GC-MS Analysis of Biological Nitrate and Nitrite Using Pentafluorobenzyl Bromide in Aqueous Acetone: A Dual Role of Carbonate/Bicarbonate as an Enhancer and Inhibitor of Derivatization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
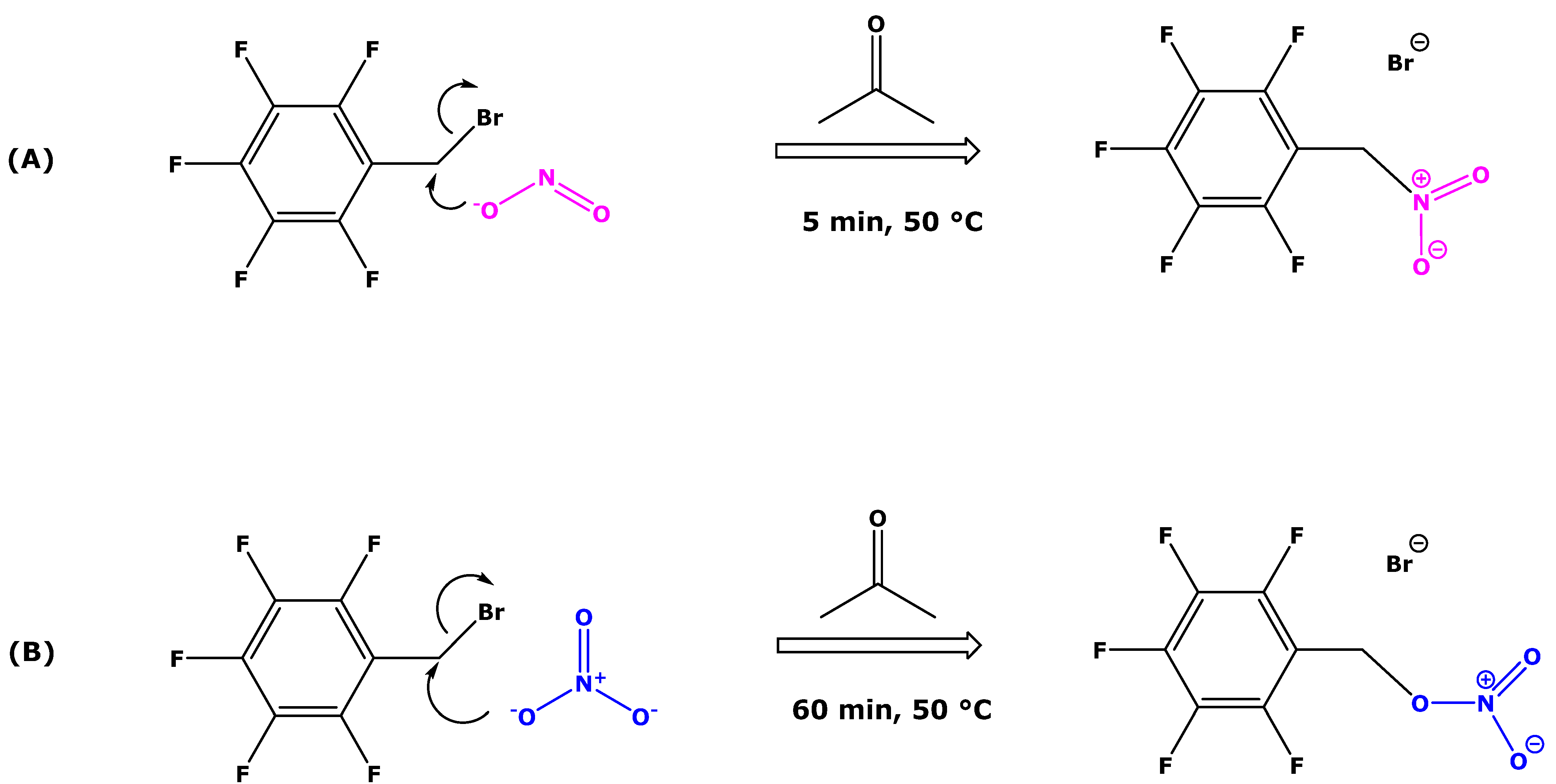
2.2. Derivatization Procedures for Nitrite and Nitrate and GC-MS Analyses
2.3. Analyses in Urine Samples Collected in Previous Studies
2.4. Statistical Analysis
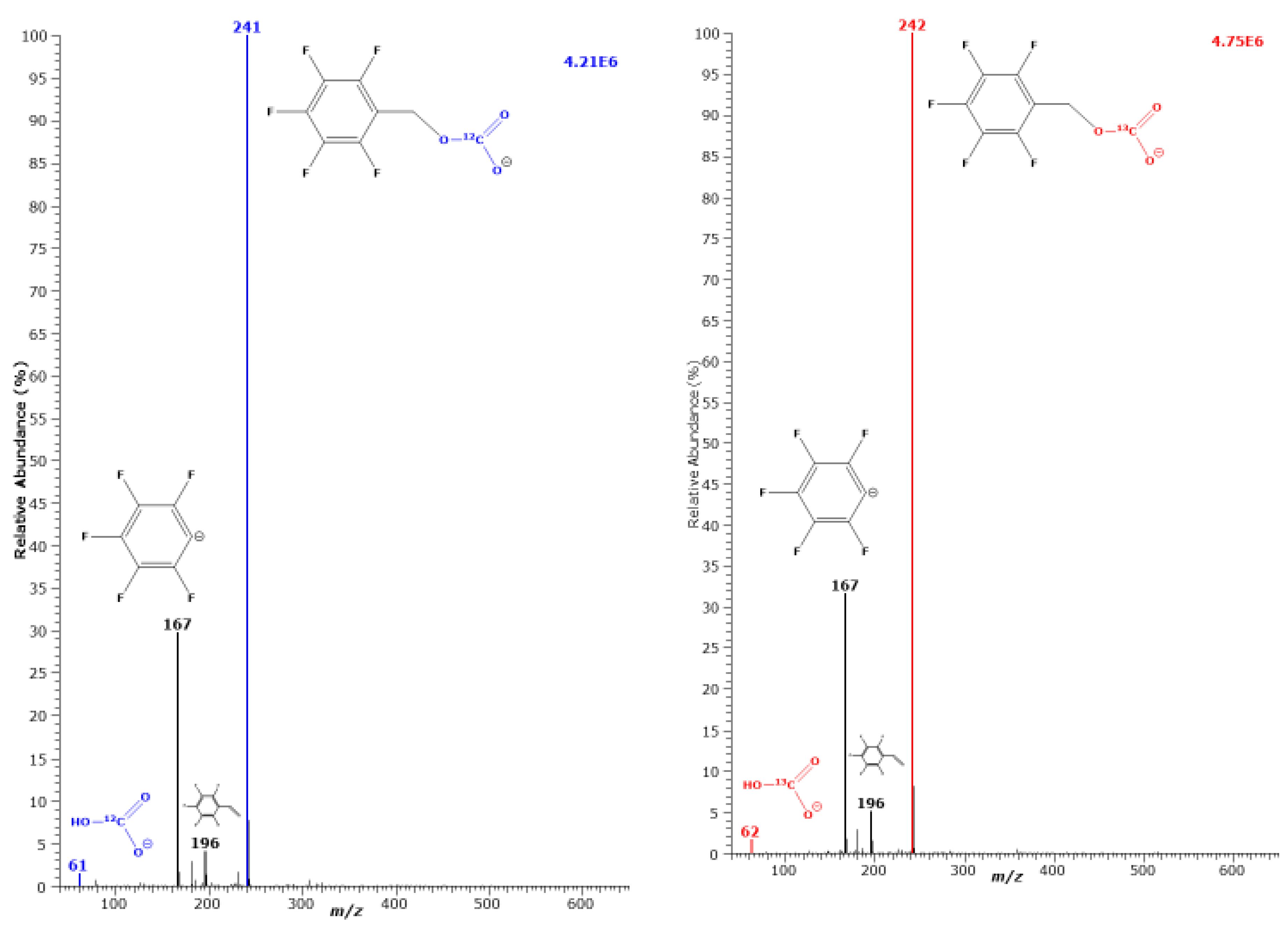
3. Results
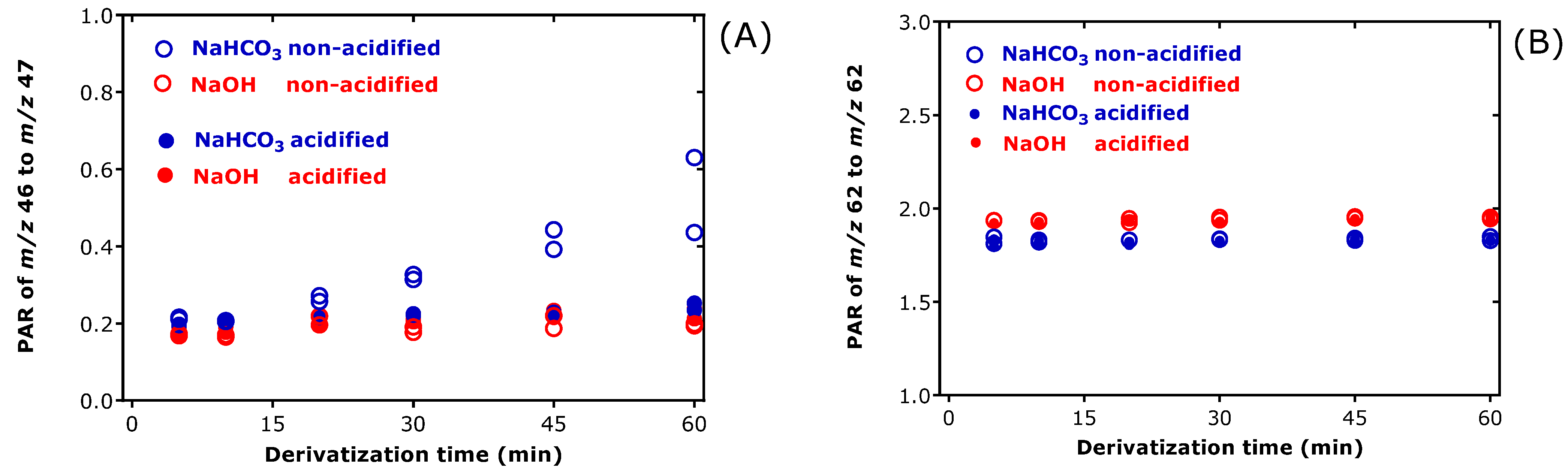
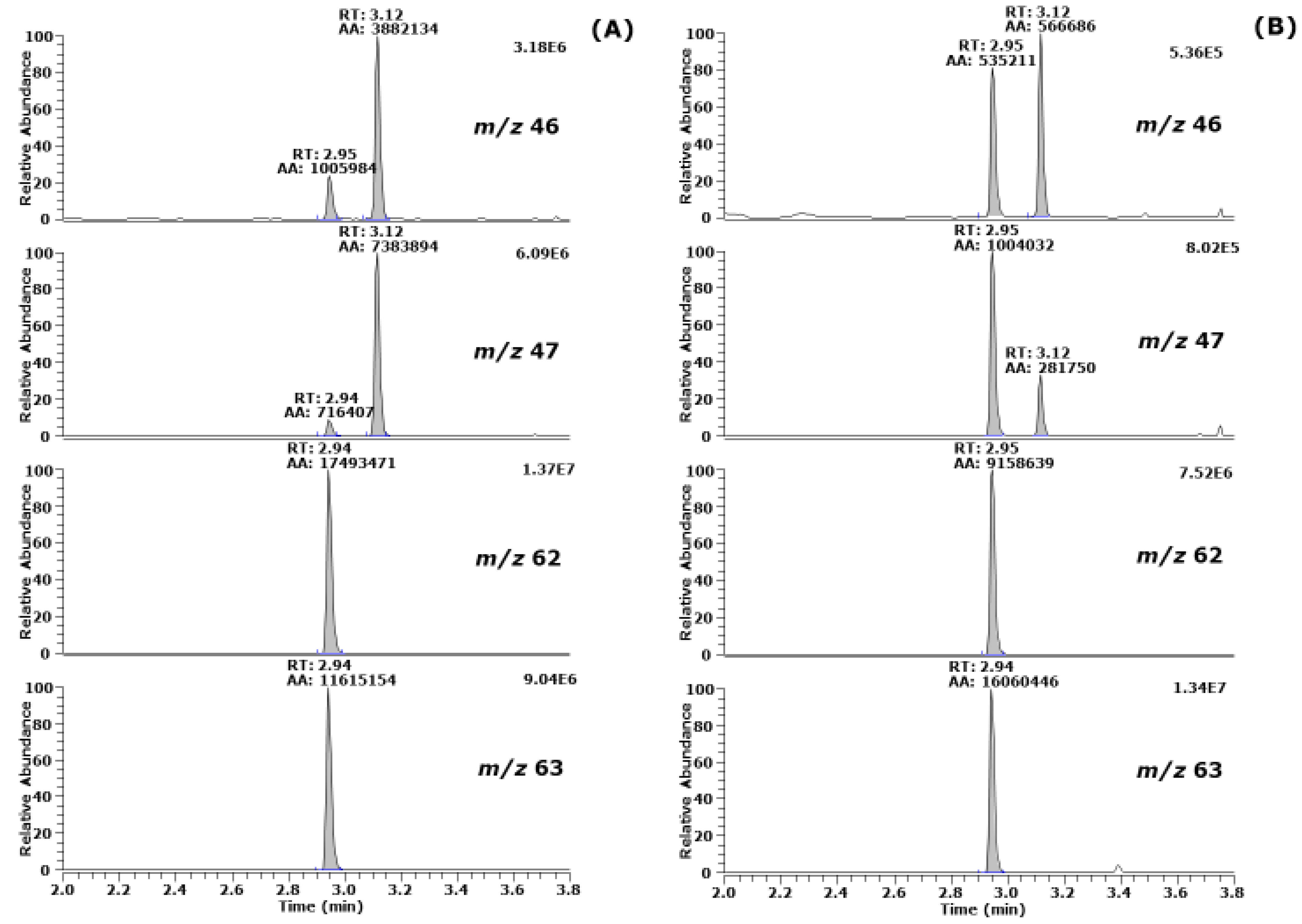
3.1. Effect of Exogenous Bicarbonate and Hydroxide on the Derivatization of [15N]nitrate, [15N]nitrite and Endogenous Nitrate and Nitrite in Human Urine
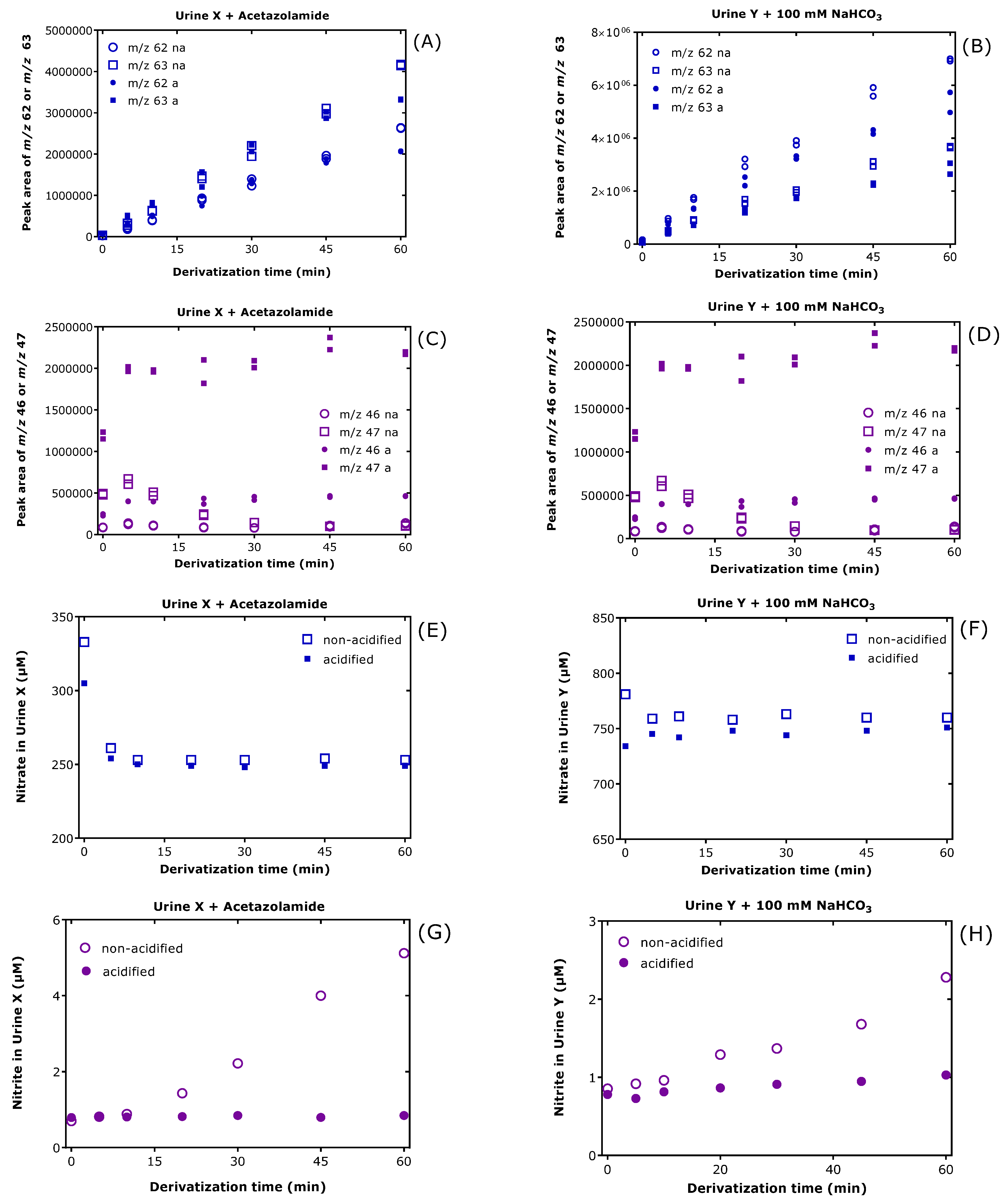
3.2. Effect of Exogenous Bicarbonate on the Derivatization of [15N]nitrate, [15N]nitrite and Endogenous Nitrate and Nitrite in Human Urine
3.3. Effects of Exogenous and Endogenous Bicarbonate, Acidification and Derivatization Time on the Derivatization of Nitrate and Nitrite in Human Urine
3.4. Effect of Exogenous Bicarbonate on the Derivatization of [15N]nitrite and Endogenous Nitrite in Human Plasma
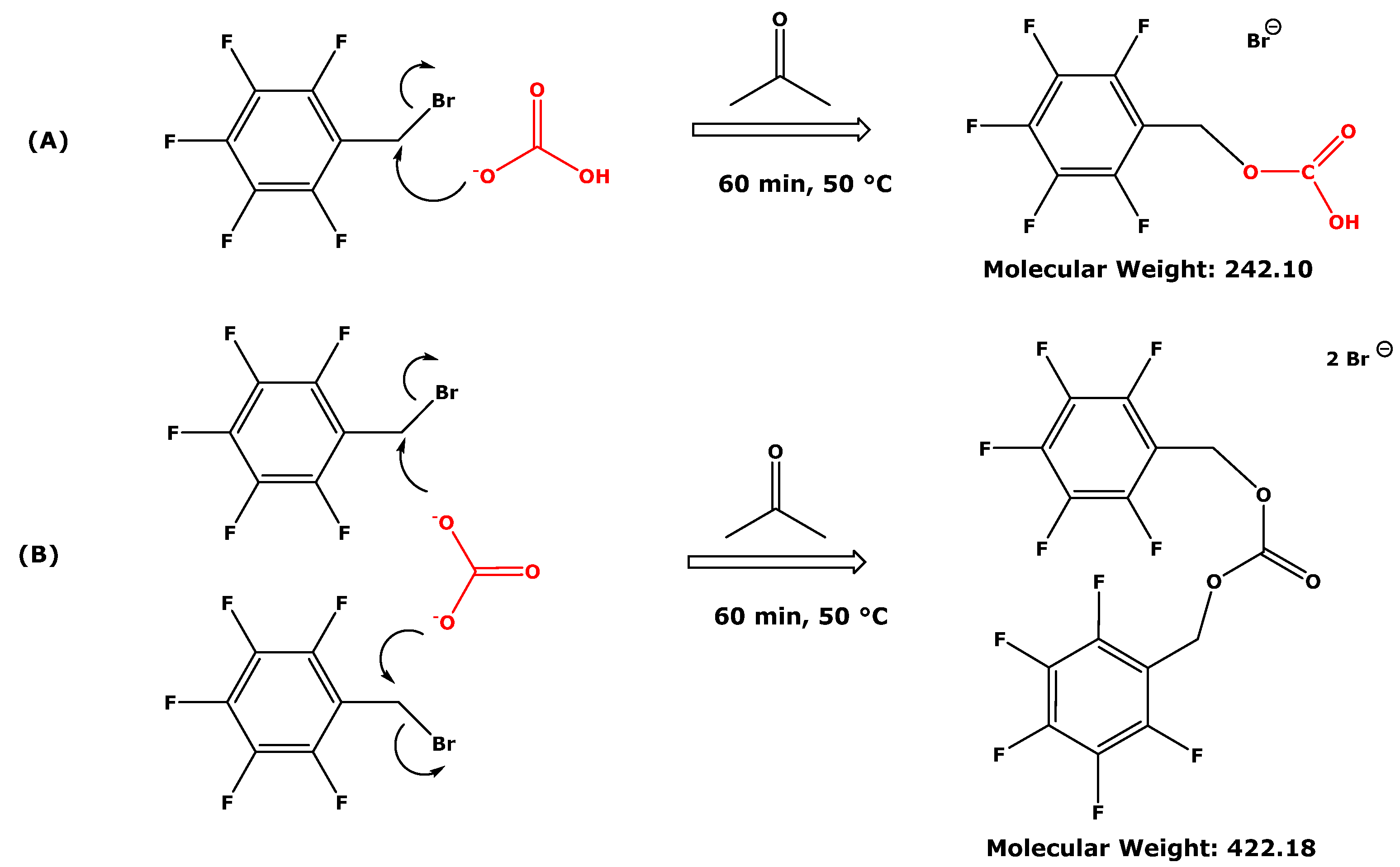
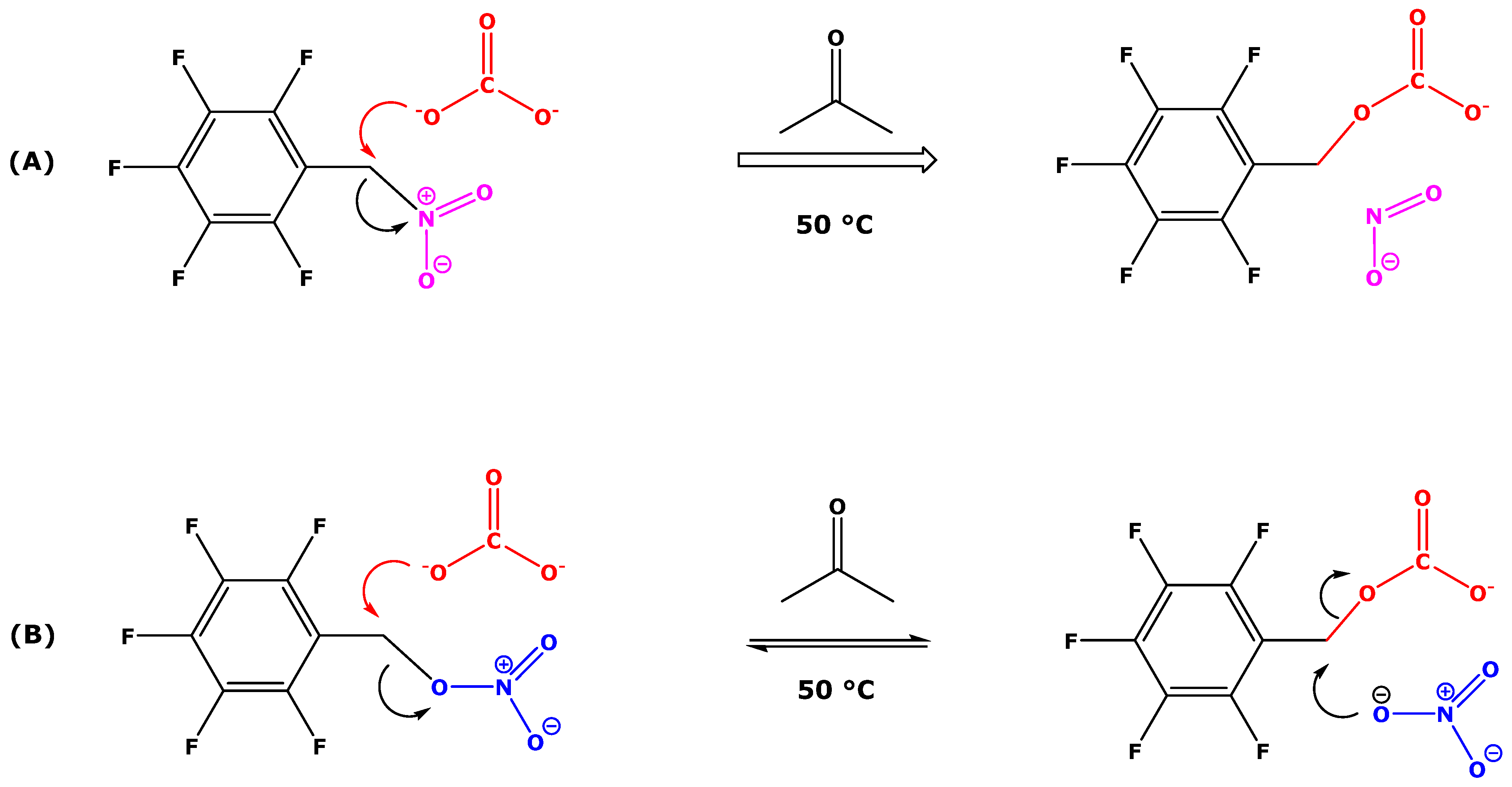
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tsikas, D. Pentafluorobenzyl bromide-A versatile derivatization agent in chromatography and mass spectrometry: I. Analysis of inorganic anions and organophosphates. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1043, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal. Chem. 2000, 72, 4064–4072. [Google Scholar] [CrossRef] [PubMed]
- Arabaci, B.; Gulcin, I.; Alwasel, S. Capsaicin: A potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2014, 19, 10103–10114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topal, F.; Gulcin, I.; Dastan, A.; Guney, M. Novel eugenol derivatives: Potent acetylcholinesterase and carbonic anhydrase inhibitors. Int. J. Biol. Macromol. 2017, 94, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Chobanyan-Jürgens, K. Quantification of carbonate by gas chromatography-mass spectrometry. Anal. Chem. 2010, 82, 7897–7905. [Google Scholar] [CrossRef] [PubMed]
- Chobanyan-Jürgens, K.; Schwarz, A.; Böhmer, A.; Beckmann, B.; Gutzki, F.M.; Michaelsen, J.T.; Stichtenoth, D.O.; Tsikas, D. Renal carbonic anhydrases are involved in the reabsorption of endogenous nitrite. Nitric Oxide 2012, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 28, 1233–1249. [Google Scholar] [CrossRef] [PubMed]
- Boztaş, M.; Çetinkaya, Y.; Topal, M.; Gülçin, I.; Menzek, A.; Şahin, E.; Tanc, M.; Supuran, C.T. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxybromophenol derivatives incorporating cyclopropane moieties. J. Med. Chem. 2015, 58, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, H.L.; Kou, H.S.; Chen, S.H.; Wu, S.M. Derivatization-gas chromatographic determination of captopril. Anal. Lett. 1995, 28, 1465–1481. [Google Scholar] [CrossRef]
- Tsikas, D.; Thum, T.; Becker, T.; Pham, V.V.; Chobanyan, K.; Mitschke, A.; Beckmann, B.; Gutzki, F.M.; Bauersachs, J.; Stichtenoth, D.O. Accurate quantification of dimethylamine (DMA) in human urine by gas chromatography-mass spectrometry as pentafluorobenzamide derivative: Evaluation of the relationship between DMA and its precursor asymmetric dimethylarginine (ADMA) in health and disease. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Chobanyan, K.; Mitschke, A.; Gutzki, F.M.; Stichtenoth, D.O.; Tsikas, D. Accurate quantification of dimethylamine (DMA) in human plasma and serum by GC-MS and GC-tandem MS as pentafluorobenzamide derivative in the positive-ion chemical ionization mode. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 51, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Chen, S.H.; Lin, S.J.; Hwang, W.R.; Funazo, K.; Tanaka, M.; Shono, T. Gas chromatographic determination of inorganic anions as pentafluorobenzyl derivatives. J. Chromatogr. A 1983, 269, 183–190. [Google Scholar]
- Tsikas, D.; Böhmer, A.; Gros, G.; Endeward, V. Evidence of the chemical reaction of 18O-labeled nitrite with CO2 in aqueous buffer of neutral pH and the formation of 18OCO by isotope ratio mass spectrometry. Nitric Oxide 2016, 55, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Wolf, A.; Mitschke, A.; Gutzki, F.M.; Will, W.; Bader, M. GC-MS determination of creatinine in human biological fluids as pentafluorobenzyl derivative in clinical studies and biomonitoring: Inter-laboratory comparison in urine with Jaffe, HPLC and enzymatic assays. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Fauler, J.; Frölich, J.C. Determination of chloride in biological fluids as pentafluorobenzylchloride by reversed-phase high-performance liquid chromatography and UV detection. Chromatographia 1992, 33, 317–320. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikas, D. GC-MS Analysis of Biological Nitrate and Nitrite Using Pentafluorobenzyl Bromide in Aqueous Acetone: A Dual Role of Carbonate/Bicarbonate as an Enhancer and Inhibitor of Derivatization. Molecules 2021, 26, 7003. https://doi.org/10.3390/molecules26227003
Tsikas D. GC-MS Analysis of Biological Nitrate and Nitrite Using Pentafluorobenzyl Bromide in Aqueous Acetone: A Dual Role of Carbonate/Bicarbonate as an Enhancer and Inhibitor of Derivatization. Molecules. 2021; 26(22):7003. https://doi.org/10.3390/molecules26227003
Chicago/Turabian StyleTsikas, Dimitrios. 2021. "GC-MS Analysis of Biological Nitrate and Nitrite Using Pentafluorobenzyl Bromide in Aqueous Acetone: A Dual Role of Carbonate/Bicarbonate as an Enhancer and Inhibitor of Derivatization" Molecules 26, no. 22: 7003. https://doi.org/10.3390/molecules26227003
APA StyleTsikas, D. (2021). GC-MS Analysis of Biological Nitrate and Nitrite Using Pentafluorobenzyl Bromide in Aqueous Acetone: A Dual Role of Carbonate/Bicarbonate as an Enhancer and Inhibitor of Derivatization. Molecules, 26(22), 7003. https://doi.org/10.3390/molecules26227003





