5-Dodecanolide, a Compound Isolated from Pig Lard, Presents Powerful Anti-Inflammatory Properties
Abstract
:1. Introduction
2. Results
2.1. Effects of PL in In Vivo Animal Model
2.2. Antioxidant and Proinflammatory Markers in In Vivo Animal Model
2.3. PL Hydroalcoholic Extract Composition
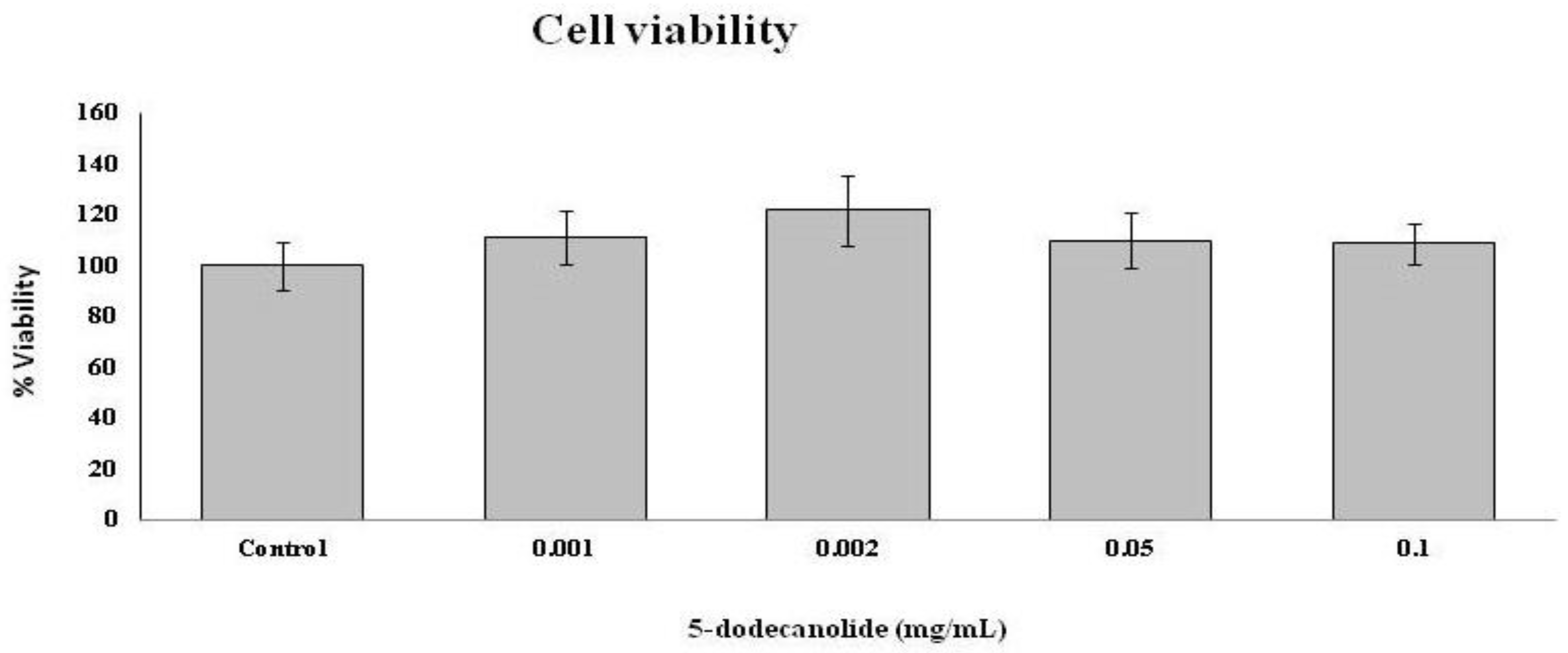
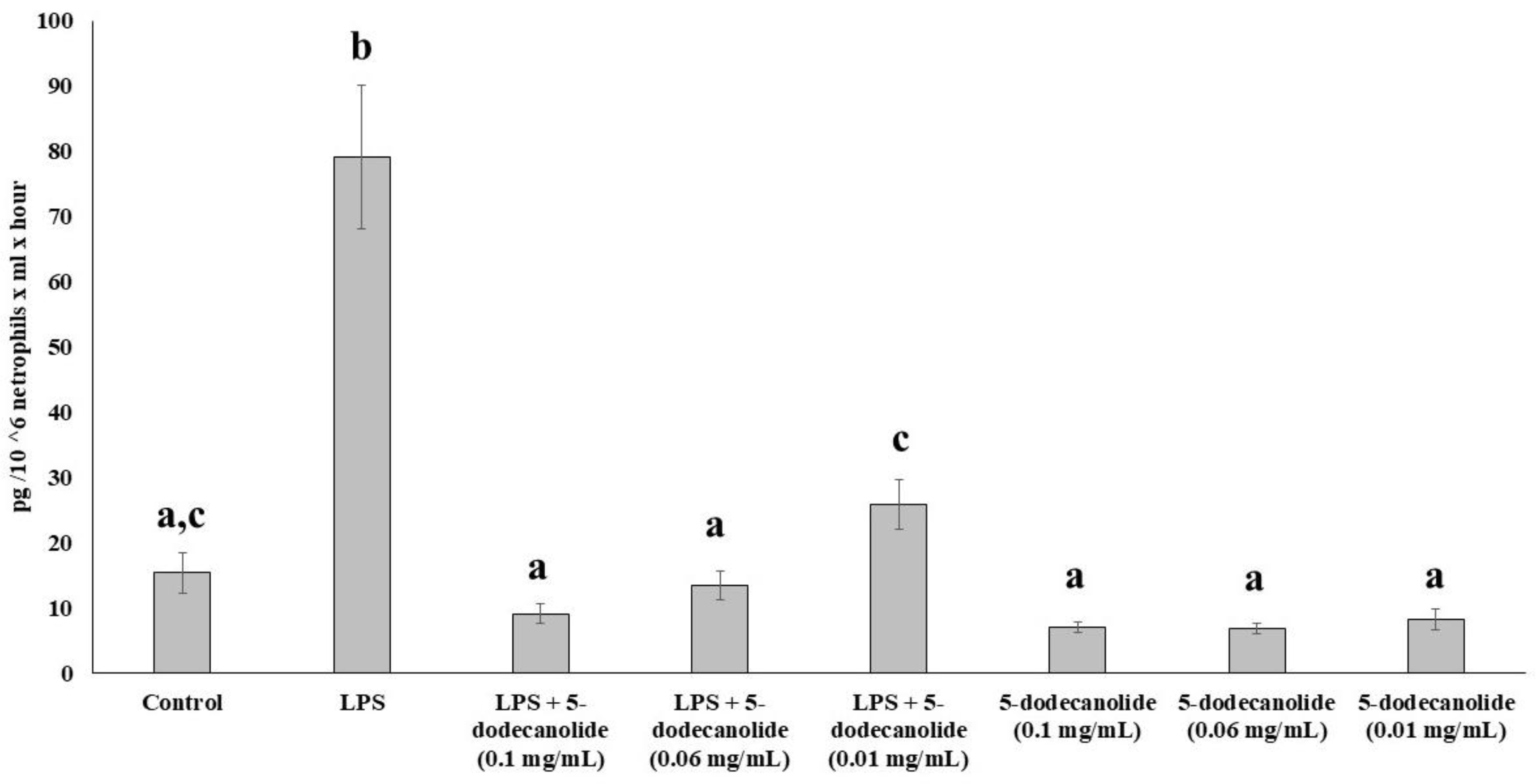
2.4. Effects of PL Hydroalcoholic Extract in Ex Vivo Immune Cell Model
2.5. Antioxidant and Anti-Inflammatory Properties of PL Components
3. Discussion
4. Materials and Methods
4.1. Animals and Zymosan-Induced Hind Paw Inflammation
4.2. Animal Sample Processing
4.3. Enzymatic Activities
4.4. Oxidative Damage Markers
4.5. Nitrite Levels
4.6. Ex Vivo Immune Cell Model of Inflammation
4.6.1. Pork Lard Hydroalcoholic Extract Obtention
4.6.2. Hydroalcoholic Extract Composition
4.6.3. Immune Cell Isolation
4.6.4. MTT Assay
4.6.5. Immune Cell Incubations
4.6.6. Cytokine Assays
4.6.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Geering, B.; Stoeckle, C.; Conus, S.; Simon, H.-U. Living and dying for inflammation: Neutrophils, eosinophils, basophils. Trends Immunol. 2013, 34, 398–409. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Cronkite, D.A.; Strutt, T.M. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J. Immunol. Res. 2018, 2018, 1467538. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Lyga, J. Inflammaging in skin and other tissues—The roles of complement system and macrophage. Inflamm. Allergy Drug Targets 2014, 13, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Cho, S.; Lee, S.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J. Lipid Res. 2006, 47, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Polte, T.; Tyrrell, R.M. Involvement of lipid peroxidation and organic peroxides in UVA-induced Matrix Metalloproteinase-1 expression. Free. Radic. Biol. Med. 2004, 36, 1566–1574. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Leitinger, N. Anti-inflammatory properties of lipid oxidation products. J. Mol. Med. 2003, 81, 613–626. [Google Scholar] [CrossRef]
- Bochkov, V.N.; Kadl, A.; Huber, J.; Gruber, F.; Binder, B.R.; Leitinger, N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nat. Cell Biol. 2002, 419, 77–81. [Google Scholar] [CrossRef]
- Erridge, C.; Kennedy, S.; Spickett, C.M.; Webb, D.J. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: Roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 2008, 283, 24748–24759. [Google Scholar] [CrossRef] [Green Version]
- Friedl, R.; Pichler, I.; Spieckermann, P.; Moeslinger, T. Oxidized phospatidylcholine but not native phosphatidylcholine inhibits inducible nitric oxide synthase in RAW 264.7 macrophages. Life Sci. 2006, 78, 1586–1591. [Google Scholar] [CrossRef]
- Ke, Y.; Zebda, N.; Oskolkova, O.; Afonyushkin, T.; Berdyshev, E.; Tian, Y.; Meng, F.; Sarich, N.; Bochkov, V.N.; Wang, J.M.; et al. Anti-Inflammatory Effects of OxPAPC Involve Endothelial Cell–Mediated Generation of LXA4. Circ. Res. 2017, 121, 244–257. [Google Scholar] [CrossRef]
- Navarro Hernandez, C. The Invention Relates to the Field of Medicine and to a Composition, a Method for Obtaining Said Composition and the Use of Same as an Anti-Inflammatory, Anti-Edematous and Anti-Erythematous Composition and a Tissue Regenerator. U.S. Patent WO2009077635A3, 6 June.
- Burattin, L. Mezcla de Ácidos Grasos Para su uso en el Tratamiento De patologías Inflamatorias. U.S. Patent WO14135529, 12 September 2014. [Google Scholar]
- González González, S. Procedimiento de Elaboración de una Crema Venoprotectora con Protector Solar y Composiciones Obtenidas por Este Procedimiento. ESPAÑA Patent ES2590229, 18 November 2016. [Google Scholar]
- Pons, A.; Sureda, A.; Tur, J.A.; Martorell, M.; Capó, X. Pharmaceutical Composition Comprising 5-Dodecanolide, Preparation Thereof and Use of Same. U.S. Patent WO/2017/121916, 20 July 2017. [Google Scholar]
- Fujiwara, M.; Miyoshi, M.; Sakai, S.; Nishiokada, A.; Ishikawa, M.; Maeshige, N.; Usami, Y.; Hamada, Y.; Takahashi, M.; Usami, M. Lard-based high-fat diet increases secretory leukocyte protease inhibitor expression and attenuates the inflammatory response of acute lung injury in endotoxemic rats. Clin. Nutr. 2015, 34, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- Hierholzer, C.; Harbrecht, B.; Menezes, J.M.; Kane, J.; MacMicking, J.; Nathan, C.F.; Peitzman, A.B.; Billiar, T.R.; Tweardy, D.J. Essential Role of Induced Nitric Oxide in the Initiation of the Inflammatory Response after Hemorrhagic Shock. J. Exp. Med. 1998, 187, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of Docosahexaenoic Supplementation andIn VitroVitamin C on the Oxidative and Inflammatory Neutrophil Response to Activation. Oxidative Med. Cell. Longev. 2015, 2015, 187849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Veen, B.S.; de Winther, M.P.; Heeringa, P. Myeloperoxidase: Molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.; Egger, J.; Shamshiev, A.; Trötzmüller, M.; Köfeler, H.; Carreira, E.M.; Kopf, M.; Freigang, S. Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO Mol. Med. 2015, 7, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chen, L.-Y.; Papadimos, T.; Huang, S.; Zuraw, B.; Pan, Z.K. Lipopolysaccharide-driven Th2 Cytokine Production in Macrophages Is Regulated by Both MyD88 and TRAM. J. Biol. Chem. 2009, 284, 29391–29398. [Google Scholar] [CrossRef] [Green Version]
- Capó, X.; Martorell, M.; Sureda, A.; Batle, J.M.; Tur, J.A.; Pons, A. Docosahexaenoic diet supplementation, exercise and temperature affect cytokine production by lipopolysaccharide-stimulated mononuclear cells. J. Physiol. Biochem. 2016, 72, 421–434. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Bishop-Bailey, D. Lipid mediators in immune regulation and resolution. Br. J. Pharmacol. 2019, 176, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Uchida, M.C.; Nosaka, K.; Ugrinowitsch, C.; Yamashita, A.; Martins, E.; Moriscot, A.S.; Aoki, M.S.; Nosaka, K. Effect of bench press exercise intensity on muscle soreness and inflammatory mediators. J. Sports Sci. 2009, 27, 499–507. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Gronert, K.; Chiang, N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: A novel mechanism for NSAID and N-3 PUFA therapeutic actions. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2000, 51, 643–654. [Google Scholar]
- Fräßdorf, J.; Luther, B.; Müllenheim, J.; Otto, F.; Preckel, B.; Schlack, W.; Thämer, V. Influence of Groin Incision, Duration of Ischemia, and Prostaglandin E1 on Ischemia-Reperfusion Injury of the Lower Limb. J. Cardiothorac. Vasc. Anesth. 2006, 20, 187–195. [Google Scholar] [CrossRef]
- Oh, Y.T.; Lee, J.Y.; Lee, J.; Lee, J.H.; Kim, J.E.; Ha, J.; Kang, I. Oleamide suppresses lipopolysaccharide-induced expression of iNOS and COX-2 through inhibition of NF-kappaB activation in BV2 murine microglial cells. Neurosci. Lett. 2010, 474, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-M.; Lee, S.A.; Hong, J.H.; Kim, J.-S.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef]
- Sureda, A.; Martorell, M.; Bibiloni, M.D.M.; Bouzas, C.; Gallardo-Alfaro, L.; Mateos, D.; Capó, X.; Tur, J.A.; Pons, A. Effect of Free Fatty Acids on Inflammatory Gene Expression and Hydrogen Peroxide Production by Ex Vivo Blood Mononuclear Cells. Nutrients 2020, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proin-flammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [Green Version]
- Capó, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of dietary Docosahexaenoic, training and acute exercise on lipid mediators. J. Int. Soc. Sports Nutr. 2016, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Verhoef, J.; Verhage, E.A.E.; Visser, M.R. A Decade of Experience with Selective Decontamination of the Digestive Tract as Prophylaxis for Infections in Patients in the Intensive Care Unit: What Have We Learned? Clin. Infect. Dis. 1993, 17, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Weeks, M.; DeSena, B.J. Topical Hazard Evaluation Program of Candidate Insect Repellent AI3-36401 US Department of Agriculture Proprietary Compound—AMIDE; United States Department of Agriculture: Washington, DC, USA, 1978. [Google Scholar]
- Gil, J.; Kim, N.; Yoon, S.-K.; Ham, J.-S.; Jang, A. Anti-Oxidative and Anti-Inflammation Activities of Pork Extracts. Food Sci. Anim. Resour. 2016, 36, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, T.R.; Wilfinger, W.W. Fluorometric quantification of DNA in cells and tissue. Anal. Biochem. 1983, 131, 538–547. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Res. Nitrification Relat. Process. Part A 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Capeillère-Blandin, C. Oxidation of guaiacol by myeloperoxidase: A two-electron-oxidized guaiacol transient species as a mediator of NADPH oxidation. Biochem. J. 1998, 336, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Batle, J.M.; Ferrer, M.D.; Mestre-Alfaro, A.; Tur, J.A.; Pons, A. Scuba Diving Activates Vascular Antioxidant System. Endosc. 2012, 33, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Martorell, M.; Sureda, A.; Llompart, I.; Tur, J.A.; Pons, A. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur. J. Nutr. 2015, 54, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Sureda, A.; Mestre, A.; Tur, J.; Pons, A. The Double Edge of Reactive Oxygen Species as Damaging and Signaling Molecules in HL60 Cell Culture. Cell. Physiol. Biochem. 2010, 25, 241–252. [Google Scholar] [CrossRef] [PubMed]
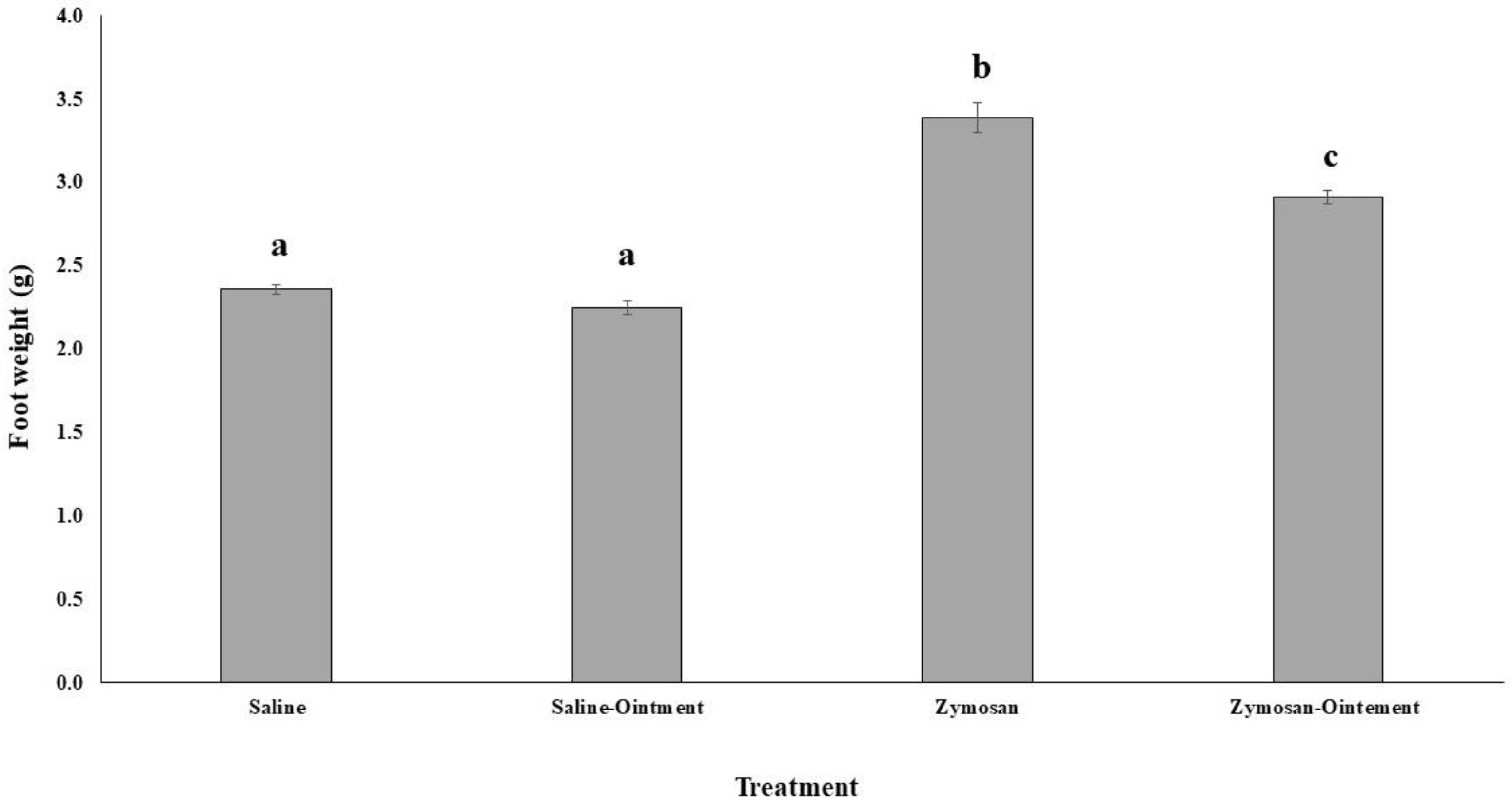
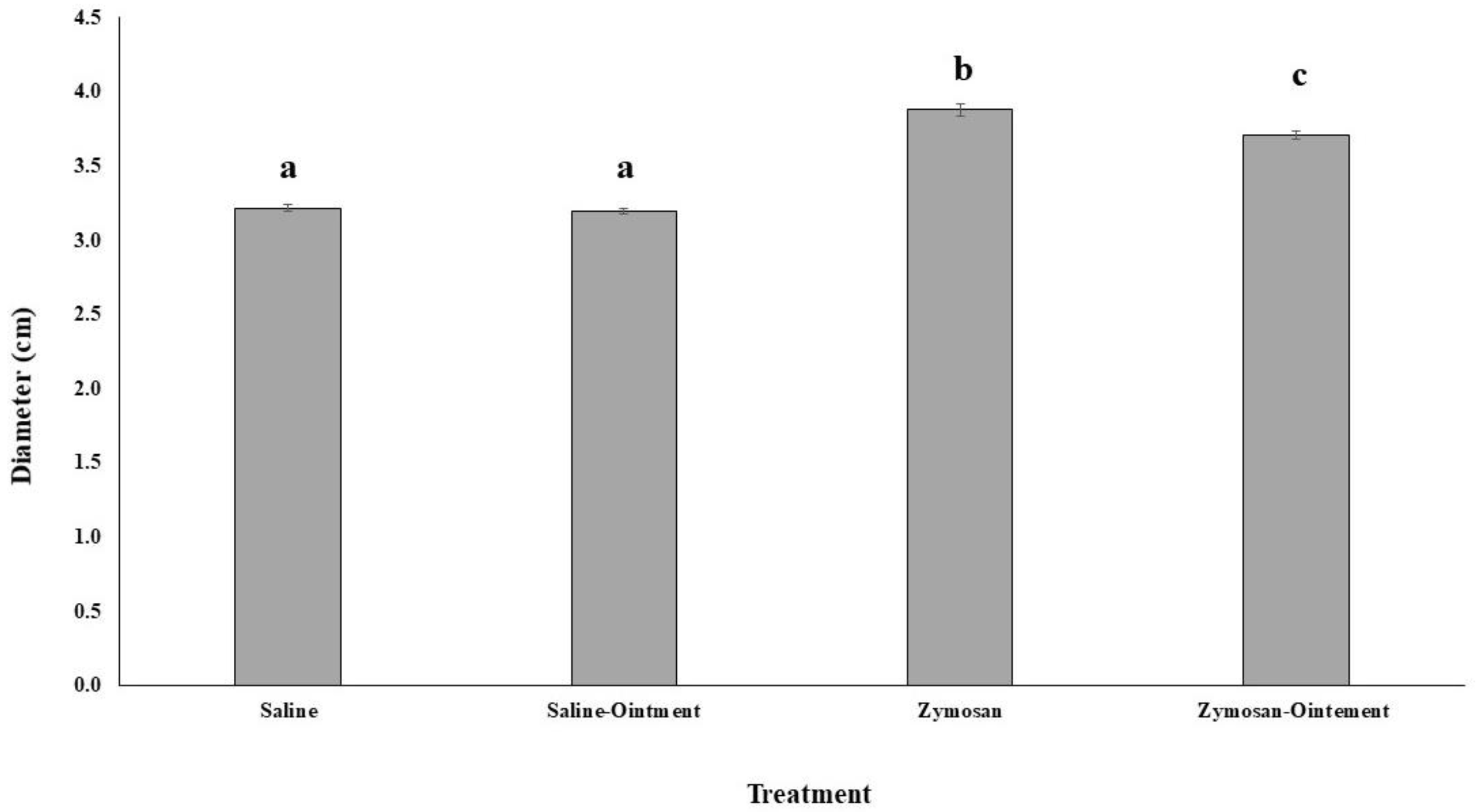
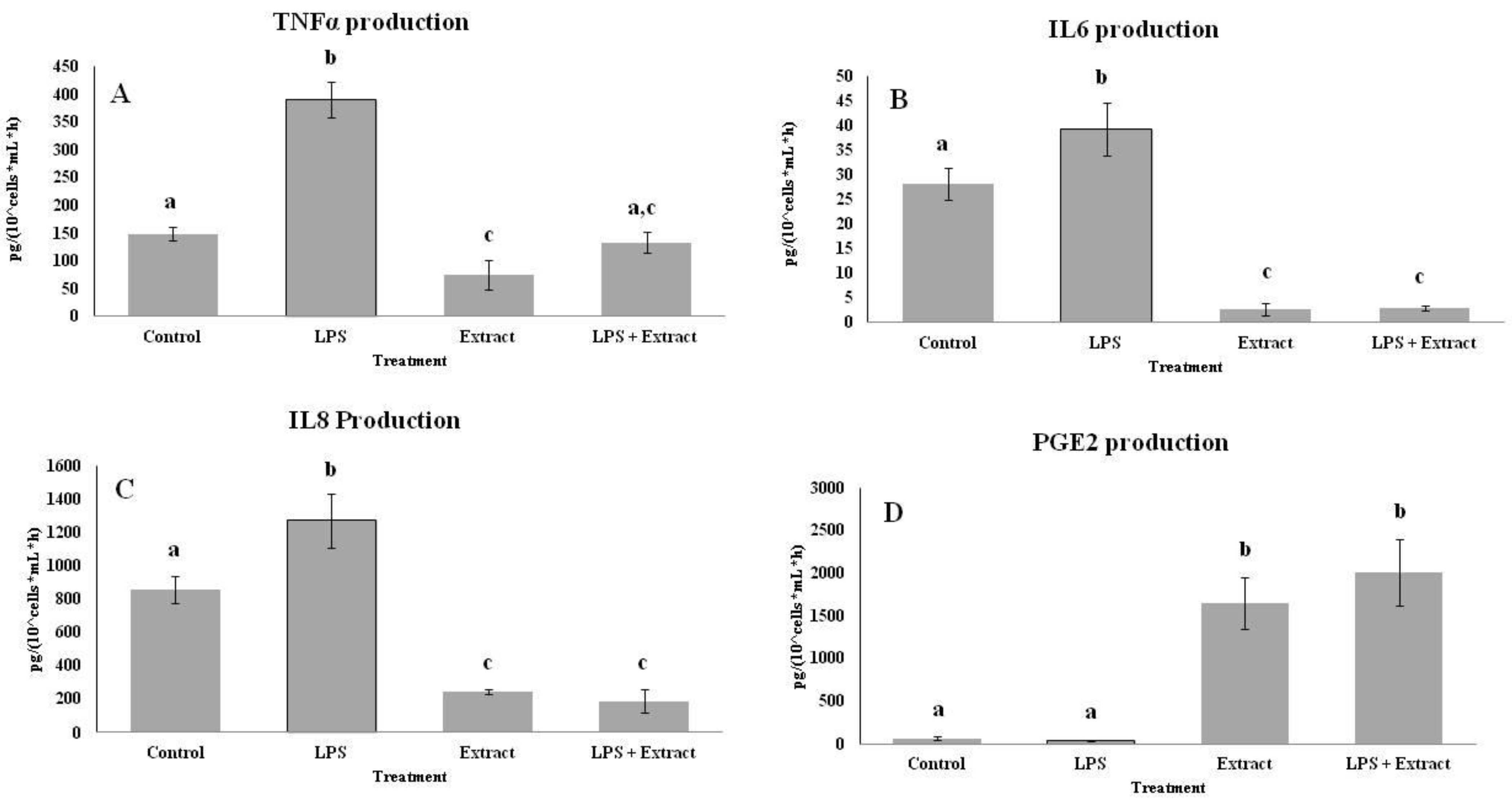
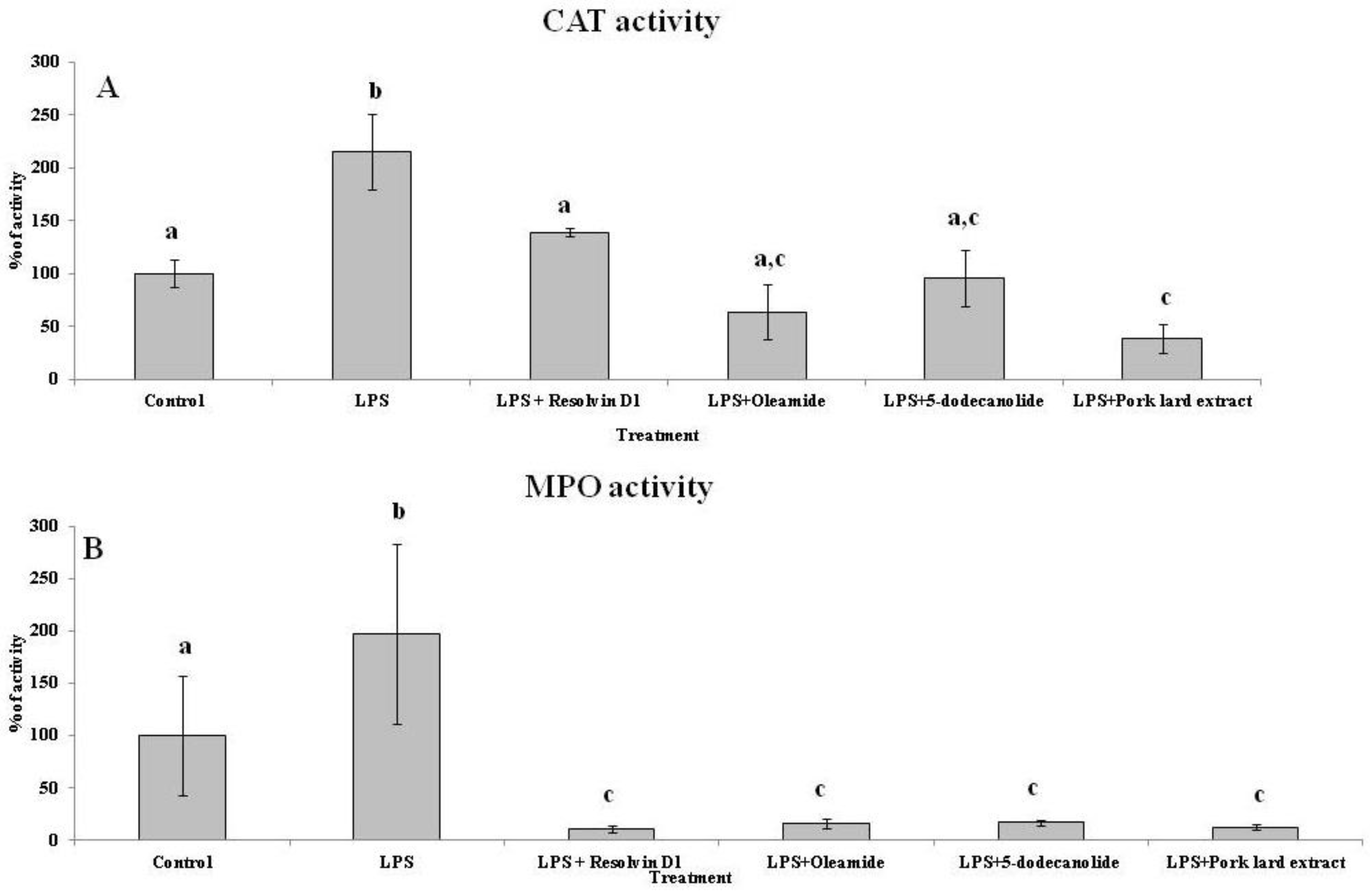
| Saline Buffer + Vaseline | Saline Buffer + Pig Lard | Zymosan + Vaseline | Zymosan + Pig Lard | |
|---|---|---|---|---|
| Enzymatic activities in subplantar tissue | ||||
| MPO (nKat/mg protein) | 2.79 ± 0.30 | 3.02 ± 0.28 | 21.8 ± 2.41 a | 17.8 ± 3.02 a |
| GPx (nKat/mg protein) | 0.29 ± 0.02 | 0.32 ± 0.04 | 0.38 ± 0.02 | 0.36 ± 0.06 |
| CAT (K/mg protein) | 11.3 ± 2.81 | 12.9 ± 1.23 | 9.17 ± 1.02 | 10.7 ± 2.23 |
| Enzymatic activities in neutrophils | ||||
| MPO (nKat/μg DNA) | 4.47 ± 0.82 a | 4.74 ± 0.44 a | 7.73 ± 1.03 b | 4.44 ± 0.67 a |
| GPx (nKat/μg DNA) | 0.80 ± 0.06 | 0.79 ± 0.06 | 0.87 ± 0.02 | 0.64 ± 0.04 |
| CAT (K/mg DNA) | 0.15 ± 0.02 a | 0.14 ± 0.01 a | 0.27 ± 0.06 b | 0.16 ± 0.03 a |
| Oxidative damage markers | ||||
| MDA (nmol/mg protein) | 523 ± 79.3 | 542 ± 86.6 | 648 ± 48.4 | 591 ± 79.2 |
| Carbonyls (μmol/mg protein) | 15.1 ± 1.13 | 16.7 ± 1.73 | 23.9 ± 0.92 a | 24.2 ± 1.13 a |
| Nitrite (μmol/mg protein) | 5.81 ± 1.06 | 6.04 ± 0.54 | 14.1 ± 1.02 a | 11.6 ± 0.75 b |
| Molecule (Certainty%) | Percentage (%) |
|---|---|
| Hexadecanoic (93%) | 0.603 ± 0.09 |
| 9-Octadecenoic (99%) | 61.6 ± 2.21 |
| 6-Octadecenoic (99%) | 13.3 ± 0.83 |
| 9,12-Octadecenoic (99%) | 0.31 ± 0.17 |
| 5,8,11,14-Eicosatetraenoic (90%) | 5.77 ± 0.42 |
| 5-Dodecanolide (81%) | 4.49 ± 0.15 |
| 7-hexadecenoic (89%) | 2.08 ± 0.72 |
| 11,14-Octadecadienoic (93%) | 5.84 ± 0.15 |
| Hexadecanamide (92%) | 1.07 ± 0.21 |
| Octadecenemide (82%) | 5.02 ± 0.52 |
| Resolvin D1 (μg/g of extract) | 2.86 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. 5-Dodecanolide, a Compound Isolated from Pig Lard, Presents Powerful Anti-Inflammatory Properties. Molecules 2021, 26, 7363. https://doi.org/10.3390/molecules26237363
Capó X, Martorell M, Tur JA, Sureda A, Pons A. 5-Dodecanolide, a Compound Isolated from Pig Lard, Presents Powerful Anti-Inflammatory Properties. Molecules. 2021; 26(23):7363. https://doi.org/10.3390/molecules26237363
Chicago/Turabian StyleCapó, Xavier, Miquel Martorell, Josep A. Tur, Antoni Sureda, and Antoni Pons. 2021. "5-Dodecanolide, a Compound Isolated from Pig Lard, Presents Powerful Anti-Inflammatory Properties" Molecules 26, no. 23: 7363. https://doi.org/10.3390/molecules26237363
APA StyleCapó, X., Martorell, M., Tur, J. A., Sureda, A., & Pons, A. (2021). 5-Dodecanolide, a Compound Isolated from Pig Lard, Presents Powerful Anti-Inflammatory Properties. Molecules, 26(23), 7363. https://doi.org/10.3390/molecules26237363








