Elucidation of the Roles of Ionic Liquid in CO2 Electrochemical Reduction to Value-Added Chemicals and Fuels
Abstract
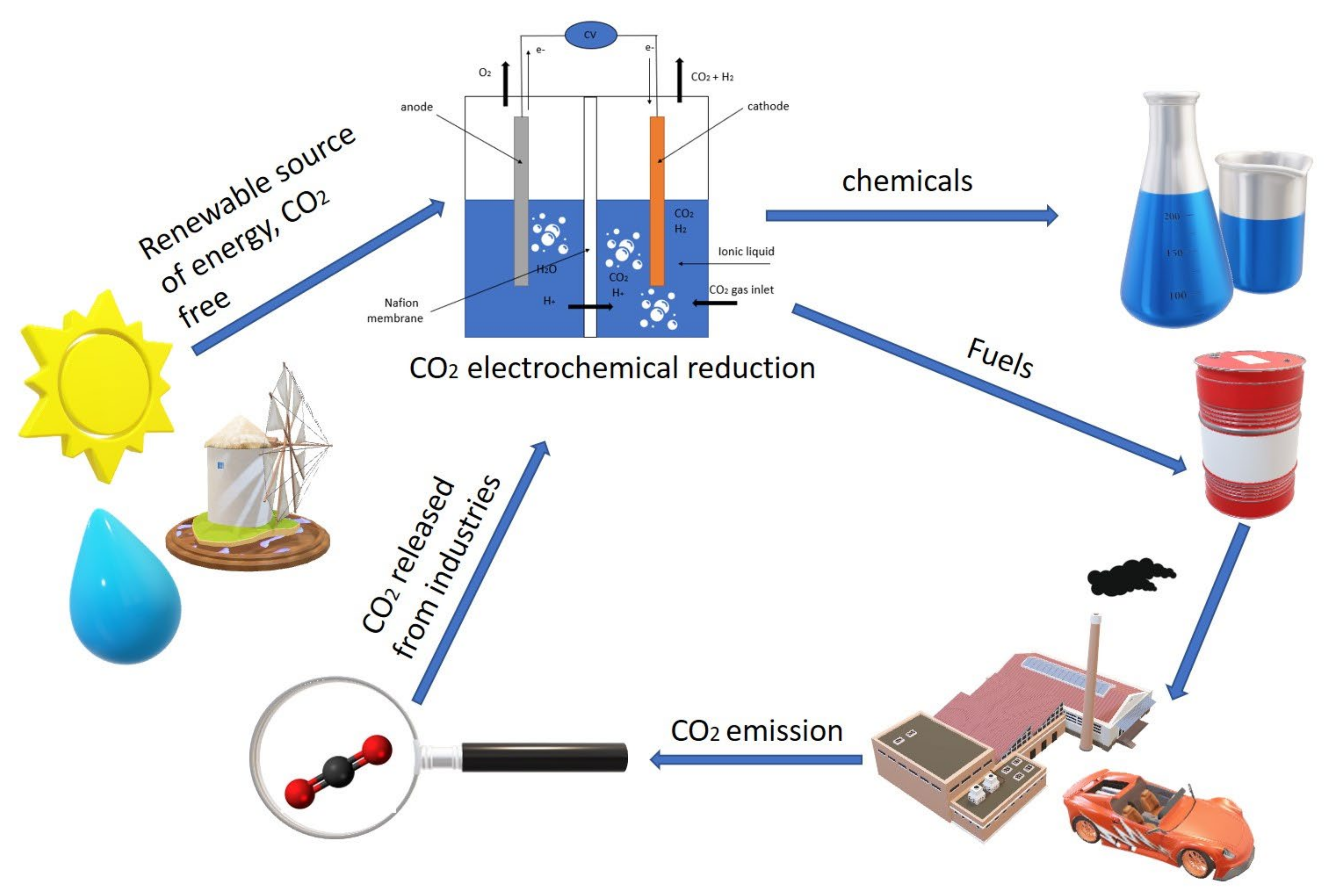
1. Introduction
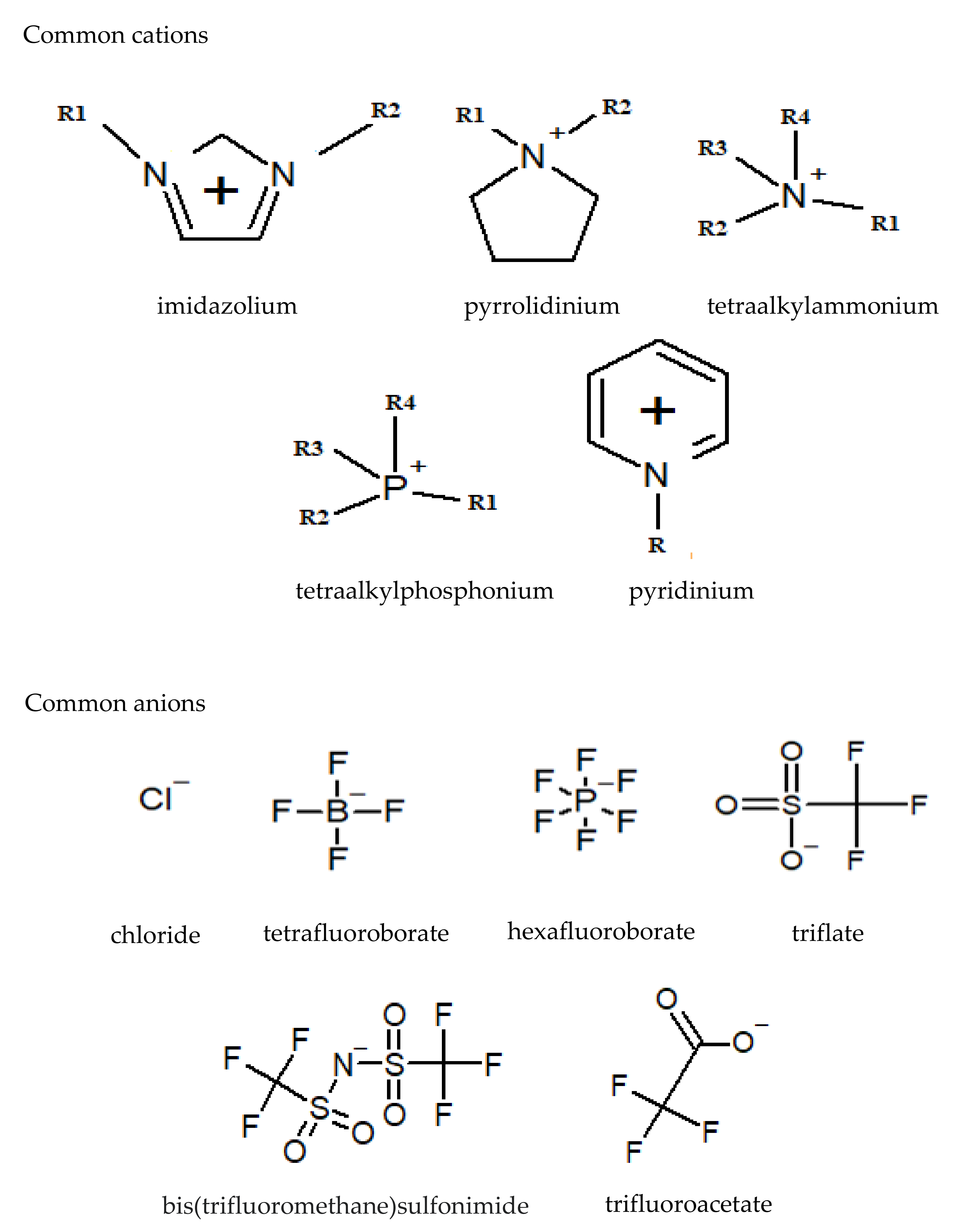
2. Introduction to Ionic Liquids
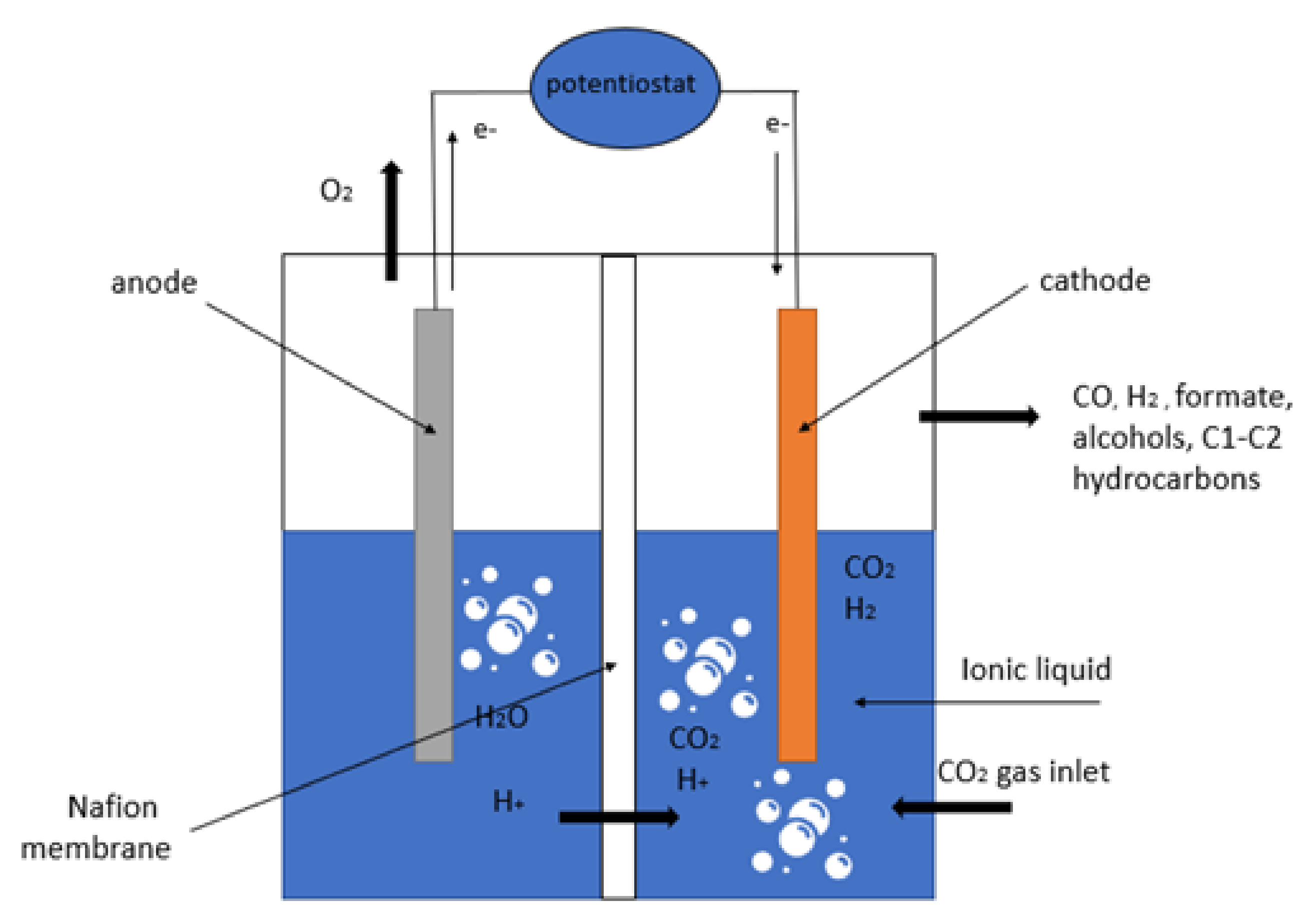
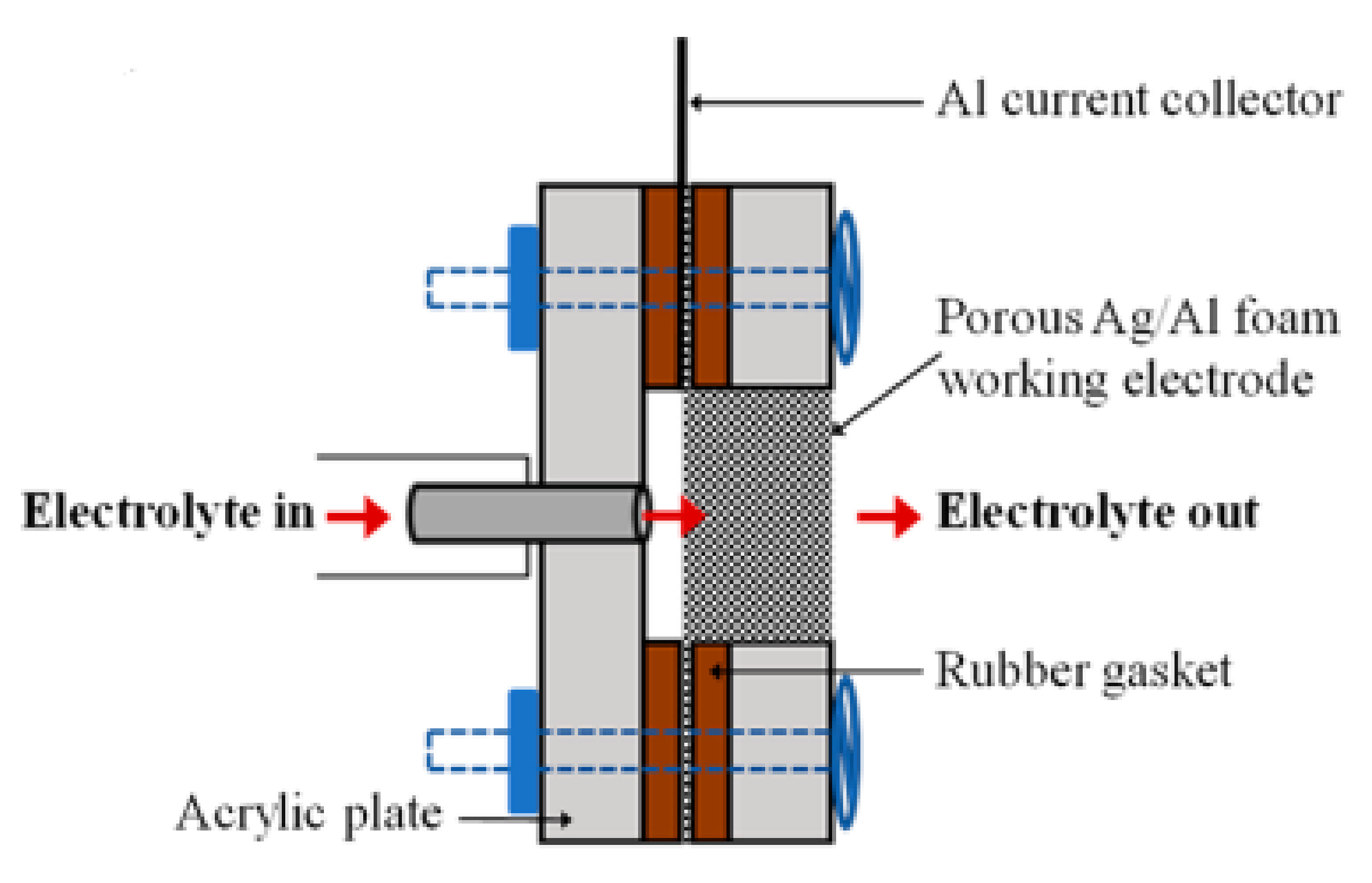
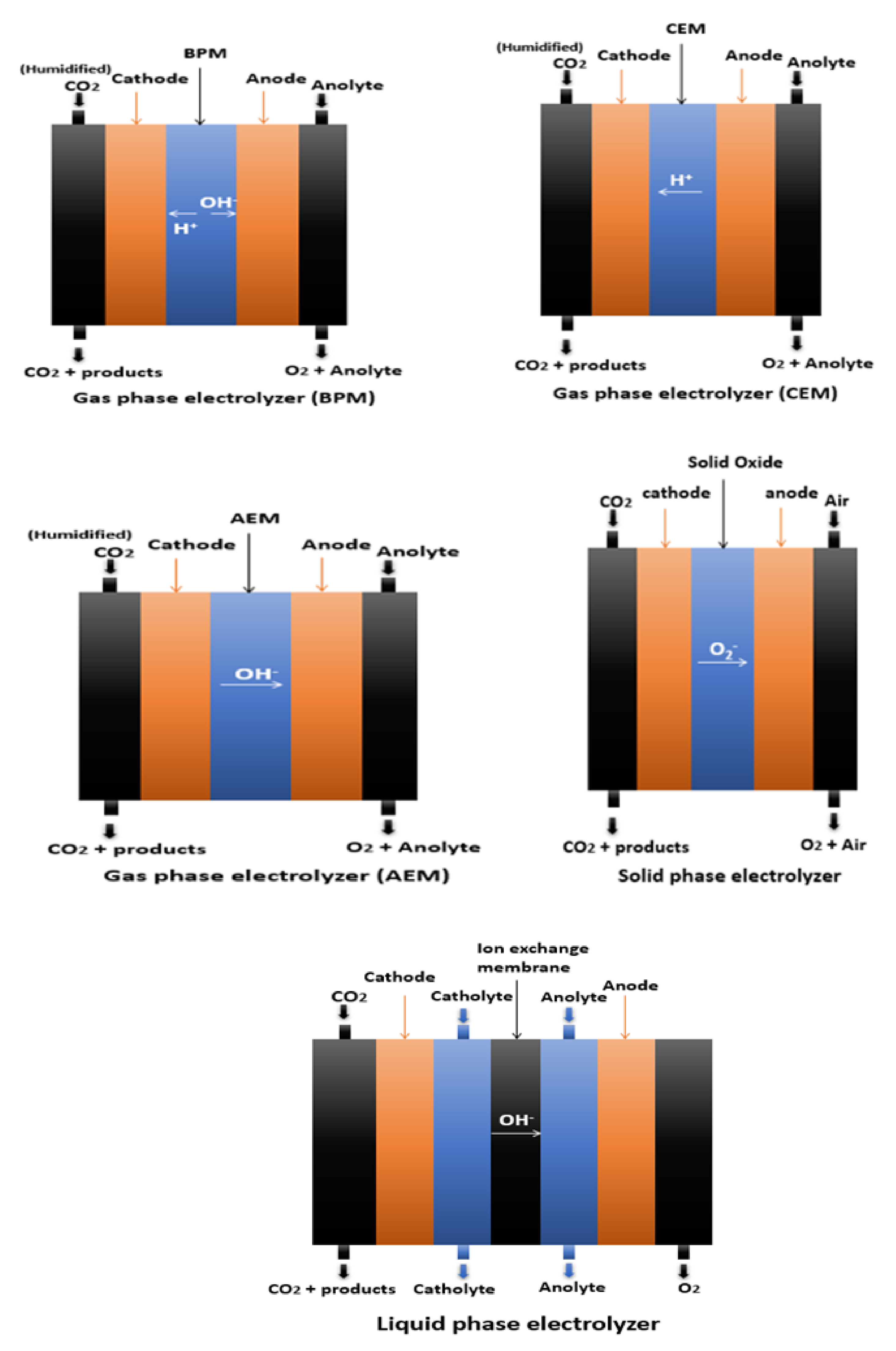
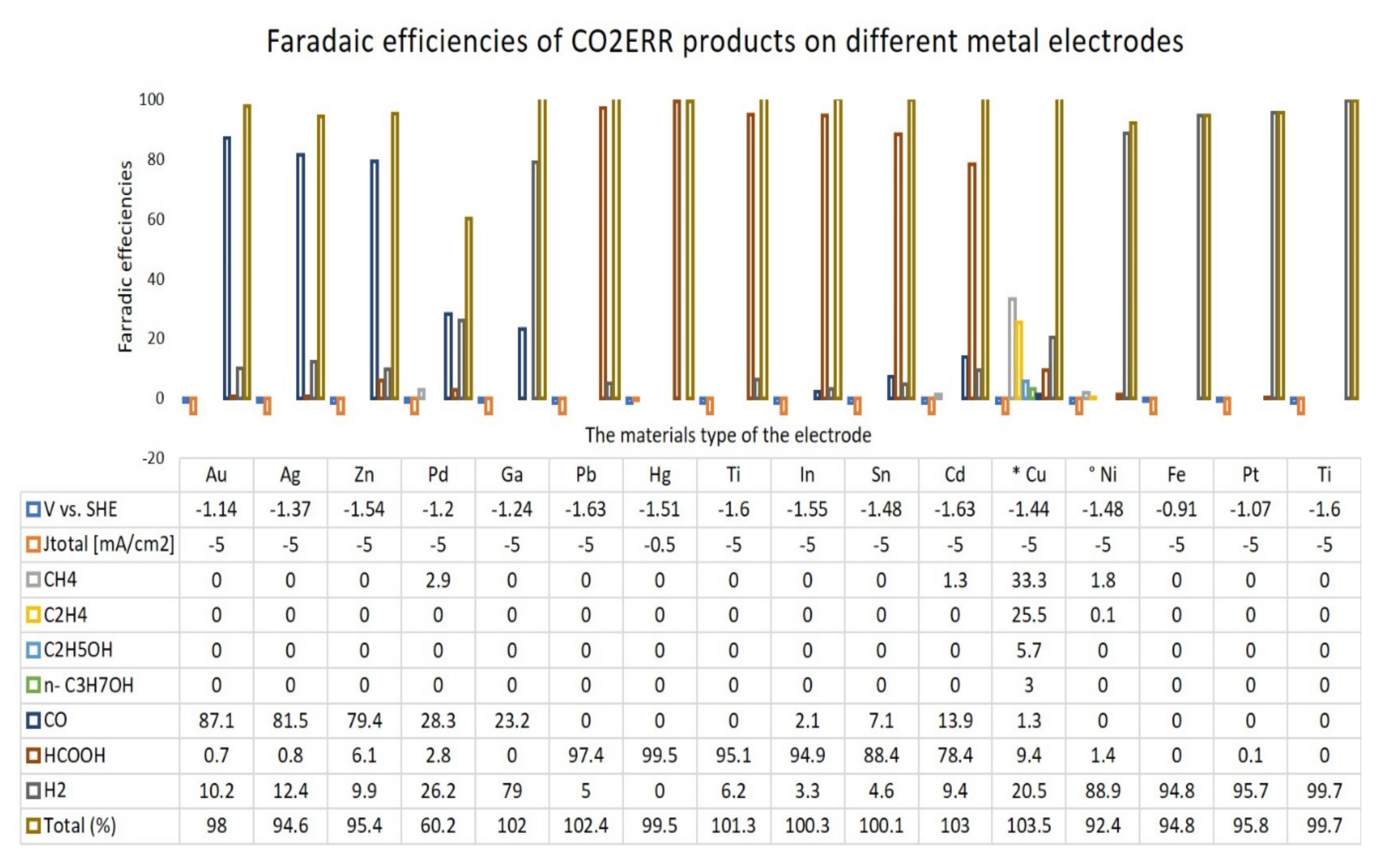
3. The Roles of the Electrode in CO2ERR
4. Ionic Liquids as Co-Catalyst for CO2ERR System
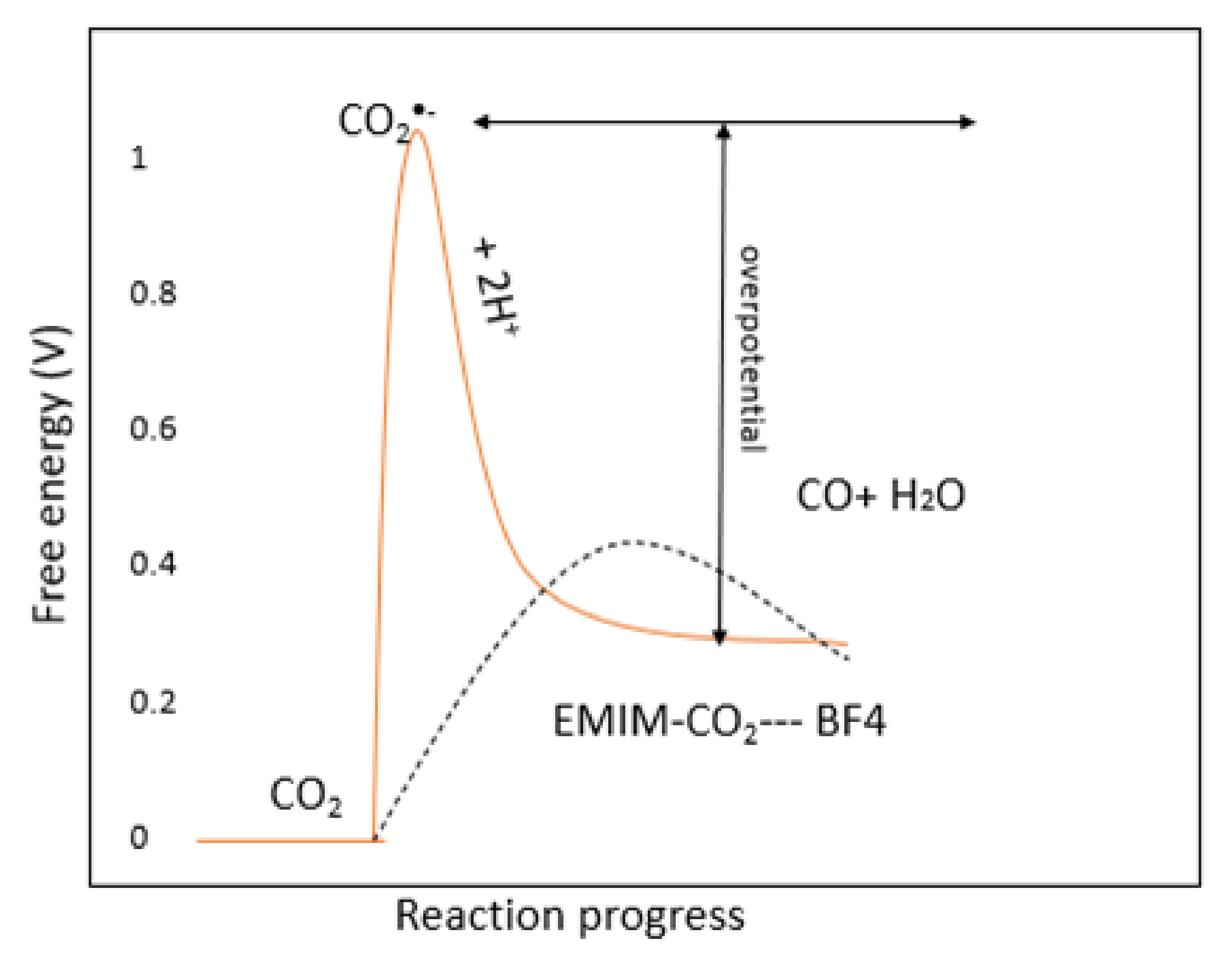
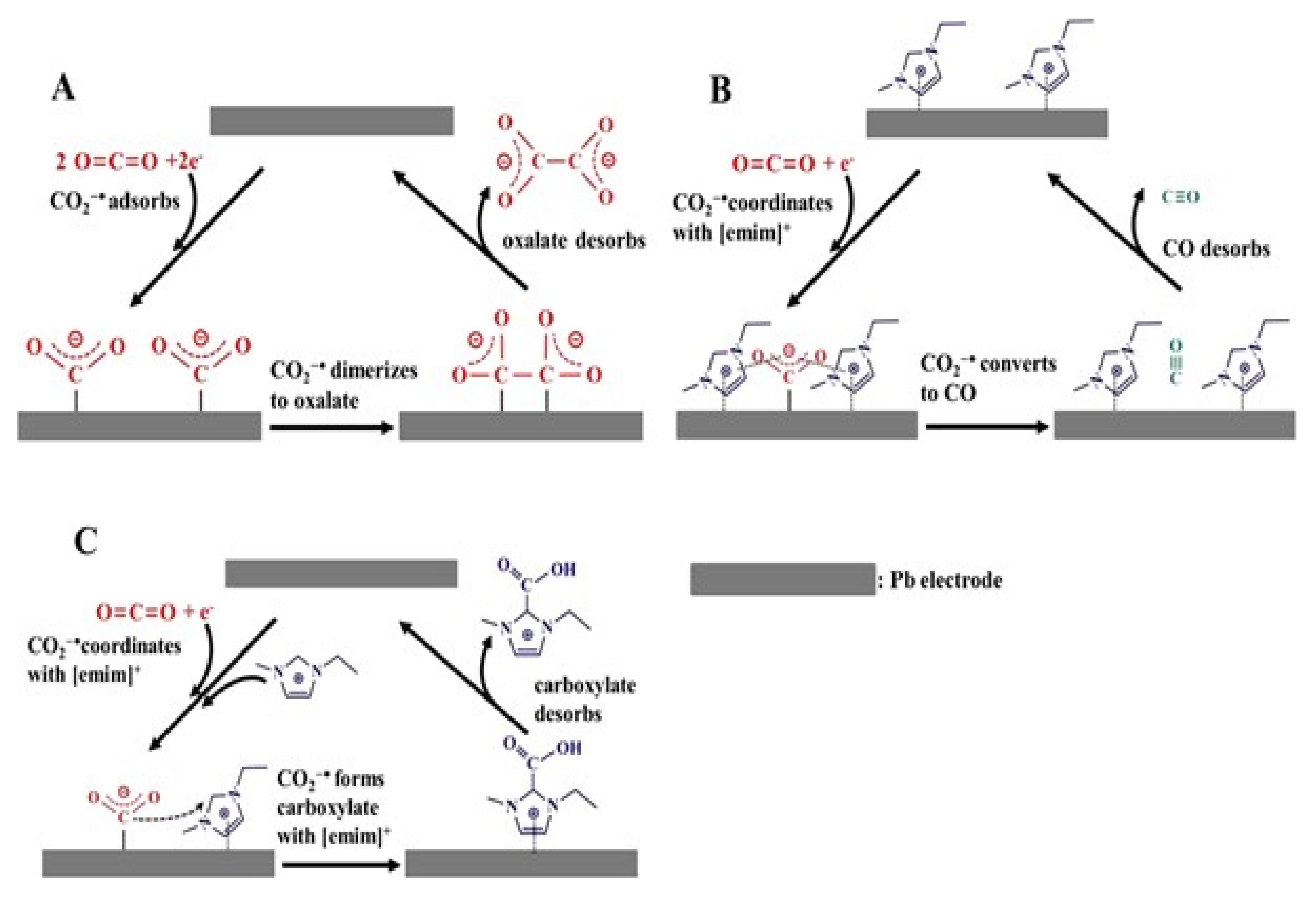
4.1. The Effect of Anions on the CO2ERR System
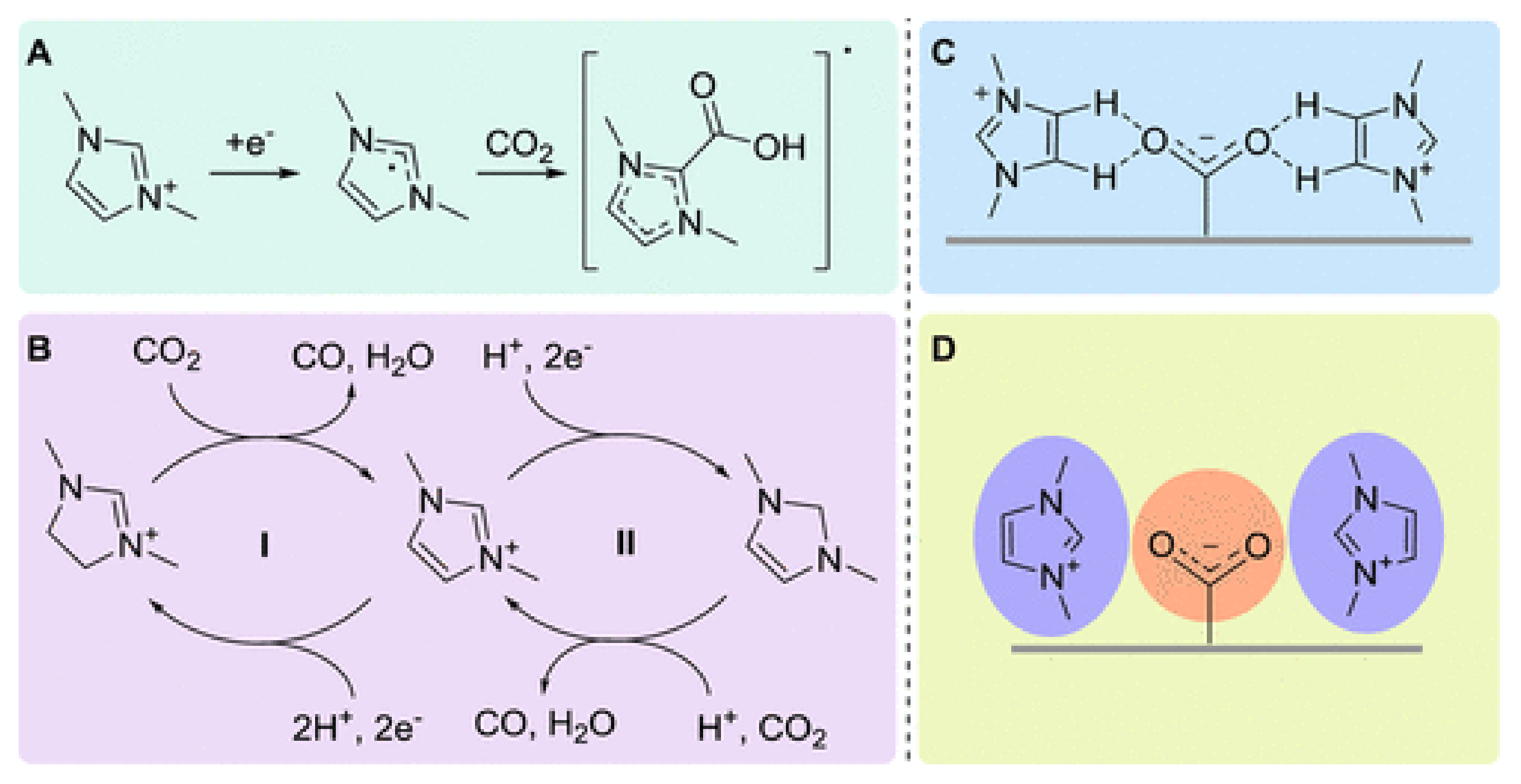
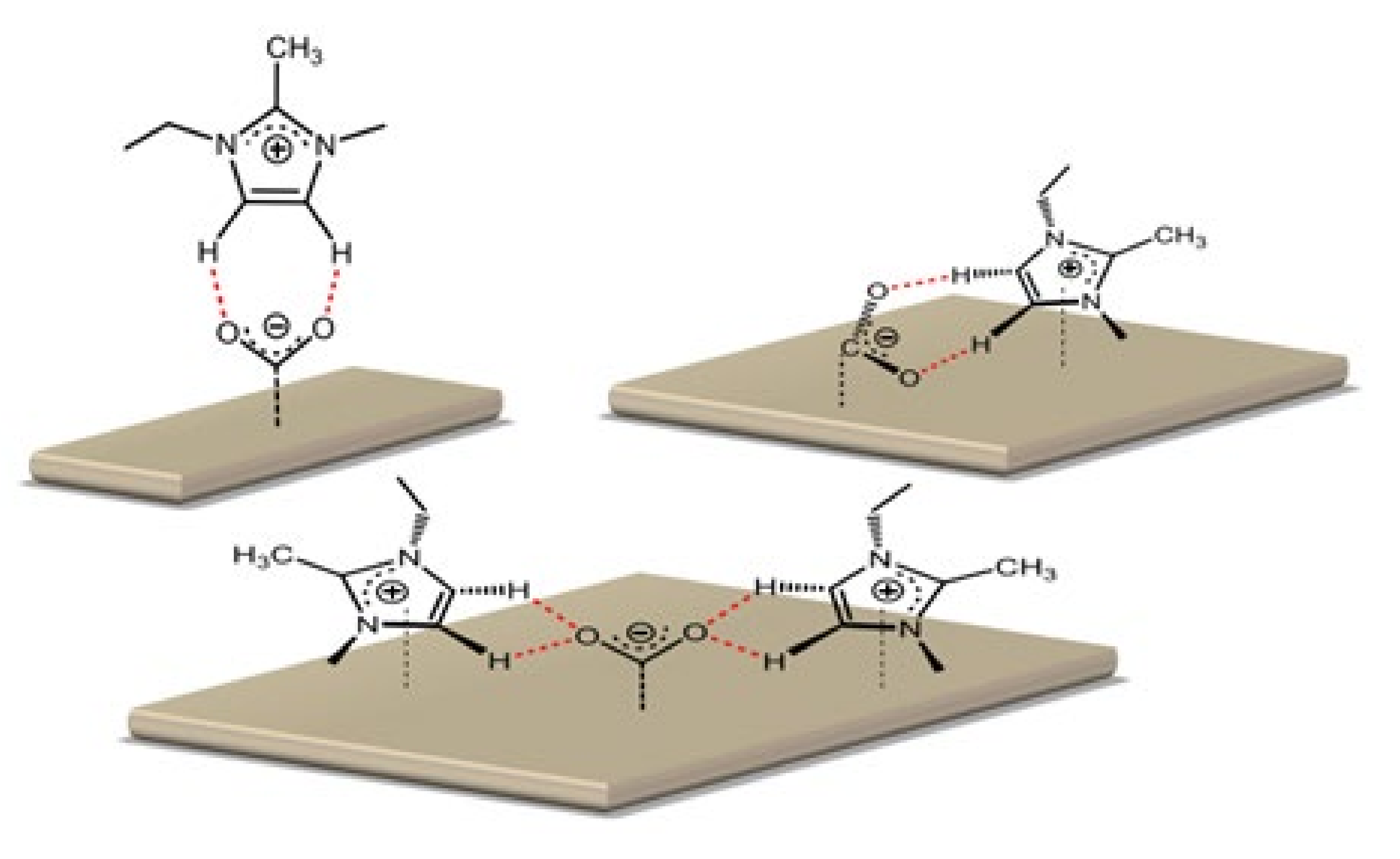
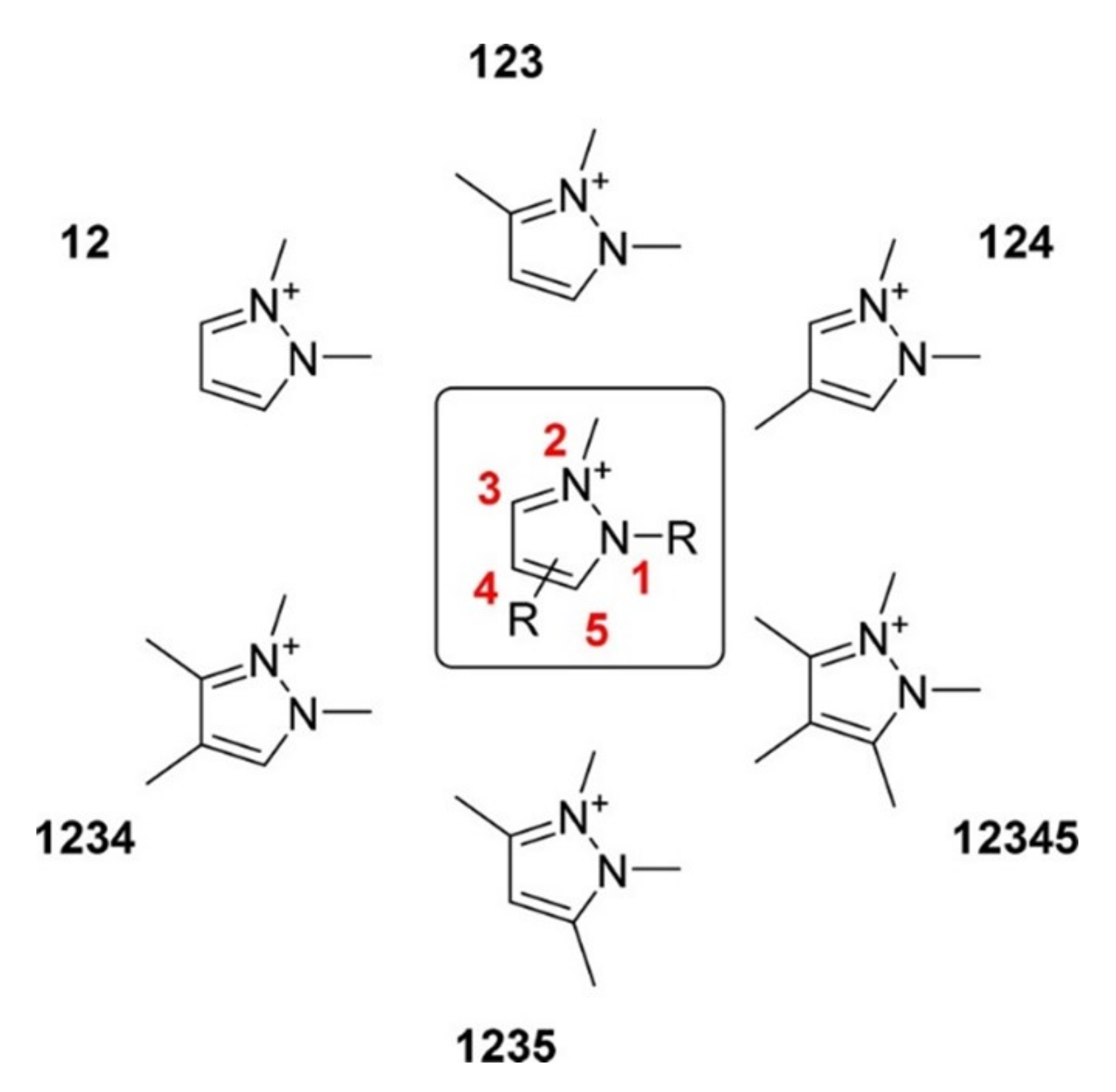
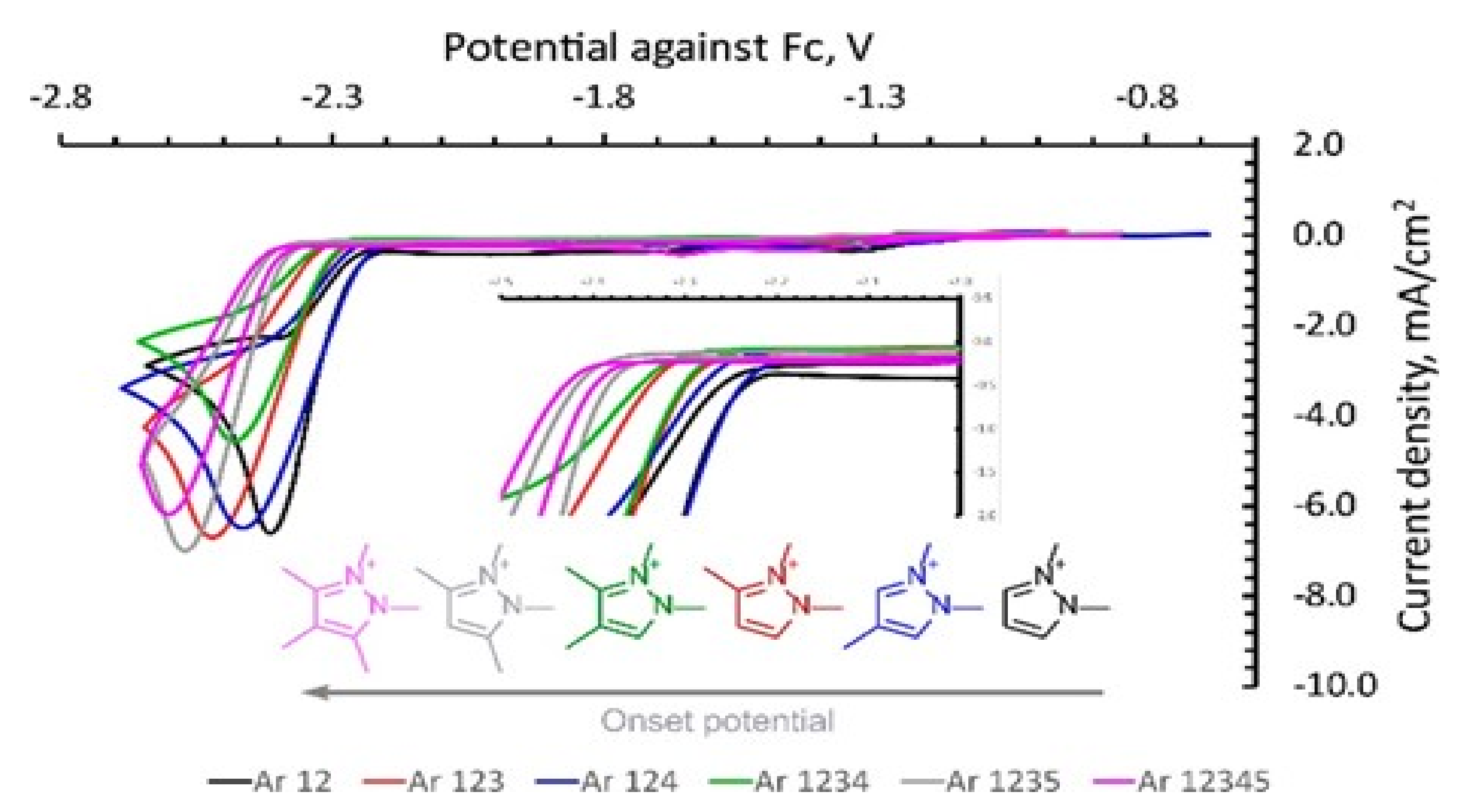
4.2. The Effect of the Cations on the Activity of CO2ERR System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldman, D.R.; Collins, W.D.; Gero, P.J.; Torn, M.S.; Mlawer, E.J.; Shippert, T.R. Observational determination of surface radiative forcing by CO2 from 2000 to 2010. Nature 2015, 519, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.E. Defining dangerous anthropogenic interference PNAS-2009-Mann-4065-6.pdf. Proc. Natl. Acad. Sci. USA 2009, 106, 4065–4066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Teng, Y.-L.; Dong, B.-X. Thermal Reduction of CO2 with Activated Alkali Metal Aluminum Hydrides for Selective Methanation. Energy Fuels 2020, 34, 11210–11218. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, C.; Wang, K.; Li, G.; Dong, X.; Song, Y.; Xue, J.; Chen, W.; Wei, W.; Sun, Y. Promotion of CO2 electrochemical reduction via Cu nanodendrites. ACS Appl. Mater. 2020, 12, 11562–11569. [Google Scholar] [CrossRef]
- Cortes, M.; McMichael, S.; Hamilton, J.; Sharma, P.; Brown, A.; Byrne, J. Photoelectrochemical reduction of CO2 with TiNT. Mater. Sci. Semicond. Process. 2020, 108, 104900. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.-H.; Lin, M.; Bhalothia, D.; Yang, S.-S.; Fan, G.-J.; Wang, J.-L.; Chan, T.-S.; Wang, Y.-l.; Tu, X. Local synergetic collaboration between Pd and local tetrahedral symmetric Ni oxide enables ultra-high-performance CO2 thermal methanation. J. Mater. Chem. A. 2020, 8, 12744–12756. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Sasirekha, N.; Basha, S.J.S.; Shanthi, K. Photocatalytic performance of Ru doped anatase mounted on silica for reduction of carbon dioxide. Appl. Catal. B Environ. 2006, 62, 169–180. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ghuman, K.K.; Popescu, R.; Duchesne, P.N.; Zhou, W.; Loh, J.Y.Y.; Jelle, A.A.; Jia, J.; Wang, D.; Mu, X.; et al. Tailoring Surface Frustrated Lewis Pairs of In2O3−x(OH)y for Gas-Phase Heterogeneous Photocatalytic Reduction of CO2 by Isomorphous Substitution of In3+ with Bi3+. Adv. Sci. 2018, 5, 1700732. [Google Scholar] [CrossRef]
- Lin, L.; Hou, C.; Zhang, X.; Wang, Y.; Chen, Y.; He, T. Highly efficient visible-light driven photocatalytic reduction of CO2 over g-C3N4 nanosheets/tetra(4-carboxyphenyl)porphyrin iron(III) chloride heterogeneous catalysts. Appl. Catal. B Environ. 2018, 221, 312–319. [Google Scholar] [CrossRef]
- Barton, E.E.; Rampulla, D.M.; Bocarsly, A.B. Selective solar-driven reduction of CO2 to methanol using a catalyzed p-GaP based photoelectrochemical cell. J. Am. Chem. Soc. 2008, 130, 6342–6344. [Google Scholar] [CrossRef] [PubMed]
- Kaneco, S.; Ueno, Y.; Katsumata, H.; Suzuki, T.; Ohta, K. Photoelectrochemical reduction of CO2 at p-InP electrode in copper particle-suspended methanol. Chem. Eng. J. 2009, 148, 57–62. [Google Scholar] [CrossRef]
- Sahara, G.; Kumagai, H.; Maeda, K.; Kaeffer, N.; Artero, V.; Higashi, M.; Abe, R.; Ishitani, O. Photoelectrochemical Reduction of CO2 Coupled to Water Oxidation Using a Photocathode with a Ru(II)-Re(I) Complex Photocatalyst and a CoOx/TaON Photoanode. J. Am. Chem. Soc. 2016, 138, 14152–14158. [Google Scholar] [CrossRef]
- Francke, R.; Schille, B.; Roemelt, M. Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts. Chem. Rev. 2018, 118, 4631–4701. [Google Scholar] [CrossRef] [PubMed]
- Resasco, J.; Lum, Y.; Clark, E.; Zeledon, J.Z.; Bell, A.T. Effects of Anion Identity and Concentration on Electrochemical Reduction of CO2. ChemElectroChem 2018, 5, 1064–1072. [Google Scholar] [CrossRef]
- Kibria, M.G.; Edwards, J.P.; Gabardo, C.M.; Dinh, C.T.; Seifitokaldani, A.; Sinton, D.; Sargent, E.H. Electrochemical CO2 reduction into chemical feedstocks: From mechanistic electrocatalysis models to system design. Adv. Mater. 2019, 31, 1807166. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhu, Q.; Han, B. Electroreduction of CO2 in ionic liquid-based electrolytes. Innovation 2020, 1, 100016. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Qi, Z.; Horwood, C.; Bourcier, B.; Stadermann, M.; Biener, J.; Biener, M. Using a 3D porous flow-through electrode geometry for high-rate electrochemical reduction of CO2 to CO in ionic liquid. ACS Catal. 2019, 9, 10605–10611. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.J.; Xie, M.; Sk, M.A.; Lee, J.-M.; Fisher, A.; Wang, X.; Lim, K.H. A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts. Catal. Today 2014, 233, 169–180. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wang, G.; Bao, X. Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: Recent advance in cathodes. J. Energy Chem. 2017, 26, 839–853. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, S.; Zhu, X.; Yang, W. Electrochemical reduction of CO2 in solid oxide electrolysis cells. J. Energy Chem. 2017, 26, 593–601. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Moure, V.R.; Dean, D.R.; Seefeldt, L.C. Carbon dioxide reduction to methane and coupling with acetylene to form propylene catalyzed by remodeled nitrogenase. Proc. Natl. Acad. Sci. USA 2012, 109, 19644–19648. [Google Scholar] [CrossRef] [PubMed]
- Reda, T.; Plugge, C.M.; Abram, N.J.; Hirst, J. Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 10654–10658. [Google Scholar] [CrossRef]
- Kuwabata, S.; Tsuda, R.; Yoneyama, H. Electrochemical Conversion of Carbon Dioxide to Methanol with the Assistance of Formate Dehydrogenase and Methanol Dehydrogenase as Biocatalysts. J. Am. Chem. Soc. 1994, 116, 5437–5443. [Google Scholar] [CrossRef]
- Modestra, J.A.; Mohan, S.V. Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: Microbial dynamics. J. CO2 Util. 2017, 20, 190–199. [Google Scholar] [CrossRef]
- Amao, Y. Viologens for coenzymes of biocatalysts with the function of CO2 reduction and utilization. Chem. Lett. 2017, 46, 780–788. [Google Scholar] [CrossRef]
- Le, Q.A.T.; Kim, H.G.; Kim, Y.H. Electrochemical synthesis of formic acid from CO2 catalyzed by Shewanella oneidensis MR-1 whole-cell biocatalyst. Enzym. Microb. Technol. 2018, 116, 1–5. [Google Scholar] [CrossRef]
- Schlager, S.; Fuchsbauer, A.; Haberbauer, M.; Neugebauer, H.; Sariciftci, N.S. Carbon dioxide conversion to synthetic fuels using biocatalytic electrodes. J. Mater. Chem. A 2017, 5, 2429–2443. [Google Scholar] [CrossRef]
- Amao, Y. Photoredox systems with biocatalysts for CO2 utilization. Sustain. Energy Fuels 2018, 2, 1928–1950. [Google Scholar] [CrossRef]
- Yin, G.; Bi, Q.; Zhao, W.; Xu, J.; Lin, T.; Huang, F. Efficient conversion of CO2 to methane photocatalyzed by conductive black titania. ChemCatChem 2017, 9, 4389–4396. [Google Scholar] [CrossRef]
- Jin, J.; He, T. Facile synthesis of Bi2S3 nanoribbons for photocatalytic reduction of CO2 into CH3OH. Appl. Surf. Sci. 2017, 394, 364–370. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of gC 3 N 4 for selective CO2 reduction to CH 3 OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Huang, Y.; Handoko, A.D.; Hirunsit, P.; Yeo, B.S. Electrochemical reduction of CO2 using copper single-crystal surfaces: Effects of CO* coverage on the selective formation of ethylene. ACS Catal. 2017, 7, 1749–1756. [Google Scholar] [CrossRef]
- Lu, W.; Jia, B.; Cui, B.; Zhang, Y.; Yao, K.; Zhao, Y.; Wang, J. Efficient Photoelectrochemical Reduction of Carbon Dioxide to Formic Acid: A Functionalized Ionic Liquid as an Absorbent and Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 11851–11854. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, C. Paths towards enhanced electrochemical CO2 reduction. Natl. Sci. Rev. 2020, 7, 7–9. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- Walker, J.; Halliday, D.; Resnick, R. Fundamentals of Physics Halliday & Resnick, 10th ed.; Wiley: Hoboken, NJ, USA, 2014; p. 1448. [Google Scholar]
- Stricker, E.A.; Ke, X.; Wainright, J.S.; Unocic, R.R.; Savinell, R.F. Current Density Distribution in Electrochemical Cells with Small Cell Heights and Coplanar Thin Electrodes as Used in ec-S/TEM Cell Geometries. J. Electrochem. Soc. 2019, 166, H126–H134. [Google Scholar] [CrossRef]
- Abdinejad, M.; Mirza, Z.; Zhang, X.A.; Kraatz, H.B. Enhanced Electrocatalytic Activity of Primary Amines for CO2 Reduction Using Copper Electrodes in Aqueous Solution. ACS Sustain. Chem. Eng. 2020, 8, 1715–1720. [Google Scholar] [CrossRef]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A.; Kikuchi, K.; Suzuki, S. Electrochemical reduction of carbon dioxides to carbon monoxide at a gold electrode in aqueous potassium hydrogen carbonate. J. Chem. Soc. Chem. Commun. 1987, 728–729. [Google Scholar] [CrossRef]
- Todoroki, M.; Hara, K.; Kudo, A.; Sakata, T. Electrochemical reduction of high pressure CO2 at Pb, Hg and In electrodes in an aqueous KHCO 3 solution. J. Electroanal. Chem. 1995, 394, 199–203. [Google Scholar] [CrossRef]
- Han, J.; Li, S.; Chen, J.; Liu, Y.; Geng, D.; Wang, D.; Zhang, L. Dendritic Ag/Pd Alloy Nanostructure Arrays for Electrochemical CO2 Reduction. ChemElectroChem 2020, 7, 2608–2613. [Google Scholar] [CrossRef]
- Bell, D.; Rall, D.; Großeheide, M.; Marx, L.; Hülsdünker, L.; Wessling, M. Tubular hollow fibre electrodes for CO2 reduction made from copper aluminum alloy with drastically increased intrinsic porosity. Electrochem. Commun. 2020, 111, 106645. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Cho, A.; Kim, S.; Han, J.W.; Lee, J. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2019, 246, 82–88. [Google Scholar] [CrossRef]
- Beheshti, M.; Kakooei, S.; Ismail, M.C.; Shahrestani, S. Investigation of CO2 electrochemical reduction to syngas on Zn/Ni-based electrocatalysts using the cyclic voltammetry method. Electrochim. Acta 2020, 341, 135976. [Google Scholar] [CrossRef]
- Lan, Y.; Niu, G.; Wang, F.; Cui, D.; Hu, Z. SnO2-Modified Two-Dimensional CuO for Enhanced Electrochemical Reduction of CO2to C2H4. ACS Appl. Mater. Interfaces 2020, 12, 36128–36136. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Hansen, H.A.; Varley, J.B.; Peterson, A.A.; Nørskov, J.K. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO. J. Phys. Chem. Lett. 2013, 4, 388–392. [Google Scholar] [CrossRef]
- Zhang, R.; Lv, W.; Li, G.; Lei, L. Electrochemical reduction of CO2 on SnO2/nitrogen-doped multiwalled carbon nanotubes composites in KHCO3 aqueous solution. Mater. Lett. 2015, 141, 63–66. [Google Scholar] [CrossRef]
- Zhong, H.; Fujii, K.; Nakano, Y. Effect of KHCO3 Concentration on Electrochemical Reduction of CO2 on Copper Electrode. J. Electrochem. Soc. 2017, 164, F923–F927. [Google Scholar] [CrossRef]
- Dunwell, M.; Lu, Q.; Heyes, J.M.; Rosen, J.; Chen, J.G.; Yan, Y.; Jiao, F.; Xu, B. The Central Role of Bicarbonate in the Electrochemical Reduction of Carbon Dioxide on Gold. J. Am. Chem. Soc. 2017, 139, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Koo, Y.M.; Macfarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Zhang, Z.; Reddy, R.G. Thermal stability of ionic liquids. TMS Annu. Meet. 2002, 33–39. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Rajavel, K.; Li, X.-M.; Wang, X.-Y.; Liao, S.-Y.; Lin, Z.-Q.; Zhu, P.-L.; Sun, R.; Wong, C.-P. Electromagnetic interference shielding of Ti3C2Tx MXene modified by ionic liquid for high chemical stability and excellent mechanical strength. Chem. Eng. J. 2021, 408, 127303. [Google Scholar] [CrossRef]
- Hilmy, N.I.M.F.; Yahya, W.Z.N.; Kurnia, K.A. Eutectic ionic liquids as potential electrolytes in dye-sensitized solar cells: Physicochemical and conductivity studies. J. Mol. Liq. 2020, 320, 114381. [Google Scholar] [CrossRef]
- Kang, S.; Chung, Y.G.; Kang, J.H.; Song, H. CO2 absorption characteristics of amino group functionalized imidazolium-based amino acid ionic liquids. J. Mol. Liq. 2020, 297, 111825. [Google Scholar] [CrossRef]
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical stability window of imidazolium-based ionic liquids as electrolytes for lithium batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef]
- Ravula, S.; Larm, N.E.; Mottaleb, M.A.; Heitz, M.P.; Baker, G.A. Vapor Pressure Mapping of Ionic Liquids and Low-Volatility Fluids Using Graded Isothermal Thermogravimetric Analysis. ChemEngineering 2019, 3, 42. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Hashmi, S.A. Ionic liquid incorporated, redox-active blend polymer electrolyte for high energy density quasi-solid-state carbon supercapacitor. J. Power Sources 2020, 451, 227771. [Google Scholar] [CrossRef]
- Shamsuri, A.A. Ionic liquids: Preparations and limitations. Makara J. Sci. 2011, 14, 101–106. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Transition metal nanoparticles in ionic liquids: Synthesis and stabilization. J. Mol. Liq. 2019, 276, 826–849. [Google Scholar] [CrossRef]
- Carlesi, C.; Carvajal, D.; Vasquez, D.; Arratia, R.S. Analysis of carbon dioxide-to-methanol direct electrochemical conversion mediated by an ionic liquid. Chem. Eng. Process. Process Intensif. 2014, 85, 48–56. [Google Scholar] [CrossRef]
- Lu, B.; Wang, X.; Li, Y.; Sun, J.; Zhao, J.; Cai, Q. Electrochemical conversion of CO2 into dimethyl carbonate in a functionalized ionic liquid. J. CO2 Util. 2013, 3, 98–101. [Google Scholar] [CrossRef]
- Zarandi, R.F.; Rezaei, B.; Ghaziaskar, H.S.; Ensafi, A.A. Electrochemical reduction of CO2 to ethanol using copper nanofoam electrode and 1-butyl-3-methyl-imidazolium bromide as the homogeneous co-catalyst. J. Environ. Chem. Eng. 2019, 7, 103141. [Google Scholar] [CrossRef]
- Bohra, D.; Chaudhry, J.H.; Burdyny, T.; Pidko, E.A.; Smith, W.A. Modeling the electrical double layer to understand the reaction environment in a CO2 electrocatalytic system. Energy Environ. Sci. 2019, 12, 3380–3389. [Google Scholar] [CrossRef]
- Karaiskakis, A.N.; Golru, S.S.; Biddinger, E. Effect of Electrode Geometry on Selectivity and Activity in CO2 Electroreduction. Ind. Eng. Chem. Res. 2019, 58, 22506–22515. [Google Scholar] [CrossRef]
- Yang, K.D.; Lee, C.W.; Jang, J.H.; Ha, T.R.; Nam, K.T. Rise of nano effects in electrode during electrocatalytic CO2 conversion. Nanotechnology 2017, 28, 352001. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, C.; Wang, N.; Jin, X.; Jin, C.; Peng, S. Electrochemical reduction of CO2 in a symmetrical solid oxide electrolysis cell with La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δelectrode. J. CO2 Util. 2019, 33, 445–451. [Google Scholar] [CrossRef]
- Song, R.B.; Zhu, W.; Fu, J.; Chen, Y.; Liu, L.; Zhang, J.R.; Lin, Y.; Zhu, J.J. Electrode Materials Engineering in Electrocatalytic CO2 Reduction: Energy Input and Conversion Efficiency. Adv. Mater. 2020, 32, 1903796. [Google Scholar] [CrossRef]
- Wang, Y.; Hayashi, T.; He, D.; Li, Y.; Jin, F.; Nakamura, R. A reduced imidazolium cation layer serves as the active site for electrochemical carbon dioxide reduction. Appl. Catal. B Environ. 2020, 264, 118495. [Google Scholar] [CrossRef]
- Zhang, L.; Merino-Garcia, I.; Albo, J.; Sánchez-Sánchez, C.M. Electrochemical CO2 reduction reaction on cost-effective oxide-derived copper and transition metal–nitrogen–carbon catalysts. Curr. Opin. Electrochem. 2020, 23, 65–73. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.; Fu, J.; Yamaguchi, A.; Li, H.; Pan, H.; Hu, J.; Miyauchi, M.; Liu, M. Recent advances in the utilization of copper sulfide compounds for electrochemical CO2 reduction. Nano Mater. Sci. 2020, 2, 235–247. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, G.; Mishra, A.; Verma, V.; Khan, A.; Pal, N.; Sinha, A.K. A Density Functional Theory and Experimental Study of CO2 Photoreduction to Methanol over α-Sulfur-TiO2 Composite. Electrocatalysis 2021, 12, 56–64. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, D.; Xu, X.; Zhang, X.; Hu, Y. Ionic Liquid [C3mim] OTf aqueous solution: Green High Efficiency Electroreduction for Carbon Dioxide at Room-Temperature. Microchem. J. 2021, 106559. [Google Scholar] [CrossRef]
- Wu, H.; Song, J.; Xie, C.; Hu, Y.; Ma, J.; Qian, Q.; Han, B. Design of naturally derived lead phytate as an electrocatalyst for highly efficient CO2 reduction to formic acid. Green Chem. 2018, 20, 4602–4606. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, H.; Feng, J.; Zeng, S.; Liu, L.; Liu, L.; Ren, B.; Li, T.; Zhang, S.; Zhang, X. Aromatic Ester-Functionalized Ionic Liquid for Highly Efficient CO2 Electrochemical Reduction to Oxalic Acid. ChemSusChem 2020, 13, 4900–4905. [Google Scholar] [CrossRef]
- Gonçalves, W.D.; Zanatta, M.; Simon, N.M.; Rutzen, L.M.; Walsh, D.A.; Dupont, J. Efficient Electrocatalytic CO2 Reduction Driven by Ionic Liquid Buffer-Like Solutions. ChemSusChem 2019, 12, 4170–4175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liu, S.; Yang, B.; Wang, P.; Alshammari, A.S.; Deng, Y. Highly selective electrocatalytic reduction of carbon dioxide to carbon monoxide on silver electrode with aqueous ionic liquids. Electrochem. Commun. 2014, 46, 103–106. [Google Scholar] [CrossRef]
- Sun, L.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the Reaction Course of Electrochemical CO2 Reduction with Ionic Liquids. Langmuir 2014, 30, 6302–6308. [Google Scholar] [CrossRef]
- Oh, Y.; Hu, X. Ionic liquids enhance the electrochemical CO2 reduction catalyzed by MoO2. Chem. Commun. 2015, 51, 13698–13701. [Google Scholar] [CrossRef]
- Lau, G.P.; Schreier, M.; Vasilyev, D.; Scopelliti, R.; Grätzel, M.; Dyson, P.J. New insights into the role of imidazolium-based promoters for the electroreduction of CO2 on a silver electrode. J. Am. Chem. Soc. 2016, 138, 7820–7823. [Google Scholar] [CrossRef]
- Vasilyev, D.; Shirzadi, E.; Rudnev, A.V.; Broekmann, P.; Dyson, P.J. Pyrazolium Ionic Liquid Co-catalysts for the Electroreduction of CO2. ACS Appl. Energy Mater. 2018, 1, 5124–5128. [Google Scholar] [CrossRef]
- Aki, S.N.; Mellein, B.R.; Saurer, E.M.; Brennecke, J.F. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J. Phys. Chem. B. 2004, 108, 20355–20365. [Google Scholar] [CrossRef]
- Snuffin, L.L.; Whaley, L.W.; Yu, L. Catalytic electrochemical reduction of CO2 in ionic liquid EMIMBF3Cl. J. Electrochem. Soc. 2011, 158, 155–158. [Google Scholar] [CrossRef]
- Almantariotis, D.; Stevanovic, S.; Fandino, O.; Pensado, A.; Padua, A.A.; Coxam, J.-Y.; Costa Gomes, M.F. Absorption of carbon dioxide, nitrous oxide, ethane and nitrogen by 1-alkyl-3-methylimidazolium (C n mim, n = 2, 4, 6) tris (pentafluoroethyl) trifluorophosphate ionic liquids (eFAP). J. Phys. Chem. B. 2012, 116, 7728–7738. [Google Scholar] [CrossRef]
- Song, Z.; Shi, H.; Zhang, X.; Zhou, T. Prediction of CO2 solubility in ionic liquids using machine learning methods. Chem. Eng. Sci. 2020, 223, 115752. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Process analysis overview of ionic liquids on CO2 chemical capture. Chem. Eng. J. 2020, 390, 124509. [Google Scholar] [CrossRef]
- Neumann, J.G.; Stassen, H. Anion Effect on Gas Absorption in Imidazolium-Based Ionic Liquids. J. Chem. Inf. Modeling 2020, 60, 661–666. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Zhang, Z.; Fei, W.; Luo, M.; Zhao, Y. Experimental and simulation study of CO2 and H2S solubility in propylene carbonate, imidazolium-based ionic liquids and their mixtures. J. Chem. Thermodyn. 2020, 142, 106017. [Google Scholar] [CrossRef]
- Yim, J.H.; Park, K.W.; Oh, B.K.; Lim, J.S. CO2 Solubility in 1,1,2,2-Tetrafluoroethanesulfonate Anion-Based Ionic Liquids: [EMIM][TFES], [BMIM][TFES], and [BNMIM][TFES]. J. Chem. Eng. Data 2020, 65, 617–627. [Google Scholar] [CrossRef]
- Darabi, M.; Pahlavanzadeh, H. Mathematical modeling of CO2 membrane absorption system using ionic liquid solutions. Chem. Eng. Process. Process Intensif. 2020, 147, 107743. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion Effects on Gas Solubility in Ionic Liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef]
- Noorani, N.; Mehrdad, A. CO2 solubility in some amino acid-based ionic liquids: Measurement, correlation and DFT studies. Fluid Phase Equilibria. 2020, 517, 112591. [Google Scholar] [CrossRef]
- Wei, L.; Guo, R.; Tang, Y.; Zhu, J.; Liu, M.; Chen, J.; Xu, Y. Properties of aqueous amine based protic ionic liquids and its application for CO2 quick capture. Sep. Purif. Technol. 2020, 239, 116531. [Google Scholar] [CrossRef]
- Feroci, M.; Orsini, M.; Rossi, L.; Sotgiu, G.; Inesi, A. Electrochemically promoted C-N bond formation from amines and CO2 in ionic liquid BMIm-BF4: Synthesis of carbamates. J. Org. Chem. 2007, 72, 200–203. [Google Scholar] [CrossRef]
- Yang, H.; Gu, Y.; Deng, Y.; Shi, F. Electrochemical activation of carbon dioxide in ionic liquid: Synthesis of cyclic carbonates at mild reaction conditions. Chem. Commun. 2002, 2, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Huang, K.; Liu, S.; Wang, X. Electrocatalytic carboxylation of 2-amino-5-bromopyridine with CO2 in ionic liquid 1-butyl-3-methyllimidazoliumtetrafluoborate to 6-aminonicotinic acid. Electrochim. Acta 2010, 55, 5741–5745. [Google Scholar] [CrossRef]
- Vasilyev, D.V.; Shyshkanov, S.; Shirzadi, E.; Katsyuba, S.A.; Nazeeruddin, M.K.; Dyson, P.J. Principal descriptors of ionic liquid co-catalysts for the electrochemical reduction of CO2. ACS Appl. Energy Mater. 2020, 3, 4690–4698. [Google Scholar] [CrossRef]
- Rosen, B.A.; Zhu, W.; Kaul, G.; Salehi-Khojin, A.; Masel, R.I. Water enhancement of CO2 conversion on silver in 1-ethyl-3- methylimidazolium tetrafluoroborate. J. Electrochem. Soc. 2013, 160, H138. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.; Masel, R.I.; Kenis, P.J.A. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Rosen, B.A.; Haan, J.L.; Mukherjee, P.; Braunschweig, B.; Zhu, W.; Salehi-Khojin, A.; Dlott, D.D.; Masel, R.I. In situ spectroscopic examination of a low overpotential pathway for carbon dioxide conversion to carbon monoxide. J. Phys. Chem. C. 2012, 116, 15307–15312. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Li, G.; Zhao, W.; Zhao, X.; Mu, T. The electrochemical stability of ionic liquids and deep eutectic solvents. Sci. China Chem. 2016, 59, 571–577. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Shang, J.; Zhang, C.; Tan, H.; Yi, X.; Pan, L.; Zhang, W.; Fan, F.; Liu, Y. An organic terpyridyl-iron polymer based memristor for synaptic plasticity and learning behavior simulation. RSC Adv. 2016, 6, 25179–25184. [Google Scholar] [CrossRef]
- Alvarez-Guerra, M.; Albo, J.; Alvarez-Guerra, E.; Irabien, A. Ionic liquids in the electrochemical valorisation of CO2. Energy Environ. Sci. 2015, 8, 2574–2599. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, J.; Zhang, X.; Xin, J.; Miao, Q.; Wang, J. Ionic liquid-based green processes for energy production. Chem. Soc. Rev. 2014, 43, 7838–7869. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Ionic liquids at electrified interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Storey, B.D.; Kornyshev, A.A. Double Layer in Ionic Liquids: Overscreening versus Crowding. Phys. Rev. Lett. 2011, 046102, 6–9. [Google Scholar] [CrossRef]
- Costa, R.; Pereira, C.M.; Silva, F. Double layer in room temperature ionic liquids: Influence of temperature and ionic size on the differential capacitance and electrocapillary curves. Phys. Chem. Chem. Phys. 2010, 12, 11125–11132. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Alam, M.T.; Okajima, T.; Ohsaka, T. Electrical double layer structure in ionic liquids: An understanding of the unusual capacitance-potential curve at a nonmetallic electrode. J. Phys. Chem. C. 2009, 113, 3386–3389. [Google Scholar] [CrossRef]
- Gutsev, G.L.; Bartlett, R.J.; Compton, R.N. Electron affinities of CO2, OCS, and CS2. J. Chem. Phys. 1998, 108, 6756–6762. [Google Scholar] [CrossRef]
- Wang, L.-S.; Reutt, J.; Lee, Y.; Shirley, D.; Phenomena, R. High resolution UV photoelectron spectroscopy of CO+2, COS+ and CS+2 using supersonic molecular beams. J. Electron Spectrosc. 1988, 47, 167–186. [Google Scholar] [CrossRef]
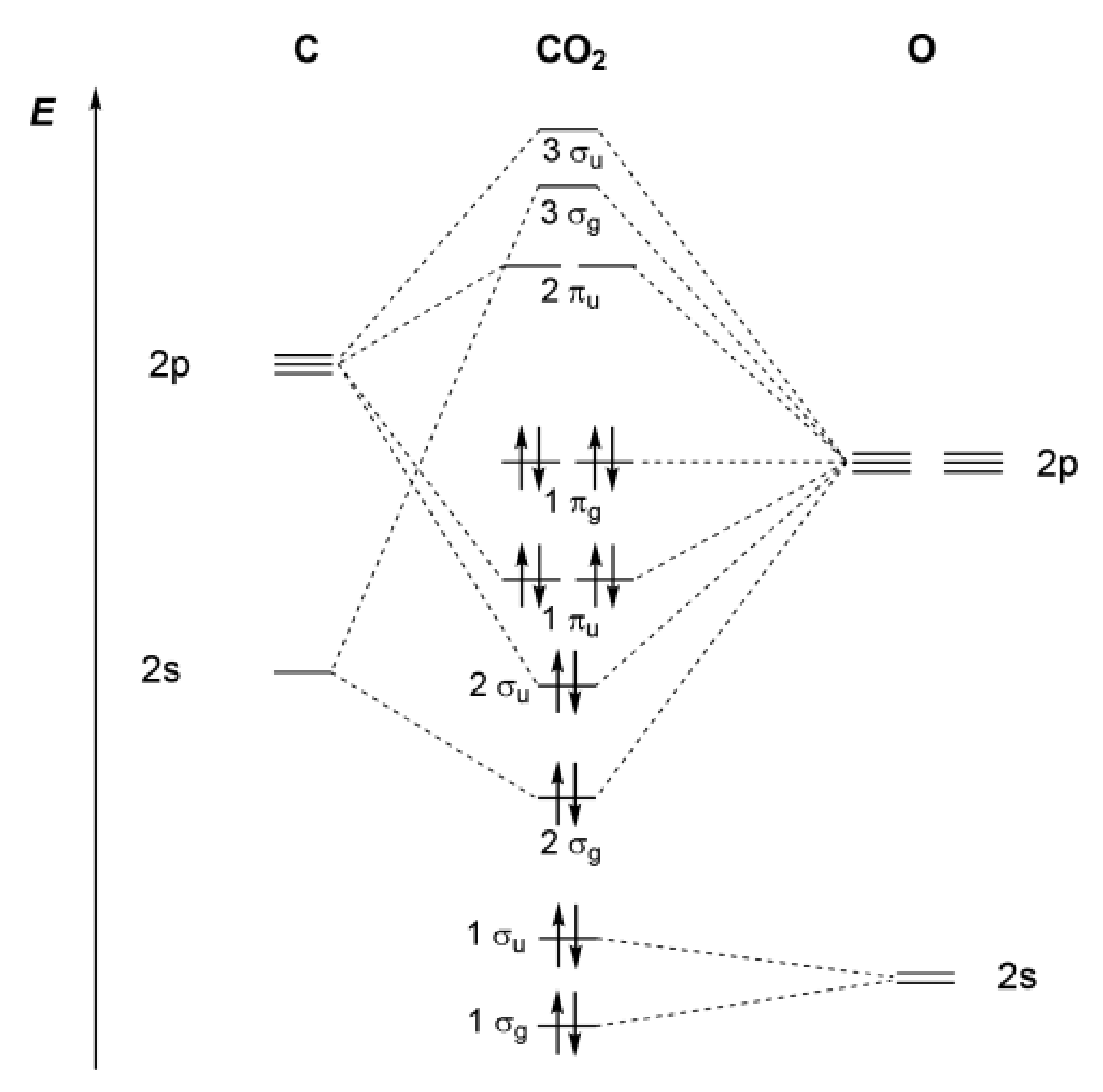
- Walsh, A. 467. The electronic orbitals, shapes, and spectra of polyatomic molecules. Part II. Non-hydride AB 2 and BAC molecules. J. Chem. Soc. 1953, 2266–2288. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. Reaction Mechanisms in Carbon Dioxide Conversion; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Steudel, R. Chemie der Nichtmetalle. 2; Auflage, De Gruyter: Berlin, Germany, 1998. [Google Scholar]
- Klapötke, T.M.; Tornieporth-Oetting, I.C.; Oetting, I.C.T. Nichtmetallchemie; Wiley VCH: Weinheim, Germany, 1994. [Google Scholar]
- Buijs, W.; Witkamp, G.-J.; Kroon, M.C. Correlation between Quantumchemically Calculated LUMO Energies and the Electrochemical Window of Ionic Liquids with Reduction-Resistant Anions. Int. J. Electrochem. 2012, 2012, 589050. [Google Scholar] [CrossRef][Green Version]
- Picquet, M.; Tkatchenko, I.; Tommasi, I.; Wasserscheid, P.; Zimmermann, J. Ionic Liquids, 3. Synthesis and Utilisation of Protic Imidazolium Salts in Homogeneous Catalysis. Adv. Synth. Catal. 2003, 345, 959–962. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hagiwara, R.; Ito, Y.; Letters, S.S. Room-temperature ionic liquids with high conductivities and wide electrochemical windows: N-Alkyl-N-methylpyrrolidinium and N-alkyl-N-methylpiperidinium fluorohydrogenates. Electrochemical 2004, 7, E41. [Google Scholar]
- Sakaebe, H.; Matsumoto, H. N-Methyl-N-propylpiperidinium bis (trifluoromethanesulfonyl) imide (PP13–TFSI)–novel electrolyte base for Li battery. Electrochem. Commun. 2003, 5, 594–598. [Google Scholar] [CrossRef]
- Huynh, P.N.; Walvoord, R.R.; Kozlowski, M.C. Rapid Quantification of the activating effects of hydrogen-bonding catalysts with a colorimetric sensor. J. Am. Chem. Soc. 2012, 134, 15621–15623. [Google Scholar] [CrossRef]
- Mohammed, S.A.S.; Yahya, W.Z.N.; Bustam, M.A. Computational studies of ionic liquids as co-catalyst for CO2 electrochemical reduction to produce syngas using COSMO-RS. E3S Web Conf. 2021, 287, 02016. [Google Scholar] [CrossRef]
- Safavi, M.; Ghotbi, C.; Taghikhani, V.; Jalili, A.H.; Mehdizadeh, A. Study of the solubility of CO2, H2S and their mixture in the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate: Experimental and modelling. J. Chem. Thermodyn. 2013, 65, 220–232. [Google Scholar] [CrossRef]
- Xie, J.; Huang, Y.; Yu, H. Tuning the catalytic selectivity in electrochemical CO2 reduction on copper oxide-derived nanomaterials. Front. Environ. Sci. Eng. 2015, 9, 861–866. [Google Scholar] [CrossRef]
- Park, Y.; Lin, K.Y.A.; Park, A.H.A.; Petit, C. Recent advances in anhydrous solvents for CO2 capture: Ionic liquids, switchable solvents, and nanoparticle organic hybrid materials. Front. Energy Res. 2015, 3, 42. [Google Scholar] [CrossRef]
- Gennaro, A.; Isse, A.A.; Severin, M.G.; Vianello, E.; Bhugun, I.; Savéant, J.M. Mechanism of the electrochemical reduction of carbon dioxide at inert electrodes in media of low proton availability. J. Chem. Soc. Faraday Trans. 1996, 92, 3963–3968. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef] [PubMed]

| Reaction | E0/(V vs. Reversible Hydrogen Electrode RHE) | (Product) Name, Abbreviation |
|---|---|---|
| 2H2O → O2 + 4H+ + 4e− | 1.23 | Oxygen Evolution Reaction, OER |
| 2H+ + 2e− → 2H2 | 0 | Hydrogen Evolution Reaction, HER |
| xCO2+ nH+ + ne− →product + yH2O | CO2 Reduction, CO2R | |
| CO2 + 2H+ + 2e− → HCOOH (aq) | −0.12 | Formic acid |
| CO2 + 2H+ + 2e− → CO(g) + H2O | −0.10 | Carbon monoxide |
| CO2 + 6H+ + 6e− → CH3OH (aq) + H2O | 0.03 | Methanol, MeOH |
| CO2 + 4H+ + 4e− → C(s) + 2H2O | 0.21 | Graphite |
| CO2 + 8H+ + 8e− → CH4(g) + 2H2O | 0.17 | Methane |
| 2CO2 + 2H+ + 2e− → (COOH)2 (s) | −0.47 | Oxalic acid |
| 2CO2 + 8H+ + 8e− → CH3COOH (aq) + 2H2O | 0.11 | Acetic acid |
| 2CO2 + 10H+ + 10e− → CH3CHO (aq) + 3H2O | 0.06 | Acetaldehyde |
| 2CO2 + 12H+ + 12e− → C2H5OH (aq) + 3H2O | 0.09 | Ethanol, EtOH |
| 2CO2 + 12H+ +12e−→ C2H4(g) + 4H2O | 0.08 | Ethylene |
| 2CO2+ 14H+ + 14e−→ C2H6(g) + 4 H2O | 0.14 | Ethane |
| 3CO2+ 16H+ + 16e− → C2H5CHO (aq)+ 5H2O | 0.09 | Propionaldehyde |
| 3CO2 + 18H+ + 18e− → C3H7OH (aq) + 5H2O | 0.10 | Propanol, PrOH |
| xCO + nH+ + ne− → product + yH2O | CO Reduction, COR | |
| CO+ 6H+ + 6e−→ CH4(g) + H2O | 0.26 | Methane |
| 2CO+ 8H+ + 8e− → CH3CH2OH (aq) +H2O | 0.19 | Ethanol, EtOH |
| 2CO + 8H+ + 8e− → C2H4(g) + 2H2O | 0.17 | Ethylene |
| Electrode | Electrolyte | Potential | Faradaic Efficiency of the Product (%) | Current Density (mA⋅cm−2) | Reference |
|---|---|---|---|---|---|
| Sn | 40 mg·mL−1 [C3mim] OTf aqueous solution | −2.1 V (vs. Ag/AgCl) | HCOOH (35.47) | 9.3 | [80] |
| In | 40 mg·mL−1 [C3mim] OTf aqueous solution | −2.1 V (vs. Ag/AgCl) | HCOOH (73.90) | 11.9 | [80] |
| Pb phytate | 12.8 wt% [Bmim]BF4/9.9 wt% H2O/acetonitrile | −2.25 V (Vs. Ag/Ag+) | HCOOH (92.7) | 30.5 | [81] |
| Pb | 4-(methoxycarbonyl) phenol tetraethylammonium ([TEA][4-MF-PhO]) | −2.6 V (vs. Ag/Ag+) | H2C2O4 (86) | 9.03 | [82] |
| Cu nanoporous foam | 0.04 mol/L[Bmim][Br] + 0.1 mol/L KHCO3 | −1.6 vs. Ag/Ag | Ethanol (49) | 20 | [70] |
| Au | 0.1 M [Bmim][OAc] + 0.2 vol% H2OinDMSO | −1.8 vs. Ag/Ag | CO (98) | 8.6 | [83] |
| Ag | [Bmim][Cl] with 20 wt% H2O | −1.5 vs. SCE | CO > 99 | 2.4 | [84] |
| Ag | [Emim]BF4/18% water | −1.50 vs. cell potential | CO (96) | n/a | [45] |
| Pb | 0.1 M [Emim][Tf2N]/AcN | −2.25 V (vs. Ag/AgNO3) | CO (44) Carboxylate(74) | - | [85] |
| Pb | 0.1 M TEAP/AcN | −2.40 V (vs. Ag/AgNO3) | CO (10) Oxalate(70) | - | [85] |
| MoO2/Pb | 0.3M [Bmim]PF6/MeCN/0.1 M H2O | −2.45 V (vs. Fc/Fc+) | H2 (12.4) CO(60.8) C2O4−2(5.3) HCOO−(17.8) | - | [86] |
| MoO2/Pb | 0.3M [Bmim]PF6/MeCN/0.2 M H2O | −2.45 V (vs. Fc/Fc+) | H2 (25.1) CO(51.7) C2O4−2(5.5) HCOO−(9.8) | - | [86] |
| MoO2/Pb | 0.3M [Bmim]PF6/MeCN/0.3 M H2O | −2.45 V (vs. Fc/Fc+) | H2 (28.9) CO(51.4) C2O4−2(4.3) HCOO−(6.2) | - | [86] |
| Ag | 0.02 M [1a][BF4]/0.1 M NBu4PF6 in dry acetonitrile | −2.46 V (vs. Ag/AgCl) | CO(99.8) | 4.2 | [87] |
| Ag | 0.02 M [2a][BF4]/0.1 M NBu4PF6 in dry acetonitrile | −2.37 V (vs. Ag/AgCl) | CO(100) | 4.2 | [87] |
| Ag | 0.02 M [3a][BF4]/0.1 M NBu4PF6 in dry acetonitrile | −2.34 V (vs. Ag/AgCl) | CO(100) | 2.8 | [87] |
| Ag | 0.02 M [4a][BF4]/0.1 M NBu4PF6 in dry acetonitrile | −2.35 V (vs. Ag/AgCl) | CO(99) | 4.2 | [87] |
| Ag | 0.02 M [5a][BF4]/0.1 M NBu4PF6 in dry acetonitrile | −2.43 V (vs. Ag/AgCl) | CO(100) | 4.2 | [87] |
| Ag | [Bmim][BF4} | −2.11 V (vs. Fc/Fc+) | CO(92) H2(<1) | - | [88] |
| Ag | 0.02 M [Pz1235B] [TFSI] /0.1 M NBu4PF6 in dry acetonitrile | −2.11 V (vs. Fc/Fc+) | CO(99) H2(4) CH4(0.9) | - | [88] |
| Name | Abbreviation | Reference |
|---|---|---|
| 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [Bmim][Tf2N] | [89,90] |
| 1-butyl-3-methylimidazolium tetrafluoroborate | [Bmim][BF4] | [89,90] |
| 1-butyl-3-methylimidazolium tris(trifluoromethylsulfonyl)methide | [Bmim][methide] | [89] |
| 1-butyl-3-methylimidazolium nitrate | [Bmim][NO3] | [89] |
| 1-butyl-3-methylimidazolium trifluoromethanesulfonate | [Bmim][OTf] | [89] |
| 1-butyl-3-methylimidazolium dicyanamide | [Bmim][DCA] | [89] |
| 1-butyl-3-methylimidazolium hexafluorophosphate | [Bmim][PF6] | [89] |
| 1-octyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [Omim][Tf2N] | [89] |
| 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [Hmim][Tf2N] | [89] |
| 2,3-dimethyl-1-hexylimidazolium bis(trifluoromethylsulfonyl)imide | [DMHxIm][Tf2N] | [89] |
| 1-ethyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate ionic liquids (FAP) | [Emim][FAP] | [91] |
| 1- butyl -3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate ionic liquids (FAP) | [Bmim][FAP] | [91] |
| 1- hexyl -3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate | [Hmim][FAP] | [91] |
| 1-ethyl-3-methylimidazolium trifluorochloroborate | [Emim][BF3Cl] | [90] |
| 1-ethyl-3-methylimidazolium tetrafluoroborate | Emim BF4 | [45] |
| 1-butyl-3-methylimidazolium hexafluorophosphate | [Bmim][PF6] | [86] |
| 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [Emim][Tf2N] | [85] |
| 1,2-dimethylpyrazolium bis(trifluoromethylsulfonyl)imide | [Pz12][Tf2N] | [88] |
| 1,2,3-trimethylpyrazolium bis(trifluoromethylsulfonyl)imide | [Pz123][Tf2N] | [88] |
| 1,2,4-trimethylpyrazolium bis(trifluoromethylsulfonyl)imide | [Pz124][Tf2N] | [88] |
| 1,2,3,4,5-pentamethylpyrazolium bis(trifluoromethylsulfonyl)imide | [Pz12345][Tf2N] | [88] |
| 1,2,3,5-tetramethylpyrazolium bis(trifluoromethylsulfonyl)imide | [Pz1235][Tf2N] | [88] |
| 1,2,3,4-tetramethylpyrazolium bis(trifluoromethylsulfonyl)imide | [Pz1234][Tf2N] | [88] |
| Tetrabutylammonium hexafluorophosphate | NBu4PF6 | [88] |
| 3-ethyl-1-methyl-1H-imidazolium tetrafluoroborate | [1a][BF4] | [87] |
| 3-ethyl-1,2-dimethyl-imidazolium tetrafluoroborate | [2a][BF4] | [87] |
| 3-ethyl-1,2,4,5-tetramethyl-imidazolium tetrafluoroborate | [3a][BF4] | [87] |
| 1,3-dimethyl-2-phenyl-1H-imidazolium tetrafluoroborate | [4a][BF4] | [87] |
| 1,3-dimethyl-2-(4-methoxyphenyl)-imidazolium tetrafluoroborate | [5a][BF4] | [87] |
| 1-butyl-2,3,5-trimethyl-pyrazolium | [Pz1235B] [TFSI] | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, S.A.S.; Yahya, W.Z.N.; Bustam, M.A.; Kibria, M.G. Elucidation of the Roles of Ionic Liquid in CO2 Electrochemical Reduction to Value-Added Chemicals and Fuels. Molecules 2021, 26, 6962. https://doi.org/10.3390/molecules26226962
Mohammed SAS, Yahya WZN, Bustam MA, Kibria MG. Elucidation of the Roles of Ionic Liquid in CO2 Electrochemical Reduction to Value-Added Chemicals and Fuels. Molecules. 2021; 26(22):6962. https://doi.org/10.3390/molecules26226962
Chicago/Turabian StyleMohammed, Sulafa Abdalmageed Saadaldeen, Wan Zaireen Nisa Yahya, Mohamad Azmi Bustam, and Md Golam Kibria. 2021. "Elucidation of the Roles of Ionic Liquid in CO2 Electrochemical Reduction to Value-Added Chemicals and Fuels" Molecules 26, no. 22: 6962. https://doi.org/10.3390/molecules26226962
APA StyleMohammed, S. A. S., Yahya, W. Z. N., Bustam, M. A., & Kibria, M. G. (2021). Elucidation of the Roles of Ionic Liquid in CO2 Electrochemical Reduction to Value-Added Chemicals and Fuels. Molecules, 26(22), 6962. https://doi.org/10.3390/molecules26226962






