Plasmonic Spherical Nanoparticles Coupled with Titania Nanotube Arrays Prepared by Anodization as Substrates for Surface-Enhanced Raman Spectroscopy Applications: A Review
Abstract
:1. Introduction
- (1)
- Provide a background on the SERS principles that pave the way to further discussion within the review.
- (2)
- Justify the use of titania nanotubes prepared by anodization as an appropriate substrate for metal nanoparticles in SERS.
- (3)
- Discuss the main features of the synthesis of titania nanotubes that influence the metal nanoparticle deposition.
- (4)
- Present information about the metal deposition techniques on the TiO2 nanotubes substrate and the resulting SERS performance.
2. Raman Spectroscopy
3. Surface-Enhanced Raman Spectroscopy (SERS)
4. SERS Substrates and TiO2/MNPs Arrays
- (1)
- Reproducibility, providing similar enhancements across the whole surface by arranging metal nanoparticles regularly. In the same sense, the fabrication of SERS substrate must be reproducible from array to array or from batch to batch.
- (2)
- Stable and unresponsive to environmental conditions like humidity, oxygen, and light.
- (3)
- Cost-effective and easy to handle.
- (4)
- Highly stable and biocompatible if biological molecules or specimens are to be detected.
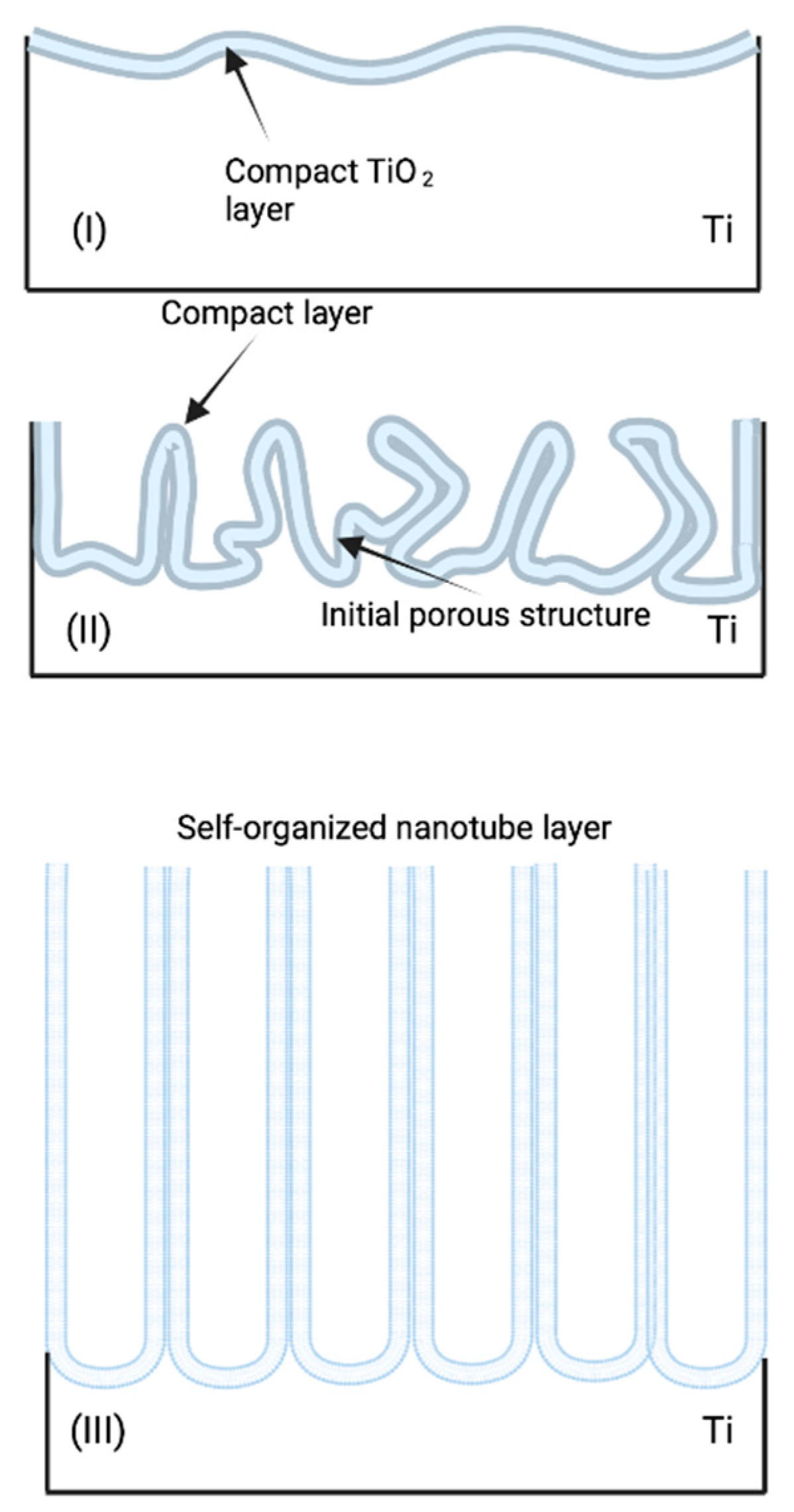
Titanium Dioxide Nanotubes (TiO2 NTs) Prepared by Anodization
5. Noble Metal Nanoparticles
5.1. Silver Nanoparticles (Ag NPs)
Silver Nanoparticle Deposition on TiO2 NTs
5.2. Gold Nanoparticles (Au NPs)
Gold Nanoparticle Deposition on TiO2 NTs
5.3. Platinum Nanoparticles (Pt NPs)
Platinum Nanoparticle Deposition on TiO2 NTs
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Kumar, P.; Das, A.; Pathak, C.S. Surface-enhanced raman scattering: Introduction and applications. In Recent Advances in Nanophotonics. Fundamentals and Applications; Kahrizi, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Li, R.; Zhou, A.; Lu, Q.; Yang, C.; Zhang, J. In situ monitoring and analysis of the photocatalytic degradation process and mechanism on recyclable Au NPs-TiO2 NTs substrate using surface-enhanced Raman scattering. Colloid Surf. A Physicochem. Eng. Asp. 2013, 436, 270–278. [Google Scholar] [CrossRef]
- Li, D.W.; Zhai, W.L.; Li, Y.T.; Long, Y.T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23–43. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef]
- Krajczewski, J.; Ambroziak, R.; Kudelski, A. Substrates for Surface-Enhanced Raman Scattering Formed on Nanostructured Non-Metallic Materials: Preparation and Characterization. Nanomaterials 2021, 11, 75. [Google Scholar] [CrossRef]
- Procházka, M. Basics of Surface-enhanced raman scattering (SERS). In Surface-Enhanced Raman Spectroscopy. Bioanalytical, Biomolecular and Medical Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.; Zhen, D.; Gu, W.; Liu, Y.; Cai, Q. Au nanoparticle-modified WO3 nanoflowers/TiO2 nanotubes used for the SERS detection of dyes. New J. Chem. 2017, 41, 13968–13973. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Ngo, Y.H.; Li, D.; Simon, G.P.; Garnier, G. Effect of cationic polyacrylamides on the aggregation and SERS performance of gold nanoparticles-treated paper. J. Colloid Interface Sci. 2012, 392, 237–246. [Google Scholar] [CrossRef]
- Ambroziak, R.; Krajczewski, J.; Pisarek, M.; Kudelski, A. Immobilization of cubic silver plasmonic nanoparticles on TiO2 nanotubes, reducing the coffee ring effect in surface-enhanced raman spectroscopy applications. ACS Omega 2020, 5, 13963–13972. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, R.; Liu, G.K.; Wang, Y.H.; Liu, J.Y.; Ding, S.Y.; Li, J.F.; Wu, D.Y.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Bottlenecks and future directions. Chem. Commun. 2017, 54, 10–25. [Google Scholar] [CrossRef]
- Lin, J.; Wu, A. Surface-Enhanced Raman spectrum of TiO2 nanoparticle for biosensing (TiO2 nanoparticle served as SERS sensing substrate). In TiO2 Nanoparticles: Applications in Nanobiotechnology and Nanomedicine; Wu, A., Ren, W., Eds.; Wiley-VCH: Weinhein, Germany, 2020; pp. 133–152. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of surface-enhanced Raman scattering in semiconductors. J. Phys. Chem. 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Yang, B.; Jin, S.; Guo, S.; Park, Y.; Chen, L.; Zhao, B.; Jung, Y.M. Recent development of SERS technology: Semiconductor-based study. ACS Omega 2019, 4, 20101–20108. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.; Ge, M.; Iocozzia, J.; Lin, Z.; Zhang, K.Q.; Lai, Y. Immobilization of Pt nanoparticles via rapid and reusable electropolymerization of dopamine on TiO2 nanotube arrays for reversible SERS substrates and nonenzymatic glucose sensors. Small 2017, 13, 1604240. [Google Scholar] [CrossRef]
- Gupta, R.K.; Bills, B.; Dubey, M.; Galipeau, D.; Fan, Q.H. Light scattering behavior of oxide nanoparticles. In Proceedings of the IEEE International Conference on Electro-Information Technology, Rapid City, SD, USA, 9–11 May 2013. [Google Scholar] [CrossRef]
- Liu, L.; Pan, F.; Liu, C.; Huang, L.; Li, W.; Lu, X. TiO2 nanofoam-nanotube array for surface-enhanced raman scattering. ACS Appl. Nano Mater. 2018, 1, 6563–6566. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Jin, Y.; Zhou, Y.; Wang, Y. SERS activity of self-cleaning silver/titania nanoarray. Appl. Surf. Sci. 2014, 313, 549–557. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Lin, X.; Rong, F.; Yuan, C.; Fu, D. Surface-enhanced Raman scattering study of Au nanoparticles electrodeposited on TiO2 nanotube film. Nanosci. Nanotechnol. Lett. 2013, 5, 243–247. [Google Scholar] [CrossRef]
- Pisarek, M.; Holdynski, M.; Roguska, A.; Kudelski, A.; Janik-Czachor, M. TiO2 and Al2O3 nanporous oxide layers decorated with silver nanoparticles—Active substrates for SERS measurements. J. Solid State Electrochem. 2014, 18, 3099–3109. [Google Scholar] [CrossRef] [Green Version]
- Pisarek, M.; Krajczewski, J.; Holdynski, M.; Plocinski, T.; Krawczyk, M.; Kudelski, A.; Janik-Czachr, M. Titanium (lV) oxide nanotubes in design of active SERS substrates for high sensitivity analytical applications: Effect of geometrical factors in nanotubes and in Ag-n deposits. In Raman Spectroscopy; Morari Do Nascimento, G., Ed.; IntechOpen: London, UK, 2018; pp. 37–54. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Ji, W.; Mao, Z.; Li, Z.; Guo, Z.; Zhao, B.; Zhao, C. SERS study of Co-doped TiO2 nanoparticles. Chem. Res. Chin. 2013, 29, 751–754. [Google Scholar] [CrossRef]
- Feng, J.; Bao, W.; Li, L.; Cheng, H.; Huang, W.; Kong, H.; Li, Y. The synergistic effect of nitrogen-doped titanium dioxide/mercaptobenzoic acid/silver nanocomplexes for surface-enhanced Raman scattering. J. Nanopart. Res. 2018, 20, 3–15. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.E.; Vallejo-Loazada, W.A.; Martinez-Ortega, F. Synthesis and characterization of TiO2 thin films doped with copper to be used in photocatalysis. ITECKNE 2013, 10, 16–20. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Efrima, S.; Zeiri, L. Understanding SERS of bacteria. J. Raman Spectrosc. 2009, 40, 277–288. [Google Scholar] [CrossRef]
- Dong, R.; Weng, S.; Yang, L.; Liu, J. Detection and direct readout of drugs in human urine using dynamic surface-enhanced Raman spectroscopy and support vector machines. Anal. Chem. 2015, 87, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.A.; Vikesland, P.J. Surface-enhanced Raman spectroscopy (SERS) for environmental analyses. Environ. Sci. Technol. 2010, 44, 7749–7755. [Google Scholar] [CrossRef]
- Hernández-Arteaga, D.; Zermeño Nava, J.J.; Kolosovas-Machuca, E.S.; Velázquez-Salazar, J.J.; Vinogradova, E.; José-Yacamán, M.; Navarro-Contreras, H.R. Diagnosis of breast cancer by analysis of sialic acid concentrations in human saliva by surface-enhanced Raman spectroscopy of silver nanoparticles. Nano Res. 2017, 10, 3662–3670. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Das, R.S.; Agrawal, Y.K. Raman spectroscopy: Recent advancements, techniques and applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Cai, W. Imaging with Raman Spectroscopy. Curr. Pharm. Biotechnol. 2010, 11, 654–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostron, P.; Gaber, S.; Gaber, D. Raman Spectroscopy, Review. IJETR 2016, 6, 50–63. [Google Scholar]
- Vašková, H. A powerful tool for material identification: Raman spectroscopy. Math. Models Methods Appl. Sci. 2011, 5, 1205–1212. [Google Scholar]
- Jeanmaire, D.L.; Van Duyne, R.P.J. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Han, D.; Huang, H.; Du, D.; Lang, X.; Long, K.; Hao, Q.; Qiu, T. Facile synthesis of gold-capped TiO2 nanocomposites for surface-enhanced Raman scattering. Mater. Chem. Phys. 2015, 153, 88–92. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Zhou, W.M.; Wang, J.; Wang, X.G.; Li, J.F.; Li, Y.; Wang, C.W. Ag loaded TiO2 nanotube photonic crystals self-doped Ti3+ periodically by anodization process and their performance of surface enhanced Raman scattering. Opt. Mater. 2019. [Google Scholar] [CrossRef]
- Haynes, C.; McFarland, A.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2005, 77, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and applications of surface-enhanced Raman spectroscopy (SERS) based biosensors. Curr. Opin. Biomed. Eng. 2019, 13, 51–59. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 2–42. [Google Scholar] [CrossRef]
- Lamberti, A.; Virga, A.; Chiadó, A.; Chiodoni, A.; Bejtka, K.; Rivolo, P.; Giorgis, F. Ultrasensitive Ag-coated TiO2 nanotube arrays for flexible SERS-based optofluidic devices. J. Mater. Chem. C 2015, 3, 6868–6875. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, Y.; Wu, M. Visible-light driven self-cleaning SERS substrate of silver nanoparticles and graphene oxide decorated nitrogen-doped titania nanotube array. Surf. Interface Anal. 2016, 48, 334–340. [Google Scholar] [CrossRef]
- Xie, W.; Qiu, P.; Mao, C. Bio-imaging, detection and analysis by using nanostructures as SERS substrates. J. Mater. Chem 2011, 21, 5190–5202. [Google Scholar] [CrossRef]
- Bora, T. Recent developments on metal nanoparticles for SERS applications. In Noble Metals—Properties, Nanoscale Effects and Applications; Seehra, M., Ed.; IntechOpen: London, UK, 2018; pp. 117–135. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, A.; Virga, A.; Giorgis, F. Microfluidic electrochemical growth of vertically aligned TiO2 nanotubes for SERS optofluidic devices. RSC Adv. 2015, 5, 105484–105488. [Google Scholar] [CrossRef]
- Mosier Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, J.; Jia, H.; Li, M.; Zeng, J.; Yang, B.; Zhao, B.; Xu, W.; Lombardi, J.R. Mercaptopyridine Surface-functionalized CdTe quantum dots with enhanced raman scattering properties. J. Phys. Chem. C 2008, 112, 996–1000. [Google Scholar] [CrossRef]
- Jiang, L.; You, T.; Ying, P.; Shang, Y.; Zhang, S.; Guo, L.; Yang, S. Surface-enhanced Raman scattering spectra of adsorbates on Cu2O nanospheres: Charge-transfer and electromagnetic enhancement. Nanoscale 2013, 5, 2784–2789. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Considine, C.R.; Menon, V.; Lin, W.C.; Lee, Y.H.; Lombardi, J.R. Ultra-high raman enhancement on monolayer MoS. ACS Photonics 2016, 3, 1164–1169. [Google Scholar] [CrossRef]
- Christie, D.; Lombardi, J.; Kretzschmar, I. Two-dimensional array of silica particles as a SERS substrate. J. Phys. Chem. C 2014, 118, 9114–9118. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, P.; You, T.; Wang, H.; Lang, X.; Guo, L.; Yang, S. Highly reproducible surface-enhanced raman spectra on semiconductor SnO2 octahedral nanoparticles. ChemPhysChem 2012, 13, 3932–3936. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS sensitivity on plasmon-free TiO2 photonic microarray by enhancing light-matter coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Wang, S.; Zhao, H.; Lin, J.; Yang, Z.; Chen, M.; Guo, L. Two-dimensional amorphous TiO2 nanosheets enabling high-efficiency photoinduced charge transfer for excellent SERS activity. J. Am. Chem. Soc. 2019, 141, 5856–5862. [Google Scholar] [CrossRef]
- Xue, X.; Ji, W.; Mao, Z.; Mao, H.; Wang, Y.; Wang, X.; Ruan, W.; Zhao, B.; Lombardi, J.R. Raman investigation of nanosized TiO2: Effect of crystallite size and quantum confinement. J. Phys. Chem. 2012, 116, 8792–9897. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Guo, L. Precursor-directed self-assembly of porous ZnO nanosheets as high-performance surface-enhanced raman scattering substrate. Small 2014, 10, 48–51. [Google Scholar] [CrossRef]
- Islam, S.K.; Tamargo, M.; Moug, R.; Lombardi, J.R. Surface-enhanced raman scattering on a chemically etched ZnSe surface. J. Phys. Chem. C 2013, 117, 23372–23377. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Hu, H.; Jing, S.; Zhao, B.; Xu, W.; Zhao, C.; Lombardi, J.R. Raman scattering study of molecules adsorbed on ZnS nanocrystals. J. Raman Spectrosc. 2007, 38, 34–38. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Lai, W.; Jiang, T.; Zhou, J. Bifunctional 4MBA mediated recyclable SERS-based immunoassay induced by photocatalytic activity of TiO2 nanotube arrays. Phys. Chem. Chem. Phys. 2016, 18, 23795–23802. [Google Scholar] [CrossRef]
- Rupa, A.V.; Manikandan, D.; Divakar, D.; Sivakumar, T. Effect of deposition of Ag on TiO2 nanoparticles on the photodegradation of reactive yellow-17. J. Hazard. Mater. 2007, 147, 906–913. [Google Scholar] [CrossRef]
- Wang, Y.H.; Rahman, K.H.; Wu, C.C.; Chen, K.C. A review on the pathways of the improved structural characteristics and photocatalytic performance of titanium dioxide (TiO2) thin films fabricated by the magnetron-sputtering technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.; Deng, C.; Liu, Z.; Wang, R.; Zhao, H. Design and preparation of a recyclable microfluidic SERS chip with integrated Au@Ag/TiO2 NTs. RSC Adv. 2016, 6, 113115–113122. [Google Scholar] [CrossRef]
- Ling, Y.; Zhuo, Y.; Huang, L.; Mao, D. Using Ag-embedded TiO2 nanotubes array as recyclable SERS substrate. Appl. Surf. Sci. 2016, 388, 169–173. [Google Scholar] [CrossRef]
- Wang, J.; Fan, H.; Zhang, H.; Chen, Q.; Liu, Y.; Ma, W. Anodizing process of titanium and formation mechanism of anodic TiO2 nanotubes. Prog. Chem. 2016, 28, 284–295. [Google Scholar]
- Fu, Y.; Mo, A. A review on the electrochemically self-organized titania nanotube arrays: Synthesis, modifications, and biomedical applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cao, S.; Li, C.; Qi, J.; Jiang, L.; Zhang, J.; Zhu, X. Rapid growth of TiO2 nanotubes under the compact oxide layer: Evidence against the digging manner of dissolution reaction. Electrochem. Commun. 2019, 103, 88–93. [Google Scholar] [CrossRef]
- Apolinário, A.; Quitério, P.; Sousa, C.T.; Ventura, J.; Sousa, J.B.; Andrade, L.; Mendes, A.M.; Araújo, J.P. Modeling the growth kinetics of anodic TiO2 nanotubes. J. Phys. Chem. Lett. 2015, 6, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Shah, U.H.; Deen, K.M.; Asgar, H.; Rahman, Z.; Haider, W. Understanding the mechanism of TiO2 nanotubes formation at low potentials (≤8 V) through electrochemical methods. J. Electroanal. Chem. 2017, 807, 228–234. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Bio-Tribos-Corros. 2015, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, D.; Gao, M.; Li, D.; Song, Y.; Jin, R.; Ma, W.; Zhu, X. Growth of anodic TiO2 nanotubes in mixed electrolytes and novel method to extend nanotube diameter. Electrochim. Acta 2015, 160, 33–42. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Opara, M.; Janik-Czachor, M. Raman investigations of SERS activity of Ag nanoclusters on a TiO2-nanotubes/Ti substrate. Vib. Spectrosc. 2011, 55, 38–43. [Google Scholar] [CrossRef]
- Ambroziak, R.; Holdynski, M.; Plocinski, T.; Pisarek, M.; Kudelski, A. Cubic silver nanoparticles fixed on TiO2 nanotubes as simple and efficient substrates for surface enhanced raman scattering. Materials 2019, 12, 3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yang, L.; Liao, F.; Dang, Q.; Shao, M. Parameter optimization for Ag-coated TiO2 nanotube arrays as recyclable SERS substrates. Appl. Surf. Sci. 2018, 443, 613–618. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–879. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Jiang, X.; Du, J.; Li, X.; Han, X.; Yang, L.; Zhao, B. Anatase TiO2 nanoparticles with controllable crystallinity as a substrate for SERS: Improved charge-transfer contribution. RSC Adv. 2015, 5, 80269–80275. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, H.; Xu, Y.; Zhang, J.; Wang, L. Improving SERS sensitivity of TiO2 through utilizing the heterogeinity of the facets potentials. J. Mater. Chem. C 2020, 8, 13836–13842. [Google Scholar] [CrossRef]
- Yang, L.; Gong, M.; Jiang, X.; Yin, D.; Qin, X.; Zhao, B.; Ruan, W. Investigation on SERS of different phase structure TiO2 nanoparticles. J. Raman Spectrosc. 2015, 46, 287–292. [Google Scholar] [CrossRef]
- Barone, P.; Stranges, F.; Barberio, M.; Renzelli, D.; Bonanno, A.; Xu, F. Study of band gap of silver nanoparticles—Titanium dioxide nanocomposites. J. Chem. 2014, 2014, 589707. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Wei, W.; Wu, Y.; Li, T. Synthesis, characterization and application of TiO2/Ag recyclable SERS substrates. RSC Adv. 2017, 7, 26704–26709. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Smirniotis, P.G. Interaction of anatase and rutile TiO2 particles in aqueous photooxidation. Catal. Today 2003, 88, 49–59. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, F.; Li, Y. A review on the effects of TiO2 surface point defects on CO2 photoreduction with H2O. J. Mater. 2017, 3, 17–32. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Zhang, M.; Jiang, X.; Wang, Y.; Lv, J.; He, G.; Sun, Z. Three-dimensional hierarchical anatase@rutile TiO2 nanotree array films decorated by silver nanoparticles as ultrasensitive recyclable surface-enhanced Raman scattering substrates. J. Alloys Compd. 2017, 725, 1166–1174. [Google Scholar] [CrossRef]
- Boehme, M.; Ensinger, W. Mixed phase anatase/rutile titanium dioxide nanotubes for enhanced photocatalytic degradation of methylene-blue. Nano-Micro Lett. 2011, 3, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shen, X.; Liu, Y.; Wang, L.; Lei, J.; Zhang, J. Facile phase control for hydrothermal synthesis of anatase-rutile TiO2 with enhanced photocatalytic activity. J. Alloys Compd. 2015, 646, 380–386. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Pan, W. Enhanced photocatalytic activity of electrospun TiO2 nanofibers with optimal anatase/rutile ratio. J. Am. Ceram. Soc. 2011, 94, 3184–3187. [Google Scholar] [CrossRef]
- Chang, T.H.; Chang, Y.C.; Chen, C.M.; Chuang, K.W.; Chou, C.M. A facile method to directly deposit the large-scale Ag nanoparticles on a silicon substrate for sensitive, uniform, reproducible and stable SERS substrate. J. Alloys Compd. 2019, 782, 887–892. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, H.; Zang, L.; Jin, S.; Guo, S.; Park, E.; Mo, Z.; Jung, Y.M. Flexible and reusable Ag coated TiO2 nanotube arrays for highly sensitive SERS detection of formaldehyde. Molecules 2020, 25, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.X.; Liang, R.; Peng, P.; Zhou, Y.N. Effect of the size of silver nanoparticles on SERS signal enhancement. J. Nanopart. Res. 2017, 19, 696–703. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Garcia Ramos, J.V.; Sanchez Cortes, A.; Castillejo, M.; Oujja, M. Comparative SERS effectiveness of silver nanoparticles prepared by different methods: A study of the enhancement factor and the interfacial properties. J. Colloid Interface Sci. 2008, 326, 103–109. [Google Scholar] [CrossRef]
- Chong, X.; Zhao, B.; Li, R.; Ruan, W.; Yang, W. Photocatalytic degradation of rhodamine 6G on Ag modified TiO2 nanotubes: Surface-enhanced Raman scattering study on catalytic kinetics and substrate recyclability. Colloid. Surf. A-Physicochem. Eng. Asp. 2015, 481, 7–12. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Onishi, K.; Nishikawa, K. Effects of sputtering conditions on formation of gold nanoparticles in sputter deposition technique. RSC Adv. 2011, 1, 1815–1821. [Google Scholar] [CrossRef]
- Kudelski, A.; Pisarek, M.; Roguska, A.; Holdynski, M.; Janik-Czachor, M. Surface-enhanced Raman scattering investigations on silver nanoparticles deposited on alumina and titania nanotubes: Influence of the substrate material on surface-enhanced Raman scattering activity of Ag nanoparticles. J. Raman Spectrosc. 2012, 43, 1360–1366. [Google Scholar] [CrossRef]
- Rezaee, S. The effect of deposition time on the structural properties of silver nanoparticles deposited on anodic alumina templates. Results Phys. 2018, 9, 1521–1524. [Google Scholar] [CrossRef]
- Zhang, T.; Ye, J.; Arul, R.; Yang, T.; Wang, Y.; Yue, X.; Schaefer, M.; Simpson, C.; Nieuwoudt, M.K.; Huang, S.; et al. Surface-enhanced Raman scattering (SERS) by Ag nanoparticles on anodized TiO2-x nanotubes. Int. J. Mod. Phys. 2020, 34, 2040009. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Shi, W.; Li, Y.; Wu, Y.; Liu, Q.; Zhu, J.; Zhao, N.; Zhang, L.; Yang, J.; et al. Ecofriendly and environment-friendly synthesis of size-controlled silver nanoparticles/graphene composites for antimicrobial and SERS actions. Appl. Surf. Sci. 2018, 457, 1000–1008. [Google Scholar] [CrossRef]
- Pisarek, M.; Roguska, A.; Kudelski, A.; Andrzejczuk, M.; Janik-Czachor, M.; Kuryzydklowski, K.J. The role of Ag particles deposited on TiO2 or Al2O3 self-organized nanoporous layers in their behavior as SERS-active and biomedical substrates. Mater. Chem. Phys. 2013, 139, 55–65. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Lewandwska, M.; Kurzydlowski, K.J.; Janik-Czachor, M. In situ spectroelectrochemical surface-enhanced Raman scattering (SERS) investigations on composite Ag/TiO2-nanotubes/Ti substrates. Surf. Sci. 2009, 603, 2820–2824. [Google Scholar] [CrossRef]
- Calderon Velasco, S.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef] [Green Version]
- Cobley, C.M.; Skrabalak, S.W.; Campbell, D.J.; Xia, Y. Shape-controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L. Synthesis and SERS performance of a recyclable SERS substrate based on Ag NPs coated TiO2 NT arrays. Integr. Ferroelectr. 2013, 147, 17–23. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Art. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Slepicka, P.; Kasálková, N.S.; Siegel, J.; Kolská, Z.; Svorcik, V. Methods of gold and silver nanoparticles preparation. Materials 2020, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, N.; Pandikumar, A.; Ramaraj, R.; Lim, H.N.; Huang, N.M. Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim. Acta 2015, 182, 2091–2114. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Zhang, A.P.; Tam, H.-Y. Direct printing of micropatterned plasmonic substrates of size-controlled gold nanoparticles by precision photoreduction. Adv. Opt. Mater. 2021, 9, 2001368. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Zhang, T.; Guo, X.L.; Wang, Q.-L.; Liu, X.; Zhang, X.-Y. Gold nanoparticle thin films fabricated by electrophoretic deposition method for highly sensitive SERS application. Nanoscale Res. Lett. 2012, 7, 613. [Google Scholar] [CrossRef] [Green Version]
- Markelonis, A.R.; Wang, J.S.; Ullrich, B.; Wai, C.M.; Brown, G.J. Nanoparticle film deposition using a simple and fast centrifuge sedimentation method. Appl. Nanosci. 2015, 5, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Ding, L.; Chen, L.; Di, J. Deposition of gold nanoparticles upon bare and indium tin oxide film coated glass based on annealing process. J. Exp. Nanosci. 2019, 14, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Kho, K.W.; Shen, Z.X.; Zeng, H.C.; Soo, K.C.; Olivo, M. Deposition method for preparing SERS-active gold nanoparticle substrates. Anal. Chem. 2005, 77, 7462–7471. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wang, N.; Zhu, G.; Yadav, T.P.; Shuai, X.; Bao, D.; Yang, G.; Li, D.; Li, H. Facile fabrication of ternary TiO2-gold nanoparticle-graphene oxide nanocomposites for recyclable surface enhanced Raman scattering. Talanta 2018, 186, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, G.; Yang, L.; Jin, Z.; Liu, J. Multifunctional Au-coated TiO2 nanotube arrays as recyclable SERS substrates for multifold organic pollutants detection. Adv. Funct. Mater. 2010, 20, 2815–2824. [Google Scholar] [CrossRef]
- Brognara, A.; Mohamad Ali Nasri, I.F.; Bricchi, B.R.; Li Bassi, A.; Gauchotte-Lindsay, C.; Ghidelli, M.; Lidgi-Guigui, N. Highly sensitive detection of estradiol by a SERS sensor based on TiO2 covered with gold nanoparticles. Beilstein J. Nanotechnol. 2020, 11, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liang, X.; You, T.; Yin, P.; Wang, H.; Guo, L.; Yang, S. A sensitive SERS substrate based on Au/TiO2/Au nanosheets. Spectrochim. Acta A 2015, 142, 50–53. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Wang, B.; Jiang, T.; Ho, H.-P.; Petti, L.; Mormile, P. Au@Ag core-shell nanocubes: Epitaxial growth synthesis and surface-enhanced Raman scattering performance. Phys. Chem. Chem. Phys. 2015, 17, 6819–6826. [Google Scholar] [CrossRef]
- Gharibshani, E.; Saion, E. Influence of dose on particle size and optical properties of colloidal platinum nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. A comprehensive review on the synthesis, characterization and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [Green Version]
- Kunwar, S.; Sui, M.; Pandey, P.; Gu, Z.; Pandit, S.; Lee, J. Improved configuration and LSPR response of platinum nanoparticles via enhanced solid state dewetting of In-Pt biayers. Sci. Rep. 2019, 9, 1329. [Google Scholar] [CrossRef]
- Dong, J.; Huang, J.; Wang, A.; Biesold-McGee, G.V.; Zhang, X.; Gao, S.; Wang, S.; Lai, Y.; Lin, Z. Vertically-aligned Pt decorated MoS2 nanosheets coated on TiO2 nanotube arrays enable high-efficiency solar-light energy utilization for photocatalysis and self-cleaning SERS devices. Nano Energy 2020, 71, 104579. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pelluite, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO2 nanoparticles. Appl. Catal. B Environ. 2017, 218, 80–87. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, K. Surface-enhanced Raman scattering at Pt nanoaggregates. Chem. Phys. Lett. 2004, 396, 478–482. [Google Scholar] [CrossRef]
- Bai, Y.; Li, W.; Liu, C.; Yang, Z.; Feng, X.; Lu, X.; Chan, K.Y. Stability of Pt nanoparticles and enhanced photocatalytic performance in mesoporous Pt-(anatase/TiO2(B)) nanoarchitecture. J. Mater. Chem. 2009, 9, 7055–7061. [Google Scholar] [CrossRef]
- Wei, W.; Gong, X.; Sun, J.; Pan, D.; Huang, Q.; Wang, C. Cellophane paper-based surface-enhanced Raman scattering (SERS) substrates for detecting Rhodamine 6G in water and chili powder. Vib. Spectrosc. 2019, 102, 52–56. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Carmichael, E.; McCall, D. Fabrication of SERS substrate for the detection od rhodamine 6G, glyphosate, melamine and salicylic acid. Vib. Spectrosc. 2016, 83, 159–169. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, C.; Mu, X.; Pang, H.; Chen, X.; Liu, D. Ultrasensitive SERS detection of rhodamine 6G and p-nitrophenol based on electrochemically roughened nano-Au film. Talanta 2020, 210, 120631. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, K. Surface-enhanced resonance Raman scattering of Rhodamine 6G on Pt nanoaggregates. J. Raman Spectrosc. 2005, 36, 623–628. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; He, L.; Tang, J.; Zhou, Y.; Li, J.; Ding, K. One-pot synthesis of multifunctional magnetic N-doped graphene composite for SERS detection, adsorption separation and photocatalytic degradation of Rhodamine 6G. Chem. Eng. J. 2017, 327, 694–704. [Google Scholar] [CrossRef]
- Zheng, Z.; Cong, S.; Gong, W.; Xuan, J.; Li, G.; Lu, W.; Geng, F.; Zhao, Z. Semiconductor SERS enhancement enabled by oxygen incorporation. Nat. Commun. 2017, 8, 1993. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Shang, Y.; Li, X.; Yu, J.; Wang, X.; Guo, L. Ultrasensitive SERS detection by defect engineering on single Cu2O superstructure particle. Adv. Mater. 2016, 29, 1604797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Yang, J.; Zheng, X. Improved SERS sensitivity of TiO2 nanorod films by annealing in vacuum. Vacuum 2021, 194, 110579. [Google Scholar] [CrossRef]
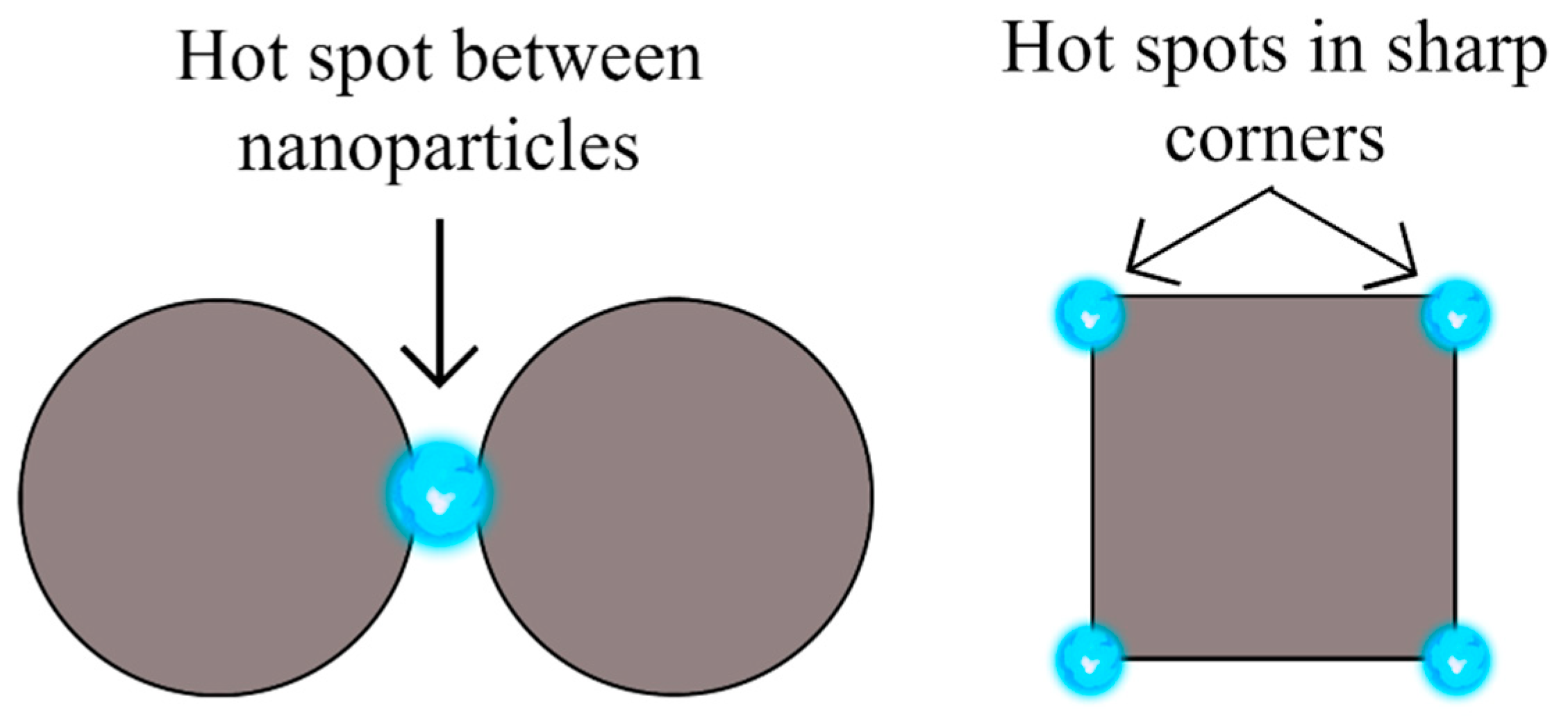
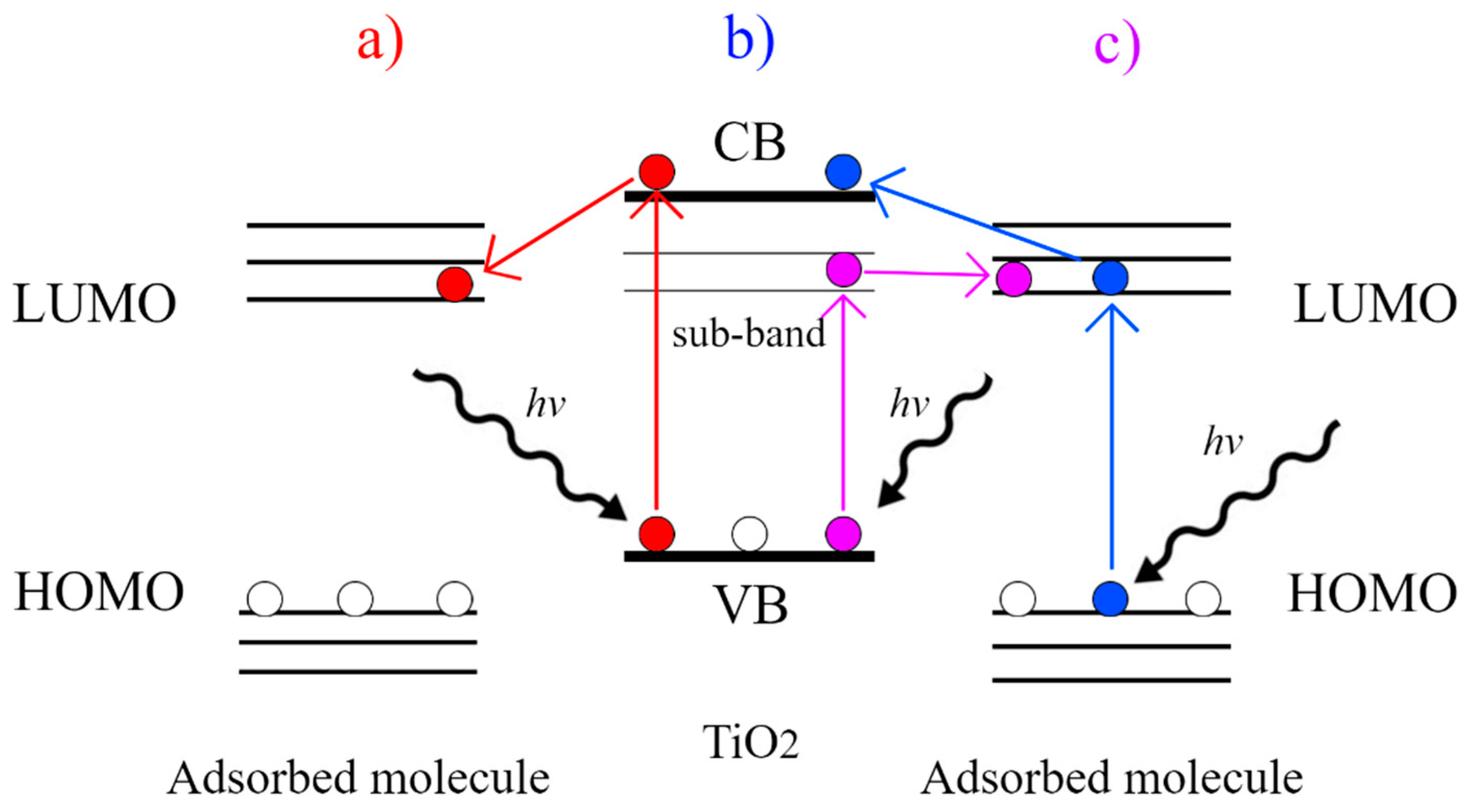
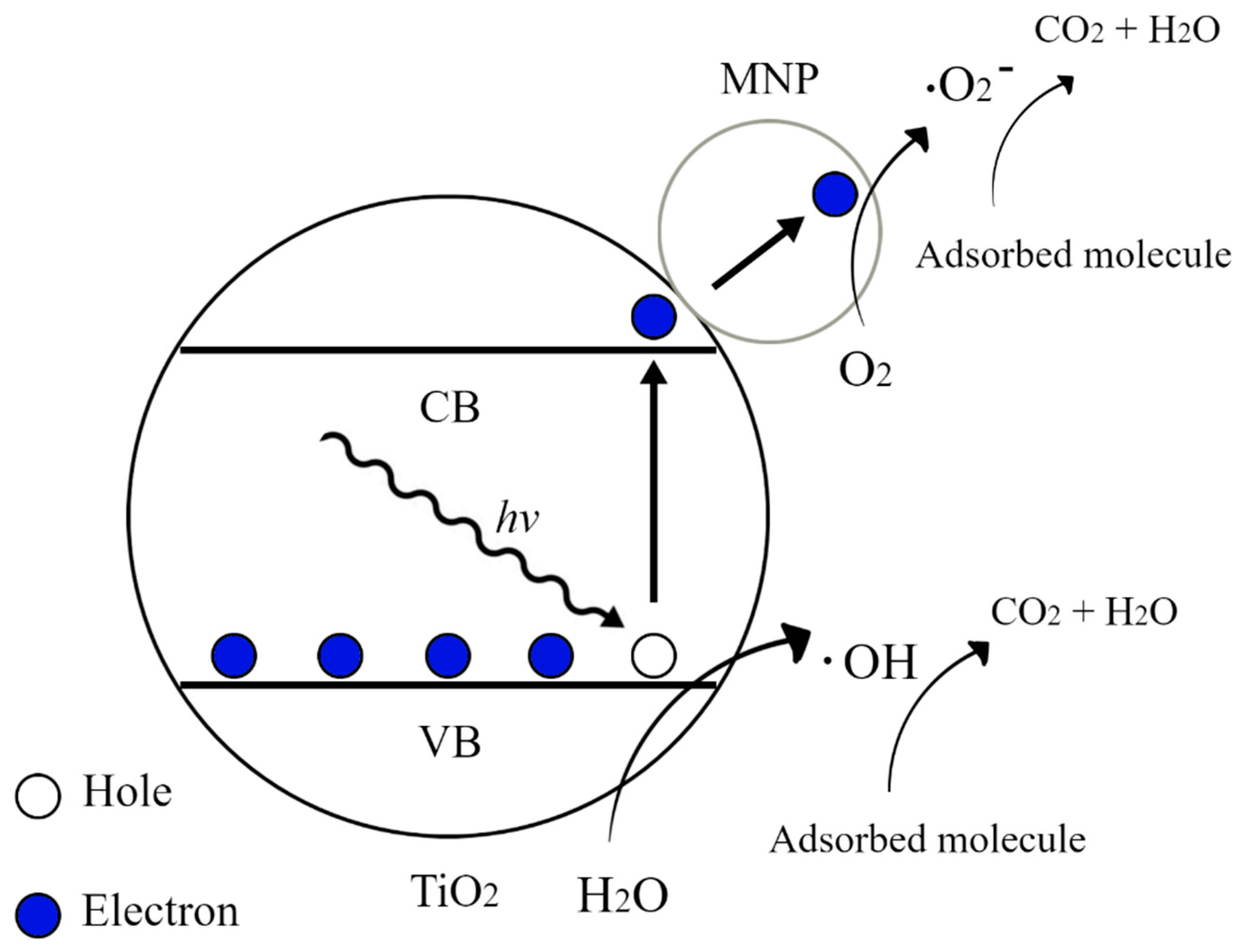
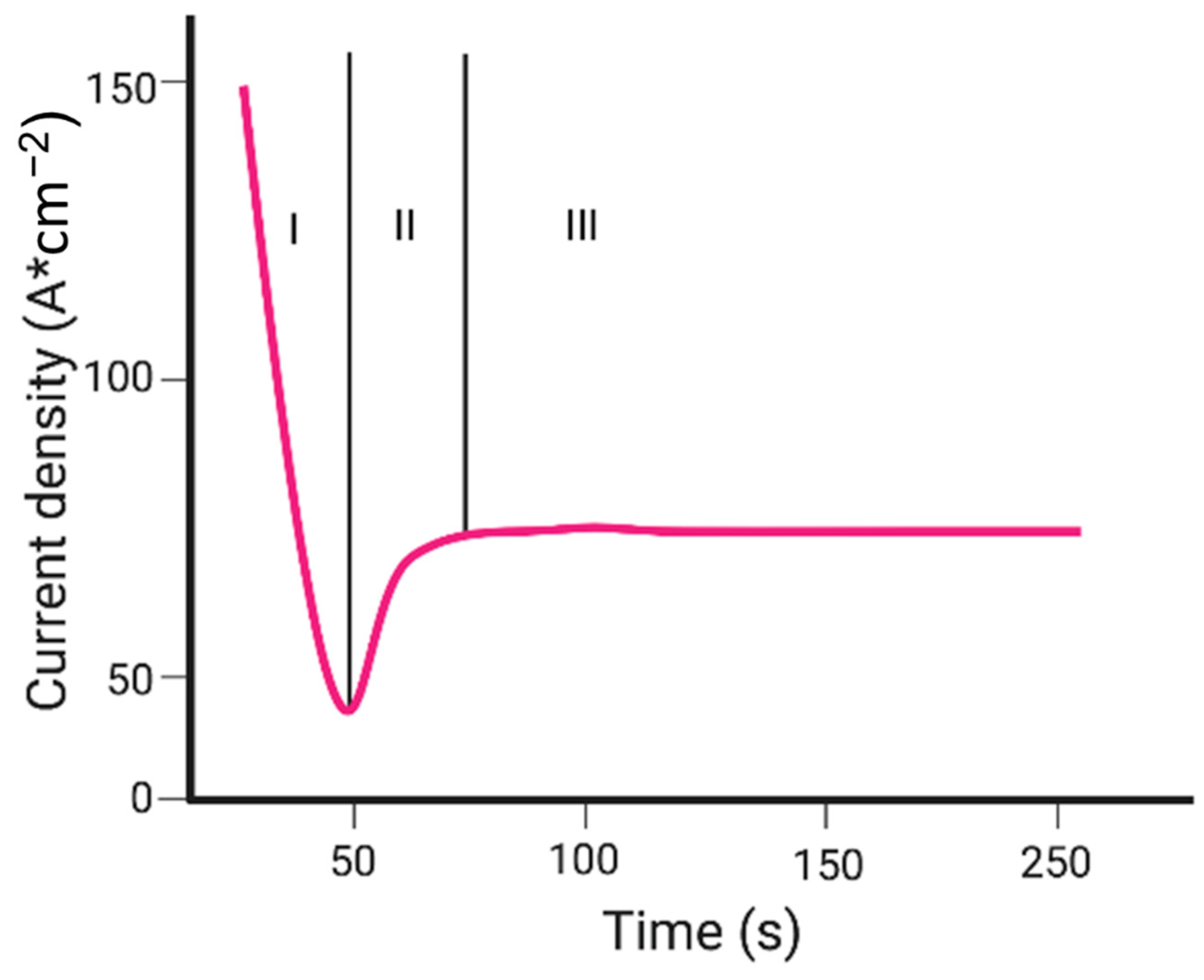
| Semiconductor | Synthesis Method | Probe Molecule | Enhancement Factor | LOD | Reference |
|---|---|---|---|---|---|
| CdTe quantum dots | 4-Mpy | 104 | [50] | ||
| Cu2O nanospheres | 4-MBA | ≈105 | [51] | ||
| MoS2 monolayer | APCVD | 4-Mpy | 3.8 × 105 | [52] | |
| SiO2 particles | CV | 2.2 × 104 | [53] | ||
| SnO2 octahedral nanoparticles | Self-assembly | 4-MBA | 103 | [54] | |
| TiO2 inverse opal photonic microarray | Casting and calcination | MB | 2 × 104 | 6 × 10−6 M | [55] |
| TiO2 nanosheets | 4-MBA | 1.86 × 106 | [56] | ||
| TiO2 nanoparticles | Sol-hydrothermal | 4-MBA | 3.5 × 103 | [57] | |
| ZnO nanosheets | Self-assembly | 4-MBA | 103 | 1 × 10−6 M | [58] |
| ZnSe nanoparticles | MBE | 4-Mpy | 2 × 106 | [59] | |
| ZnS nanocrystals | 4-Mpy | 103 | [60] |
| Probe Molecule | Excitation Wavelength | LOD (M) | EF | Recyclability | Reference |
|---|---|---|---|---|---|
| Formaldehyde | 532 nm | 1.21 × 10−7 | 3 h, 3 times | [90] | |
| 2-mercaptobenzoxazole | 514 nm | ~10−9 | 2.26 × 108 | 20 min, 3 times | [89] |
| Rhodamine G | 633 nm | 10−8 | 140 min, 3 times | [94] | |
| Rhodamine G | 532 nm | 10−7 | 20 min, 3 times | [104] |
| Molecular Probe | Excitation Wavelength | LOD (M) | EF | Recyclability | Reference |
|---|---|---|---|---|---|
| 4-CP | 785 nm | 1 × 10−9 | --------- | 30 min, three times | [116] |
| R6G | 514 nm | 1 × 10−5 | 5 × 104 | 270 min, 4 times | [38] |
| 4-MBA | 647 nm | 1 × 10−9 | 1 × 107 | ---------------- | [118] |
| Estradiol | 633 nm | 1 × 10−9 | 1 × 106 | ---------------- | [117] |
| Substrate | Synthesis Method | EF | LOD | Relative Standard Deviation | Stability | Reference |
|---|---|---|---|---|---|---|
| Ag NPs-coated CP | Chemical Reduction on CP by hydrazine | 10−11 M | 7.6% six for 6 batches | No obvious change after 1 month | [127] | |
| Ag NPs—Cu grid | Chemical reduction, drop casting deposition, glow discharge treatment on Cu-grids | 6.1 × 105 | 240 ppb | 5–10% signal reduction after 3 weeks | [128] | |
| Ag NPs/TiO2 NTs | Photochemical reduction | 10−8 | [94] | |||
| Ag NPs/TiO2 NTs | Chemical reduction by Sn2+ | 10−7 | [104] | |||
| Au NPs film | EBE Au deposition on Si wafer followed by ER | 2.45 × 108 | 7.08 × 10−11 M | 6.88% for 12 measurements | 26.5% signal reduction in 30 days | [129] |
| Au NPs/TiO2 nanopores | Au evaporation on anodized Ti | 5 × 104 | 1 × 10−5 M | [38] | ||
| Pt nanoaggregates on Si wafers | Pt solution dropping on Si wafer | 5 × 104 | [130] | |||
| Pt@TiO2 NTs | Reduction at 90 °C of adsorbed Pt ions on PDA-modified TiO2 NTAs | 4.3 × 104 | ~10−8 M | [16] | ||
| Pt NPs/MoS2/TiO2NTs | Pt NPs deposited by CV on MoS2 nanosheets deposited on TiO2NTs | 2.5 × 105 | [123] | |||
| Fe2O3 NPs/N-rGO | Fe2O3 NPs grown in situ on N-rGO | 5 × 10−7 M | <9.43% for 10 measurements | [131] | ||
| Partially oxidized MoS2 nanosheets | Thermal oxygen incorporation in MoS2 | 1.4 × 105 | 10−7 M | [132] | ||
| Cu2O mesoporous spheres | Recrystallization induced self-assembly | ~105 | 10−9 M | [133] | ||
| TiO2−x nanorod films | Hydrothermal method | 1.8 × 104 | 10−6 M | Reduced signals after 2 months | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez-Cisneros, J.; Galindo-Lazo, J.P.; Mendez-Rojas, M.A.; Campos-Delgado, J.R.; Cerro-Lopez, M. Plasmonic Spherical Nanoparticles Coupled with Titania Nanotube Arrays Prepared by Anodization as Substrates for Surface-Enhanced Raman Spectroscopy Applications: A Review. Molecules 2021, 26, 7443. https://doi.org/10.3390/molecules26247443
Jimenez-Cisneros J, Galindo-Lazo JP, Mendez-Rojas MA, Campos-Delgado JR, Cerro-Lopez M. Plasmonic Spherical Nanoparticles Coupled with Titania Nanotube Arrays Prepared by Anodization as Substrates for Surface-Enhanced Raman Spectroscopy Applications: A Review. Molecules. 2021; 26(24):7443. https://doi.org/10.3390/molecules26247443
Chicago/Turabian StyleJimenez-Cisneros, Jorge, Juan Pablo Galindo-Lazo, Miguel Angel Mendez-Rojas, Jessica Rosaura Campos-Delgado, and Monica Cerro-Lopez. 2021. "Plasmonic Spherical Nanoparticles Coupled with Titania Nanotube Arrays Prepared by Anodization as Substrates for Surface-Enhanced Raman Spectroscopy Applications: A Review" Molecules 26, no. 24: 7443. https://doi.org/10.3390/molecules26247443
APA StyleJimenez-Cisneros, J., Galindo-Lazo, J. P., Mendez-Rojas, M. A., Campos-Delgado, J. R., & Cerro-Lopez, M. (2021). Plasmonic Spherical Nanoparticles Coupled with Titania Nanotube Arrays Prepared by Anodization as Substrates for Surface-Enhanced Raman Spectroscopy Applications: A Review. Molecules, 26(24), 7443. https://doi.org/10.3390/molecules26247443







