Towards a Comprehensive Characterization of the Low-Temperature Autoxidation of Di-n-Butyl Ether
Abstract
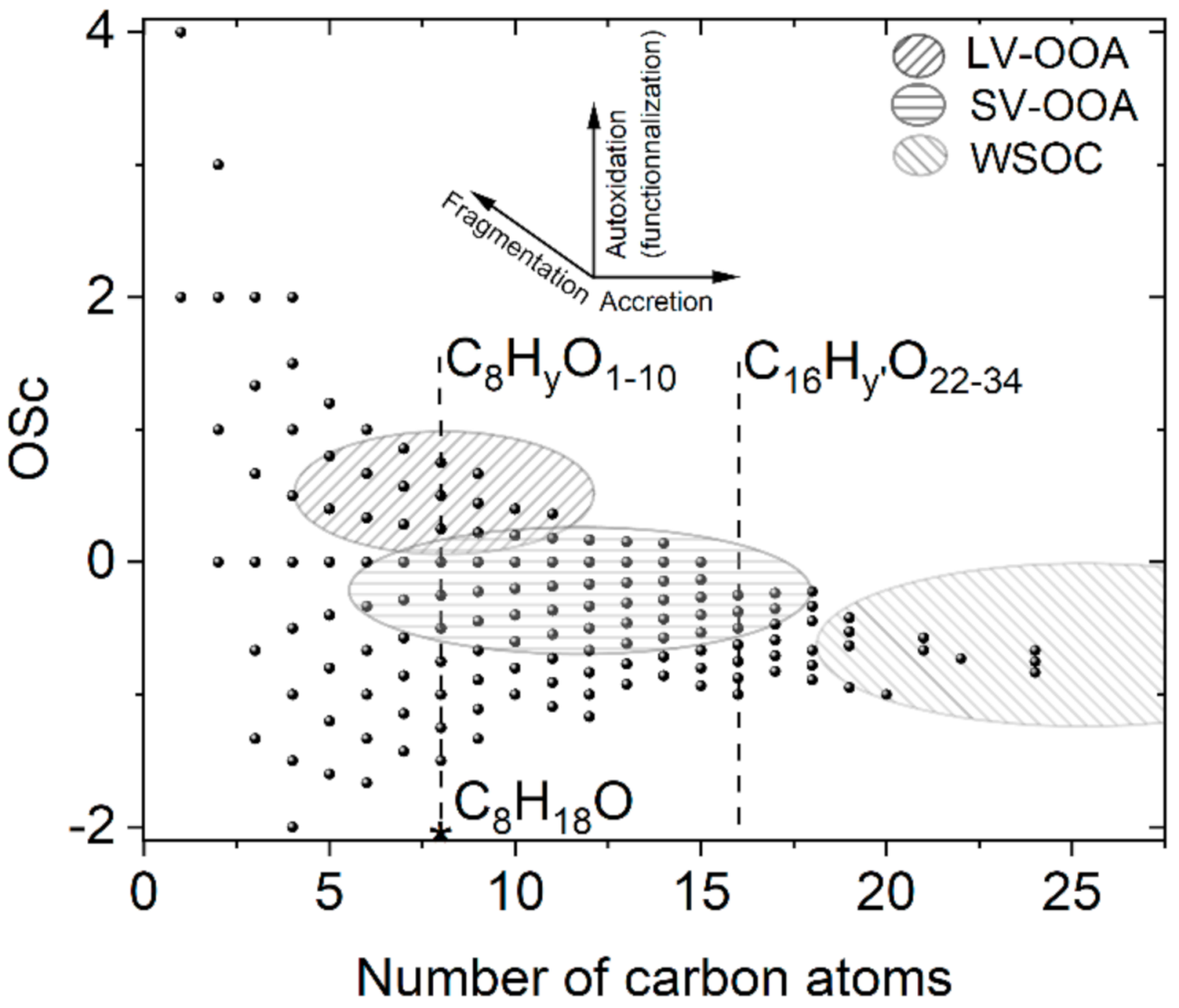
:1. Introduction
2. Results and Discussion
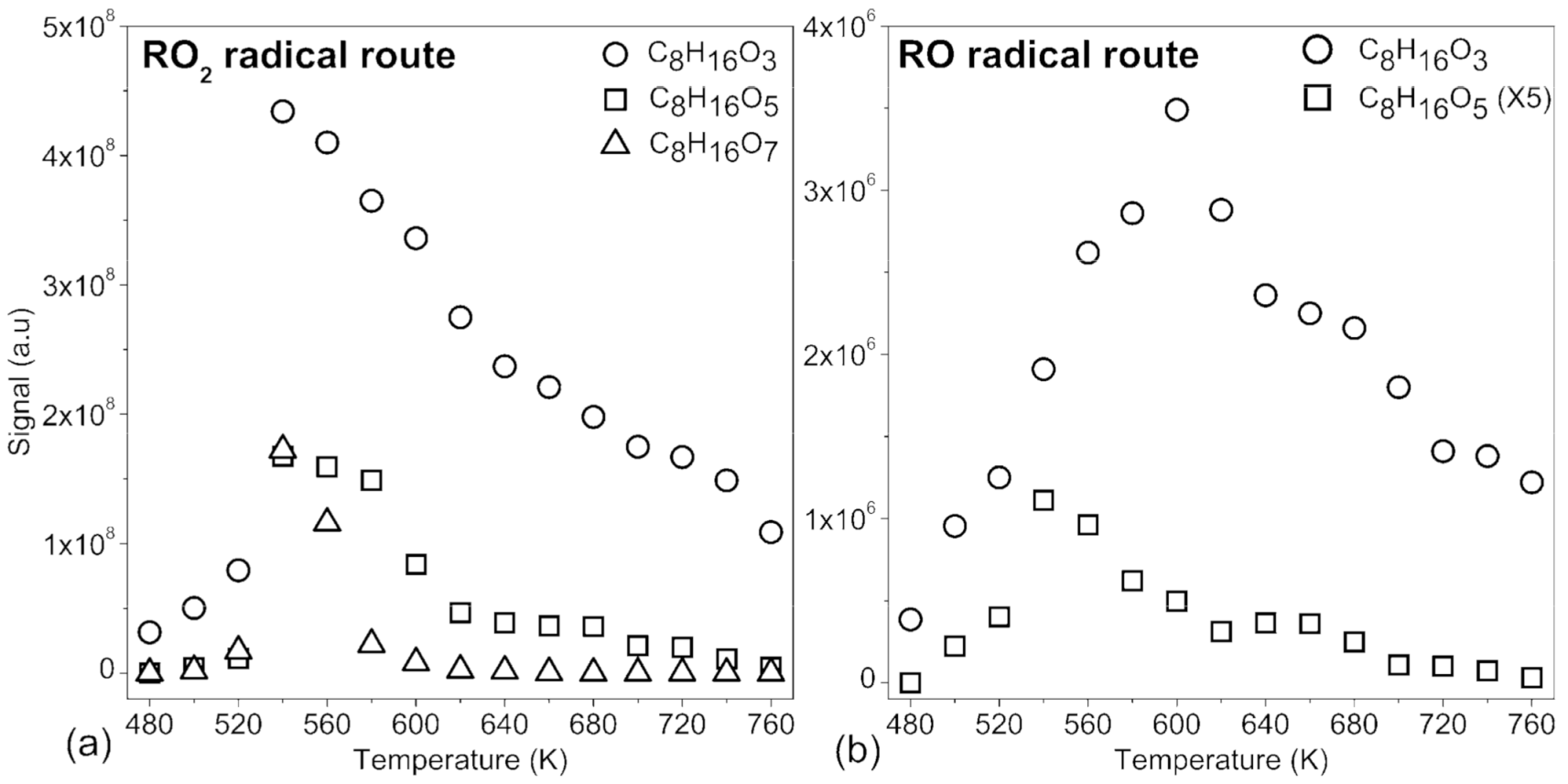
2.1. Oxygenated Intermediates Formed in Combustion via the RO2• Route
2.1.1. Hydroperoxides (C8H18O3), Dihydroperoxides (C8H18O5), Unsaturated Hydroperoxides (C8H16O3), Unsaturated Dihydroperoxides (C8H16O5), and Unsaturated Trihydroperoxides (C8H16O7)
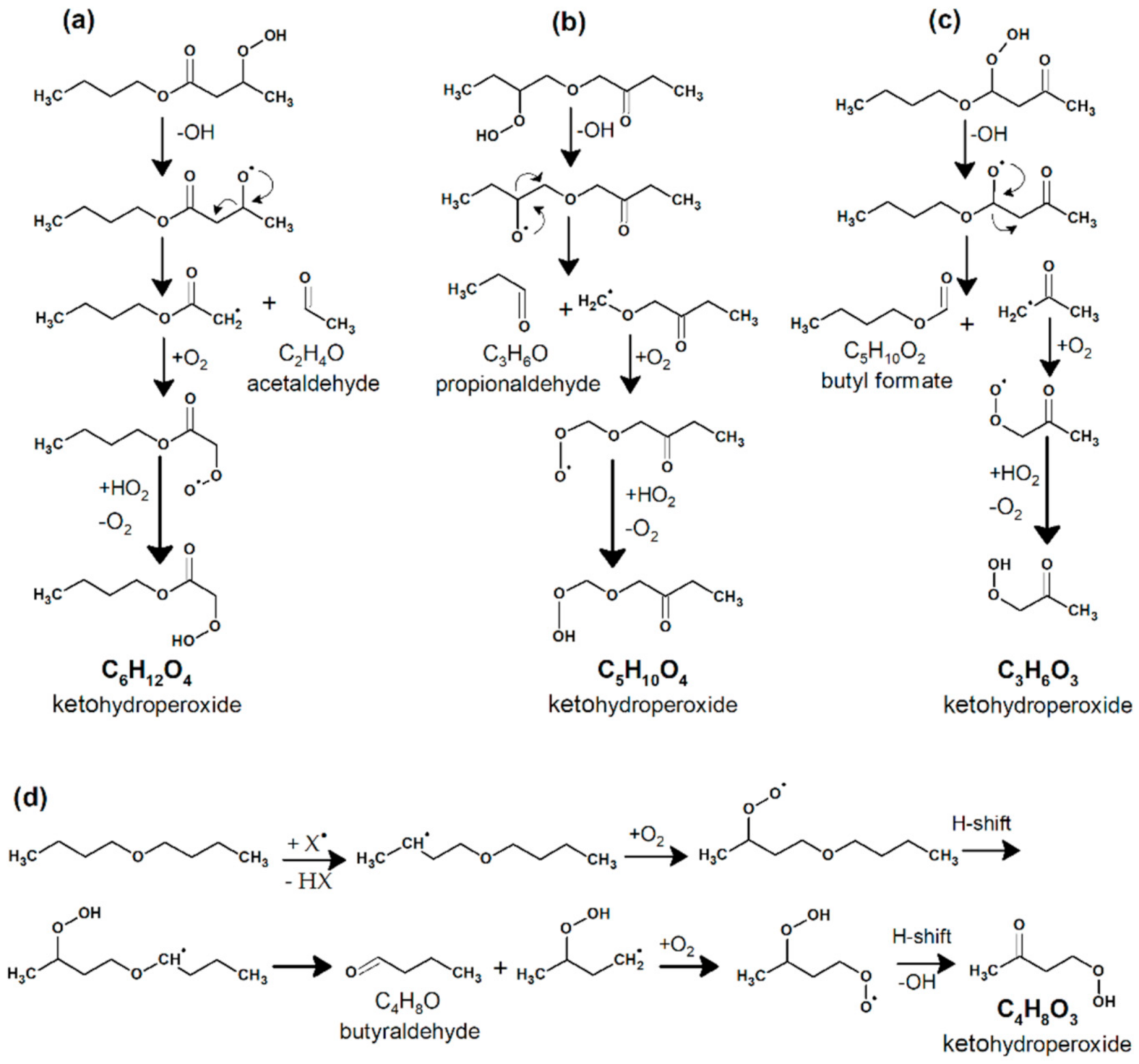
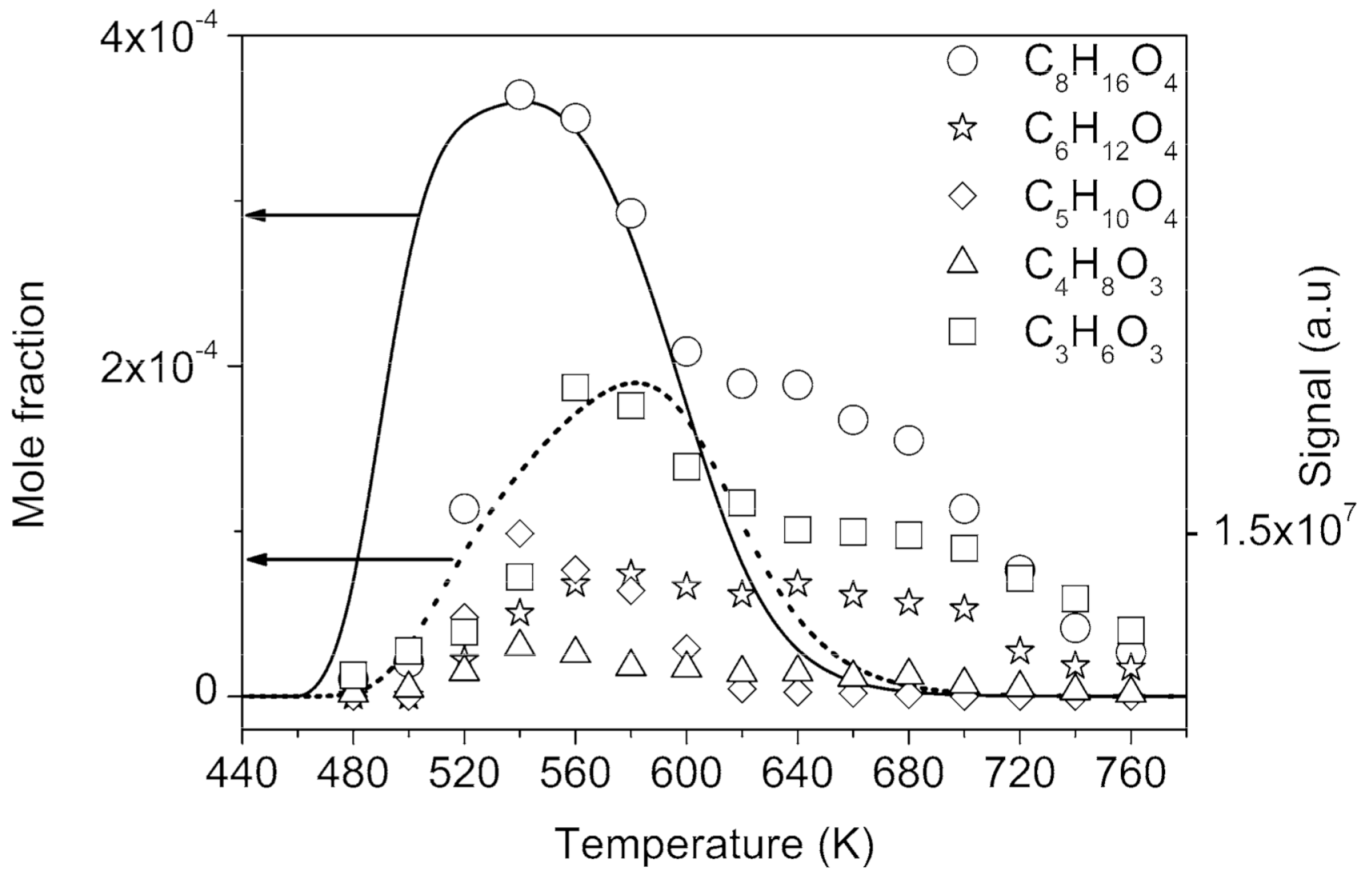
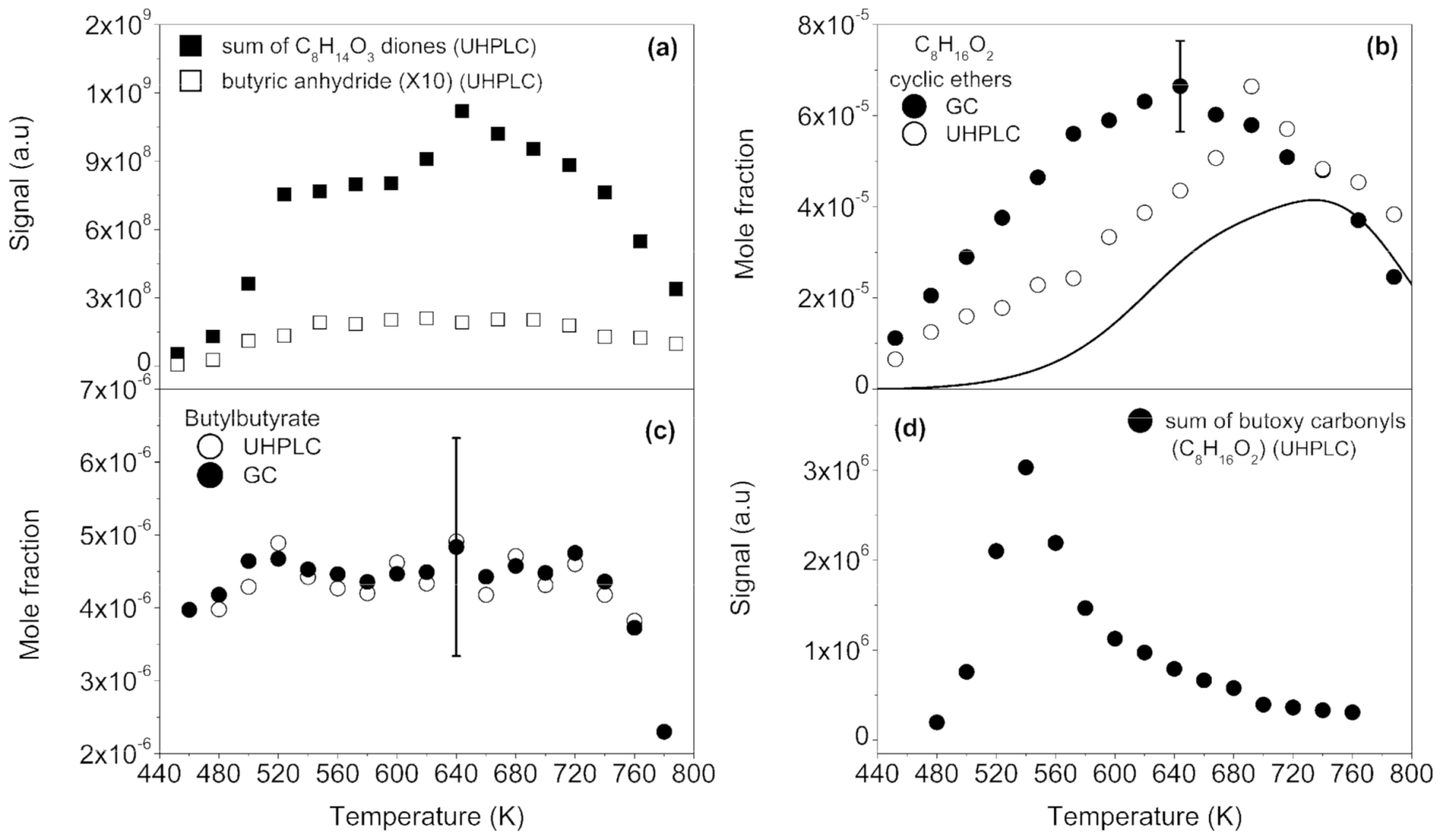
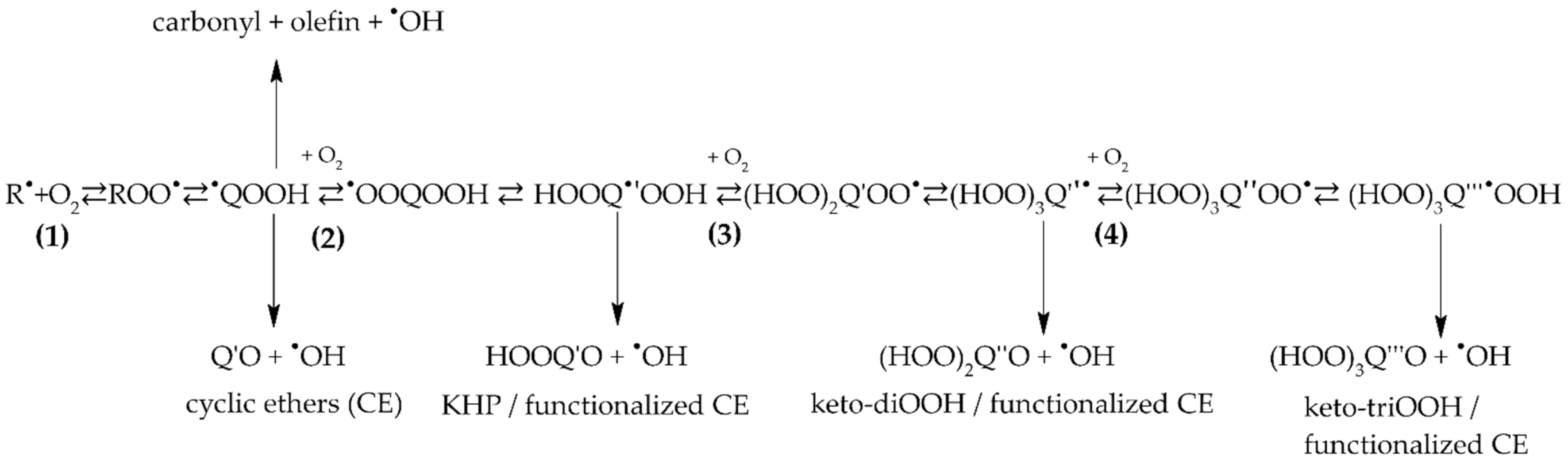
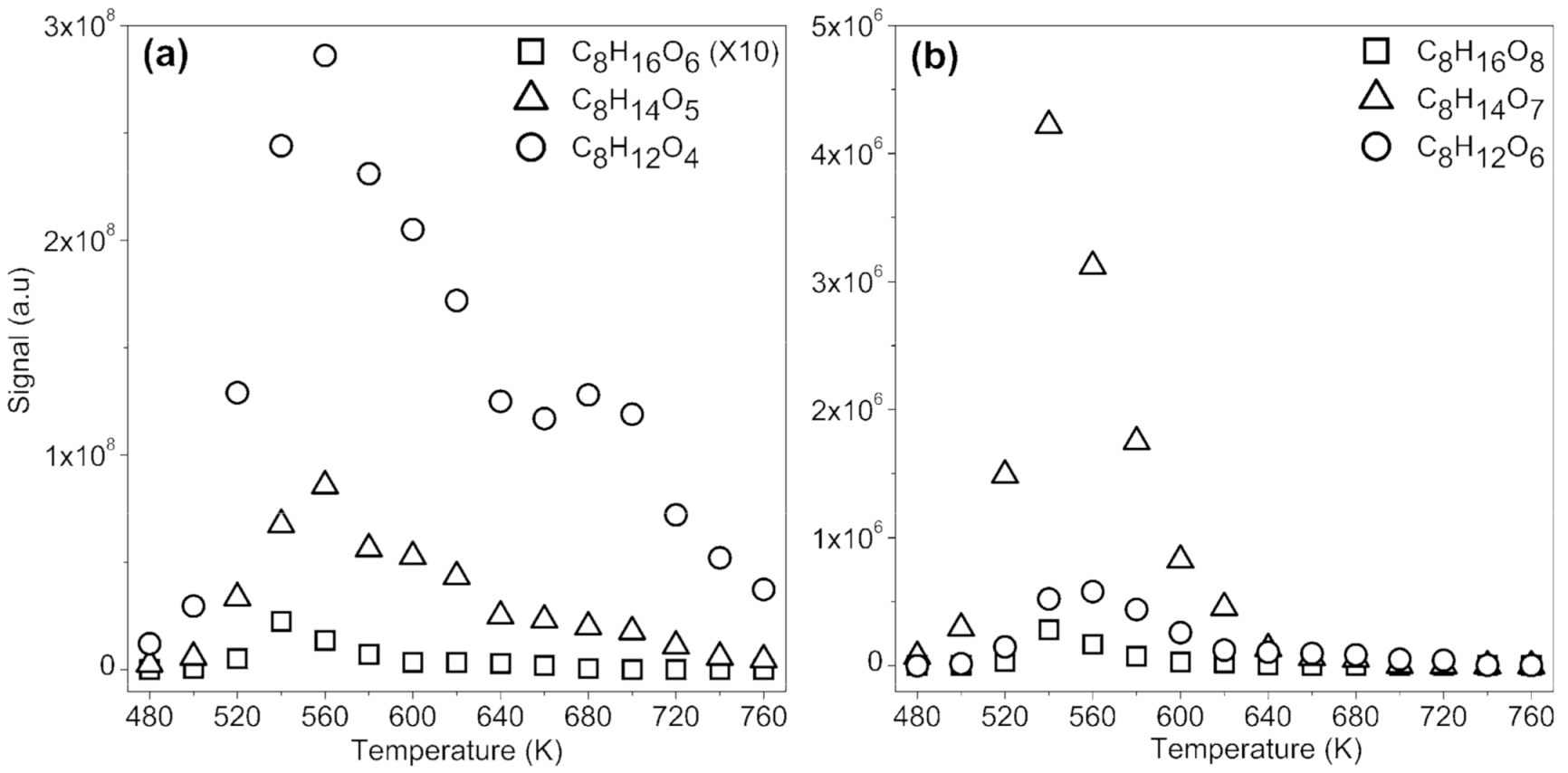
2.1.2. Formation of Ketohydroperoxides (C3H6O3, C4H8O3, C5H10O4, C6H12O4, and C8H16O4), Diketones (C8H14O3), Cyclic Ethers (C8H16O2), and Highly Oxygenated Molecules (C8H12O4,6, C8H14O5,7, and C8H16O6,8)
- C8H16O4 (keto-hydroperoxides) + X → HX + OH + C8H14O3 (diketones)
- C8H16O6 (keto-di-hydroperoxides) + X → HX + OH + C8H14O5 (di-keto-hydroperoxides)
- C8H14O5 (di-keto-hydroperoxides) + X → HX + OH + C8H12O4 (tri-ketones)
- C8H16O8 (keto-tri-hydroperoxides) + X → HX + OH + C8H14O7 (di-keto-di-hydroperoxides)
- C8H14O7 (di-keto-di-hydroperoxides) + X → HX + OH + C8H12O6 (tri-keto-hydroperoxides)
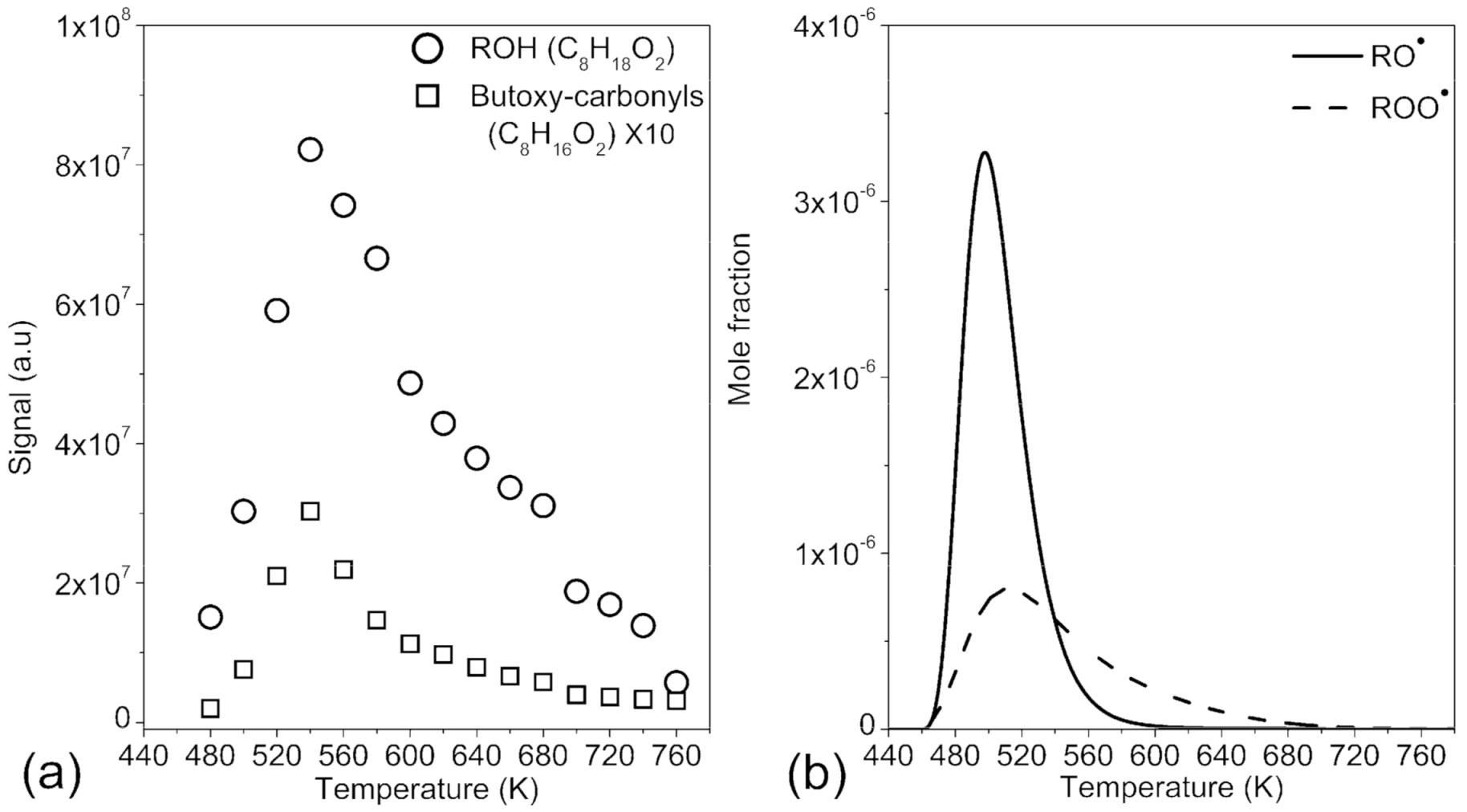
2.2. Oxygenated Products Formed via Atmospheric Oxidation Routes (RO2• and RO•)
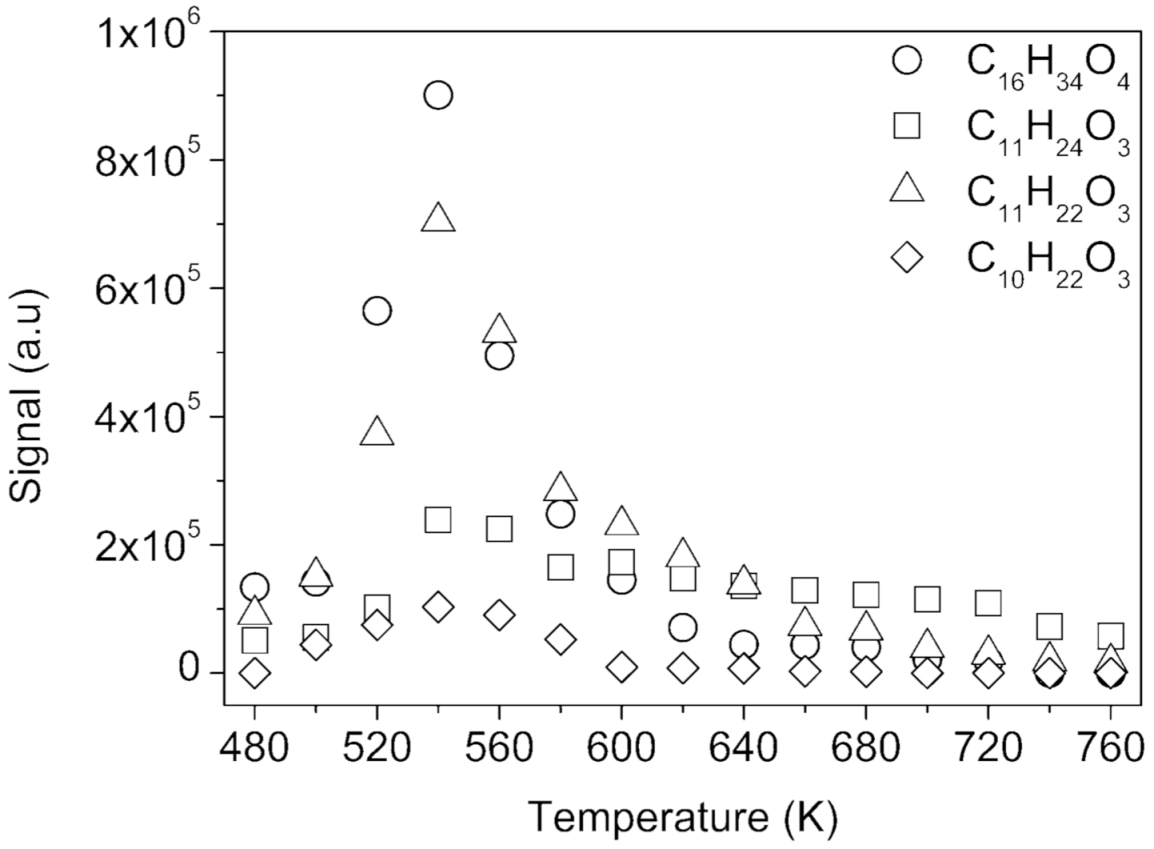
2.3. Further Characterization of Oxygenated Products
3. Materials and Methods
3.1. JSR Experiments
3.2. Analytical Procedures
3.3. Kinetic Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Burnett, R.T.; Haines, A.; Ramanathan, V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. USA 2019, 116, 7192–7197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, C.; Lenzmann, F. The social and economic consequences of the fossil fuel supply chain. MRS Energy Sustain. 2016, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Muscat, A.; de Olde, E.M.; de Boer, I.J.M.; Ripoll-Bosch, R. The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Secur. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Rotavera, B.; Taatjes, C.A. Influence of functional groups on low-temperature combustion chemistry of biofuels. Prog. Energy Combust. Sci. 2021, 86, 100925. [Google Scholar] [CrossRef]
- Martinez, M.; Duret, X.; Pham Minh, D.; Nzihou, A.; Lavoie, J.-M. Conversion of lignocellulosic biomass in biobutanol by a NOVEL thermal process. Int. J. Energy Prod. Manag. 2019, 4, 298–310. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.; Tang, C.; Yang, K.; Jun, M. Effect of di-n-butyl ether blending with soybean-biodiesel on spray and atomization characteristics in a common-rail fuel injection system. Fuel 2015, 140, 116–125. [Google Scholar] [CrossRef]
- Guan, L.; Tang, C.; Yang, K.; Mo, J.; Huang, Z. Experimental and Kinetic Study on Ignition Delay Times of Di-n-butyl Ether at High Temperatures. Energy Fuels 2014, 28, 5489–5496. [Google Scholar] [CrossRef]
- Yuanhang, G.; Liu, W.; Han, D. Comparative Study on Spray Auto-Ignition of Di-n-Butyl Ether and Diesel Blends at Engine-Like Conditions. J. Energy Resour. Technol. 2021, 143, 042302. [Google Scholar]
- Garcia, A.; Monsalve-Serrano, J.; Villalta, D.; Zubel, M.; Pischinger, S. Potential of 1-octanol and di-n-butyl ether (DNBE) to improve the performance and reduce the emissions of a direct injected compression ignition diesel engine. Energy Convers. Manag. 2018, 177, 563–571. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, L.; Zou, X.; Liu, C.; Tian, B.; Huang, Z. Soot reduction effects of dibutyl ether (DBE) addition to a biodiesel surrogate in laminar coflow diffusion flames. Proc. Combust. Inst. 2019, 37, 1265–1272. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, I.; Cracknell, R.; Parkes, M.; Megaritis, T.; Ganippa, L. Experimental study of the effect of C8 oxygenates on sooting processes in high pressure spray flames. Combust. Flame 2020, 220, 235–246. [Google Scholar] [CrossRef]
- Wullenkord, J.; Tran, L.-S.; Böttchers, J.; Graf, I.; Kohse-Höinghaus, K. A laminar flame study on di-n-butyl ether as a potential biofuel candidate. Combust. Flame 2018, 190, 36–49. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Yang, J.; Yang, B. Pyrolysis of Lignocellulosic Biofuel Di-n-butyl Ether (DBE): Flow Reactor Experiments and Kinetic Modeling. Energy Fuels 2021, 35, 14077–14086. [Google Scholar] [CrossRef]
- Thion, S.; Togbe, C.; Serinyel, Z.; Dayma, G.; Dagaut, P. A chemical kinetic study of the oxidation of dibutyl-ether in a jet-stirred reactor. Combust. Flame 2017, 185, 4–15. [Google Scholar] [CrossRef]
- Tran, L.S.; Wullenkord, J.; Li, Y.Y.; Herbinet, O.; Zeng, M.R.; Qi, F.; Kohse-Hoinghaus, K.; Battin-Leclerc, F. Probing the low-temperature chemistry of di-n-butyl ether: Detection of previously unobserved intermediates. Combust. Flame 2019, 210, 9–24. [Google Scholar] [CrossRef]
- Belhadj, N.; Benoit, R.; Dagaut, P.; Lailliau, M.; Serinyel, Z.; Dayma, G.; Khaled, F.; Moreau, B.; Foucher, F. Oxidation of di-n-butyl ether: Experimental characterization of low-temperature products in JSR and RCM. Combust. Flame 2020, 222, 133–144. [Google Scholar] [CrossRef]
- Wang, Z.; Popolan-Vaida, D.M.; Chen, B.; Moshammer, K.; Mohamed, S.Y.; Wang, H.; Sioud, S.; Raji, M.A.; Kohse-Höinghaus, K.; Hansen, N.; et al. Unraveling the structure and chemical mechanisms of highly oxygenated intermediates in oxidation of organic compounds. Proc. Natl. Acad. Sci. USA 2017, 114, 13102–13107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.D.; Sarathy, S.M. Third O-2 addition reactions promote the low-temperature auto-ignition of n-alkanes. Combust. Flame 2016, 165, 364–372. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Moshammer, K.; Popolan-Vaida, D.M.; Shankar, V.S.B.; Lucassen, A.; Hemken, C.; Taatjes, C.A.; Leone, S.R.; Kohse-Hoeinghaus, K.; et al. Additional chain-branching pathways in the low-temperature oxidation of branched alkanes. Combust. Flame 2016, 164, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Glarborg, P.; Kee, R.J.; Grcar, J.F.; Miller, J.A. PSR: A Fortran Program for Modeling Well-Stirred Reactors; SAND86-8209; Sandia National Laboratories: Livermore, CA, USA, 1986. [Google Scholar]
- Fan, X.F.; Sun, W.Y.; Gao, Y.; Hansen, N.; Chen, B.J.; Pitsch, H.; Yang, B. Chemical insights into the multi-regime low-temperature oxidation of di-n-propyl ether: Jet-stirred reactor experiments and kinetic modeling. Combust. Flame 2021, 233, 11592. [Google Scholar] [CrossRef]
- Blin-Simiand, N.; Jorand, F.; Sahetchian, K.; Brun, M.; Kerhoas, L.; Malosse, C.; Einhorn, J. Hydroperoxides with zero, one, two or more carbonyl groups formed during the oxidation of n-dodecane. Combust. Flame 2001, 126, 1524–1532. [Google Scholar] [CrossRef]
- Belhadj, N.; Lailliau, M.; Benoit, R.; Dagaut, P. Experimental and kinetic modeling study of n-hexane oxidation. Detection of complex low-temperature products using high-resolution mass spectrometry. Combust. Flame 2021, 233, 111581. [Google Scholar] [CrossRef]
- Bianchi, F.; Kurtén, T.; Riva, M.; Mohr, C.; Rissanen, M.P.; Roldin, P.; Berndt, T.; Crounse, J.D.; Wennberg, P.O.; Mentel, T.F.; et al. Highly Oxygenated Organic Molecules (HOM) from Gas-Phase Autoxidation Involving Peroxy Radicals: A Key Contributor to Atmospheric Aerosol. Chem. Rev. 2019, 119, 3472–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, R.; Belhadj, N.; Lailliau, M.; Dagaut, P. On the similarities and differences between the products of oxidation of hydrocarbons under simulated atmospheric conditions and cool flames. Atmos. Chem. Phys. 2021, 21, 7845–7862. [Google Scholar] [CrossRef]
- Kroll, J.H.; Donahue, N.M.; Jimenez, J.L.; Kessler, S.H.; Canagaratna, M.R.; Wilson, K.R.; Altieri, K.E.; Mazzoleni, L.R.; Wozniak, A.S.; Bluhm, H.; et al. Carbon oxidation state as a metric for describing the chemistry of atmospheric organic aerosol. Nat. Chem. 2011, 3, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dagaut, P.; Cathonnet, M.; Boettner, J.C.; Gaillard, F. Kinetic modeling of ethylene oxidation. Combust. Flame 1988, 71, 295–312. [Google Scholar] [CrossRef]
- Dagaut, P.; Cathonnet, M.; Boettner, J.C.; Gaillard, F. Kinetic Modeling of Propane Oxidation. Combust. Sci. Technol. 1987, 56, 23–63. [Google Scholar] [CrossRef]
- Dagaut, P.; Cathonnet, M.; Rouan, J.P.; Foulatier, R.; Quilgars, A.; Boettner, J.C.; Gaillard, F.; James, H. A Jet-Stirred Reactor for Kinetic-Studies of Homogeneous Gas-Phase Reactions at Pressures up to 10-Atmospheres (~1 MPa). J. Phys. E-Sci. Instrum. 1986, 19, 207–209. [Google Scholar] [CrossRef]
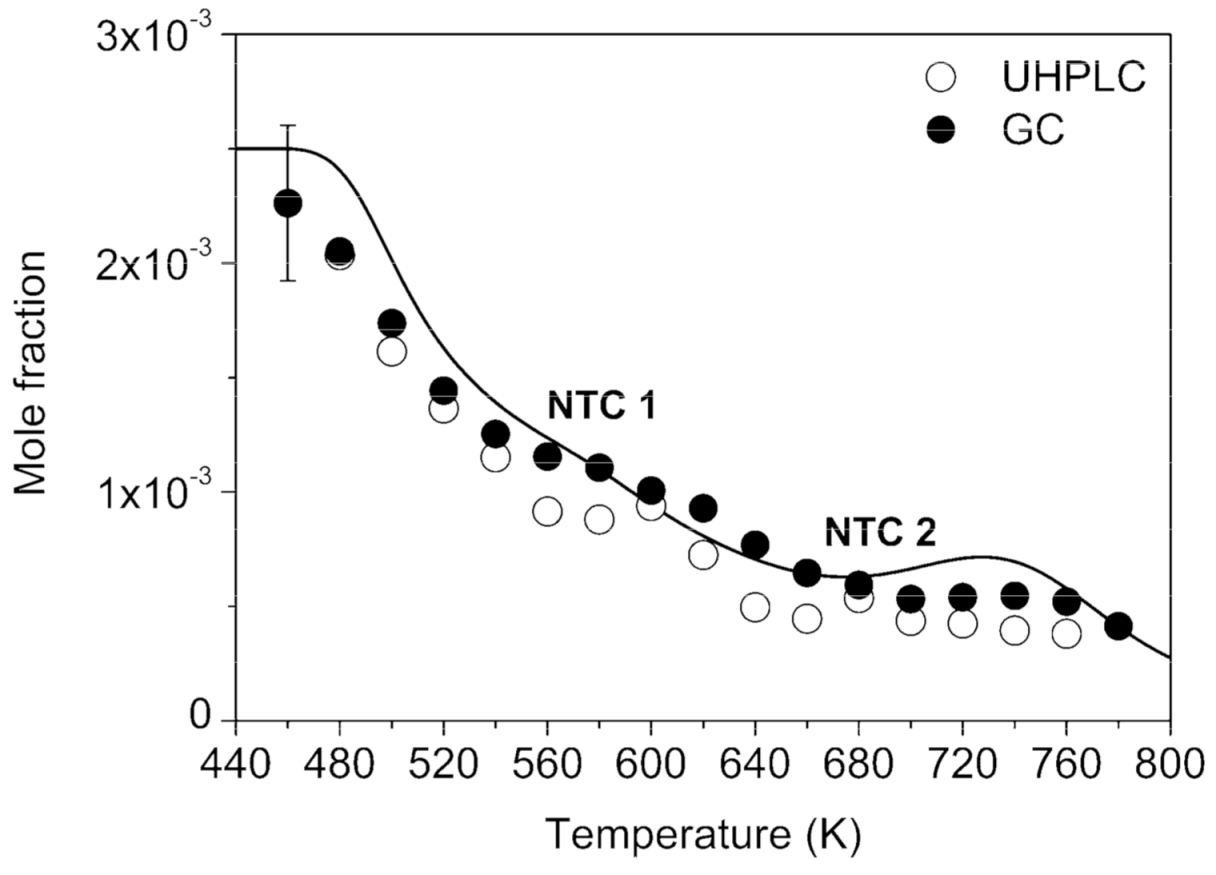

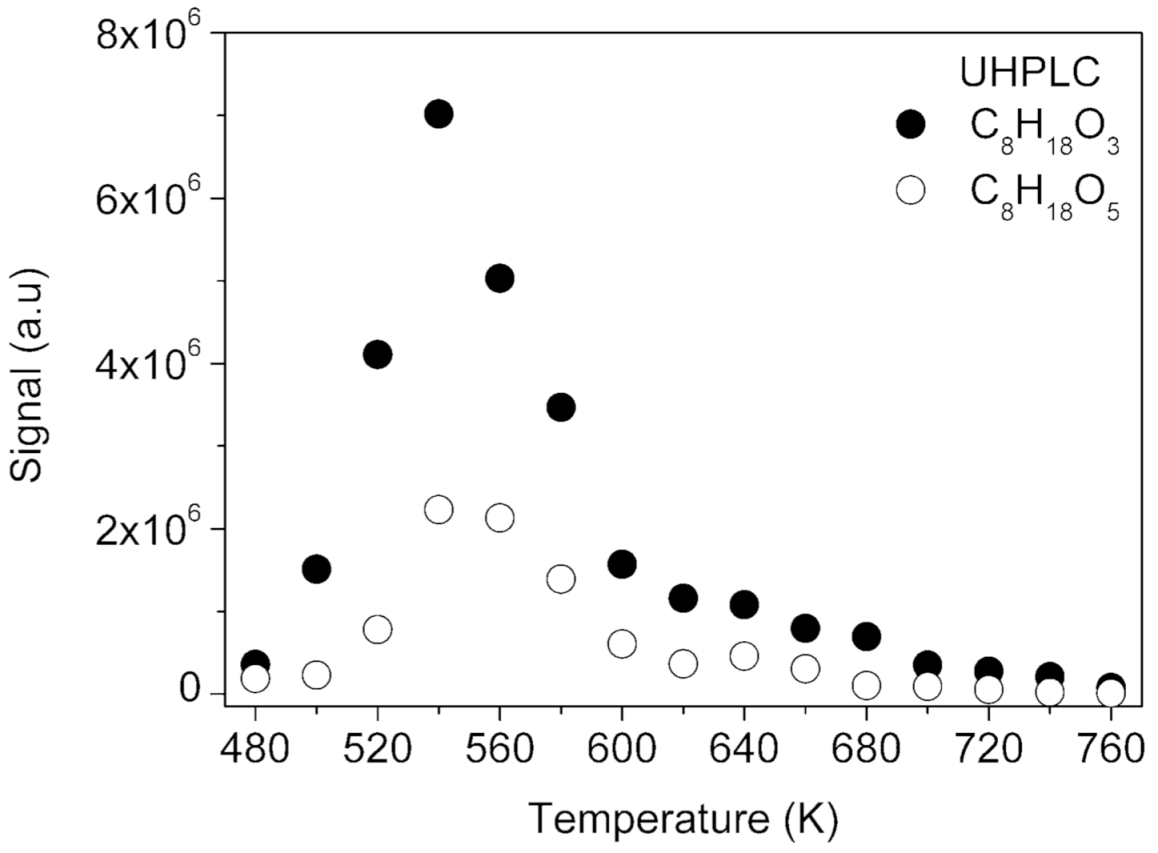
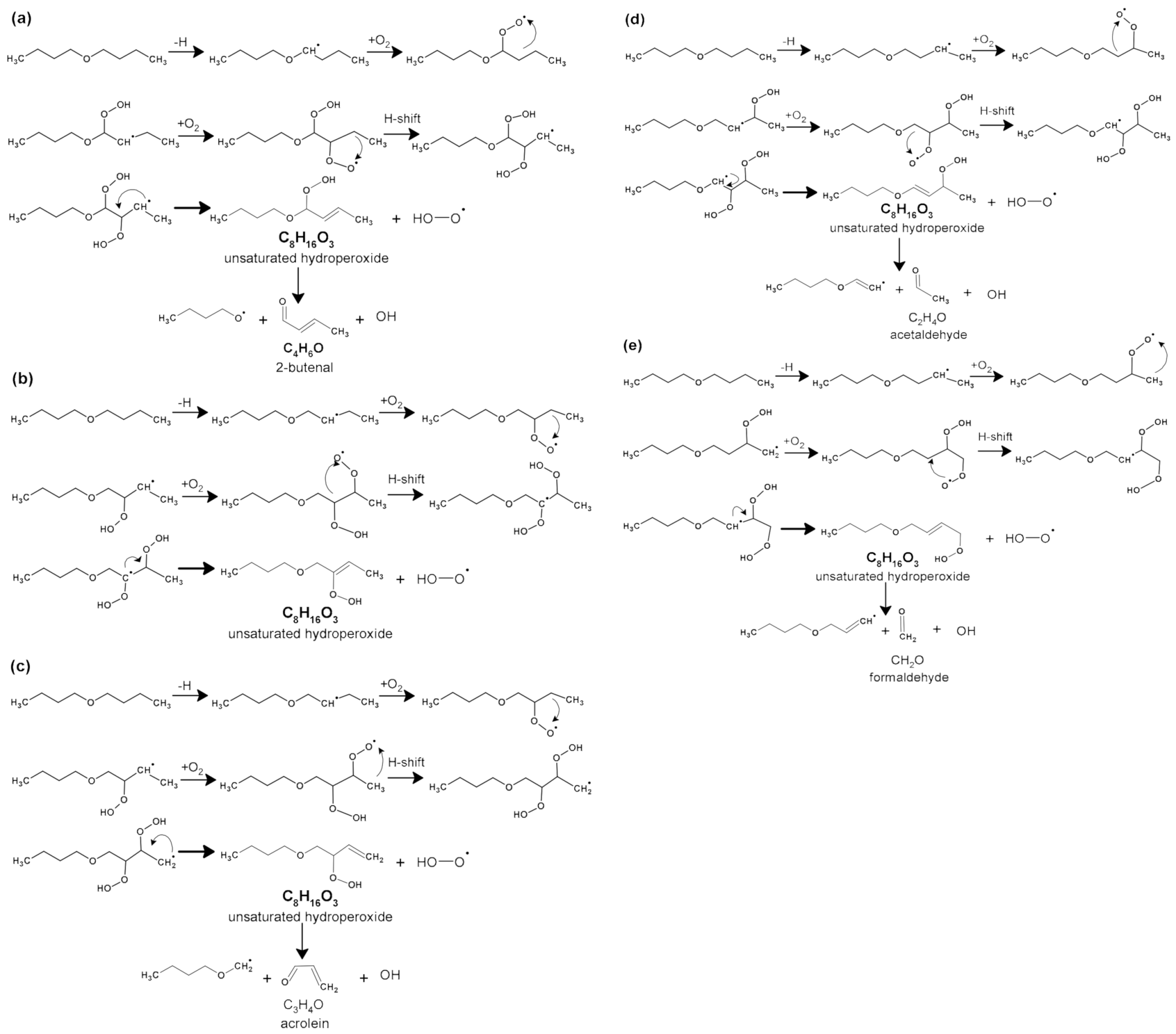
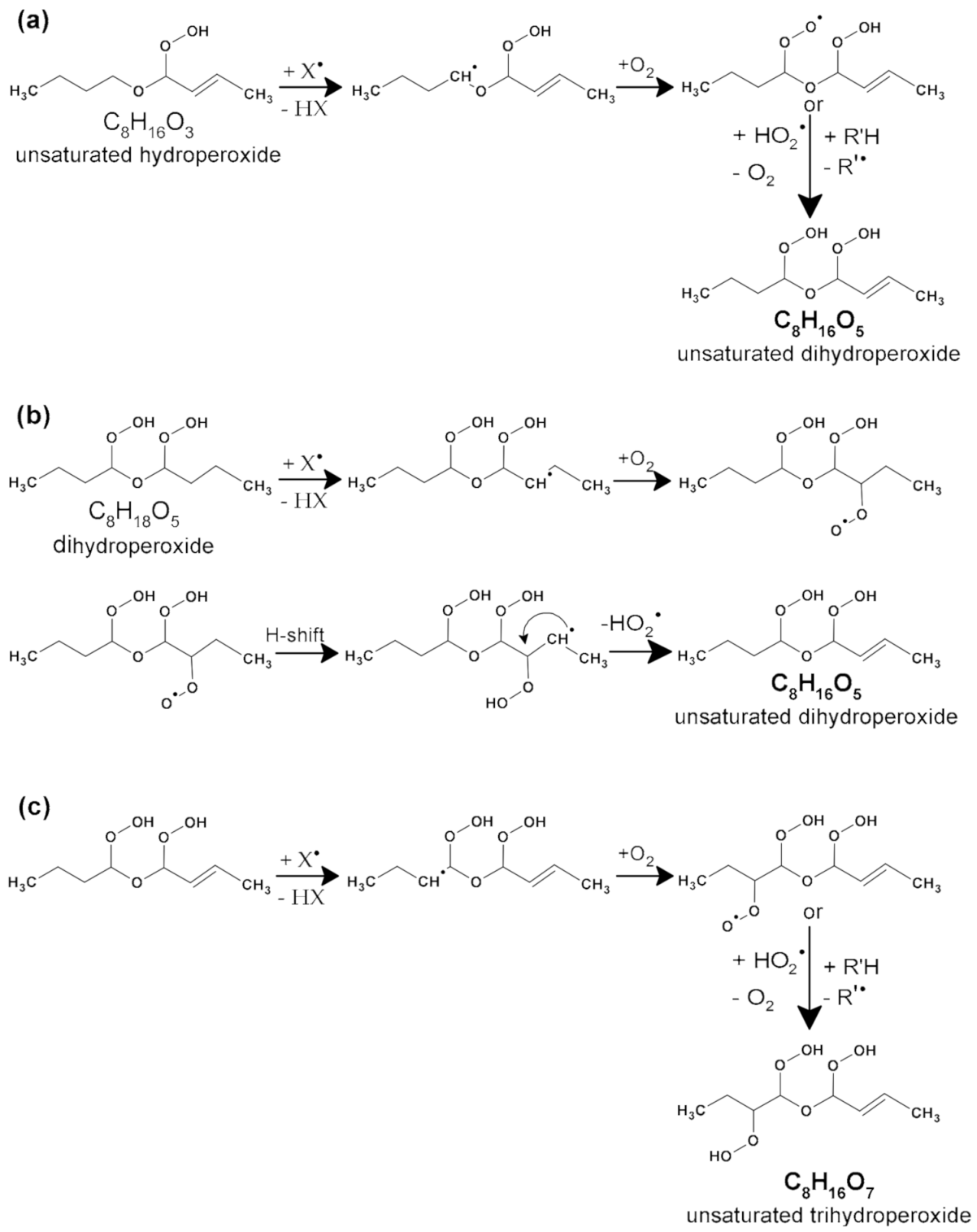

| Parameters | Values |
|---|---|
| Reactive mixture | 0.25% of DBE, 1.5% of O2, 98.25% of N2 |
| Equivalence ratio (φ) | 2 |
| Residence time (s) | 1 |
| Pressure (atm) | 10 |
| Temperature (K) | 460–780 |
| Volume of acetonitrile (mL) | 20 |
| Bubbling time (min) | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhadj, N.; Lailliau, M.; Benoit, R.; Dagaut, P. Towards a Comprehensive Characterization of the Low-Temperature Autoxidation of Di-n-Butyl Ether. Molecules 2021, 26, 7174. https://doi.org/10.3390/molecules26237174
Belhadj N, Lailliau M, Benoit R, Dagaut P. Towards a Comprehensive Characterization of the Low-Temperature Autoxidation of Di-n-Butyl Ether. Molecules. 2021; 26(23):7174. https://doi.org/10.3390/molecules26237174
Chicago/Turabian StyleBelhadj, Nesrine, Maxence Lailliau, Roland Benoit, and Philippe Dagaut. 2021. "Towards a Comprehensive Characterization of the Low-Temperature Autoxidation of Di-n-Butyl Ether" Molecules 26, no. 23: 7174. https://doi.org/10.3390/molecules26237174
APA StyleBelhadj, N., Lailliau, M., Benoit, R., & Dagaut, P. (2021). Towards a Comprehensive Characterization of the Low-Temperature Autoxidation of Di-n-Butyl Ether. Molecules, 26(23), 7174. https://doi.org/10.3390/molecules26237174






