Molecular Simulation of the Adsorption Characteristics of Methane in Pores of Coal with Different Metamorphic Degrees
Abstract
:1. Introduction
2. Coal Structure Model and Simulation Scheme
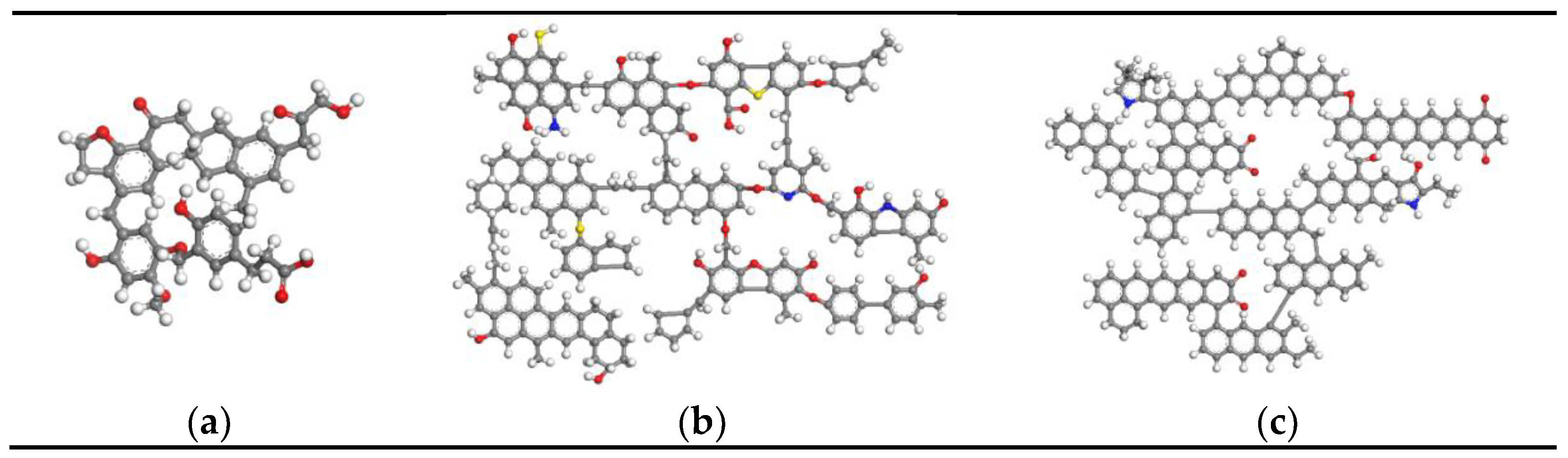
2.1. Establishment of Coal Model
2.2. Type of Force Field
2.3. Simulation Scheme
3. Results and Discussion
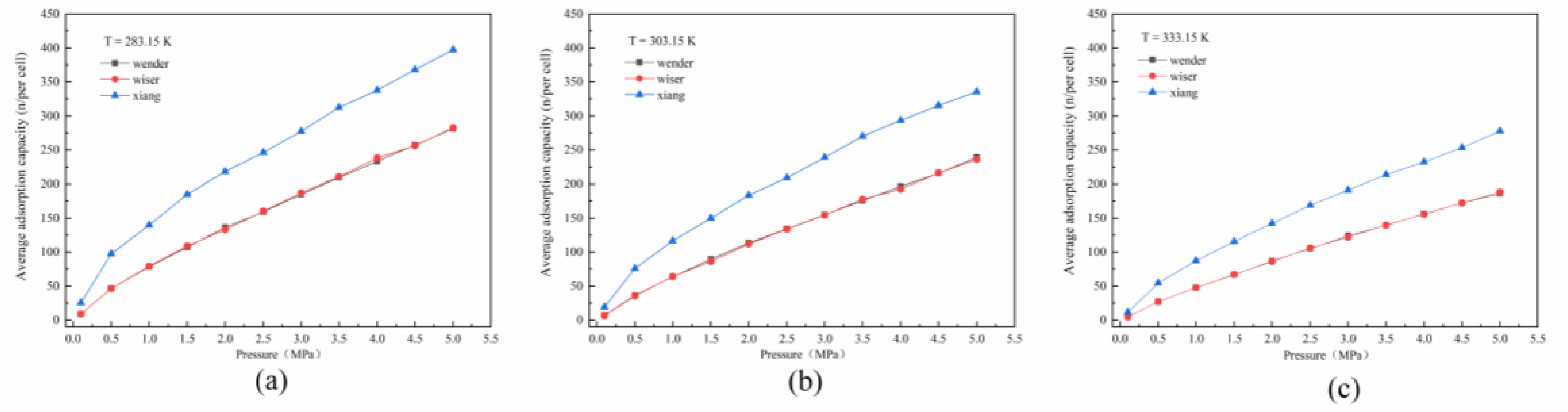
3.1. Adsorption Isotherm
3.2. Energy Analysis
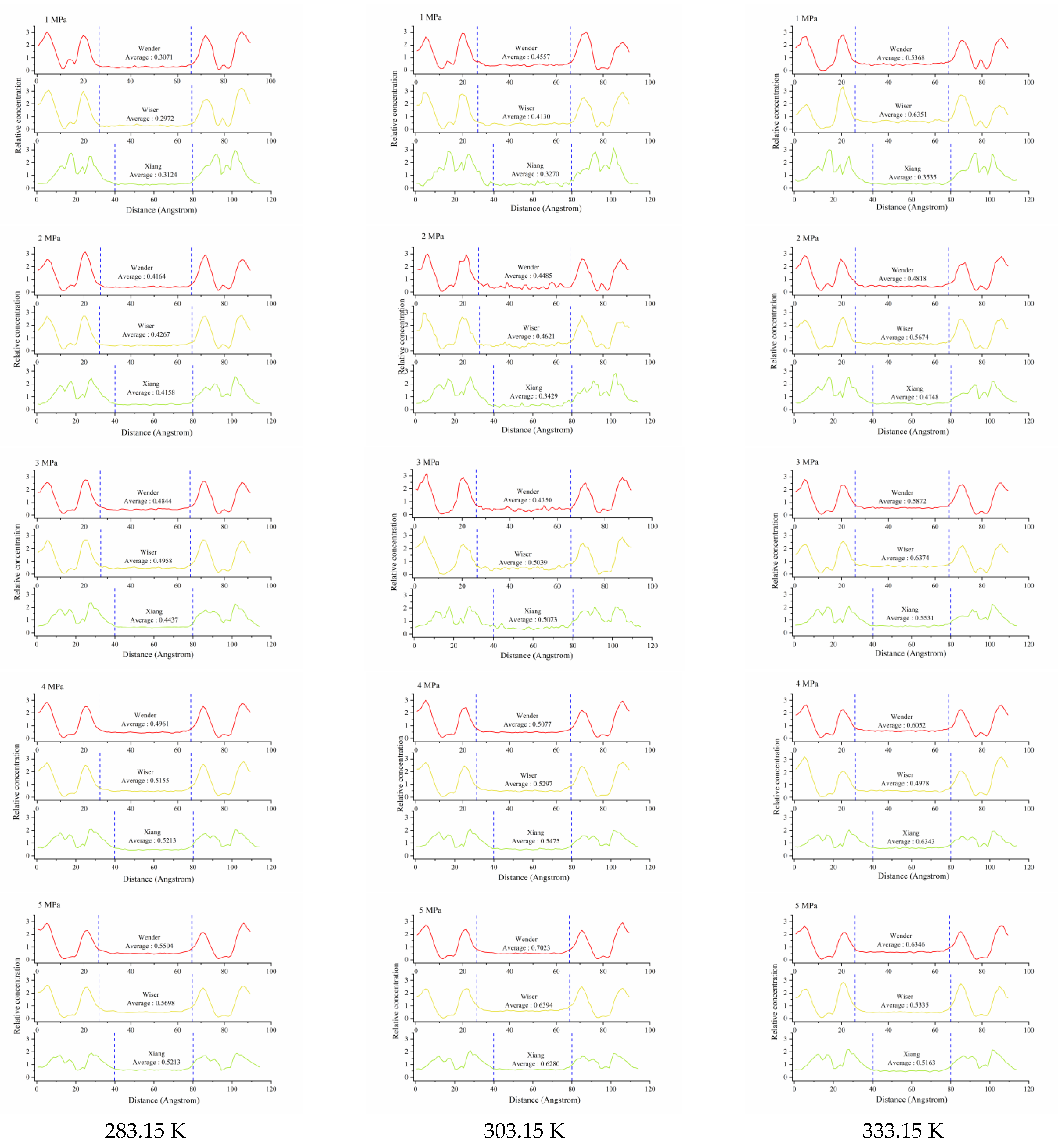
3.3. Relative Concentration Distribution
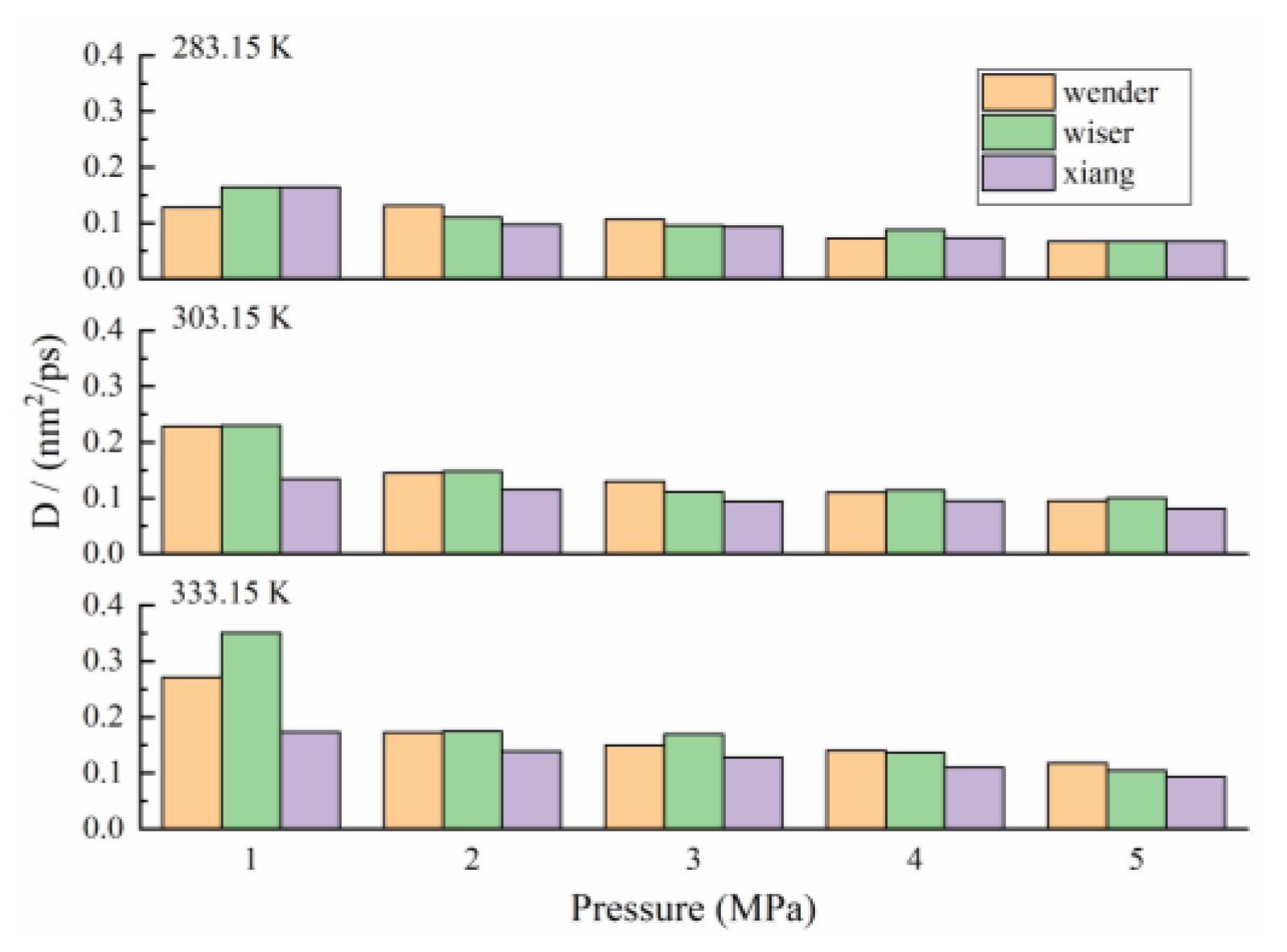
3.4. Diffusion Laws of CH4 in Coal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Wang, H.; Zhang, Z.; Chen, H.; Liu, X. Molecular simulation of CO2/CH4/H2O competitive adsorption and diffusion in brown coal. RSC Adv. 2019, 9, 3004–3011. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yang, H.; Wang, W.; Cheng, W.; Xin, L. Experimental study on the pore structure fractals and seepage characteristics of a coal sample around a borehole in coal seam water infusion. Transp. Porous Media 2018, 125, 289–309. [Google Scholar] [CrossRef]
- Kong, B.; Wang, E.; Li, Z. The effect of high temperature environment on rock properties—an example of electromagnetic radiation characterization. Environ. Sci. Pollut. Res. 2018, 25, 29104–29114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Liu, S.; Li, W.; Tang, X. Temperature effect on gas adsorption capacity in different sized pores of coal: Ex-periment and numerical modeling. J. Pet. Sci. Eng. 2018, 165, 821–830. [Google Scholar] [CrossRef]
- Dang, Y.; Zhao, L.; Lu, X.; Xu, J.; Sang, P.; Guo, S.; Zhu, H.; Guo, W. Molecular simulation of CO2/CH4 adsorption in brown coal: Effect of oxygen-, nitrogen-, and sulfur-containing functional groups. Appl. Surf. Sci. 2017, 423, 33–42. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Chen, S.; Tao, S.; Tang, D.; Xu, H.; Li, S.; Zhao, J.; Jiang, Q.; Yang, H. Pore structure characterization of different rank coals using N2 and CO2 adsorption and its effect on CH4 adsorption capacity: A case in Panguan Syncline, Western Guizhou, China. Energy Fuels 2017, 31, 6034–6044. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Cai, Y.; Li, X. Variation of petrophysical properties and adsorption capacity in different rank coals: An experimental study of coals from the Junggar, Ordos and Qinshui Basins in China. Energies 2019, 12, 986. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, B.; Xia, Y.; Liu, S. Wettability modification of Wender lignite by adsorption of dodecyl poly ethoxylated sur-factants with different degree of ethoxylation: A molecular dynamics simulation study. J. Mol. Graph. Model. 2017, 76, 106–117. [Google Scholar] [CrossRef]
- Shinn, J. From coal to single-stage and two-stage products: A reactive model of coal structure. Fuel 1984, 63, 1187–1196. [Google Scholar] [CrossRef]
- Hatcher, P.G. Chemical structural models for coalified wood (vitrinite) in low rank coal. Org. Geochem. 1990, 16, 959–968. [Google Scholar] [CrossRef]
- Wiser, W.H. Conversion of bituminous coal to liquids and gases: Chemistry and representative processes. In Magnetic Resonance; Petrakis, L., Fraissard, J.P., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 124, pp. 325–350. [Google Scholar] [CrossRef]
- Fuchs, W.; Sandhoff, A.G. Theory of coal pyrolysis. Ind. Eng. Chem. 1942, 34, 567–571. [Google Scholar] [CrossRef]
- Given, P.H. Structure of bituminous coals: Evidence from distribution of hydrogen. Nature 1959, 184, 980–981. [Google Scholar] [CrossRef]
- Hill, G.B.; Lyon, L.B. A new chemical structure for coal. Ind. Eng. Chem. 1962, 54, 36–41. [Google Scholar] [CrossRef]
- Hirsch, P.B. X-ray scattering from coals. Proc. Royal Soc. Lond. 1954, 226, 143–169. [Google Scholar]
- Pappano, P.J.; Mathews, J.P.; Schobert, H.H. Structural determinations of Pennsylvania anthracites. Fuel Energy Abstr. 2002, 43, 7. [Google Scholar]
- Xiang, J.-H.; Zeng, F.-G.; Li, B.; Zhang, L.; Li, M.-F.; Liang, H.-Z. Construction of macromolecular structural model of anthracite from Chengzhuang coal mine and its molecular simulation. J. Fuel Chem. Technol. 2013, 41, 391–400. [Google Scholar] [CrossRef]
- Gao, D.; Hong, L.; Wang, J.; Zheng, D. Molecular simulation of gas adsorption characteristics and diffusion in micropores of lignite. Fuel 2020, 269, 117443. [Google Scholar] [CrossRef]
- Mosher, K.; He, J.; Liu, Y.; Rupp, E.; Wilcox, J. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol. 2013, 109–110, 36–44. [Google Scholar] [CrossRef]
- Yin, T.; Liu, D.; Cai, Y.; Liu, Z.; Gutierrez, M. A new constructed macromolecule-pore structure of anthracite and its related gas adsorption: A molecular simulation study. Int. J. Coal Geol. 2020, 220, 103415. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, L.; Liu, Q.; Zhao, Y.; Feng, Q. Competitive adsorption and selective diffusion of CH4 and the intruding gases in coal vitrinite. Energy Fuels 2019, 33, 6971–6982. [Google Scholar] [CrossRef]
- Asif, M.; Naveen, P.; Panigrahi, D.; Kumar, S.; Ojha, K. Adsorption isotherms of CO2 – CH4 binary mixture using IAST for optimized ECBM recovery from sub-bituminous coals of Jharia coalfield: An experimental and modeling approach. Int. J. Coal Prep. Util. 2018, 39, 403–420. [Google Scholar] [CrossRef]
- Bai, Y.; Lin, H.-F.; Li, S.-G.; Yan, M.; Long, H. Molecular simulation of N2 and CO2 injection into a coal model containing adsorbed methane at different temperatures. Energy 2021, 219, 119686. [Google Scholar] [CrossRef]
- Wu, S.; Deng, C.; Wang, X. Molecular simulation of flue gas and CH4 competitive adsorption in dry and wet coal. J. Nat. Gas Sci. Eng. 2019, 71, 102980. [Google Scholar] [CrossRef]
- Wu, S.; Jin, Z.; Deng, C. Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel 2019, 239, 87–96. [Google Scholar] [CrossRef]
- Xiang, J.H.; Zeng, F.G.; Liang, H.Z.; Bin, L.I.; Song, X.X. Molecular simulation of the CH4/CO2/ H2O adsorption onto the molecular structure of coal. Sci. China (Earth Sci.) 2014, 57, 1749–1759. [Google Scholar] [CrossRef]
- Wiser, W.H.; Hill, G.R.; Kertamus, N.J. Kinetic study of pyrolysis of high volatile bituminous coal. Ind. Eng. Chem. Process Des. Dev. 1967, 6, 133–138. [Google Scholar] [CrossRef]
- Wender, I. Catalytic synthesis of chemicals from coal. Catal. Rev. Sci. Eng. 1976, 14, 97–129. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Merkel, A.; Gensterblum, Y.; Krooss, B.; Amann-Hildenbrand, A. Competitive sorption of CH4, CO2 and H2O on natural coals of different rank. Int. J. Coal Geol. 2015, 150–151, 181–192. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Liu, J.; Chen, X.; Li, Z. Effect of the pore structure on adsorption and diffusion migration of different rank coal samples. Energy Fuels 2020, 34, 12486–12504. [Google Scholar] [CrossRef]
- Graff, M.; Bukowska, J. Surface-enhanced Raman scattering (SERS) spectroscopy of enantiomeric and racemic methionine on a silver electrode-evidence for chiral discrimination in interactions between adsorbed molecules. Chem. Phys. Lett. 2011, 509, 58–61. [Google Scholar] [CrossRef]
- Yang, J.Z.; Liu, Q.L.; Wang, H.T. Analyzing adsorption and diffusion behaviors of ethanol/water through silicalite membranes by molecular simulation. J. Membr. Sci. 2007, 291, 1–9. [Google Scholar] [CrossRef]

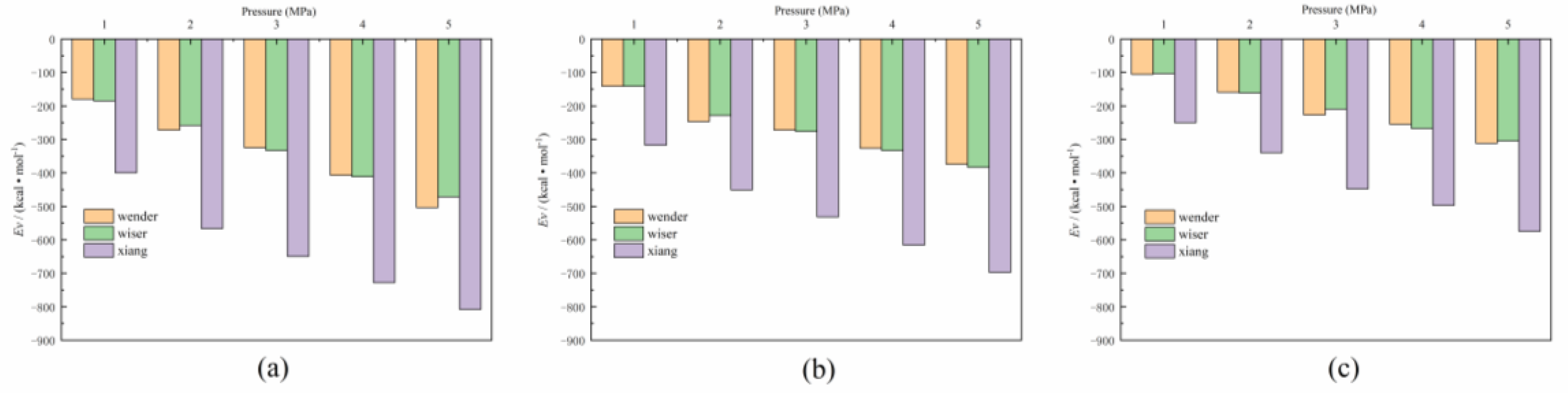
| Coal Samples | T 1 | P 2 | Q 3 | EvdW 4 | T | Q | EvdW | T | Q | EvdW |
|---|---|---|---|---|---|---|---|---|---|---|
| Wender | 283.15 | 1 | 78.4002 | −179.5581 | 303.15 | 63.6366 | −141.04313 | 333.15 | 47.7494 | −105.4962 |
| Wiser | 79.4713 | −185.0184 | 63.9987 | −141.0229 | 47.8132 | −102.8283 | ||||
| Xiang | 139.5387 | −399.5742 | 116.6083 | −317.6438 | 87.4664 | −249.4977 | ||||
| Wender | 2 | 136.1117 | −271.6324 | 113.5411 | −246.6959 | 87.0326 | −158.7563 | |||
| Wiser | 132.9195 | −258.2213 | 111.8657 | −228.2721 | 85.9271 | −160.3511 | ||||
| Xiang | 218.7014 | −566.3772 | 183.4784 | −451.2131 | 142.2486 | −339.69111 | ||||
| Wender | 3 | 184.5834 | −325.0988 | 154.8854 | −271.6868 | 124.0636 | −226.7707 | |||
| Wiser | 186.8574 | −332.8752 | 154.6254 | −275.7122 | 121.8778 | −209.4812 | ||||
| Xiang | 277.7498 | −649.6994 | 239.2534 | −531.8532 | 191.3180 | −447.8494 | ||||
| Wender | 4 | 233.1210 | −407.0464 | 196.5561 | −326.6914 | 156.0592 | −254.8214 | |||
| Wiser | 238.1569 | −411.5075 | 192.4109 | −332.7546 | 155.7692 | −266.9878 | ||||
| Xiang | 337.8066 | −727.7002 | 293.3652 | −615.4051 | 232.2581 | −496.8911 | ||||
| Wender | 5 | 281.5214 | −503.7776 | 239.0463 | −374.3551 | 186.2385 | −311.3128 | |||
| Wiser | 282.7054 | −471.6480 | 236.0646 | −383.1802 | 188.2769 | −304.0934 | ||||
| Xiang | 397.5014 | −807.9002 | 335.8903 | −696.9941 | 277.8015 | −574.7722 |
| Coal Samples | T 1 | P 2 | D 3 | T | D | T | D |
|---|---|---|---|---|---|---|---|
| Wender | 283.15 | 1 | 0.1285 | 303.15 | 0.2282 | 333.15 | 0.2713 |
| Wiser | 0.1642 | 0.2301 | 0.3511 | ||||
| Xiang | 0.1598 | 0.1341 | 0.1736 | ||||
| Wender | 2 | 0.1311 | 0.1454 | 0.1726 | |||
| Wiser | 0.1103 | 0.1484 | 0.1748 | ||||
| Xiang | 0.0975 | 0.1153 | 0.1387 | ||||
| Wender | 3 | 0.1065 | 0.1299 | 0.1494 | |||
| Wiser | 0.0962 | 0.1112 | 0.1698 | ||||
| Xiang | 0.0939 | 0.0943 | 0.1282 | ||||
| Wender | 4 | 0.0733 | 0.1102 | 0.1404 | |||
| Wiser | 0.0884 | 0.1147 | 0.1365 | ||||
| Xiang | 0.0731 | 0.0949 | 0.1098 | ||||
| Wender | 5 | 0.0675 | 0.0948 | 0.1178 | |||
| Wiser | 0.0672 | 0.1004 | 0.1049 | ||||
| Xiang | 0.0673 | 0.0811 | 0.0933 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Deng, C.; Jin, Z.; Gao, T. Molecular Simulation of the Adsorption Characteristics of Methane in Pores of Coal with Different Metamorphic Degrees. Molecules 2021, 26, 7217. https://doi.org/10.3390/molecules26237217
Han Q, Deng C, Jin Z, Gao T. Molecular Simulation of the Adsorption Characteristics of Methane in Pores of Coal with Different Metamorphic Degrees. Molecules. 2021; 26(23):7217. https://doi.org/10.3390/molecules26237217
Chicago/Turabian StyleHan, Qing, Cunbao Deng, Zhixin Jin, and Tao Gao. 2021. "Molecular Simulation of the Adsorption Characteristics of Methane in Pores of Coal with Different Metamorphic Degrees" Molecules 26, no. 23: 7217. https://doi.org/10.3390/molecules26237217
APA StyleHan, Q., Deng, C., Jin, Z., & Gao, T. (2021). Molecular Simulation of the Adsorption Characteristics of Methane in Pores of Coal with Different Metamorphic Degrees. Molecules, 26(23), 7217. https://doi.org/10.3390/molecules26237217





