New Sustainable Synthetic Routes to Cyclic Oxyterpenes Using the Ecocatalyst Toolbox
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Ecocatalyts Toolbox
2.1.1. Preparation of the Ecocatalysts
2.1.2. Characterization of the Ecocatalysts
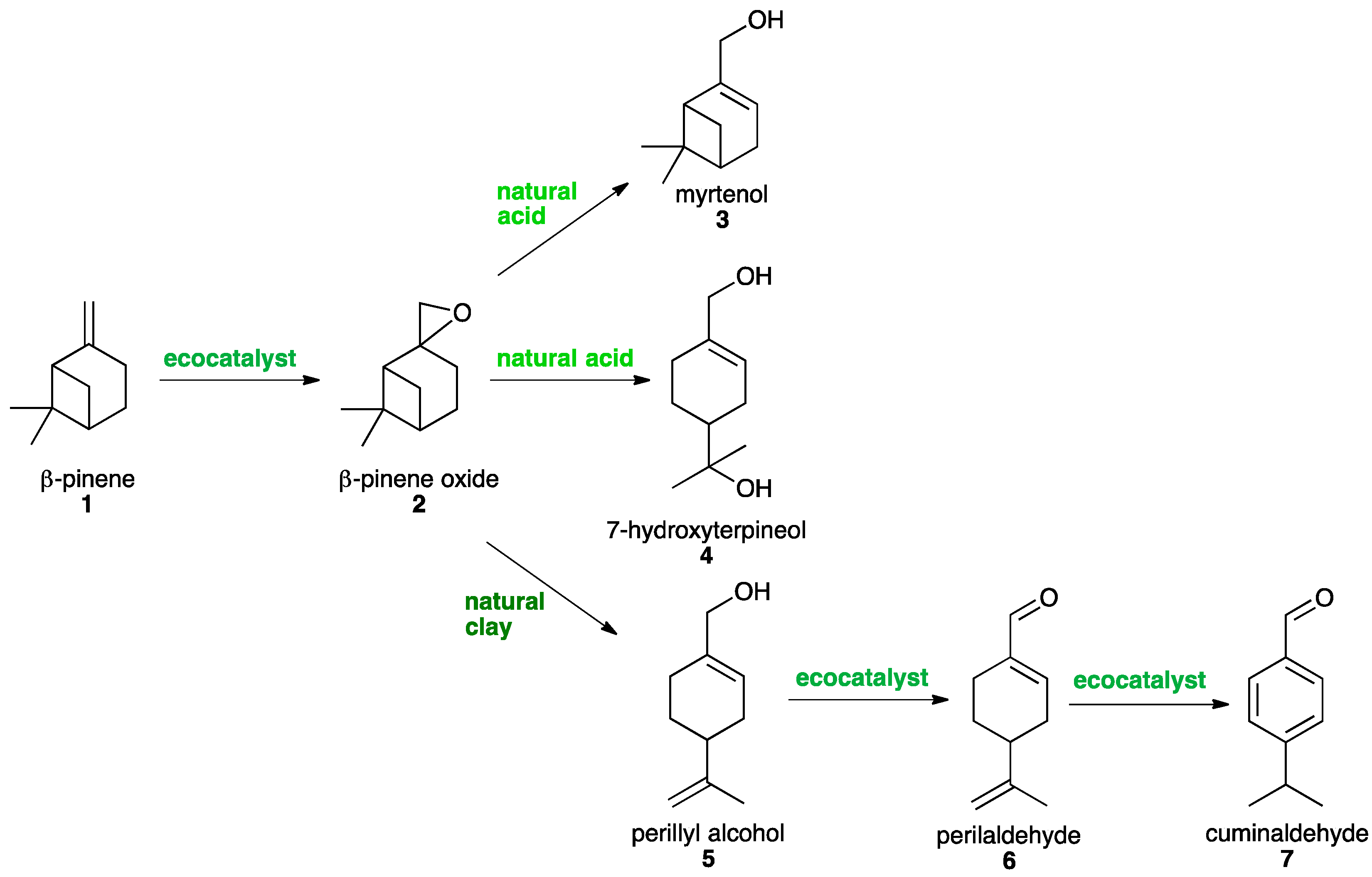
2.2. Syntheses of Oxyterpenes
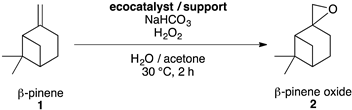
Selective Epoxidation of ß-Pinene 1 into ß-Pinene Oxide 2
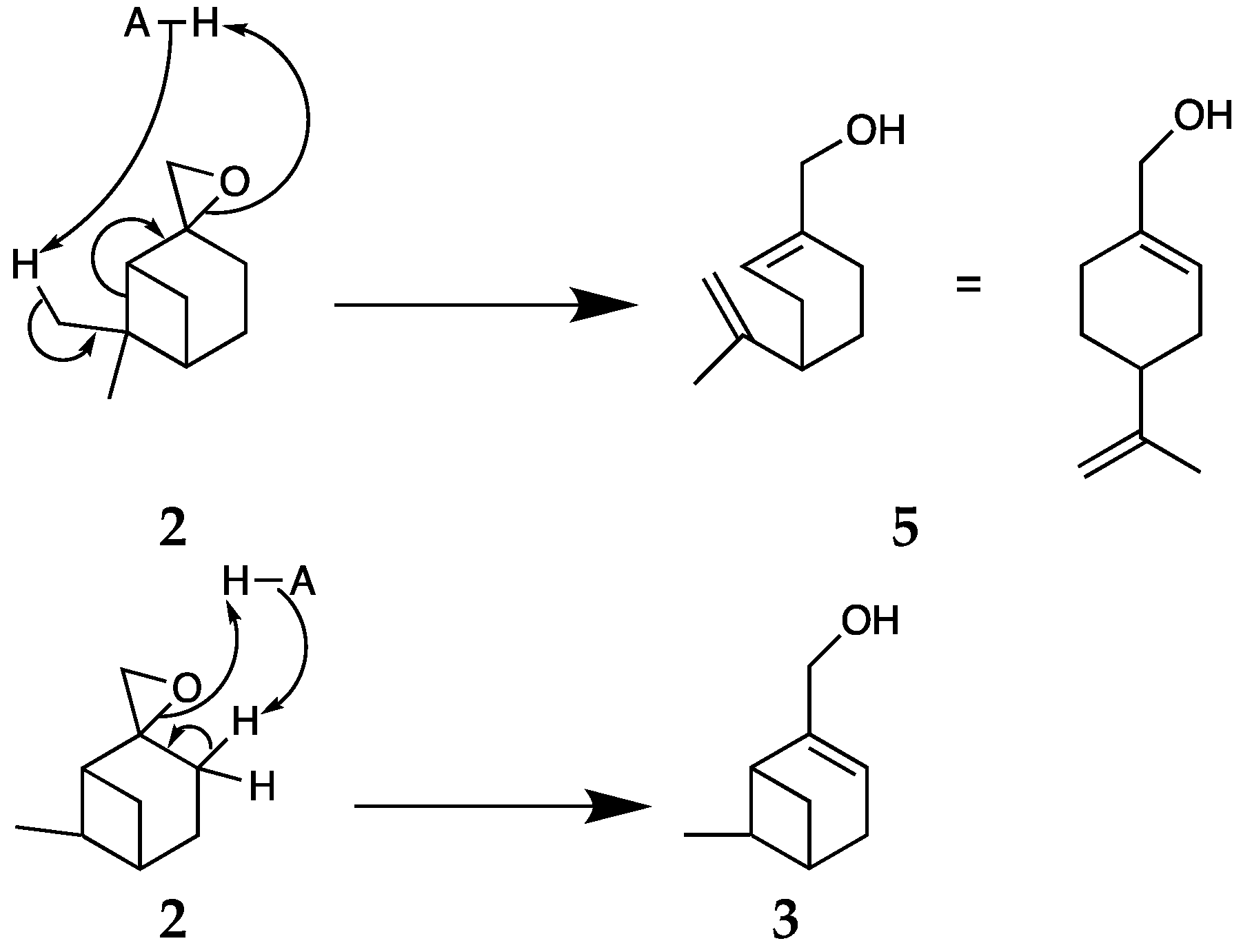
2.3. Selective Opening of ß-Pinene Oxide 2
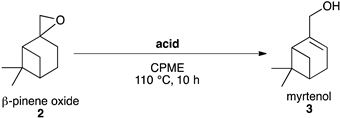
2.3.1. Synthesis of Myrtenol 3
2.3.2. Synthesis of 7-Hydroxy-α-terpineol 4
2.3.3. Synthesis of Perillyl Alcohol 5
2.4. Synthesis of Perillaldehyde 6
2.5. Synthesis of Cuminaldehyde 7
3. Materials and Methods
3.1. General Information
3.2. General Procedures
3.2.1. Preparation of the Ecocatalysts
Harvest of Pistia Stratiotes
Transformation of Biomass into Eco-CaMnOx-Gg and Eco-MnOx-Ps
Preparation of Green HCl (6 M)
Preparation of Eco-MnCl-Gg and Eco-MnCl-Ps
Preparation of Eco-NaMnOx-Gg and Eco-NaMnOx-Ps
Preparation of Eco-MnOx-Ps Supported on MK10
3.2.2. Preparation of Citric Acid Functionalised Coffee Grounds
3.2.3. General Procedures for the Synthesis of Oxyterpenes
Synthesis of ß-Pinene Oxide 2
Synthesis of Myrtenol 3
One pot Synthesis of 7-Hydroxy-α-terpineol 4 from β-Pinene 1
Sequential Synthesis of 7-Hydroxy-α-terpineol 4 from β-Pinene Oxide 2
Synthesis of Perillyl Alcohol 5
Synthesis of Perillaldehyde 6
Synthesis of Cuminaldehyde 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gomes, B.S.; Neto, B.P.S.; Lopes, E.M.; Cunha, F.V.M.; Araújo, A.R.; Wanderley, C.W.S.; Wong, D.V.T.; Júnior, R.C.P.L.; Ribeiro, R.A.; Sousa, D.P.; et al. Anti-Inflammatory Effect of the Monoterpene Myrtenol Is Dependent on the Direct Modulation of Neutrophil Migration and Oxidative Stress. Chem. Biol. Interact. 2017, 273, 73–81. [Google Scholar] [CrossRef]
- Moreira, M.R.; Salvadori, M.G.; de Almeida, A.A.; de Sousa, D.P.; Jordán, J.; Satyal, P.; de Freitas, R.M.; de Almeida, R.N. Anxiolytic-like Effects and Mechanism of (-)-Myrtenol: A Monoterpene Alcohol. Neurosci. Lett. 2014, 579, 119–124. [Google Scholar] [CrossRef] [PubMed]
- de Britto, R.M.; da Silva-Neto, J.A.; Mesquita, T.R.R.; de Vasconcelos, C.M.L.; de Almeida, G.K.M.; de Jesus, I.C.G.; dos Santos, P.H.; Souza, D.S.; Miguel-dos-Santos, R.; de Sá, L.A.; et al. Myrtenol Protects against Myocardial Ischemia-Reperfusion Injury through Antioxidant and Anti-Apoptotic Dependent Mechanisms. Food Chem. Toxicol. 2018, 111, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Belanger, J.T. Perillyl Alcohol: Applications in Oncology. Altern. Med. Rev. 1998, 3, 448–457. [Google Scholar] [PubMed]
- Chen, T.C.; Fonseca, C.O.D.; Schönthal, A.H. Preclinical Development and Clinical Use of Perillyl Alcohol for Chemoprevention and Cancer Therapy. Am. J. Cancer Res. 2015, 5, 1580–1593. [Google Scholar]
- Benedito, R.B.; Alves, M.F.; Pereira, W.B.; de Arruda Torres, P.; Costa, J.P.; da Rocha Tomé, A.; de Cássia da Silveira e Sá, R.; de Sousa, D.P.; Ferreira, P.M.P.; de Freitas, R.M.; et al. Perillyl Alcohol: Antinociceptive Effects and Histopathological Analysis in Rodent Brains. Nat. Prod. Commun. 2017, 12, 1934578X1701200902. [Google Scholar] [CrossRef]
- Chastain, D.E.; Sanders, W.E.S., Jr.; Sanders, C.C. Using Perillyl Alcohol to Kill Bacteria and Yeasts. U.S. Patent US5110832A, 5 May 1992. [Google Scholar]
- Zafeer, M.F.; Firdaus, F.; Ahmad, F.; Ullah, R.; Anis, E.; Waseem, M.; Ali, A.; Hossain, M.M. Perillyl Alcohol Alleviates Amyloid-β Peptides-Induced Mitochondrial Dysfunction and Cytotoxicity in SH-SY5Y Cells. Int. J. Biol. Macromol. 2018, C, 1029–1038. [Google Scholar] [CrossRef]
- Rodriguez, A.A.M.; Carvalho, L.J.M.; Kimura, E.A.; Katzin, A.M. Perillyl Alcohol Exhibits in Vitro Inhibitory Activity against Plasmodium Falciparum and Protects against Experimental Cerebral Malaria. Int. J. Antimicrob. Agents 2018, 51, 370–377. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; John Wiley & Sons: New York, NY, USA, 2016; ISBN 978-3-527-33160-4. [Google Scholar]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on P-Mentha-1,8-Dien-7-Ol. Food Chem. Toxicol. 2008, 46, S197–S200. [Google Scholar] [CrossRef] [PubMed]
- O’Brien-Nabors, L. Alternative Sweeteners; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4398-4615-5. [Google Scholar]
- Omari-Siaw, E.; Zhu, Y.; Wang, H.; Peng, W.; Firempong, C.K.; Wang, Y.W.; Cao, X.; Deng, W.; Yu, J.; Xu, X. Hypolipidemic Potential of Perillaldehyde-Loaded Self-Nanoemulsifying Delivery System in High-Fat Diet Induced Hyperlipidemic Mice: Formulation, in Vitro and in Vivo Evaluation. Eur. J. Pharm. Sci. 2016, 85, 112–122. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Fu, Q.; Ma, S. Perillaldehyde Attenuates Cerebral Ischemia-Reperfusion Injury-Triggered Overexpression of Inflammatory Cytokines via Modulating Akt/JNK Pathway in the Rat Brain Cortex. Biochem. Biophys. Res. Commun. 2014, 454, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-W.; Wang, S.-Y.; Ma, Z.-Q.; Li, R.-P.; Li, S.-S.; Xue, J.-S.; Li, W.; Niu, X.-X.; Yan, L.; Zhang, X.; et al. Effects of Perillaldehyde on Alternations in Serum Cytokines and Depressive-like Behavior in Mice after Lipopolysaccharide Administration. Pharmacol. Biochem. Behav. 2014, 116, 1–8. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zeng, H.; Li, Z.; Zhang, P.; Tessema, A.; Peng, X. Efficacy and Possible Mechanisms of Perillaldehyde in Control of Aspergillus Niger Causing Grape Decay. Int. J. Food Microbiol. 2015, 202, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Krist, S.; Buchbauer, G. Antimicrobial Effect of Trans-Cinnamaldehyde, (−)-Perillaldehyde, (−)-Citronellal, Citral, Eugenol and Carvacrol on Airborne Microbes Using an Airwasher. Biol. Pharm. Bull. 2006, 29, 2292–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, M.; Mandal, S. Chapter 42—Cumin (Cuminum cyminum L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 377–383. ISBN 978-0-12-416641-7. [Google Scholar]
- Hajlaoui, H.; Mighri, H.; Noumi, E.; Snoussi, M.; Trabelsi, N.; Ksouri, R.; Bakhrouf, A. Chemical Composition and Biological Activities of Tunisian Cuminum Cyminum L. Essential Oil: A High Effectiveness against Vibrio Spp. Strains. Food Chem. Toxicol. 2010, 48, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Rihawy, M.S.; Bakraji, E.H.; Odeh, A. PIXE and GC–MS Investigation for the Determination of the Chemical Composition of Syrian Cuminum cyminum L. Appl. Radiat. Isot. 2014, 86, 118–125. [Google Scholar] [CrossRef]
- Sahana, K.; Nagarajan, S.; Mohan Rao, L.J. Chapter 50—Cumin (Cuminum cyminum L.) Seed Volatile Oil: Chemistry and Role in Health and Disease Prevention. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 417–427. ISBN 978-0-12-375688-6. [Google Scholar]
- Wongkattiya, N.; Sanguansermsri, P.; Fraser, I.H.; Sanguansermsri, D. Antibacterial Activity of Cuminaldehyde on Food-Borne Pathogens, the Bioactive Component of Essential Oil from Cuminum Cyminum L. Collected in Thailand. J. Complement. Integr. Med. 2019, 16, 20180195. [Google Scholar] [CrossRef] [PubMed]
- Ebada, M.E. Cuminaldehyde: A Potential Drug Candidate. J. Pharmcol. Clin. Res. 2017, 2, 555585. [Google Scholar] [CrossRef]
- Corma, A.; Renz, M.; Susarte, M. Transformation of Biomass Products into Fine Chemicals Catalyzed by Solid Lewis- and Brønsted-Acids. Top. Catal. 2009, 52, 1182–1189. [Google Scholar] [CrossRef]
- Vyskočilová, E.; Malý, M.; Aho, A.; Krupka, J.; Červený, L. The Solvent Effect in β-Pinene Oxide Rearrangement. Reac. Kinet. Mech. Cat. 2016, 118, 235–246. [Google Scholar] [CrossRef]
- Vyskočilová, E.; Dušek, J.; Babirádová, M.; Krupka, J.; Paterová, I.; Červený, L. Perillyl Alcohol Preparation from β-Pinene Oxide Using Fe-Modified Zeolite Beta. Res. Chem. Intermed. 2018, 44, 3971–3984. [Google Scholar] [CrossRef]
- Luštická, I.; Jansa, P.; Abelová, L.; Vyskočilová-Leitmannová, E.; Červený, L. Immobilization of Pyridinium Nitrate and Its Application for the Catalytic Converting of β-Pinene Oxide. Reac. Kinet. Mech. Cat. 2013, 108, 69–79. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Gelves, J.-F.; Dorkis, L.; Márquez, M.-A.; Villa, A.-L. Ring-Opening of β-Pinene Epoxide into High-Added Value Products over Colombian Natural Zeolite. Microporous Mesoporous Mater. 2019, 287, 114–123. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Mejía, S.M.; Villa, A.L. Reaction Mechanism of the Isomerization of Monoterpene Epoxides with Fe3+ as Active Catalytic Specie: A Computational Approach. J. Phys. Chem. A 2020, 124, 3761–3769. [Google Scholar] [CrossRef]
- Escande, V.; Poullain, C.; Clavé, G.; Petit, E.; Masquelez, N.; Hesemann, P.; Grison, C. Bio-Based and Environmental Input for Transfer Hydrogenation Using EcoNi(0) Catalyst in Isopropanol. Appl. Catal. B 2017, 210, 495–503. [Google Scholar] [CrossRef]
- Bihanic, C.; Richards, K.; Olszewski, T.K.; Grison, C. Eco-Mn Ecocatalysts: Toolbox for Sustainable and Green Lewis Acid Catalysis and Oxidation Reactions. ChemCatChem 2019, 12, 1529–1545. [Google Scholar] [CrossRef]
- Bihanic, C.; Diliberto, S.; Pelissier, F.; Petit, E.; Boulanger, C.; Grison, C. Eco-CaMnOx: A Greener Generation of Eco-Catalysts for Eco-Friendly Oxidation Processes. ACS Sustain. Chem. Eng. 2020, 8, 4044–4057. [Google Scholar] [CrossRef]
- Clavé, G.; Garoux, L.; Boulanger, C.; Hesemann, P.; Grison, C. Ecological Recycling of a Bio-Based Catalyst for Cu Click Reaction: A New Strategy for a Greener Sustainable Catalysis. ChemistrySelect 2016, 1, 1410–1416. [Google Scholar] [CrossRef]
- Clavé, G.; Pelissier, F.; Campidelli, S.; Grison, C. Ecocatalyzed Suzuki Cross Coupling of Heteroaryl Compounds. Green Chem. 2017, 19, 4093–4103. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Adler, P.; Grison, C. Bio-Based Catalysts from Biomass Issued after Decontamination of Effluents Rich in Copper—An Innovative Approach towards Greener Copper-Based Catalysis. Catalysts 2019, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Stanovych, A.; Balloy, M.; Olszewski, T.K.; Petit, E.; Grison, C. Depollution of Mining Effluents: Innovative Mobilization of Plant Resources. Environ. Sci. Pollut. Res. 2019, 26, 19327–19334. [Google Scholar] [CrossRef] [PubMed]
- Grison, C.; Adler, P.; Deyris, P.-A.; Diliberto, S.; Boulanger, C. A Green Approach for the Reduction of Representative Aryl Functional Groups Using Palladium Ecocatalysts. Green Chem. Lett. Rev. 2021, 14, 234–245. [Google Scholar] [CrossRef]
- Cases, L.; Adler, P.; Pelissier, F.; Diliberto, S.; Boulanger, C.; Grison, C. New Biomaterials for Ni Biosorption Turned into Catalysts for Suzuki–Miyaura Cross Coupling of Aryl Iodides in Green Conditions. RSC Adv. 2021, 11, 28085–28091. [Google Scholar] [CrossRef]
- Grison, C.; Lock Toy Ki, Y. Ecocatalysis, a New Vision of Green and Sustainable Chemistry. Curr. Opin. Green Sustain. Chem. 2021, 29, 100461. [Google Scholar] [CrossRef]
- Deyris, P.-A.; Grison, C. Nature, Ecology and Chemistry: An Unusual Combination for a New Green Catalysis, Ecocatalysis. Curr. Opin. Green Sustain. Chem. 2018, 10, 6. [Google Scholar] [CrossRef]
- Grison, C. Special Issue in Environmental Science and Pollution Research: Combining Phytoextraction and EcoCatalysis: An Environmental, Ecological, Ethic and Economic Opportunity. Environ. Sci. Pollut. Res. 2015, 22, 5589–5698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grison, C.; Escande, V. Use of Certain Manganese-Accumulating Plants for Carrying out Organic Chemistry Reactions. U.S. Patent US10702860B2, 7 July 2020. [Google Scholar]
- Grison, C.; Carrasco, D.; Stanovych, A. Procede De Preparation De Materiau D’origine Vegetale Riche En Acides Phenoliques, Comprenant Au Moins Un Metal, Pour La Mise En Oeuvre De Reactions De Synthese Organique. WO2018178374A1, 4 October 2018. [Google Scholar]
- Grison, C. Procédé de Préparation D’acides Mineraux D’origine Naturelle et Utilisation des Acides Obtenus en Synthèse Organique. WO2015159033A1, 22 October 2015. [Google Scholar]
- Garel, C.; Fonda, E.; Michalowicz, A.; Diliberto, S.; Boulanger, C.; Petit, E.; Legrand, Y.M.; Poullain, C.; Grison, C. Structure and Composition of First Biosourced Mn-Rich Catalysts with a Unique Vegetal Footprint. Mater. Today Sustain. 2019, 5, 100020. [Google Scholar] [CrossRef]
- Neuenschwander, U.; Meier, E.; Hermans, I. Peculiarities of β-Pinene Autoxidation. ChemSusChem 2011, 4, 1613–1621. [Google Scholar] [CrossRef]
- Coelho, J.V.; Oliveira, L.C.A.; Moura, F.C.C.; de Souza, P.P.; Silva, C.A.; Batista, K.B.; Silva, M.J. da β-Pinene Oxidation by Hydrogen Peroxide Catalyzed by Modified Niobium-MCM. Appl. Catal. A-Gen. 2012, 419–420, 215–220. [Google Scholar] [CrossRef]
- da Silva, M.J.; Vieira, L.M.M.; Oliveira, A.A.; Ribeiro, M.C. Novel Effect of Palladium Catalysts on Chemoselective Oxidation of β-Pinene by Hydrogen Peroxide. Monatsh. Chem. 2013, 144, 321–326. [Google Scholar] [CrossRef]
- Schröder, K.; Junge, K.; Spannenberg, A.; Beller, M. Design of a Bio-Inspired Imidazole-Based Iron Catalyst for Epoxidation of Olefins: Mechanistic Insights. Catal. Today 2010, 157, 364–370. [Google Scholar] [CrossRef]
- Constantino, M.G.; Júnior, V.L.; Invernize, P.R.; Filho, L.C.; José da Silva, G.V. Opening of Epoxide Rings Catalyzed by Niobium Pentachloride. Synth. Commun. 2007, 37, 3529–3539. [Google Scholar] [CrossRef]
- Fomenko, V.V.; Bakhvalov, O.V.; Kollegov, V.F.; Salakhutdinov, N.F. Catalytic Epoxidation of β-Pinene with Aqueous Hydrogen Peroxide. Russ. J. Gen. Chem. 2017, 87, 1675–1679. [Google Scholar] [CrossRef]
- Zheng, W.; Tan, R.; Zhao, L.; Chen, Y.; Xiong, C.; Yin, D. Mn2+/Graphene Oxide Nanocomposite Efficiently Catalyzes the Epoxidation of Alkenes with H2O2. RSC Adv. 2014, 4, 11732–11739. [Google Scholar] [CrossRef]
- McKillop, A.; Kabalka, G.W.; Reddy, M.S. Sodium Perborate. In Encyclopedia of Reagents for Organic Synthesis; American Cancer Society: Atlanta, GA, USA, 2008; ISBN 978-0-470-84289-8. [Google Scholar]
- Zheng, W.; Hu, H.; Chen, Y.; Tan, R.; Yin, D. Diamine-Decorated Graphene Oxide with Immobilized Gold Nanoparticles of Small Size for Alkenes Epoxidation with H2O2. Catal. Lett. 2019, 149, 3328–3337. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; da Silva, M.L.; da Silva, M.J. Palladium-Catalysed Oxidation of Bicycle Monoterpenes by Hydrogen Peroxide in Acetonitrile Solutions: A Metal Reoxidant-Free and Environmentally Benign Oxidative Process. Catal. Lett. 2009, 130, 424–431. [Google Scholar] [CrossRef]
- Mouret, A.; Leclercq, L.; Mühlbauer, A.; Nardello-Rataj, V. Eco-Friendly Solvents and Amphiphilic Catalytic Polyoxometalate Nanoparticles: A Winning Combination for Olefin Epoxidation. Green Chem. 2013, 16, 269–278. [Google Scholar] [CrossRef]
- Martinez Q, H.; Amaya, Á.A.; Paez-Mozo, E.A.; Martinez O, F.; Valange, S. Photo-Assisted O-Atom Transfer to Monoterpenes with Molecular Oxygen and a DioxoMo(VI) Complex Immobilized on TiO2 Nanotubes. Catal. Today 2021, 375, 441–457. [Google Scholar] [CrossRef]
- Li, Q.; Kung, L.; Peng, S.; Li, X.; Wang, L. Synthesis of Perillaldehyde via Selective Oxidation of Perilla Alcohol. J. Nat. Sci.-Hunan Norm. Univ. 2007, 79, 64–66. [Google Scholar]
- Escande, V.; Renard, B.-L.; Grison, C. Lewis Acid Catalysis and Green Oxidations: Sequential Tandem Oxidation Processes Induced by Mn-Hyperaccumulating Plants. Environ. Sci. Pollut. Res. 2015, 22, 5633–5652. [Google Scholar] [CrossRef]


| Composition (Weight% (±%RSD)) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Ecocatalyst | Mn | Ca | Fe | Mg | Na | K | Al |
| 1 | Roots of P. stratiotes | 5.20 (4.97) | 5.65 (4.60) | 1.66 (1.64) | 1.58 (0.83) | 2.85 (2.06) | 5.19 (2.60) | 0.36 (0.36) |
| 2 | Eco-MnOx-Ps | 7.53 (2.45) | 10.56 (4.14) | 2.37 (0.77) | 3.54 (3.15) | 4.57 (0.37) | 9.64 (3.32) | 0.56 (0.04) |
| 3 | Eco-MnCl-Ps | 7.03 (2.07) | 5.53 (2.67) | 2.08 (1.02) | 1.47 (0.74) | 2.18 (0.88) | 5.50 (1.74) | 0.40 (0.20) |
| 4 | Eco-NaMnOx-Ps | 15.94 (2.70) | 13.75 (4.75) | 5.27 (0.39) | 3.59 (0.56) | 1.24 (0.76) | 0.04 (0.05) | 0.89 (0.32) |
| Entry | Ecocatalyst | Mn | Na | Ca | Si |
|---|---|---|---|---|---|
| 1 | Eco-MnOx-Ps | MnO2, K2Mn4O8 | NaCl, KCl, K3Na(SO4)2 | CaCO3 | SiO2 |
| 2 | Eco-MnCl-Ps | K3NaMnCl6 | NaCl | - | - |
| 3 [45] | Eco-CaMgOx-Gg | Ca2Mn3O8 | K3Na(SO4)2, KCl | CaCO3 | SiO2 |
| 4 [45] | Eco-MnCl-Gg | KMnCl3 | NaCl | CaCl2 | - |
| Entry | Ecocatalyst | Catalytic Loading (Eq.) | Support | Conv. (%) b | Yield (%) b |
|---|---|---|---|---|---|
| 1 | Eco-CaMnOx-Gg | 0.005 | / | 89 | 33 |
| 2 | Eco-MnOx-Ps | 0.005 | / | >99 | 63 c |
| 3 | 0.005 | MK10 | 97 | 26 | |
| 4 | 0.01 | / | >99 | 43 | |
| 5 | Eco-MnCl-Ps | 0.005 | / | >99 | 63 |
| 6 | 0.005 | MK10 | 79 | 35 | |
| 7 | / | / | MK10 | 0 | 0 |
| Entry | Acid | Conv. (%) b | Yield (%) b | ||
|---|---|---|---|---|---|
| 3 | 4 | 5 | |||
| 1 | betaine hydrochloride | >99 | 45 (44) c | 0 | 5 |
| 2 | thiamine hydrochloride | >99 | 33 | 0 | 12 |
| 3 | acetic acid | 97 | 21 | 17 | 3 |
| 4 | ascorbic acid | >99 | 10 | 0 | 20 |

| Entry | Acid | Eq. of Acid | Conv. (%) b | Yield (%) b |
|---|---|---|---|---|
| 1 | HCl | 1 | 66 | 31 |
| 2 | 3 | >99 | 76 | |
| 3 | oxalic acid | 3 | >99 | 76(72) c |
| 4 | formic acid | 3 | >99 | 73 |
| 5 | citric acid | 3 | >99 | 65 |
| 6 | betaine hydrochloride | 3 | >99 | 75 |
| 7 | thiamine hydrochloride | 3 | >99 | 73 |
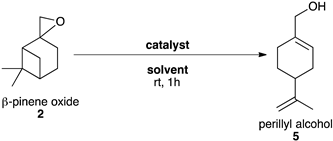
| Entry | Catalyst | Catalytic Loading | Solvent | Conv. (%) b | Yield (%) b |
|---|---|---|---|---|---|
| 1 | Eco-MnCl-Ps | 85 mg | CPME (2 mL) | >99 | 4 |
| 2 | MK10 | 85 mg | DCM (2 mL) | >99 | 26 |
| 3 | Me-THF (2 mL) | >99 | 34 | ||
| 4 | CPME (2 mL) | >99 | 28 | ||
| 5 | MK10 | 85 mg | Me-THF (50 mL) | >99 | 27 |
| 6 | CPME (50 mL) | >99 | 40(38) c | ||
| 7 | MK10 | 8.5 mg | CPME (50 mL) | >99 | 35 |
| 8 | 850 mg | >99 | 36 |
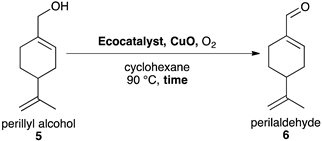
| Entry | Ecocatalyst | Catalytic Loading (Eq.) | CuO (Eq.) | Time (h) | Conv. (%) b | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | Eco-NaMnOx-Ps | 1 | - | 2 | 41 | 38 |
| 2 | 2 | - | 81 | 61 | ||
| 3 | 2 | 4 | 80 | 66(63) c | ||
| 4 | 4 | 8 | 94 | 45 | ||
| 5 | - | 4 | 0 | 0 | ||
| 6 | 2 | 4 | 4 | 84 | 61 | |
| 7 | 2 | 4 | 7 | 78 | 49 |
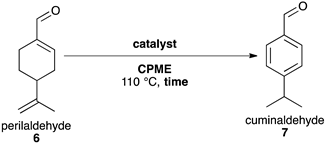
| Entry | Catalyst | CPME (mL) | Time (h) c | Conv. (%) b | Yield (%) b |
|---|---|---|---|---|---|
| 1 | Eco-MnCl-Ps | 0 | 2 | >99 | 6 |
| 2 | MK10 | >99 | 5 | ||
| 3 | Eco-MnCl-Ps + MK10 | >99 | 5 | ||
| 4 | Coffee ground-CA d | 0 | - | ||
| 5 | Eco-MnCl-Ps + Coffee ground- CA d | >99 | 9 | ||
| 6 | Eco-MnCl-Ps | 2 | 5 | >99 | 27 |
| 7 | Eco-MnCl-Ps + Coffee ground-CA d | >99 | 29 | ||
| 8 | Eco-MnCl-Ps | 10 | 27 | 96 | 22 |
| 9 | Eco-MnCl-Ps + Coffee ground-CA d | >99 | 55(53) e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bihanic, C.; Lasbleiz, A.; Regnier, M.; Petit, E.; Le Blainvaux, P.; Grison, C. New Sustainable Synthetic Routes to Cyclic Oxyterpenes Using the Ecocatalyst Toolbox. Molecules 2021, 26, 7194. https://doi.org/10.3390/molecules26237194
Bihanic C, Lasbleiz A, Regnier M, Petit E, Le Blainvaux P, Grison C. New Sustainable Synthetic Routes to Cyclic Oxyterpenes Using the Ecocatalyst Toolbox. Molecules. 2021; 26(23):7194. https://doi.org/10.3390/molecules26237194
Chicago/Turabian StyleBihanic, Camille, Arthur Lasbleiz, Morgan Regnier, Eddy Petit, Pierre Le Blainvaux, and Claude Grison. 2021. "New Sustainable Synthetic Routes to Cyclic Oxyterpenes Using the Ecocatalyst Toolbox" Molecules 26, no. 23: 7194. https://doi.org/10.3390/molecules26237194
APA StyleBihanic, C., Lasbleiz, A., Regnier, M., Petit, E., Le Blainvaux, P., & Grison, C. (2021). New Sustainable Synthetic Routes to Cyclic Oxyterpenes Using the Ecocatalyst Toolbox. Molecules, 26(23), 7194. https://doi.org/10.3390/molecules26237194






