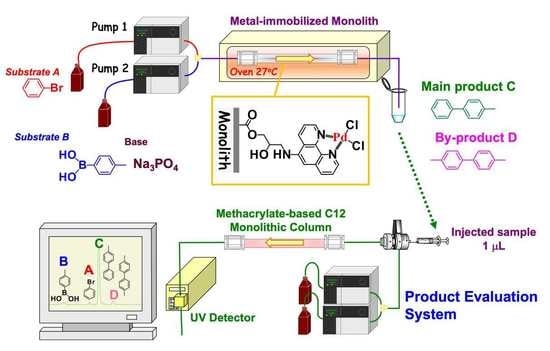
Preparation of Metal-Immobilized Methacrylate-Based Monolithic Columns for Flow-Through Cross-Coupling Reactions
Abstract
:1. Introduction
2. Results and Discussion
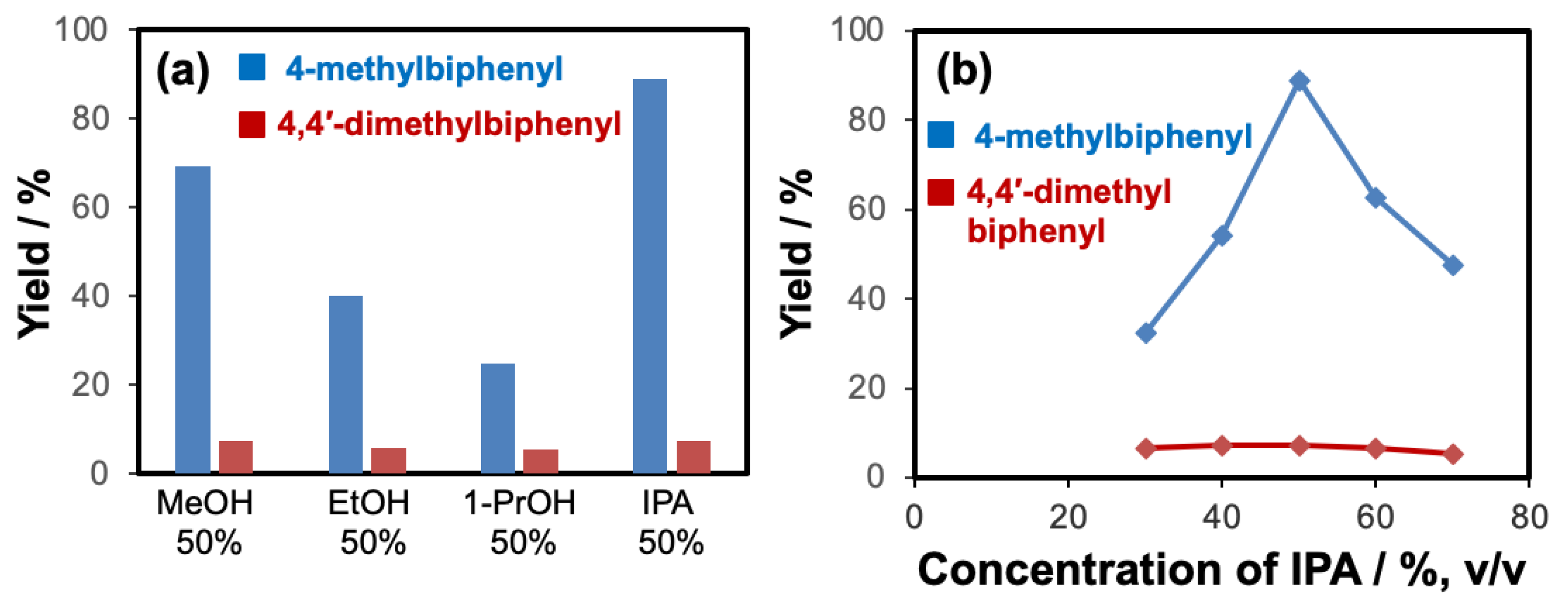
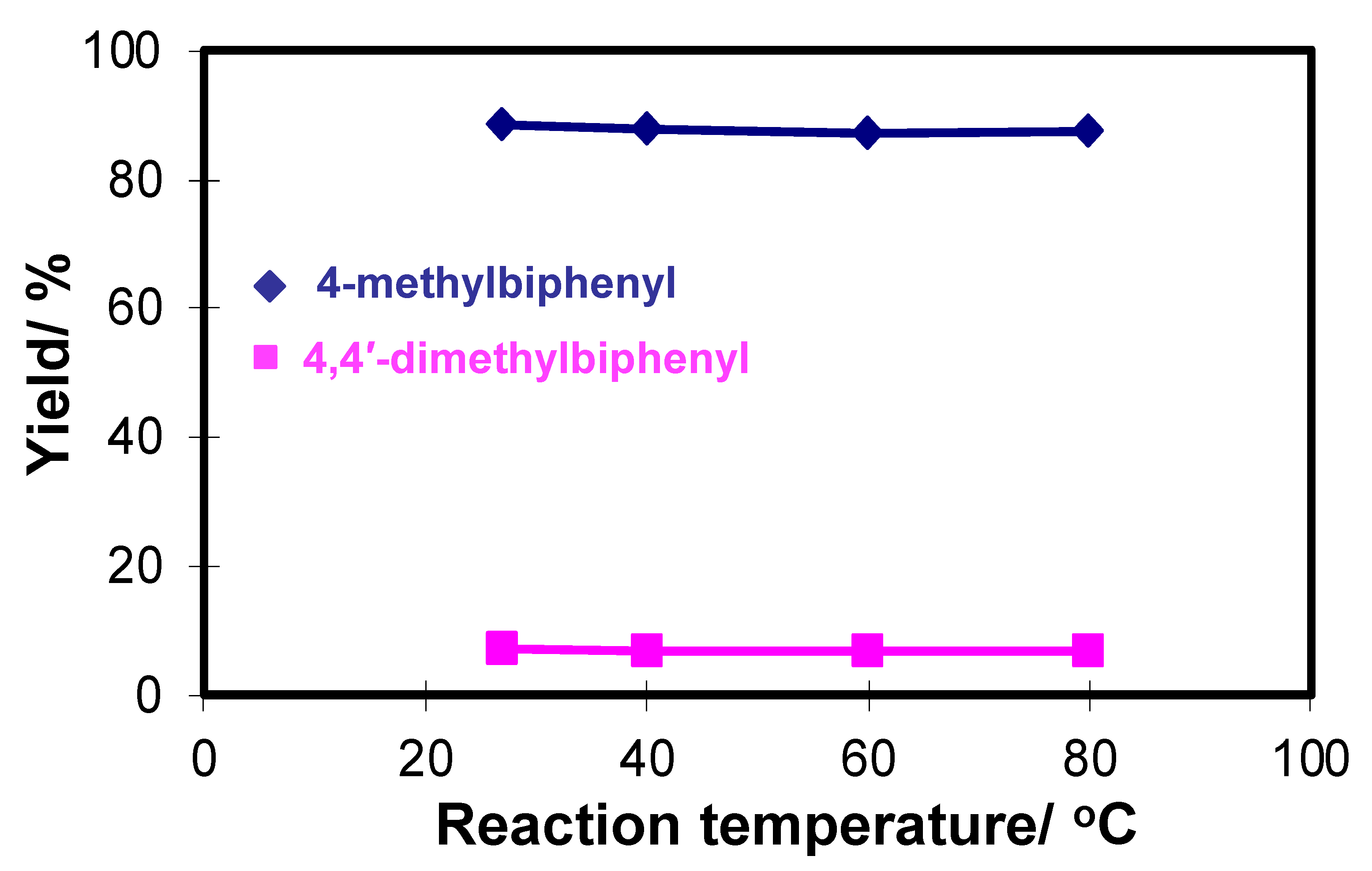
2.1. Optimization of Chemical Reagents for Flow-Through Cross-Coupling Reactions
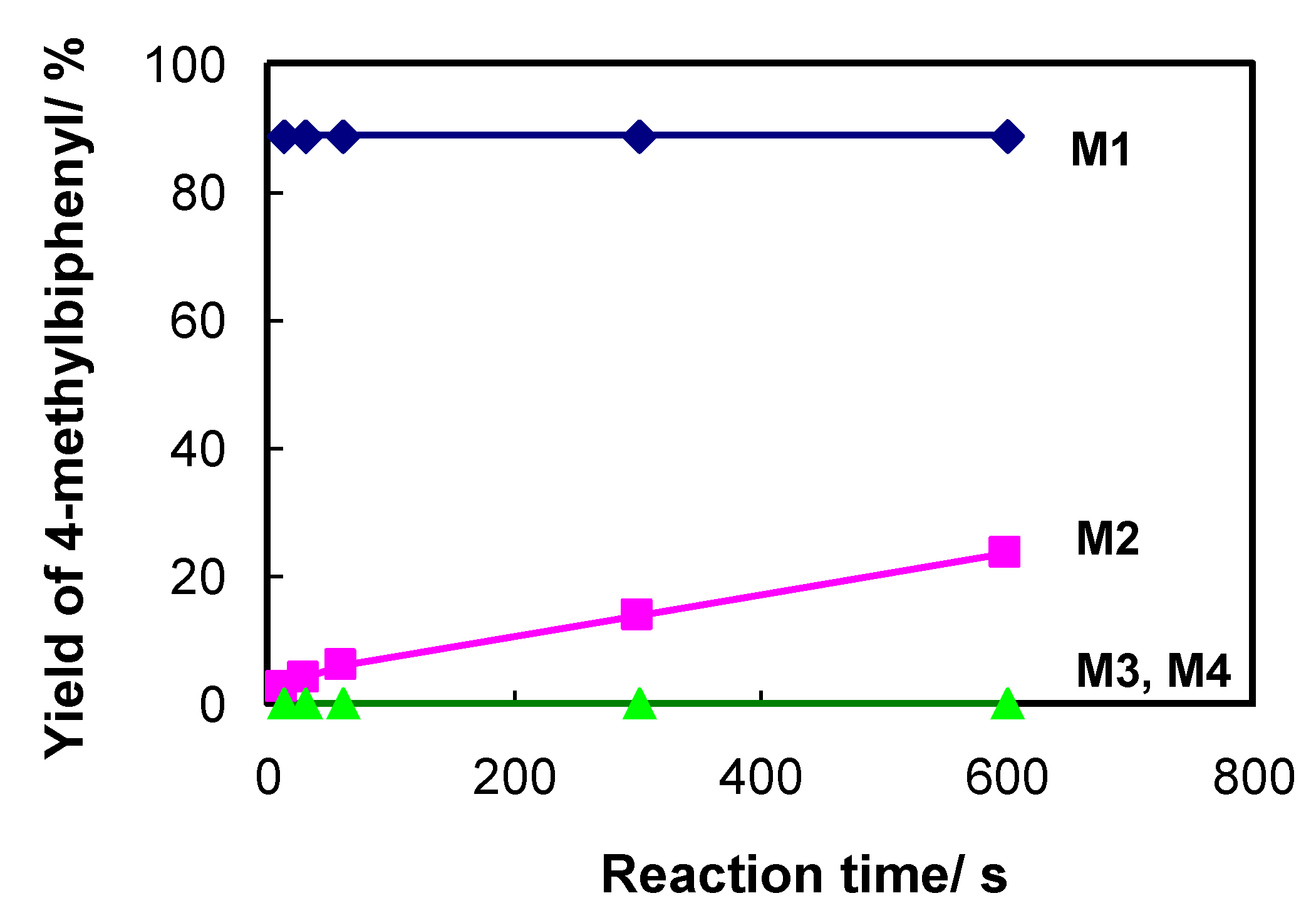
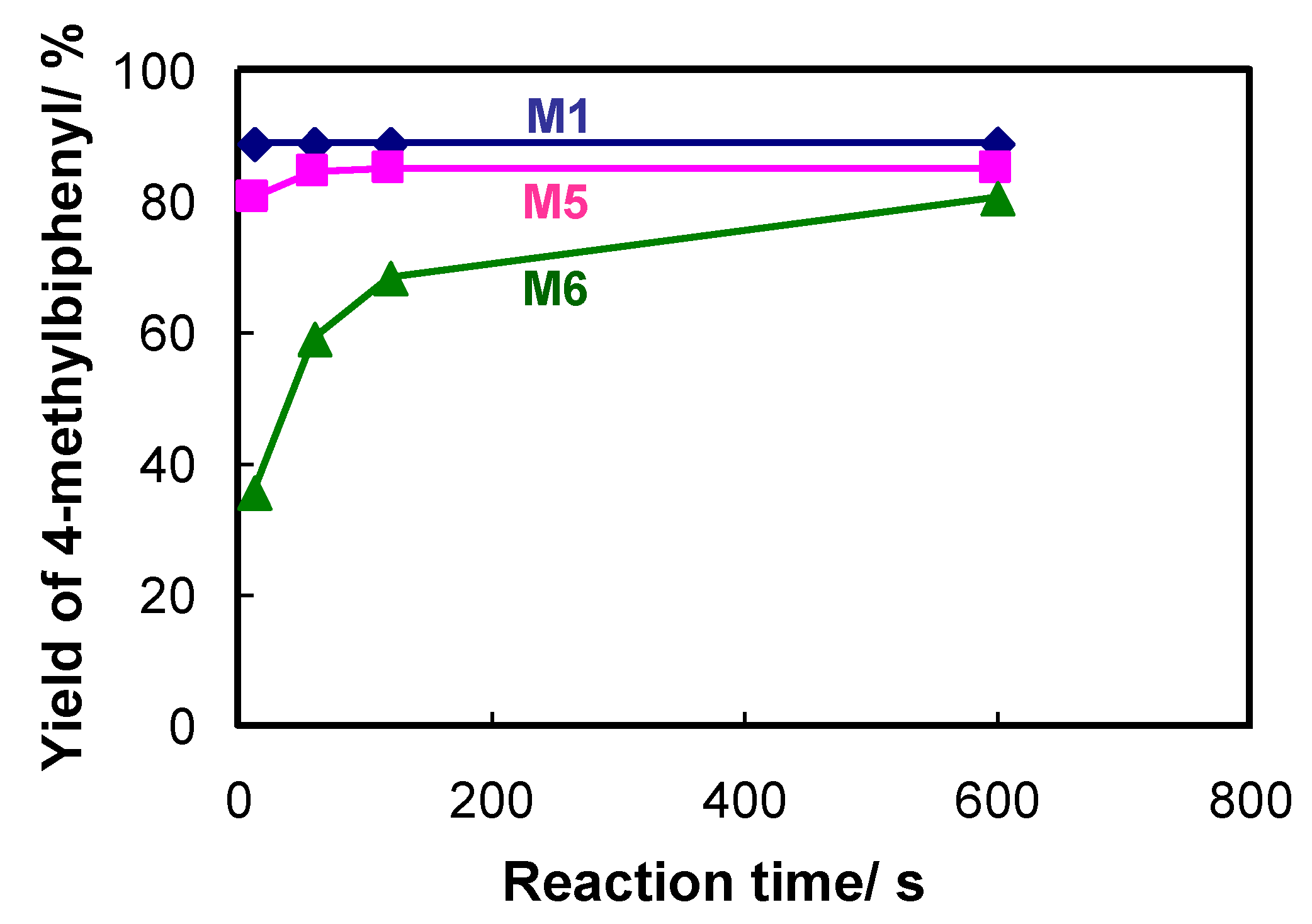
2.2. Effect of the Metal Catalyst and Ligands on the Efficiency of Flow-Through Cross-Coupling Reactions
3. Materials and Methods
3.1. Reagents
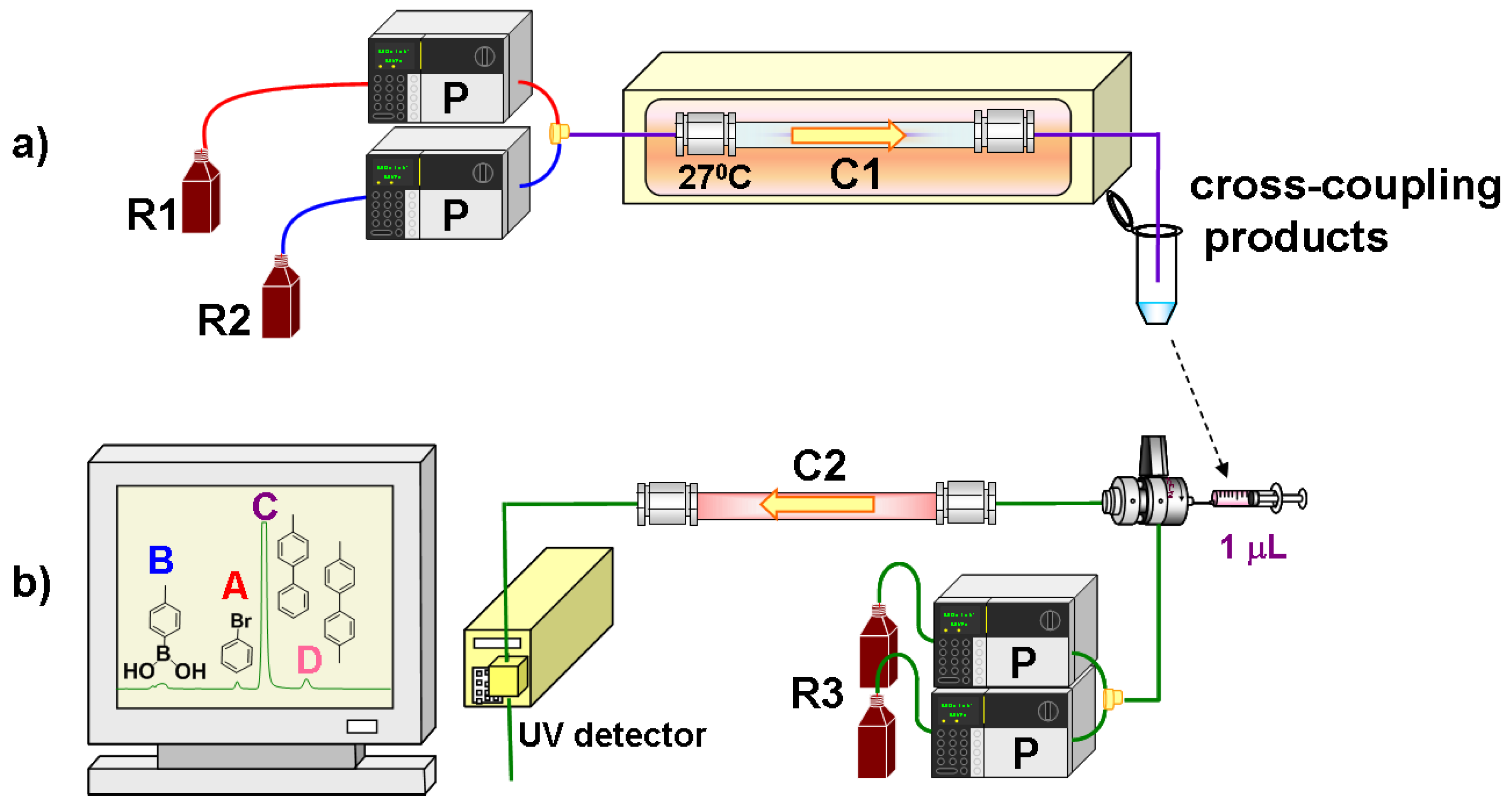
3.2. Procedure for the Preparation of Flow-Through Monolithic Microreactors
3.3. Instrumentation and Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jin, J.; Cai, M.-M.; Li, J.-X. Highly Efficient Suzuki Coupling of Aryl Chlorides in a Continuous Flow Capillary Microreactor. Synlett 2009, 15, 2534–2538. [Google Scholar]
- Shi, G.; Hong, F.; Liang, Q.; Fang, H.; Nelson, S.; Weber, S.G. Capillary-based, serial-loading, parallel microreactor for catalyst screening. Anal. Chem. 2006, 78, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Basheer, C.; Hussain, F.S.J.; Lee, H.K.; Valiyaveettil, S. Design of a capillary-microreactor for efficient Suzuki coupling reactions. Tetrahedron Lett. 2004, 45, 7297–7300. [Google Scholar] [CrossRef]
- Uozumi, Y.; Yamada, Y.M.A.; Beppu, T.; Fukuyama, N.; Ueno, M.; Kitamori, T. Instantaneous Carbon-Carbon Bond Formation Using a Microchannel Reactor with a Catalytic Membrane. J. Am. Chem. Soc. 2006, 128, 15994–15995. [Google Scholar] [CrossRef]
- Min, K.-I.; Lee, T.-H.; Park, C.P.; Wu, Z.-Y.; Girault, H.H.; Ryu, I.; Fukuyama, T.; Mukai, Y.; Kim, D.-P. Monolithic and flexible polyimide film microreactors for organic microchemical applications fabricated by laser ablation. Angew. Chem. Int. Ed. 2010, 49, 7063–7067. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, A.; Kenmoku, A.; Moriwaki, Y.; Hayashi, A.; Yoshida, J. Cross-Coupling in a Flow Microreactor: Space Integration of Lithiation and Murahashi Coupling. Angew. Chem. Int. Ed. 2010, 49, 7543–7547. [Google Scholar] [CrossRef] [PubMed]
- Usutani, H.; Tomida, Y.; Nagaki, I.; Okamoto, H.; Nokami, T.; Yoshida, J. Generation and Reactions of o-Bromophenyllithium without Benzyne Formation Using a Microreactor. J. Am. Chem. Soc. 2007, 129, 3046–3047. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Hamakawa, S.; Tanaka, S.; Sato, K.; Esashi, M.; Mizukami, F. A one-step conversion of benzene to phenol using mems-based Pd membrane microreactors. Chem. Eng. J. 2009, 155, 829–837. [Google Scholar] [CrossRef]
- Nagaki, A.; Togai, M.; Suga, S.; Aoki, N.; Mae, K.; Yoshida, J. Control of Extremely Fast Competitive Consecutive Reactions using Micromixing. Selective Friedel-Crafts Aminoalkylation. J. Am. Chem. Soc. 2005, 127, 11666–11675. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, T.; Kitamura, Y.; Sako, S.; Udzu, T.; Sakurai, A.; Tanaka, A.; Kobayashi, Y.; Endo, K.; Bora, U.; Kurita, T.; et al. Heterogeneous Pd/C-Catalyzed Ligand-Free, Room-Temperature Suzuki-Miyaura Coupling Reactions in Aqueous Media. Chem. Eur. J. 2007, 13, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Sako, S.; Tsutsui, A.; Monguchi, Y.; Maegawa, T.; Kitade, Y.; Sajiki, H. Ligand-Free and Heterogeneous Palladium on Carbon-Catalyzed Hetero-Suzuki-Miyaura Cross-Coupling. Adv. Synth. Catal. 2010, 352, 718–730. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Wang, Y.; Zhang, M. Palladium-Iminodiacetic Acid Immobilized on pH-Responsive Polymeric Microspheres: Efficient Quasi-Homogeneous Catalyst for Suzuki and Heck Reactions in Aqueous Solution. Adv. Synth. Catal. 2008, 350, 2065–2076. [Google Scholar] [CrossRef]
- Altava, B.; Burguete, M.I.; Garcia-Verdugo, E.; Karbass, N.; Luis, S.V.; Puzary, A.; Sans, V. Palladium N-methylimidazolium supported complexes as efficient catalysts for the Heck reaction. Tetrahedron Lett. 2006, 47, 2311–2314. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tanaka, D.; Danjo, H.; Uozumi, Y. A Combinatorial Approach to Heterogeneous Asymmetric Aquacatalysis with Amphiphilic Polymer-Supported Chiral Phosphine-Palladium Complexes. Adv. Synth. Catal. 2006, 348, 1561–1566. [Google Scholar] [CrossRef]
- Hocke, H.; Uozumi, Y. PS-PEG resin-supported palladium-MOP complexes. Application in asymmetric π-allylic reduction. Tetrahedron 2004, 60, 9297–9306. [Google Scholar] [CrossRef]
- Oe, Y.; Uozumi, Y. Highly Efficient Heterogeneous Aqueous Kharasch Reaction with an Amphiphilic Resin-Supported Ruthenium Catalyst. Adv. Synth. Catal. 2008, 350, 1771–1775. [Google Scholar] [CrossRef]
- Greenway, G.M.; Haswell, S.J.; Morgan, D.O.; Skelton, V.; Styring, P. The use of a novel microreactor for high throughput continuous flow organic synthesis. Sens. Actuators B Chem. 2000, 63, 153–158. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Khan, J.; Styring, P. Polymer-supported palladium catalysed Suzuki-Miyaura reactions in batch and a mini-continuous flow reactor system. Tetrahedron 2005, 61, 12065–12073. [Google Scholar] [CrossRef]
- Mennecke, K.; Kirschning, A. Polyionic polymers—Heterogeneous media for metal nanoparticles as catalyst in Suzuki-Miyaura and Heck-Mizoroki reactions under flow conditions. Beilstein J. Org. Chem. 2009, 5, 21. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, M.; Zhang, W.; Yang, L. Enhanced Pd-catalyzed hydrogenation of olefins within polymeric microreactors under organic/aqueous biphasic conditions. Chem. Eur. J. 2009, 15, 3670–3673. [Google Scholar] [CrossRef]
- Lee, C.K.Y.; Holmes, A.B.; Ley, S.V.; McConvey, I.F.; Al-Duri, B.; Leeke, G.A.; Santos, R.C.D.; Seville, J.P.K. Efficient batch and continuous flow Suzuki cross-coupling reactions under mild conditions, catalysed by polyurea-encapsulated palladium (II) acetate and tetra-n-butylammonium salts. Chem. Commun. 2005, 16, 2175–2177. [Google Scholar] [CrossRef]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported Catalysis in Continuous-Flow Microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Sachse, A.; Galarneau, A.; Coq, B.; Fajula, F. Monolithic flow microreactors improve fine chemicals synthesis. New J. Chem. 2011, 35, 259–264. [Google Scholar] [CrossRef]
- Zhao, X.S.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32–39. [Google Scholar] [CrossRef]
- Lu, L.; Zou, S.; Fang, B. The Critical Impacts of Ligands on Heterogeneous Nanocatalysis: A Review. ACS Catal. 2021, 11, 6020–6058. [Google Scholar] [CrossRef]
- Zou, Y.-Q.; Chakraborty, S.; Nerush, A.; Oren, D.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Highly Selective, Efficient Deoxygenative Hydrogenation of Amides Catalyzed by a Manganese Pincer Complex via Metal-Ligand Cooperation. ACS Catal. 2018, 8, 8014–8019. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Toy, P.H. Organic Polymer Supports for Synthesis and for Reagent and Catalyst Immobilization. Chem. Rev. 2009, 109, 815–838. [Google Scholar] [CrossRef]
- Arrua, R.D.; Strumia, M.C.; Igarzabal, C.I.A. Macroporous Monolithic Polymers: Preparation and Applications. Materials 2009, 2, 2429–2466. [Google Scholar] [CrossRef] [Green Version]
- Shu, S.; Kobayashi, H.; Kojima, N.; Sabarudin, A.; Umemura, T. Preparation and characterization of lauryl methacrylate-based monolithic microbore column for reversed-phase liquid chromatography. J. Chromatogr. A 2011, 1218, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Tasfiyati, A.N.; Iftitah, E.D.; Sakti, S.P.; Sabarudin, A. Evaluation of glycidyl methacrylate-based monolith functionalized with weak anion exchange moiety inside 0.5 mm id column for liquid chromatographic separation of DNA. Anal. Chem. Res. 2016, 7, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Sabarudin, A.; Huang, J.; Shu, S.; Sakagawa, S.; Umemura, T. Preparation of methacrylate-based anion-exchange monolithic microbore column for chromatographic separation of DNA fragments and oligonucleotides. Anal. Chim. Acta 2012, 736, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gömann, A.; Deverell, J.A.; Munting, K.F.; Jones, R.C.; Rodemann, T.; Canty, A.J.; Smith, J.A.; Guijt, R.M. Palladium-mediated organic synthesis using porous polymer monolith formed in situ as a continuous catalyst support structure for application in microfluidic devices. Tetrahedron 2009, 65, 1450–1454. [Google Scholar] [CrossRef]
- Bolton, K.F.; Canty, A.J.; Deverell, J.A.; Guijt, R.M.; Hilder, E.F.; Rodemann, T.; Smith, J.A. Macroporous monolith supports for continuous flow capillary microreactors. Tetrahedron Lett. 2006, 47, 9321–9324. [Google Scholar] [CrossRef]
- Jones, R.C.; Canty, A.J.; Deverell, J.A.; Gardiner, M.G.; Guijt, R.M.; Rodemann, T.; Smith, J.A.; Tolhurst, V.A. Supported palladium catalysis using a heteroleptic 2-methylthiomethylpyridine-N,S-donor motif for Mizoroki-Heck and Suzuki-Miyaura coupling, including continuous organic monolith in capillary microscale flow-through mode. Tetrahedron 2009, 65, 7474–7481. [Google Scholar] [CrossRef]
- Karbass, N.; Sans, V.; Garcia-Verdugo, E.; Burguete, M.I.; Luis, S.V. Pd(0) supported onto monolithic polymers containing IL-like moieties. Continuous flow catalysis for the Heck reaction in near-critical EtOH. Chem. Commun. 2006, 29, 3095–3097. [Google Scholar] [CrossRef] [PubMed]
- Burguete, M.I.; Cornejo, A.; GarcíaVerdugo, E.; García, J.; Gil, M.J.; Luis, S.V.; Martínez-Merino, V.; Mayoral, J.A.; Sokolova, M. Bisoxazoline-functionalised enantioselective monolithic mini-flow-reactors: Development of efficient processes from batch to flow conditions. Green Chem. 2007, 9, 1091–1096. [Google Scholar] [CrossRef]
- Burguete, M.I.; Cornejo, A.; GarcíaVerdugo, E.; Gil, M.J.; Luis, S.V.; Mayoral, J.A.; Martínez-Merino, V.; Sokolova, M. Pybox monolithic miniflow reactors for continuous asymmetric cyclopropanation reaction under conventional and supercritical conditions. J. Org. Chem. 2007, 72, 4344–4350. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N.; Smith, C.D. Azide monoliths as convenient flow reactors for efficient Curtius rearrangement reactions. Org. Biomol. Chem. 2008, 6, 1587–1593. [Google Scholar] [CrossRef]
- Burguete, M.I.; Garcia-Verdugo, E.; Karbass, N.; Luis, S.V.; Sans, V.; Sokolova, M. Development of efficient processes under flow conditions based on catalysts immobilized onto monolithic supported ionic liquid-like phases. Pure Appl. Chem. 2009, 81, 1991–2000. [Google Scholar] [CrossRef] [Green Version]
- Lozano, P.; García-Verdugo, E.; Piamtongkam, R.; Karbass, N.; de Diego, T.; Burguete, M.I.; Luis, S.V.; Iborra, J.L. Bioreactors Based on Monolith-Supported Ionic Liquid Phase for Enzyme Catalysis in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2007, 349, 1077–1084. [Google Scholar] [CrossRef]
- Zhang, G. Ligand-free Suzuki-Miyaura reaction catalysed by Pd/C at room temperature. J. Chem. Res. 2004, 9, 593–595. [Google Scholar] [CrossRef]
- Basavaraju, G.; Rajanna, R. Flow Process Development and Optimization of A Suzuki-Miyaura Cross Coupling Reaction using Response Surface Methodology. Bull. Chem. React. Eng. Catal. 2020, 15, 604–616. [Google Scholar] [CrossRef]
- Nagaki, A.; Hirose, K.; Moriwaki, Y.; Takumi, M.; Takahashi, Y.; Mitamura, K.; Matsukawa, K.; Ishizuka, N.; Yoshida, J. Suzuki-Miyaura Coupling Using Monolithic Pd Reactors and Scaling-Up by Series Connection of the Reactors. Catalysts 2019, 9, 300. [Google Scholar] [CrossRef] [Green Version]
- Reizman, B.J.; Wang, Y.-M.; Buchwald, S.L.; Jensen, K.F. Suzuki-Miyaura cross-coupling optimization enabled by automated feedback. React. Chem. Eng. 2016, 1, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.A.; Davis, B.A.; Ramezani, M.; Genzer, J.; Efimenko, K.; Abolhasani, M. Continuous Ligand-Free Suzuki-Miyaura Cross-Coupling Reactions in a Cartridge Flow Reactor Using a Gel-Supported Catalyst. Ind. Eng. Chem. Res. 2021, 60, 9418–9428. [Google Scholar] [CrossRef]
- Yan, L.; Wang, H.; Bai, L.; Fu, Y.; Cheng, Y. Suzuki-Miyura cross-coupling reaction in droplet-based microreactor. Chem. Eng. Sci. 2019, 207, 352–357. [Google Scholar] [CrossRef]
- Shusuke, O.; Takuma, U.; Takashi, K.; Kohsuke, M.; Hiromi, Y. Synthesis of Pd-supported Nanosized Mesoporous Silica as a Spherical Nanocatalyst for Suzuki-Miyaura Coupling Reaction. Chem. Lett. 2011, 40, 609–611. [Google Scholar]
- Yamamoto, K.; Komiyama, R.; Umemura, T. Numerical Simulation on Flow in Column Chromatography. Int. J. Mod. Phys. C 2013, 24, 1340003. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tajima, Y. Numerical Simulation of Fluid Dynamics in a Monolithic Column. Separations 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Percec, V.; Bae, J.-Y.; Hill, D.H. Aryl Mesylates in Metal Catalyzed Homocoupling and Cross-Coupling Reactions. 2. Suzuki-Type Nickel-Catalyzed Cross-Coupling of Aryl Arenesulfonates and Aryl Mesylates with Arylboronic Acids. J. Org. Chem. 1995, 60, 1060–1065. [Google Scholar] [CrossRef]
- Galland, J.-C.; Savignac, M.; Genet, J.-P. Cross-coupling of chloroarenes with boronic acids using a water-soluble nickel catalyst. Tetrahedron Lett. 1999, 40, 2323–2326. [Google Scholar] [CrossRef]


| Polymeric Support | Ligand | Catalyst | Microreactor |
|---|---|---|---|
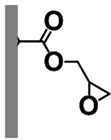 Poly(GMA-co-EDMA) Monolith | 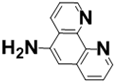 APHEN | Pd(II) | 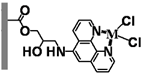 M1 = Pd(II), M2 = Ni(II) M3 = Fe(II), M4 = Cu(II) |
| Ni(II) | |||
| Fe(II) | |||
| Cu(II) | |||
| HN(CH2COOH)2 IDA | Pd(II) |  M5 M5 | |
| HN(CH2PO3H2)2 IDP | Pd(II) |  M6 M6 |
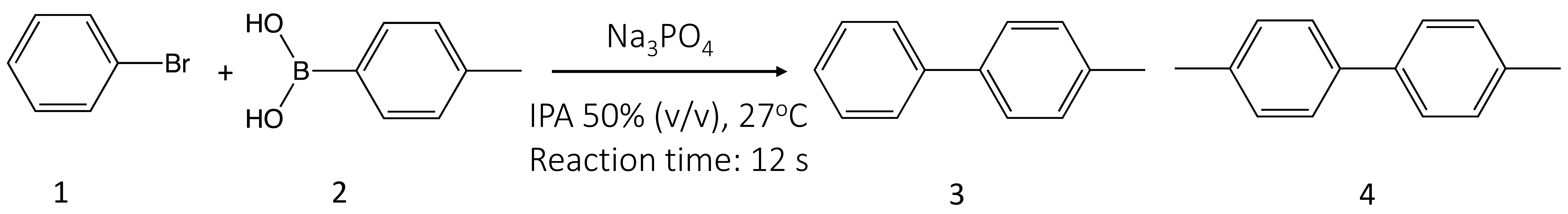
| Mol 1 | Mol 2 | Mol Na3PO4 | Yield 3 (%) | Yield 4 (%) |
|---|---|---|---|---|
| 1 | 1 | 0.1 | 7 | 1 |
| 1 | 1 | 0.5 | 50 | 5 |
| 1 | 1 | 1 | 89 | 7 |
| 1 | 1 | 1.5 | 90 | 10 |
| 1 | 1.2 | 1 | 97 | 9 |
| 1 | 1.5 | 1 | 99 | 11 |
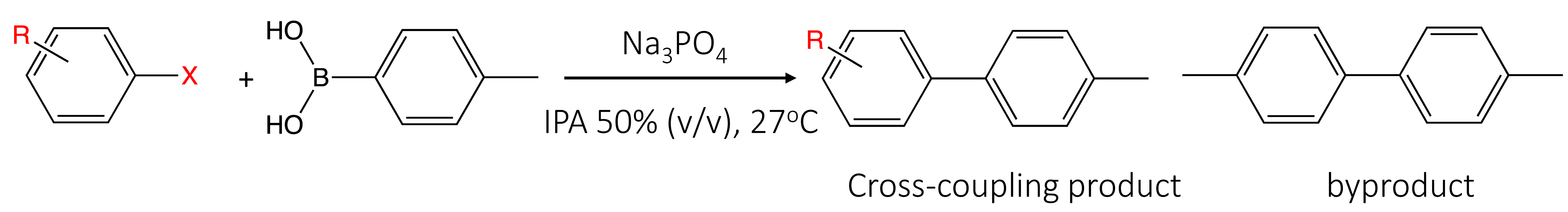
| Entry | Aryl–X | Cross-Coupling Product | Yield (%), [] Indicates the Yield of Byproduct | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 s *a | 60 s *a | 300 s *a | 600 s *a | |||||||
| 1 |  | 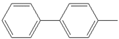 3 | 93 | [4] | - | - | - | |||
| 2 | 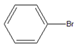 |  3 | 89 | [7] | - | - | - | |||
| 3 |  |  3 | 74 | [13] | - | - | - | |||
| 4 | 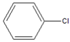 | 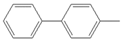 3 | 2 | [16] | - | - | - | |||
| 5 | 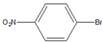 | 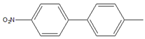 5 | 93 | [4] | 99 | - | - | |||
| 6 | 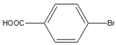 |  6 | 88 | [6] | 99 | - | - | |||
| 7 |  |  7 | 46 | [8] | 50 | [9] | 62 | [14] | 69 | [15] |
| 8 | 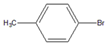 |  8 | 95 | 99 | - | - | ||||
| 9 | 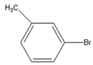 | 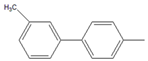 9 | 84 | [6] | 99 | - | - | |||
| 10 | 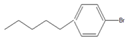 | 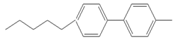 10 | 60 | [7] | 63 | [10] | 74 | [13] | 99 | |
| 11 | 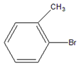 |  11 | 47 | [4] | 55 | [5] | 69 | [7] | 75 | [9] |
| 12 |  | 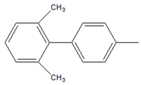 12 | 12 | [3] | 21 | [4] | 39 | [8] | 52 | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabarudin, A.; Shu, S.; Yamamoto, K.; Umemura, T. Preparation of Metal-Immobilized Methacrylate-Based Monolithic Columns for Flow-Through Cross-Coupling Reactions. Molecules 2021, 26, 7346. https://doi.org/10.3390/molecules26237346
Sabarudin A, Shu S, Yamamoto K, Umemura T. Preparation of Metal-Immobilized Methacrylate-Based Monolithic Columns for Flow-Through Cross-Coupling Reactions. Molecules. 2021; 26(23):7346. https://doi.org/10.3390/molecules26237346
Chicago/Turabian StyleSabarudin, Akhmad, Shin Shu, Kazuhiro Yamamoto, and Tomonari Umemura. 2021. "Preparation of Metal-Immobilized Methacrylate-Based Monolithic Columns for Flow-Through Cross-Coupling Reactions" Molecules 26, no. 23: 7346. https://doi.org/10.3390/molecules26237346
APA StyleSabarudin, A., Shu, S., Yamamoto, K., & Umemura, T. (2021). Preparation of Metal-Immobilized Methacrylate-Based Monolithic Columns for Flow-Through Cross-Coupling Reactions. Molecules, 26(23), 7346. https://doi.org/10.3390/molecules26237346