Theoretical Investigation of the Fusion Process of Mono-Cages to Tri-Cages with CH4/C2H6 Guest Molecules in sI Hydrates
Abstract
:1. Introduction
2. Models and Methods
3. Discussion
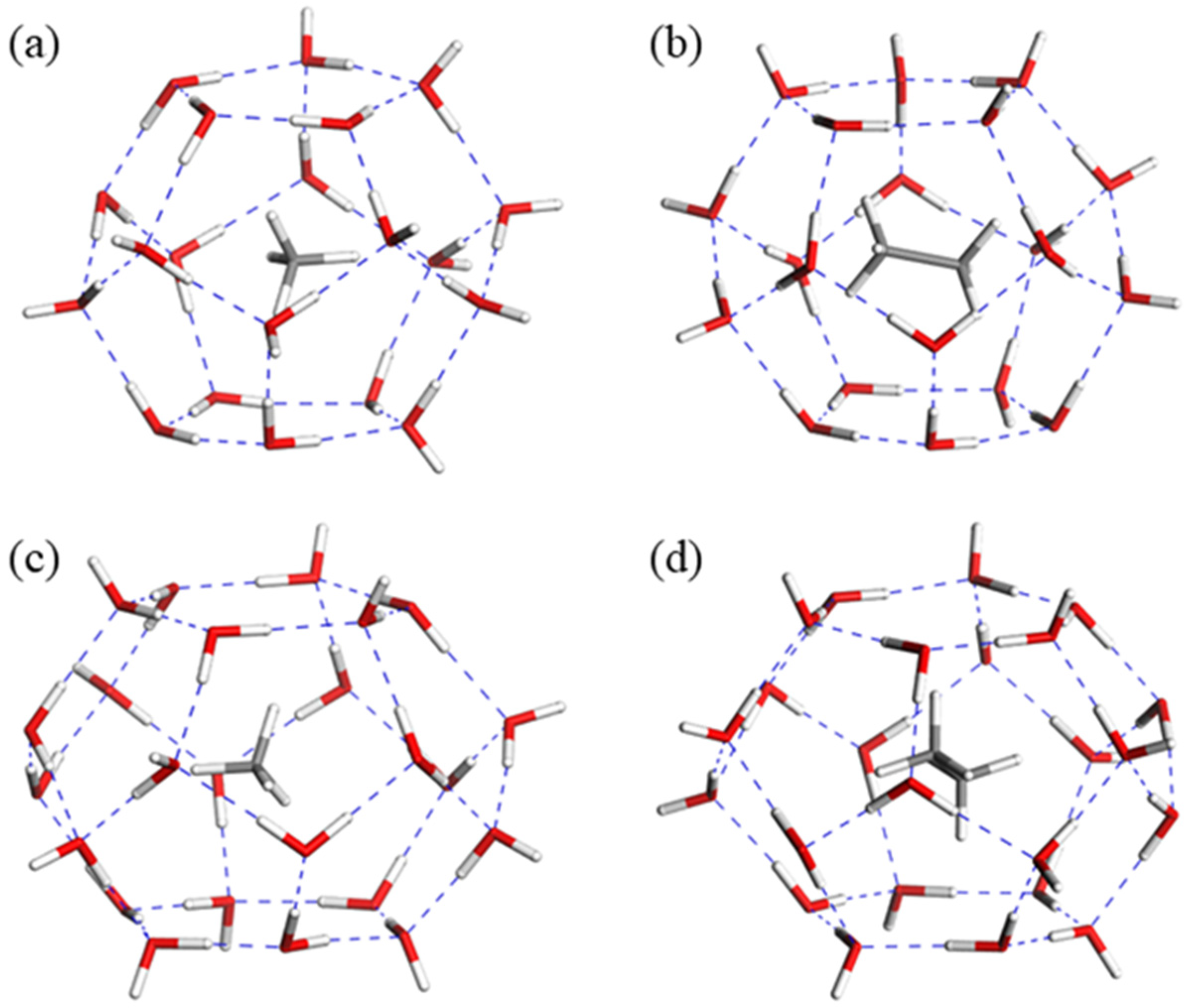
3.1. The Influence of CH4/C2H6 Guest Molecules on Single Cage
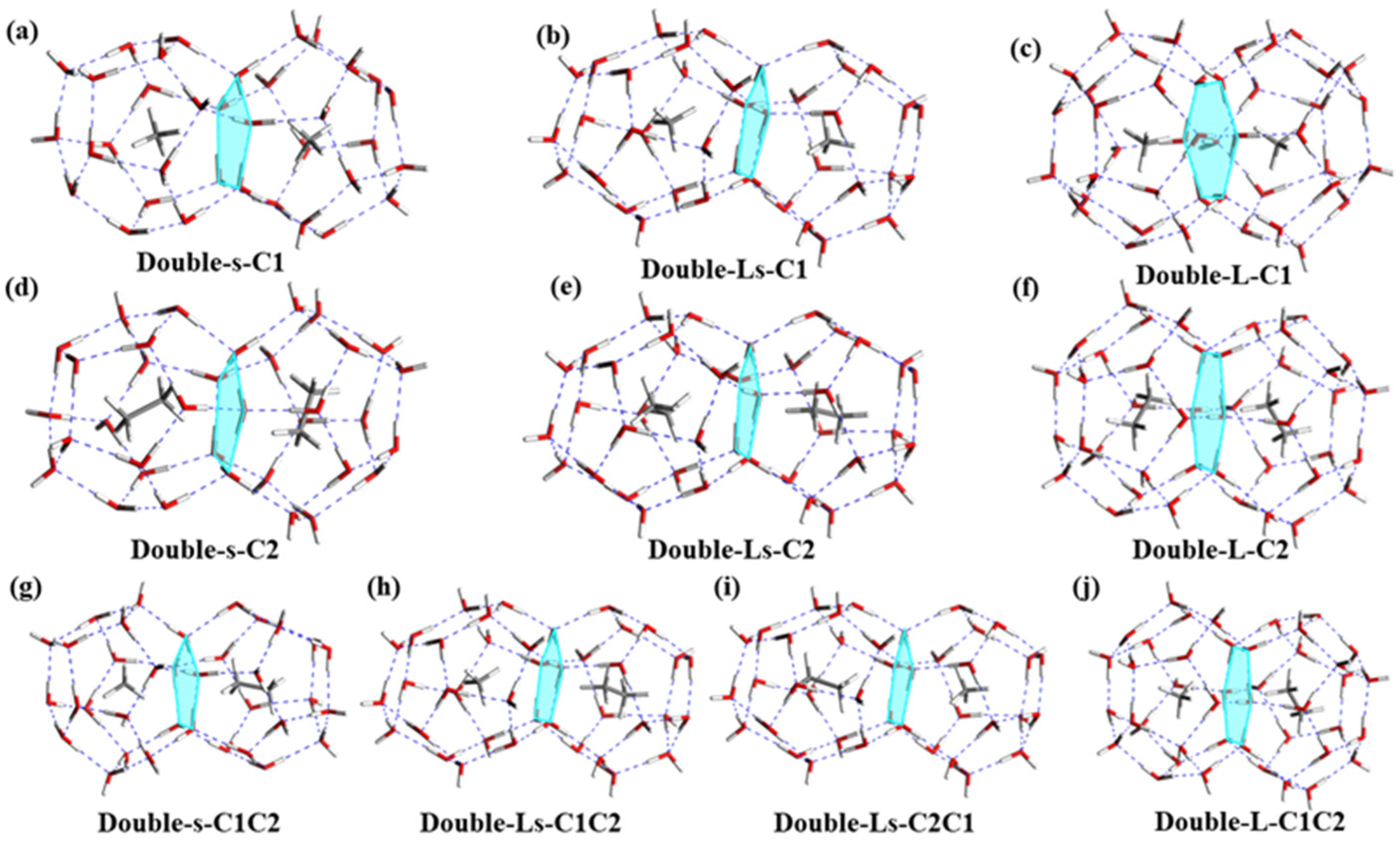
3.2. The Stabilities of Double Cages with CH4/C2H6 Guest Molecules
3.3. The Fusion of Double Cages with CH4/C2H6 Guest Molecules
3.4. The Stabilities of Triple Cages with CH4/C2H6 Guest Molecules
3.5. The Fusion of Double Cages to Triple Cages with CH4/C2H6 Guest Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casco, M.E.; Silvestre-Albero, J.; Ramirez-Cuesta, A.J.; Rey, F.; Jorda, J.L.; Bansode, A.; Urakawa, A.; Peral, I.; Martinez-Escandell, M.; Kaneko, K.; et al. Methane hydrate formation in confined nanospace can surpass nature. Nat. Commun. 2015, 6, 6432–6439. [Google Scholar] [CrossRef] [Green Version]
- Makogon, Y.; Holditch, S.; Makogon, T.Y. Natural gas-hydrates―A potential energy source for the 21st Century. J. Petrol. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, G.; Zhang, X. Modeling and molecular simulation of natural gas hydrate stabilizers. Eur. J. Remote Sens. 2020, 54, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef]
- Takeuchi, F.; Hiratsuka, M.; Ohmura, R.; Alavi, S.; Sum, A.K.; Yasuoka, K. Water proton configurations in structures I, II, and H clathrate hydrate unit cells. J. Chem. Phys. 2013, 138, 124504. [Google Scholar] [CrossRef] [PubMed]
- Linga, P.; Kumar, R.; Englezos, P. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures. Chem. Eng. Sci. 2007, 62, 4268–4276. [Google Scholar] [CrossRef]
- Cao, X.; Huang, Y.; Li, W.; Zheng, Z.; Jiang, X.; Su, Y.; Zhao, J.; Liu, C. Phase diagrams for clathrate hydrates of methane, ethane, and propane from first-principles thermodynamics. Phys. Chem. Chem. Phys. 2016, 18, 3272–3279. [Google Scholar] [CrossRef]
- He, Z.; Gupta, K.M.; Linga, P.; Jiang, J. Molecular Insights into the Nucleation and Growth of CH4 and CO2 Mixed Hydrates from Microsecond Simulations. J. Phys. Chem. C 2016, 120, 25225–25236. [Google Scholar] [CrossRef]
- Giricheva, N.I.; Ischenko, A.A.; Yusupov, V.I.; Bagratashvili, V.N.; Girichev, G.V. Structure and energetic characteristics of methane hydrates. From single cage to triple cage: A DFT-D study. J. Mol. Struct. 2017, 1132, 157–166. [Google Scholar] [CrossRef]
- Mondal, S.; Goswami, T.; Jana, G.; Misra, A.; Chattaraj, P.K. A possible reason behind the initial formation of pentagonal dodecahedron cavities in sI-methane hydrate nucleation: A DFT study. Chem. Phys. Lett. 2018, 691, 415–420. [Google Scholar] [CrossRef]
- Petuya, C.; Martin-Gondre, L.; Aurel, P.; Damay, F.; Desmedt, A. Unraveling the metastability of the SI and SII carbon monoxide hydrate with a combined DFT-neutron diffraction investigation. J. Chem. Phys. 2019, 150, 184705. [Google Scholar] [CrossRef]
- Li, K.; Wang, P.; Tang, L.; Shi, R.; Su, Y.; Zhao, J. Stability and NMR Chemical Shift of Amorphous Precursors of Methane Hydrate: Insights from Dispersion-Corrected Density Functional Theory Calculations Combined with Machine Learning. J. Phys. Chem. B 2021, 125, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Roman-Perez, G.; Moaied, M.; Soler, J.M.; Yndurain, F. Stability, adsorption, and diffusion of CH(4), CO(2), and H(2) in clathrate hydrates. Phys. Rev. Lett. 2010, 105, 145901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Liu, Z.Y.; Zhang, X. Molecular Insights into Cage Occupancy of Hydrogen Hydrate: A Computational Study. Processes 2019, 7, 699. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, H.K.; Sastry, G.N. Viability of clathrate hydrates as CO2 capturing agents: A theoretical study. J. Phys. Chem. A 2011, 115, 7633–7637. [Google Scholar] [CrossRef]
- Liu, J.; Hou, J.; Xu, J.; Liu, H.; Chen, G.; Zhang, J. Ab initio study of the molecular hydrogen occupancy in pure H2 and binary H2-THF clathrate hydrates. Int. J. Hydro. Energy 2017, 42, 17136–17143. [Google Scholar] [CrossRef]
- Izquierdo-Ruiz, F.; Otero-de-la-Roza, A.; Contreras-Garcia, J.; Prieto-Ballesteros, O.; Recio, J.M. Effects of the CO(2) Guest Molecule on the sI Clathrate Hydrate Structure. Materials 2016, 9, 777. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hou, J.; Liu, H.; Liu, M.; Xu, J.; Chen, G.; Zhang, J. Molecular mechanism of formation of the face-sharing double cages in structure-I methane hydrate. Chem. Phys. Lett. 2018, 691, 155–162. [Google Scholar] [CrossRef]
- Li, K.; Shi, R.; Tang, L.; Huang, Y.; Cao, X.; Su, Y. Cage fusion from bi-cages to tri-cages during nucleation of methane hydrate: A DFT-D simulation. Phys. Chem. Chem. Phys. 2019, 21, 9150–9158. [Google Scholar] [CrossRef]
- Tang, L.L.; Shi, R.L.; Su, Y.; Zhao, J.J. Structures, Stabilities, and Spectra Properties of Fused CH4 Endohedral Water Cage (CH4)m(H2O)n Clusters from DFT-D Methods. J. Phys. Chem. A 2015, 119, 10971–10979. [Google Scholar] [CrossRef]
- Uchida, T.; Takeya, S.; Kamata, Y.; Ikeda, I.Y.; Nagao, J.; Ebinuma, T.; Narita, H.; Zatsepina, O.; Buffett, B.A. Spectroscopic Observations and Thermodynamic Calculations on Clathrate Hydrates of Mixed Gas Containing Methane and Ethane: Determination of Structure, Composition and Cage Occupancy. J. Phys. Chem. B 2002, 106, 12426–12431. [Google Scholar] [CrossRef]
- Fuseya, G.; Takeya, S.; Hachikubo, A. Temperature effects on the C–H symmetric stretching vibrational frequencies of guest hydrocarbon molecules in 512, 51262 and 51264 cages of sI and sII clathrate hydrates. RSC Adv. 2020, 10, 37582–37587. [Google Scholar] [CrossRef]
- Kamata, R.; Hachikubo, A.; Takeya, S. Hydrogen isotopic fractionation of methane at the formation of synthetic mixed-gas hydrate composed of methane and propane. Limnol. Freshw. Biol. 2020, 4, 920–921. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Bhattacharjee, G.; Liao, J.; Linga, P. Macroscopic Kinetic Investigations on Mixed Natural Gas Hydrate Formation for Gas Storage Application. Energy Fuels 2020, 34, 15257–15269. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision d. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Gordon, M.S. The isomers of silacyclopropane. Chem. Phys. Lett. 1980, 76, 163–168. [Google Scholar] [CrossRef]
- Khan, A. Theoretical studies of CH4(H2O)20,(H2O)21,(H2O)20, and fused dodecahedral and tetrakaidecahedral structures: How do natural gas hydrates form? J. Chem. Phys. 1999, 110, 11884–11889. [Google Scholar] [CrossRef]
- Hou, J.; Liu, J.; Xu, J.; Zhong, J.; Yan, Y.; Zhang, J. Two-dimensional methane hydrate: Plum-pudding structure and sandwich structure. Chem. Phys. Lett. 2019, 725, 38–44. [Google Scholar] [CrossRef]
- Khan, A. Stabilization of hydrate structure H by N2 and CH4 molecules in 435663 and 512 cavities, and fused structure formation with 51268 cage: A theoretical study. J. Phys. Chem. A 2001, 105, 7429–7434. [Google Scholar] [CrossRef]
- Liu, J.; Hou, J.; Xu, J.; Liu, H.; Chen, G.; Zhang, J. Formation of clathrate cages of sI methane hydrate revealed by ab initio study. Energy 2017, 120, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.L.; Su, Y.; Liu, Y.; Zhao, J.J.; Qiu, R.F. Nonstandard cages in the formation process of methane clathrate: Stability, structure, and spectroscopic implications from first-principles. J. Chem. Phys. 2012, 136, 224508. [Google Scholar] [CrossRef] [PubMed]


| Method | Basis Set | dO-C (Å) | Reference |
|---|---|---|---|
| MP2 | Aug-cc-PVTZ | 3.508 | [33] |
| PBE-TS | TNP | 3.473 | |
| B3LYP | 6-311++g(2d,2p) | 3.671 | |
| B97-D | 6-311++g(2d,2p) | 3.491 | |
| B3LYP | 6-31+g(d,p) | 3.684 | This work |
| Guest Molecule | Structure | Esta | Esta-p | Ec | Ecp | Efusion |
|---|---|---|---|---|---|---|
| CH4 | Double-s | 1780.12 | 50.86 | −55.41 | −27.71 | 130.00 |
| Double-Ls | 2141.74 | 54.92 | −51.08 | −25.54 | 155.81 | |
| Double-L | 2339.38 | 55.70 | −49.64 | −24.82 | 101.58 | |
| C2H6 | Double-s | 1789.15 | 51.12 | −64.45 | −32.23 | 128.27 |
| Double-Ls | 2152.63 | 55.20 | −61.97 | −30.99 | 150.77 | |
| Double-L | 2364.07 | 56.29 | −74.32 | −37.16 | 106.47 | |
| CH4/C2H6 | Double-s | 1783.62 | 50.96 | −58.92 | −29.46 | 127.35 |
| Ls-C1C2 | 2140.82 | 54.89 | −50.16 | −25.08 | 150.27 | |
| Ls-C2C1 | 2153.12 | 55.21 | −62.46 | −31.23 | 155.87 | |
| Double-L | 2351.56 | 55.99 | −61.81 | −30.91 | 102.45 |
| Guest Molecule | Structures | Esta | Esta-p | Efusion | |
|---|---|---|---|---|---|
| CH4 | tri-LsL | C1C1C1 | 3070.29 | 56.86 | 235.90 |
| tri-sLs | C1C1C1 | 2776.97 | 54.45 | 163.77 | |
| C2H6 | tri-LsL | C2C2C2 | 3092.03 | 57.26 | 240.62 |
| tri-sLs | C2C2C2 | 2795.97 | 54.82 | 168.80 | |
| CH4/C2H6 | tri-LsL | C1C1C2 | 3083.75 | 57.11 | 243.23 |
| C1C2C1 | 3065.12 | 56.76 | 231.65 | ||
| C1C2C2 | 3078.14 | 57.00 | 238.55 | ||
| C2C1C2 | 3097.27 | 57.36 | 245.37 | ||
| tri-sLs | C1C1C2 | 2779.89 | 54.51 | 163.61 | |
| C1C2C1 | 2791.20 | 54.73 | 166.62 | ||
| C1C2C2 | 2794.62 | 54.80 | 166.96 | ||
| C2C1C2 | 2780.55 | 54.52 | 165.19 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Liu, S.; Cao, S.; Zhou, S.; Chen, Y.; Wang, Z.; Lu, X. Theoretical Investigation of the Fusion Process of Mono-Cages to Tri-Cages with CH4/C2H6 Guest Molecules in sI Hydrates. Molecules 2021, 26, 7071. https://doi.org/10.3390/molecules26237071
Wei S, Liu S, Cao S, Zhou S, Chen Y, Wang Z, Lu X. Theoretical Investigation of the Fusion Process of Mono-Cages to Tri-Cages with CH4/C2H6 Guest Molecules in sI Hydrates. Molecules. 2021; 26(23):7071. https://doi.org/10.3390/molecules26237071
Chicago/Turabian StyleWei, Shuxian, Siyuan Liu, Shoufu Cao, Sainan Zhou, Yong Chen, Zhaojie Wang, and Xiaoqing Lu. 2021. "Theoretical Investigation of the Fusion Process of Mono-Cages to Tri-Cages with CH4/C2H6 Guest Molecules in sI Hydrates" Molecules 26, no. 23: 7071. https://doi.org/10.3390/molecules26237071
APA StyleWei, S., Liu, S., Cao, S., Zhou, S., Chen, Y., Wang, Z., & Lu, X. (2021). Theoretical Investigation of the Fusion Process of Mono-Cages to Tri-Cages with CH4/C2H6 Guest Molecules in sI Hydrates. Molecules, 26(23), 7071. https://doi.org/10.3390/molecules26237071








