In Situ Stability of Anthocyanins in Lycium ruthenicum Murray
Abstract
:1. Introduction
2. Results
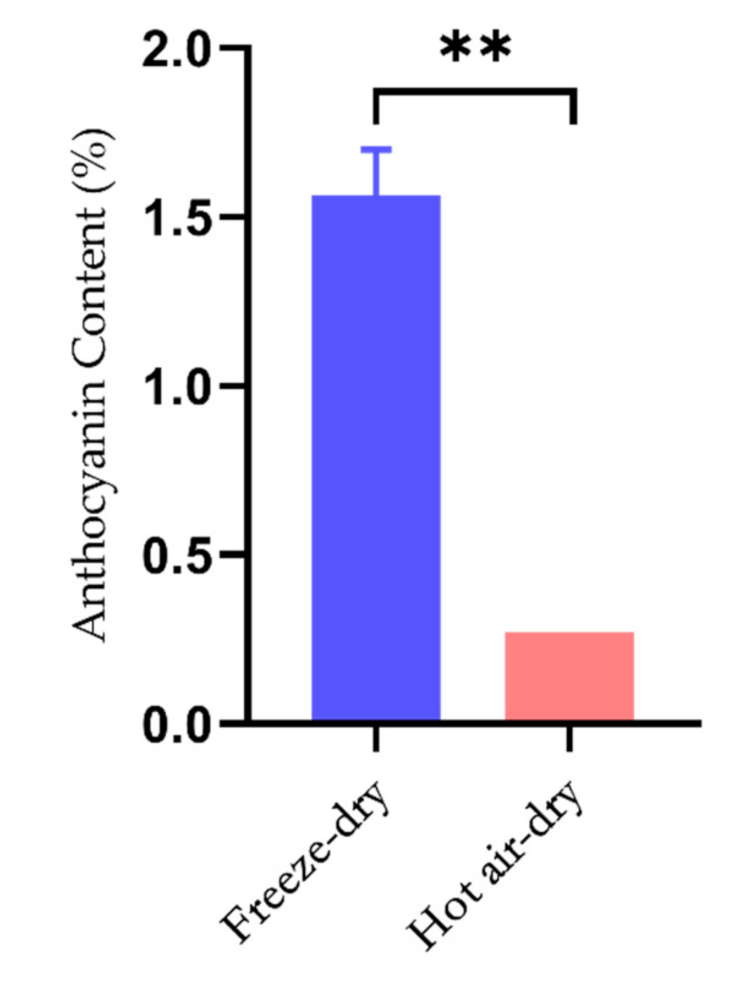
2.1. Effect of Different Drying Methods on the Stability of Anthocyanins in Lycium ruthenicum Murr.
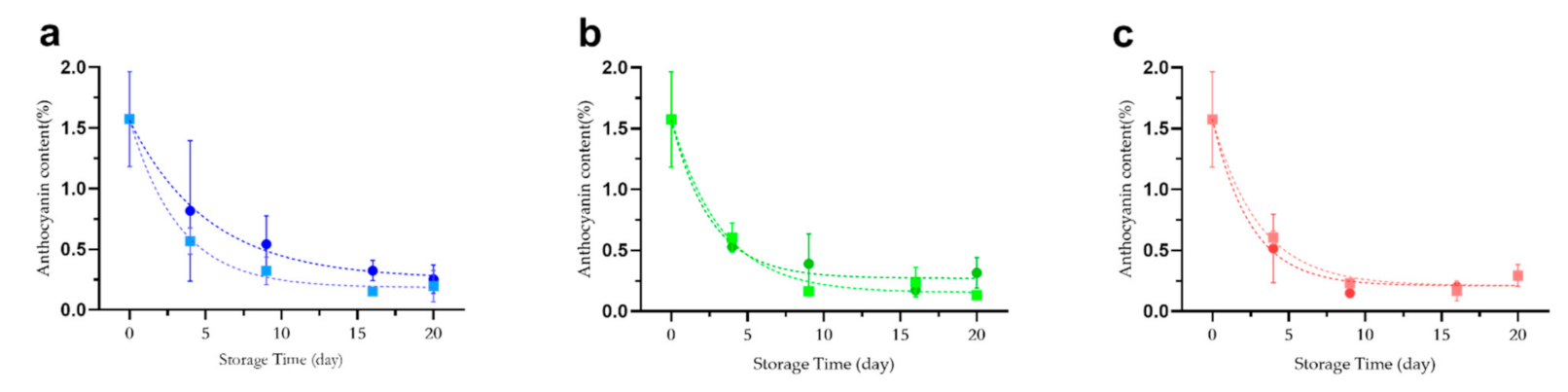
2.2. Effect of Temprature and Glucose on the Stability of Anthocyanins in Lycium ruthenicum Murr.
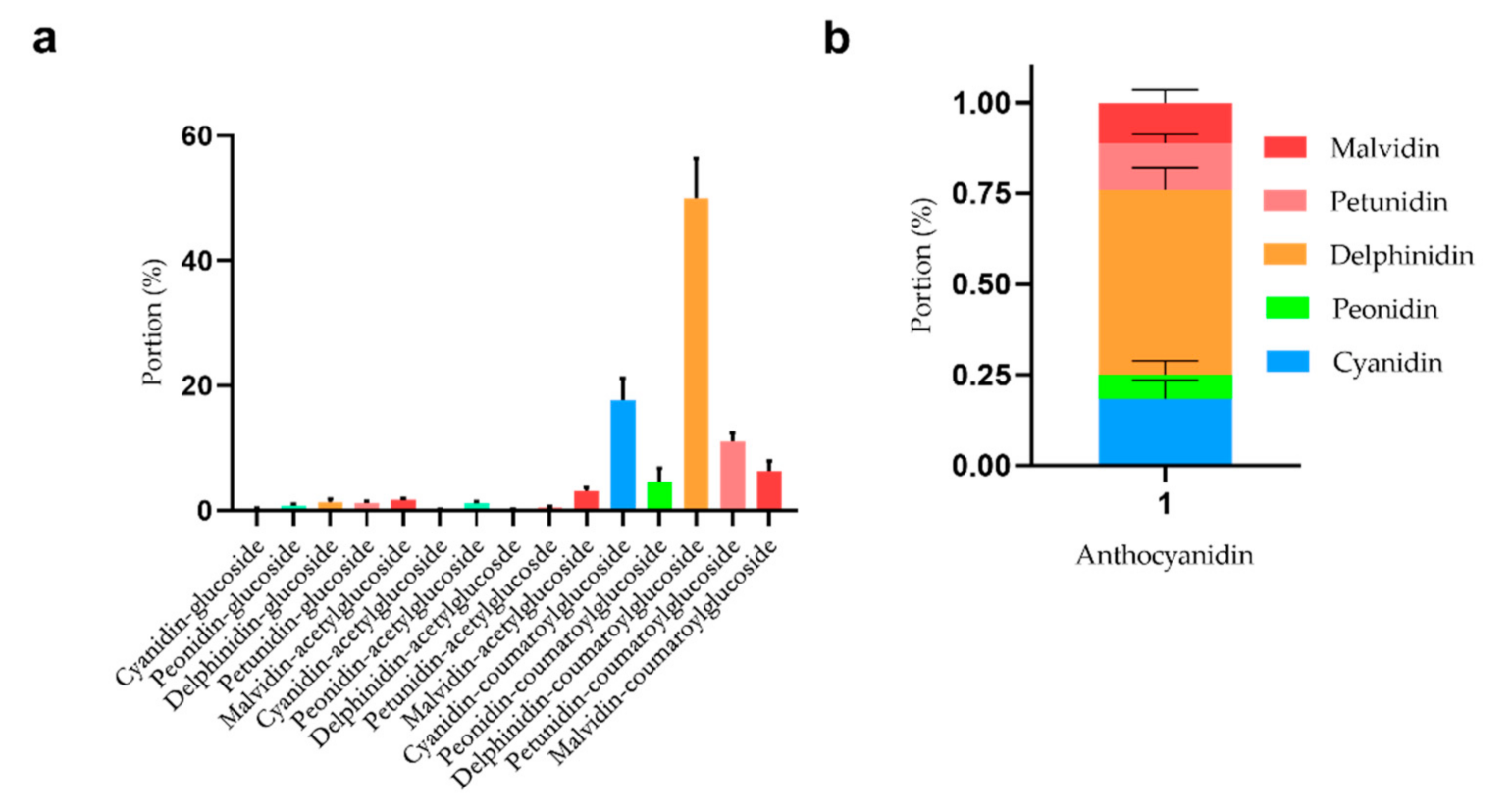
2.3. Composition Analysis and Quantification of Anthocyanins in Freeze-Dried Lycium ruthenicum Murr.
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Drying Methods
4.2.2. Storage Conditions
4.2.3. Anthocyanin Content Determination
4.2.4. Anthocyanin Composition and Quantification
4.2.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, N.; Zheng, J.; Li, W.; Suo, Y. Isolation, Stability, and Antioxidant Activity of Anthocyanins from Lycium ruthenicum Murray and Nitraria Tangutorum Bobr of Qinghai-Tibetan Plateau. Sep. Sci. Technol. 2014, 49, 2897–2906. [Google Scholar] [CrossRef]
- Tang, P.P.; Giusti, M. Black goji as a potential source of natural color in a wide pH range. Food Chem. 2018, 269, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wang, J.; Yu, Y.; Wu, Y.; He, Z. Lycium ruthenicum Murr. alleviates nonalcoholic fatty liver in mice. Food Sci. Nutr. 2020, 8, 2588–2597. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.; Li, Y.; Xiao, M.; Wang, F.; Zhou, P.; Zhang, W.; Heep, A.; Li, X. Lycium ruthenicum Murr polysaccharide protects cortical neurons against oxygen-glucose deprivation/reperfusion in neonatal hypoxic-ischemic encephalopathy. Int. J. Biol. Macromol. 2020, 158, 562–568. [Google Scholar] [CrossRef]
- Luo, Z.; Yu, G.; Chen, X.; Liu, Y.; Zhou, Y.; Wang, G.; Shi, Y. Integrated phytochemical analysis based on UHPLC-LTQ-Orbitrap and network pharmacology approaches to explore the potential mechanism of Lycium ruthenicum Murr. for ameliorating Alzheimer’s disease. Food Funct. 2020, 11, 1362–1372. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, H.; Zhang, G.; Meng, J.; Deng, K.; Zhou, W.; Wang, H.; Wang, Z.; Hu, N.; Suo, Y. Anthocyanins from Lycium ruthenicum Murr. Ameliorated d-Galactose-Induced Memory Impairment, Oxidative Stress, and Neuroinflammation in Adult Rats. J. Agric. Food Chem. 2019, 67, 3140–3149. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, F.; Yao, X.; Zhu, J.; Wang, C.; Zhang, J.; Li, X. Protective Effect of Lycium ruthenicum Murr. Against Radiation Injury in Mice. Int. J. Environ. Res. Public Health 2015, 12, 8332–8347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Q.; Xu, Q.; Yin, H.; Huang, L.; Du, Y. Characterization of an immunologically active pectin from the fruits of Lycium ruthenicum. Int. J. Biol. Macromol. 2014, 64, 69–75. [Google Scholar] [CrossRef]
- He, F.; Zhang, S.; Li, Y.; Chen, X.; Du, Z.; Shao, C.; Ding, K. The structure elucidation of novel arabinogalactan LRP1-S2 against pancreatic cancer cells growth in vitro and in vivo. Carbohydr. Polym. 2021, 267, 118172. [Google Scholar] [CrossRef]
- Xiong, L.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Goji berry (Lycium spp.) extracts exhibit antiproliferative activity via modulating cell cycle arrest, cell apoptosis, and the p53 signaling pathway. Food Funct. 2021, 12, 6513–6525. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Xia, H.; Shi, Y.; Yang, C.; Shah, M.W.; Guo, B.; Wang, L.; Sun, G. Fatty acid and mineral contents of Lycium ruthenicum Murr. and antioxidant activity against isoproterenol-induced acute myocardial ischemia in mice. Food Sci. Nutr. 2020, 8, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Liu, B.; Liu, S.; Xie, S.Z.; Pan, L.H.; Zha, X.Q.; Li, Q.M.; Luo, J.P. Structural features of an acidic polysaccharide with the potential of promoting osteoblast differentiation from Lycium ruthenicum Murr. Nat. Prod. Res. 2020, 34, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Bian, L.-h.; Yao, Z.-w.; Wang, X.-m.; Li, Q.-y.; Guo, J.-y.; Shi, J.-l. Anthocyanins in Lycium ruthenicum Murray reduce nicotine withdrawal-induced anxiety and craving in mice. Neurosci. Lett. 2021, 763, 136152. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar]
- Liu, Z.; Tang, X.; Liu, C.; Dong, B.; Shao, Y.; Liu, B.; Yue, H. Ultrasonic extraction of anthocyanins from Lycium ruthenicum Murr. and its antioxidant activity. Food Sci. Nutr. 2020, 8, 2642–2651. [Google Scholar] [CrossRef]
- Shen, M.; Liu, K.; Liang, Y.; Liu, G.; Sang, J.; Li, C. Extraction optimization and purification of anthocyanins from Lycium ruthenicum Murr. and evaluation of tyrosinase inhibitory activity of the anthocyanins. J. Food Sci. 2020, 85, 696–706. [Google Scholar] [CrossRef]
- Wang, Y.; Luan, G.; Zhou, W.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Subcritical water extraction, UPLC-Triple-TOF/MS analysis and antioxidant activity of anthocyanins from Lycium ruthenicum Murr. Food Chem. 2018, 249, 119–126. [Google Scholar] [CrossRef]
- Qin, B.; Liu, X.; Cui, H.; Ma, Y.; Wang, Z.; Han, J. Aqueous two-phase assisted by ultrasound for the extraction of anthocyanins from Lycium ruthenicum Murr. Prep. Biochem. Biotechnol. 2017, 47, 881–888. [Google Scholar] [CrossRef]
- Tang, P.; Giusti, M.M. Metal Chelates of Petunidin Derivatives Exhibit Enhanced Color and Stability. Foods 2020, 9, 1426. [Google Scholar] [CrossRef]
- Luan, G.; Wang, Y.; Ouyang, J.; He, Y.; Zhou, W.; Dong, Q.; Wang, H.; Hu, N. Stabilization of Lycium ruthenicum Murr. anthocyanins by natural polyphenol extracts. J. Food Sci. 2021, 86, 4365–4375. [Google Scholar] [CrossRef]
- Lu, Y.; Kong, X.; Zhang, J.; Guo, C.; Qu, Z.; Jin, L.; Wang, H. Composition Changes in Lycium ruthenicum Fruit Dried by Different Methods. Front. Nutr. 2021, 8, 737521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Zhao, X.Y.; Mu, J. Influence of Copigmentation on Stability of Anthocyanins from Purple Potato Peel in both Liquid State and Solid State. J. Agric. Food Chem. 2009, 57, 9503–9508. [Google Scholar] [CrossRef] [PubMed]
- Song, H.N.; Ji, S.A.; Park, H.R.; Kim, H.H.; Hogstrand, C. Impact of Various Factors on Color Stability of Fresh Blueberry Juice during Storage. Prev. Nutr. Food Sci. 2018, 23, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidot, K.; Achir, N.; Mertz, C.; Sinela, A.; Rawat, N.; Prades, A.; Dangles, O.; Fulcrand, H.; Dornier, M. Effect of Temperature on Acidity and Hydration Equilibrium Constants of Delphinidin-3-O- and Cyanidin-3-O-sambubioside Calculated from Uni- and Multiwavelength Spectroscopic Data. J. Agric. Food Chem. 2016, 64, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ding, C.X.; Wang, L.S.; Li, G.L.; Shi, J.Y.; Li, H.; Wang, H.L.; Suo, Y.R. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Rimpapa, Z.; Toromanovic, J.; Tahirovic, I.; Sapcanin, A.; Sofic, E. Total content of phenols and anthocyanins in edible fruits from Bosnia. Bosn. J. Basic Med. Sci. 2007, 7, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Lang, Y.; Li, B.; Gong, E.; Shu, C.; Si, X.; Gao, N.; Zhang, W.; Cui, H.; Meng, X. Effects of alpha-casein and beta-casein on the stability, antioxidant activity and bioaccessibility of blueberry anthocyanins with an in vitro simulated digestion. Food Chem. 2021, 334, 127526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Duan, C.Q.; Wang, J. Anthocyanins profile of grape berries of Vitis amurensis, its hybrids and their wines. Int. J. Mol. Sci. 2010, 11, 2212–2228. [Google Scholar] [CrossRef] [Green Version]
- He, J.J.; Liu, Y.X.; Pan, Q.H.; Cui, X.Y.; Duan, C.Q. Different anthocyanin profiles of the skin and the pulp of Yan7 (Muscat Hamburg × Alicante Bouschet) grape berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef]
- Jin, Z.M.; He, J.J.; Bi, H.Q.; Cui, X.Y.; Duan, C.Q. Phenolic compound profiles in berry skins from nine red wine grape cultivars in northwest China. Molecules 2009, 14, 4922–4935. [Google Scholar] [CrossRef]
| Temp | Half-Life (Day) | |
|---|---|---|
| No Addition | Glucose Addition | |
| 4 °C | 2.246 | 3.596 |
| 20 °C | 2.263 | 1.801 |
| 37 °C | 2.123 | 1.734 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fu, J.; Yang, D. In Situ Stability of Anthocyanins in Lycium ruthenicum Murray. Molecules 2021, 26, 7073. https://doi.org/10.3390/molecules26237073
Wang Y, Fu J, Yang D. In Situ Stability of Anthocyanins in Lycium ruthenicum Murray. Molecules. 2021; 26(23):7073. https://doi.org/10.3390/molecules26237073
Chicago/Turabian StyleWang, Yanping, Jingxian Fu, and Dong Yang. 2021. "In Situ Stability of Anthocyanins in Lycium ruthenicum Murray" Molecules 26, no. 23: 7073. https://doi.org/10.3390/molecules26237073
APA StyleWang, Y., Fu, J., & Yang, D. (2021). In Situ Stability of Anthocyanins in Lycium ruthenicum Murray. Molecules, 26(23), 7073. https://doi.org/10.3390/molecules26237073







