.F.; Hosni, H.M.; Fayed, A.A.; Elsayed, E.A. In vitro and in vivo anti-breast cancer activities of some newly synthesized 5-(thiophen-2-yl)thieno-[2,3-d]pyrimidin-4-one candidates. Molecules 2019, 24, 2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Wang, Y.; Dong, F.; Wang, Z.; Zhao, Y.; Shan, Y.; Gu, W.; Wang, S. Synthesis and antitumor activity of isolongifoleno[7,8-d] thiazolo[3,2-a] pyrimidine derivatives via enhancing ROS level. Chem. Biol. Drug Des. 2019, 94, 1457–1466. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, Y.; Gu, W.; Zhu, Y.; Wang, S. Novel camphor-based pyrimidine derivatives induced cancer cell death through a ROS-mediated mitochondrial apoptosis pathway. RSC Adv. 2019, 9, 29711–29720. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.H.; Zheng, M.W.; Li, Y.P.; Lin, X.D.; Huang, M.; Zhong, L.; Li, G.B.; Zhang, R.J.; Lin, W.T.; Jiao, Y.; et al. Design, synthesis, and structure–activity relationship studies of 3-(phenylethynyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine derivatives as a new class of Srcinhibitors with potent activities in models of triple negative breast cancer. J. Med. Chem. 2015, 58, 3957–3974. [Google Scholar] [CrossRef]
- Abd El-Salam, H.A.; Yakout, E.S.M.; El-Hashash, M.A.; Nawwar, G.A. Facile synthesis of 6-(heptadec-8-enyl) thiopyrimidines incorporating glycosyl moiety and their antitumor activity. Mon. Chem. 2013, 144, 1893–1901. [Google Scholar] [CrossRef]
- Sharaky, M.; Kamel, M.; Aziz, M.A.; Omran, M.; Rageh, M.M.; Abouzid, K.A.M.; Shouman, S.A. Design, synthesis and biological evaluation of a new thieno[2,3-d]pyrimidinebased urea derivative with potential antitumor activity against tamoxifen sensitive and resistant breast cancer cell lines. J. Enzyme Inhib. Med. Chem. 2020, 35, 1641–1656. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Manickam, M.; Pillaiyar, T. Exploration of imidazole and imidazopyridine dimers as anticancer agents: Design, synthesis, and structure–activityrelationship study. Arch. Pharm. Chem. Life Sci. 2019, 352, 1900011. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.C.; Akkoc, S.; Sarıpınar, E. The cytotoxic activities of imidazole derivatives preparedfrom various guanylhydrazone and phenylglyoxalmonohydrate. Synth. Commun. 2019, 49, 3198–3209. [Google Scholar] [CrossRef]
- Yadav, S.; Lim, S.M.; Ramasamy, K.; Vasudevan, M.; Shah SA, A.; Mathur, A.; Narasimhan, B. Synthesis and evaluation of antimicrobial, antitubercular and anticancer activities of 2-(1-benzoyl-1H-benzo[d]imidazol-2-ylthio)-N-substituted acetamides. Chem. Cent. J. 2018, 12, 66. [Google Scholar] [CrossRef]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Saha, S.T.; Gu, L.; Palma, G.; Perumal, S.; Singh-Pillay, A.; Singh, P.; Anand, A.; Kaur, M.; Kumar, V. 1H 1,2,3-triazole tethered nitroimidazole−isatin conjugates: Synthesis, docking, and anti-proliferative evaluation against breast cancer. ACS Omega 2018, 3, 12106–12113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, C.; Solomon, V.R.; Lee, H.; Trivedi, P. Synthesis and biological evaluation of 2-(phenyl)-3H-benzo[d]imidazole-5-carboxylic acids and its methyl esters as potent anti-breast cancer agents. Arab. J. Chem. 2017, 10, S1788–S1794. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.Y.; Latif NA, A.; El-Mansy, M.F.; Elserwy, W.S.; Abdelhafez, O.M. VEGFR-2 inhibiting effect and molecular modeling of newly synthesized coumarin derivatives as anti-breast cancer agents. Bioorg. Med. Chem. 2020, 28, 115328. [Google Scholar] [CrossRef] [PubMed]
- El-Samahy, F.A.; Abd El Salam, H.A.; El-Sayed, N.F.; Shalaby, E.M.; Dondeti, M.F. Synthesis of unexpected novel bis-coumarin derivatives via three component reactions of 4-hydroxycoumarin, aldehydes and cyclic secondary amines. Conformation in the solid state and pharmacological evaluation. Z. Nat. B 2017, 72, 705–716. [Google Scholar] [CrossRef]
- Batran, R.B.; Dawood, D.H.; El-Seginy, S.A.; Ali, M.M.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. New coumarin derivatives as anti-breast and anti-cervical cancer agents targeting VEGFR-2 and p38aMAPK. Arch. Pharm. Chem. Life Sci. 2017, 350, e1700064. [Google Scholar] [CrossRef]
- Sabt, A.; Abdelhafez, O.M.; El-Haggar, R.S.; Madkour, H.M.; Eldehna, W.M.; El-Khrisy, E.E.D.A.; Abdel-Rahman, M.A.; Rashed, L.A. Novel coumarin-6-sulfonamides as apoptotic anti-proliferative agents: Synthesis, in vitro biologicalevaluation, and QSAR studies. J. Enzyme Inhib. Med. Chem. 2018, 33, 1095–1107. [Google Scholar] [CrossRef] [Green Version]
- Dileep, K.; Polepalli, S.; Jain, N.; Buddana, S.K.; Prakasham, R.S.; Murty, M.S.R. Synthesis of novel tetrazole containing hybrid ciprofloxacin and pipemidic acid analogues and preliminary biological evaluation of their antibacterial and antiproliferative activity. Mol. Divers. 2018, 22, 83–93. [Google Scholar] [CrossRef]
- Arshad, M.; Bhat, A.R.; Pokharel, S.; Kim, J.E.; Lee, E.J.; Athar, F.; Choi, I. Synthesis, characterization and anticancer screening of some novel piperonyl–tetrazole derivatives. Eur. J. Med. Chem. 2014, 71, 229–236. [Google Scholar] [CrossRef]
- Kohler, S.C.; Wiese, M. HM30181 derivatives as novel € potent and selective inhibitors of the breast cancer resistance protein (BCRP/ABCG2). J. Med. Chem. 2015, 58, 3910–3921. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Singla, R.; Jaitak, V. Indole derivatives as anticancer agents for breast cancer therapy: A review. Anti-Cancer Agents Med. Chem. 2016, 16, 160–173. [Google Scholar] [CrossRef]
- Mady, M.S.; Mohyeldin, M.M.; Ebrahim, H.Y.; Elsayed, H.E.; Houssen, W.E.; Haggag, E.G.; Soliman, R.F.; El Sayed, K.A. The indole alkaloid meleagrin, from the olive tree endophytic fungus Penicillium chrysogenum, as a novel lead for the control of c-Met-dependent breast cancer proliferation, migration and invasion. Bioorg. Med. Chem. 2016, 24, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Diao, Q.P. The anti-breast cancer potential of bis-isatin scaffolds. Curr. Top. Med. Chem. 2020, 20, 1499–1503. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Abo-Ashour, M.F.; Ibrahim, H.S.; Al-Ansary, G.H.; Ghabbour, H.A.; Elaasser, M.M.; Ahmedf, H.Y.A.; Safwat, N.A. Novel [(3-indolylmethylene)hydrazono]indolin-2-ones as apoptotic anti-proliferative agents: Design, synthesis and in vitro biological evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 686–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Singh, J.; Narasimhan, B. Indole hybridized diazenyl derivatives: Synthesis, antimicrobial activity, cytotoxicity evaluation and docking studies. BMC Chem. 2019, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, G.J.; Bouaziz, Z.; Winter, E.; Daflon-Yunes, N.; Aichele, D.; Nacereddine, A.; Marminon, C.; Valdameri, G.; Zeinyeh, W.; Bollacke, A.; et al. Converting potent indeno[1,2-b]indole inhibitors of protein kinase CK2 into selective inhibitors of the breast cancer resistance protein ABCG2. J. Med. Chem. 2014, 58, 265–277. [Google Scholar] [CrossRef]
- Ma, J.; Bao, G.; Wang, L.; Li, W.; Xu, B.; Du, B.; Lv, J.; Zhai, X.; Gong, P. Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur. J. Med. Chem. 2015, 96, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zheng, S.L.; Li, X.; Li, J.L.; Qiang, O.; Liu, R.; He, L. Synthesis and anti-breast cancer activity of new indolylquinone derivatives. Eur. J. Med. Chem. 2012, 54, 42–48. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ghosh, S.; Banerjee, B.; Santra, A.; Adhikary, A.; Misra, A.K.; Sen, P.C. Phemindole, a synthetic di-indole derivative maneuvers the store operated calcium entry (SOCE) to induce potent anticarcinogenic activity in human triple negative breast cancer cells. Front. Pharmacol. 2016, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Debnath, B.; Ganguly, S. Synthesis, biological evaluation, in silico docking, and virtual ADME studies of 2-[2-oxo-3-(arylimino)indolin-1-yl]-N-arylacetamides as potent antibreast cancer agents. Mon. Chem.—Chem. Mon. 2016, 147, 565–574. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Solomon, V.R.; Lee, H.; Trivedi, P. Design, synthesis and biological evaluation of some isatin-linked chalcones as novel anti-breast cancer agents: A molecular hybridization approach. Biomed. Prev. Nutr. 2013, 3, 325–330. [Google Scholar] [CrossRef]
- El-Faham, A.; Farooq, M.; Almarhoon, Z.; Alhameed, R.A.; Wadaan, M.A.M.; de la Torre, B.G.; Albericio, F. Di- and tri-substituted s-triazine derivatives: Synthesis, characterization, anticancer activity in human breast-cancer cell lines, and developmental toxicity in zebrafish embryos. Bioorg. Chem. 2019, 94, 103397. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Pillai, G.G.; Bhat, H.R.; Verma, A.; Singh, U.P. Design and discovery of novel monastrol-1,3,5-triazines as potent anti-breast cancer agent via attenuating epidermal growth factor receptor tyrosine kinase. Sci. Rep. 2017, 7, 5851. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, A.; Xiao, M.; Sun, L.; Mao, J.; Luo, G.; Xiang, H. A new class of 1,3,5-triazine-based selective estrogen receptor degraders (SERDs): Lead optimization, molecular docking and dynamic simulation. Bioorg. Chem. 2020, 97, 1036662. [Google Scholar] [CrossRef]
- Junaid, A.; Lim, F.P.L.; Chuah, L.H.; Dolzhenko, A.V. 6,N2-Diaryl-1,3,5-triazine-2,4-diamines: Synthesis, antiproliferative activity and 3D-QSAR modeling. RSC Adv. 2020, 10, 12135–12144. [Google Scholar] [CrossRef]
- Lakshmithendral, K.; Saravanan, K.; Elancheran, R.; Archana, K.; Manikandan, N.; Arjun, H.A.; Ramanathan, M.; Lokanath, N.K.; Kabilan, S. Design, synthesis and biological evaluation of 2-(phenoxymethyl)-5-phenyl-1,3,4-oxadiazole derivatives as anti-breast cancer agents. Eur. J. Med. Chem. 2019, 168, 1–10. [Google Scholar] [CrossRef]
- El-Din, M.M.G.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Oh, C.-H. Synthesis and in vitro antiproliferative activity of new 1,3,4-oxadiazole derivatives possessing sulfonamide moiety. Eur. J. Med. Chem. 2015, 90, 45–52. [Google Scholar] [CrossRef]
- Dhawan, S.; Kerru, N.; Awolade, P.; Singh-Pillay, A.; Saha, S.T.; Kaur, M.; Singh, P. Synthesis, computational studies and antiproliferative activities of coumarin-tagged 1,3,4-oxadiazole conjugates against MDA-MB-231 and MCF-7 human breast cancer cells. Bioorg. Med. Chem. 2018, 26, 5612–5623. [Google Scholar] [CrossRef] [PubMed]
- Tahmasvand, R.; Bayat, P.; Vahdaniparast, S.M.; Dehghani, S.; Kooshafar, Z.; Khaleghi, S.; Almasirad, A.; Salimi, M. Design and synthesis of novel 4-thiazolidinone derivatives with promising anti-breast cancer activity: Synthesis, characterization, in vitro and in vivo results. Bioorg. Chem. 2020, 104, 104276. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Chimento, A.; Saturnino, C.; Gomez-Monterrey, I.M.; Musella, S.; Milite, C.; Sinicropi, M.S.; Caruso, A.; Sirianni, R.; Tortorella, P.; et al. Synthesis and cytotoxic activity evaluation of 2,3-thiazolidin-4-one derivatives on human breast cancer cell lines. Bioorg. Med. Chem. Lett. 2013, 23, 4990–4995. [Google Scholar] [CrossRef] [PubMed]
- El-Kashef, H.; Badr, G.; El-Maali, N.A.; Sayed, D.; Melnyk, P.; Lebegue, N.; El-Khalek, R.A. Synthesis of a novel series of (Z)-3,5-disubstituted thiazolidine-2,4-diones as promising anti-breast cancer agents. Bioorg. Chem. 2020, 96, 103569. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, W.; Huang, Y.; Li, Y.; Peng, Z. Design, synthesis and biological evaluation of isoxazole-naphthalene derivatives as anti-tubulin agents. Arab. J. Chem. 2020, 13, 5765–5775. [Google Scholar] [CrossRef]
- Wang, G.; Liu, W.; Peng, Z.; Huang, Y.; Gong, Z.; Li, Y. Design, synthesis, molecular modeling, and biological evaluation of pyrazole-naphthalene derivatives as potential anticancer agents on MCF-7 breast cancer cells by inhibiting tubulin polymerization. Bioorg. Chem. 2020, 103, 104141. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Yadav, Y.; Naidu, A.B.; Rao, V.K.; Kumar, A.; Parmar, V.S.; MacDonald, W.J.; Too, C.K.; Balzarini, J.; Barden, C.J.; et al. Design, synthesis and bioevaluation of novel 6-(4-hydroxypiperidino)naphthalen-2-ol-based potential selective estrogen receptor modulators for breast cancer. Eur. J. Med. Chem. 2015, 92, 103–114. [Google Scholar] [CrossRef]
- Reddy, S.T.; Mendonza, J.J.; Makani, V.K.K.; Bhadra, M.P.; Uppuluri, V.M. Synthesis of some novel methyl β-orsellinate based 3, 5-disubstituted isoxazoles and their anti-proliferative activity: Identification of potent leads active against MCF-7 breast cancer cell. Bioorg. Chem. 2020, 105, 104374. [Google Scholar] [CrossRef] [PubMed]
- Dawood, D.H.; Nossier, E.S.; Ali, M.M.; Mahmoud, A.E. Synthesis and molecular docking study of new pyrazole derivatives as potent anti-breast cancer agents targeting VEGFR-2 kinase. Bioorg. Chem. 2020, 101, 103916. [Google Scholar] [CrossRef]
- Coskun, D.; Tekin, S.; Sandal, S.; Coskun, M.F. Synthesis, characterization, and anticancer activity of new benzofuran substituted chalcones. J. Chem. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Jina, L.P.; Xiea, Q.; Huanga, E.F.; Wanga, L.; Zhanga, B.-Q.; Hub, J.-S.; Wanb, D.C.C.; Jina, Z.; Hua, C. Design, synthesis, and biological activity of a novel series of benzofuran derivatives against oestrogen receptor-dependent breast cancer cell lines. Bioorg. Chem. 2020, 95, 103566. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Nocentini, A.; Elsayed, Z.M.; Al-Warhi, T.; Aljaeed, N.; Alotaibi, O.J.; Al-Sanea, M.M.; Abdel-Aziz, H.A.; Supuran, C.T. Benzofuran-based carboxylic acids as carbonic anhydrase inhibitors and antiproliferative agents against breast cancer. ACS Med. Chem. Lett. 2020, 11, 1022–1027. [Google Scholar] [CrossRef]
- Shaldam, M.; Eldehna, W.M.; Nocentini, A.; Elsayed, Z.M.; Ibrahim, T.M.; Salem, R.; El-Domany, R.A.; Capasso, C.; Abdel-Aziz, H.A.; Supuran, C.T. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur. J. Med. Chem. 2021, 216, 113283. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Al Rashood, S.T.; Al-Warhi, T.; Eskandrani, R.O.; Alharbi, A.; El Kerdawy, A.M. Novel oxindole/benzofuran hybrids as potential dual CDK2/GSK-3β inhibitors targeting breast cancer: Design, synthesis, biological evaluation, and in silico studies. J. Enzyme Inhib. Med. Chem. 2021, 36, 270–285. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Ana, G.; Kelly, P.M.; Nathwani, S.M.; Noorani, S.; Fayne, D.; Bright, S.A.; Twamley, B.; Zisterer, D.M.; Meegan, M.J. Synthesis and evaluation of antiproliferative microtubule-destabilising combretastatin A-4 piperazine conjugates. Org. Biomol. Chem. 2019, 17, 6184. [Google Scholar] [CrossRef]
- Bhat, M.A.; Al-Dhfyan, A.; Khan, A.A.; Al-Harbi, N.; Manogaran, P.S.; Alanazi, A.M.; Fun, H.-K.; AlOmar, M.A. Targeting HER-2 over expressed breast cancer cells with 2-cyclohexyl-N-[(Z)-(substituted phenyl/furan-2-yl/thiophene-2-yl)methylidene]hydrazine-carbothioamide. Bioorg. Med. Chem. Lett. 2015, 25, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Weldon, D.J.; Saulsbury, M.D.; Goh, J.; Rowland, L.; Campbell, P.; Robinson, L.; Miller, C.; Christian, J.; Amis, L.; Taylor, N.; et al. One-pot synthesis of cinnamylideneacetophenones and their in vitro cytotoxicity in breast cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 3381–3384. [Google Scholar] [CrossRef] [Green Version]
- Varela, C.L.; Amaral, C.; da Silva, E.T.; Lopes, A.; Correiada-Silva, G.; Carvalho, R.A.; Costa, S.C.; Roleira, F.M.; Teixeira, N. Exemestane metabolites: Synthesis, stereochemical elucidation, biochemical activity and antiproliferative effects in a hormone-dependent breast cancer cell line. Eur. J. Med. Chem. 2014, 87, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Hussain, M.K.; Arun, A.; Chakravarti, B.; Konwar, R.; Hajela, K. Synthesis of targeted dibenzo [b,f]thiepines and dibenzo[b,f]oxepines as potential leadmolecules with promising anti-breast cancer activity. Eur. J. Med. Chem. 2015, 99, 113–124. [Google Scholar] [CrossRef]
- Nikolic, A.R.; Petri, E.T.; Klisuric, O.R.; Celic, A.S.; Jakimov, D.S.; Djurendic, E.A.; Gasi, K.M.P.; Sakac, M.N. Synthesis and anticancer cell potential of steroidal 16,17-seco-16,17a-dinitriles: Identification of a selective inhibitor of hormone-independent breast cancer cells. Bioorg. Med. Chem. 2015, 23, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Mahajan, M.P.; Pandey, M.K.; Singh, P.; Ramisetti, S.R.; Sharma, A.K. Design, synthesis and evaluation of ospemifene analogs as anti-breast cancer agents. Eur. J. Med. Chem. 2014, 86, 211–218. [Google Scholar] [CrossRef]
- Eldehna, W.M.; El Hassab, M.A.; Abo-Ashour, M.F.; Al-Warhi, T.; Elaasser, M.M.; Safwat, N.A.; Suliman, H.; Ahmed, M.F.; Al-Rashood, S.T.; Abdel-Aziz, H.A.; et al. Development of isatin-thiazolo[3,2-a]benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic antiproliferative activity: Synthesis, biological and molecular dynamics investigations. Bioorg. Chem. 2021, 110, 104748. [Google Scholar] [CrossRef]
- Alkhaldi, A.A.; Al-Sanea, M.M.; Nocentini, A.; Eldehna, W.M.; Elsayed, Z.M.; Bonardi, A.; Abo-Ashour, M.F.; El-Damasy, A.K.; Abdel-Maksoud, M.S.; Al-Warhi, T.; et al. 3-Methylthiazolo[3,2-a]benzimidazole-benzenesulfonamide conjugates as novel carbonic anhydrase inhibitors endowed with anticancer activity: Design, synthesis, biological and molecular modeling studies. Eur. J. Med. Chem. 2020, 207, 112745. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Yang, Y.; Liu, X. Ferrocenyl compounds derived from the reaction of phenylamines with ferrocene carbonyl chloride: Synthesis, characterization and their biological activity. Inorg. Chim. Acta 2016, 447, 121–126. [Google Scholar] [CrossRef]
- Tan, Y.L.K.; Pigeon, P.; Top, S.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessières, A.; Amatore, C.; Leong, W.K.; Jaouen, G. Ferrocenyl catechols: Synthesis, oxidationchemistry and anti-proliferative effects on MDA-MB-231breast cancer cells. Dalton Trans. 2012, 41, 7537–7549. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, C.; Li, C.; Qiao, J.; Zhang, F.; Huang, M.; Ren, W.; Dong, C.; Huang, J.; Zhou, H.B. Discovery ofnovel SERMs with a ferrocenyl entity based on theoxabicyclo[2.2.1]heptene scaffold and evaluation of their antiproliferative effects in breast cancer cells. Org. Biomol. Chem. 2012, 10, 9689–9699. [Google Scholar] [CrossRef]
- de Jesús Cázares-Marinero, J.; Top, S.; Jaouen, G. Synthesis and characterization of new ferrocenylcompounds with different alkyl chain lengths andfunctional groups to target breast cancer cells. J. Organomet. Chem. 2014, 751, 610–619. [Google Scholar] [CrossRef]
- Wambang, N.; Schifano-Faux, N.; Aillerie, A.; Baldeyrou, B.; Jacquet, C.; Bal-Mahieu, C.; Bousquet, T.; Pellegrini, S.; Ndifon, P.T.; Meignan, S.; et al. Synthesis and biological activity of ferrocenylindeno[1,2-c]isoquinolines as topoisomerase II inhibitors. Bioorg. Med. Chem. 2016, 15, 651–660. [Google Scholar] [CrossRef]
- Wu, D.; Guo, L.; Li, S.-J. Synthesis, structural characterization and anti-breast cancer activity evaluation of three new Schiff base metal (II) complexes and their nanoparticles. J. Mol. Struct. 2020, 1199, 126938. [Google Scholar] [CrossRef]
- Stojkovic, D.L.; Jevtic, V.V.; Radic, G.P.; Đacic, D.S.; Curcic, M.G.; Markovic, S.D.; Đinovic, V.M.; Petrovic, V.P.; Trifunovic, S.R. Stereospecifc ligands and their complexes. Part XII. Synthesis, characterization and in vitro antiproliferative activity of platinum(IV) complexes with some O,O’-dialkyl esters of (S,S)-ethylenediamineN,N’-di-2-propanoic acid against colon cancer (HCT-116) and breast cancer (MDA-MB-231) cell lines. J. Mol. Struct. 2014, 1062, 21–28. [Google Scholar]
- Varela, J.G.; De Chatterjee, A.; Guevara, P.; Ramirez, V.; Metta-Magana, A.J.; Villagran, D.; Varela-Ramirez, A.; Das, S.; Nunez, J.E. Synthesis, characterization, and evaluation of cis-diphenyl pyridineamine platinum(II) complexes as potential anti-breast cancer agents. JBIC J. Biol. Inorg. Chem. 2014, 19, 967–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutlu, T.; Yıldırım, I.; Karabıyık, H.; Kılınçlı, A.; Tekedereli, İ.; Gök, Y.; Dikmen, M.; Akta, A. Cytotoxic activity and apoptosis induction by a series Ag(I)-NHC complexes on human breast cancer cells and non-tumorigenic epithelial cell line. J. Mol. Struct. 2020, 1228, 129462. [Google Scholar] [CrossRef]



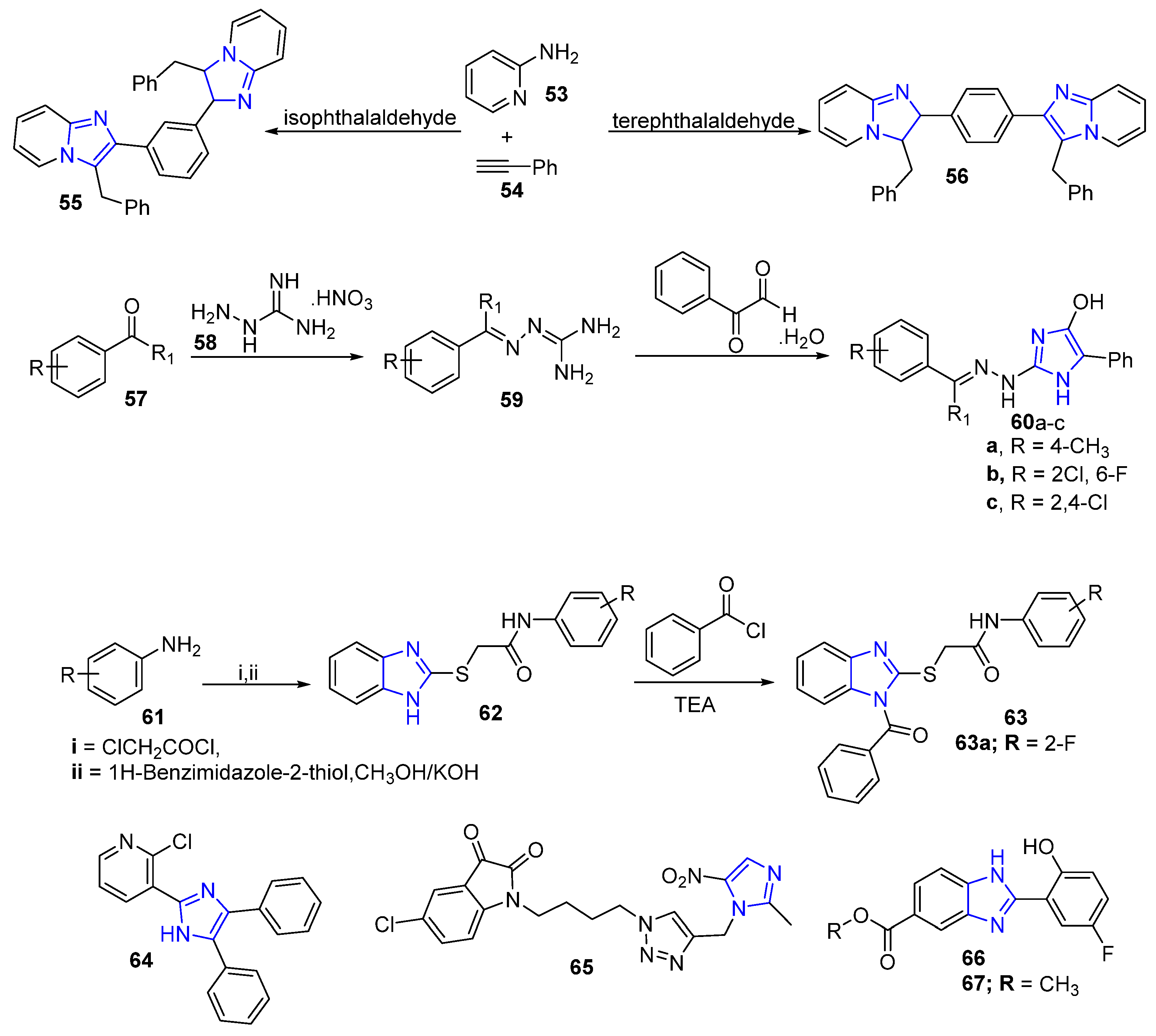


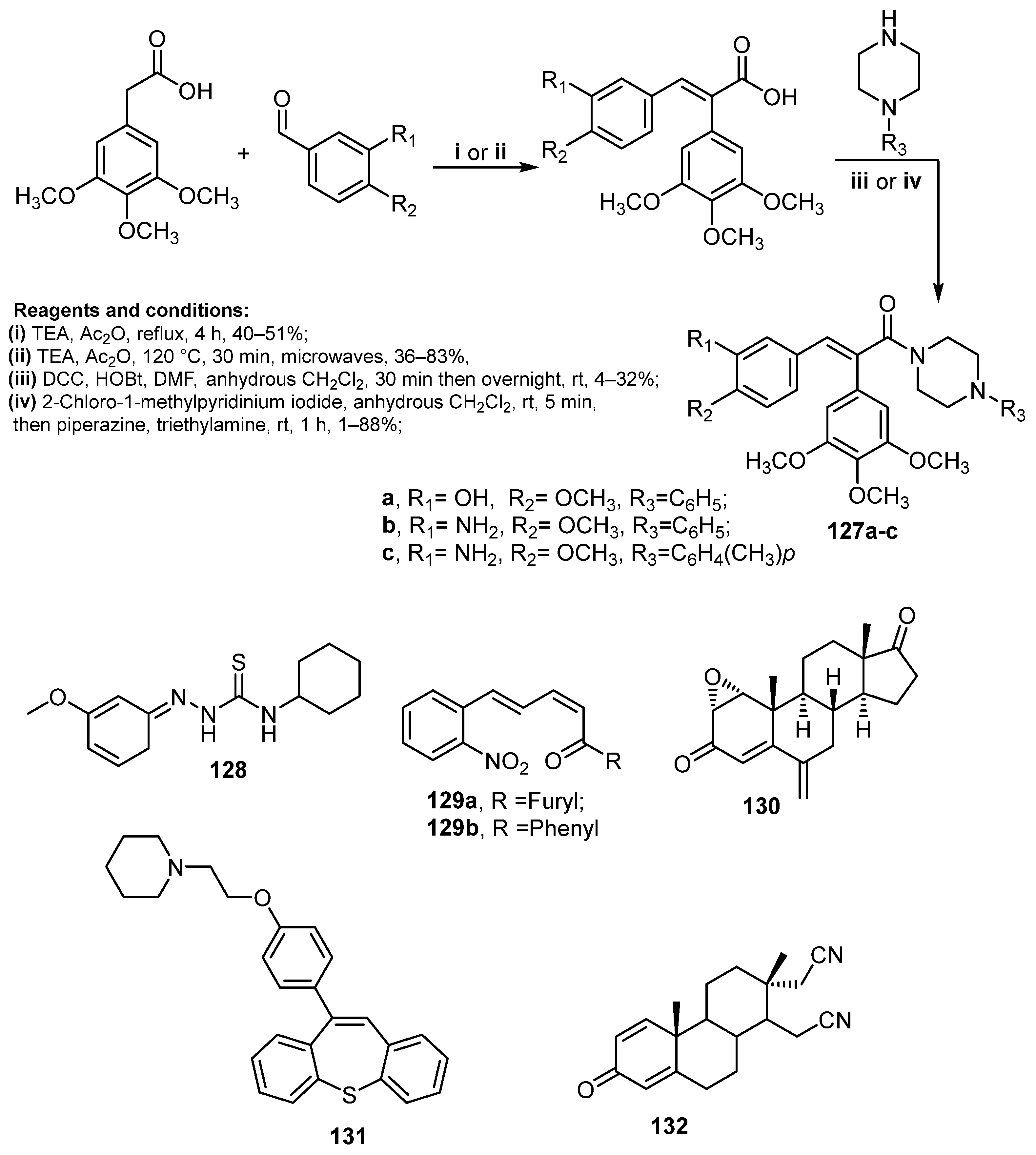
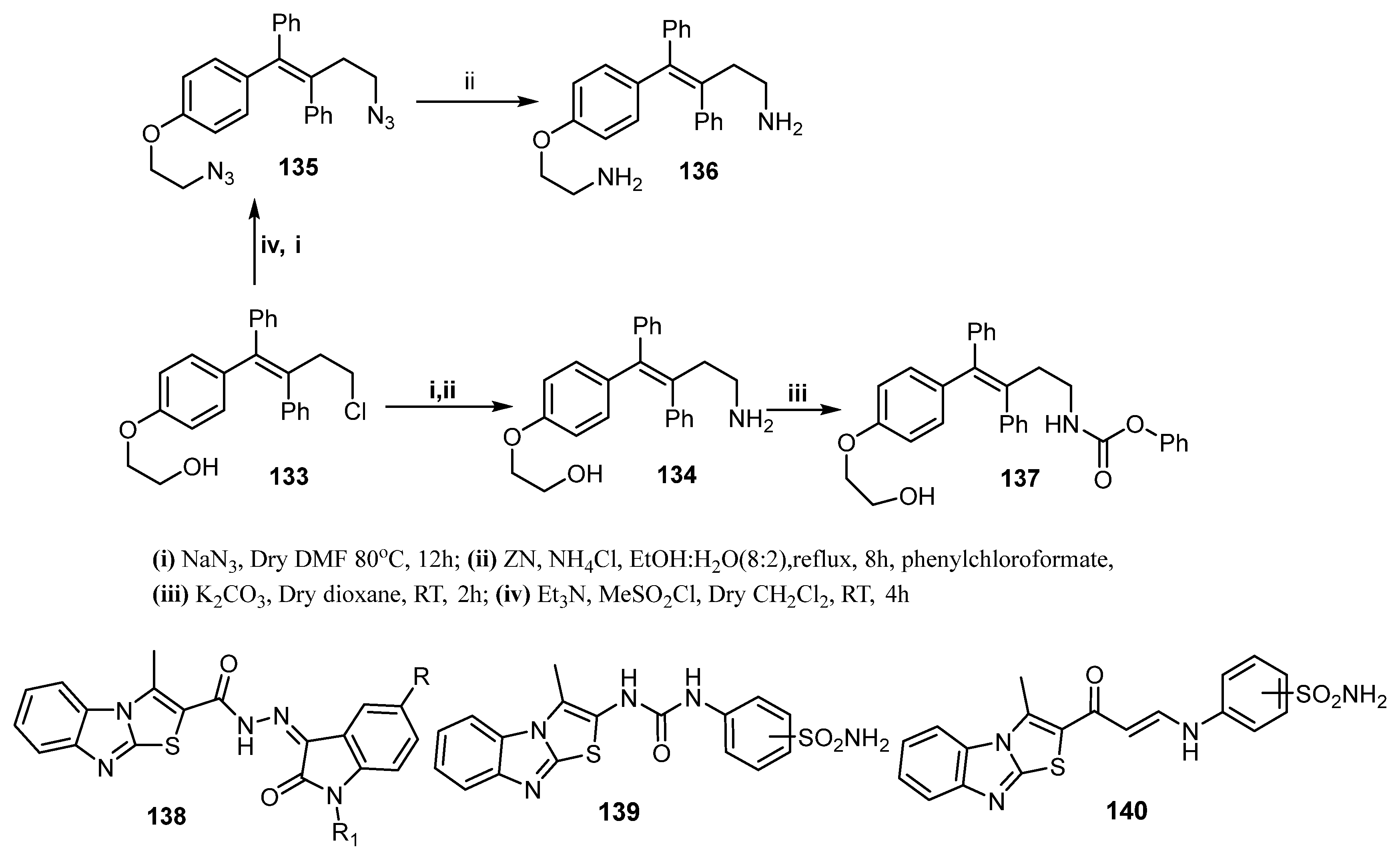
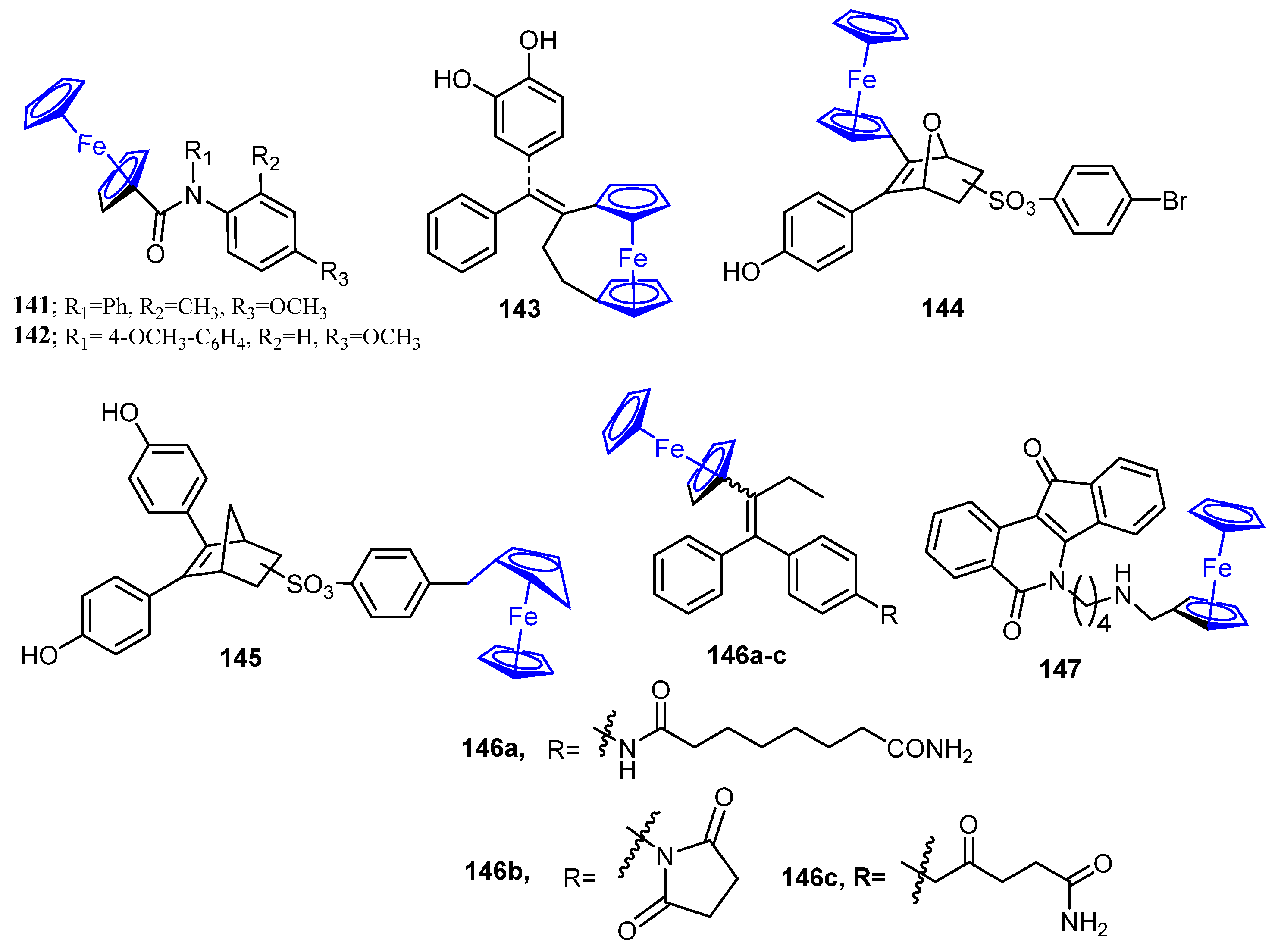
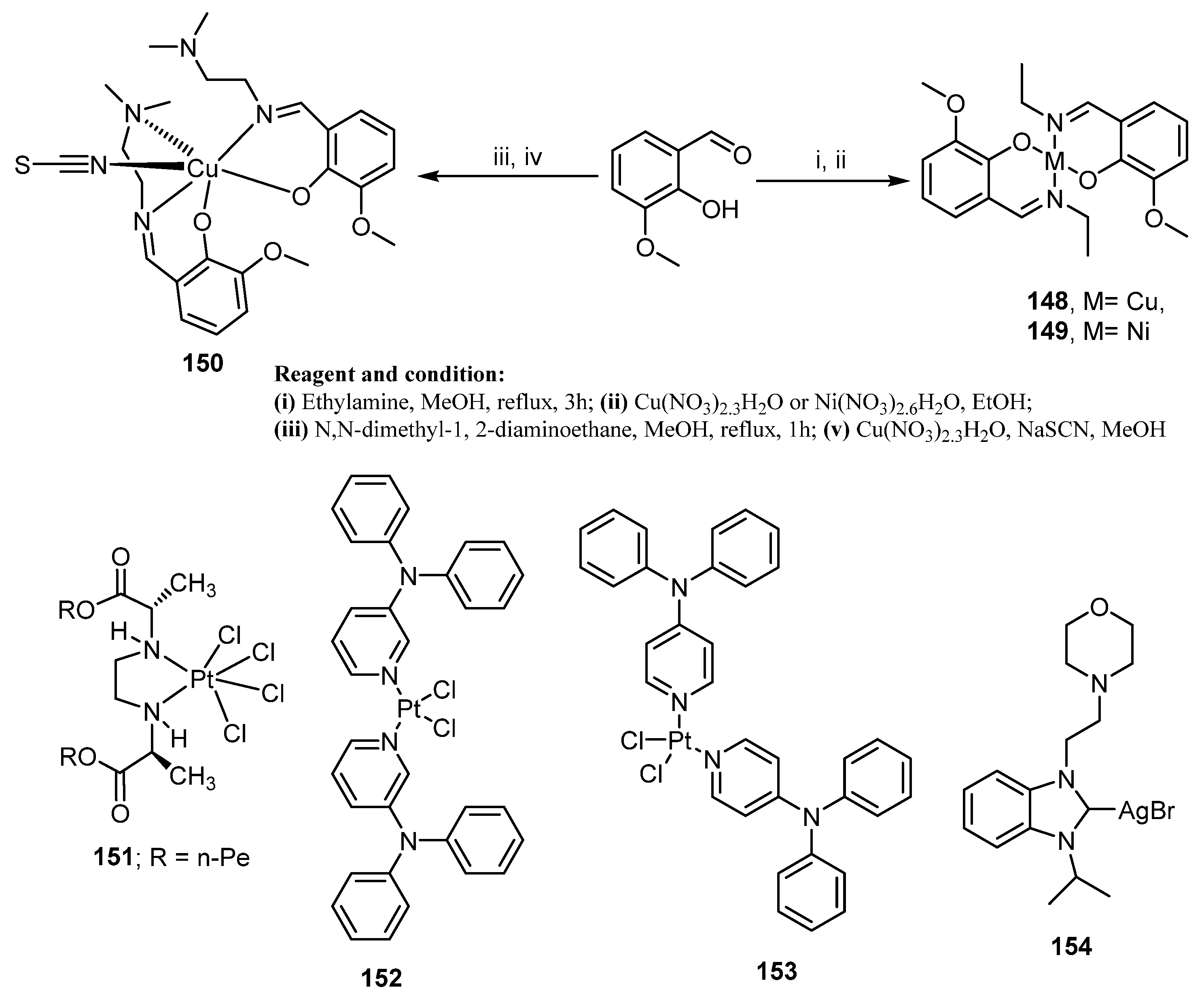
| Comp. | Enzyme Inhibition IC50 (nM) | Cell Growth Inhibition IC50 (μM) | ||
|---|---|---|---|---|
| EGFR | HER2 | A549 | SK-Br3 | |
| 18 | 8 ± 0.4 | 33 ± 0.10 | 2.03 ± 0.54 | 12.50 ± 2.41 |
| 19 | 10 ± 0.2 | 21 ± 0.7 | 3.60 ± 0.89 | 2.30 ± 0.37 |
| 20 | 20 ± 0.11 | 9 ± 0.10 | 1.22 ± 0.60 | 25.1 ± 8.54 |
| 21 | 19 ± 0.10 | 35 ± 0.8 | 4.49 ± 2.68 | 0.47 ± 0.35 |
| Lapatinib | 26 ± 0.12 | 17 ± 0.10 | 6.74 ± 1.33 | 0.49 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkaeed, E.B.; Salam, H.A.A.E.; Sabt, A.; Al-Ansary, G.H.; Eldehna, W.M. Recent Advancements in the Development of Anti-Breast Cancer Synthetic Small Molecules. Molecules 2021, 26, 7611. https://doi.org/10.3390/molecules26247611
Elkaeed EB, Salam HAAE, Sabt A, Al-Ansary GH, Eldehna WM. Recent Advancements in the Development of Anti-Breast Cancer Synthetic Small Molecules. Molecules. 2021; 26(24):7611. https://doi.org/10.3390/molecules26247611
Chicago/Turabian StyleElkaeed, Eslam B., Hayam A. Abd El Salam, Ahmed Sabt, Ghada H. Al-Ansary, and Wagdy M. Eldehna. 2021. "Recent Advancements in the Development of Anti-Breast Cancer Synthetic Small Molecules" Molecules 26, no. 24: 7611. https://doi.org/10.3390/molecules26247611
APA StyleElkaeed, E. B., Salam, H. A. A. E., Sabt, A., Al-Ansary, G. H., & Eldehna, W. M. (2021). Recent Advancements in the Development of Anti-Breast Cancer Synthetic Small Molecules. Molecules, 26(24), 7611. https://doi.org/10.3390/molecules26247611








