Abstract
A simple and efficient method was developed for the one-pot synthesis of 3-aryl derivatives of ortho-carborane with sensitive functional groups using 3-iodo-ortho-carborane and aryl zinc bromides that were generated in situ. A series of 3-aryl-ortho-carboranes, including those containing nitrile and ester groups, 3-RC6H4-1,2-C2B10H11 (R = p-Me, p-NMe2, p-OCH2OMe, p-OMe, o-CN, p-CN, o-COOEt, m-COOEt, p-COOEt) was synthesized using this approach. The solid-state structures of 3-RC6H4-1,2-C2B10H11 (R = p-OMe, o-CN, and p-CN) were determined by single crystal X-ray diffraction. The intramolecular hydrogen bonding involving the ortho-substituents of the aryl ring and the CH and BH groups of carborane was discussed.
1. Introduction
Aryl derivatives of icosahedral carboranes C2B10H12 are of interest for a variety of applications, from the development of new materials [1,2,3,4,5,6,7,8,9,10,11,12] to the design of pharmaceuticals [13,14,15,16,17]. This dictates the need to develop convenient methods for their synthesis. Methods for the synthesis of C-arylcarboranes have been well developed and are widely used in the synthesis of a wide variety of aryl derivatives [18]. The general method includes the reaction of decaborane nido-B10H14 with Lewis bases L (L = SR2, NR3, MeCN), resulting in the 6,9-arachno-B10H12L2 derivatives. These derivatives react with arylacetylenes to form the corresponding C-aryl-ortho-carboranes [19]. However, this reaction gives very low yields when used with some sterically hindered alkynes, especially those containing two aromatic moieties [10,20,21,22,23,24,25], and it cannot be used with arylacetylenes that have acidic or easily reducible substituents. This method is also unsuitable for use in the synthesis of the aryl derivatives of meta- and para-carboranes. Therefore, for the synthesis of aryl derivatives of meta- and para-carboranes, the Ullmann-type copper-coupling reactions are used. In this way, C-mono and C,C’-diaryl derivatives of meta- and para-carboranes can be obtained, as well as C-aryl derivatives of ortho-carborane [20,26,27,28,29,30]. An alternative method is based on the Ni-catalyzed cross-coupling reactions of aryl iodides with carboranyl Grignard reagents. In this way, both monoaryl and diaryl derivatives of ortho-carborane can be prepared [31,32,33].
The synthesis of the B-aryl derivatives of carboranes is mainly based on the Pd-catalyzed cross-coupling reactions of their iodo derivatives with aryl Grignard reagents (Kumada cross-coupling). In this way, various 9-aryl and 9,12-diaryl derivatives of ortho-carborane, 9-aryl and 9,10-diaryl derivatives of meta-carborane, and 2-aryl derivatives of para-carborane were synthesized [34,35,36,37,38,39,40,41,42]. However, the available substituents on the aromatic ring in these reactions are strictly limited due to the high reactivity of the Grignard reagents. Mild B-arylation of carboranes via the Suzuki cross-coupling reactions of aryl boronic acids with 9-iodo-meta- and 2-iodo-para-carboranes were reported [43,44]. These cross-coupling reactions can be used for the direct introduction of functionalized aryl substituents that are not compatible with the Kumada reaction conditions. However, this approach turned out to be ineffective for ortho-carborane because in order to facilitate the transmetallation step in the Suzuki cross-coupling reactions, inorganic bases such as F− or OH− are usually used, which are strong nucleophiles that can lead to the deboronation of the ortho-carborane cage.
4,5-Diphenyl- and 3,6-diphenyl-ortho-carboranes were obtained from the Pd- and Rh-catalyzed B-H activation reactions that started from ortho-carborane derivatives containing removable carboxylic- [45] and imine-directing [46] functional groups, respectively. 3,6-Diphenyl-ortho-carborane was prepared as well by the Ir-catalyzed borylation of ortho-carborane via direct B-H activation followed by the Pd-catalyzed Suzuki cross-coupling of the resulting 3,6-(Bpin)2-ortho-carborane with phenyl bromide [47]. 3-Phenyl-ortho-carborane and some its C-substituted analogues can be obtained via the insertion of a BPh fragment into the nido-carborane cage by the reaction with PhBCl2 under basic conditions [48,49,50]. However, this approach cannot be applied to the synthesis of a wide range of 3-aryl derivatives due to the practical unavailability of many ArBCl2 reagents, as well as because of their high reactivity, which excludes the use of aryls with sensitive functional groups. 3-Phenyl- and 3-(9-anthracenyl)-ortho-carboranes were prepared by the Pd-catalyzed cross-coupling of 3-iodo-ortho-carborane with the corresponding aryl Grignard reagents [51]. However, the range of available substituents on the aromatic ring in these reactions is also very limited due to the high reactivity of the Grignard reagents. Moreover, it was found that reactions 3-iodo-ortho-carborane with organometallic reagents, which are “hard” nucleophiles, in the presence of a catalytic amount of [Pd(PPh3)4] can lead to the loss of a halogen with the formation of 1,3-dehydro-ortho-carboryne [52], whereas the reaction with an equimolar amount of [Pd(PPh3)4] in the presence of K2CO3 in DMF proceeds with the decapitation of the carborane cage resulting in nido-carborane [7,8-C2B9H12]− as the final product [53]. The preparation of 3-phenyl-ortho-carborane by the reaction of the diazonium derivative of ortho-carborane [3-N2-1,2-C2B10H11]BF4 with the Grignard reagent was reported [54]; however, in our hands these reactions led exclusively to the 3-arylazo derivatives of ortho-carborane [55]. The attempt to use the Suzuki cross-coupling reaction of 3-iodo-ortho-carborane with aryl boronic acids only gave good results for aryls containing electron-donating substituents, while reactions with aryl boronic acids containing electron-withdrawing substituents (-CN, -NO2) only led to the desired carboranes in low yields [56]. Recently, the direct arylation of ortho-carborane via Pd-catalyzed B-H activation has been reported, but this approach has been optimized for the synthesis of the 3,6-diaryl derivatives rather than the 3-aryl derivatives [57]. A series of 3-aryl derivatives of ortho-carborane were prepared by Pd-catalyzed B-H activation reactions with aryl iodides under functional group assistance; however, these reactions are currently only of academic interest rather than real synthetic methods [58,59]. Recently, we proposed a convenient and mild one-pot method for the synthesis of 9-aryl- and 9,12-diaryl-ortho-carboranes with sensitive functional groups, including esters and nitriles, using sequential Co- and Pd-catalyzed reactions [60].
In this contribution, we describe the application of this method for the synthesis of a series of 3-aryl-ortho-carboranes, including those containing sensitive functional groups.
2. Results and Discussion
The method proposed is based on the mild generation of aryl zinc reagents followed by their Pd-catalyzed cross-coupling with 3-iodo-ortho-carborane. The aryl zinc reagents were prepared via the Co-catalyzed reaction of aryl bromides containing various functional groups with zinc dust [61,62,63]. The organozinc compounds that are obtained in this way can be easily coupled with various aryl iodides in the presence of a catalytic amount of (Ph3P)2PdCl2 [63]. Previously, we had successfully used this approach for the synthesis of 9-aryl-ortho-carboranes containing functional groups that were sensitive to organolithium and organomagnesium reagents [60].
Aryl zinc bromides containing various substituents, including sensitive functional groups (-CN, -COOEt), were prepared by the reaction of the corresponding aryl bromides with allyl zinc chloride/bromide that was generated from allyl chloride and zinc metal in the presence of 25 mol.% of CoBr2 and a catalytic amount of trifluoroacetic acid in acetonitrile at ambient temperature (Scheme 1).
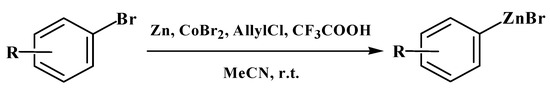
Scheme 1.
In situ synthesis of aryl zinc bromides.
The reactions of the prepared aryl zinc bromides with 3-iodo-ortho-carborane in the presence of 2 mol.% of [(Ph3P)2PdCl2] in acetonitrile at room temperature results in the corresponding 3-aryl-ortho-carboranes (Scheme 2).
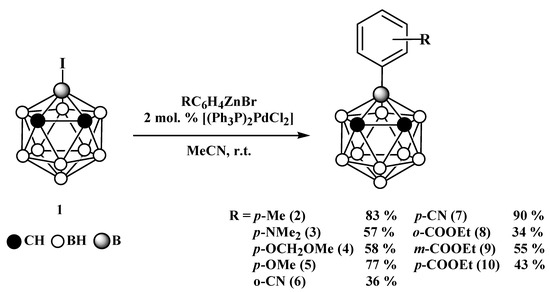
Scheme 2.
Synthesis of 3-aryl-ortho-carboranes.
The synthesized 3-aryl-ortho-carboranes were characterized by 1H, 13C and 11B NMR spectroscopy. The 1H and 13C NMR spectra of all compounds contain signals of the corresponding aryl substituents as well as the signals of the carborane cage. It should be noted that the signals of the aromatic hydrogens in the 1H NMR spectra of 3-aryl-ortho-carboranes in CDCl3 are noticeably shifted to the downfield region in comparison with the signals of the corresponding 9-aryl-ortho-carboranes [60], with a difference in the weighted average chemical shift of aromatic hydrogens in the range of 0.11 to 0.34 ppm. This is in agreement with the transition from the markedly electron-donating ortho-carboran-9-yl group to the slightly electron-accepting ortho-carboran-3-yl group [64]. In general, the chemical shift of the carborane CH groups depends slightly on the presence of electron-donating (3.65–3.69 ppm) or electron-accepting (3.74–3.77 ppm) substituents in the aromatic ring. However, most notable is the strong downfield shift of the CH carborane signals in the case of ortho-substituted aryl groups (4.38 and 4.37 ppm for 3-(2′-EtOOCC6H4)-1,2-C2B10H11 (8) and 3-(2′-NCC6H4)-1,2-C2B10H11 (6), respectively). In the first case, it can be explained by the formation of a hydrogen bond between the carborane CH group and the ester carbonyl group. It is known that the chemical shifts of the CH groups of carboranes and metallacarboranes are very sensitive to the formation of intramolecular hydrogen bonds [65,66]. On the other hand, the signals of the carbonyl group in the 13C NMR spectra are also sensitive to the formation of hydrogen bonds [67,68]. Indeed, the signal of the carbonyl group in 3-(2′-EtOOCC6H4)-1,2-C2B10H11 (8) (170.0 ppm) demonstrates a significant downfield shift compared to those found in 3-(3′-EtOOCC6H4)-1,2-C2B10H11 (9) (166.5 ppm) and 3-(4′-EtOOCC6H4)-1,2-C2B10H11 (10) (166.4 ppm), as well as in 9-(2′-EtOOCC6H4)-1,2-C2B10H11 (161.5 ppm) [60], which is a clear confirmation of the formation of an intramolecular hydrogen bond. It is also worth noting that the mass spectrum of 8 in the negative mode, in contrast to the mass spectra of other 3-aryl-ortho-carboranes, exhibits a peak which, in addition to the loss of the carborane proton, corresponds to the abstraction of the ethanol molecule. This is caused by the attack of the carbonyl group by the nucleophile, which is formed upon the loss of the carborane CH proton, leading to intramolecular cyclization with the elimination of ethanol and the formation of carboranyl fluorenone 1,3-µ-C(O)C6H4-1,2-C2B10H10. In the case of 3-(2′-NCC6H4)-1,2-C2B10H11, the nature of the interactions involving the carborane CH group is less clear.
This prompted us to perform an X-ray diffraction study of the synthesized 3-aryl-ortho-carboranes. The solid-state structures of carboranes 3, 5–7, 9 and 10 were determined by single crystal X-ray diffraction (Figure 1). The most characteristic feature of the structure of 3-aryl-ortho-carboranes is the deviation of the exo-polyhedral B-C bond from the B(3)-B(10) axis of the carborane cage towards the carborane C(1)-C(2) bond (See Table 1).
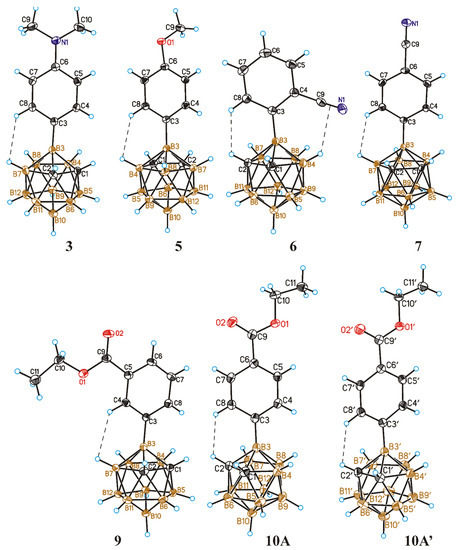
Figure 1.
General views of 3-(p-Me2NC6H4)-1,2-C2B10H11 (3), 3-(p-MeOC6H4)-1,2-C2B10H11 (5), 3-(o-NCC6H4)-1,2-C2B10H11 (6), 3-(p-NCC6H4)-1,2-C2B10H11 (7), 3-(m-EtOOCC6H4)-1,2-C2B10H11 (9), and 3-(p-EtOOCC6H4)-1,2-C2B10H11 (10) (both symmetrically independent molecules are presented) showing atomic numbering. Thermal ellipsoids are drawn at 50% probability level. For each structure, the shortest H⋯H and H⋯π contacts are shown by dashed lines. The H⋯H distances are equal to 2.43, 2.43, 2.18, 2.44, 2.47, 2.27, and 2.37 Å for compounds 3, 5, 6, 7, 9, 10A, and 10A′, respectively. The distance of the H⋯π contact for 6 (from H atom δ to the center of the C≡N bond) is equal to 2.80(2) Å.

Table 1.
Some selected angles in 3-aryl-ortho-carboranes 3, 5–7, 9 and 10.
This can be related to the fact that the B(3)-C bonds are somewhat shorter than the B(3)-B bonds, as well as to the participation of the aryl substituents in intermolecular interactions. The orientation of the aryl ring with respect to the carborane cage in the obtained derivatives is different. For compounds 3, 5, 7, and 9, the projection line of the phenyl ring onto the C2B3 plane passes through the B(7) or B(4) atom and the center of the opposite B-C bond, while for compounds 6 and 10, it passes through the C(2) atom and the center of the B(4)-B(8) bond. As shown in Figure 1, a Cphen-H⋯H-B(C) contact is observed in all of the compounds. In some cases, it is slightly longer than the sum of the van-der-Waals radii (2.4 Å [69]), while it is somewhat shorter in the other cases. The shortest distance (2.18 Å) is observed for compound 6 with an ortho-cyanophenyl substituent. In structures 5 and 7, there are no short contacts between the aryl ring and the carborane cage, while in structure 6 the aryl ring is rotated in such a way that leads to the formation of a short (2.174 Å) C(2)H⋯HC(8) contact between the aryl and carborane hydrogens. It should be noted that the presence of such contacts was previously found in the structure of 1-phenyl-ortho-carborane [70]. In addition, there is a slightly shortened contact of the B-H⋯π(N≡C-) type between the B(4)-H group of carborane and the cyano group of the aryl substituent. It should be mentioned that the existence of the intramolecular B-H⋯π(N≡C-) hydrogen bonds in the nido-carborane derivative 10-N≡CCH2(Me)S-7,8-C2B9H11 was postulated earlier based on the NMR spectroscopy data [71].
In order to understand in more detail the observed differences in molecular conformation, we carried out a comparative quantum chemical study for compounds 7 and 6, which have para- and ortho-cyanophenyl substituents and significantly differ in their molecular conformation and intramolecular contacts. The calculation was done using the GAUSSIAN program [72] at the PBE0/def2tzvp level of theory that was shown to provide realistic geometrical and energetic properties for different types of compounds [73,74,75]. In order to search for preferential molecular conformation, the aryl substituent was rotated about the B(3)-C(3) bond with a step of 5°. The results are shown in Figure 2.
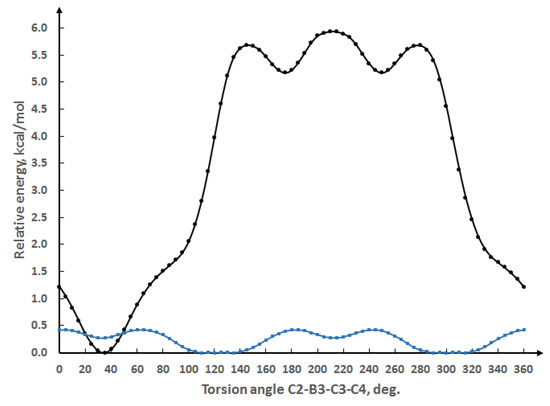
Figure 2.
Dependence of conformational energy on C(2)-B(3)-C(3)-C(4) torsion angle for compounds 6 (black curve) and 7 (blue curve).
As expected, for the para-cyanophenyl derivative (7), the barrier to rotation is small and the conformational curve is symmetrical with respect to the plane passing through the B(3) and B(8) atoms and the center of the C(1)-C(2) bond. The experimental structure corresponds to the global minimum. In the case of the ortho-cyanophenyl derivative (6), there are two equivalent local minima and a global minimum, which probably corresponds to structure 6 in solution, and which is ~5 kcal/mol lower in energy. Using the geometries of the local and global minima as starting points, we carried out an additional optimization without any restrictions. Both optimizations converged to true minima, global and local, respectively. The QTAIM theory [76] was utilized to analyze the intramolecular noncovalent interactions. A search for the bond critical points (using the AIMALL program [77]) revealed the presence of two intramolecular attractive interactions between the carborane cage and the aryl substituent for both local and global minima (Figure 3). The energies of the observed noncovalent interactions were estimated using the empirical correlation between interaction energy and potential energy density at the bond critical point (E = 1/2V(r)) [78], which is frequently utilized for energetic analysis [73,79,80].
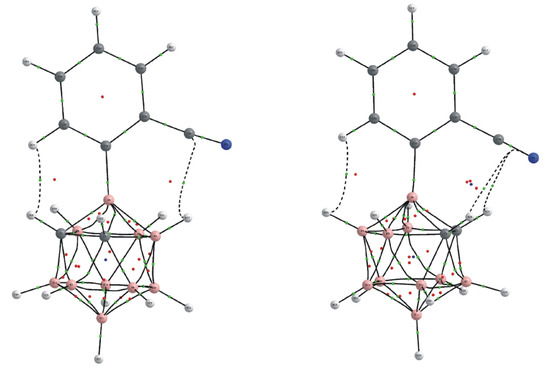
Figure 3.
View of the molecular conformations corresponding to local (left) and global (right) minima for compound 6.
The stabilization of the global minimum was provided by the B-H⋯H-Cphen (−1.6 kcal/mol) and Ccarb-H⋯π (−3.8 kcal/mol) nonbonded interactions, while Ccarb-H⋯H-Cphen (−2.4 kcal/mol) and B-H⋯π (−1.3 kcal/mol) contacts were observed for the local minimum which, evidently, was weaker. At the same time, the experimentally observed solid-state structure of compound 6 corresponds to the local minima which should be caused by the crystal-packing influence. Indeed, in the crystal structure of 6, a relatively strong intermolecular hydrogen bond C(2)-H(2)⋯N(1) is formed (Figure S1 in the Supplementary Materials). Such an interaction cannot exist for a molecular conformation corresponding to the global minima due to steric reasons.
Based on these results we can suggest that experimental molecular conformation of compound 10, for which intramolecular Cphen-H⋯H-Ccarb shortened contacts are observed, is also influenced by the crystal-packing effect (Figure S2 in the Supplementary Materials).
In summary, the efficient method for the one-pot synthesis of 3-aryl-ortho-carboranes with sensitive functional groups using sequential Co- and Pd-catalyzed reaction was proposed. A series of functional aryl derivatives, including esters and nitriles, were synthesized and characterized by methods of the NMR spectroscopy and single crystal X-ray diffraction.
3. Experimental Part
General Synthetic Procedure and Characterization of 3-Aryl-ortho-carboranes
Allyl chloride (82 μL, 77 mg, 1.00 mmol) and trifluoroacetic acid (25 μL, catalytic amount) were added to a blue mixture of zinc powder (490 mg, 7.50 mmol) and anhydrous cobalt dibromide (55 mg, 0.25 mmol) in 2.5 mL of fresh distilled acetonitrile. The resulting dark orange mixture was stirred at room temperature for 15 min. Then corresponding aryl bromide (2.50 mmol) was added, and reaction was stirred at room temperature for an additional 1 h. Then, 3-iodo-ortho-carborane (1) (270 mg, 1.00 mmol) with bis(triphenylphosphine)palladium dichloride (14 mg, 0.02 mmol) were added. The reaction was stirred at room temperature overnight. After the removal of volatiles under reduced pressure, the residue was washed with water (25 mL), dichloromethane (3 × 25 mL) and acetone (until no trace of carborane appeared on TLC). The organic phases were combined, dried over Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography on silica to give the corresponding 3-aryl-ortho-carborane.
3-(4′-Methylphenyl)-ortho-carborane (2): 4-methylphenyl bromide (315 μL, 435 mg, 2.50 mmol) was used; diethyl ether was used as the eluent for column chromatography; a pale-yellow crystalline solid was obtained (195 mg, yield 83%). 1H NMR (400 MHz, CDCl3): δ 7.49 (2H, d, J = 7.7 Hz, CHAr), 7.19 (2H, d, J = 7.7 Hz, CHAr), 3.69 (2H, br s, CHCarb), 2.37 (3H, s, CH3) ppm; 11B NMR (128 MHz, CDCl3): δ −2.4 (2B, d, J = 148 Hz), −5.0 (1B, s, B-C), −8.5 (1B, d, J = 145 Hz), −12.9 (3B, d, J = 162 Hz), −13.7 (3B, d, J = 174 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 139.7 (CAr-CH3), 133.2 (CArH), 129.2 (CArH), 56.8 (CCarbH), 21.5 (CH3) ppm. MS (DUIS): m/z for C9H18B10: calcd. 233.2 [M−H]−, obsd. 233.2 [M−H]−.
3-(4′-N,N-Dimethylaminophenyl)-ortho-carborane (3): 4-N,N-dimethylaminophenyl bromide (500 mg, 2.50 mmol) was used; a mixture of chloroform and hexane (3:1, v/v) was used as the eluent for column chromatography; a pale-pink crystalline solid was obtained (150 mg, yield 57%). 1H NMR (400 MHz, CDCl3): δ 7.46 (2H, d, J = 8.5 Hz, CHAr), 6.72 (2H, d, J = 8.5 Hz, CHAr), 3.65 (2H, br s, CHCarb), 3.00 (6H, s, N(CH3)2) ppm; 11B NMR (128 MHz, CDCl3): δ −2.7 (2B, d, J = 149 Hz), −4.0 (1B, s, B-C), −8.6 (1B, d, J = 150 Hz), −13.1 (3B, d, J = 152 Hz,), −13.7 (3B, d, J = 164 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 151.5 (CAr-N), 134.3 (CArH), 117.4 (CAr-B), 112.0 (CArH), 56.8 (CCarbH), 40.3 (N(CH3)2) ppm. MS (DUIS): m/z for C10H21B10N: calcd. 262.3 [M−H]−, obsd. 262.3 [M−H]−; calcd. 305.3 [M+H+MeCN]+, obsd. 305.3 [M+H+MeCN]+.
3-(4′-Methoxymethoxyphenyl)-ortho-carborane (4): 4-methoxymethoxy bromide (381 μL, 543 mg, 2.50 mmol) was used; a mixture of chloroform and hexane (3:1, v/v) and a mixture of diethyl ether and hexane (1:2, v/v) were used as the eluent for column chromatography; a pale-yellow solid was obtained (164 mg, yield 58%). 1H NMR (400 MHz, CDCl3): δ 7.52 (2H, d, J = 8.5 Hz, CHAr), 7.04 (2H, d, J = 8.5 Hz, CHAr), 5.20 (2H, s, OCH2O), 3.67 (2H, br s, CHCarb), 3.48 (3H, s, OCH3) ppm; 11B NMR (128 MHz, CDCl3): δ −2.5 (2B, d, J = 149 Hz), −4.8 (1B, s, B-C), −8.5 (1B, d, J = 150 Hz), −13.0 (3B, d, J = 156 Hz), −13.6 (3B, d, J = 159 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 158.7 (CAr-O), 134.6 (CArH), 122.7 (CAr-B), 116.2 (CArH), 94.2 (OCH2O), 56.8 (CCarbH), 56.2 (OCH3) ppm. MS (DUIS): m/z for C10H20B10O2: calcd. 279.2 [M−H]−, obsd. 279.3 [M−H]−. Crystallographic data (CCDC number 2124596): C10H21B10N are monoclinic, space group P21/c: a = 12.9798(5) Å, b = 9.8799(4) Å, c = 12.5908(5) Å, β = 109.130(2)°, V = 1525.47(11) Å3, Z = 4, M = 263.38, dcryst = 1.147 g·cm−3. wR2 = 0.1159 calculated on F2hkl for all 3666 independent reflections with 2θ < 56.0°, (GOF = 1.074, R = 0.0417 calculated on Fhkl for 2986 reflections with I > 2σ(I)).
3-(4′-Methoxyphenyl)-ortho-carborane (5): 4-methoxyphenyl bromide (312 μL, 468 mg, 2.50 mmol) was used; a mixture of diethyl ether and hexane (1:2, v/v) was used as the eluent for column chromatography; a yellow solid was obtained (193 mg, yield 77%). 1H NMR (400 MHz, CDCl3): δ 7.52 (2H, d, J = 8.6 Hz, CHAr), 6.90 (2H, d, J = 8.6 Hz, CHAr), 3.83 (3H, s, OCH3), 3.67 (2H, br s, CHCarb) ppm; 11B NMR (128 MHz, CDCl3): δ −2.5 (2B, d, J = 149 Hz), −4.7 (1B, s, B-C), −8.5 (1B, d, J = 149 Hz), −13.0 (3B, d, J = 156 Hz), −13.6 (3B, d, J = 159 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 161.1 (CAr-O), 134.7 (CArH), 122.3 (CAr-B), 114.0 (CArH), 56.8 (CCarbH), 56.1 (OCH3), 55.4 (CCarbH) ppm. MS (DUIS): m/z for C9H18B10O: calcd. 249.2 [M−H]−, obsd. 249.3 [M−H]−. Crystallographic data (CCDC number 2118711): C9H18B10O are orthorhombic, space group P212121: a = 9.5602(2) Å, b = 11.2642(3) Å, c = 12.9598(3) Å, V = 1395.62(6) Å3, Z = 4, M = 250.33, dcryst = 1.191 g⋅cm−3. wR2 = 0.0857 calculated on F2hkl for all 3377 independent reflections with 2θ < 56.0°, (GOF = 1.048, R = 0.0308 calculated on Fhkl for 3200 reflections with I > 2σ(I)).
3-(2′-Cyanophenyl)-ortho-carborane (6): 2-cyanophenyl bromide (455 mg, 2.50 mmol) was used; a mixture of chloroform and hexane (1:1, v/v) was used as the eluent for column chromatography; a pale-yellow crystalline solid was obtained (88 mg, yield 36%). 1H NMR (400 MHz, CDCl3): δ 8.14 (1H, d, J = 7.6 Hz, CHAr), 7.69 (2H, m, CHAr), 7.54 (1H, dd, J1 = 7.6 Hz, J2 = 7.5 Hz, CHAr), 4.37 (2H, br s, CHCarb) ppm; 11B NMR (128 MHz, CDCl3): δ −2.4 (2B, d, J = 149 Hz), −7.0 (1B, s, B-C), −8.6 (1B, d, J = 153 Hz), −10.7 (1B, d, J = 152 Hz), −12.1 (2B, d, J = 161 Hz), −13.0 (2B, d, J = 150 Hz), −13.9 (1B, d, J = 176 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 138.3 (CArH), 133.8 (CArH), 132.8 (CArH), 130.0 (CArH), 119.7 (CN), 113.5 (CAr-CN), 55.6 (CCarbH) ppm. MS (DUIS): m/z for C9H15B10N: calcd. 244.2 [M−H]−, obsd. 244.3 [M−H]−. Crystallographic data (CCDC number 2118710): C9H15B10N are monoclinic, space group P21/c: a = 6.7263(3) Å, b = 19.8472(8) Å, c = 10.5592(4) Å, β = 105.7989(17)°, V = 1356.38(10) Å3, Z = 4, M = 245.32, dcryst = 1.201 g⋅cm−3. wR2 = 0.1139 calculated on F2hkl for all 3247 independent reflections with 2θ < 55.9°, (GOF = 1.045, R = 0.0406 calculated on Fhkl for 2535 reflections with I > 2σ(I)).
3-(4′-Cyanophenyl)-ortho-carborane (7): 4-cyanophenyl bromide (455 mg, 2.50 mmol) was used; a mixture of chloroform and hexane (3:1, v/v) was used as the eluent for column chromatography; a pale-yellow crystalline solid was obtained (221 mg, yield 90%). 1H NMR (400 MHz, CDCl3): δ 7.69 (2H, m, CHAr), 7.63 (2H, m, CHAr), 3.74 (2H, br s, CHCarb) ppm; 11B NMR (128 MHz, CDCl3): δ −2.0 (2B, d, J = 149 Hz), −6.3 (1B, s, B-C), −8.5 (1B, d, J = 151 Hz), −11.4 (1B, d, J = 148 Hz), −13.0 (5B, d, J = 164 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 133.8 (CArH), 131.7 (CArH), 118.5 (CN), 113.6 (CAr-CN), 56.6 (CCarbH) ppm. MS (DUIS): m/z for C9H15B10N: calcd. 244.2 [M−H]−, obsd. 244.3 [M−H]−. Crystallographic data (CCDC number 2118709): C9H15B10N are monoclinic, space group P21/n: a = 7.1000(10) Å, b = 17.782(2) Å, c = 10.7239(14) Å, β = 93.146(5)°, V = 1351.9(3) Å3, Z = 4, M = 245.32, dcryst = 1.205 g⋅cm−3. wR2 = 0.2020 calculated on F2hkl for all 2949 independent reflections with 2θθ < 54.2°, (GOF = 1.323, R = 0.0792 calculated on Fhkl for 2335 reflections with I > 2σ(I)).
3-(2′-Ethoxycarbonylphenyl)-ortho-carborane (8): 2-ethoxycarbonyl bromide (398 μL, 573 mg, 2.50 mmol) was used; a mixture of chloroform and hexane (2:1, v/v) was used as the eluent for column chromatography; a colorless oil was obtained (100 mg, yield 34%). 1H NMR (400 MHz, CDCl3): δ 8.24 (1H, d, J = 7.5 Hz, CHAr), 7.81 (1H, dd, J3 = 7.8 Hz, J4 = 1.3 Hz, CHAr), 7.55 (1H, ddd, J31 = 7.5 Hz, J32 = 7.5 Hz, J4 = 1.3 Hz, CHAr), 7.46 (1H, ddd, J31 = 7.8 Hz, J32 = 7.5 Hz, J4 = 1.3 Hz, CHAr), 4.38 (2H, br s, CHCarb), 4.33 (2H, q, J = 7.1 Hz, OCH2CH3), 1.40 (3H, t, J = 7.1 Hz, OCH2CH3) ppm; 11B NMR (128 MHz, CDCl3): δ −3.3 (2B, d, J = 147 Hz), −6.1 1B, (s, B-C), −8.2 (1B, d, J = 148 Hz), −11.2 (1B, d, J = 134 Hz), −11.9 (2B, d, J = 163 Hz), −13.9 (3B, d, J = 169 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 170.0 (CO), 139.8 (CArH), 134.5 (CAr-CO), 131.3 (CArH), 130.3 (CArH), 129.2 (CArH), 62.0 (OCH2CH3), 57.0 (CCarbH), 14.3 (OCH2CH3) ppm. MS (DUIS): m/z for C11H20B10O2: calcd. 245.2 [M−H−EtOH]−, obsd. 245.3 [M−H−EtOH]−.
3-(3′-Ethoxycarbonylphenyl)-ortho-carborane (9): 3-ethoxycarbonyl bromide (401 μL, 573 mg, 2.50 mmol) was used; a mixture of chloroform and hexane (2:1, v/v) was used as the eluent for column chromatography; a pale-yellow crystalline solid was obtained (161 mg, yield 55%). 1H NMR (400 MHz, CDCl3): δ 8.17 (1H, s, CHAr), 8.08 (1H, d, J = 7.8 Hz, CHAr), 7.85 (1H, d, J = 7.6 Hz, CHAr), 7.46 (1H, dd, J1 = 7.8 Hz, J2 = 7.6 Hz, CHAr), 4.39 (2H, q, J = 7.1 Hz, OCH2CH3), 3.77 (2H, br s, CHCarb), 1.40 (3H, t, J = 7.1 Hz, OCH2CH3) ppm; 11B NMR (128 MHz, CDCl3): δ −2.2 (2B, d, J = 149 Hz), −5.5 (1B, s, B-C), −8.6 (1B, d, J = 150 Hz), −12.0 (1B, d, J = 134 Hz), −12.9 (2B, d, J = 154 Hz), −13.4 (3B, d, J = 168 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 166.5 (CO), 138.1 (CArH), 133.5 (CArH), 130.9 (CArH), 130.5 (CAr-CO), 128.6 (CArH), 61.4 (OCH2CH3), 56.8 (CCarbH), 14.5 (OCH2CH3) ppm. MS (DUIS): m/z for C11H20B10O2: calcd. 291.2 [M−H]−, obsd. 291.3 [M−H]−. Crystallographic data (CCDC number 2124597): C11H20B10O2 are monoclinic, space group P21/n: a = 7.0480(7) Å, b = 10.3342(10) Å, c = 21.972(2) Å, β = 90.515(5)°, V = 1600.3(3) Å3, Z = 4, M = 292.37, dcryst = 1.214 g·cm−3. wR2 = 0.1601 calculated on F2hkl for all 3144 independent reflections with 2θ < 52.0°, (GOF = 1.149, R = 0.0512 calculated on Fhkl for 2555 reflections with I > 2σ(I)).
3-(4′-Ethoxycarbonylphenyl)-ortho-carborane (10): 4-ethoxycarbonyl bromide (408 μL, 573 mg, 2.50 mmol) was used; a mixture of chloroform and hexane (1:5, v/v) and diethyl ether were used as the eluent for column chromatography; a pale-yellow crystalline solid was obtained (126 mg, yield 43%). 1H NMR (400 MHz, CDCl3): δ 8.01 (2H, d, J = 8.0 Hz, CHAr), 7.67 (2H, d, J = 8.0 Hz, CHAr), 4.39 (2H, q, J = 7.1 Hz, OCH2CH3), 3.75 (2H, br s, CHCarb), 1.40 (3H, t, J = 7.1 Hz, OCH2CH3) ppm; 11B NMR (128 MHz, CDCl3): δ −2.2 (2B, d, J = 150 Hz), −5.7 (1B, s, B-C), −8.5 (1B, d, J = 149 Hz), −11.8 (1B, d, J = 146 Hz), −12.9 (5B, d, J = 157 Hz) ppm; 13C NMR (100 MHz, CDCl3): δ 166.4 (CO), 149.9 (CAr-B), 133.2 (CArH), 132.9 (CAr-CO), 129.2 (CArH), 61.3 (OCH2CH3), 56.7 (CCarbH), 14.5 (OCH2CH3) ppm. MS (DUIS): m/z for C11H20B10O2: calcd. 291.2 [M−H]−, obsd. 291.3 [M−H]−. Crystallographic data (CCDC number 2124598): C11H20B10O2 are monoclinic, space group P21/n: a = 16.8494(13) Å, b = 7.0272(6) Å, c = 28.549(2) Å, β = 105.387(2)°, V = 3259.2(5) Å3, Z = 8, M = 292.37, dcryst = 1.192 g·cm−3. wR2 = 0.1266 calculated on F2hkl for all 7113 independent reflections with 2θ < 54.2°, (GOF = 1.006, R = 0.0503 calculated on Fhkl for 4786 reflections with I > 2σ(I)).
Supplementary Materials
The following are available online. Figure S1: H-bonded chain in the crystal structure of compound 6. Figure S2: Fragment of the crystal packing of compound 10. Reagents and equipment used for the synthesis and characterization of 3-aryl-ortho-carboranes, NMR and mass-spectra of compounds 2–10. References [54,81,82,83,84,85] are cited in the supplementary materials.
Author Contributions
Experiment design, synthesis, NMR spectroscopy and mass-spectrometry studies, S.A.A.; synthesis, A.V.S.; single crystal X-ray diffraction experiments and quantum-chemical calculations, K.Y.S.; supervision and manuscript concept, I.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant No. 21-13-00345).
Data Availability Statement
The data presented in this study are available from the authors.
Acknowledgments
The NMR spectroscopic and X-ray diffraction data were obtained using the equipment of the Center for Molecular Structure Studies at the A.N. Nesmeyanov Institute of Organoelement Compounds operating with the support of the Ministry of Science and Higher Education of the Russian Federation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds 2–10 are available from the authors.
References
- Spokoyny, A.M.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T.; Mirkin, C.A. Porosity tuning of carborane-based metal–organic frameworks (MOFs) via coordination chemistry and ligand design. Inorg. Chim. Acta 2010, 364, 266–271. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.; Sarjeant, A.A.; Snurr, R.; Hupp, J.T.; Yildirim, T.; et al. Carborane-based metal–organic framework with high methane and hydrogen storage capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.-C.; Han, G.; Wang, H.-R.; Li, N.; Zhang, X.-A.; Jiang, S.-L.; Lu, Y.-F. Synthesis and characterization of carborane bisphenol resol phenolic resins with ultrahigh char yield. Chin. J. Polym. Sci. 2015, 33, 1606–1617. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, G.; Feng, C.; Yang, J. High Tg and thermo-oxidatively stable thermosetting polyimides derived from a carborane-containing diamine. Macromol. Rapid Commun. 2018, 39, e1800484. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, L.; Lou, P.; Zhang, X.; Han, X.; Zhang, Z.; Zhu, S. Study on thermal degradation mechanism of heat-resistant epoxy resin modified with carboranes. Polym. Degrad. Stab. 2020, 176, 109143. [Google Scholar] [CrossRef]
- Li, X.; Yan, H.; Zhao, Q. Carboranes as a tool to tune phosphorescence. Chem. A Eur. J. 2015, 22, 1888–1898. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2015, 52, 1070–1093. [Google Scholar] [CrossRef] [PubMed]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent progress in the development of solid-state luminescent o-carboranes with stimuli responsivity. Angew. Chem. Int. Ed. 2020, 132, 9925–9939. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Mun, M.S.; Yi, S.; Yoo, E.; Hwang, H.; Lee, K.M. Influence of electronic environment on the radiative efficiency of 9-phenyl-9H-carbazole-based ortho-carboranyl luminophores. Molecules 2021, 26, 1763. [Google Scholar] [CrossRef]
- You, D.K.; So, H.; Ryu, C.H.; Kim, M.; Lee, K.M. Strategic molecular design of closo-ortho-carboranyl luminophores to manifest thermally activated delayed fluorescence. Chem. Sci. 2021, 12, 8411–8423. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, J.; Yan, L.; Lu, C.; Yan, H. A “flexible” carborane-cored luminogen: Variable emission behaviours in aggregates. Dalton Trans. 2021, 50, 8029–8035. [Google Scholar] [CrossRef]
- Kim, M.; Im, S.; Ryu, C.H.; Lee, S.H.; Hong, J.H.; Lee, K.M. Impact of deboronation on the electronic characteristics of closo-o-carborane: Intriguing photophysical changes in triazole-appended carboranyl luminophores. Dalton Trans. 2021, 50, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Endo, Y. Utility of boron clusters for drug design. Hansch–Fujita hydrophobic parameters π of dicarba-closo-dodecaboranyl groups. Bioorganic Med. Chem. Lett. 2001, 11, 2389–2392. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y. Carboranes as hydrophobic pharmacophores: Applications for design of nuclear receptor ligands. In Boron-Based Compounds: Potential and Emerging Applications in Medicine; Hey-Hawkins, E., Viñas Teixidor, C., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2018; Volume 1, pp. 3–19. [Google Scholar] [CrossRef]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.B.; Hey-Hawkins, E. New keys for old locks: Carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016; pp. 283–502. [Google Scholar] [CrossRef]
- Heying, T.L.; Ager, J.W.; Clark, S.L.; Mangold, D.J.; Goldstein, H.L.; Hillman, M.; Polak, R.J.; Szymanski, J.W. A new series of organoboranes. I. Carboranes from the reaction of decaborane with acetylenic compounds. Inorg. Chem. 1963, 2, 1089–1092. [Google Scholar] [CrossRef]
- Ohta, K.; Goto, A.T.; Endo, Y. 1,2-Dicarba-closo-dodecaboran-1-yl naphthalene derivatives. Inorg. Chem. 2005, 44, 8569–8573. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Morisaki, Y.; Chujo, Y. o-Carborane-based anthracene: A variety of emission behaviors. Angew. Chem. Int. Ed. 2015, 54, 5084–5087. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; So, H.; Lee, J.H.; Hwang, H.; Kwon, H.; Park, M.H.; Lee, K.M. Photophysical properties of spirobifluorene-based o-carboranyl compounds altered by structurally rotating the carborane cages. Molecules 2019, 24, 4135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, K.; Tanaka, K.; Chujo, Y. Tuning of sensitivity in thermochromic luminescence by regulating molecular rotation based on triphenylamine-substituted o-carboranes. Asian J. Org. Chem. 2019, 8, 2228–2232. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.H.; So, H.; Kim, M.; Mun, M.S.; Hwang, H.; Park, M.H.; Lee, K.M. Insights into the effects of substitution position on the photophysics of mono-o-carborane-substituted pyrenes. Inorg. Chem. Front. 2020, 7, 2949–2959. [Google Scholar] [CrossRef]
- Wada, K.; Hashimoto, K.; Ochi, J.; Tanaka, K.; Chujo, Y. Rational design for thermochromic luminescence in amorphous polystyrene films with bis-o-carborane-substituted enhanced conjugated molecule having aggregation-induced luminochromism. Aggregate 2021, 2, e93. [Google Scholar] [CrossRef]
- Coult, R.; Fox, M.A.; Gill, W.R.; Herbertson, P.L.; MacBride, J.A.H.; Wade, K. C-arylation and C-heteroarylation of icosahedral carboranes via their copper(I) derivatives. J. Organomet. Chem. 1993, 462, 19–29. [Google Scholar] [CrossRef]
- Colquhoun, H.M.; Herbertson, P.L.; Wade, K.; Baxter, I.; Williams, D.J. A carborane-based analogue of poly(p-phenylene). Macromolecules 1998, 31, 1694–1696. [Google Scholar] [CrossRef]
- Fujii, S.; Yamada, A.; Nakano, E.; Takeuchi, Y.; Mori, S.; Masuno, H.; Kagechika, H. Design and synthesis of nonsteroidal progesterone receptor antagonists based on C,C′-diphenylcarborane scaffold as a hydrophobic pharmacophore. Eur. J. Med. Chem. 2014, 84, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Solid-state emission of the anthracene-o-carborane dyad from the twisted-intramolecular charge transfer in the crystalline state. Angew. Chem. 2016, 129, 260–265. [Google Scholar] [CrossRef]
- Marsh, A.V.; Dyson, M.; Cheetham, N.J.; Bidwell, M.; Little, M.; White, A.J.P.; Warriner, C.N.; Swain, A.C.; McCulloch, I.; Stavrinou, P.N.; et al. Correlating the structural and photophysical properties of ortho-, meta-, and para-carboranyl–anthracene dyads. Adv. Electron. Mater. 2020, 6, 2000312. [Google Scholar] [CrossRef]
- Tang, C.; Xie, Z. Nickel-catalyzed cross-coupling reactions of o-carboranyl with aryl iodides: Facile synthesis of 1-aryl-o-carboranes and 1,2-diaryl-o-carboranes. Angew. Chem. Int. Ed. 2015, 54, 7662–7665. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, J.; Cao, Y.; Zhao, J.; Jia, W.; Chen, Y.; Jia, D. Mechanically triggered reversible stepwise tricolor switching and thermochromism of anthracene-o-carborane dyad. Chem. Sci. 2018, 9, 5270–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Guo, J.; Lv, Y.; Jia, D.; Zhao, J.; Shan, H.; Jin, X.; Ma, Y. Aggregation-induced emission characteristics of o-carborane-functionalized fluorene and its heteroanalogs: The influence of heteroatoms on photoluminescence. Mater. Chem. Front. 2019, 4, 257–267. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Kovredov, A.I.; Ol’shevskaya, V.A.; Shaugumbekova, Z.S. Synthesis of B-organo-substituted 1,2-, 1,7-, and 1,12-dicarbaclosododecarboranes(12). J. Organomet. Chem. 1982, 226, 217–222. [Google Scholar] [CrossRef]
- Zheng, Z.; Jiang, W.; Zinn, A.A.; Knobler, C.B.; Hawthorne, M.F. Facile electrophilic iodination of icosahedral carboranes. Synthesis of carborane derivatives with boron-carbon bonds via the palladium-catalyzed reaction of diiodocarboranes with Grignard reagents. Inorg. Chem. 1995, 34, 2095–2100. [Google Scholar] [CrossRef]
- Jiang, W.; Knobler, C.B.; Curtis, C.E.; Mortimer, M.D.; Hawthorne, M.F. Iodination reactions of icosahedral para-carborane and the synthesis of carborane derivatives with boron-carbon bonds. Inorg. Chem. 1995, 34, 3491–3498. [Google Scholar] [CrossRef]
- Lee, H.; Knobler, C.B.; Hawthorne, M.F. Supramolecular self-assembly directed by carborane C–H‥F interactions. Chem. Commun. 2000, 2485–2486. [Google Scholar] [CrossRef]
- Fox, M.A.; Wade, K. Model compounds and monomers for phenylene ether carboranylene ketone (PECK) polymer synthesis: Preparation and characterization of boron-arylated ortho-carboranes bearing carboxyphenyl, phenoxyphenyl or benzoylphenyl substituents. J. Mater. Chem. 2002, 12, 1301–1306. [Google Scholar] [CrossRef]
- Bayer, M.J.; Herzog, A.; Diaz, M.; Harakas, G.A.; Lee, H.; Knobler, C.B.; Hawthorne, M.F. The synthesis of carboracycles derived from B,B′-bis(aryl) derivatives of icosahedral ortho-carborane. Chem. A Eur. J. 2003, 9, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Spokoyny, A.M.; Li, T.C.; Farha, O.K.; Machan, C.W.; She, C.; Stern, C.L.; Marks, T.J.; Hupp, J.T.; Mirkin, C.A. Electronic tuning of nickel-based bis(dicarbollide) redox shuttles in dye-sensitized solar cells. Angew. Chem. Int. Ed. 2010, 49, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of 9,9′,12,12′-substituted cobalt bis(dicarbollide) derivatives. Russ. Chem. Bull. 2015, 64, 712–717. [Google Scholar] [CrossRef]
- Anderson, K.P.; Mills, H.A.; Mao, C.; Kirlikovali, K.O.; Axtell, J.C.; Rheingold, A.L.; Spokoyny, A.M. Improved synthesis of icosahedral carboranes containing exopolyhedral B-C and C-C bonds. Tetrahedron 2018, 75, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Zakharkin, L.I.; Balagurova, E.V.; Lebedev, V.N. Suzuki cross-coupling in the carborane series. Russ. J. Gen. Chem. 1998, 68, 922–924. [Google Scholar]
- Eriksson, L.; Beletskaya, I.P.; Bregadze, V.I.; Sivaev, I.B.; Sjöberg, S. Palladium-catalyzed cross-coupling reactions of arylboronic acids and 2-I-p-carborane. J. Organomet. Chem. 2002, 657, 267–272. [Google Scholar] [CrossRef]
- Quan, Y.; Xie, Z. Palladium-catalyzed regioselective diarylation of o-Carboranes by direct cage B−H activation. Angew. Chem. Int. Ed. 2015, 55, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Ning, Q.; Zhao, W.; Cao, H.J.; Wang, Y.-P.; Yan, H.; Lu, C.-S.; Liang, Y. Rh-Catalyzed decarbonylative cross-coupling between o-carboranes and twisted amides: A regioselective, additive-free, and concise late-stage carboranylation. Chem. A Eur. J. 2020, 27, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Qiu, Z.; Xie, Z. Iridium-catalysed regioselective borylation of carboranes via direct B–H activation. Nat. Commun. 2017, 8, 14827. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Wegner, P.A. Reconstruction of the 1,2-dicarbaclovododecaborane(12) structure by boron-atom insertion with (3)-1,2-dicarbollide ions. J. Am. Chem. Soc. 1968, 90, 896–901. [Google Scholar] [CrossRef]
- Shmal’ko, A.V.; Anufriev, S.A.; Anisimov, A.A.; Stogniy, M.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis of cobalt and nickel 6,6′-diphenylbis(dicarbollides). Russ. Chem. Bull. 2019, 68, 1239–1247. [Google Scholar] [CrossRef]
- Jin, G.F.; Hwang, J.-H.; Lee, J.-D.; Wee, K.-R.; Suh, I.-H.; Kang, S.O. A three-dimensional π-electron acceptor, tri-phenyl-o-carborane, bearing a rigid conformation with end-on phenyl units. Chem. Commun. 2013, 49, 9398–9400. [Google Scholar] [CrossRef] [PubMed]
- Viñas, C.; Barberà, G.; Oliva, J.M.; Teixidor, F.; Welch, A.J.; Rosair, G.M. Are halocarboranes suitable for substitution reactions? The case for 3-I-1,2-closo-C2B10H11: Molecular orbital calculations, aryldehalogenation reactions, 11B NMR Interpretation of closo-carboranes, and molecular structures of 1-Ph-3-Br-1,2-closo-C2B10H10 and 3-Ph-1,2-closo-C2B10H11. Inorg. Chem. 2001, 40, 6555–6562. [Google Scholar] [CrossRef]
- Qiu, Z.; Xie, Z. Palladium/Nickel-cocatalyzed cycloaddition of 1,3-dehydro-o-carborane with alkynes. Facile synthesis of C,B-substituted carboranes. J. Am. Chem. Soc. 2010, 132, 16085–16093. [Google Scholar] [CrossRef]
- Viñas, C.; Barberà, G.; Teixidor, F. The B-I activation in o-carborane clusters: Their fate towards B-H. Easy synthesis of [7,10-C2B10H13]−. J. Organomet. Chem. 2002, 642, 16–19. [Google Scholar] [CrossRef]
- Zhao, D.; Xie, Z. [3-N2-o-C2B10H11][BF4]: A useful synthon for multiple cage boron functionalizations of o-carborane. Chem. Sci. 2016, 7, 5635–5639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmalko, A.V.; Anufriev, S.A.; Stogniy, M.Y.; Suponitsky, K.Y.; Sivaev, I.B. Synthesis and structure of 3-arylazo derivatives of ortho-carborane. New J. Chem. 2020, 44, 10199–10202. [Google Scholar] [CrossRef]
- Aizawa, K.; Ohta, K.; Endo, Y. Synthesis of 3-aryl-1,2-dicarba-closo-dodecaboranes by Suzuki-Miyaura coupling reaction. Heterocycles 2010, 80, 369. [Google Scholar] [CrossRef]
- Xu, T.-T.; Cao, K.; Zhang, C.-Y.; Wu, J.; Ding, L.-F.; Yang, J. Old key opens the lock in carborane: The in situ NHC-Palladium catalytic system for selective arylation of B(3,6)–H bonds of o-carboranes via B–H activation. Org. Lett. 2019, 21, 9276–9279. [Google Scholar] [CrossRef]
- Qiu, Z.; Xie, Z. A strategy for selective catalytic B–H functionalization of o-carboranes. Accounts Chem. Res. 2021, 54, 4065–4079. [Google Scholar] [CrossRef] [PubMed]
- Sivaev, I.B. Functional group directed B–H activation of polyhedral boron hydrides by transition metal complexes (Review). Russ. J. Inorg. Chem. 2021, 66, 1289–1342. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Shmal’ko, A.V.; Suponitsky, K.Y.; Sivaev, I.B. One-pot synthesis of B-aryl carboranes with sensitive functional groups using sequential cobalt- and palladium-catalyzed reactions. Catalysts 2020, 10, 1348. [Google Scholar] [CrossRef]
- Fillon, H.; Gosmini, C.; Périchon, J. New chemical synthesis of functionalized arylzinc compounds from aromatic or thienyl bromides under mild conditions using a simple cobalt catalyst and zinc dust. J. Am. Chem. Soc. 2003, 125, 3867–3870. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, I.; Gosmini, C.; Paris, J.-M.; Périchon, J. New progress in the cobalt-catalysed synthesis of aromatic organozinc compounds by reduction of aromatic halides by zinc dust. Tetrahedron Lett. 2003, 44, 6417–6420. [Google Scholar] [CrossRef]
- Gosmini, C.; Moncomble, A. cobalt-catalyzed cross-coupling reactions of aryl halides. Isr. J. Chem. 2010, 50, 568–576. [Google Scholar] [CrossRef]
- Kalinin, V.N.; Ol’shevskaya, V.A. Some aspects of the chemical behavior of icosahedral carboranes. Russ. Chem. Bull. 2008, 57, 815–836. [Google Scholar] [CrossRef]
- Ohta, K.; Yamazaki, H.; Endo, Y. NMR study of 1-aryl-1,2-dicarba-closo-dodecaboranes: Intramolecular C–H⋯O hydrogen bonding in solution. Tetrahedron Lett. 2006, 47, 1937–1940. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Kosenko, I.D. Rotational conformation of 8,8′-dihalogenated derivatives of cobalt bis(dicarbollide) in solution. Russ. Chem. Bull. 2021, 70, 753–756. [Google Scholar] [CrossRef]
- Patterson-Elenbaum, S.; Stanley, J.T.; Dillner, D.K.; Lin, S.; Traficante, D. 13C NMR chemical shifts of carbonyl groups in substituted benzaldehydes and acetophenones: Substituent chemical shift increments. Magn. Reson. Chem. 2006, 44, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kashid, S.M.; Bagchi, S. Experimental determination of the electrostatic nature of carbonyl hydrogen-bonding interactions using IR-NMR correlations. J. Phys. Chem. Lett. 2014, 5, 3211–3215. [Google Scholar] [CrossRef] [PubMed]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Glukhov, I.V.; Lyssenko, K.A.; Korlyukov, A.; Antipin, M.Y. Nature of weak inter- and intramolecular contacts in crystals 2. Character of electron delocalization and the nature of X—H...H—X (X = C, B) contacts in the crystal of 1-phenyl-o-carborane. Russ. Chem. Bull. 2005, 54, 547–559. [Google Scholar] [CrossRef]
- Erokhina, S.A.; Stogniy, M.Y.; Suponitsky, K.Y.; Kosenko, I.D.; Sivaev, I.B.; Bregadze, V.I. Synthesis of new nido-carborane based carboxylic acids and amines. Polyhedron 2018, 153, 145–151. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B–H⋯π interactions between the B–H–B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 2017, 4436–4443. [Google Scholar] [CrossRef] [Green Version]
- Suponitsky, K.Y.; Anisimov, A.A.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. 1,12-Diiodo-ortho-carborane: A classic textbook example of the dihalogen bond. Crystals 2021, 11, 396. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Tafur, S.; Masunov, A. Applicability of hybrid density functional theory methods to calculation of molecular hyperpolarizability. J. Chem. Phys. 2008, 129, 044109. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules. A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Keith, T.A. AIMAll (Version 15.05.18); TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Semyakin, S.S.; Suponitsky, K.Y.; Lempert, D.B. Synthesis and characterization of 3-(5-(fluorodinitromethyl)-1H-1,2,4-triazol-3-yl)-4-nitrofurazan: A novel promising energetic component of boron-based fuels for rocket ramjet engines. Chem. Asian J. 2019, 14, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Godovikov, I.A.; Filippov, O.A.; Fabrizi de Biani, F.; Corsini, M.; Chizhov, A.O.; Sivaev, I.B. Methylsulfanyl-stabilized rotamers of cobalt bis(dicarbollide). Eur. J. Inorg. Chem. 2017, 2017, 4444–4451. [Google Scholar] [CrossRef] [Green Version]
- Itatani, H.; Bailar, J.C. Homogenous catalysis in the reactions of olefinic substances. V. Hydrogenation of soybean oil methyl ester with triphenylphosphine and triphenylarsine palladium catalysts. J. Am. Oil Chem. Soc. 1967, 44, 147–151. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Burlington, NJ, USA, 2009. [Google Scholar]
- Scientific Instrument Services (SIS). Available online: https://www.sisweb.com/mstools/isotope.htm (accessed on 24 November 2021).
- Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).