A UPLC-DAD-Based Bio-Screening Assay for the Evaluation of the Angiotensin Converting Enzyme Inhibitory Potential of Plant Extracts and Compounds: Pyrroquinazoline Alkaloids from Adhatoda vasica as a Case Study
Abstract
:1. Introduction
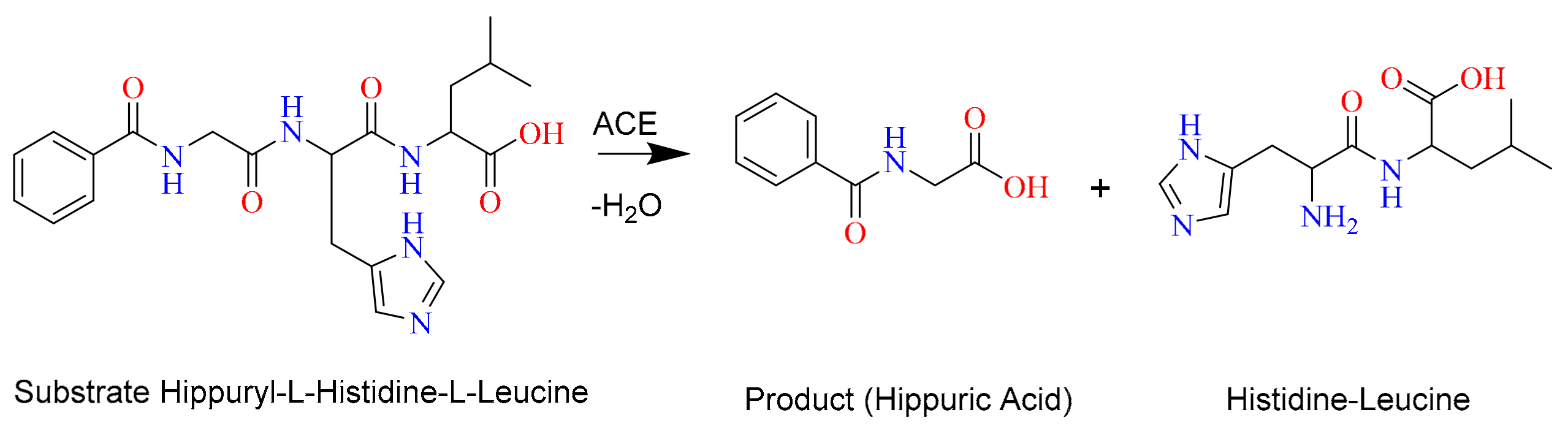
2. Results and Discussion
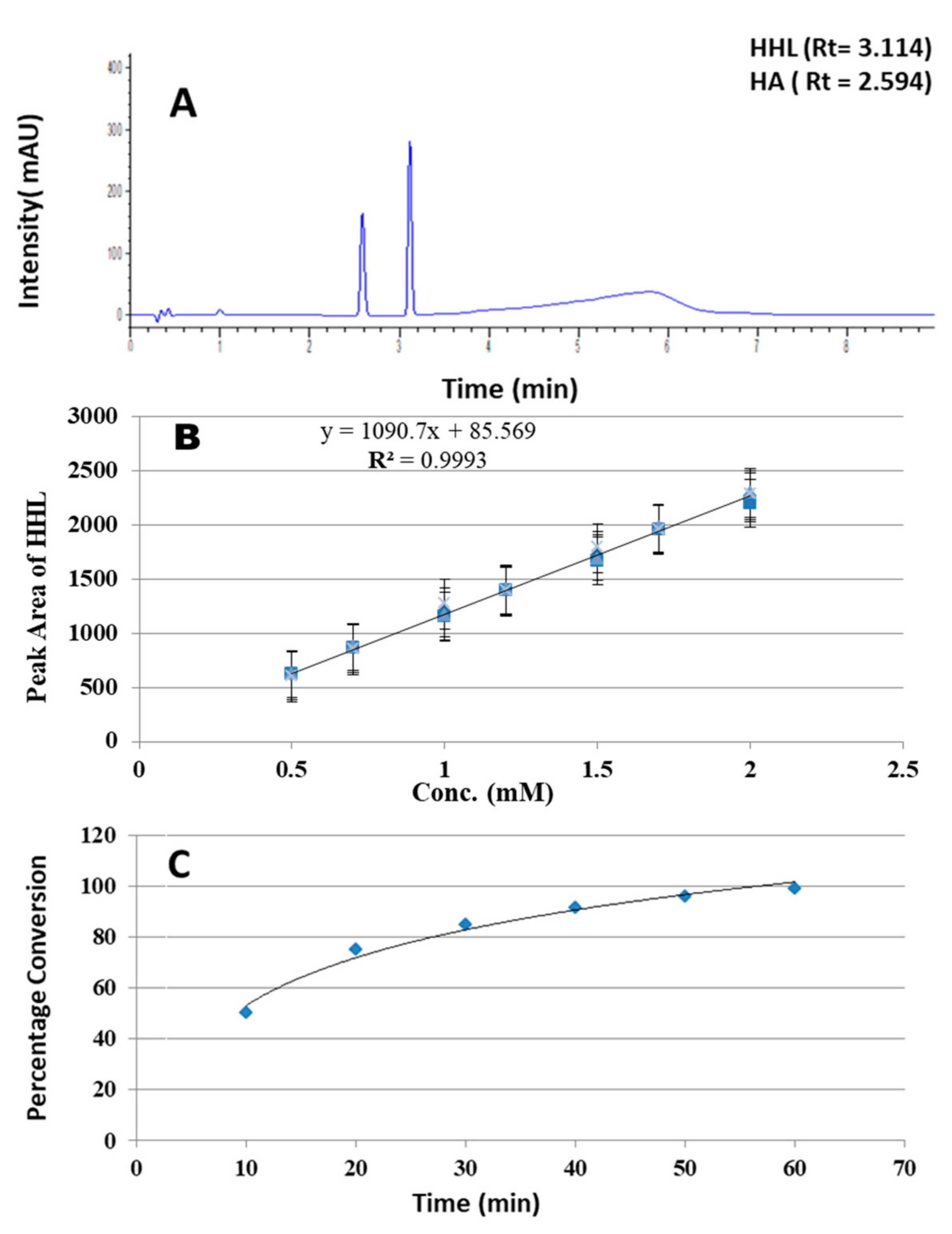
2.1. Optimization of UPLC Conditions for the ACE Inhibitory Assay
2.2. Optimization of Angiotensin Converting Enzyme Assay
2.3. ACE Inhibitory Activity of Medicinal Plants and Purified Compounds
2.4. Characterization of Pyrroquinazoline Alkaloids
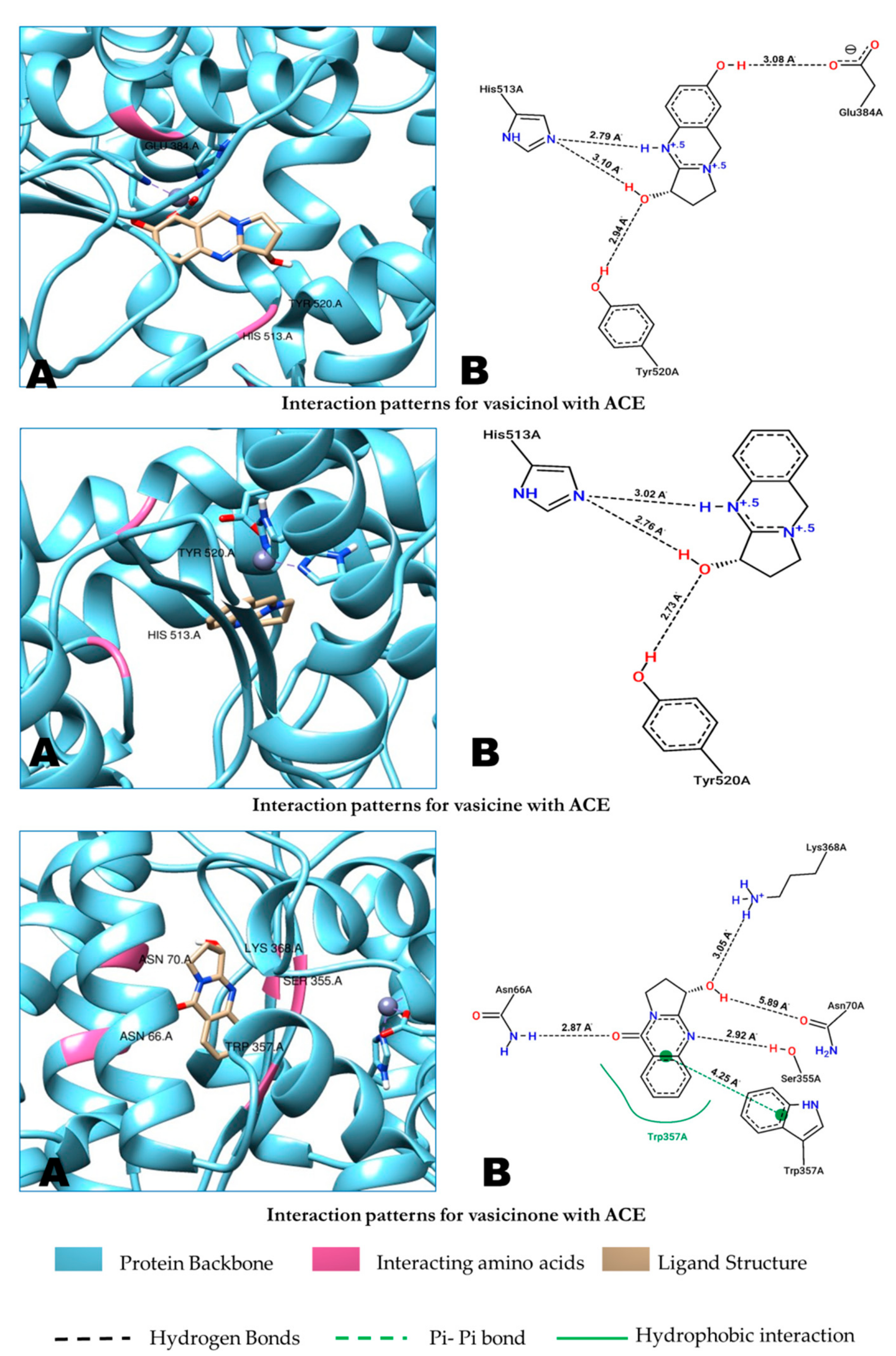
2.5. Molecular Docking
2.6. ADME/Tox Screening of Compounds
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. UPLC-Based ACE Assay
3.3. Enzymatic Assay
3.4. Method Validation
3.5. Extraction and Isolation of Compounds from Adhatoda vasica
3.6. Characterization of Pyrroquinazoline Alkaloids
3.7. Molecular Docking Screening
3.8. ADME/Tox Screening of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Chu, C.-C.; Lin, Y.-C.; Duh, P.-D. Djulis (Chenopodium formosanum) and its bioactive compounds for management of hyperlipidemia and hyperglycemia in high-fat diet-fed mice. J. Food Nutr. Res. 2019, 7, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. PTR 2007, 21, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shankar, R.; Singh, G.P. Prevalence and Associated Risk Factors of Hypertension: A Cross-Sectional Study in Urban Varanasi. Int. J. Hypertens. 2017, 2017, 5491838. [Google Scholar] [CrossRef] [Green Version]
- Chockalingam, A. Impact of World Hypertension Day. Can. J. Cardiol. 2007, 23, 517–519. [Google Scholar] [CrossRef] [Green Version]
- Nileeka Balasuriya, B.W.; Vasantha Rupasinghe, H.P. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Funct. Foods Health Dis. 2011, 1, 172. [Google Scholar] [CrossRef]
- Ghassem, M.; Arihara, K.; Babji, A.S.; Said, M.; Ibrahim, S. Purification and identification of ACE inhibitory peptides from Haruan (Channa striatus) myofibrillar protein hydrolysate using HPLC–ESI-TOF MS/MS. Food Chem. 2011, 129, 1770–1777. [Google Scholar] [CrossRef]
- Elased, K.M.; Cunha, T.S.; Gurley, S.B.; Coffman, T.M.; Morris, M. New mass spectrometric assay for angiotensin-converting enzyme 2 activity. Hypertension 2006, 47, 1010–1017. [Google Scholar] [CrossRef] [Green Version]
- Musharraf, S.G.; Bhatti, M.S.; Choudhary, M.I.; Rahman, A.U. Screening of inhibitors of angiotensin-converting enzyme (ACE) employing high performance liquid chromatography-electrospray ionization triple quadrupole mass spectrometry (HPLC-ESI-QqQ-MS). Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 101, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J.E. Lange) Imbach identified by LC-MS/MS. Food Chem. 2014, 148, 396–401. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, J. LC-MS/MS coupled with QSAR modeling in characterising of angiotensin I-converting enzyme inhibitory peptides from soybean proteins. Food Chem. 2013, 141, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Ghanta, P.; Sinha, S.; Doble, M.; Ramaiah, B. Potential of pyrroquinazoline alkaloids from Adhatoda vasica Nees. as inhibitors of 5-LOX—A computational and an in vitro study. J. Biomol. Struct. Dyn. 2020, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Claeson, U.P.; Malmfors, T.; Wikman, G.; Bruhn, J.G. Adhatoda vasica: A critical review of ethnopharmacological and toxicological data. J. Ethnopharmacol. 2000, 72, 1–20. [Google Scholar] [CrossRef]
- P Singh, T.; M Singh, O.; B Singh, H. Adhatoda vasica Nees: Phytochemical and pharmacological profile. Nat. Prod. J. 2011, 1, 29–39. [Google Scholar] [CrossRef]
- Nyman, U.; Joshi, P.; Madsen, L.B.; Pedersen, T.B.; Pinstrup, M.; Rajasekharan, S.; George, V.; Pushpangadan, P. Ethnomedical information and in vitro screening for angiotensin-converting enzyme inhibition of plants utilized as traditional medicines in Gujarat, Rajasthan and Kerala (India). J. Ethnopharmacol. 1998, 60, 247–263. [Google Scholar] [CrossRef]
- Lahogue, V.; Réhel, K.; Taupin, L.; Haras, D.; Allaume, P. A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2010, 118, 870–875. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Muir, A.D. Improved method for direct high-performance liquid chromatography assay of angiotensin-converting enzyme-catalyzed reactions. J. Chromatogr. A 2002, 950, 125–130. [Google Scholar] [CrossRef]
- Hernandez-Ledesma, B.; Martin-Alvarez, P.J.; Pueyo, E. Assessment of the spectrophotometric method for determination of angiotensin-converting-enzyme activity: Influence of the inhibition type. J. Agric. Food Chem. 2003, 51, 4175–4179. [Google Scholar] [CrossRef]
- Tiwari, M.; Gupta, P.S.; Sharma, N. Ethnopharmacological, Phytochemical and Pharmacological review of Plant Cissus quadrangularis L. Res. J. Pharmacogn. Phytochem. 2018, 10, 81–90. [Google Scholar] [CrossRef]
- Verma, R.K.; Garima, M.; Pradeep, S.; Jha, K.; Khosa, R. Alpinia galanga—An important medicinal plant: A review. Der Pharm. Sin. 2011, 2, 142–154. [Google Scholar]
- Kouchmeshky, A.; Jameie, S.B.; Amin, G.; Ziai, S.A. Investigation of Angiotensin-Convertings Enzyme Inhibitory Effects of Medicinal Plants Used in Traditional Persian Medicine for Treatment of Hypertension: Screening Study. Thrita 2012, 1, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.K.; Hamed, A.R.; Soltan, M.M.; El-Halawany, A.M.; Hegazy, U.M.; Hussein, A.A. Kinetics and molecular docking of vasicine from Adhatoda vasica: An acetylcholinesterase inhibitor for Alzheimer’s disease. S. Afr. J. Bot. 2016, 104, 118–124. [Google Scholar] [CrossRef]
- Rahman, S.; Haq, F.U.; Ali, A.; Khan, M.N.; Shah, S.M.Z.; Adhikhari, A.; El-Seedi, H.R.; Musharraf, S.G. Combining untargeted and targeted metabolomics approaches for the standardization of polyherbal formulations through UPLC–MS/MS. Metabolomics 2019, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Geng, M.; Liu, C.; Wang, J.; Min, W.; Liu, J. Structural and molecular basis of angiotensin-converting enzyme by computational modeling: Insights into the mechanisms of different inhibitors. PLoS ONE 2019, 14, e0215609. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Azminah, A.; Mulia, K.; Yanuar, A.; Mun’im, A. Angiotensinconverting enzyme inhibitory activity of polyphenolic compounds from Peperomia pellucida(L) Kunth: An in silico molecular docking study. J. Appl. Pharm. Sci. 2019, 9, 25–31. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natesh, R.; Schwager, S.L.; Sturrock, E.D.; Acharya, K.R. Crystal structure of the human angiotensin-converting enzyme–lisinopril complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akif, M.; Georgiadis, D.; Mahajan, A.; Dive, V.; Sturrock, E.D.; Isaac, R.E.; Acharya, K.R. High-resolution crystal structures of Drosophila melanogaster angiotensin-converting enzyme in complex with novel inhibitors and antihypertensive drugs. J. Mol. Biol. 2010, 400, 502–517. [Google Scholar] [CrossRef]
- Miteva, M.A.; Guyon, F.; Tuffïry, P. Frog2: Efficient 3D conformation ensemble generator for small compounds. Nucleic Acids Res. 2010, 38, W622–W627. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.M.D.; Shakil, S.; Haneef, M. A simple click by click protocol to perform docking: AutoDock 4.2 made easy for non-bioinformaticians. Excli J. 2013, 12, 831. [Google Scholar]
- Stierand, K.; Rarey, M. PoseView--molecular interaction patterns at a glance. J. Cheminform. 2010, 2, P50. [Google Scholar] [CrossRef] [Green Version]
- Lagorce, D.; Bouslama, L.; Becot, J.; Miteva, M.A.; Villoutreix, B.O. FAF-Drugs4: Free ADME-tox filtering computations for chemical biology and early stages drug discovery. Bioinformatics 2017, 33, 3658–3660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Irwin, J.J.; Shoichet, B.K. ZINC--a free database of commercially available compounds for virtual screening. J. Chem. Inf. Modeling 2005, 45, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Oprea, T.I.; Davis, A.M.; Teague, S.J.; Leeson, P.D. Is there a difference between leads and drugs? A historical perspective. J. Chem. Inf. Comput. Sci. 2001, 41, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Pihan, E.; Colliandre, L.; Guichou, J.F.; Douguet, D. e-Drug3D: 3D structure collections dedicated to drug repurposing and fragment-based drug design. Bioinformatics 2012, 28, 1540–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oprea, T.I. Property distribution of drug-related chemical databases. J. Comput.-Aided Mol. Des. 2000, 14, 251–264. [Google Scholar] [CrossRef]

| S.No. | HHL Conc. (mM) | Intraday | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| Mean Conc. (mM) | RSD % | %Error | Mean Conc. (mM) | RSD % | %Error | ||
| 1. | 0.8 | 0.8023 | 1.0850 | 0.2929 | 0.8137 | 2.2019 | 1.7230 |
| 2. | 1.8 | 1.8232 | 0.0841 | 1.2921 | 1.8160 | 0.6852 | 0.8932 |
| S.No. | ACE Assay | Intraday | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| ACE %Conversion | RSD % | %Error | ACE %Conversion | RSD % | %Error | ||
| 1. | Control | 70.0940 | 1.8826 | 3.0795 | 69.5008 | 2.7430 | 2.2071 |
| 2. | Captopril (100 nM) | 28.0923 | 2.6146 | 4.0457 | 27.8100 | 3.0499 | 3.0000 |
| 3. | Lisinopril (100 nM) | 21.8837 | 3.9631 | 4.0187 | 21.8887 | 3.9927 | 3.9969 |
| Inhibitors/Compounds | Binding Free Energy Kcal/mol | Types of Interactions Along with Interacting Amino Acids of ACE |
|---|---|---|
| Lisinopril | −8.48 kcal/mol | Hydrogen Bonds Glu162, Gln281, His353, Lys511, His513, Tyr520 and Tyr523 Hydrophobic Interactions His 353, Ala354, Ala356, Val380 and His387 |
| Captopril | −6.97 kcal/mol | Hydrogen Bonds Gln 281, His353, Glu384, Lys511, Tyr520, Tyr523 and His513 Hydrophobic Interactions His383 and Tyr 523 |
| Vasicine | −5.89 kcal/mol | Hydrogen Bonds His513 and Tyr520 |
| Vasicinol | −6.40 kcal/mol | Hydrogen Bonds Glu384, His513, and Tyr520 |
| Vasicinone | −5.79 kcal/mol | Hydrogen Bonds Asn66, Asn70, Ser355, Trp357 and Lys368 Hydrophobic Interactions Trp357 |
| Ligand | Parameters Standards | Lisinopril | Captopril | Vasicinol | Vasicine | Vasicinone |
|---|---|---|---|---|---|---|
| PubChem ID | – | 5362119 | 44093 | 442934 | 72610 | 442935 |
| Mol. Weight | 100.0–600.0 | 405.49 | 217.29 | 204.23 | 188.23 | 202.21 |
| LogP | −3.0–6.0 | −1.22 | 0.34 | 0.09 | 0.44 | 0.52 |
| HBA | ≤12 | 8 | 4 | 4 | 3 | 4 |
| HBD | ≤7 | 5 | 1 | 2 | 1 | 1 |
| Tpsa | ≤11 | 144.82 | 99.24 | 57.67 | 37.44 | 55.12 |
| RotatableB | ≤11 | 12 | 3 | 0 | 0 | 0 |
| RigidB | ≤30 | 15 | 8 | 15 | 15 | 16 |
| Rings | ≤6 | 2 | 1 | 1 | 1 | 1 |
| Max Size Ring | ≤18 | 6 | 5 | 13 | 13 | 13 |
| Carbon Atoms | 3–35 | 21 | 9 | 11 | 11 | 11 |
| Hetero Atoms | 1–15 | 8 | 5 | 4 | 3 | 4 |
| Ratio H/C | 0.1–1.1 | 0.38 | 0.56 | 0.36 | 0.27 | 0.36 |
| NumCharges | ≤4 | 4 | 1 | 1 | 1 | 0 |
| Total Charge | −8 | 0 | −1 | |||
| Lipinski Violation | – | 0 | 0 | 0 | ||
| Solubility(mg/L) | 170317.77 | 65224.97 | 54156.75 | 43933.32 | 36306.82 | |
| Solubility Forecast Index | Good Solubility | Good Solubility | Good Solubility | Good Solubility | Good Solubility | |
| Oral Bioavailability (VEBER) | Low | Good | ||||
| Oral Bioavailability (EGAN) | ||||||
| StereoCenters | 3 | 2 | 1 | |||
| Result | Accepted | |||||
| Position | Compound 1 | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| δH (mult., J in Hz) | δC13 | δH (mult., J in Hz) | δC13 | δH (mult., J in Hz) | δC13 | |
| 1 | 3.75–3.61 (m) | 53.02 | 3.33–3.45 (m) | 47.52 | 4.29–4.02 (m) | 44.58 |
| 2 | 2.12–2.06 (m) | 31.76 | 2.41–2.18 (m) | 30.78 | 2.66–2.18 (m) | 31.08 |
| 3 | 5.11 (t, 8.0) | 73.46 | 4.66 (t, 6.4) | 72.54 | 5.12 (t, 7.8) | 73.26 |
| 3a | --- | 163.77 | --- | 164.76 | --- | 161.84 |
| 4a | 126.22 | 132.43 | 150.47 | |||
| 5 | 6.98 (d, 8.8) | 121.38 | 7.18 (d, 8.1) | 130.47 | 8.24 (dd, 8.2, 1.2) | 135.67 |
| 6 | 6.75 (dd, 8.8, 2.4) | 118.54 | 7.33 (dt, 8.0, 4.0) | 128.35 | 7.84 (dt, 8.3, 1.4) | 128.00 |
| 7 | --- | 157.75 | 7.25 (dt, 8.0, 4.0) | 128.26 | 7.55 (dt, 8.1, 1.2) | 127.16 |
| 8 | 6.62 (d, 2.8) | 116.23 | 7.13 (d, 8.0) | 118.42 | 7.73 (d, 7.8) | 128.01 |
| 8a | --- | 121.18 | --- | 118.24 | --- | 121.91 |
| 9 | 4.81–4.77, 2H (d, 16.0) | 48.89 | 5.08 (d, 15) | 51.95 | 162.59 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tehreem, S.; Rahman, S.; Bhatti, M.S.; Uddin, R.; Khan, M.N.; Tauseef, S.; El-Seedi, H.R.; Bin Muhsinah, A.; Uddin, J.; Musharraf, S.G. A UPLC-DAD-Based Bio-Screening Assay for the Evaluation of the Angiotensin Converting Enzyme Inhibitory Potential of Plant Extracts and Compounds: Pyrroquinazoline Alkaloids from Adhatoda vasica as a Case Study. Molecules 2021, 26, 6971. https://doi.org/10.3390/molecules26226971
Tehreem S, Rahman S, Bhatti MS, Uddin R, Khan MN, Tauseef S, El-Seedi HR, Bin Muhsinah A, Uddin J, Musharraf SG. A UPLC-DAD-Based Bio-Screening Assay for the Evaluation of the Angiotensin Converting Enzyme Inhibitory Potential of Plant Extracts and Compounds: Pyrroquinazoline Alkaloids from Adhatoda vasica as a Case Study. Molecules. 2021; 26(22):6971. https://doi.org/10.3390/molecules26226971
Chicago/Turabian StyleTehreem, Syeda, Saeedur Rahman, Muhammad Salman Bhatti, Reaz Uddin, Muhammad Noman Khan, Saba Tauseef, Hesham R. El-Seedi, Abdullatif Bin Muhsinah, Jalal Uddin, and Syed Ghulam Musharraf. 2021. "A UPLC-DAD-Based Bio-Screening Assay for the Evaluation of the Angiotensin Converting Enzyme Inhibitory Potential of Plant Extracts and Compounds: Pyrroquinazoline Alkaloids from Adhatoda vasica as a Case Study" Molecules 26, no. 22: 6971. https://doi.org/10.3390/molecules26226971
APA StyleTehreem, S., Rahman, S., Bhatti, M. S., Uddin, R., Khan, M. N., Tauseef, S., El-Seedi, H. R., Bin Muhsinah, A., Uddin, J., & Musharraf, S. G. (2021). A UPLC-DAD-Based Bio-Screening Assay for the Evaluation of the Angiotensin Converting Enzyme Inhibitory Potential of Plant Extracts and Compounds: Pyrroquinazoline Alkaloids from Adhatoda vasica as a Case Study. Molecules, 26(22), 6971. https://doi.org/10.3390/molecules26226971







