The Ability of Near Infrared (NIR) Spectroscopy to Predict Functional Properties in Foods: Challenges and Opportunities
Abstract
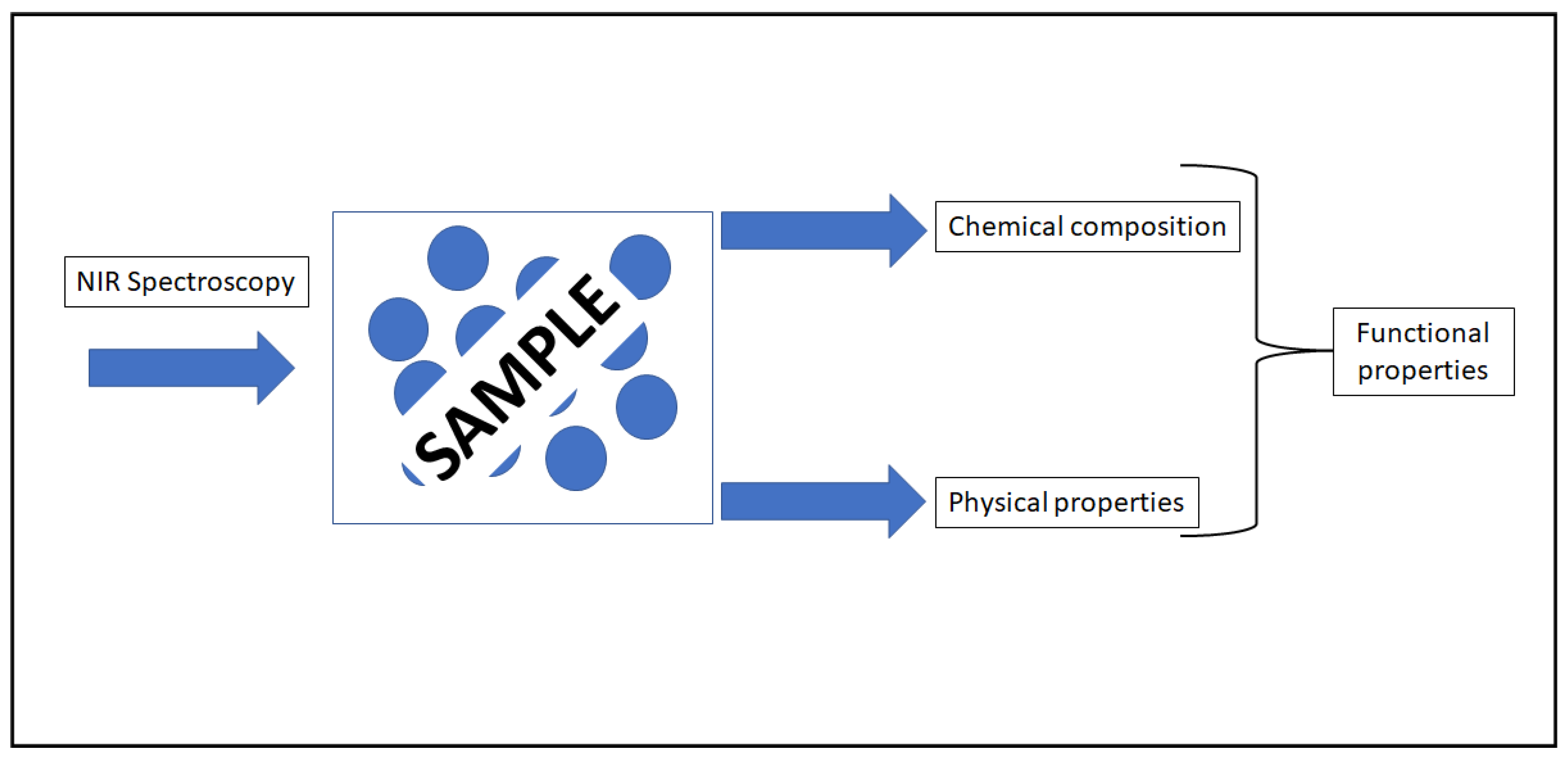
:1. Introduction
2. Near Infrared Spectroscopy
3. Examples on the Use of Near Infrared Spectroscopy to Measure Food Functionality
3.1. Dairy Products
3.2. Cereals and Starchy Foods
4. Challenges and Opportunities
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lock, K.; Pomerleau, J.; Causer, L.; Altmann, D.R.; McKee, M. The global burden of disease attributable to low consumption of fruit and vegetables: Implications for the global strategy on diet. Bull. World Health Organ. 2005, 83, 100–108. [Google Scholar]
- Fjeld, C.R.; Lawson, R.H. Food, phytonutrients, and health. Nutr. Rev. 1999, 57, S1–S2. [Google Scholar] [CrossRef]
- Cozzolino, D. Food for thought: The digital disruption and the future of food production. Curr. Res. Nutr. Food Sci. 2019, 7, 607–609. [Google Scholar] [CrossRef]
- Truong, V.K.; Dupont, M.; Elbourne, A.; Gangadoo, S.; Rajapaksha Pathirannahalage, P.; Cheeseman, S.; Chapman, J.; Cozzolino, D. From academia to reality check: A theoretical framework on the use of chemometric. Foods 2019, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Bec, K.B.; Grabska, J.; Huck, C.W. Review near-infrared spectroscopy in bio-applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Bec, K.B.; Huck, C.W. Breakthrough potential in near-infrared spectroscopy: Spectra simulation. A review of recent developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, T.M.P.; Stellari, A. Review: NIR spectroscopy as a suitable tool for the investigation of the horticultural field. Agronomy 2019, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Cozzolino, D. The sample, the spectra and the maths—The critical pillars in the development of robust and sound vibrational spectroscopy applications. Molecules 2020, 25, 3674. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Agelet, L.; Hurburgh, C.H., Jr. A Tutorial on Near Infrared Spectroscopy and its’ Calibration. Crit. Rev. Anal. Chem. 2010, 40, 246–260. [Google Scholar] [CrossRef]
- Walsh, K.B.; McGlone, V.A.; Hanc, D.H. The uses of near infra-red spectroscopy in postharvest decision support: A review. Postharvest Biol. Technol. 2020, 163, 111139. [Google Scholar] [CrossRef]
- Saeys, W.; Do Trong, N.N.; Van Beers, R.; Nicolai, B.M. Multivariate calibration of spectroscopic sensors for postharvest quality evaluation: A review. Postharvest Biol. Technol. 2019, 158, 110981. [Google Scholar] [CrossRef]
- Cozzolino, D.; Roberts, J.J. Applications and developments on the use of vibrational spectroscopy imaging for the analysis, monitoring and characterisation of crops and plants. Molecules 2016, 21, 755. [Google Scholar] [CrossRef] [Green Version]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Non-destructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Amigo, J.M.; Martí, I.; Gowen, A. Hyperspectral imaging and chemometrics: A perfect combination for the analysis of food structure, composition and quality. Data Handl. Sci. Technol. 2013, 28, 343–370. [Google Scholar]
- Crocombe, R.A. Portable spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef]
- Cortes, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talensa, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Kaavya, R.; Pandiselvam, R.; Mohammed, M.; Dakshayani, R.; Kothakota, A.; Ramesh, S.V.; Cozzolino, D.; Ashokkumar, C. Application of infrared spectroscopy techniques for assessment of quality and safety in spices—State-of-the-art and future trends spectroscopy techniques—An emerging tool for spices and herbs authentication. Appl. Spectrosc. Rev. 2021, 55, 593–611. [Google Scholar] [CrossRef]
- Sorak, D.; Herberholz, L.; Iwascek, S.; Altinpinar, S.; Pfeifer, F.; Siesler, H.W. New developments and applications of handheld Raman, mid-infrared, and near infrared spectrometers. Appl. Spectrosc. Rev. 2012, 47, 83–115. [Google Scholar] [CrossRef]
- Wang, C.; Reis, M.G.; Reis, M.M. Prediction of dairy powder functionality attributes using diffuse reflectance in the visible and near infrared (Vis-NIR) region. Int. Dairy J. 2021, 117, 104981. [Google Scholar] [CrossRef]
- Khan, A.; Munir, M.T.; Yu, W.; Young, B.R. Near-infrared spectroscopy and data analysis for predicting milk powder quality attributes. Int. J. Dairy Technol. 2021, 74, 235–245. [Google Scholar] [CrossRef]
- Aernouts, B.; Van Beers, R.; Saeys, W. Effect of ultrasonic homogenization on the Vis/NIR bulk optical properties of milk. Colloids Surf. B Biointerfaces 2015, 126, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Strani, L.; Grassi, S.; De Juan, A. Effect of physicochemical factors and use of milk powder on milk rennet-coagulation: Process understanding by near infrared spectroscopy and chemometrics. Food Control 2021, 119, 107494. [Google Scholar] [CrossRef]
- Muncan, J.; Kovacs, Z.; Tsenkova, R. Near infrared aquaphotomics study on common dietary fatty acids in cow’s liquid, thawed milk. Food Control 2021, 122, 107805. [Google Scholar] [CrossRef]
- Dowell, F.E.; Maghirang, E.B.; Xie, F.; Lookhart, G.L.; Pierce, R.O.; Seabourn, W.; Bean, S.R.; Wilson, J.D.; Chung, O.K. Predicting Wheat Quality Characteristics and Functionality Using Near-Infrared Spectroscopy. Cereal Chem. 2006, 83, 529–553. [Google Scholar] [CrossRef]
- Jerome, R.E.; Singh, S.K.; Dwivedi, M. Process analytical technology for bakery industry: A review. J. Food Process Eng. 2019, 42, e13143. [Google Scholar] [CrossRef]
- Schopf, M.; Wehrli, M.C.; Becker, T.; Jekle, M.; Scherf, K.A. Fundamental characterization of wheat gluten. Eur. Food Res. Technol. 2021, 247, 985–997. [Google Scholar] [CrossRef]
- Thanathornvarakul, N.; Anuntagool, J.; Tananuwong, K. Aging of low and high amylose rice at elevated temperature: Mechanism and predictive modelling. J. Cereal Sci. 2016, 70, 155–163. [Google Scholar] [CrossRef]
- Tonning, E.; Thybo, A.K.; Norgaard, L. Bulk Functionality Diversification by Unsupervised Single-Kernel Near-Infrared (SKNIR) Sorting of Wheat. Cereal Chem. 2009, 86, 706–713. [Google Scholar] [CrossRef]
- Liu, Y.L.; Delwiche, S.R.; Graybosch, R.A. Two-dimensional correlation analysis of near infrared spectral intensity variations of ground wheat. J. Near Infrared Spectrosc. 2009, 17, 41–50. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Weaver, G. Bread quality of wheat-flour by near-infrared spectrophotometry—Feasibility of modelling. J. Food Sci. 1994, 59, 410–415. [Google Scholar] [CrossRef]
- Izso, E.; Bartalne-Berceli, M.; Gergely, S. Monitoring of heat-treated wheat milling fractions by near infrared spectroscopic method. Qual. Assur. Saf. Crop. Foods 2018, 10, 93–101. [Google Scholar] [CrossRef]
- Chen, J.; Ye, F.Y.; Zhao, G.H. Rapid determination of farinograph parameters of wheat flour using data fusion and a forward interval variable selection algorithm. Anal. Methods 2017, 9, 6341–6348. [Google Scholar] [CrossRef]
- Flinn, P.C.; Black, R.G.; Meares, C. Estimating the food processing characteristics of pulses by near infrared spectroscopy, using ground or whole samples. J. Near Infrared Spectrosc. 1998, 6, 213–220. [Google Scholar] [CrossRef]
- Pasha, I.; Anjum, F.M.; Morris, C.F. Grain Hardness: A Major Determinant of Wheat Quality. Food Sci. Technol. Int. 2010, 16, 511–522. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Higginbotham, R.W.; Steber, C.M. Falling number of soft white wheat by near-infrared spectroscopy: A challenge revisited. Cereal Chem. 2018, 95, 469–477. [Google Scholar] [CrossRef]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Exploring the use of near infrared (NIR) reflectance spectroscopy to predict starch pasting properties in whole grain barley. Food Biophys. 2013, 8, 256–261. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid visco analyser (RVA) as a tool for measuring starch-related physiochemical properties in cereals: A review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzolino, D. The Ability of Near Infrared (NIR) Spectroscopy to Predict Functional Properties in Foods: Challenges and Opportunities. Molecules 2021, 26, 6981. https://doi.org/10.3390/molecules26226981
Cozzolino D. The Ability of Near Infrared (NIR) Spectroscopy to Predict Functional Properties in Foods: Challenges and Opportunities. Molecules. 2021; 26(22):6981. https://doi.org/10.3390/molecules26226981
Chicago/Turabian StyleCozzolino, Daniel. 2021. "The Ability of Near Infrared (NIR) Spectroscopy to Predict Functional Properties in Foods: Challenges and Opportunities" Molecules 26, no. 22: 6981. https://doi.org/10.3390/molecules26226981
APA StyleCozzolino, D. (2021). The Ability of Near Infrared (NIR) Spectroscopy to Predict Functional Properties in Foods: Challenges and Opportunities. Molecules, 26(22), 6981. https://doi.org/10.3390/molecules26226981





