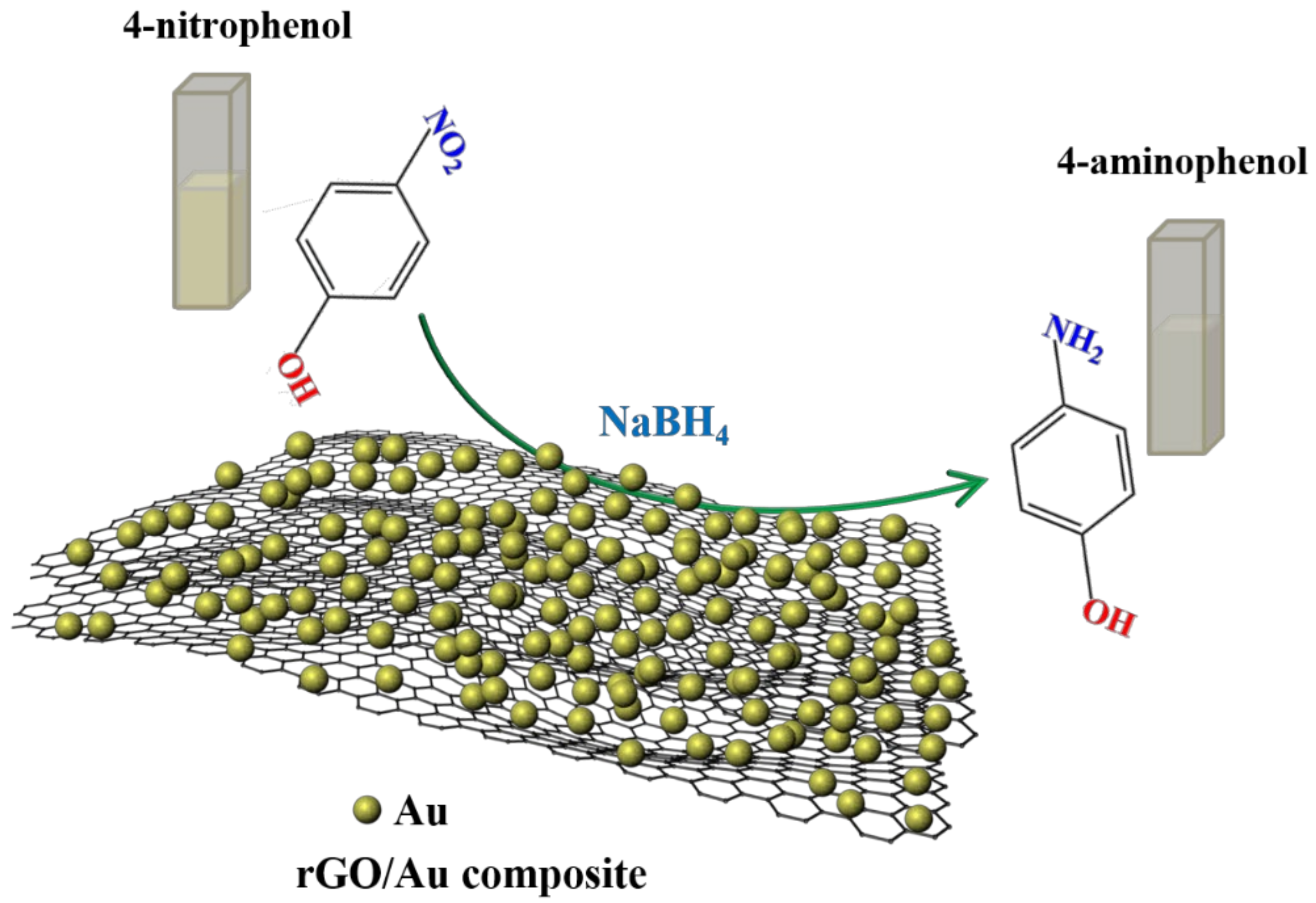
One-Pot Facile Synthesis of Noble Metal Nanoparticles Supported on rGO with Enhanced Catalytic Performance for 4-Nitrophenol Reduction
Abstract
:1. Introduction
2. Results and Discussion
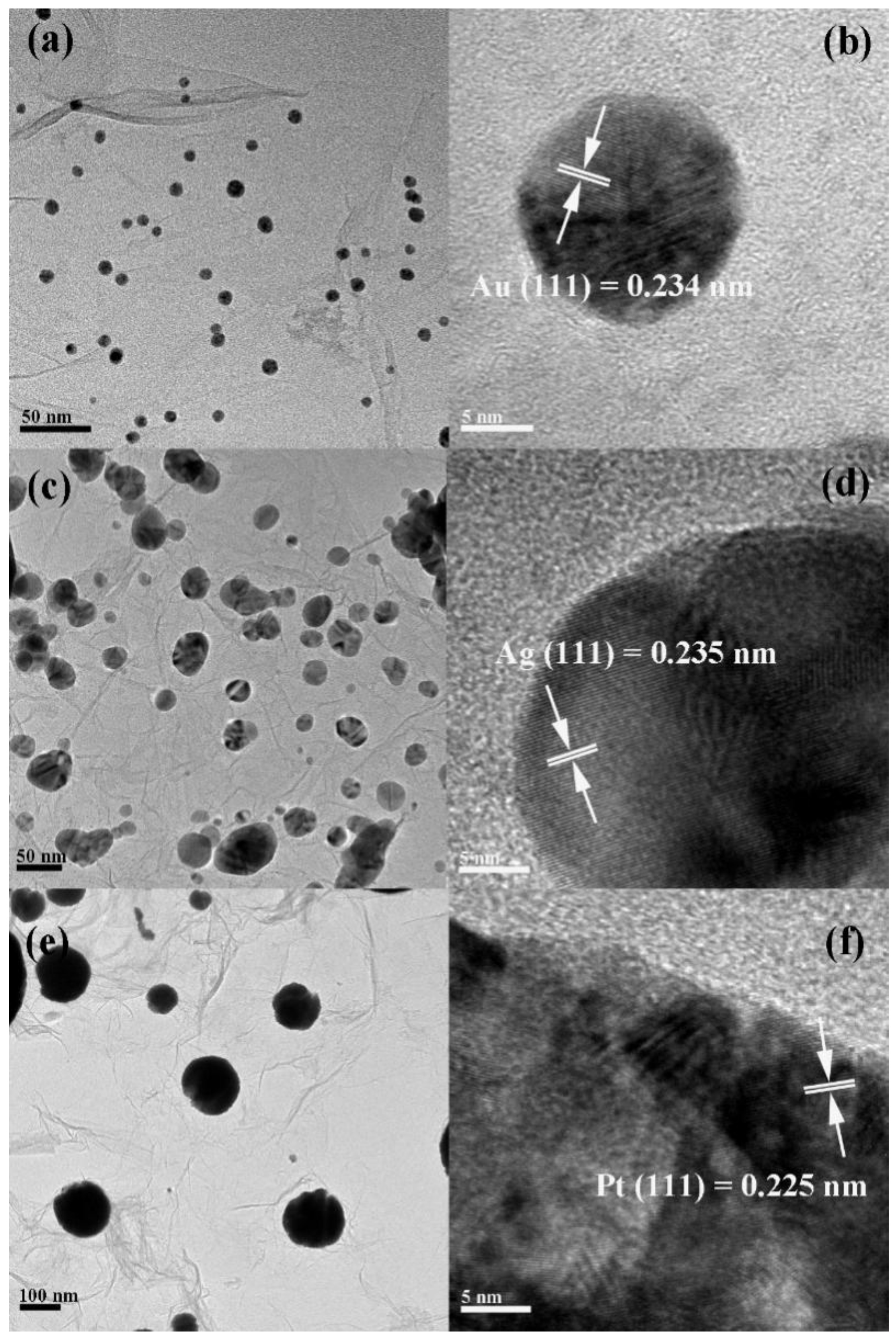
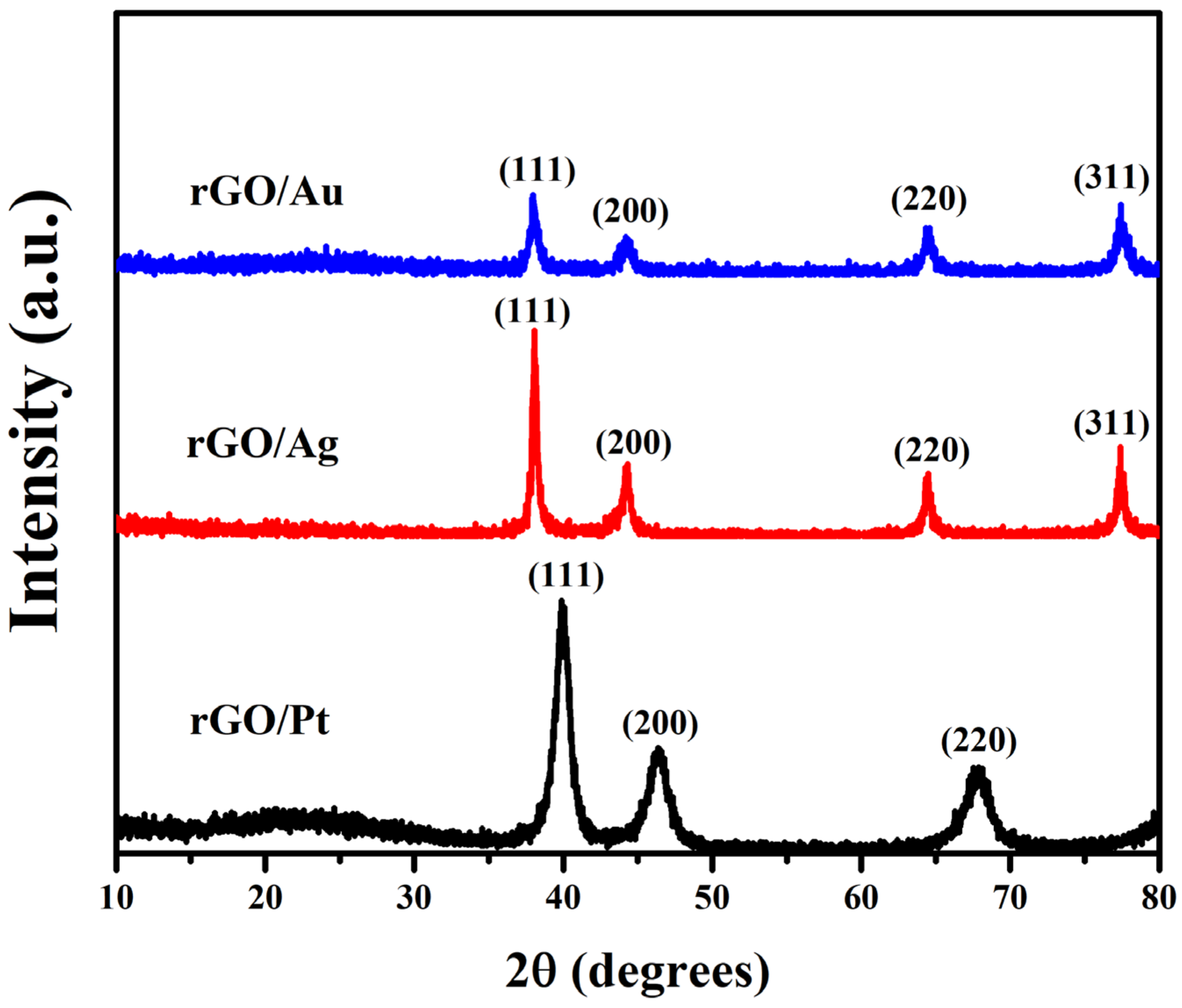
2.1. Characterization and Properties of the rGO/Au, rGO/Ag, and rGO/Pt Composites
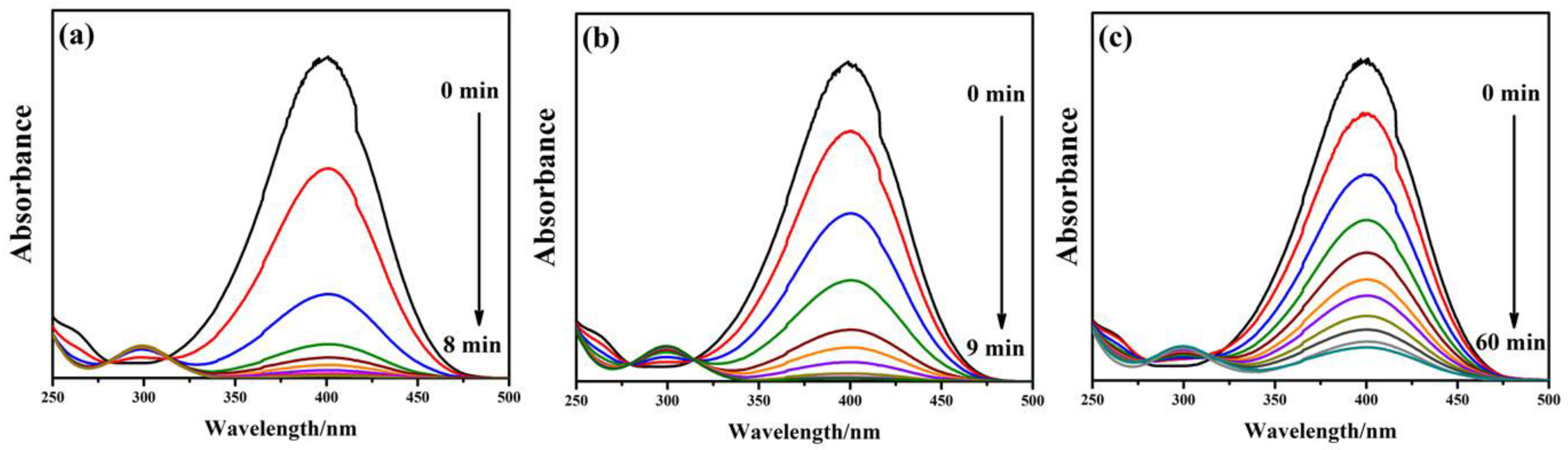
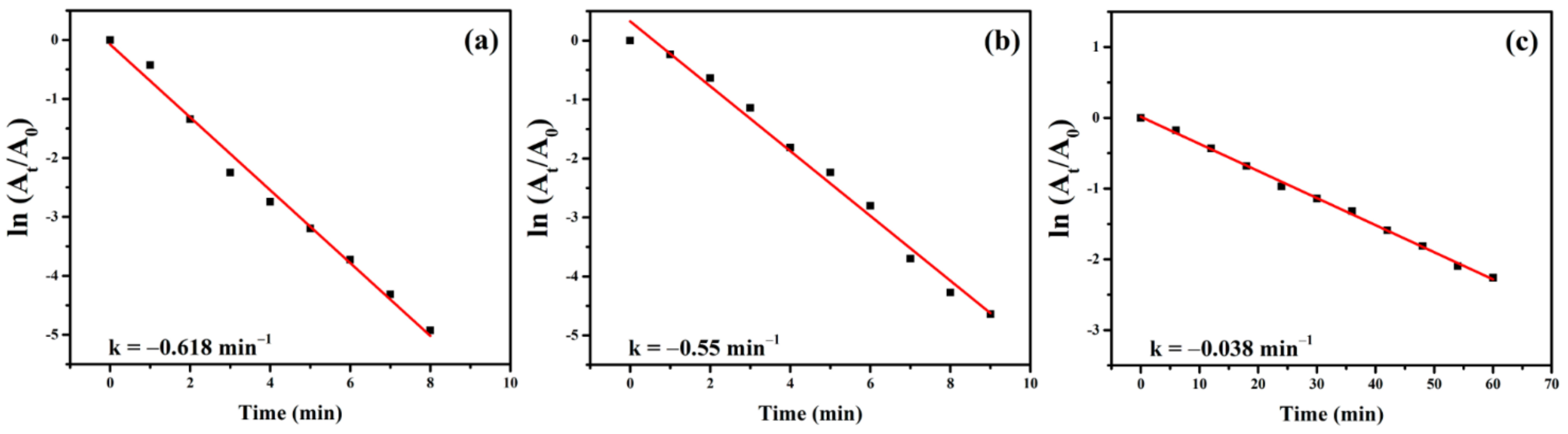
2.2. Catalytic Applications of the rGO/Au, rGO/Ag, and rGO/Pt Catalysts
2.3. Possible Mechanism of 4-NP Reduction
3. Materials and Methods
3.1. Materials
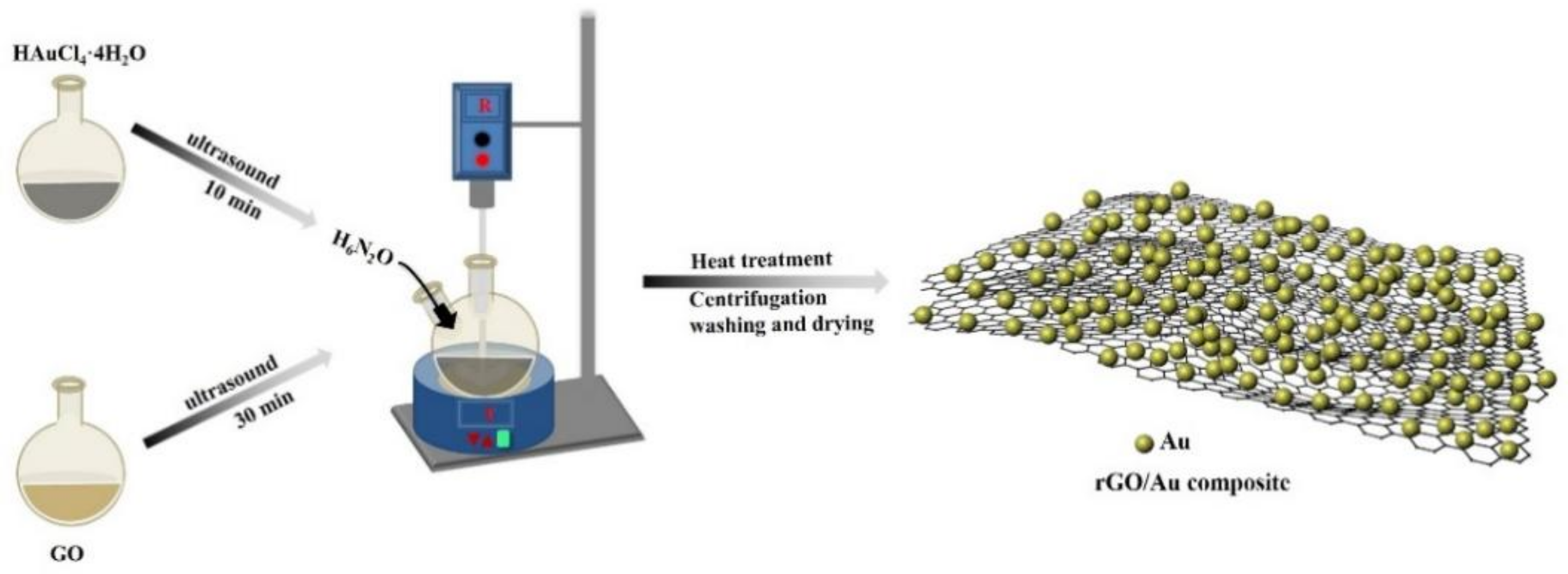
3.2. Fabrication of rGO-Supported Noble Metal Composites
3.3. Applications of rGO-Supported Noble Metal Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cui, Y.; Chen, K.B.; Ma, Z.; Yang, J.L.; Geng, Z.G.; Zeng, J. Atomic-level insights into strain effect on p-nitrophenol reduction via Au@Pd core–shell nanocubes as an ideal platform. J. Catal. 2020, 381, 427–433. [Google Scholar] [CrossRef]
- Ma, M.L.; Yang, Y.Y.; Li, W.T.; Feng, R.J.; Li, Z.W.; Lyu, P.; Ma, Y. Gold nanoparticles supported by amino groups on the surface of magnetite microspheres for the catalytic reduction of 4-nitrophenol. J. Mater. Sci. 2019, 54, 323–334. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical-Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Cao, Z.M.; Chen, Z.T.; Zhao, M.; Xie, M.H.; Lyu, Z.H.; Zhu, Z.H.; Chi, M.F.; Xia, Y.N. Catalytic System Based on Sub-2 nm Pt Particles and Its Extraordinary Activity and Durability for Oxygen Reduction. Nano Lett. 2019, 19, 4997–5002. [Google Scholar] [CrossRef]
- Wang, Y.N.; Dou, L.G.; Zhang, H. Nanosheet Array-Like Palladium-Catalysts Pdx/rGO@CoAl-LDH via Lattice Atomic-Confined in Situ Reduction for Highly Efficient Heck Coupling Reaction. ACS Appl. Mater. Interfaces 2017, 9, 38784–38795. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.F.; Yaseen, M.; Zada, A.; Ikram, M. Fabrication of highly dispersed Pt NPs in nanoconfined spaces of as-made KIT-6 for nitrophenol and MB catalytic reduction in water. Sep. Purif. Technol. 2021, 265, 118532. [Google Scholar] [CrossRef]
- Wu, T.; Zheng, H.; Kou, Y.C.; Su, X.Y.; Kadasala, N.R.; Gao, M.; Chen, L.; Han, D.L.; Liu, Y.; Yang, J.H. Self-sustainable and recyclable ternary Au@Cu2O-Ag nanocomposites: Application in ultra-sensitive SERS detection and highly efficient photocatalysis of organic dyes under visible light. Microsyst. Nanoeng. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, Y.H.; Jiang, B.; Guo, W.; Qin, W.; Xie, Y.M.; Zhao, B.; Zhao, L.; Liang, Z.Q.; Jiang, L. Pd nanoparticles decorated 3D printed hierarchically porous TiO2 scaffolds for efficient reduction of highly concentrated 4-nitrophenol solution. ACS Appl. Mater. Interfaces 2020, 12, 28100–28109. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Akhtar, M.S.; Algadi, H.; Ibrahim, A.A.; Alhamami, M.A.M.; Baskoutas, S. Highly Sensitive and Selective Eco-Toxic 4-Nitrophenol Chemical Sensor Based on Ag-Doped ZnO Nanoflowers Decorated with Nanosheets. Molecules 2021, 26, 4619. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.J.; Ma, T.; Liang, F. Syntheses of magnetic blackberry-like Ni@Cu@Pd nanoparticles for efficient catalytic reduction of organic pollutants. J. Alloy. Compd. 2021, 873, 159802. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, X.Y.; Feng, J.J.; Mei, L.P.; Wang, A.J. Simple fabrication of bimetallic platinum-rhodium alloyed nano-multipods: A highly effective and recyclable catalyst for reduction of 4-nitrophenol and rhodamine B. J. Colloid Interface Sci. 2021, 582, 701–710. [Google Scholar] [CrossRef]
- Li, Y.G.; Quan, X.J.; Hu, C.Y.; Li, C.P. Effective Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol over Etched Halloysite Nanotubes@α-Ni(OH)2. ACS Appl. Energy Mater. 2020, 5, 4756–4766. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhu, X.Y.; Feng, J.J.; Wang, A.J. Solvothermal synthesis of N-doped graphene supported PtCo nanodendrites with highly catalytic activity for 4-nitrophenol reduction. Appl. Surf. Sci. 2018, 428, 798–808. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Suh, J.M.; Choi, J.W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, R.D.; Hughes, R.A.; Sapkota, P.; Ptasinska, S.; Neretina, S. Effect of nanoparticle ligands on 4-nitrophenol reduction: Reaction rate, induction time, and ligand desorption. ACS Catal. 2020, 10, 10040–10050. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewasters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.S.; Zhu, X.Y.; Meng, H.B.; Feng, J.J.; Wang, A.J. One pot synthesis of highly branched Pt@Ag core–shell nanoparticles as a recyclable catalyst with dramatically boosting the catalytic performance for 4-nitrophenol reduction. J. Colloid Interface Sci. 2019, 538, 349–356. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Wei, X.; Chen, M. Modifiers-assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction. CrystEngComm 2013, 15, 560–569. [Google Scholar] [CrossRef]
- Hasan, Z.; Ok, Y.S.; Rinklebe, J.; Tsang, Y.F.; Cho, D.W.; Song, H. N doped cobalt-carbon composite for reduction of p-nitrophenol and pendimethaline. J. Alloy. Compd. 2017, 703, 118–124. [Google Scholar] [CrossRef]
- Hsan, Z.; Cho, D.W.; Chon, C.M.; Yoon, K.; Song, H. Reduction of p-nitrophenol by magnetic Co-carbon composites derived from metal organic frameworks. Chem. Eng. J. 2016, 298, 183–190. [Google Scholar] [CrossRef]
- Zarei, M.; Seyedi, N.; Maghsoudi, S.; Nejad, M.S.; Sheibani, H. Green synthesis of Ag nanoparticles on the modified grapheme oxide using Capparis spinosa fruit extract for catalytic reduction of organic dyes. Inorg. Chem. Commun. 2021, 123, 108327. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Entezari, M.H. Sono-synthesis approach in uniform loading of ultrafine Ag nanoparticles on reduced graphene oxide nanosheets: An efficient catalyst for the reduction of 4-Nitrophenol. Ultrason. Sonochem. 2018, 44, 1–13. [Google Scholar] [CrossRef]
- Yan, Z.; Fu, L.; Zuo, X.; Yang, H. Green assembly of stable and uniform silver nanoparticles on 2D silica nanosheets for catalytic reduction of 4-nitrophenol. Appl. Catal. B Environ. 2018, 226, 23–30. [Google Scholar] [CrossRef]
- Fu, Q.; Fu, C.; Teng, L.; Li, W.J.; Sheng, Y.P.; Wang, S. Rapid synthesis and growth process deconvolution of Au nanoflowers with ultrahigh catalytic activity based on microfluidics. J. Mater. Sci. 2021, 56, 6315–6326. [Google Scholar] [CrossRef]
- Han, X.W.; Bi, S.D.; Zhang, W.Q.; Yang, Z.W. One-step fabrication of highly dispersed Ag nanoparticles decorated N-doped reduced grapheme oxide heterogeneous nanostructure for the catalytic reduction of 4-nitrophenol. Colloid Surf. A. 2019, 574, 69–77. [Google Scholar] [CrossRef]
- Deng, C.Y.; Li, Y.H.; Sun, W.L.; Liu, F.L.; Zhang, Y.X.; Qian, H. Supported AuPd nanoparticles with high catalytic activity and excellent separability based on the magnetic polymer carriers. J. Mater. Sci. 2019, 54, 11435–11447. [Google Scholar] [CrossRef]
- Gong, H.; Liu, M.R.; Li, H.L. In situ green preparation of silver nanoparticles/chemical pulp fiber composites with excellent catalytic performance. J. Mater. Sci. 2019, 54, 6895–6907. [Google Scholar] [CrossRef]
- Cao, M.W.; Feng, L.; Yang, P.P.; Wang, H.X.; Liang, X.; Chen, X.W. Fabriation of reduced graphene oxide decorated with gold and nickel for the catalytic reduction of 4-nitrophenol. J. Mater. Sci. 2018, 53, 4874–4883. [Google Scholar] [CrossRef]
- Revathy, T.A.; Dhanavel, S.; Sivaranjani, T.; Narayanan, V.; Maiyalagan, T.; Stephen, A. Highly active graphene-supported palladium-nickel alloy nanoparticles for catalytic reduction of 4-nitrophenol. Appl. Surf. Sci. 2018, 449, 764–771. [Google Scholar] [CrossRef]
- Wei, Z.J.; Li, Y.G.; Dou, L.G.; Ahmad, M.; Zhang, H. Cu3–xNixAl-Layered Double Hydroxide-Reduced Graphene Oxide Nanosheet Array for the Reduction of 4-Nitrophenol. ACS Appl. Nano Mater. 2019, 4, 2383–2396. [Google Scholar] [CrossRef]
- Liu, Y.G.; Jiang, Y.Q.; Lv, Y.R.; He, Z.X.; Dai, L.; Wang, L. Synergistic Catalysis of SnO2/Reduced Graphene Oxide for VO2+/VO2+ and V2+/V3+ Redox Reactions. Molecules 2021, 26, 5085. [Google Scholar] [CrossRef]
- Cong, R.Y.; Park, H.H.; Jo, M.S.; Lee, H.C.; Lee, C.S. Synthesis and Electrochemical Performance of Electrostatic Self-Assembled Nano-Silicon@N-Doped Reduced Graphene Oxide/Carbon Nanofibers Composite as Anode Material for Lighium-Ion Batteries. Molecules 2021, 26, 4831. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xing, Y.; Song, Y.; Gu, Y.; Yan, X.Y.; Lu, N.N.; Liu, H.; Xu, Z.Q.; Xu, H.X.; Zhang, Z.Q.; et al. AuPt/MOF-Graphene: A Synergistic Catalyst with Surprisingly High Peroxidase-Like Activity and Its Application for H2O2 Detection. Anal. Chem. 2019, 91, 10589–10595. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, Y.; Qin, H.Y.; Wu, J.M.; Lu, Y.F.; Chi, H.Z.; Yang, F.; Zhou, B.; Yu, H.L.; Liu, J.B. Construction of rGO wrapping octahedral Ag-Cu2O heterostructure for enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2018, 227, 132–144. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, Q.C.; Zhang, P.; O’Connor, D.; Varma, R.S.; Yu, M.; Hou, D.Y. One-pot green synthesis of bimetallic hollow palladium-platinum nanotubes for enhanced catalytic reduction of p-nitrophenol. J. Colloid Interface Sci. 2019, 539, 161–167. [Google Scholar] [CrossRef]
- Li, B.P.; Song, C.Y.; Huang, X.M.; Ye, K.; Cheng, K.; Zhu, K.; Yan, J.; Cao, D.X.; Wang, G.L. A Novel Anode for Direct Borohydride-Hydrogen Peroxide Fuel Cell: Au Nanoparticles Decorated 3D Self-Supported Reduced Graphene Oxide Foam. ACS Sustain. Chem. Eng. 2019, 7, 11129–11137. [Google Scholar] [CrossRef]
- Alshanberi, A.M.; Satar, R.; Ansari, S.A. Stabilization of β-Galactosidase on Modified Gold Nanoparticles: A Preliminary Biochemical Study to Obtain Lactose-Free Dairy Products for Lactose-Intolerant Individuals. Molecules 2021, 26, 1226. [Google Scholar] [CrossRef]
- Han, X.W.; Pan, H.; Liu, M. In situ green growth of uniform and naked Ag nanoparticles on graphene oxide at room temperature and its enhanced catalytic performance. J. Nanopart. Res. 2020, 22, 166. [Google Scholar] [CrossRef]
- Huang, B.Y.; He, Y.; Wang, Z.H.; Zhu, Y.Q.; Zhang, Y.W.; Cen, K.F. High-Performance Pt Catalyst with Graphene/Carbon Black as a Hybrid Support for SO2 Electrocatalytic Oxidation. Langmuir 2020, 36, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Maarisetty, D.; Mahanta, S.; Sahoo, A.K.; Mohapatra, P.; Baral, S.S. Steering the Charge Kinetics in Dual-Functional Photocatalysis by Surface Dipole Moments and Band Edge Modulation: A Defect Study in TiO2-ZnS-rGO Composites. ACS Appl. Mater. Interfaces 2020, 12, 11679–11692. [Google Scholar] [CrossRef]
- Lin, J.H.; Pan, K.Y.; Wei, D.H.; Chung, R.J. FePt nanoparticles embedded-rGO nanocomposites for magnetic fluid hyperthermia. Surf. Coat. Tech. 2018, 350, 868–873. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.Y.; Kou, Q.W.; Liu, Y.; Han, D.L.; Wang, D.D.; Sun, Y.T.; Zhang, Y.J.; Wang, Y.X.; Lu, Z.Y.; et al. Enhanced Catalytic Reduction of 4-Nitrophenol Driven by Fe3O4-Au Magnetic Nanocomposite Interface Engineering: From Facile Preparation to Recyclable Application. Nanomaterials 2018, 8, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wang, H.; Ma, X.; Jin, J.; Ji, W.; Wang, X.; Song, W.; Zhao, B.; He, C. Fabrication of Ag-Cu2O/reduced graphene oxide nanocomposites as SERS substrates for in situ monitoring of peroxidase-like catalytic reaction and biosensing. ACS Appl. Mater. Interfaces 2017, 9, 19074. [Google Scholar] [CrossRef]
- Basavegowda, N.; Mishra, K.; Lee, Y.R. Trimetallic FeAgPt alloy as a nanocatalyst for the reduction of 4-nitroaniline and decolorization of rhodamine B: A comparative study. J. Alloy. Compd. 2017, 701, 456–464. [Google Scholar] [CrossRef]
- Park, J.; Cha, S.H.; Cho, S.; Park, Y. Green synthesis of gold and silver nanoparticles using gallic acid: Catalytic activity and conversion yield toward the 4-nitrophenol reduction reaction. J. Nanopart. Res. 2016, 18, 166. [Google Scholar] [CrossRef]
- Qian, Y.L.; Zhou, C.; Zhou, J.J.; Huang, A.S. Synthesis of silver-nanoparticles composite with highly catalytic activity supported on the reduced graphene oxide. Appl. Surf. Sci. 2020, 525, 146597. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhong, L.X.; Tong, L.M.; Yu, Y.; Liu, Q.; Zhang, S.C.; Yin, C.; Qiao, L.; Li, S.Z.; Si, R.; et al. Atomic Pd on Graphdiyne/Graphene Heterostructure as Efficient Catalyst for Aromatic Nitroreduction. Adv. Funct. Mater. 2019, 29, 1905423. [Google Scholar] [CrossRef]
- Fang, J.L.; Chen, X.; Wu, Y.L.; Liu, H.Y. Facile and green synthesis of Au nanorods/grapheme oxide nanocomposite with excellent catalytic properties for reduction of 4-nitrophenol. J. Mater. Sci. 2020, 55, 5880–5891. [Google Scholar] [CrossRef]
- Yao, W.; Li, F.L.; Li, X.H.; Lang, J.P. Fabrication of hollow Cu2O@CuO-supported Au-Pd alloy nanoparticles with high catalytic activity through the galvanic replacement reaction. J. Mater. Chem. A 2015, 3, 4578–4585. [Google Scholar] [CrossRef]
- Yao, T.J.; Zuo, Q.; Wang, H.; Wu, J.; Zhang, X.; Sun, J.M.; Cui, T.Y. Preparation of PdxAuy bimetallic nanostructures with controllable morphologies supported on reduced grapheme oxide nanosheets and wrapped in a polypyrrole layer. RSC Adv. 2015, 5, 87831–87837. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Liu, S.; Lu, W.B.; Wang, L.; Tian, J.Q.; Sun, X.P. In situ green synthesis of Au nanostructures on graphene oxide and their application for catalytic reduction of 4-nitrophenol. Catal. Sci. Technol. 2011, 1, 1142–1144. [Google Scholar] [CrossRef]
- Arora, N.; Mehta, A.; Mishra, A.; Basu, S. 4-Nitrophenol reduction catalysed by Au-Ag bimetallic nanoparticles supported on LDH: Homogeneous vs. heterogeneous catalysis. Appl. Clay Sci. 2018, 151, 1–9. [Google Scholar] [CrossRef]
- Shi, G.M.; Li, S.T.; Shi, F.N.; Shi, X.F.; Lv, S.H.; Cheng, X.B. A facile strategy for synthesis of Ni@C(N) nanocapsules with enhanced catalytic activity for 4-nitrophenol reduction. Colloid. Surf. A 2018, 555, 170–179. [Google Scholar] [CrossRef]
- Tran, X.T.; Hussain, M.; Kim, H.T. Facile and fast synthesis of a reduced grapheme oxide/carbon nanotube/iron/silver hybrid and its enhanced performance in catalytic reduction of 4-nitrophenol. Solid State Sci. 2020, 100, 106107. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, H.; Liu, Y.G.; Ren, Z.P.; Lin, C.P.; Tao, J.L.; Zhai, Y.P. Facile synthesis of PdNiP/Reduced graphene oxide nanocomposites for catalytic reduction of 4-nitrophenol. Mater. Chem. Phys. 2019, 222, 391–397. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Jin, S.; Zhang, Y.; Wang, L.; Liu, Y.; Duan, Q. One-Pot Facile Synthesis of Noble Metal Nanoparticles Supported on rGO with Enhanced Catalytic Performance for 4-Nitrophenol Reduction. Molecules 2021, 26, 7261. https://doi.org/10.3390/molecules26237261
Zhang X, Jin S, Zhang Y, Wang L, Liu Y, Duan Q. One-Pot Facile Synthesis of Noble Metal Nanoparticles Supported on rGO with Enhanced Catalytic Performance for 4-Nitrophenol Reduction. Molecules. 2021; 26(23):7261. https://doi.org/10.3390/molecules26237261
Chicago/Turabian StyleZhang, Xiaolong, Shilei Jin, Yuhan Zhang, Liyuan Wang, Yang Liu, and Qian Duan. 2021. "One-Pot Facile Synthesis of Noble Metal Nanoparticles Supported on rGO with Enhanced Catalytic Performance for 4-Nitrophenol Reduction" Molecules 26, no. 23: 7261. https://doi.org/10.3390/molecules26237261
APA StyleZhang, X., Jin, S., Zhang, Y., Wang, L., Liu, Y., & Duan, Q. (2021). One-Pot Facile Synthesis of Noble Metal Nanoparticles Supported on rGO with Enhanced Catalytic Performance for 4-Nitrophenol Reduction. Molecules, 26(23), 7261. https://doi.org/10.3390/molecules26237261





