Artemisia santolinifolia-Mediated Chemosensitization via Activation of Distinct Cell Death Modes and Suppression of STAT3/Survivin-Signaling Pathways in NSCLC
Abstract
:1. Introduction
2. Results
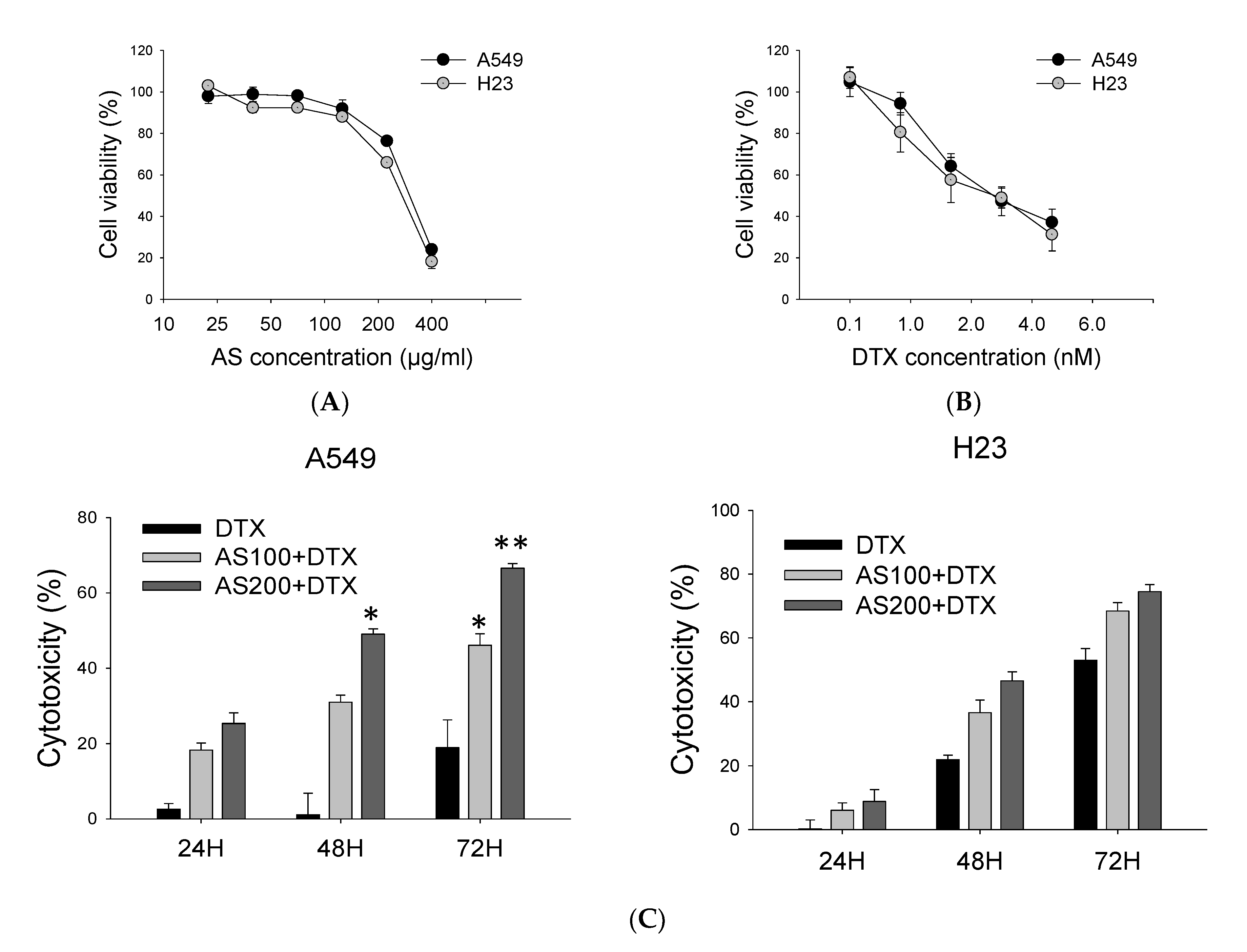
2.1. AS Combined with DTX Synergistically Inhibits A549 and H23 Cells Proliferation
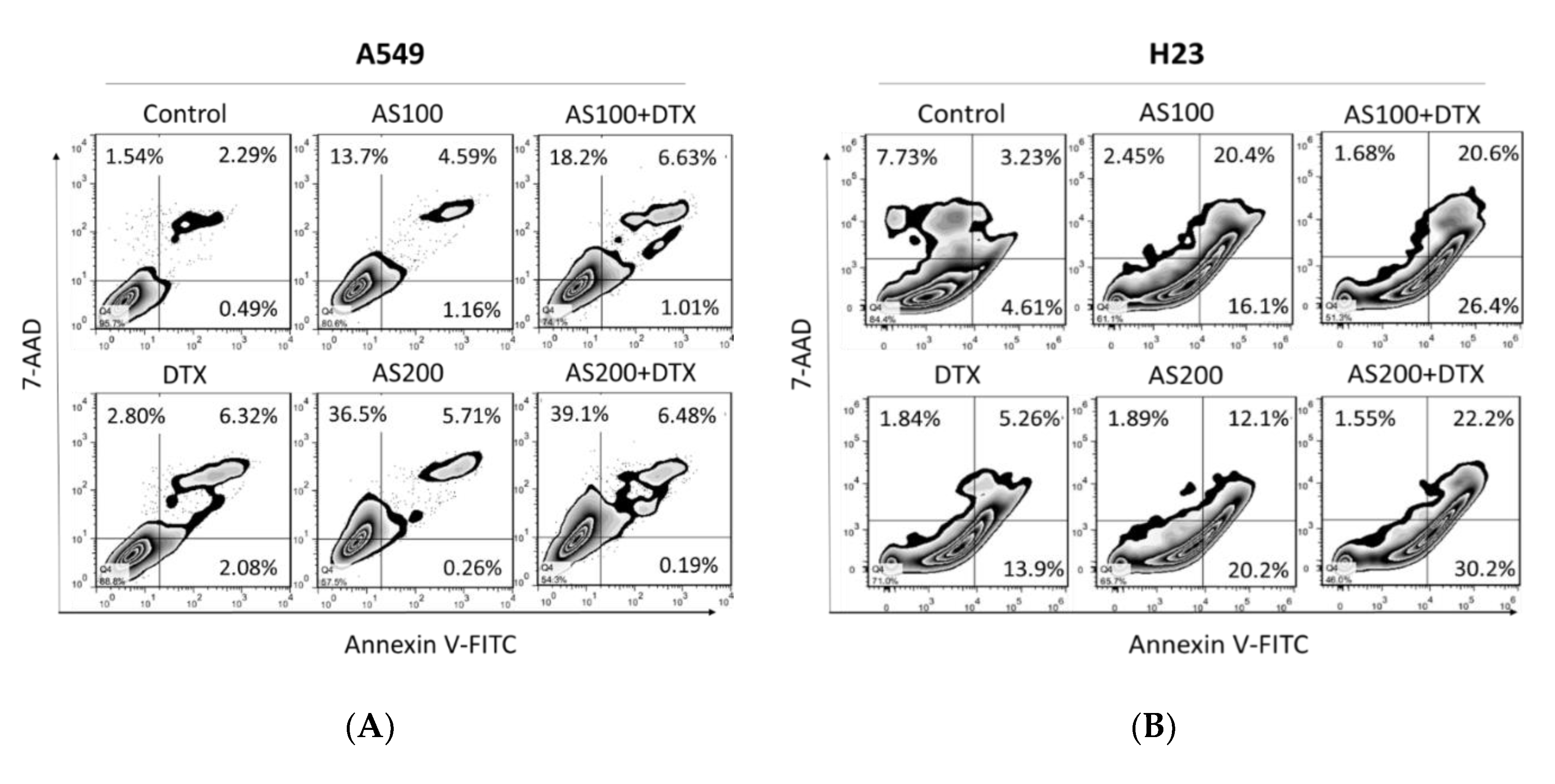
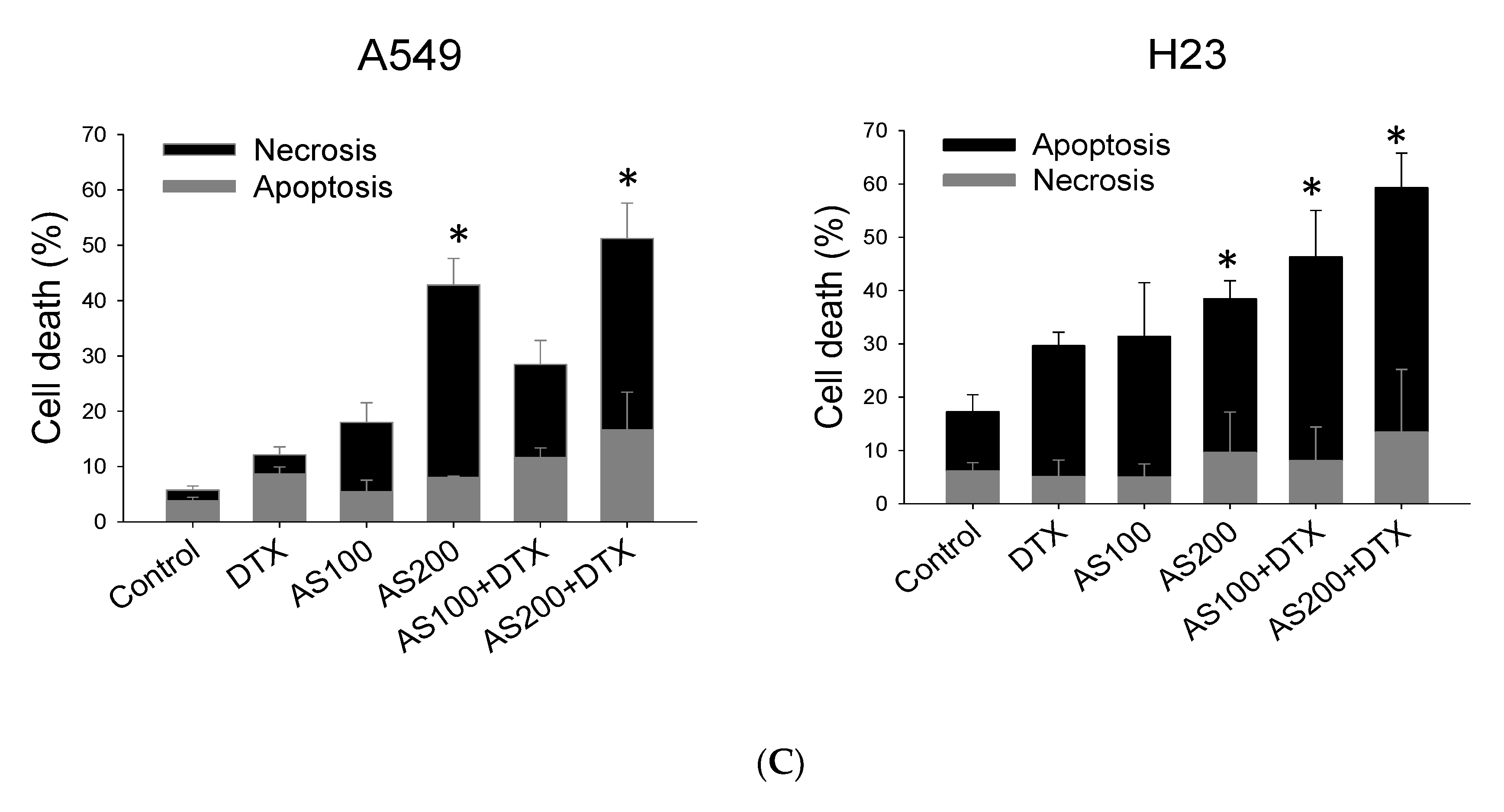
2.2. Enhancement of DTX-Induced Cytotoxicity Effect through Distinct Cell Death Modalities
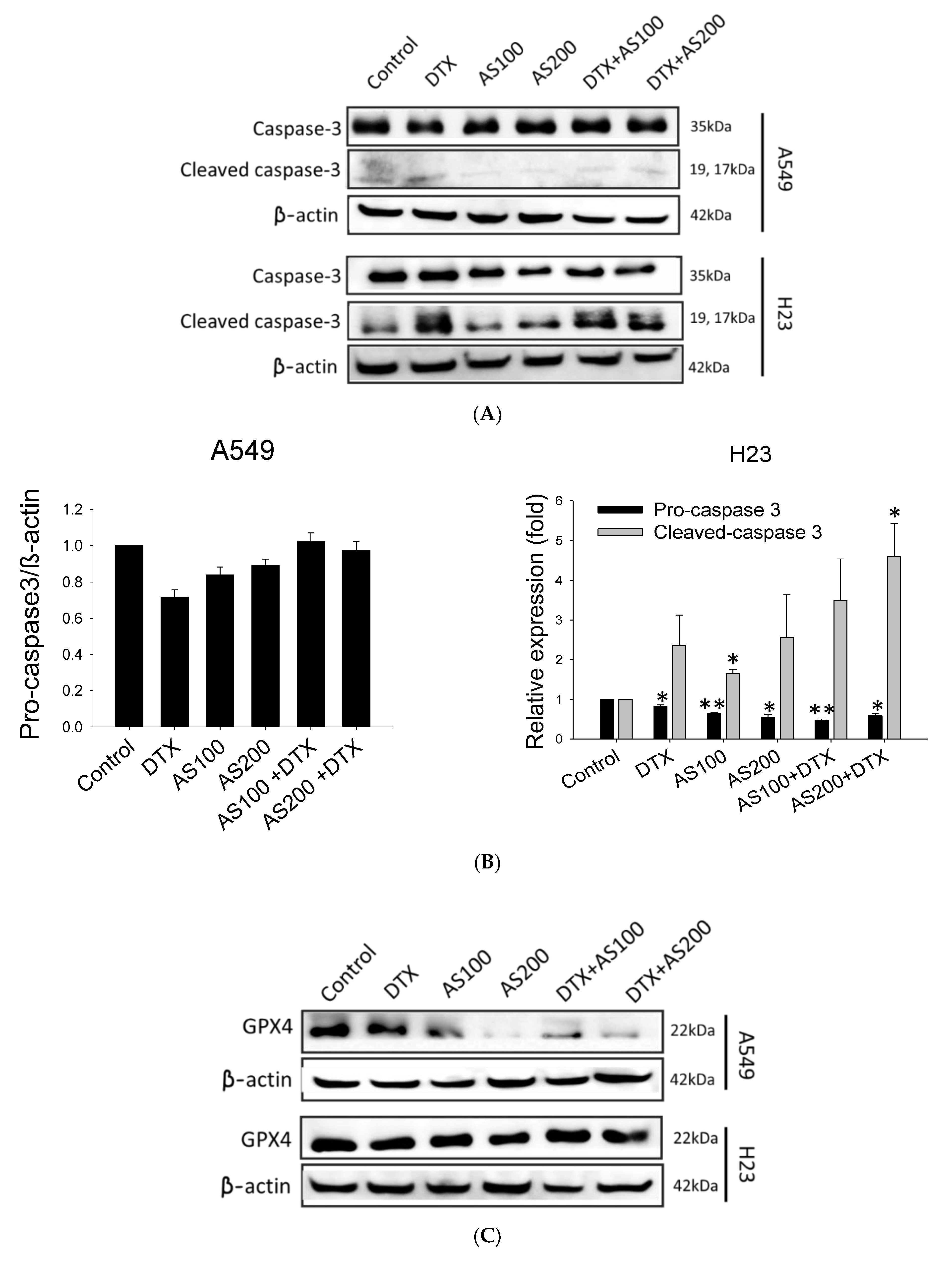
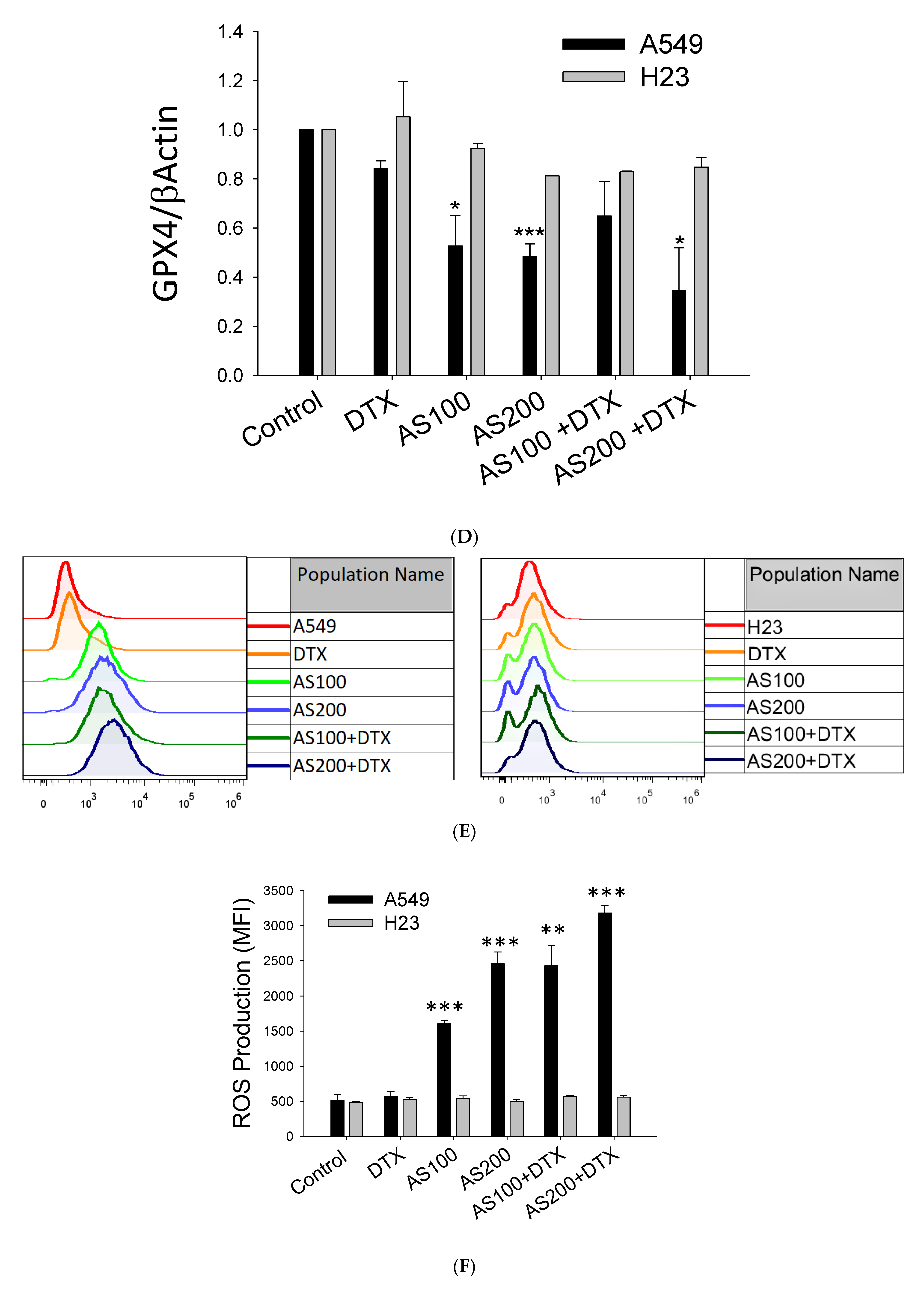
2.3. AS-Mediated Chemosensitization in H23 Is Primarily through Activation of Apoptosis, While in A549 via Ferroptosis
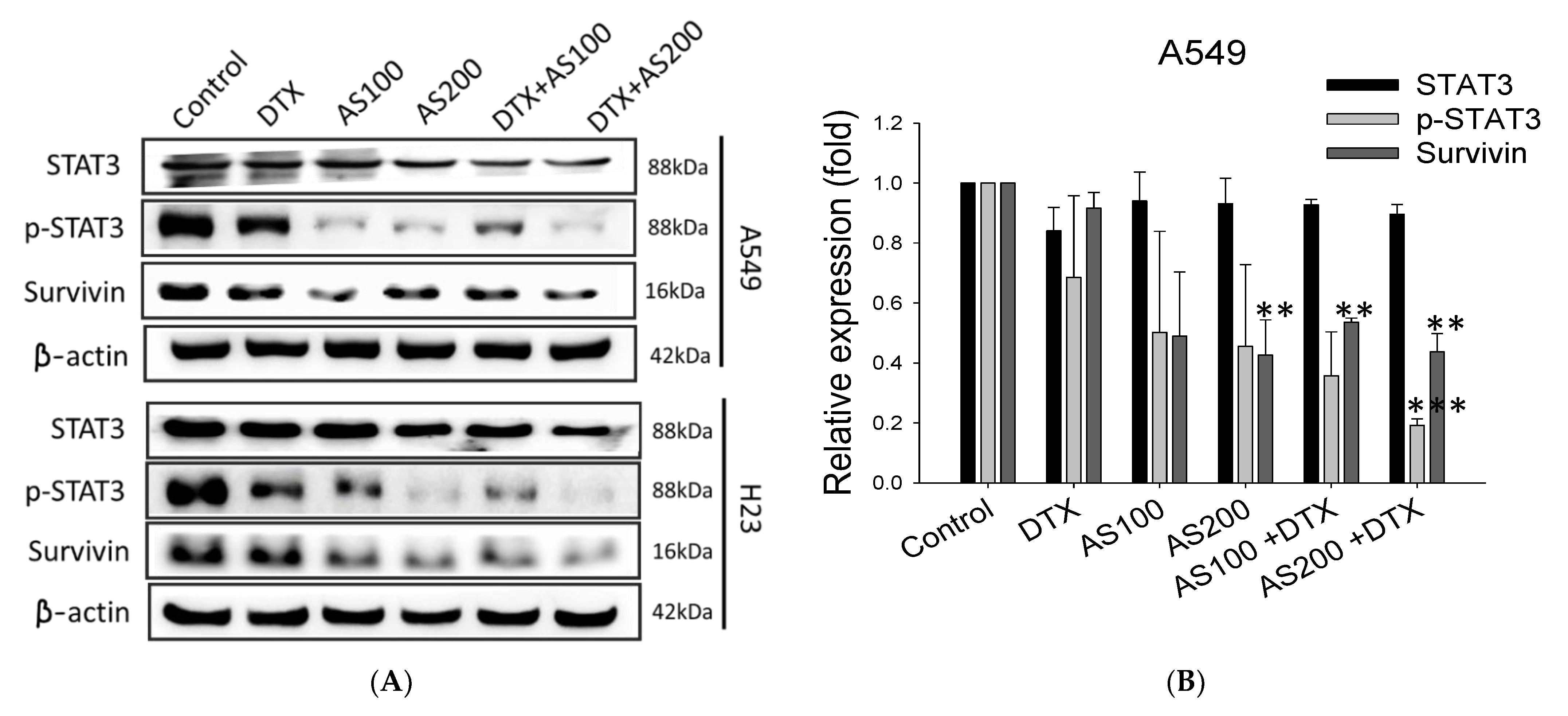
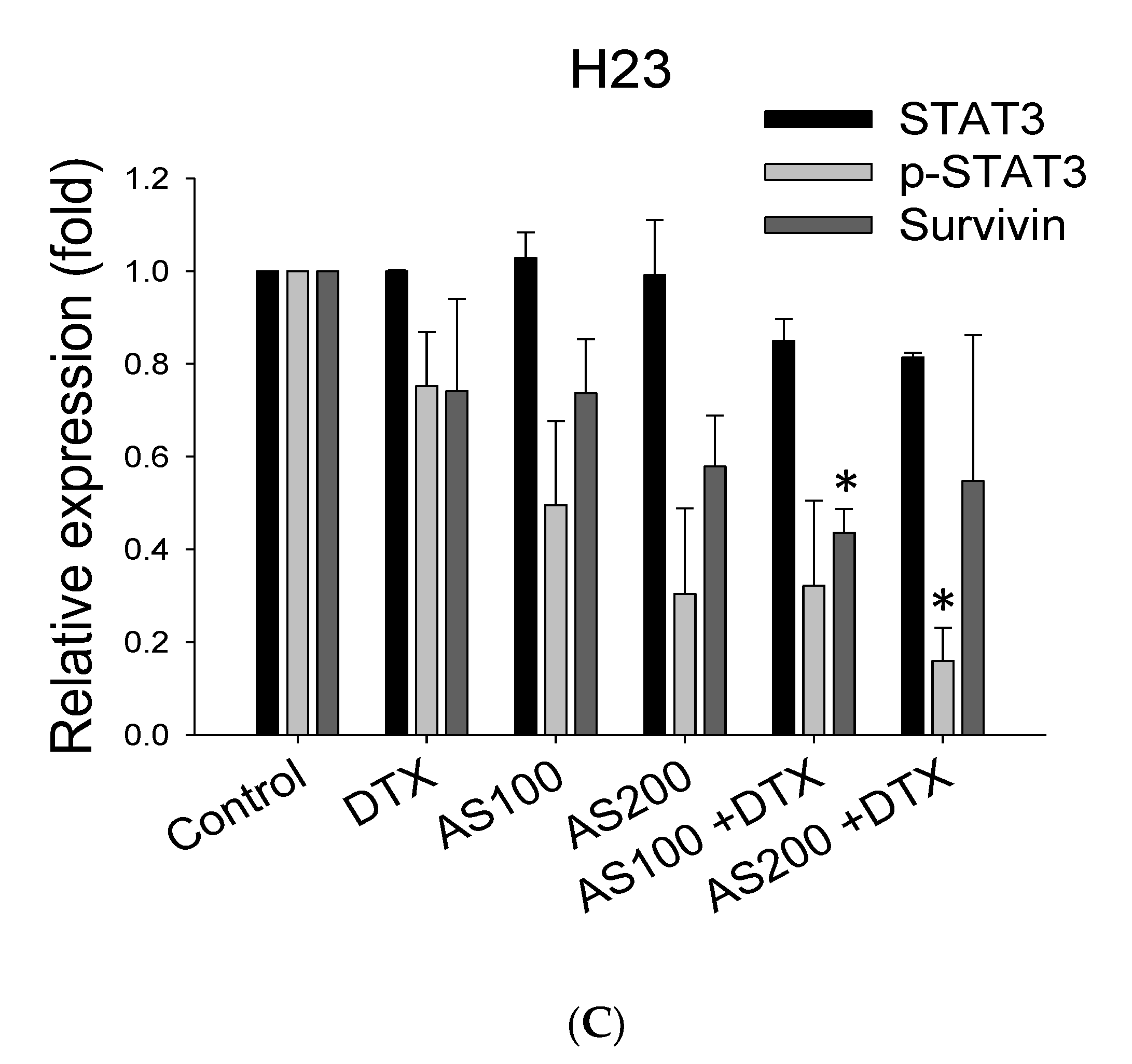
2.4. The Enhancement Functioning of AS on DTX-Induced Cell Death Associated with Inhibition of STAT3/Survivin Signaling
2.5. LC-QTOF Analysis of Ethanol Extract of A. santolinifolia
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Preparation of Extract
4.3. Cell Culture
4.4. SRB Assay
4.5. Drug Synergy
4.6. Cell Cycle Analysis
4.7. Flow Cytometric Analysis of Early and Late Apoptosis
4.8. Analysis of ROS
4.9. Western Blot Analysis
4.10. LC-MS/MS Analysis
4.11. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Smeltzer, M.P.; Wynes, M.W.; Lantuejoul, S.; Soo, R.; Ramalingam, S.S.; Varella-Garcia, M.; Meadows Taylor, M.; Richeimer, K.; Wood, K.; Howell, K.E.; et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J. Thorac. Oncol. 2020, 15, 1434–1448. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.; Cormier, J.N.; Xing, Y.; Liu, C.C.; Xia, R.; Du, X.L. Chemotherapy-associated toxicity in a large cohort of elderly patients with non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Newman, D.J. Modern traditional Chinese medicine: Identifying, defining and usage of TCM components. Adv. Pharmacol. 2020, 87, 113–158. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, B.; Puhalla, S. Docetaxel: A tubulin-stabilizing agent approved for the management of several solid tumors. Drugs Today 2006, 42, 265–279. [Google Scholar] [CrossRef]
- Saloustros, E.; Georgoulias, V. Docetaxel in the treatment of advanced non-small-cell lung cancer. Expert Rev. Anticancer Ther. 2008, 8, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.; Paganelli, G.; Tesei, A.; Zoli, W.; Giordano, E.; Calistri, D. Specific Biomarkers Are Associated with Docetaxeland Gemcitabine-Resistant NSCLC Cell Lines. Transl. Oncol. 2012, 5, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Ronnenberg, K. Reproductive Ecology of Two Common Woody Species, Juniperus Sabina and Artemisia Santolinifolia, in Mountain Steppes of Southern Mongolia; Institut für Biologie der Martin-Luther-Universität Halle-Wittenberg at Digital Commons@ University of Nebraska-Lincoln: Halle, German, 2005. [Google Scholar]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatar, S.; Dung, N.X.; Karashawa, D. Essential oil composition of some Mongolian Artemisia species. J. Essent. Oil Bear. Plants 2003, 6, 203–206. [Google Scholar] [CrossRef]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.C.; Seca, A.M.L. Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef]
- Tan, R.X.; Zheng, W.F.; Tang, H.Q. Biologically active substances from the genus Artemisia. Planta Med. 1998, 64, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A promising plant for the treatment of cancer. Bioorg. Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Jung, E.J.; Go, S.I.; Jeong, B.K.; Kim, G.S.; Jung, J.M.; Hong, S.C.; Kim, C.W.; Kim, H.J.; Lee, W.S. Polyphenols Extracted from Artemisia annua L. Exhibit Anti-Cancer Effects on Radio-Resistant MDA-MB-231 Human Breast Cancer Cells by Suppressing Stem Cell Phenotype, beta-Catenin, and MMP-9. Molecules 2020, 25, 1916. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.; Mirzaie-Asl, A.; Saidijam, M.; Moradi, M. Methanolic extract of Artemisia absinthium prompts apoptosis, enhancing expression of Bax/Bcl-2 ratio, cell cycle arrest, caspase-3 activation and mitochondrial membrane potential destruction in human colorectal cancer HCT-116 cells. Mol. Biol. Rep. 2020, 47, 8831–8840. [Google Scholar] [CrossRef]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Somintara, S.; Leardkamolkarn, V.; Suttiarporn, P.; Mahatheeranont, S. Anti-Tumor and Immune Enhancing Activities of Rice Bran Gramisterol on Acute Myelogenous Leukemia. PLoS ONE 2016, 11, e0146869. [Google Scholar] [CrossRef] [Green Version]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, K.; Nahar, K.; Khan, M.; Hasan, C. Phytochemical and biological studies on Nephelium longan. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2007, 6, 68–72. [Google Scholar]
- Zhang, Q.; Huang, N.; Wang, J.; Luo, H.; He, H.; Ding, M.; Deng, W.Q.; Zou, K. The H+/K+-ATPase inhibitory activities of Trametenolic acid B from Trametes lactinea (Berk.) Pat, and its effects on gastric cancer cells. Fitoterapia 2013, 89, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wang, J.; He, H.; Liu, H.; Yan, X.; Zou, K. Trametenolic acid B reverses multidrug resistance in breast cancer cells through regulating the expression level of P-glycoprotein. Phytother. Res. 2014, 28, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, A.; He, H.; She, X.; He, Y.; Li, S.; Liu, L.; Luo, T.; Huang, N.; Luo, H.; et al. Trametenolic acid B protects against cerebral ischemia and reperfusion injury through modulation of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed. Pharmacother. 2019, 112, 108692. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Daaboul, H.; Hajj, G.; Jabbour, A.; Kassem, N. Antitumor activity of a new cholesterol derivative (24-ethyl-cholestane-3β, 5α, 6α-triol) in solid tumors. J. Clin. Oncol. 2009, 27, e13541. [Google Scholar] [CrossRef]
- Loumouamou, A.N.; Bikindou, K.; Ntalani, H.; Silou, T.; Chalard, P.; Danton, O.; Delort, L.; Decombat, C.; Caldefie-Chezet, F.; Rubat-Coudert, C. Evaluation of the Correlationbetween The Chemical Profile And The Antalgic And Anti-Proliferative Activities Of Essential Oil Of Elionurus Hensii K. Schum 2017, 5, 41–46. [Google Scholar]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Harada, D.; Takigawa, N.; Kiura, K. The Role of STAT3 in Non-Small Cell Lung Cancer. Cancers 2014, 6, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Mohrherr, J.; Uras, I.Z.; Moll, H.P.; Casanova, E. STAT3: Versatile Functions in Non-Small Cell Lung Cancer. Cancers 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Gritsko, T.; Williams, A.; Turkson, J.; Kaneko, S.; Bowman, T.; Huang, M.; Nam, S.; Eweis, I.; Diaz, N.; Sullivan, D.; et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin. Cancer Res. 2006, 12, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altieri, D.C. Validating survivin as a cancer therapeutic target. Nat. Rev. Cancer 2003, 3, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, D.; Perez-Hernandez, M.; Korrodi-Gregorio, L.; Quesada, R.; Ramos, R.; Baixeras, N.; Perez-Tomas, R.; Soto-Cerrato, V. The Natural-Based Antitumor Compound T21 Decreases Survivin Levels through Potent STAT3 Inhibition in Lung Cancer Models. Biomolecules 2019, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Yan, X.M.; Wang, J.Z.; She, X.X.; Yu, H.L.; He, H.B.; Shi, L.; Li, L.E. Trametenolic acid B induces the programmed death of human gastric cancer HGC-27 cells. Chin. Tradit. Pat. Med. 2017, 39, 33–39. [Google Scholar]
- Houghton, P.; Fang, R.; Techatanawat, I.; Steventon, G.; Hylands, P.J.; Lee, C.C. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 2007, 42, 377–387. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cell Line | AS (µg/mL) | DTX 0.0 nM | DTX 0.5 nM | DTX 1 nM | DTX 2.5 nM |
|---|---|---|---|---|---|
| A549 | 0.0 | 8.8 ± 6.5 | 23.7 ± 2.7 | 47.1 ± 1.9 | |
| 100 | 18.4 ± 3.4 | 30.9 ± 1.9 (0.96) | 40.2 ± 1.1 (0.97) | 45.7 ± 3.5(1.09) | |
| 200 | 37.3 ± 1.4 | 58.7 ± 3.5 (0.68) | 59.3 ± 1.5 (0.81) | 65.4 ± 0.5 (1.04) | |
| H23 | 0.0 | 20.8 ± 4.4 | 36.1 ± 5.7 | 42.2 ± 5.8 | |
| 100 | 14.8 ± 3.3 | 37.2 ± 3.0 (0.74) | 42.1 ± 1.7 (0.82) | 49.3 ± 4.4 (1.01) | |
| 200 | 31.2 ± 2.7 | 47.2 ± 2.8 (0.78) | 53.1 ± 2.2 (0.74) | 54.4 ± 1.4 (1.02) |
| No. | Compound Name | Compound Formula | Observed RT (min) | Observed m/z | Mass Error (mDa) | References |
|---|---|---|---|---|---|---|
| 1. | Gramisterol | C29H48O | 25.97 | 413.3777 | −0.1 | [23,24] |
| 2. | Momor-cerebroside I | C48H93NO10 | 25.97 | 844.6872 | 0 | [25] |
| 3. | Δ7-Stigmasterol | C29H46O | 22.48 | 411.3622 | 0 | - |
| 4. | Trametenolic acid | C30H48O3 | 22.49 | 457.3677 | 0.1 | [26,27,28] |
| 5. | 24-Ethyl cholesterol | C29H52O | 25.98 | 415.3921 | −1.3 | [29] |
| 6. | Parkeol | C30H50O | 26 | 427.3925 | −0.9 | - |
| 7. | Aristolone | C15H22O | 25.29 | 219.1745 | 0.2 | [30] |
| 8. | Siraitic acid C | C29H44O5 | 26 | 441.298 | −1.9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batbold, U.; Liu, J.-J. Artemisia santolinifolia-Mediated Chemosensitization via Activation of Distinct Cell Death Modes and Suppression of STAT3/Survivin-Signaling Pathways in NSCLC. Molecules 2021, 26, 7200. https://doi.org/10.3390/molecules26237200
Batbold U, Liu J-J. Artemisia santolinifolia-Mediated Chemosensitization via Activation of Distinct Cell Death Modes and Suppression of STAT3/Survivin-Signaling Pathways in NSCLC. Molecules. 2021; 26(23):7200. https://doi.org/10.3390/molecules26237200
Chicago/Turabian StyleBatbold, Uyanga, and Jun-Jen Liu. 2021. "Artemisia santolinifolia-Mediated Chemosensitization via Activation of Distinct Cell Death Modes and Suppression of STAT3/Survivin-Signaling Pathways in NSCLC" Molecules 26, no. 23: 7200. https://doi.org/10.3390/molecules26237200
APA StyleBatbold, U., & Liu, J.-J. (2021). Artemisia santolinifolia-Mediated Chemosensitization via Activation of Distinct Cell Death Modes and Suppression of STAT3/Survivin-Signaling Pathways in NSCLC. Molecules, 26(23), 7200. https://doi.org/10.3390/molecules26237200







