Application of HPTLC Multiwavelength Imaging and Color Scale Fingerprinting Approach Combined with Multivariate Chemometric Methods for Medicinal Plant Clustering According to Their Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Plant Material and Extraction Procedure
2.3. HPTLC Procedure
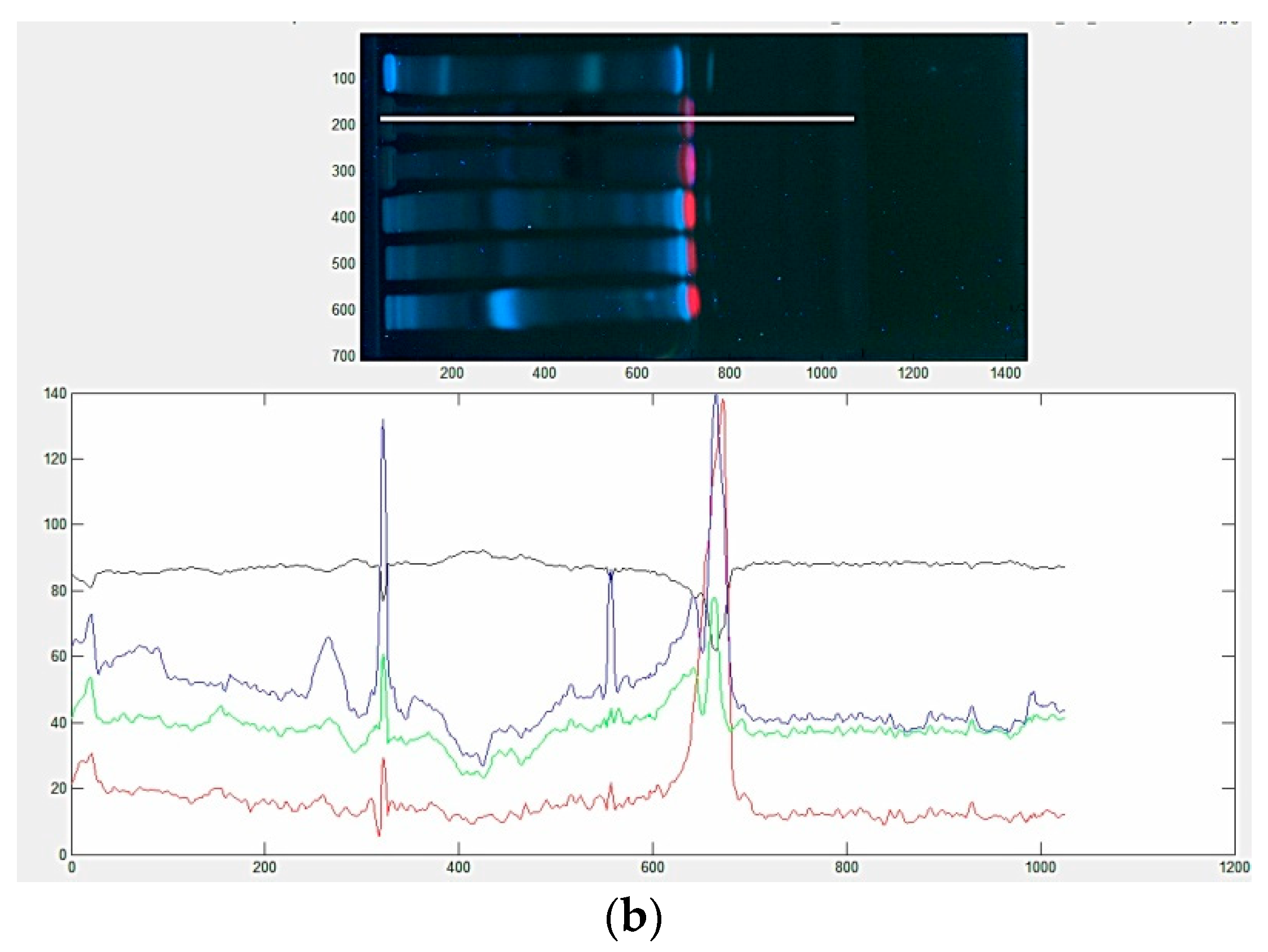
2.4. Image Processing
2.5. Chemometric Analysis of Data from Color Scale Fingerprints
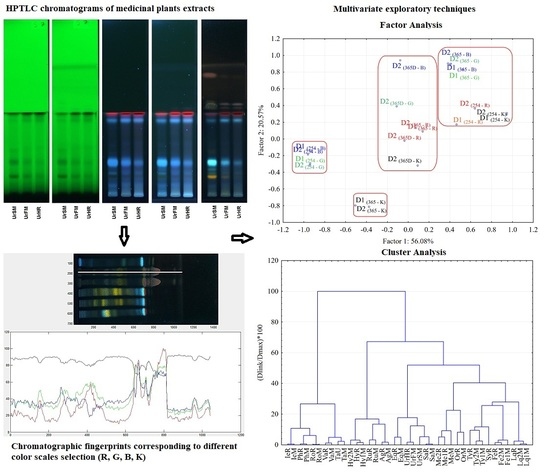
3. Results and Discussion
3.1. Analysis of the HPTLC Chromatograms
3.2. Evaluation of Color Scales HPTLC Fingerprints
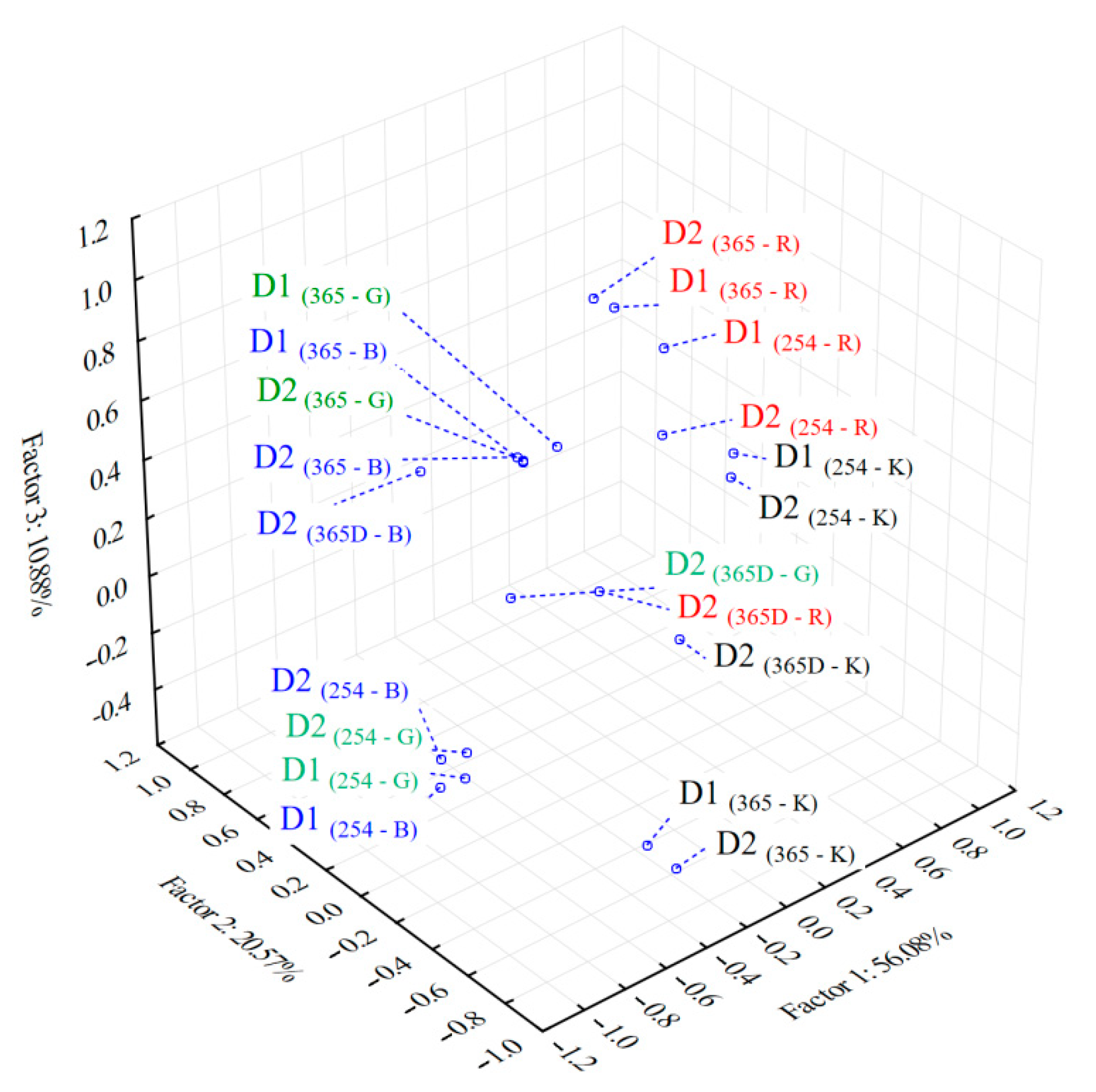
3.3. Chemometric Analysis of the Color Scale Fingerprints
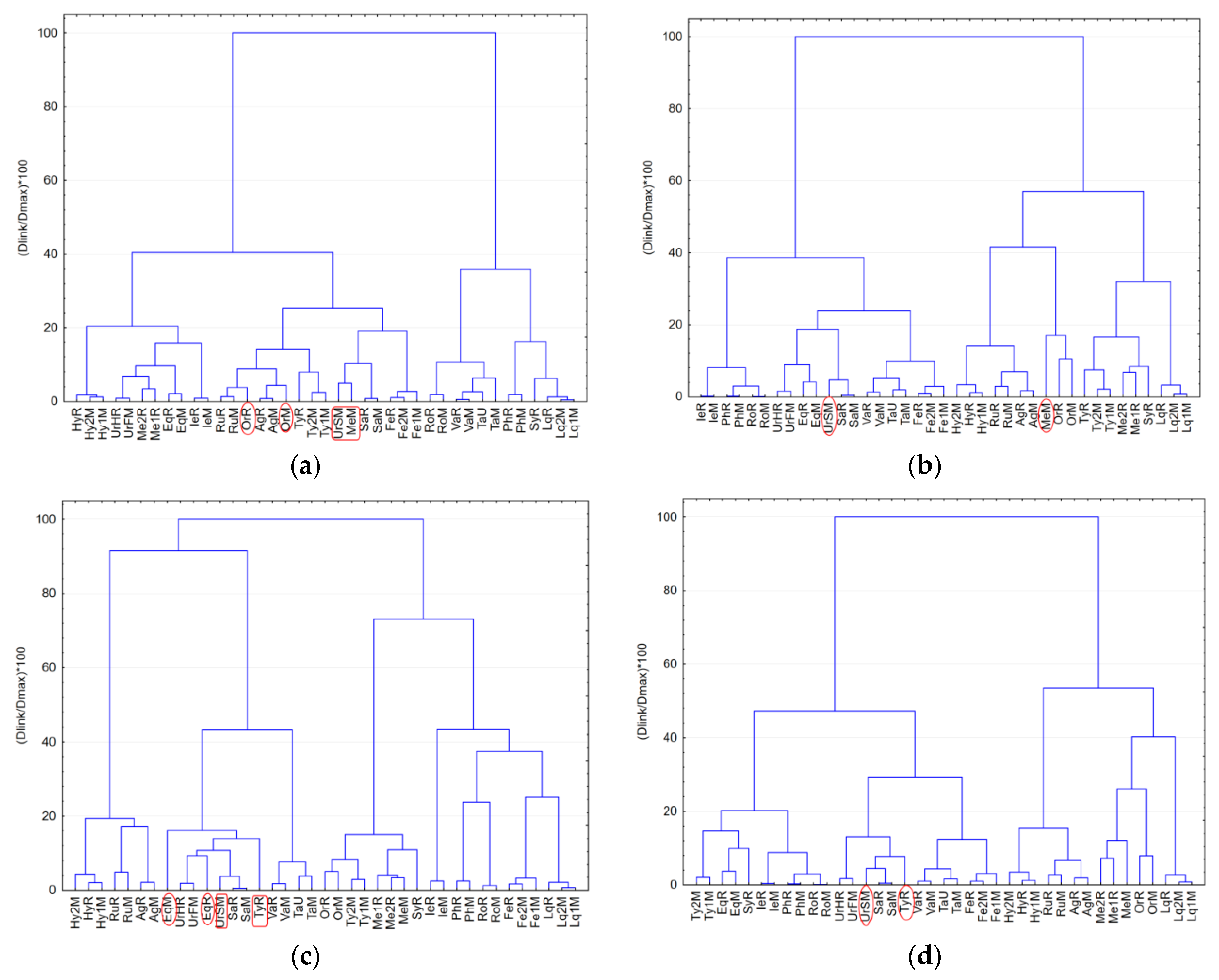
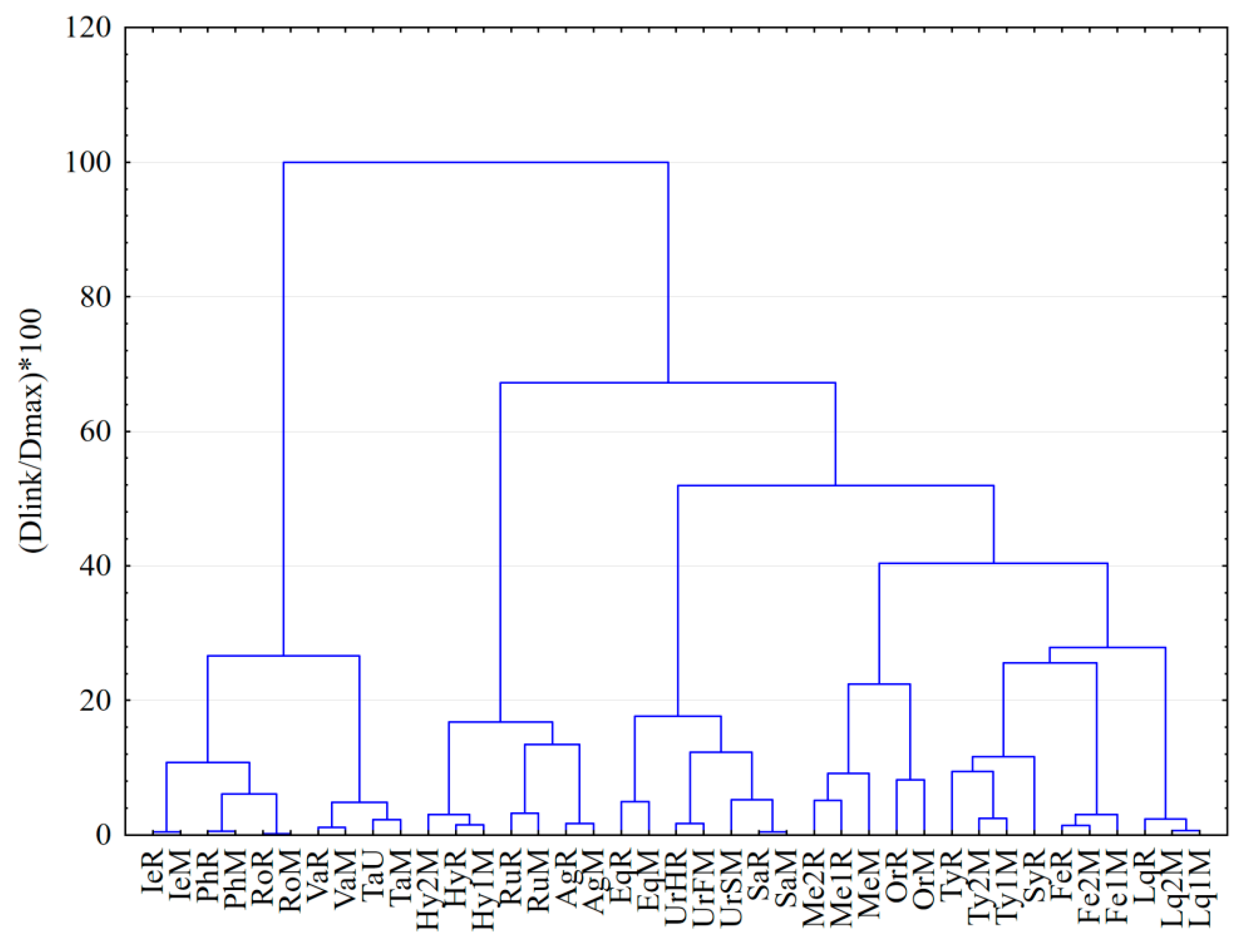
3.4. Hierarchical Clustering Analysis of Medicinal Plants Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jin, L.; Li, X.-B.; Tian, D.-Q.; Fang, X.-P.; Yu, Y.-M.; Zhu, H.-Q.; Ge, Y.-Y.; Ma, G.-Y.; Wang, W.-Y.; Xiao, W.-F.; et al. Antioxidant properties and color parameters of herbal teas in China. Ind. Crop. Prod. 2016, 87, 198–209. [Google Scholar] [CrossRef]
- Gunalan, G.; Saraswathy, A.; Vijayalakshmi, K. HPTLC Fingerprint Profile of Bauhinia Variegata Linn. Leaves. Asian Pac. J. Trop. Dis. 2012, 2, 21–25. [Google Scholar] [CrossRef]
- Srinivasan, S.; Wankhar, W.; Rathinasamy, S.; Rajan, R. Free Radical Scavenging Potential and HPTL Canalysis of Indigofera tinctorialinn (Fabaceae). J. Pharm. Anal. 2016, 6, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Embuscado, M.E. Handbook of antioxidants for food preservation. In Herbs and Spices as Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 251–281. [Google Scholar]
- Dhivya, S.M.; Kalaichelvi, K. UV-VIS Spectroscopic and FTIR analysis of Sarcostemma Brevistigma, WIGHT and ARN. Int. J. Curr. Pharm. Res. 2017, 9, 46–49. [Google Scholar] [CrossRef] [Green Version]
- Kalaichelvi, K.; Dhivya, S.M. Screening of phytoconstituents, UV-VIS Spectrum and FTIR analysis of Micrococca mercurialis (L.) Benth. Int. J. Herb. Med. 2017, 5, 40–44. [Google Scholar]
- Rodionova, O.Y.; Titova, A.V.; Polmerantsev, A.L. Discriminant analysis is an inappropriate method of authentication. Trends Anal. Chem. 2016, 78, 17–22. [Google Scholar] [CrossRef]
- Fibigr, J.; Šatínský, D.; Solich, P. Current trends in the analysis and quality control of food supplements based on plant extracts. Anal. Chim. Acta 2018, 1036, 1–15. [Google Scholar] [CrossRef]
- Chavan, S.S.; Jadhav, V.M.; Kadam, V.J. A review on spectroscopic analysis of phytopharmaceuticals. Int. J. Pharm. Sci. Rev. Res. 2017, 43, 161–168. [Google Scholar]
- Zhang, X.; Zhang, J.; Zhang, S.; Jiang, Z.; Wu, S.; Liu, J.; Ye, Y.; Si, J. Chemical fingerprint analysis and identification of Dendranthema lavandulifolium buds. Ind. Crop. Prod. 2018, 124, 707–718. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trend. Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Simion, I.M.; Casoni, D.; Sârbu, C. Classification of Romanian medicinal plant extracts according to the therapeutic effects using thin layer chromatography and robust chemometrics. J. Pharm. Biomed. Anal. 2019, 163, 137–143. [Google Scholar] [CrossRef]
- Bent, S. Herbal Medicine in the United States: Review of Efficacy, Safety, and Regulation: Grand Rounds at University of California, san Francisco Medical Center. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills, R.B.; Bone, K.; Morgan, M. Herbal products: Active constituents, modes of action and quality control. Nutr. Res. Rev. 2000, 13, 47–77. [Google Scholar] [CrossRef] [PubMed]
- Tistaert, C.; Dejaegher, B.; Heyden, Y.V. Chromatographic Separation Techniques and Data Handling Methods for Herbal Fingerprints: A Review. Anal. Chim. Acta 2011, 690, 148–161. [Google Scholar] [CrossRef]
- WHO. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- USFDA. FDA Guidance for Industry-Botanical Drug Products (Draft Guidance); US Food and Drug Administration: Rockville, MD, USA, 2000; pp. 18–22.
- EMA. Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products; Committee on Herbal Medicinal Products (HMPC) European Medicines Agency: London, UK, 2011.
- Fan, X.-H.; Cheng, Y.-Y.; Ye, Z.-L.; Lin, R.-C.; Qian, Z.-Z. Multiple Chromatographic Fingerprinting and its Application to the Quality Control of Herbal Medicines. Anal. Chim. Acta 2006, 555, 217–224. [Google Scholar] [CrossRef]
- Soponar, F.; Moţ, A.C.; Sârbu, C. High-Performance Thin-Layer Chromatography and Three-Dimensional Image Analysis for the Determination of Rutin in Pharmaceutical Preparations. J. AOAC Int. 2010, 93, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.E.; Olesik, S.V. Electrospun glassy carbon ultra-thin layer chromatography devices. J. Chromatogr. A. 2010, 1217, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Sârbu, C.; Moţ, A.C. Ecosystem discrimination and fingerprinting of Romanian propolis by hierarchical fuzzy clustering and image analysis of TLC patterns. Talanta 2011, 85, 1112–1117. [Google Scholar] [CrossRef]
- Komsta, L.; Ciesla, L.; Bogucka-Kocka, A.; Jozefczyk, A.; Kryszen, J.; Waksmundzka-Hajnos, M. The start-to-end chemometric image processing of 2D Thin-Layer videoscans. J. Chromatogr. A. 2011, 1218, 2820–2825. [Google Scholar] [CrossRef]
- Casoni, D.; Sima, I.A.; Sârbu, C. Simultaneous determination of parabens in pharmaceutical preparations using High-Performance Thin-Layer Chromatography and image analysis. J. Liquid Chromatogr. Relat. Technol. 2011, 34, 805–816. [Google Scholar] [CrossRef]
- Phattanawasin, P.; Sotanaphun, U.; Sukwattanasinit, T.; Akkarawaranthorn, J.; Kitchaiya, S. Quantitative determination of sibutramine in adulterated herbal slimming formulations by TLC-image analysis method. Forensic Sci. Int. 2012, 219, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ristivojevića, P.; Trifkovićb, J.; Vovkc, I.; Milojković-Opsenica, D. Comparative study of different approaches for multivariate image analysis in HPTLC fingerprinting of natural products such as plant resin. Talanta 2017, 162, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ciésla, Ł. Thin-layer chromatography with biodetection in the search for new potential drugs to treat neurodegenerative diseases—State of the art and future perspectives. Med. Chem. 2012, 8, 102–111. [Google Scholar] [CrossRef]
- Sima, I.A.; Casoni, D.; Sârbu, C. High sensitive and selective HPTLC method assisted by digital image processing for simultaneous determination of catecholamines and related drugs. Talanta 2013, 114, 117–123. [Google Scholar] [CrossRef]
- Casoni, D.; Sima, I.A.; Sârbu, C. Thin-layer chromatography—An image processing method for the determination of acidic catecholamine metabolites. J. Sep. Sci. 2014, 37, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Komsta, Ł.; Nowak, R.; Cieśla, Ł.; Waksmundzka-Hajnos, M. Investigation of antiradical activity of plant material by thin-layer chromatography with image processing. Food Chem. 2012, 132, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, Ł.M.; Kryszen, J.; Stochmal, A.; Oleszek, W.; Waksmundzka-Hajnos, M. Approach to develop a standardized TLC-DPPH test for assessing free radical scavenging properties of selected phenolic compounds. J. Pharm. Biomed. Anal. 2012, 70, 126–135. [Google Scholar] [CrossRef]
- Głod, B.K.; Wantusiak, P.M.; Piszcz, P.; Lewczuk, E.; Zarzycki, P.K. Application of micro-TLC to the total antioxidant potential (TAP) measurement. Food Chem. 2015, 173, 749–754. [Google Scholar] [CrossRef]
- Gökbulut, A. High Performance Thin Layer Chromatography (HPTLC) for the investigation of medicinal plants. Curr. Anal. Chem. 2021, 17, 1252–1259. [Google Scholar] [CrossRef]
- Xie, P.S.; Sun, S.; Xu, S.; Guo, L. Value the unique merit of HPTLC image analysis and extending its performance by digitalization for herbal medicines quality control. J. Chromatogr. Sep. Tech. 2014, 5, 249–258. [Google Scholar]
- Ristivojević, P.; Andrić, F.L.; Trifković, J.Đ.; Vovk, I.; Stanisavljević, L.Ž.; Tešić, Ž.L.; Milojković-Opsenica, D.M. Pattern recognition methods and multivariate image analysis in HPTLC fingerprinting of propolis extracts. J. Chemom. 2014, 28, 301–310. [Google Scholar] [CrossRef]
- Sudberg, S.; Sudberg, E.M.; Terrazas, J.; Sudberg, S.; Patel, K.; Pineda, J.; Fine, B. Fingerprint analysis and the application of HPTLC to the determination of identity and quality of botanicals, from an industry perspective. J. AOAC Int. 2010, 93, 1367–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi-Najafabadi, H.; Kazemeini Seyed, S.; Pasdaran, A.; Hamedi, A. A novel similarity search approach for high-performance thin-layer chromatography (HPTLC) fingerprinting of medicinal plants. Phytochem. Anal. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Simion, I.M.; Casoni, D.; Sârbu, C. Multivariate color scale image analysis—Thin layer chromatography for comprehensive evaluation of complex samples fingerprint. J. Chromatogr. B 2021, 1170, 122590. [Google Scholar] [CrossRef] [PubMed]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics, 2nd ed.; Taylor & Francis Group LLC, Broken Sound Parkway NW: Boca Raton, FL, USA, 2008. [Google Scholar]
- Calixto, M.G.; Ramos, H.A.; Veríssimo, L.S.; Alves Dantas, V.; Medeiros, A.C.D.; Fernandes, A.F.H.; Veras, G. Trends and application of chemometric pattern recognition techniques in medicinal plants analysis. Crit. Rev. Anal. Chem. 2021, in press. [Google Scholar] [CrossRef]
- Matevski, V. Flora of the Republic of Macedonia, 1st ed.; Macedonian Academy of Science and Arts: Skopje, North Macedonia, 2010. [Google Scholar]
- Rural Development. A Romania of medicinal and aromatic herb products. Rural Romania. Natl. Rural. Dev. Netw. 2015, 15, 8–22.
- Wagner, H.; Bladt, S.; Zgainski, E.M. Drogenanalyse Dunnschichtchromatographische Analyse von Arzneidrogen; Springer: New York, NY, USA, 1983. [Google Scholar]
- Guideline on Declaration of Herbal Substances and Herbal Preparations in Herbal Medicinal Products/Traditional Herbal Medicinal Products; Post-Authorisation Evaluation of Medicines for Human Use; European Medicines Agency: London, UK, 2009.


| No. | Common Name | Genus (Taxonomic Rank) | Familly | Part/Plant | Code Label 1 | Provenience | Herbarium No. |
|---|---|---|---|---|---|---|---|
| 1 | Liquorice | Glycyrrhiza glabra L. | Fabaceae | Radix | Lq1M | North Macedonia | 1/2018 |
| Lq2M | North Macedonia | 2/2018 | |||||
| LqR | Romania/Fares | 22/2018 | |||||
| 2 | Sweet fennel | Foeniculum vulgare var.dulcis Mill. | Apiaceae | Fructus | Fe1M | North Macedonia | 3/2018 |
| Fe2M | North Macedonia | 4/2018 | |||||
| FeR | Romania/Dacia Plant | 23/2018 | |||||
| 3 | Dandelion | Taraxacum officinale F.H.Wigg. | Asteraceae | Radix | TaM | North Macedonia | 5/2018 |
| TaU | Hungary/ Gyogyfu KFT | 39/2018 | |||||
| 4 | Valerian | Valeriana officinalis L. | Caprifoliaceae | Radix | ValM | North Macedonia | 6/2018 |
| ValR | Romania/ | 24/2018 | |||||
| 5 | Dog rose | Rosa canina L. | Rosaceae | Fructus | RoM | North Macedonia | 7/2018 |
| RoR | Romania/Fares | 25/2018 | |||||
| 6 | Comfrey | Symphytum officinale L. | Boraginaceae | Radix | SyR | Romania/Fares | 26/2018 |
| 7 | Juniper | Juniperus communis L. | Cupresaceae | Fructus | JuM | North Macedonia | 8/2018 |
| JuR | Romania/Plafar | 27/2018 | |||||
| 8 | Wild thyme | Thymus serpyllum L. | Lamiaceae | Herba | Ty1M | North Macedonia | 9/2018 |
| Ty2M | North Macedonia | 10/2018 | |||||
| TyR | Romania/Plafar | 28/2018 | |||||
| 9 | Bean | Phaseolus vulgaris L. | Fabaceae | Pericarpum | PhM | North Macedonia | 11/2018 |
| PhR | Romania/Fares | 29/2018 | |||||
| 10 | Elderberry | Sambucus nigra L. | Adoxaceae | Flowers | SaM | North Macedonia | 12/2018 |
| SaR | Romania/Fares | 30/2018 | |||||
| 11 | Horsetail | Equisetum arvense L. | Equisetaceae | Herba | EqM | North Macedonia | 13/2018 |
| EqR | Romania/Fares | 31/2018 | |||||
| 12 | Oregano | Origanum vulgare L. | Lamiaceae | Herba | OrM | North Macedonia | 14/2018 |
| OrR | Romania/Fares | 32/2018 | |||||
| 13 | Agrimony | Agrimonia eupatoria L. | Rosaceae | Herba | AgM | North Macedonia | 15/2018 |
| AgR | Romania/Fares | 33/2018 | |||||
| 14 | Blackberry | Rubus fruticosus L. | Rosaceae | Folium | RuM | North Macedonia | 16/2018 |
| RuR | Romania/Plafar | 34/2018 | |||||
| 15 | Peppermint | Mentha x piperita L. | Lamiaceae | Folium | MeM | North Macedonia | 17/2018 |
| Herba | Me1R | Romania/Fares | 35/2018 | ||||
| Folium | Me2R | Romania/Plafar | 36/2018 | ||||
| 16 | St.John’s wort | Hypericum perforatum L. | Hypericaceae | Herba | Hy1M | North Macedonia | 18/2018 |
| Hy2M | North Macedonia | 19/2018 | |||||
| HyR | Romania/Fares | 37/2018 | |||||
| 17 | Stinging nettle | Urtica dioica L. | Urticaceae | Seeds | UrSM | North Macedonia | 20/2018 |
| Folium | UrFM | North Macedonia | 21/2018 | ||||
| Herba | UrHR | Romania/Fares | 38/2018 |
| Variables * | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| D1 (254-R) | 0.46 | 0.17 | 0.68 |
| D1 (254-G) | −0.94 | −0.30 | −0.14 |
| D1 (254-B) | −0.93 | −0.15 | −0.24 |
| D1 (254-K) | 0.94 | 0.29 | 0.14 |
| D1 (365-R) | 0.14 | 0.09 | 0.94 |
| D1 (365-G) | 0.52 | 0.83 | 0.07 |
| D1 (365-B) | 0.4 | 0.89 | 0.04 |
| D1 (365-K) | −0.51 | −0.80 | −0.30 |
| D2 (254-R) | 0.64 | 0.37 | 0.26 |
| D2 (254-G) | −0.95 | −0.31 | −0.05 |
| D2 (254-B) | −0.96 | −0.19 | −0.12 |
| D2 (254-K) | 0.95 | 0.31 | 0.04 |
| D2 (365-R) | 0.07 | 0.13 | 0.98 |
| D2 (365-G) | 0.41 | 0.9 | 0.03 |
| D2 (365-B) | 0.38 | 0.9 | 0.05 |
| D2 (365-K) | −0.38 | −0.81 | −0.42 |
| D2 (365D-R) | −0.04 | −0.02 | 0.09 |
| D2 (365D-G) | −0.11 | 0.39 | −0.08 |
| D2 (365D-B) | −0.08 | 0.94 | 0.13 |
| D2 (365D-K) | 0.09 | −0.32 | 0 |
| Total variance (%) | 56.08 | 20.57 | 10.88 |
| Cumulative variance (%) | 56.08 | 76.66 | 87.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobzac, S.C.A.; Olah, N.K.; Casoni, D. Application of HPTLC Multiwavelength Imaging and Color Scale Fingerprinting Approach Combined with Multivariate Chemometric Methods for Medicinal Plant Clustering According to Their Species. Molecules 2021, 26, 7225. https://doi.org/10.3390/molecules26237225
Cobzac SCA, Olah NK, Casoni D. Application of HPTLC Multiwavelength Imaging and Color Scale Fingerprinting Approach Combined with Multivariate Chemometric Methods for Medicinal Plant Clustering According to Their Species. Molecules. 2021; 26(23):7225. https://doi.org/10.3390/molecules26237225
Chicago/Turabian StyleCobzac, Simona Codruța Aurora, Neli Kinga Olah, and Dorina Casoni. 2021. "Application of HPTLC Multiwavelength Imaging and Color Scale Fingerprinting Approach Combined with Multivariate Chemometric Methods for Medicinal Plant Clustering According to Their Species" Molecules 26, no. 23: 7225. https://doi.org/10.3390/molecules26237225
APA StyleCobzac, S. C. A., Olah, N. K., & Casoni, D. (2021). Application of HPTLC Multiwavelength Imaging and Color Scale Fingerprinting Approach Combined with Multivariate Chemometric Methods for Medicinal Plant Clustering According to Their Species. Molecules, 26(23), 7225. https://doi.org/10.3390/molecules26237225