Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-β-cyclodextrin and Release Kinetics of Curcumin
Abstract
:1. Introduction
2. Results and Discussion
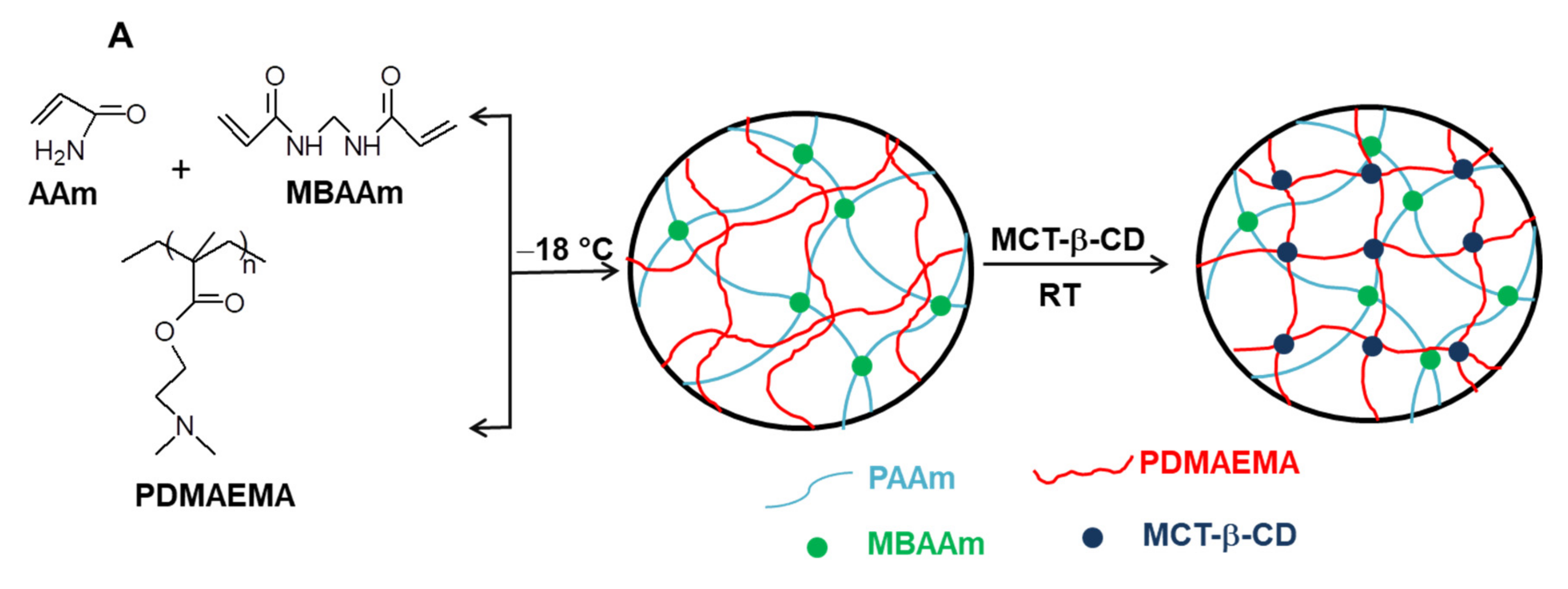
2.1. Synthesis of Composite Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate)
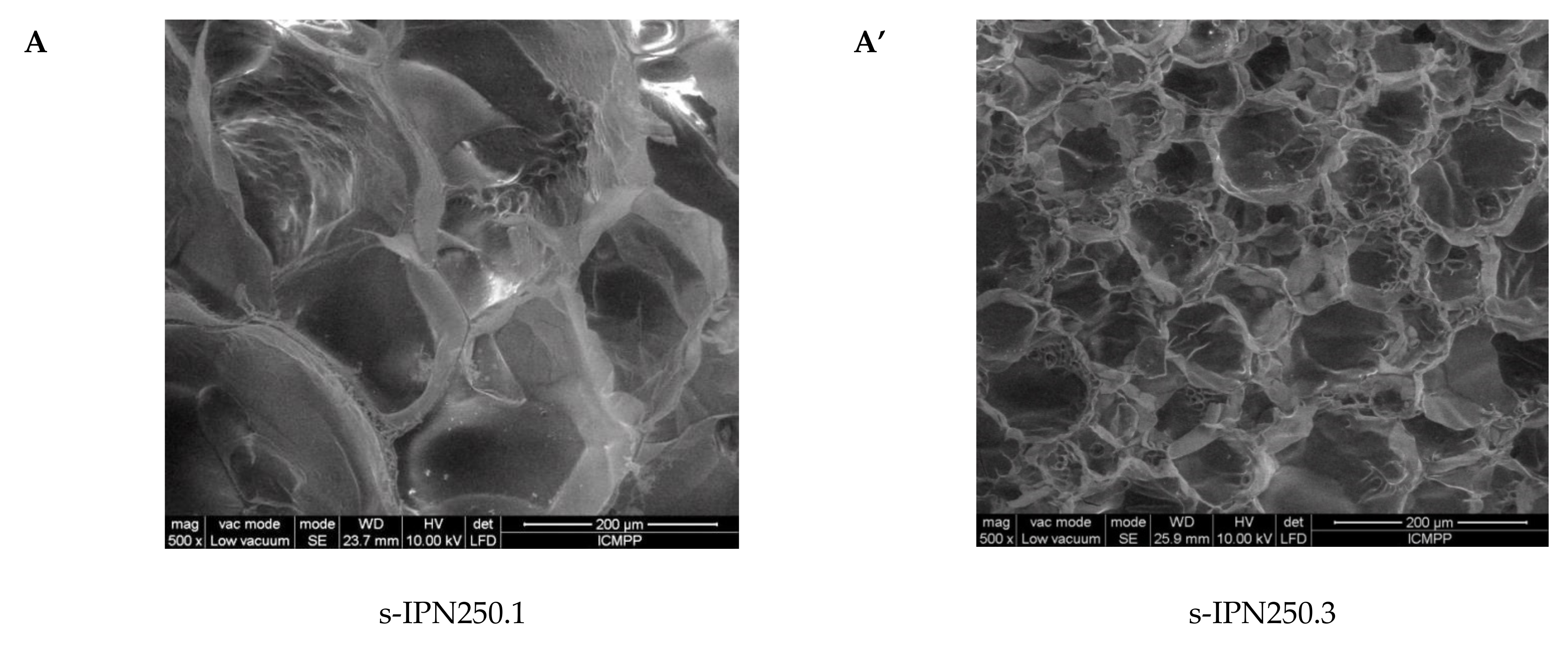
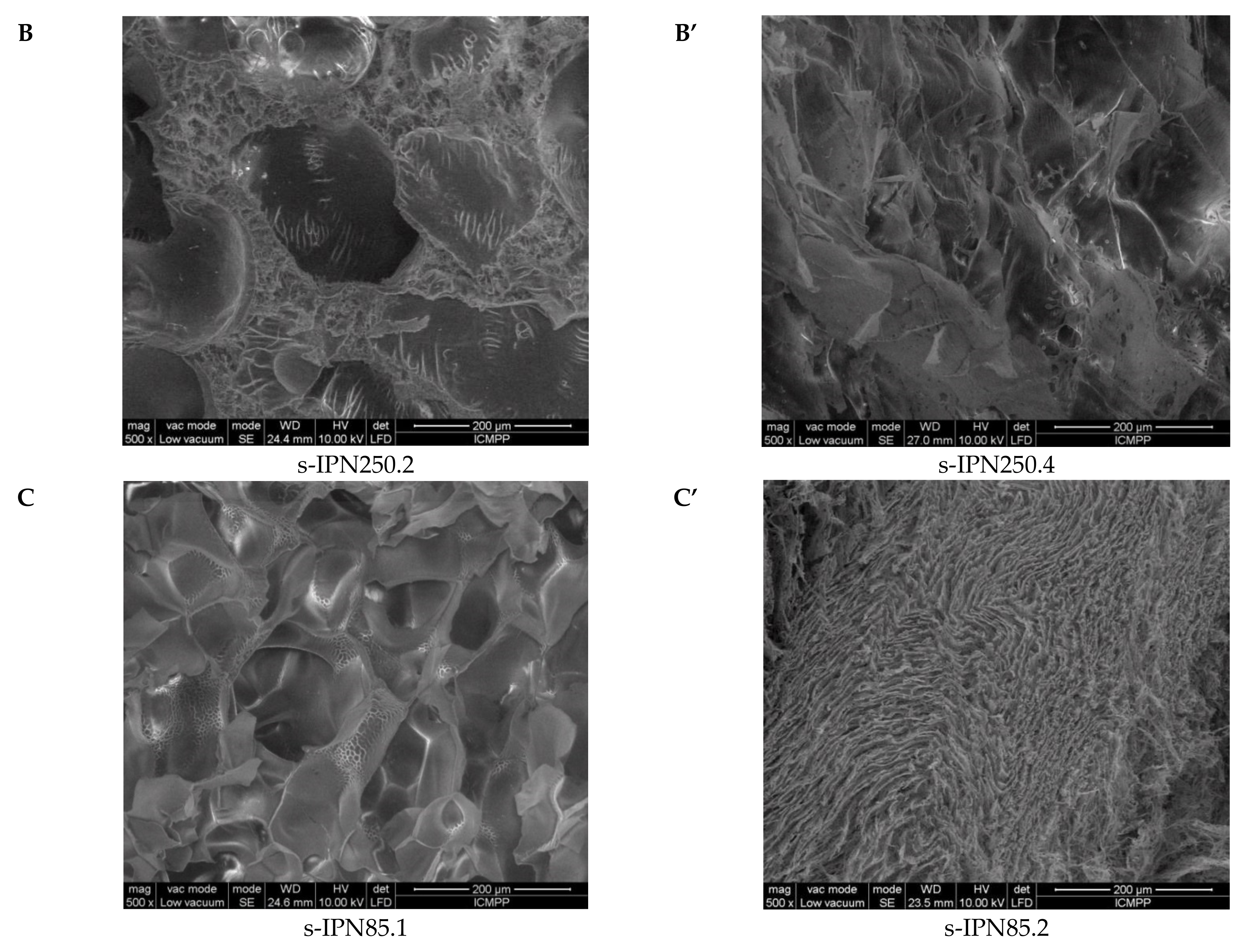
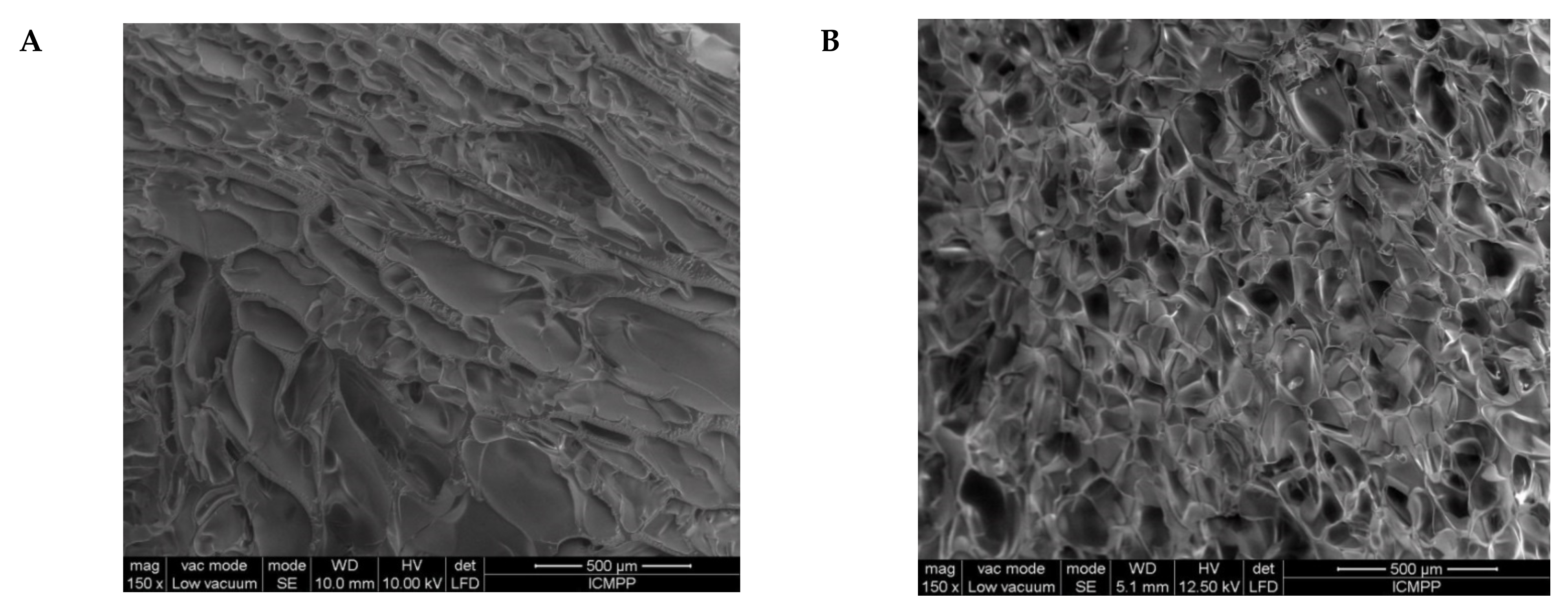
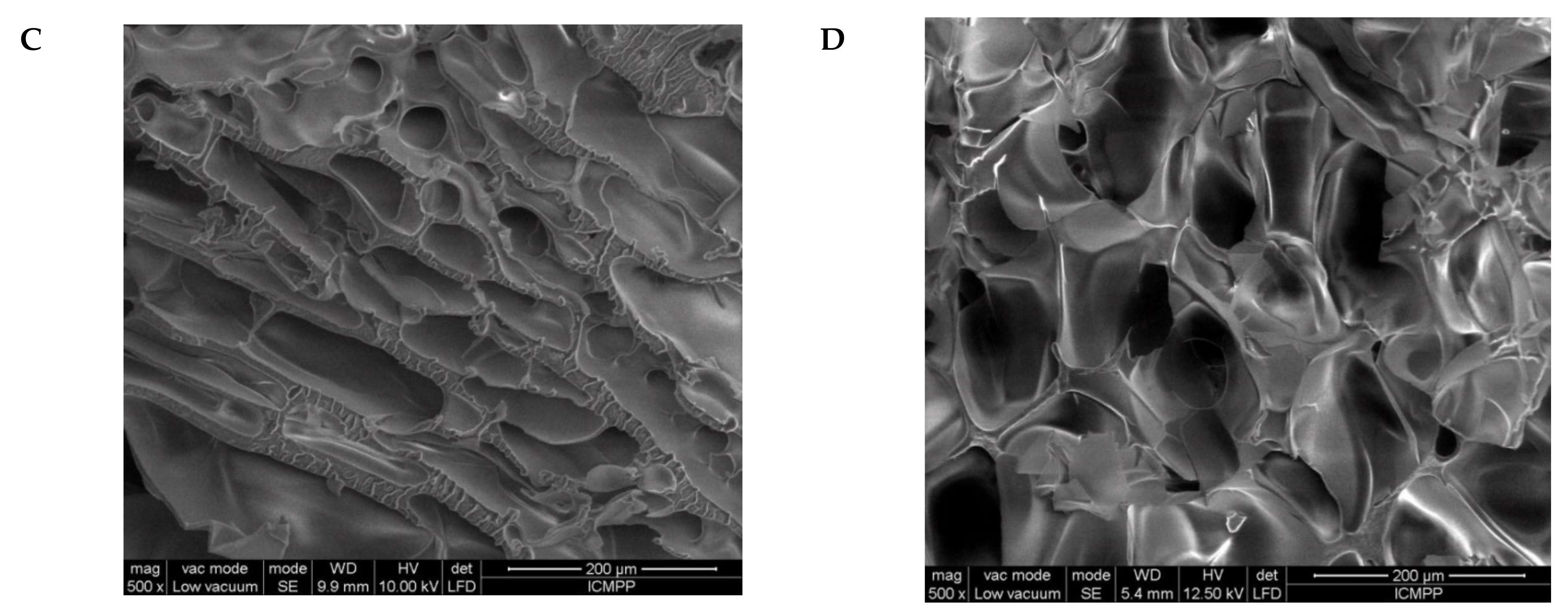
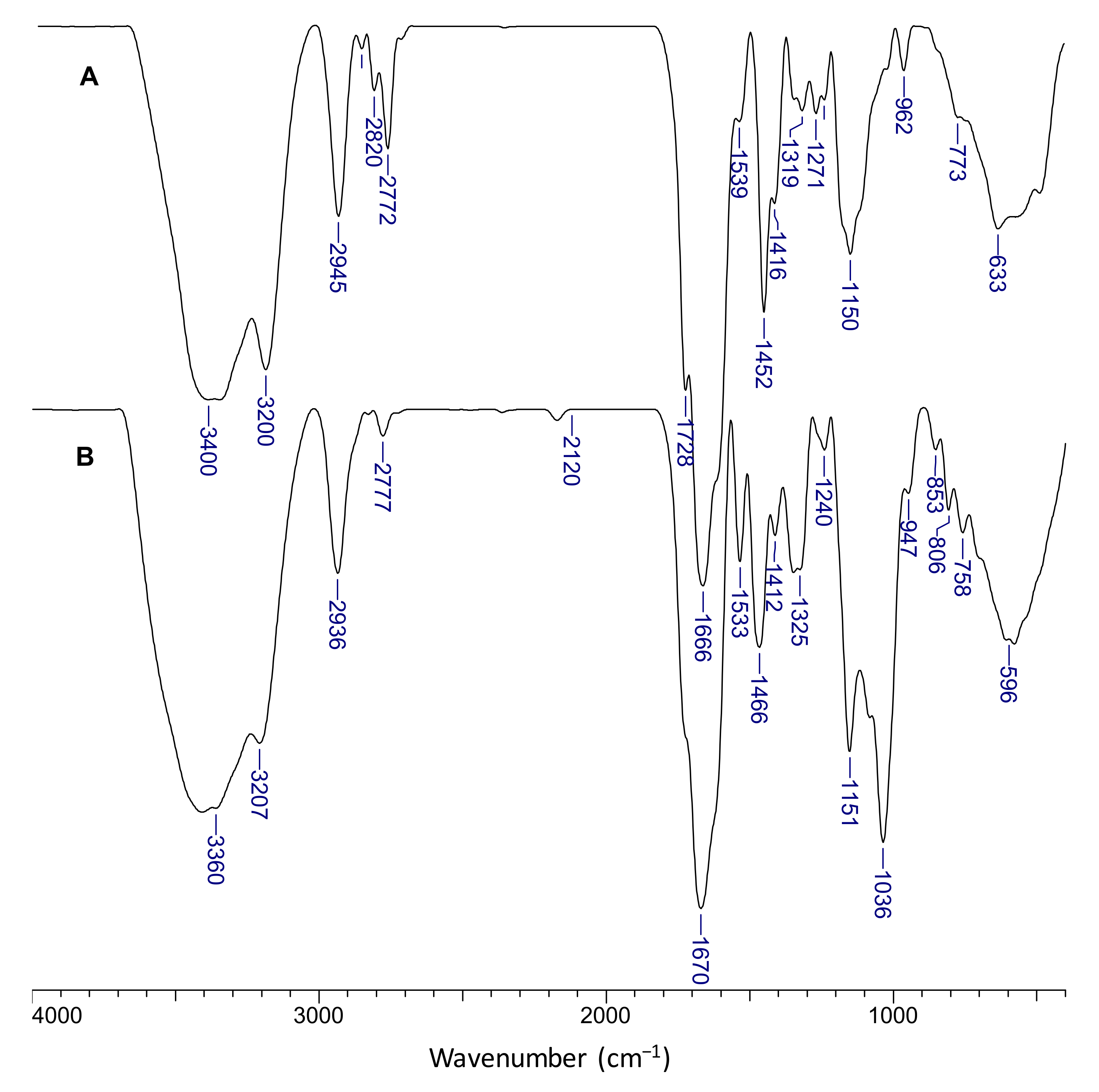
2.2. Morphological and Chemical Characterization of the Semi-IPN Cryogels
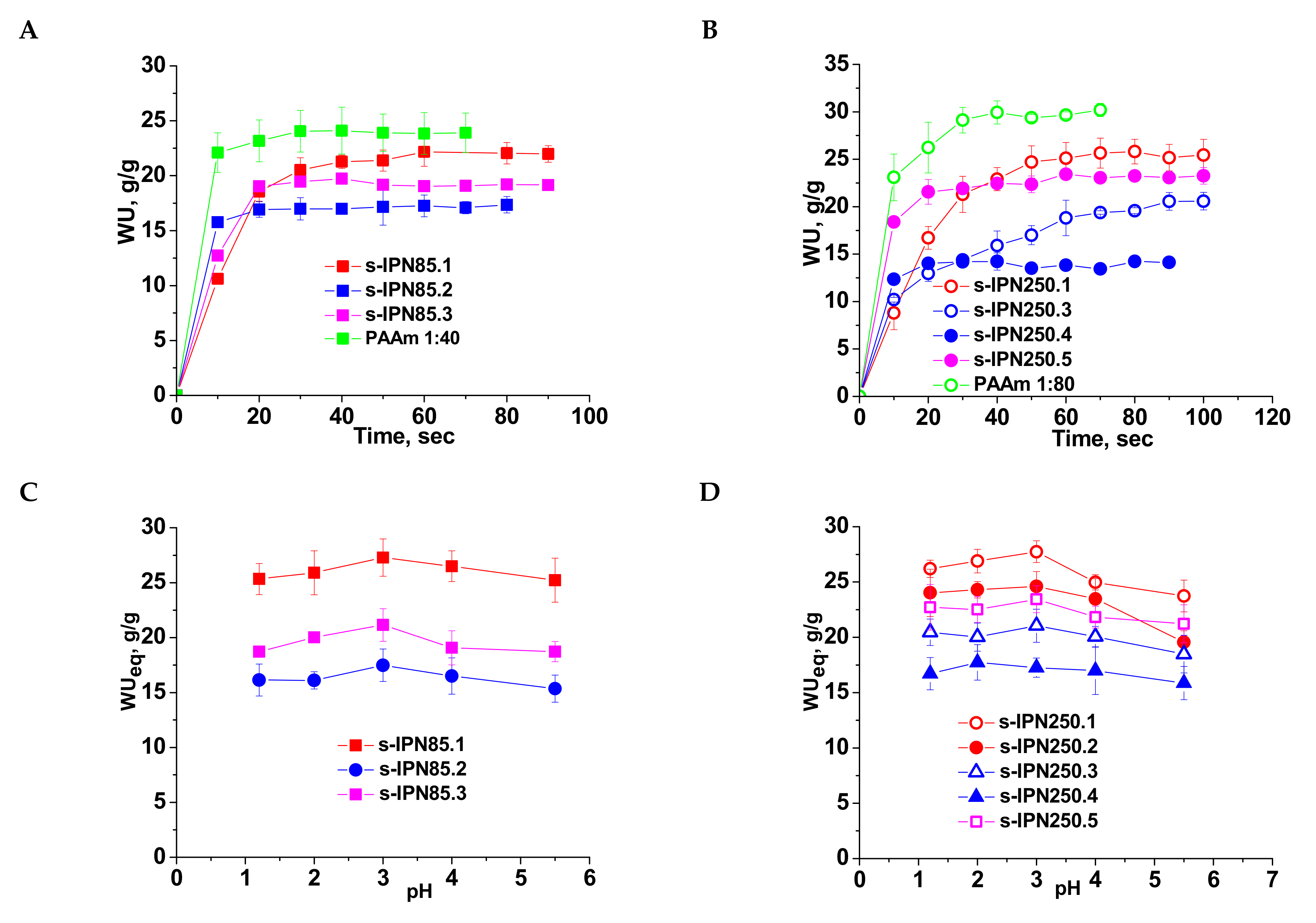
2.3. Swelling Kinetics of Semi-IPN PAAm/PDMAEMA Cryogels
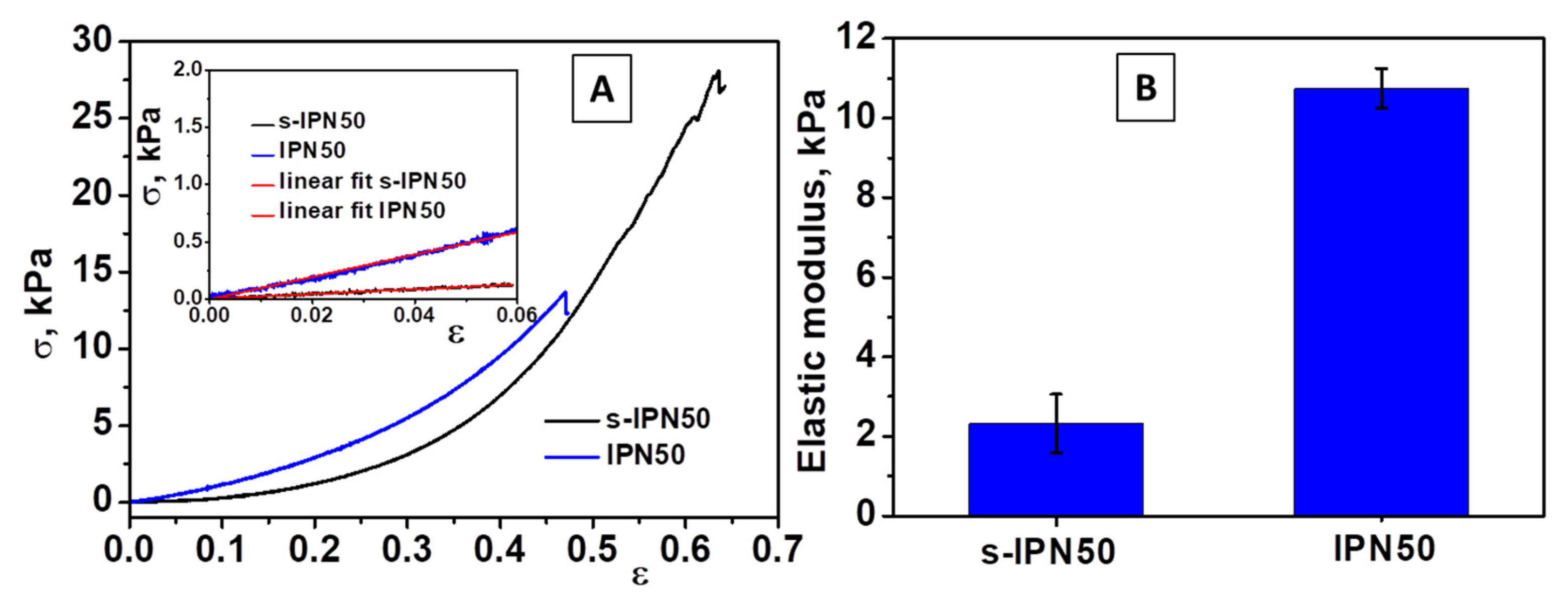
2.4. Synthesis and Characterization of IPN cryogels
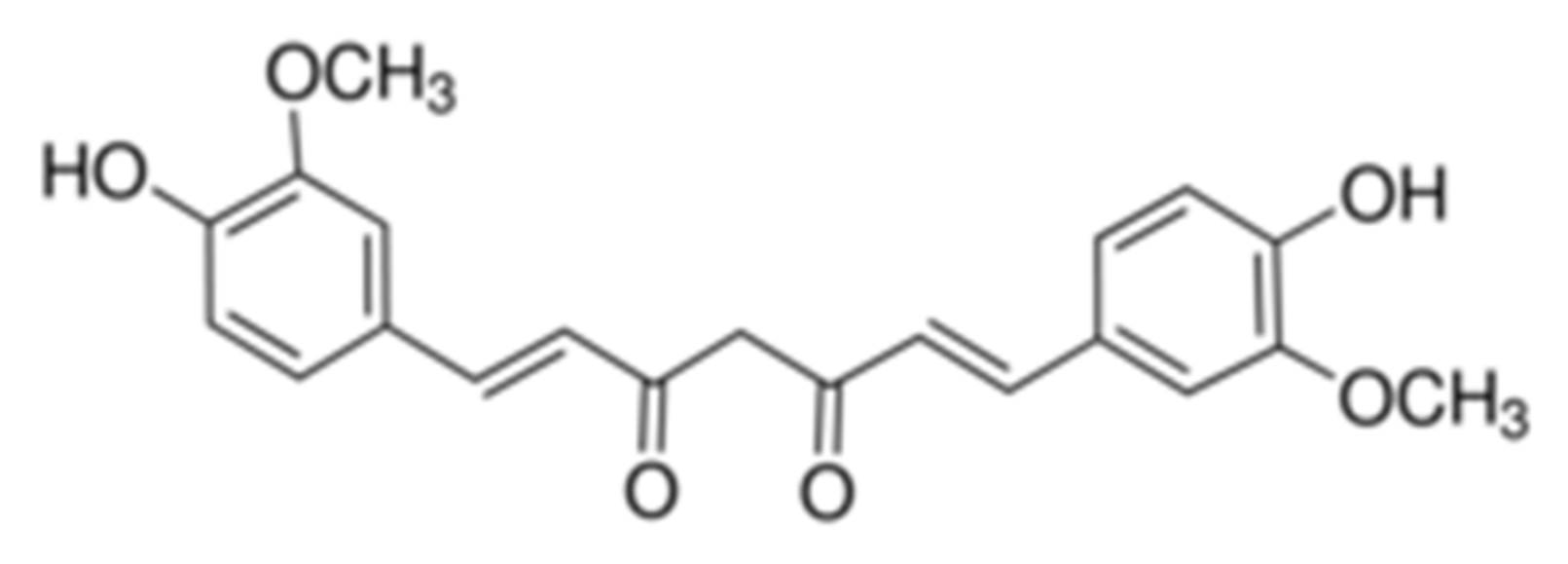
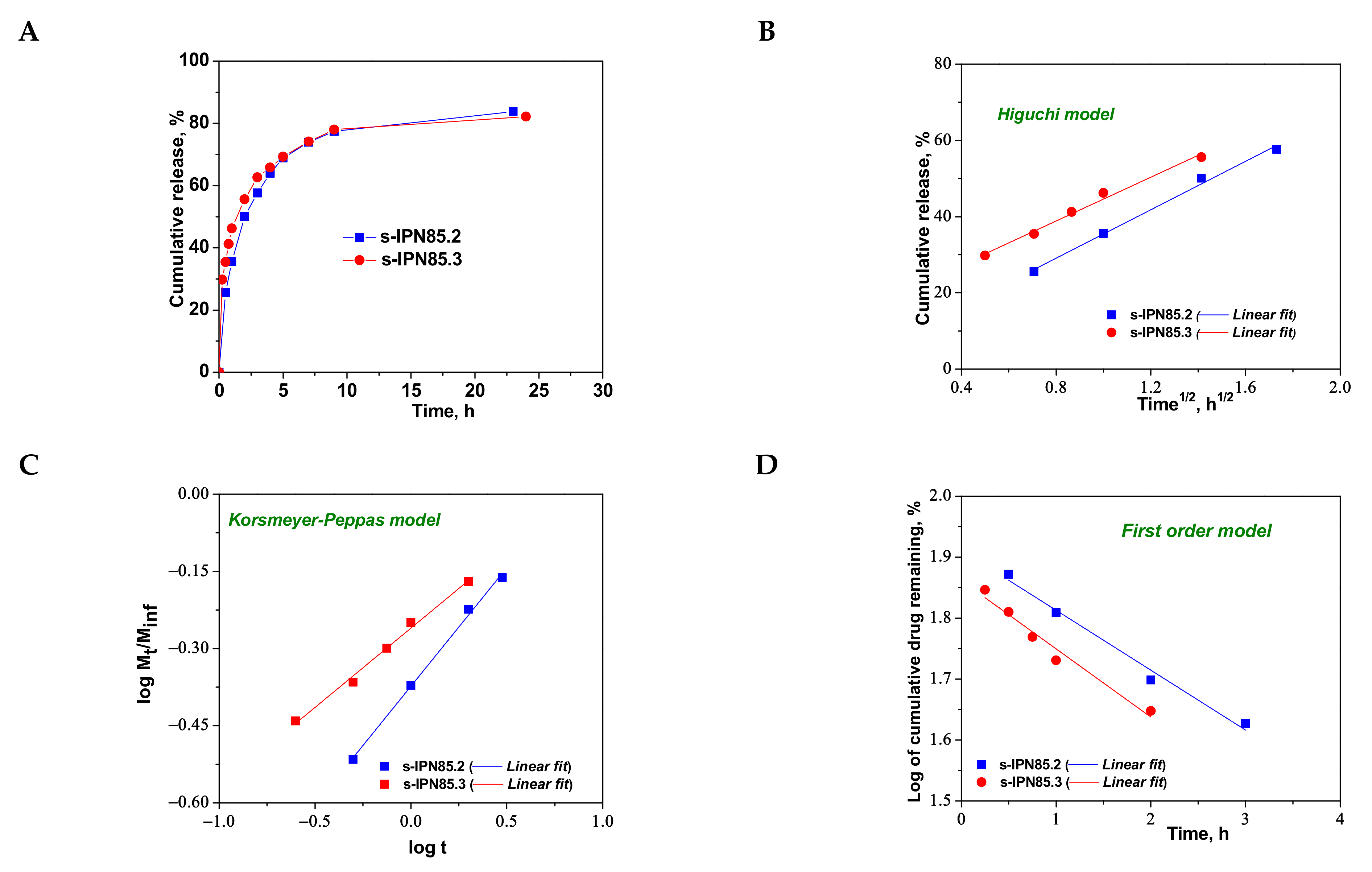
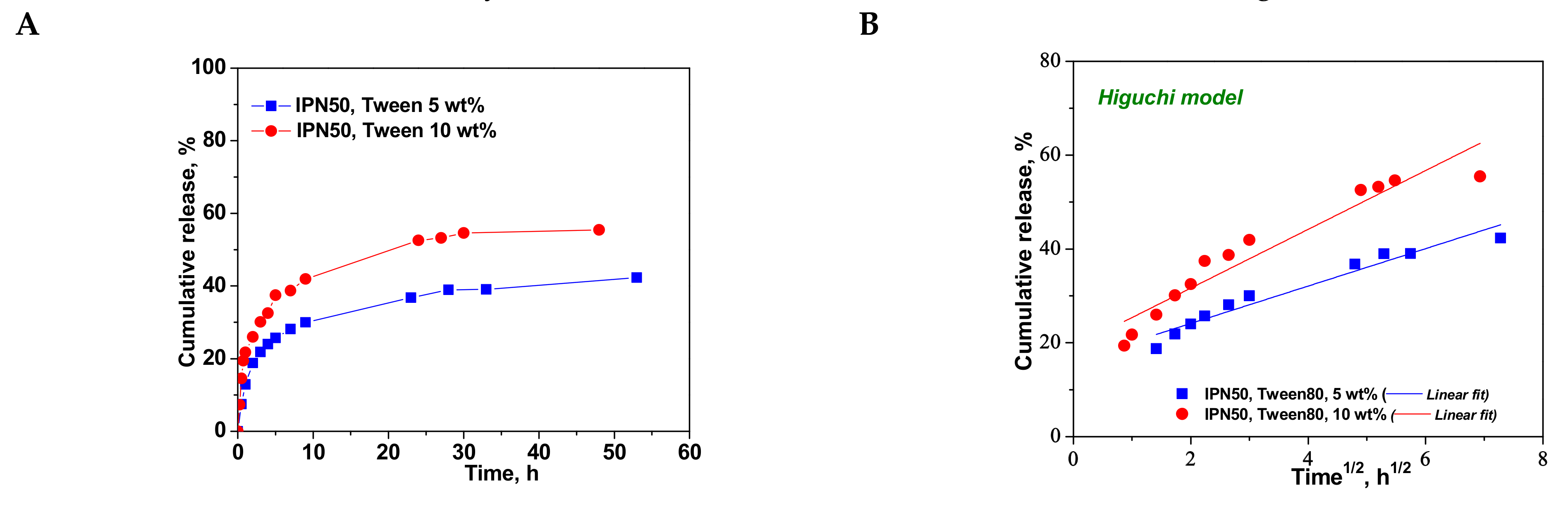
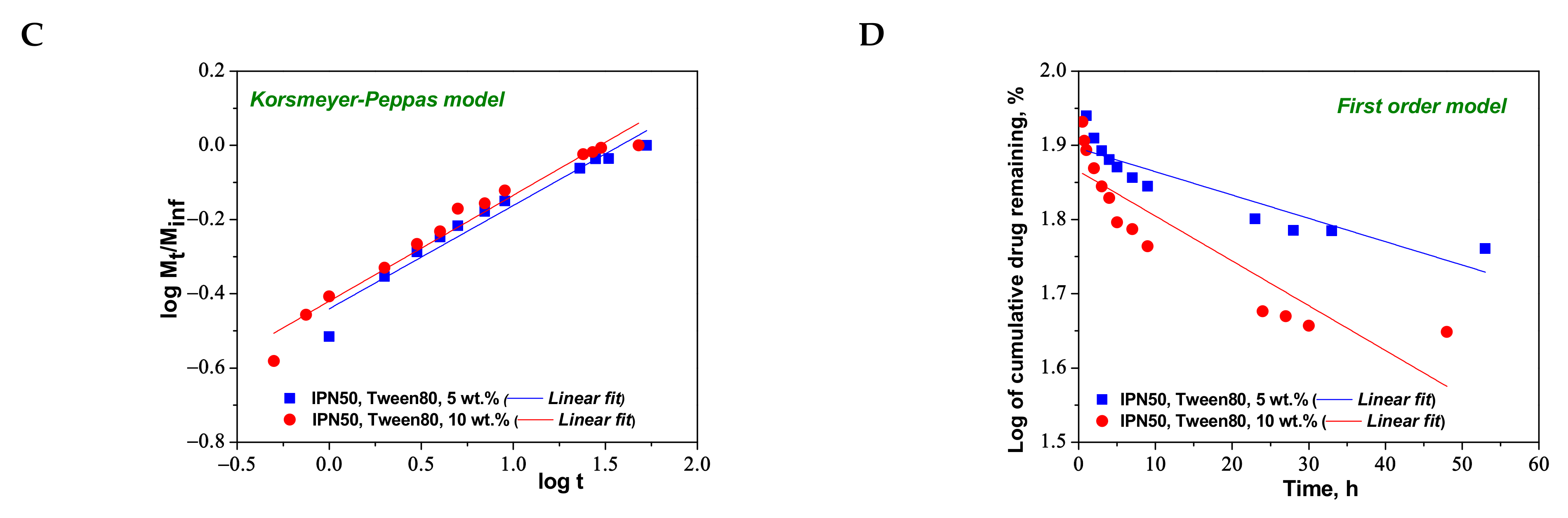
2.5. Loading and Release of CCM from PAAm/PDMAEMA Composite Cryogels
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of PDMAEMA
3.2.2. Synthesis of Semi-IPN and IPN PAAm/PDMAEMA Cryogels
3.2.3. Equipments for Characterization of Cryogels
3.2.4. Swelling Behavior of the Composite Cryogels
3.2.5. Loading and Release of Curcumin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| AAm | Acrylamide |
| APS | Ammonium persulfate |
| BAAm | N,N’-Methylenebisacrylamide |
| CCM | Curcumin |
| CD | Cyclodextrin |
| DDS | Drug delivery system |
| EDX | Energy-dispersive X-ray spectroscopy |
| GFY | Gel fraction yield |
| LCST | Lower critical solution temperature |
| MCT-β-CD | Monochlorotriazinyl-β-cyclodextrin |
| PAAm | Polyacrylamide |
| PDMAEMA | Poly(dimethylamonoethyl methacrylate) |
| s-IPN | Semi-interpenetrating polymer networks |
| TEMED | N,N,N’,N’-tetramethylethylenediamine |
| WU | Water uptake |
| UF | Unidirectional freezing |
| VPTT | Volume phase transition temperature |
References
- Tizabi, Y.; Hurley, L.L.; Qualls, Z.; Akinfiresoye, L. Relevance of the Anti-Inflammatory Properties of Curcumin in Neurodegenerative Diseases and Depression. Molecules 2014, 19, 20864–20879. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Philippot, S.; Fontanay, S.; Duval, R.E.; Lamouroux, E.; Canilho, N.; Pasc, A. pH- and glutathione-responsive release of curcumin from mesoporous silica nanoparticles coated using tannic acid–Fe(iii) complex. RSC Adv. 2015, 5, 90550–90558. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Panaro, M.A.; La Torre, M.E.; Monda, V.; Messina, G.; Porro, C. Curcumin as Prospective Anti-Aging Natural Compound: Focus on Brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef] [PubMed]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, X.; Pi, C.; Yang, H.; Zheng, X.; Zhao, L.; Wei, Y. Review of Curcumin Physicochemical Targeting Delivery System. Int. J. Nanomed. 2020, 15, 9799–9821. [Google Scholar] [CrossRef]
- AbouAitah, K.E.A.; Farghali, A.A.; Swiderska-Sroda, A.; Lojkowski, W.; Razin, A.-F.M.; Khedr, M.H. pH-controlled Release System for Curcumin based on Functionalized Dendritic Mesoporous Silica Nanoparticles. J. Nanomed. Nanotechnol. 2016, 7, 1000351. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Y.; Liu, Q.; Yang, L.; Zhu, R.; Yu, C.; Wang, S. Rational Design of Multifunctional Dendritic Mesoporous Silica Nanoparticles to Load Curcumin and Enhance Efficacy for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 8, 26511–26523. [Google Scholar] [CrossRef]
- Daryasari, M.P.; Akhgar, M.R.; Mamashli, F.; Bigdeli, B.; Khoobi, M. Chitosan-folate coated mesoporous silica nanoparticles as a smart and pH-sensitive system for curcumin delivery. RSC Adv. 2016, 6, 105578–105588. [Google Scholar] [CrossRef]
- Alavijeh, R.K.; Akhbari, K. Biocompatible MIL-101(Fe) as a Smart Carrier with High Loading Potential and Sustained Release of Curcumin. Inorg. Chem. 2020, 59, 3570–3578. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Li, R.; Mao, L.; Liu, F.; Gao, Y. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.F.; Matafwali, S.K.; Mweetwa, L.; Shandele, G.; Walker, R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a nonionic surfactant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef]
- Jardim, K.V.; Palomec-Garfias, A.F.; Andrade, B.Y.G.; Chaker, J.A.; Báo, S.; Márquez-Beltrán, C.; Moya, S.E.; Parize, A.L.; Sousa, M.H. Novel magneto-responsive nanoplatforms based on MnFe2O4 nanoparticles layer-by-layer functionalized with chitosan and sodium alginate for magnetic controlled release of curcumin. Mater. Sci. Eng. C 2018, 92, 184–195. [Google Scholar] [CrossRef]
- Hasan, M.; ElKhoury, K.; Kahn, C.J.F.; Arab-tehrany, E.; Linder, M. Preparation, Characterization, and Release Kinetics of Chitosan-Coated Nanoliposomes Encapsulating Curcumin in Simulated Environments. Molecules 2019, 24, 2023. [Google Scholar] [CrossRef] [Green Version]
- Omer, A.; Ziora, Z.; Tamer, T.; Khalifa, R.; Hassan, M.; Mohy-Eldin, M.; Blaskovich, M. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef]
- Liu, F.; Li, R.; Mao, L.; Gao, Y. Ethanol-induced composite hydrogel based on propylene glycol alginate and zein: Formation, characterization and application. Food Chem. 2018, 255, 390–398. [Google Scholar] [CrossRef]
- Gao, N.; Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Feng, C.; Liu, M. Injectable shell-crosslinked F127 micelle/hydrogel composites with pH and redox sensitivity for combined release of anticancer drugs. Chem. Eng. J. 2016, 287, 20–29. [Google Scholar] [CrossRef]
- Wu, L.; Tian, J.; Ye, X.; Fang, H.; Zhang, Z.; Xu, C.; Zhang, H. Encapsulation and Release of Curcumin with the Mixture of Porous Rice Starch and Xanthan Gum. Starch Stärke 2020, 73, 2000042. [Google Scholar] [CrossRef]
- Qi, X.; Yuan, Y.; Zhang, J.; Bulte, J.W.M.; Dong, W. Oral Administration of Salecan-Based Hydrogels for Controlled Insulin Delivery. J. Agric. Food Chem. 2018, 66, 10479–10489. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Shen, J.; Dong, W. Salecan polysaccharide-based hydrogels and their applications: A review. J. Mater. Chem. B 2019, 7, 2577–2587. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2020, 306, 125632. [Google Scholar] [CrossRef]
- Caka, M.; Türkcan, C.; Uygun, D.A.; Uygun, M.; Akgöl, S.; Denizli, A. Controlled release of curcumin from poly(HEMA-MAPA) membrane. Artif. Cells Nanomed. Biotechnol. 2017, 45, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Chiaoprakobkij, N.; Suwanmajo, T.; Sanchavanakit, N.; Phisalaphong, M. Curcumin-Loaded Bacterial Cellulose/Alginate/Gelatin as A Multifunctional Biopolymer Composite Film. Molecules 2020, 25, 3800. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocarta, A.I. Smart Macroporous IPN Hydrogels Responsive to pH, Temperature, and Ionic Strength: Synthesis, Characterization, and Evaluation of Controlled Release of Drugs. ACS Appl. Mater. Interfaces 2016, 8, 12018–12030. [Google Scholar] [CrossRef]
- Dragan, E.S.; Loghin, D.F.A.; Cocarta, A.-I.; Doroftei, M. Multi-stimuli-responsive semi-IPN cryogels with native and anionic potato starch entrapped in poly(N,N-dimethylaminoethyl methacrylate) matrix and their potential in drug delivery. React. Funct. Polym. 2016, 105, 66–77. [Google Scholar] [CrossRef]
- Dinu, M.V.; Perju, M.M.; Drăgan, E.S. Composite IPN ionic hydrogels based on polyacrylamide and dextran sulfate. React. Funct. Polym. 2011, 71, 881–890. [Google Scholar] [CrossRef]
- Risbud, M.V.; Bhonde, R.R. Polyacrylamide-Chitosan Hydrogels: In Vitro Biocompatibility and Sustained Antibiotic Release Studies. Drug Deliv. 2000, 7, 69–75. [Google Scholar] [CrossRef]
- Ekici, S.; Saraydin, D. Synthesis, Characterization and Evaluation of IPN Hydrogels for Antibiotic Release. Drug Deliv. 2004, 11, 381–388. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Dragan, E.S.; Lazar, M.M.; Dinu, M.V.; Doroftei, F. Macroporous composite IPN hydrogels based on poly(acrylamide) and chitosan with tuned swelling and sorption of cationic dyes. Chem. Eng. J. 2012, 204–206, 198–209. [Google Scholar] [CrossRef]
- Rawlinson, L.-A.B.; Ryan, S.M.; Mantovani, G.; Syrett, J.A.; Haddleton, D.; Brayden, D. Antibacterial Effects of Poly(2-(dimethylamino ethyl)methacrylate) against Selected Gram-Positive and Gram-Negative Bacteria. Biomacromolecules 2010, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-L.; Tsai, H.-M.; Yang, S.-J.; Luo, T.-Y.; Lin, C.-F.; Lin, W.-J.; Shieh, M.-J. Development of thermosensitive poly(n-isopropylacrylamide-co-((2-dimethylamino) ethyl methacrylate))-based nanoparticles for controlled drug release. Nanotechnology 2011, 22, 265608. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Jhon, M.S.; Yuk, S.H.; Lee, H.B. Temperature-induced phase transition of poly(N,N-dimethylaminoethyl methac-rylate-co-acrylamide). J. Polym. Sci. Part B Polym. Phys. 1997, 35, 595–598. [Google Scholar] [CrossRef]
- Popat, A.; Karmakar, S.; Jambhrunkar, S.; Xu, C.; Yu, C. Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity. Colloids Surf. B Biointerfaces 2014, 117, 520–527. [Google Scholar] [CrossRef]
- Guo, S. Encapsulation of curcumin into β-cyclodextrins inclusion: A review. E3S Web Conf. 2019, 131, 01100. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A. Evaluation of Release Kinetics and Mechanisms of Curcumin and Curcumin-β-Cyclodextrin Inclusion Complex Incorporated in Electrospun Almond Gum/PVA Nanofibers in Simulated Saliva and Simulated Gastrointestinal Conditions. BioNanoScience 2019, 9, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef]
- Jain, E.; Damania, A.; Shakya, A.K.; Kumar, A.; Sarin, S.K.; Kumar, A. Fabrication of macroporous cryogels as potential hepatocyte carriers for bioartificial liver support. Colloids Surf. B 2015, 136, 761–771. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Idil, N.; Perçin, I.; Denizli, A. Biomedical Applications of Polymeric Cryogels. Appl. Sci. 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbohydr. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocarta, A.I.; Gierszewska, M. Designing novel macroporous composite hydrogels based on methacrylic acid copolymers and chitosan and in vitro assessment of lysozyme controlled delivery. Colloids Surf. B Biointerfaces 2016, 139, 33–41. [Google Scholar] [CrossRef]
- Loghin, D.F.A.; Biliuta, G.; Coseri, S.; Dragan, E.S. Preparation and characterization of oxidized starch/poly(N,N-dimethylaminoethyl methacrylate) semi-IPN cryogels and in vitro controlled release evaluation of indomethacin. Int. J. Biol. Macromol. 2017, 96, 589–599. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr. Polym. 2019, 225, 115210. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Ice segregation induced self-assembly of salecan and grapheme oxide nanosheets into ion-imprinted aerogel with superior selectivity for cadmium (II) capture. Chem. Eng. J. 2021, 417, 128106. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Development of chitosan-poly(ethyleneimine) based double network cryogels and their application as superadsorbents for phosphate. Carbohydr. Polym. 2019, 210, 17–25. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Designing smart triple-network cationic cryogels with outstanding efficiency and selectivity for deep cleaning of phosphate. Chem. Eng. J. 2021, 426, 131411. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Abdel-Mohdy, F.A.; Al-Deyab, S.S.; El-Newehy, M.H. Chitosan and monochlorotriazinyl-β-cyclodextrin finishes inprove antistatic properties of cotton/polyester blend and polyester fabrics. Carbohydr. Polym. 2010, 82, 202–208. [Google Scholar] [CrossRef]
- Popescu, V.; Muresan, E.I.; Grigoriu, A.-M. Monochlorotriazinyl-β-cyclodextrin grafting onto polyester fabrics and films. Carbohydr. Polym. 2011, 86, 600–611. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Rukmani, A. Durable Antibacterial Finishing on Organic Cotton by Inclusion of Thymol into Cyclodextrin Derivative. J. Chem. 2012, 9, 1511–1517. [Google Scholar] [CrossRef]
- Khanna, S.; Chakraborty, J.N. Optimization of monochlorotriazine β-cyclodextrin grafting on cotton and assessment of release behavior of essential oils from functionalized fabric. Fash. Text. 2017, 4, 5196. [Google Scholar] [CrossRef] [Green Version]
- Elzairy, E.R.M.; Abdallah, W.A.; Osman, S.M.; Fouad, M.A.M. Recent approach for multifunctional finishing of cotton/polyester fabric blends via natural products. Int. J. Text. Sci. 2017, 6, 148–152. [Google Scholar] [CrossRef]
- Setthayanond, J.; Sodsangchan, C.; Suwanruji, P.; Tooptompong, P.; Avinc, O. Influence of MCT-β-cyclodextrin treatment on strength, reactive dyeing and third-hand cigarette smoke odor release properties of cotton fabric. Cellulose 2017, 24, 5233–5250. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; Da Silva, J.G.D.; Valle, R.D.C.S.C.; Valle, J.A.B.; Scacchetti, F.A.P.; Tessaro, A.L. The Role of β-Cyclodextrin in the Textile Industry—Review. Molecules 2020, 25, 3624. [Google Scholar] [CrossRef]
- Manolova, Y.; Deneva, V.; Antonov, L.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Dragan, E.S.; Dinu, I.A. Solution behavior of progressively quaternized poly(dimethylaminoethyl methacrylate) as a function of charge density. Eur. Polym. J. 2011, 47, 1065–1072. [Google Scholar] [CrossRef]
- Dinu, M.V.; Přádný, M.; Drăgan, E.S.; Michálek, J. Ice-templated hydrogels based on chitosan with tailored porous morphology. Carbohydr. Polym. 2013, 94, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, R.; Das, G.; Singh, S.; Pathak, L.; Ghosh, R.N.; Venkataraman, B.; Krishnamurthy, R. Study on influence of porosity, pore size, spatial and topological distribution of pores on microhardness of as plasma sprayed ceramic coatings. Mater. Sci. Eng. A 2007, 445–446, 269–274. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Ak, F.; Oztoprak, Z.; Karakutuk, I.; Okay, O. Macroporous Silk Fibroin Cryogels. Biomacromolecules 2013, 14, 719–727. [Google Scholar] [CrossRef]
- Ja’Far, M.H.; Kamal, N.N.S.N.M.; Hui, B.Y.; Kamaruzzaman, M.F.; Zain, N.N.M.; Yahaya, N.; Raoov, M. Inclusion of Curcumin in β-cyclodextrins as Potential Drug Delivery System: Preparation, Characterization and Its Preliminary Cytotoxicity Approaches. Sains Malays. 2018, 47, 977–989. [Google Scholar] [CrossRef]
- Martel, B.; Devassine, M.; Crini, G.; Weltrowski, M.; Bourdonneau, M.; Morcellet, M. Preparation and sorption properties of a β-cyclodextrin-linked chitosan derivative. J. Polym. Sci. A Polym. Chem. 2001, 39, 169–176. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Morozova, S.A.; Vainerman, E.S.; Titova, E.F.; Shtilman, M.I.; Belatseva, E.M.; Rogozhin, S.V. Study of cryo-structuration of polymer systems. VIII. Characteristic features of the formation of crosslinked poly(acryl amide) cryogels under different thermal conditions. Acta Polym. 1989, 40, 8–15. [Google Scholar] [CrossRef]


| Code of PDMAEMA | Mw, kg mol−1 | Mw/Mn | Lc a, nm |
|---|---|---|---|
| PDMAEMA50 | 50 | 2.2 | 80 |
| PDMAEMA85 | 85 | 2 | 135 |
| PDMAEMA250 | 250 | 2.4 | 398 |
| Sample Code | PAAm | PDMAEMA | GFY *, % | ||
|---|---|---|---|---|---|
| Conc., wt.% | Cross-Linking, Mole BAAm:Moles AAm | Mv, kDa | Conc., wt.% | ||
| s-IPN250.1 | 5 | 1:80 | 250 | 0.225 | 82 |
| s-IPN250.2 | 5 | 1:80 | 250 | 0.45 | 88 |
| s-IPN250.3 | 5 | 1:40 | 250 | 0.225 | 87 |
| s-IPN250.4 | 5 | 1:40 | 250 | 0.45 | 86 |
| s-IPN250.5 ** | 5 | 1:40 | 250 | 0.225 | 84 |
| s-IPN85.1 | 5 | 1:80 | 85 | 0.45 | 80 |
| s-IPN85.2 | 5 | 1:40 | 85 | 0.45 | 84 |
| s-IPN85.3 ** | 5 | 1:40 | 85 | 0.45 | 82 |
| s-IPN50 | 5 | 1:40 | 50 | 2.5 | - |
| Code Sample | Average Pore Diameter, μm |
|---|---|
| s-IPN250.1 | 123.92 ± 34.69 |
| s-IPN250.2 | 151.30 ± 16.56 |
| s-IPN250.3 | 68.68 ± 16.42 |
| s-IPN250.4 | 82.55 ± 12.11 |
| s-IPN250.5 | 80.41 ± 7.72 |
| s-IPN85.1 | 61.20 ± 8.48 |
| s-IPN85.2 | 11.42 ± 1.37 |
| s-IPN85.3 | 45.69 ± 13.22 |
| Sample Code | s-IPN250.1 | s-IPN250.3 | s-IPN250.5 | s-IPN250.4 | s-IPN85.2 | s-IPN85.3 | IPN50 |
|---|---|---|---|---|---|---|---|
| Loading, mg CCM/g CG | 81 | 54.17 | 79 | 74.35 | 84.6 | 73.53 | 62 |
| Model Name | Parameters | Sample Code | |||||
|---|---|---|---|---|---|---|---|
| s-IPN250.1 | s-IPN250.3 | s-IPN250.5 | s-IPN250.4 | s-IPN85.2 | s-IPN85.3 | ||
| Higuchi | kH | 18.8525 | 26.3783 | 23.69.9 | 26.9382 | 31.7601 | 28.7244 |
| R2 | 0.9328 | 0.9811 | 0.9606 | 0.9790 | 0.9911 | 0.9848 | |
| Korsmeyer–Peppas | nr | 0.2991 | 0.2479 | 0.2893 | 0.17531 | 0.4607 | 0.3083 |
| kKP (min−nr) | 7.5199 | 8.0734 | 8.1536 | 9.1081 | 6.8844 | 7.7077 | |
| R2 | 0.9567 | 0.9946 | 0.9723 | 0.9937 | 0.9956 | 0.9911 | |
| First order | k1 | −0.0050 | −0.1140 | −0.0825 | −0.1663 | −0.0982 | −0.1119 |
| R2 | 0.8993 | 0.9513 | 0.9495 | 0.9492 | 0.9796 | 0.9594 | |
| Model Name | Parameters | Sample Code | |
|---|---|---|---|
| IPN50 Tween 5 wt.% | IPN50 Tween 10 wt.% | ||
| Higuchi | kH | 3.9860 | 6.2642 |
| R2 | 0.9449 | 0.9126 | |
| Korsmeyer–Peppas | nr | 0.2785 | 0.2857 |
| kKP (min−nr) | 6.4369 | 6.5699 | |
| R2 | 0.9516 | 0.9644 | |
| First-order | k1 | −0.0032 | −0.0060 |
| R2 | 0.8145 | 0.7964 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A.; Lazar, M.M.; Doroftei, F. Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-β-cyclodextrin and Release Kinetics of Curcumin. Molecules 2021, 26, 6975. https://doi.org/10.3390/molecules26226975
Dragan ES, Dinu MV, Ghiorghita CA, Lazar MM, Doroftei F. Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-β-cyclodextrin and Release Kinetics of Curcumin. Molecules. 2021; 26(22):6975. https://doi.org/10.3390/molecules26226975
Chicago/Turabian StyleDragan, Ecaterina Stela, Maria Valentina Dinu, Claudiu Augustin Ghiorghita, Maria Marinela Lazar, and Florica Doroftei. 2021. "Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-β-cyclodextrin and Release Kinetics of Curcumin" Molecules 26, no. 22: 6975. https://doi.org/10.3390/molecules26226975
APA StyleDragan, E. S., Dinu, M. V., Ghiorghita, C. A., Lazar, M. M., & Doroftei, F. (2021). Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-β-cyclodextrin and Release Kinetics of Curcumin. Molecules, 26(22), 6975. https://doi.org/10.3390/molecules26226975







