Abstract
Syzygium cumini (Pomposia) is a well-known aromatic plant belonging to the family Myrtaceae, and has been reported for its various traditional and pharmacological potentials, such as its antioxidant, antimicrobial, anti-inflammatory, and antidiarrheal properties. The chemical composition of the leaf essential oil via gas chromatography–mass spectrometry (GC/MS) analysis revealed the identification of fifty-three compounds representing about 91.22% of the total oil. The identified oil was predominated by α-pinene (21.09%), followed by β-(E)-ocimene (11.80%), D-limonene (8.08%), β-pinene (7.33%), and α-terpineol (5.38%). The tested oil revealed a moderate cytotoxic effect against human liver cancer cells (HepG2) with an IC50 value of 38.15 ± 2.09 µg/mL. In addition, it effectively inhibited acetylcholinesterase with an IC50 value of 32.9 ± 2.1 µg/mL. Furthermore, it showed inhibitory properties against α-amylase and α-glucosidase with IC50 values of 57.80 ± 3.30 and 274.03 ± 12.37 µg/mL, respectively. The molecular docking studies revealed that (E)-β-caryophyllene, one of the major compounds, achieved the best docking scores of −6.75, −5.61, and −7.75 for acetylcholinesterase, α-amylase, and α-glucosidase, respectively. Thus, it is concluded that S. cumini oil should be considered as a food supplement for the elderly to enhance memory performance and for diabetic patients to control blood glucose.
1. Introduction
Syzygium cumini (L.) is an aromatic evergreen plant belonging to the family Myrtaceae, originating in Asia and widely distributed in America [1]. It is commonly known as jamum or jambul in Asia and as jambolão or jamelão in Brazil [2]. Traditionally, it has acquired great value in the Ayurveda and Unani systems of medication for possessing various treatment applications such as digestive, carminative, anthelmintic, antiulcer, bronchitis, antiasthma, antiallergic, diuretic, antiscorbutic, and wound healing [3,4]. Further, a wide range of pharmacological studies have been carried out on S. cumini that have proven its therapeutic potential as an antioxidant, antidiabetic, antimicrobial, anti-HIV, anti-inflammatory, and antidiarrheal [5,6,7]. Phytochemical investigations have shown that the plant is rich in flavonoids, tannins, anthocyanins, triterpenoids, essential oil, vitamins, and fatty acids [8,9]. Interestingly, the fruits of Syzygium cumini are purplish black in color with a pleasant odor, are edible with a high nutritional value, and are involved in many food products such as ice cream, jam, jellies, and yogurt [10]. Previously, the oil isolated from S. cumini leaves growing in Brazil exerted antioxidant, molluscicidal, and leishmanicidal effects [11,12].
Recently, the inhibition of key enzymes involved in the pathogenesis of disease has become a most effective therapeutic technique [13]. For instance, the most widely accepted strategy for the management of Alzheimer’s disease is to inhibit acetylcholinesterase (AChE) [14]. Furthermore, the inhibition of carbohydrate-metabolizing enzymes such as α-amylase and α-glucosidase is an effective mechanism to control hyperglycemia in diabetic patients [15]. Interestingly, synthetic drugs such as galantamine and acarbose are well-developed enzyme inhibitors in the pharmaceutical market for the management of Alzheimer’s disease and diabetes mellitus, but these drugs are known for side effects, such as hepatic injury and bowel disorders [16,17]. Consequently, medicinal plants have great value in an era of natural enzyme inhibitors to overcome the struggles of synthetic drugs [18]. Several essential oils from the genus Syzygium exploit enzyme inhibitory activities [19]. For example, S. aromaticum oil has shown remarkable antidiabetic activity via its inhibitory effect against α-amylase [20].
Molecular docking was chosen as the most suitable method to assess the underlying mechanism of inhibitory action for the pharmacologically active components, thus helping us understand the interactions of the enzyme with the major oil components [21]. In turn, molecular modeling provides information about the most appropriate geometry and binding affinity of the tested components (ligand) to the active site of the enzymes (target macromolecules) [22].
The present study was designed to investigate the chemical composition and enzyme inhibitory properties of the essential oil isolated from Syzygium cumini (L.) leaves grown in Egypt. The enzyme inhibitory assays were evaluated against AChE, α-amylase, and α-glucosidase. An added objective was to evaluate the binding affinities between the characterized major oil components and the tested enzymes using molecular docking studies.
As the essential oils are mostly safe with few complications, they can be a valuable non-medicinal option or coupled with conventional care for some health conditions, provided safety and quality issues are taken into consideration [23].
2. Results and Discussion
2.1. GC/MS Analysis of Essential Oil
The GC/MS analysis revealed identification of fifty-three compounds, representing about 91.22% of total oil as shown in Figure 1 and Table 1. The major components of the oil were found to be α-pinene (21.09%), followed by β-(E)-ocimene (11.80%), D-limonene (8.08%), β-pinene (7.33%), α-terpineol (5.38%), (E)-β-caryophyllene (4.51%), and myrcene (3.90%). The oil was predominated by hydrocarbon monoterpenes representing about 61.82%, followed by oxygenated monoterpenes (15.17%), hydrocarbon sesquiterpenes (8.18%), and oxygenated sesquiterpenes (6.02%).
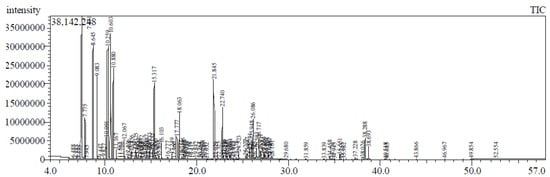
Figure 1.
GC chromatogram of essential oil isolated from Syzigium cumini leaves grown in Egypt.

Table 1.
Chemical composition (%) of essential oil identified from Syzigium cumini leaves grown in Egypt using GC/MS analysis.
Previous reports concerning the essential oil of Syzygium revealed differences in its chemical composition relative to its geographical collection area. The major compounds of the essential oil of leaves of S. cumini (L.) Skeels, collected from the southwestern region of Brazil are sesquiterpenes, namely, α-caryophyllene and β-caryophyllen, α-terpineol, and iso-caryophyllene, along with caryophyllenyl alcohol and oxide [1]. The essential oil of S. cumini collected from India showed τ-cadinol and τ-muurolol to be the major compounds, followed by τ-globulol, caryophyllene, δ-cadinene, and α-pinene [24]. The collected S. cumini leaves from Pakistan revealed the major compounds to be β-farnesene, cis-β-ocimene, terpinen-4-ol, fenchol, β-myrcene, and γ-cadinene [25]. Another report revealed that the principal components of the essential oil isolated from S. jambos collected from Brazil to be (E)-caryophyllene, α-humulene, α-zingibirene, hydroxytoluene butylated, caryophyllene alcohol, caryolan-8-ol, caryophyllene oxide, thujopsan-2-α-ol, and n-heneicosane [26].
Previous studies of the essential oil content of S. cumini leaves from Egypt revealed components that were comparable to our results, α-pinene, β-pinene, trans-caryophyllene, and α-limonene [12]. The chemical constituents of another species collected from a private garden on the Cairo–Alexandria desert road, Egypt, namely, S. aqueum and S. samarangense, showed differences from S. cumini, whereby the essential oil of leaves of S. aqueum showed a high percentage of α-selinene followed by β-caryophyllene and β-selinene, and Germacrene D was found to be the major constituent in S. samarangense essential oil [27]. From these previous reports, it is obvious that the essential oil composition shows variability between different Syzygium species along with their geographical distribution.
2.2. Cytotoxic Activity
Essential oils are well known for their richness with oxygenated and non-oxygenated components, such as monoterpenes and sesquiterpenes, and previous reports have revealed their impact on several cancer cell lines [28,29]. The results of the cytotoxicity of the essential oil of S. cumini leaves revealed its inhibitory effect on human liver cancer cells (HepG2), with an IC50 value of 38.15 ± 2.09 µg/mL as compared to staurosporine (IC50 = 8.637 ± 0.47 µg/mL) as a reference drug.
In agreement with our results, α-pinene, one of the major components of S. cumini oil, showed inhibition toward hepatoma carcinoma BEL-7402 cells with an inhibitory rate of 79.3% in vitro and 69.1% in vivo through the suppression of growth of tumor cells in tumor-bearing mice. Additionally, α-pinene induced a significant increase in the G2/M population of the hepatoma cell line (BEL-7402) [30]. One of the major components of S. cumini oil is β-caryophyllene, which has shown potent cytotoxicity towards human oral squamous (OEC-M1) cells, human hepatocellular carcinoma (J5) cells, human lung adenocarcinoma (A549) cells, human colon (HT-29) cells, human melanoma (UACC-62) cells, and human leukemic (K562) cells with IC50 values of 24.0, 111.2, 31.3, 9.8, 3.2, and 4.6 μg/mL, respectively (Su and Ho 2013). In addition, it has exhibited cytotoxicity towards MCF-7, MDA-MB-468, UACC257, A549, Hela, and HT-29 cancer cell lines [31,32].
2.3. Acetylcholinesterase Inhibition
The acetylcholinesterase enzyme is responsible for the hydrolysis of acetylcholine, which is considered a key enzyme in the treatment of Alzheimer’s disease. Several plants have been reported for their inhibitory activity against acetylcholinesterase [33,34,35]. The essential oil of S. cumini showed moderate inhibitory ability against AChE with an IC50 value of 32.90 ± 2.10 µg/mL as compared to donepezil (IC50 = 7.89 ± 1.30 µg/mL) as a reference drug.
The essential oil of S. cumini leaves showed promising anti-cholinesterase inhibitory activity that could be attributed to the presence of some volatile compounds such as monoterpenes: α-pinene, β-pinene, and β-(E)-ocimene being major compounds; along with the presence of sesquiterpenes, such as (E)-β-caryophyllene, which has been supported by previous investigations [36,37,38,39]. Mohamed et al. reported on the antioxidant activity of the essential oil of S. cumini leaves using ferric reducing power (FRAP) assays [12]. They showed that the highest ferric reducing power property was 0.47 mg/100 mg of essential oil, which correlated with the presence of a mixture of monoterpene hydrocarbons and oxygen containing mono- and sesquiterpenes. In addition, S. cumini leaves have been reported for their traditional use as a natural antioxidant agent [12]. The essential oil of S. cumini leaves isolated from the mature trees in Pakistan showed DPPH radical scavenging activity with an IC50 value of 1.2 mg/mL [24]. The potential activity of the essential oil components of S. cumini leaves, as new AChE inhibitors and antioxidants, could be considered a potential strategy for treating and decreasing the progress of Alzheimer’s disease [39,40].
2.4. α-Amylase and α-Glucosidase Inhibition
Inhibition of the α-amylase enzyme has been reported in previous studies to be one of the effective approaches for diabetes management through reduction in the postprandial hyperglycemia associated with type 2 diabetes mellitus [41,42]. Regarding our results, the essential oil of S. cumini leaves showed α-amylase inhibitory ability with an IC50 value of 57.80± 3.30 µg/mL as compared to acarbose (IC50 = 34.71 ± 2.30 µg/mL) as a reference drug. In addition, it showed moderate α-glucosidase inhibitory properties with an IC50 value of 274.03 ± 12.37 µg/mL as compared to acarbose (IC50 = 138.76 ± 7.59 µg/mL).
The antidiabetic effects of the S. cumini volatile components as enzymes inhibitors could be attributed to the presence of oxygenated compounds, such as monoterpene and sesquiterpene, which can bind non-selectively to amino and sulfhydryl groups of enzymes and cause a conformational change and loss of activity [43]. Previous reports concerning the seed extract of S. cumini in south India have shown an α-amylase enzyme inhibitory effect of up to 95.4% [44]. In accordance with these previous reports, we found that α- and β-pinene showed α-amylase inhibitory ability that suggests their responsibility for the observed inhibitory activity of the essential oil of S. cumini [45,46].
2.5. Molecular Docking
In this section, we aimed to identify the molecular mechanism of action as well as the binding mode of the identified compounds. Therefore, the crystal structures of the acetylcholinesterase, α-amylase, and α-glucosidase were downloaded from the PDB and prepared for docking. The docking protocol was roughly validated by re-docking each o-crystalized ligand into its corresponding active site. The calculated RMSD between the co-crystalized pose and the docked pose was 0.53, 0.82, and 0.76 for AChE, α-amylase, and α-glucosidase, respectively, highlighting the validity of the docking. To benchmark our docking results so far, the docking score of each crystal reference was taken into consideration when comparing the docking scores of the seven selected compounds. As depicted in Table 2, all the major compounds in the isolated oil achieved favorable-accepted scores with the three targeted enzymes. Taking into account the hydrophobic nature of the isolated oil—composed entirely from hydro-carbonic compounds—all the interactions formed between any compounds within the three targets were found to be hydrophobic in nature (see Figure 2). Among the tested seven compounds, (E)-β-caryophyllene was the best compound to interact with the three enzymes, achieving docking scores of −6.75, −5.61, and −7.75 for AChE, α-amylase, and α-glucosidase, respectively. On the second rank after (E)-β-caryophyllene came β-(E)-ocimene, myrcene, and α-terpineol for AChE, α-amylase, and α-glucosidase, respectively (see Figure 3).

Table 2.
The docking scores achieved by the major identified compounds against different enzymes.
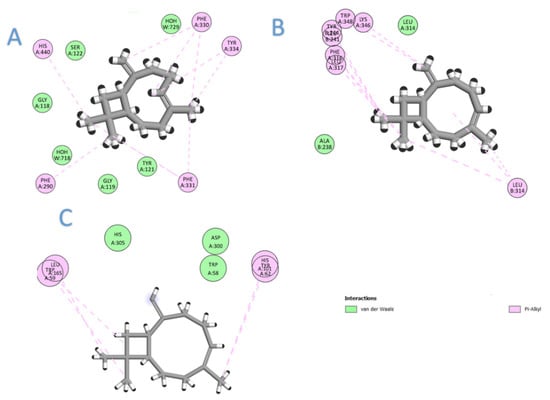
Figure 2.
The 2D interaction diagram of (E)-β-caryophyllene with the three targets: (A) acetylcholinesterase; (B) α-glucosidase; (C) α-amylase.
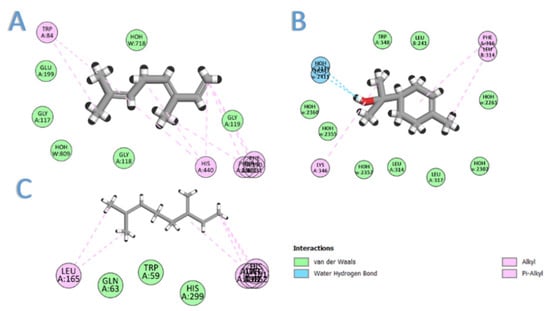
Figure 3.
The 2D interaction diagram of: (A) β-(E)-ocimene with acetylcholinesterase; (B) α-terpineol with α-glucosidase; (C) myrcene with α-amylase.
In conclusion, from the major oils, (E)-β-caryophyllene was the best compound to interact with the three enzymes, achieving docking scores of −6.75, −5.61, and −7.75 for AChE, α-amylase, and α-glucosidase, respectively. Compounds achieved acceptable scores with these three enzymes through the establishment of many hydrophobic interactions. It is worth mentioning that no compound was able to single-handedly overcome the scores achieved by any co-crystalized ligand, which highlights that their biological effects come from synergistic contributions from all the compounds.
3. Materials and Methods
3.1. Plant Material
Fresh leaves of Syzygium cumini (L.) were collected from a private garden in the Abo-Zabal area (N 30°17′43.386′′, E 31°22′27.9804′′), Qualiobya, Egypt, in February 2021. They were kindly authenticated by taxonomy specialist engineer, Therease Labib, the taxonomy specialist at El-Orman Botanical Garden, Giza, Egypt. A voucher specimen, PHG-P-SC-348, was deposited at the Pharmacognosy Department, Faculty of Pharmacy, Ain Shams University.
3.2. Isolation of the Essential Oil
The fresh leaves were finely cut and hydrodistilled for 5 h using a Clevenger apparatus. After hydrodistillation, the essential oil was isolated and kept in a sealed glass tube at −4 °C until GC/MS analysis.
3.3. Gas Chromatography–Mass Spectrometry (GC/MS)
The GC/MS analysis of the resulting oil was carried out at the Department of Medicinal and Aromatic Plants Research, National Research Centre, with the following specifications. Instrument: a TRACE GC Ultra Gas Chromatographs (THERMO Scientific Corp., Waltham, MA, USA), coupled with a thermo mass spectrometer detector (ISQ Single Quadrupole Mass Spectrometer). The GC–MS system was equipped with a TG-5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness). Analysis was carried out using helium as carrier gas at a flow rate of 1.0 mL/min and a split ratio of 1:10 using the following temperature program: 80 °C for 2 min; rising 5.0 °C/min to 300 °C; and held for 5 min. The injector and detector were held at 280 °C, and 0.2 μL of diluted samples (1:10 hexane, v/v) was injected. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z 35–500.
3.4. Identification of Oil Components
The components of the essential oil were characterized by comparison of their GC/MS spectra, fragmentation patterns, and retention indices with those reported in the literature data [28,47]. The retention indices were calculated relative to a homologous series of n-alkanes (C8-C28) injected under the same conditions.
3.5. Assessment of Cytotoxic Activity
The cytotoxicity of the essential oil of S. cumini leaves was evaluated against human liver cancer cells (HepG2) using MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay [28,48,49]. The cell lines were obtained from American Type Culture Collection. The cell viability was evaluated based on the reduction in MTT by the metabolically active cells using staurosporine as a reference drug (Sigma-Aldrich chemicals). The results were expressed as the concentration that induces 50% of maximum inhibition of cell proliferation (IC50) from graphic plots of the dose–response curve for each concentration using Graphpad Prism software (San Diego, CA, USA).
3.6. Assessment of Enzyme Inhibitory Activities
3.6.1. Acetylcholinesterase Inhibition Assay
Cholinesterase (ChE) inhibitory activity was evaluated using acetylcholinesterase activity colorimetric assay kit (Bio-vision company; K197-100), following Ellman’s method as previously described [17]. About 10 μL of essential oil was mixed with colorimetric substrate (DTNB) and AChE solution in Tris–HCl buffer (pH 8.0) in a 96-well microplate and incubated for 10–15 min at room temperature away from light. The reaction was based on the ability of an active human AchE enzyme to hydrolyze the provided colorimetric substrate, producing a yellow compound. Similarly, a blank was prepared by addition of a sample solution to all reaction reagents without enzyme solution. Donepezil was used as the positive control (Sigma-Aldrich, St. Louis, MO, USA). The absorbance of the sample, blank, and standard was measured at 412 nm after 10 min incubation at room temperature. The absorbance of the blank was subtracted from the value of the sample, and the results were recorded as IC50.
3.6.2. α-Amylase Inhibition Assay
The inhibitory activity of the tested oil was performed according to the standard method with minor modifications [50]. The enzyme solution was prepared by dissolving α-amylase in 20 mM phosphate buffer (PH = 6.9) at a concentration of 0.50 mg/mL. Then, 1 mL of the tested oil of various concentrations (1000–7.81 μg/mL) and 1 mL of enzyme solution were mixed and incubated at 25 °C for 10 min. After incubation, 1 mL of starch (0.50%) solution was added to the mixture and further incubated at 25 °C for 10 min. The reaction was then stopped by adding 2 mL of dinitro salicylic acid (3,5-dinitrosalicylic acid, color reagent) and heating the reaction mixture in a boiling water bath for 5 min. After cooling, the absorbance was measured colorimetrically at 565 nm. Acarbose was used as a standard drug. The inhibition percentage was calculated using the given formula: % inhibition = (1 − As/Ac) × 100, where As was the absorbance of the tested compound, and Ac was the absorbance of the control reaction (containing all reagents except the test sample). The IC50 value was defined as the concentration of the α-amylase inhibitor to inhibit 50% of its activity under the assay’s conditions.
3.6.3. α-Glucosidase Inhibition Assay
The α-glucosidase inhibitory activity was performed according to the previously reported method using BioVision’s α-glucosidase inhibitor screening kit (K938-100) [17]. About 10 µL of leaf oil was mixed with glutathione (10 µL), α-glucosidase solution (10 µL) in phosphate buffer (pH 6.8), and PNPG (4-nitrophenyl-α-D-glucopyranoside) (10 µL) in a 96-well microplate and incubated for 15–20 min at room temperature. Similarly, a blank was prepared by adding the sample solution to all reaction reagents without α-glucosidase solution. The reaction utilized the ability of an active α-glucosidase to cleave a synthetic substrate (PNPG), releasing a chromophore (p-nitrophenol; OD = 410 nm). The reaction was then stopped with the addition of sodium carbonate (50 µL, 0.2 M). The absorbance of the tested oil and blank was read at 410 nm. The absorbance of the blank was subtracted from the values of the tested oil, and the results were reported as IC50.
3.7. Molecular Docking Studies
The crystal structures of acetylcholinesterase, α-amylase, and α-glucosidase were downloaded from the protein data bank (www.pdb.org) with the following IDs 1DX6, 4GQR, and 2V3E [51,52,53], respectively. All the docking studies were conducted using MOE 2019 [54], and the results were visualized by the open-source Discovery studio. Firstly, all the target enzymes, co-crystalized ligands, and the isolated compounds were prepared using the default settings. The active site of each target was determined from the binding of the corresponding co-crystalized ligand. Prior to commencing the docking of the seven identified compounds, a pose retrieval step for the co-crystalized ligands was performed and followed by RMSD calculation. Finally, the identified compounds were docked at the predetermined active site of the three target enzymes.
4. Conclusions
In our study, we described the detailed chemical composition of leaf oil isolated from S. cumini grown in Egypt. The tested oil was found to be rich in α-pinene (21.09%), β-(E)-ocimene (11.80%), D-limonene (8.08%), β-pinene (7.33%), α-terpineol (5.38%), and (E)-β-caryophyllene (4.51%). Further, we investigated its cytotoxic effects against the human liver cell line. In addition, we explored its effective inhibitory properties against acetylcholinesterase, α-amylase, and α-glucosidase. Our findings show that we should consider this oil for use as a food supplement or adjuvant therapy for the elderly to enhance memory performance and for diabetic patients to control blood glucose. Furthermore, the essential oils exhibited stronger toxicity towards the different pathogens documented in the literature [55,56,57,58], making the essential oil and its constituents, α-pinene, β-caryophyllene, and α-terpineol, a good candidate as an antimicrobial, antifungal, and insecticidal agent. Further in vivo neuroprotective and antihyperglycemic investigations are recommended to construct a molecular mechanistic profile for the isolated essential oil in the management of Alzheimer’s disease and diabetic mellitus. Lastly, without further investigations regarding its toxicity, the in vitro results in the present study should not be considered as encouraging the use of S. cumini essential oil as a herbal medicinal product.
Author Contributions
Conceptualization, methodology, software, writing—original draft preparation, H.A.S.E.-N. and S.H.A.; funding acquisition, review and editing, S.T.A.-R., A.A. and R.O.E.; methodology, review and editing, W.M.E. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from the Researchers Supporting Project number (RSP-2021/103), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the first author.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RG-1439-065.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the plant material and tested essential oil may be available from the authors.
References
- Machado, R.R.; Jardim, D.F.; Souza, A.R.; Scio, E.; Fabri, R.L.; Carpanez, A.G.; Grazul, R.M.; de Mendonça, J.P.R.; Lesche, B.; Aarestrup, F.M. The Effect of essential oil of Syzygium Cumini on the development of granulomatous inflammation in mice. Rev. Bras. Farmacogn. 2013, 23, 488–496. [Google Scholar] [CrossRef]
- Faria, A.F.; Marques, M.C.; Mercadante, A.Z. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. 2011, 126, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Ilavarasan, R.; Jayachandran, T.; Decaraman, M.; Aravindhan, P.; Padmanabhan, N.; Krishnan, M. Phytochemicals investigation on a tropical plant, Syzygium cumini from Kattuppalayam, Erode district, Tamil Nadu, South India. Pak. J. Nutr. 2009, 8, 83–85. [Google Scholar] [CrossRef] [Green Version]
- Balakrishna, G.; Sowmya, K.; Bollapalli, V.R.; Rao, M.R. Anti-allergic studies of Alibizzia Lebbeck and Syzygium cumini (L. Syzygium gambolana). J. Microbiol. Biotechnol. 2016, 1, 103. [Google Scholar]
- Bhuiyan, M.; Mia, M.Y.; Rashid, M. Antibacterial principles of the seeds of Eugenia jambolana. Bangladesh J. Bot. 1996, 25, 239–241. [Google Scholar]
- Ravi, K.; Sekar, D.S.; Subramanian, S. Hypoglycemic activity of inorganic constitutents in Eugenia jambolana seed on streptozotocin-induced diabetes in rats. Biol. Trace Elem. Res. 2004, 99, 145–155. [Google Scholar] [CrossRef]
- Kumar, A.; Ilavarasan, R.; Jayachandran, T.; Deecaraman, M.; Kumar, R.M.; Aravindan, P.; Padmanabhan, N.; Krishan, M. Anti-inflammatory activity of Syzygium cumini seed. Afr. J. Biotechnol. 2008, 7, 941–943. [Google Scholar]
- Benherlal, P.S.; Arumughan, C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J. Sci. Food. Agric. 2007, 87, 2560–2569. [Google Scholar] [CrossRef]
- Srivastava, S.; Chandra, D. Pharmacological potentials of Syzygium cumini: A review. J. Sci. Food. Agric. 2013, 93, 2084–2093. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds and pharmacological and food applications of Syzygium cumini-A review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef]
- Dias, C.N.; Rodrigues, K.A.; Carvalho, F.A.; Carneiro, S.M.; Maia, J.G.; Andrade, E.H.; Moraes, D.F. Molluscicidal and leishmanicidal activity of the leaf essential oil of Syzygium cumini (L.) Skeels from Brazil. Chem. Biodivers. 2013, 10, 1133–1141. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ali, S.I.; El-Baz, F.K. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS ONE 2013, 8, 60269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Tundis, R. Natural products for Alzheimer’s disease therapy: Basic and application. J. Pharm. Pharmacol. 2013, 65, 1679–1680. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Li, J.; Wei, H.; Huang, L.; Li, X. Tacrine-propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2015, 30, 995–1001. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martínez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Kraus, B.; Lehmann, J.; Heilmann, J.; Zhang, Y.; Decker, M. Design and synthesis of tacrine-ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008, 18, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef]
- Ceylan, R.; Zengin, G.; Uysal, S.; Ilhan, V.; Aktumsek, A.; Kandemir, A.; Anwar, F. GC-MS analysis and in vitro antioxidant and enzyme inhibitory activities of essential oil from aerial parts of endemic Thymus spathulifolius Hausskn. et Velen. J. Enzyme Inhib. Med. Chem. 2016, 31, 983–990. [Google Scholar] [CrossRef]
- da Costa, J.S.; da Cruz, E.N.S.; Setzer, W.N.; da Silva, J.; Maia, J.G.S.; Figueiredo, P.L.B. Essentials oils from Brazilian Eugenia and Syzygium species and their biological activities. Biomolecules 2020, 10, 1155. [Google Scholar] [CrossRef]
- Tahir, H.U.; Sarfraz, R.A.; Ashraf, A.; Adil, S. Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). Int. J. Food Prop. 2016, 19, 2156–2164. [Google Scholar] [CrossRef]
- Ramya, L.N.; Pulicherla, K.K. Molecular insights into cold active polygalacturonase enzyme for its potential application in food processing. J. Food Sci. Technol. 2015, 52, 5484–5496. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, R.T. Structure-based drug design: Docking and scoring. Curr. Protein Pept. Sci. 2007, 8, 312–328. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Sarma, N.; Begum, T.; Pandey, S.K.; Gogoi, R.; Munda, S.; Lal, M. Chemical Composition of Syzygium cumini (L.) Skeels leaf essential oil with respect to its uses from North East Region of India. J. Essent. Oil-Bear. Plants 2020, 23, 601–607. [Google Scholar] [CrossRef]
- Hanif, M.U.; Hussain, A.I.; Aslam, N.; Kamal, G.M.; Chatha, S.A.; Shahida, S.; Khalid, M.; Hussain, R. Chemical composition and bioactivities of essential oil from leaves of Syzygium cumini (L.) Skeels native to Punjab, Pakistan. Chem. Biodivers. 2020, 17, 1900733. [Google Scholar] [CrossRef]
- Rezende, W.P.; Borges, L.L.; Alves, N.M.; Ferri, P.H.; Paula, J.R. Chemical variability in the essential oils from leaves of Syzygium jambos. Rev. Bras. Farmacogn. 2013, 23, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Sobeh, M.; Braun, M.S.; Krstin, S.; Youssef, F.S.; Ashour, M.L.; Wink, M. Chemical profiling of the essential oils of Syzygium aqueum, Syzygium samarangense and Eugenia uniflora and their discrimination using chemometric analysis. Chem. Biodivers. 2016, 13, 1537–1550. [Google Scholar] [CrossRef]
- Taha, A.M.; Eldahshan, O.A. Chemical characteristics, antimicrobial, and cytotoxic activities of the essential oil of egyptian Cinnamomum glanduliferum bark. Chem. Biodivers. 2017, 14, 1600443. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Badry, M.A.; Eldahshan, O.A.; Singab, A.N.B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2020, 21, 1–4. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Cole, R.A.; Bansal, A.; Moriarity, D.M.; Haber, W.A.; Setzer, W.N. Chemical composition and cytotoxic activity of the leaf essential oil of Eugenia zuchowskiae from Monteverde, Costa Rica. J. Nat. Med. 2007, 61, 414–417. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Chaar, J.D.S.; Figueiredo, P.D.; Yano, T. Cytotoxic evaluation of essential oil from Casearia sylvestris Sw on human cancer cells and erythrocytes. Acta Amazon. 2008, 38, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Adewusi, E.A.; Steenkamp, V. In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac. J. Trop. Med. 2011, 4, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Aly, S.H.; Elissawy, A.M.; Fayez, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Neuroprotective effects of Sophora secundiflora, Sophora tomentosa leaves and formononetin on scopolamine-induced dementia. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- El-Hawarym, S.S.; Fathym, F.I.; Sleemm, A.A.; Morsym, F.A.; Khadarm, M.S.; Mansourm, M.K. Anticholinesterase activity and metabolite profiling of Syagrus romanzoffiana (Cham.) Glassman leaves and fruits via UPLC–QTOF–PDA–MS. Nat. Prod. Res. 2021, 35, 1671–1675. [Google Scholar] [CrossRef]
- Owokotomo, I.A.; Ekundayo, O.; Abayomi, T.G.; Chukwuka, A.V. In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol. Rep. 2015, 2, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deveci, E.; Tel-çayan, G.; Usluer, Ö.; Duru, M.E. Chemical composition, antioxidant, anticholinesterase and anti-tyrosinase activities of essential oils of two Sideritis species from Turkey. Iran. J. Pharm. Sci. 2019, 18, 903–913. [Google Scholar]
- El Bishbishy, M.H.; Gad, H.A.; Aborehab, N.M. Chemometric discrimination of three Pistacia species via their metabolic profiling and their possible in vitro effects on memory functions. J. Pharm. Biomed. Anal. 2020, 177, 112840. [Google Scholar] [CrossRef] [PubMed]
- Panamito, M.F.; Bec, N.; Valdivieso, V.; Salinas, M.; Calva, J.; Ramírez, J.; Larroque, C.; Armijos, C. Chemical composition and anticholinesterase activity of the essential oil of leaves and flowers from the Ecuadorian plant Lepechinia paniculata (Kunth) epling. Molecules 2021, 26, 3198. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Basha, S.K.; Kumari, V.S. In vitro antidiabetic activity of Psidium guajava leaves extracts. Asian Pac. J. Trop. Dis. 2012, 2, 98–100. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Badry, M.A.; Eldahshan, O.A.; Singab, A.N.B. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Nat. Prod. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Gad, H.A.; Mamadalieva, N.Z.; Böhmdorfer, S.; Rosenau, T.; Zengin, G.; Mamadalieva, R.Z.; Al Musayeib, N.M.; Ashour, M.L. GC-MS based identification of the volatile components of six Astragalus species from Uzbekistan and their biological activity. Plants 2021, 10, 124. [Google Scholar] [CrossRef]
- Prabakaran, K.; Shanmugave, G. Antidiabetic activity and phytochemical constituents of Syzygium cumini seeds in Puducherry region, South India. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 985–989. [Google Scholar] [CrossRef]
- Oboh, G.; Akinbola, I.A.; Ademosun, A.O.; Sanni, D.M.; Odubanjo, O.V.; Olasehinde, T.A.; Oyeleye, S.I. Essential oil from clove bud (Eugenia aromatica Kuntze) Inhibit key enzymes relevant to the management of type-2 diabetes and some pro-oxidant induced lipid peroxidation in rats pancreas in vitro. J. Oleo Sci. 2015, 64, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capetti, F.; Cagliero, C.; Marengo, A.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Bio-guided fractionation driven by in vitro α-amylase inhibition assays of essential oils bearing specialized metabolites with potential hypoglycemic activity. Plants 2020, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Todirascu-Ciornea, E.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Dumitru, G.; Hritcu, L.; Singab, A.N.B. Schinus terebinthifolius essential oil attenuates Scopolamine-induced memory deficits via cholinergic modulation and antioxidant properties in a zebrafish model. Evid. Based Complement. Alternat. Med. 2019, 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Mitra, I.; Mukherjee, S.; Reddy, B.V.; Dasgupta, S.; Bose, K.J.C.; Mukherjee, S.; Linert, W.; Moi, S.C. Benzimidazole based Pt(II) complexes with better normal cell viability than cisplatin: Synthesis, substitution behavior, cytotoxicity, DNA binding and DFT study. RSC Advances 2016, 6, 76600–76613. [Google Scholar] [CrossRef] [Green Version]
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Phytochemical investigation using GC/MS analysis and evaluation of antimicrobial and cytotoxic activities of the lipoidal matter of leaves of Sophora secundiflora and Sophora tomentosa. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 207–214. [Google Scholar]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenblatt, H.M.; Kryger, G.; Lewis, T.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with (−)-galanthamine at 2.3 Å resolution. FEBS Lett. 1999, 463, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.K.; Li, C.; Withers, S.G.; Brayer, G.D. Order and disorder: Differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target. J. Med. Chem. 2012, 55, 10177–10186. [Google Scholar] [CrossRef]
- Brumshtein, B.; Greenblatt, H.M.; Butters, T.D.; Shaaltiel, Y.; Aviezer, D.; Silman, I.; Futerman, A.H.; Sussman, J.L. Crystal structures of complexes of N-butyl-and N-nonyl-deoxynojirimycin bound to acid β-glucosidase: Insights into the mechanism of chemical chaperone action in Gaucher disease. J. Bio. Chem. 2007, 282, 29052–29058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassab, M.A.; Fares, M.; Amin, M.K.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Alkahtani, H.M.; Eldehna, W.M. Toward the identification of potential α-Ketoamide covalent inhibitors for SARS-CoV-2 Main Protease: Fragment-based drug design and MM-PBSA calculations. Processes 2021, 9, 1004. [Google Scholar] [CrossRef]
- Silva, A.C.; Lopes, P.M.; Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.S.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Chemical composition and insecticidal activity against Sitophilus zeamais of the essential oils derived from Artemisia giraldii and Artemisia subdigitata. Molecules 2012, 17, 7255–7265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, D.; Levy, R.; Carroll, B. Toxicological evaluation of β-caryophyllene oil: Subchronic toxicity in rats. Int. J. Toxicol. 2016, 35, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Abdelghffar, E.A.; El-Nashar, H.A.; Al-Mohammadi, A.G.; Eldahshan, O.A. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food Function 2021, 12, 9443–9455. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).