Effect of S–Se Bioisosteric Exchange on Affinity and Intrinsic Efficacy of Novel N-acylhydrazone Derivatives at the Adenosine A2A Receptor
Abstract
:1. Introduction
2. Results and Discussion
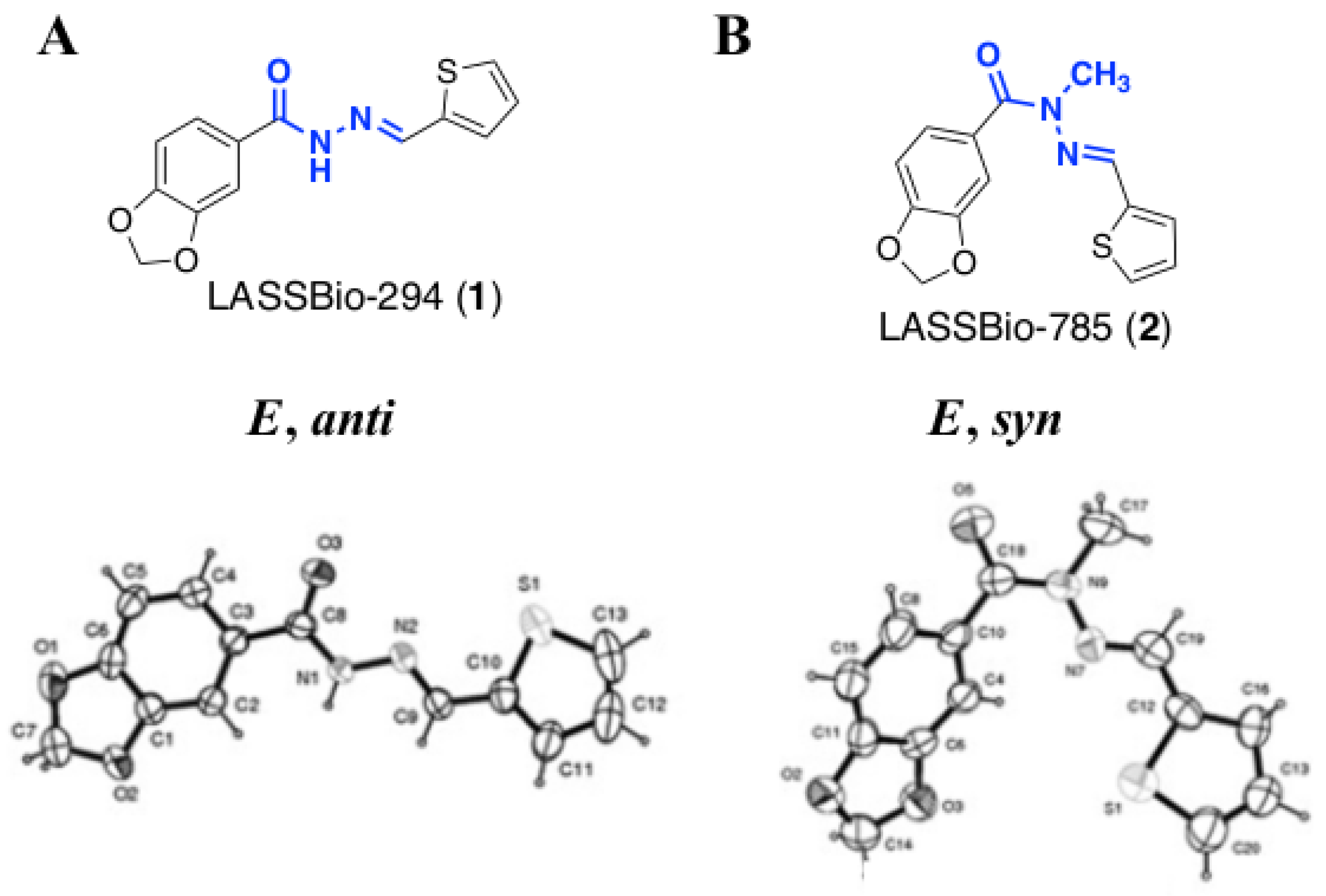
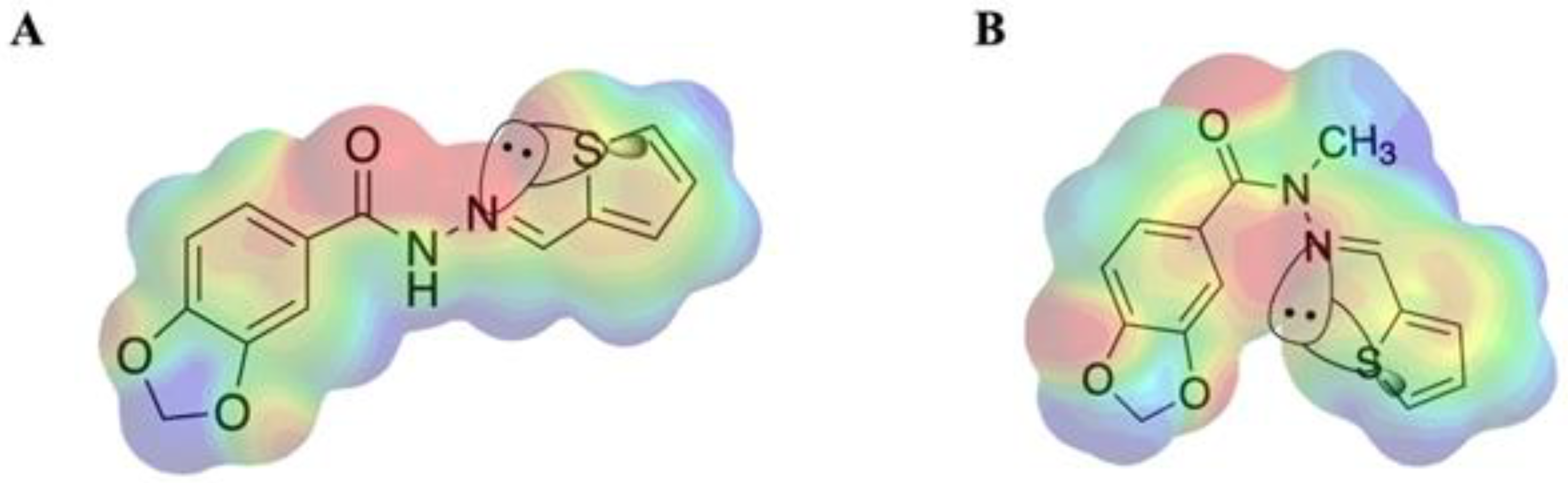
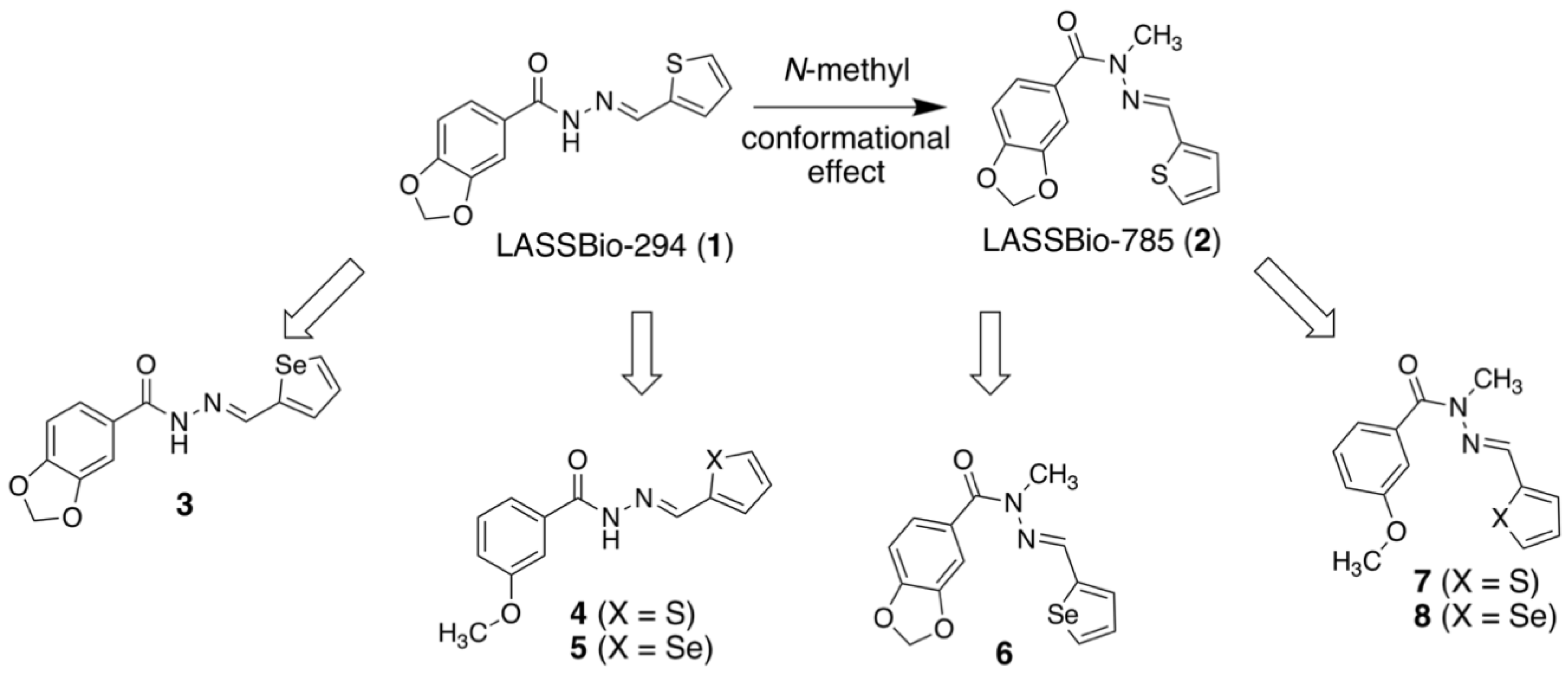
2.1. Molecular Design
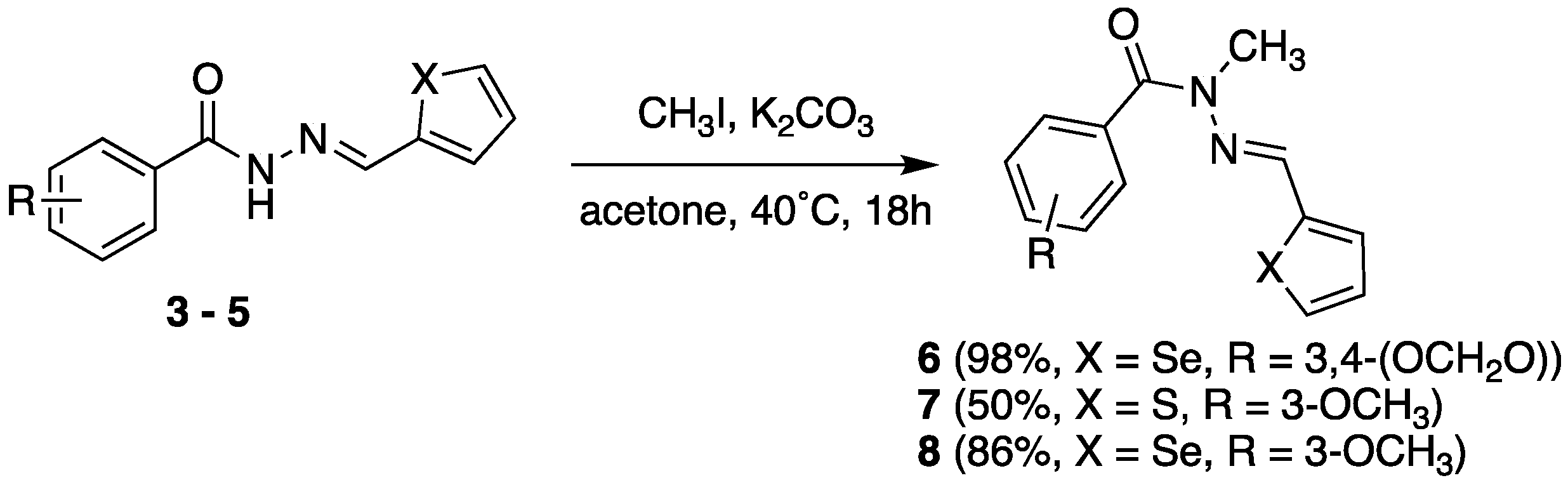
2.2. Isosteric Derivatives (3–8)
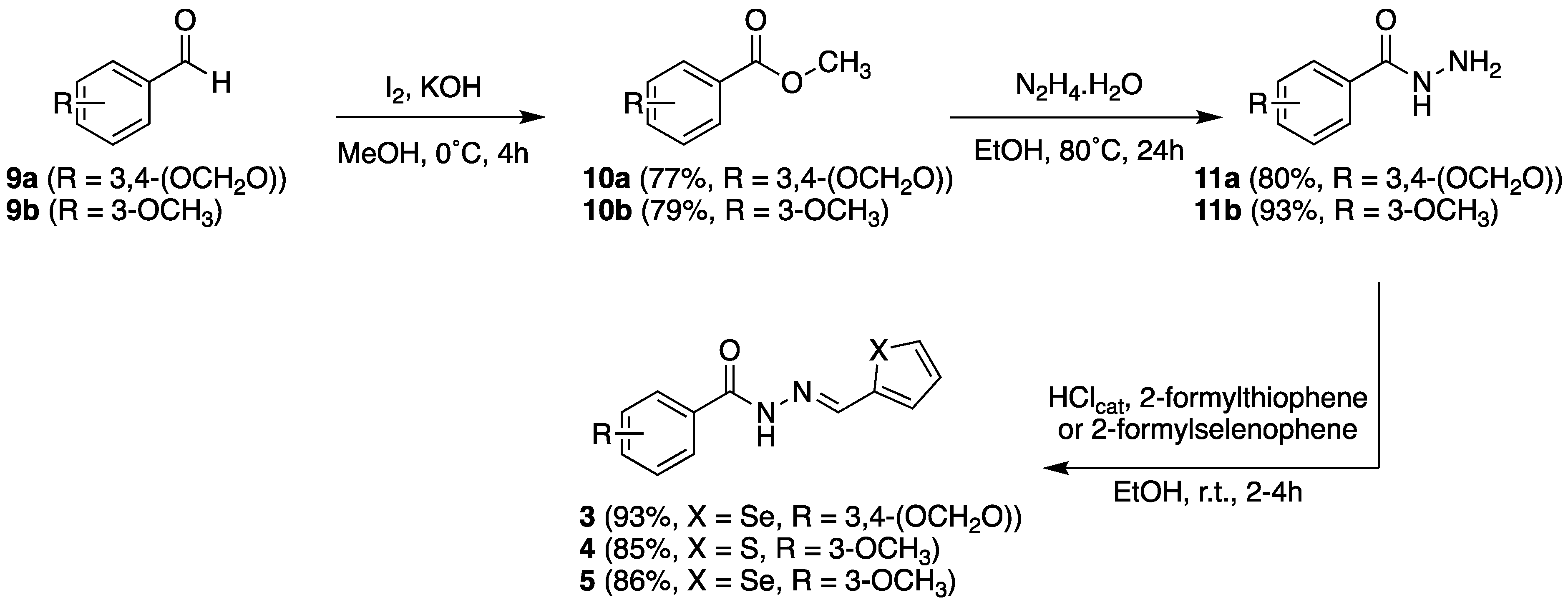
2.2.1. Chemistry
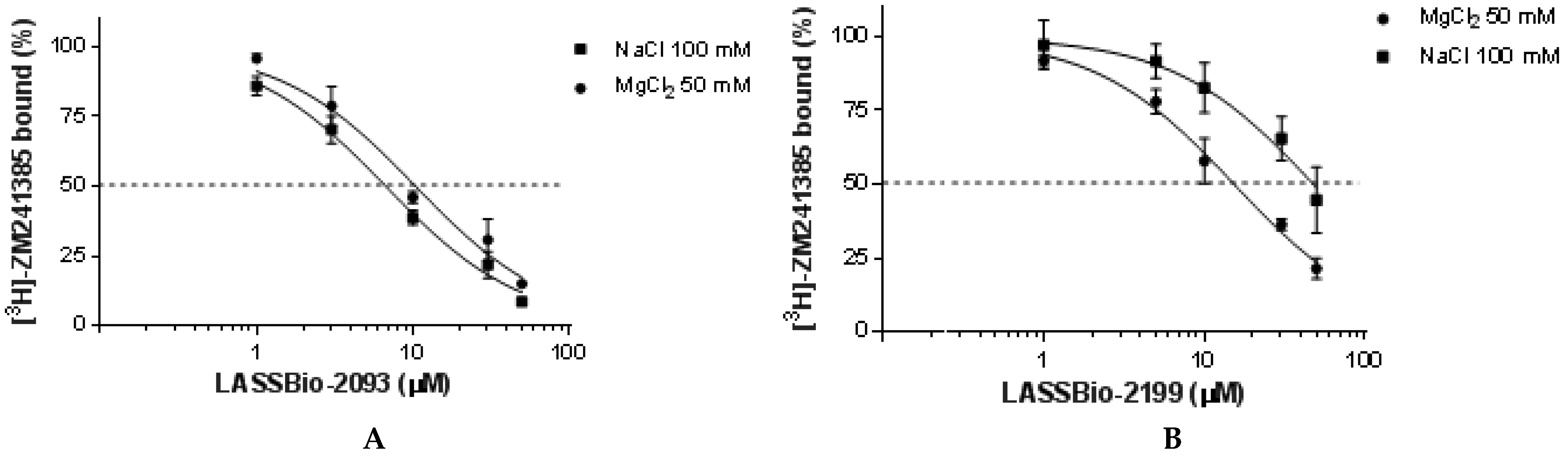
2.2.2. Pharmacological Evaluation
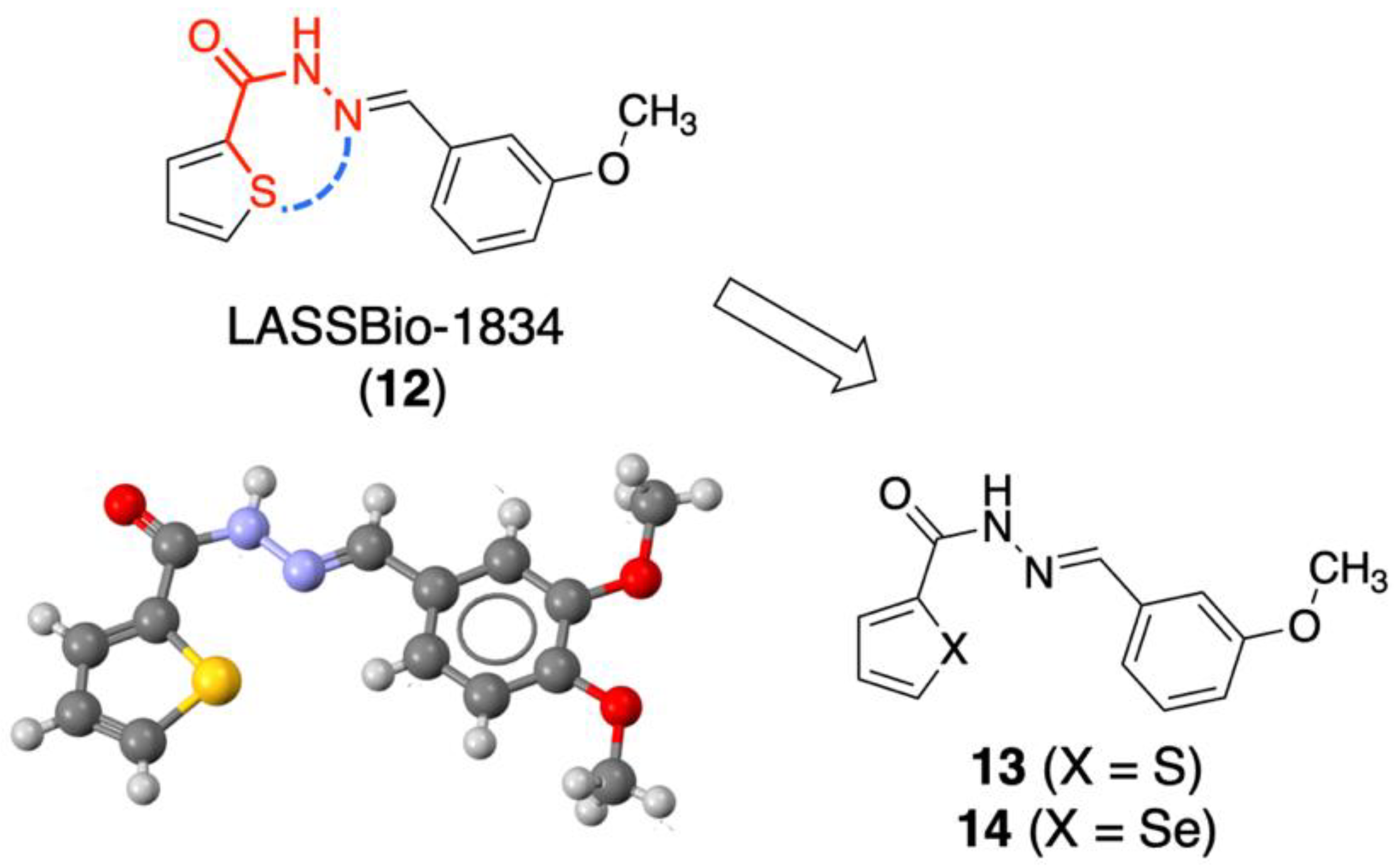
2.3. Retroisosteric Derivatives (13 and 14)
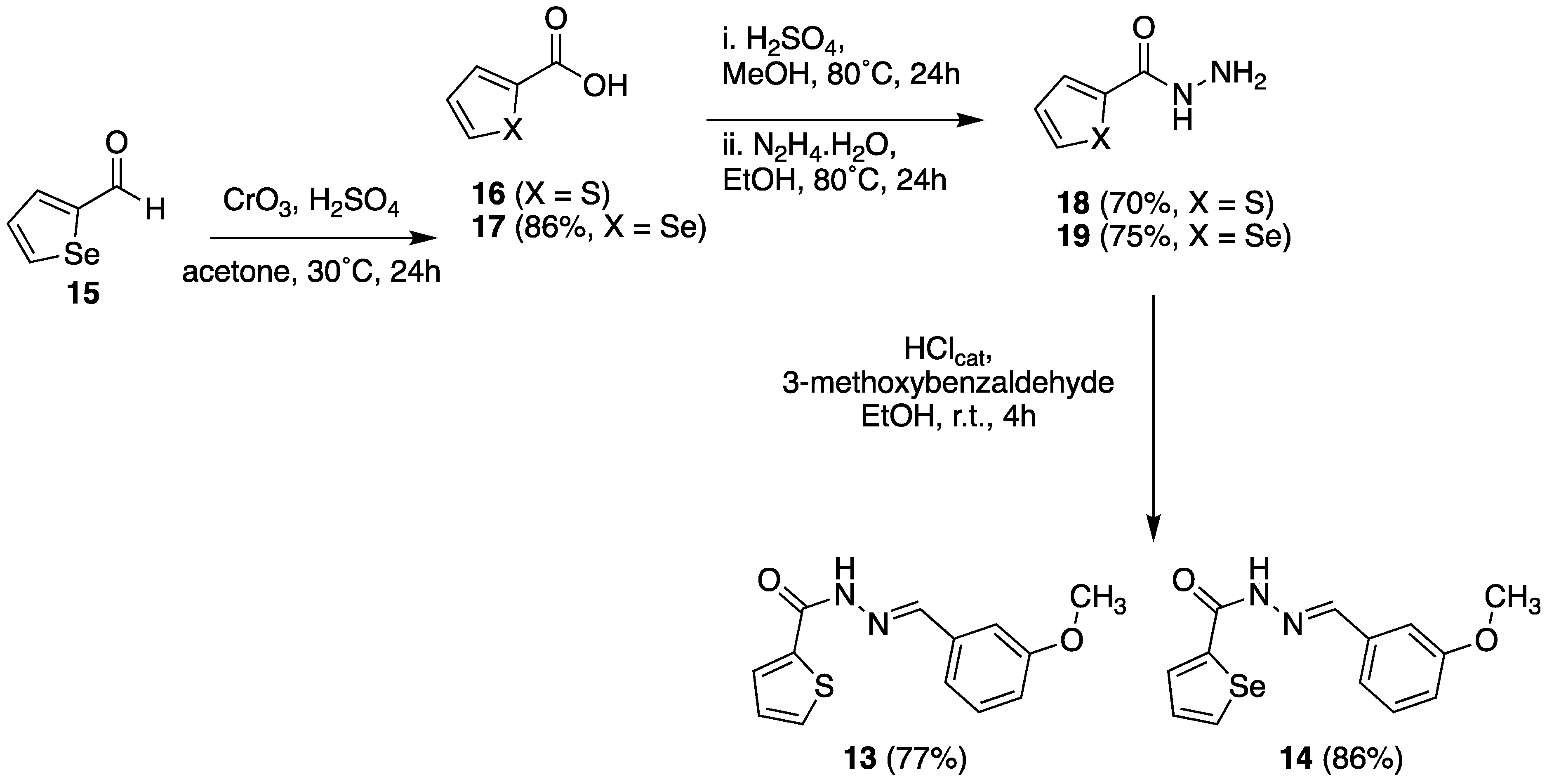
2.3.1. Chemistry
2.3.2. Pharmacological Evaluation
3. Materials and Methods
3.1. Synthesis of N-Acylhydrazones (3–8, 13, and 14)
3.1.1. N’-(Selenophen-2-yl-methylene)benzo[d][1,3] dioxole-5-carbohydrazide (3)
3.1.2. 3-Methoxy-N’-(thiophen-2-yl-methylene)benzohydrazide (4)
3.1.3. 3-Methoxy-N’-(selenophen-2-yl-methylene)benzohydrazide (5)
3.1.4. N’-(3-Methoxybenzylidene)thiophene-2-carbo-hydrazide (13)
3.1.5. N’-(3-Methoxybenzylidene)selenophene-2-ca-rbohydrazide (14)
3.2. N-Methylation of N-Acylhydrazones (6, 7 and 8)
3.2.1. N-Methyl-N’-(selenophen-2-yl-methylene) benzo[d][1,3]dioxole-5-carbohydrazide (6)
3.2.2. 3-Methoxy-N-methyl-N’-(thiophen-2-yl-methylene)benzohydrazide (7)
3.2.3. 3-Methoxy-N-methyl-N’-(thiophen-2-yl-methylene)benzohydrazide (8)
3.3. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thota, S.; Rodrigues, D.A.; Pinheiro, P.S.M.; Lima, L.M.; Fraga, C.A.M.; Barreiro, E.J. N-acylhydrazone as drugs. Bioorg. Med. Chem. Lett. 2018, 28, 2792–2806. [Google Scholar] [CrossRef]
- Kümmerle, A.E.; Schmitt, M.; Cardozo, S.V.S.; Lugnier, C.; Villa, P.; Lopes, A.B.; Romeiro, N.C.; Justiano, H.; Martins, M.A.; Fraga, C.A.M.; et al. Design, Synthesis and Pharmacological Evaluation of N-acylhydrazones and Novel Conformationally Constrained Compounds as Selective and Potent Orally Active Phosphodiesterase-4 Inhibitors. J. Med. Chem. 2012, 55, 7525–7545. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; Silva, K.C.M.D.; Léda, P.H.O.; Miranda, A.L.P.D.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Kümmerle, A.E. Síntese de Compostos Cardioativos 1,3-benzodioxolil-N-acilidrazônicos Planejados por Otimização do LASSBio-294. Master’s Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Barreiro, E.J. Strategy of molecular simplification in rational drug design: The discovery of a new cardioactive agent. Química Nova 2002, 24, 1172–1180. [Google Scholar] [CrossRef]
- Silva, A.G.; Zapata-Sudo, G.; Kummerle, A.E.; Fraga, C.A.M.; Barreiro, E.J.; Sudo, R.T. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg. Med. Chem. Lett. 2005, 13, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Sudo, R.T.; Zapata-Sudo, G.; Barreiro, E.J. The new compound, LASSBio 294, increases the contractility of intact and saponin-skinned cardiac muscle from Wistar rats. Br. J. Pharmacol. 2001, 134, 603–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Serratos, H.; Chang, R.; Pereira, E.F.R.; Castro, N.G.; Aracava, Y.; Melo, P.A.; Lima, P.C.; Fraga, C.A.M.; Barreiro, E.J.; Albuquerque, E.X. A Novel Thienylhydrazone, (2-Thiehylidiene)3,4-methylenedioxybenzoylhydrazine, Increases Inotropism and Decreases Fatigue of Skeletal Musle. J. Pharmacol. Exp. Ther. 2001, 299, 558–566. [Google Scholar] [PubMed]
- Silva, C.L.; Noël, F.; Barreiro, E.J. Cyclic GMP-dependent vasodilatory properties of LASSBio-294 in rat aorta. Br. J. Pharmacol. 2002, 135, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, E.O.; Andrade, C.H.; Braga, R.C.; Tôrres, A.C.B.; Alves, R.O.; Lião, L.M.; Fraga, C.A.M.; Barreiro, E.J.; Oliveira, V.D. Structure-based prediction and biosynthesis of the major mammalian metabolite of the cardioactive prototype LASSBio-294. Bioorg. Med. Chem. Lett. 2010, 12, 3734–3736. [Google Scholar] [CrossRef]
- Costa, D.G.; Silva, J.S.D.; Kümmerle, A.E.; Sudo, R.T.; Landgraf, S.S.; Caruso-Neves, C.; Fraga, C.A.M.; Barreiro, E.J.; Zapata-Sudo, G. LASSBio-294, A compound with inotropic and lusitropic activity, decreases cardiac remodeling and improves Ca2+ influx into sarcoplasmic reticulum after myocardial infarction. Am. J. Hypertens. 2012, 23, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.S.D.; Pereira, S.L.; Maia, R.C.; Landgraf, S.S.; Caruso-Neves, C.; Kümmerle, A.E.; Fraga, C.A.M.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. N-acylhydrazone improves exercise intolerance in rats submitted to myocardial infarction by the recovery of calcium homeostasis in skeletal muscle. Life Sci. 2014, 94, 30–36. [Google Scholar] [CrossRef]
- da Silva, J.S.; Gabriel-Costa, D.; Sudo, R.T.; Wang, H.; Groban, L.; Ferraz, E.B.; Nascimento, J.H.; Fraga, C.A.M.; Barreiro, E.J.; Zapata-Sudo, G. Adenosine A2A receptor agonist prevents cardiac remodeling and dysfunction in spontaneously hypertensive male rats after myocardial infarction. Drug. Des. Devel. Ther. 2017, 11, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, E.J.; Kümmerle, A.E.; Fraga, C.A.M. The methylation effect in medicinal chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef] [PubMed]
- Kümmerle, A.E.; Raimundo, J.M.; Leal, C.M.; da Silva, G.S.; Balliano, T.L.; Pereira, M.A.; de Simone, C.A.; Sudo, R.T.; Zapata-Sudo, G.; Fraga, C.A.; et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur. J. Med. Chem. 2009, 44, 4004–4009. [Google Scholar] [CrossRef] [PubMed]
- Pol-Pachin, L.; Fraga, C.A.M.; Barreiro, E.J.; Verli, H. Characterization of the conformational ensemble from bioactive N-acylhydrazone derivatives. J. Mol. Graph. Model. 2010, 28, 446–454. [Google Scholar] [CrossRef]
- Costa, F.N.; Ferreira, F.F.; Silva, T.F.D.; Barreiro, E.J. Structure Re-determination of LASSBio-294—a cardioactive compound of the N-acylhydrazone class—Using X-ray powder diffraction data. Powder Diff. 2013, 28, S491–S509. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Politzer, P. Sigma-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Pascoe, D.J.; Ling, K.B.; Cockroft, S.L. The Origin of Chalcogen-Bonding Interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, P.S.M.; Rodrigues, D.A.; Alves, M.A.; Tinoco, L.W.; Ferreira, G.B.; Sant’Anna, C.M.R.D.; Fraga, C.A.M. Theoretical and experimental characterization of 1,4-N⋯S σ-hole intramolecular interactions in bioactive N-acylhydrazone derivatives. New J. Chem. 2018, 42, 497–505. [Google Scholar] [CrossRef]
- Wermuth, C.G. Molecular Variations Based on Isosteric Replacement. In The Practice of Medicinal Chemistry, 2nd ed.; Wermuth, C.G., Ed.; Elsevier: London, UK, 2003; pp. 189–214. [Google Scholar]
- Lima, L.M.; Barreiro, E.J. New Concepts in Drug Discovery III. In Comprehensive Medicinal Chemistry; Chackalamnil, S., Rotella, S., Ward, S.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 186–210. [Google Scholar]
- Noël, F.; do Monte, F.M. Validation of a Na(+)-shift binding assay for estimation of the intrinsic efficacy of ligands at the A2A adenosine receptor. J. Pharmacol. Toxicol. Methods 2017, 84, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Pedreira, J.G.B. Study of the structure-property-activity relationship of bioisosteric sulfur/seleno compounds. Ph.D. Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Oertly, E.; Ester, D. Über einige Derivate der Piperoysäure. Ber. Dtsch. Chem. Ges. 1910, 43, 1336–1340. [Google Scholar] [CrossRef] [Green Version]
- Hartman, R.J.; Gassmann, A.G. Kinetics of the Estherification of Substituted Benzoic Acids. J. Am. Chem. Soc. 1940, 62, 1559–1560. [Google Scholar] [CrossRef]
- Yamada, S.; Morizono, D.; Yamamoto, K. Mild oxidation of aldehydes to the corresponding carboxylic acids and esters: Alkaline iodine oxidation revisited. Tetrahedron Lett. 1992, 33, 4329–4332. [Google Scholar] [CrossRef]
- Hutton, K. The Synthesis of Some New Phenylurethans as Potential Local Anesthetics. J. Org. Chem. 1955, 7, 855–861. [Google Scholar] [CrossRef]
- Omodei-Sale, A.; Consonni, P.; Galliani, G. A new class of nonhormonal pregnancy-terminating agents. Synthesis and contragestational activity of 3,5-diaryl-s-triazoles. J. Med. Chem. 1983, 26, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Palla, G.; Pedrieri, G.; Domiano, P.; Vignali, C.; Turner, W. Conformational behaviour and isomerization of -acyl and -aroylhydrazones. Tetrahedron 1986, 42, 3649–3654. [Google Scholar] [CrossRef]
- Akiba, K.; Tsuchiya, T.; Inamoto, N.; Yamada, K.; Tanaka, H.; Kawazura, H. A 13C-NMR study on the adduct of “hector’s base” with arylcyanamides evidence for intramolecular S⋯N interaction. Tetrahedron Lett. 1976, 17, 3819–3820. [Google Scholar] [CrossRef]
- Akiba, K.; Tsuchiya, T.; Inamoto, N.; Onuma, K.; Nagashima, N. The Structure of the 1:1 Adduct of “Hector′s Base” with Arylcyanamides Bond Switch on Hypervalent Sulfur. Chem. Chem. Lett. 1976, 5, 723–726. [Google Scholar] [CrossRef]
- Noël, F.; Pompeu, T.E.; Moura, B.C. Functional binding assays for estimation of the intrinsic efficacy of ligands at the 5-HT1A receptor: Application for screening drug candidates. J. Pharmacol. Toxicol. Methods 2014, 70, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Wittmann, H.J.; Schneider, E.H.; Seifert, R. Modulation of GPCRs by monovalent cations and anions. Naunyn-Schmiedeb. Arch. Pharmacol. 2015, 388, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Bastos, I.T.S.; Pinheiro, P.S.M.; Costa, F.N.; Rocha, M.D.; Sant’Anna, C.M.R.; Braz, D.; Souza, E.T.; Martins, M.A.; Barreiro, E.J.; Ferreira, F.F.; et al. Design, Synthesis, Experimental and Theoretical Characterization of a New Multitarget 2-Thienyl-N-Acylhydrazone Derivative. Pharmaceuticals 2018, 11, 119. [Google Scholar] [CrossRef] [Green Version]
- Carrara, G.; Chiancone, F.M.; D’Amato, V.; Ginoulhiac, E.; Martinuzzi, C.; Teotino, U.M.; Visconti, N. Initial contribution to knowledge of the antitubercular activity of hydrazides. Gazz. Chim. Ital. 1952, 82, 625–670. [Google Scholar]
- Fischer, E.; Speier, A. Darstellung der Ester. Ber. Dtsch. Chem. Ges. 1895, 28, 3252–3258. [Google Scholar] [CrossRef]
- Dubus, P.; Decroix, B.; Morel, J.; Paulmier, C. Reactions of heterocyclic cyano derivatives. C. R. Acad. Sci. 1974, 278, 61–63. [Google Scholar]
- Kornblum, N.; Powers, J.W.; Anderson, G.J.; Jones, W.J.; Larson, O.H. New and Selective Method of Oxidation. J. Am. Chem. Soc. 1957, 79, 6562–6573. [Google Scholar] [CrossRef]
- Jasiak, K.; Kudelki, A.; Zienlinki, W.; Wojciech, K.; Kuznik, N. Study on DDQ-promoted synthesis of 2,4-disubstituted 1,3,4-oxadiazoles from hydrazides and aldehydes. ARKIVOC 2017, 2, 87–106. [Google Scholar]
- Chemical Abstracts Number: 315206-29-2. In National Library of Medicine (US) National Center for Biotechnology Information; PubChem Compound Summary for CID 688040; American Chemiscal Society: Washington, DC, USA, 2004.
- Lowry, O.H.; Rosenbrough, N.J.; Farr, H.J.; Randall, R.J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
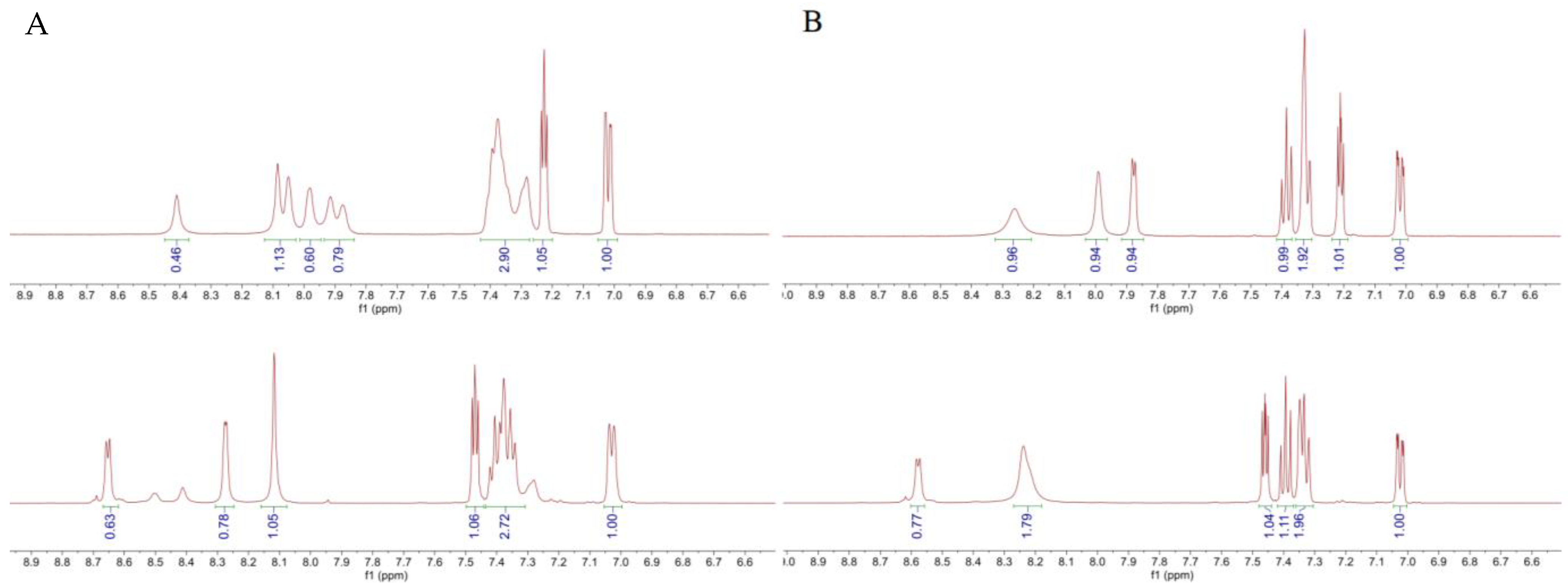
| Compound | Ar | X | 1H NMR Shift (ppm)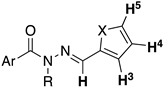 | ||||
|---|---|---|---|---|---|---|---|
| H-3 | H-4 | H-5 | OCNH | N=CH | |||
| LASSBio-294 (1) | 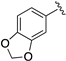 | S | 7.43–7.45 | 7.13 | 7.66 | 11.56 | 8.62 |
| LASSBio-2062 (3) | Se | 7.62 | 7.35 | 8.23 | 11.68 | 8.60 | |
| LASSBio-2092 (4) | 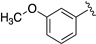 | S | 7.49–7.44 | 7.15 | 7.68 | 11.68 | 8.66 |
| LASSBio-2093(5) | Se | 7.62 | 7.36 | 8.24 | 11.85 | 8.68 | |
| LASSBio-785 (2) | 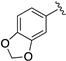 | S | 7.39 | 7.09 | 7.54 | - | 8.22 |
| LASSBio-2063 (6) | Se | 7.56 | 7.31 | 8.10 | - | 8.16 | |
| LASSBio-2198 (7) | 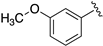 | S | 7.38 | 7.09 | 7.52 | - | 8.24 |
| LASSBio-2199 (8) | Se | 7.56 | 7.30 | 8.09 | - | 8.20 | |
| Compound | IC50 (µM) MgCl2 (I) | IC50 (µM) NaCl (II) | Na+-Shift (II/I) | P |
|---|---|---|---|---|
| LASSBio-294 (1) | 38.9 (33.1–45.4) | 24.4 (22.0–27.1) | 0.6 | 0.018 |
| LASSBio-2062 (3) | 20.9 (17.5–24.9) | 11.3 (10.0–12.8) | 0.5 | 0.010 |
| LASSBio-2092 (4) | 10.8 (9.6–12.2) | 7.3 (6.4–8.4) | 0.7 | 0.016 |
| LASSBio-2093 (5) | 10.4 (8.7–12.5) | 6.7 (5.6–8.1) | 0.6 | 0.011 |
| LASSBio-785 (2) | 47.9 (39.4–58.3) | 107 (84–135) | 2.3 | 0.043 |
| LASSBio-2063 (6) | 52.9 (43.4–64.4) | 86.9 (70.6–107) | 1.7 | 0.004 |
| LASSBio-2198 (7) | 22.3 (19.8–25.2) | 54.6 (44.7–66.8) | 2.5 | 0.010 |
| LASSBio-2199 (8) | 15.4 (13.4–17.6) | 47.9 (37.0–61.9) | 3.1 | 0.039 |
| Compound | IC50 (µM) MgCl2 (I) | IC50 (µM) NaCl (II) | Na+-Shift (II/I) | P |
|---|---|---|---|---|
| LASSBio-2278 (13) | 27.1 (24.6–30.0) | 55.6 (46.7–66.3) | 2 | 0.010 |
| LASSBio-2279 (14) | 16.6 (15.3–18.1) | 37.7 (34.5–41.2) | 2.3 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedreira, J.G.B.; Silva, R.R.; Noël, F.G.; Barreiro, E.J. Effect of S–Se Bioisosteric Exchange on Affinity and Intrinsic Efficacy of Novel N-acylhydrazone Derivatives at the Adenosine A2A Receptor. Molecules 2021, 26, 7364. https://doi.org/10.3390/molecules26237364
Pedreira JGB, Silva RR, Noël FG, Barreiro EJ. Effect of S–Se Bioisosteric Exchange on Affinity and Intrinsic Efficacy of Novel N-acylhydrazone Derivatives at the Adenosine A2A Receptor. Molecules. 2021; 26(23):7364. https://doi.org/10.3390/molecules26237364
Chicago/Turabian StylePedreira, Júlia Galvez Bulhões, Rafaela Ribeiro Silva, François G. Noël, and Eliezer J. Barreiro. 2021. "Effect of S–Se Bioisosteric Exchange on Affinity and Intrinsic Efficacy of Novel N-acylhydrazone Derivatives at the Adenosine A2A Receptor" Molecules 26, no. 23: 7364. https://doi.org/10.3390/molecules26237364
APA StylePedreira, J. G. B., Silva, R. R., Noël, F. G., & Barreiro, E. J. (2021). Effect of S–Se Bioisosteric Exchange on Affinity and Intrinsic Efficacy of Novel N-acylhydrazone Derivatives at the Adenosine A2A Receptor. Molecules, 26(23), 7364. https://doi.org/10.3390/molecules26237364






