Insecticidal, Repellent and Antifeedant Activity of Essential Oils from Blepharocalyx cruckshanksii (Hook. & Arn.) Nied. Leaves and Pilgerodendron uviferum (D. Don) Florin Heartwood against Horn Flies, Haematobia irritans (Diptera: Muscidae)
Abstract
1. Introduction
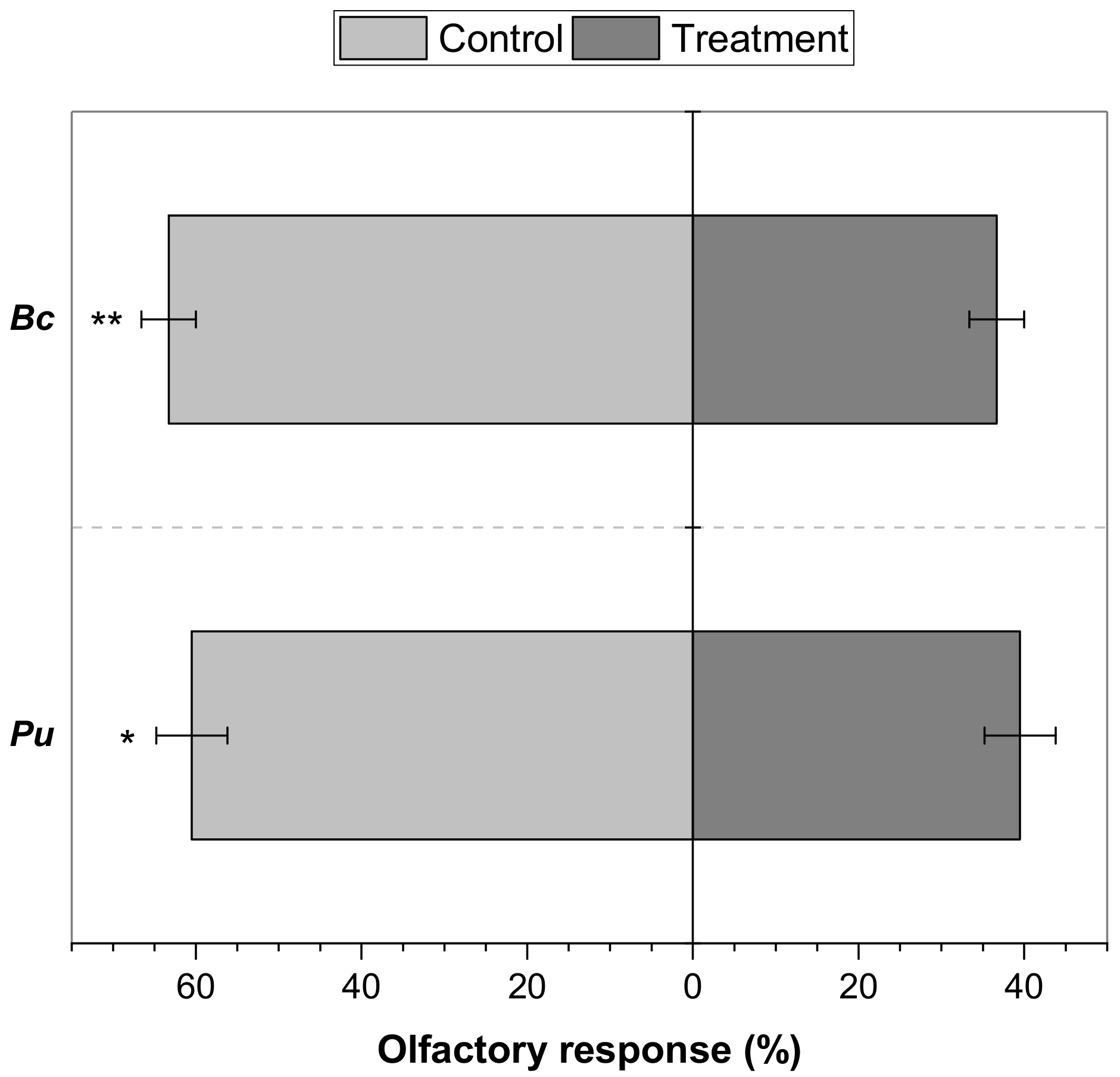
2. Results
3. Discussion
4. Materials and Methods
4.1. H. Irritans Collection
4.2. Plant Material and Essential Oil Extraction
4.3. Essential Oil Analysis by GC/MS
4.4. Laboratory Feeding Bioassay
4.4.1. Blood Collection
4.4.2. Feeding Bioassay
4.4.3. Toxicity Bioassays
4.4.4. Olfactometer Bioassays
4.4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cupp, E.W.; Cupp, M.S.; Ribeiro, J.M.; Kunz, S.E. Blood-feeding strategy of Haematobia irritans (Diptera: Muscidae). J. Med. Entomol. 1998, 35, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Pérez de León, A.A.; Mitchell, R.D.; Watson, D.W. Ectoparasites of Cattle. Vet. Clin. Food Anim. 2020, 36, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, K. Survival, ovarian development and bloodmeal size for the horn fly Haematobia irritans irritans reared in vitro. Med. Vet. Entomol. 2000, 14, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, A.; Monzón, C.M.; Bulman, G.M. Haematobia irritans: Una actualización a diez años de su introducción en Argentina (Parte I). Vet. Argent. 2001, 18, 34–46. [Google Scholar]
- Mancebo, A.; Monzón, C.M.; Bulman, G.M. Haematobia irritans: Una actualización a diez años de su introducción en Argentina (Parte II). Vet. Argent. 2001, 18, 119–135. [Google Scholar]
- James, M.T.; Harwood, R.F. Herms’ Medical Entomology, 6th ed.; The McMillan Co.: New York, NY, USA, 1969; pp. 275–277. [Google Scholar]
- Depner, K.R. The effect of temperature on development and diapause of the horn fly, Siphona irritans (L.) (Diptera: Muscidae). Can. Entomol. 1961, 10, 855–859. [Google Scholar] [CrossRef]
- Carballo, M.; Martínez, M. Hallazgo de Haematobia irritans en Uruguay. Veterinaria 1991, 27, 20–21. [Google Scholar]
- Luzuriaga, R.; Eddi, C.; Caracostantogolo, J.; Botto, E.; Pereira, J. Diagnosis of infestation of cattle by Haematobia irritans (L.) in Misiones, Argentina. Rev. Med. Vet. 1991, 72, 262–264. [Google Scholar]
- Cisternas, E.A. Mosca de los cuernos Haematobia irritans. Inf. INIA Remehue 1999, 11, 1–2. [Google Scholar]
- Fundación Para la Innovación Agraria (FIA); BTA Consultores. Resultados y Lecciones en Control. Biológico de la Mosca de los Cuernos en Bovinos con Extracto de Neem: Proyecto de Innovación en Regiones Metropolitana, de Valparaíso, del Maule y del Biobío; Fundación Para La Innovación Agraria: La Reina, Chile, 2009; pp. 1–36. [Google Scholar]
- Muñoz, M.; Serrano, E. Infestación por Haematobia irritans en el toro de lidia: “Mosca de los cuernos”. Rev. Complut. Cienc. Vet. 2007, 1, 347–351. [Google Scholar]
- Foil, L.D.; Hogsette, J.A. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. 1994, 13, 1125–1158. [Google Scholar] [CrossRef]
- Hawkins, J.A. The Horn Fly (Haematobia irritans); Merial Veterinary Bulletin No TSB-0-00028-FTB; Veterinary Professional Services, Merial: Iselin, NJ, USA, 2001. [Google Scholar]
- Schreiber, E.T.; Campbell, J.B.; Kunz, S.E.; Clanton, D.C.; Hudson, D.B. Effects of horn fly (Diptera: Muscidae) control on cows and gastrointestinal worm (Nematode: Trichostrongylidae) treatment for calves on cow and calf weight gains. J. Econ. Entomol. 1987, 80, 451–454. [Google Scholar] [CrossRef]
- DeRouen, S.M.; Foil, L.D.; MacKay, A.J.; Fraenke, D.E.; Sanson, D.W.; Wyatt, W.E. Effect of horn fly (Haematobia irritans) control on growth and reproduction of beef heifers. J. Econ. Entomol. 2003, 96, 1612–1616. [Google Scholar] [CrossRef]
- Campbell, J.B. Effect of horn fly control on cows as expressed by increased weaning weights of calves. J. Econ. Entomol. 1976, 69, 711–712. [Google Scholar] [CrossRef]
- Harvey, T.L.; Brethour, J.L. Effect of horn flies on weight gains of beef cattle. J. Econ. Entomol. 1979, 72, 516–518. [Google Scholar] [CrossRef]
- Steelman, C.D.; Brown, A.H., Jr.; Gbur, E.E.; Tolley, G. Interactive response of the horn fly (Diptera: Muscidae) and selected breeds of beef cattle. J. Econ. Entomol. 1991, 84, 1275–1282. [Google Scholar] [CrossRef]
- Foil, L.D.; Allison, M.; DeRouen, S.M.; Kimball, M.; Morrison, D.M.; Sanson, D.W.; Wyatt, W.E. Controlling horn flies. La. Agric. 2000, 43, 21–23. [Google Scholar]
- Oyarzún, M.P.; Quiroz, A.; Birkett, M.A. Insecticide resistance in the horn fly: Alternative control strategies. Med. Vet. Entomol. 2008, 22, 188–202. [Google Scholar] [CrossRef]
- Williams, J.D.; Sutherst, R.W.; Maywald, G.F.; Petherbridge, C.T. The southward spread of buffalo fly (Haematobia irritans exigua) in eastern Australia and its survival through a severe winter. Aust. Vet. J. 1985, 62, 367–369. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Metcalf, R.A. Destructive and Useful Insects, 5th ed.; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Anderson, K.L.; Lyman, R.; Moury, K.; Ray, D.; Watson, D.W.; Correa, M.T. Molecular epidemiology of Staphylococcus aureus mastitis in dairy heifers. J. Dairy Sci. 2012, 95, 4921–4930. [Google Scholar] [CrossRef]
- Edwards, J.F.; Wikse, S.E.; Field, R.W.; Hoelscher, C.C.; Herd, D.B. Bovine teat atresia associated with horn fly (Haematobia irritans irritans L.) induced dermatitis. Vet. Pathol. 2000, 37, 360–364. [Google Scholar] [CrossRef]
- Oliver, S.P.; Gillespie, B.E.; Headrick, S.J.; Lewis, M.J.; Dowlen, H.H. Prevalence, risk factors, and strategies for controlling mastitis in heifers during the periparturient period. Int. J. Appl. Res. Vet. Med. 2005, 3, 150–162. [Google Scholar]
- DeRouen, S.M.; Miller, J.E.; Foil, L.D. Control of horn flies (Haematobia irritans) and gastrointestinal nematodes and its relation with growth performance in stocker cattle. Prof. Anim. Sci. 2010, 26, 109–114. [Google Scholar] [CrossRef]
- Grisi, L.; Cerqueira, R.; de Souza, J.; Medeiros, A.; Andreotti, R.; Duarte, P.; Pérez, A.; Barros, J.; Silva, H. Reassessment of the potential economic impact of cattle parasites in Brazil. Braz. J. Vet. Parasitol. 2014, 23, 150–156. [Google Scholar] [CrossRef]
- Velasco, R.; González, J.; Morales, G.; Ortega, E. Daño económico y costos de control en bovinos mosca de los cuernos. Inf. Agropecu. Bioleche INIA Quilamapu 2001, 14, 4–7. [Google Scholar]
- Lanuza, F.; Paredes, E.; Sievers, G.; Cortázar, J.M. Manejo Sanitario y Principales Enfermedades de los Bovinos de Carne. In Producción y Manejo de Carne Bovina en Chile; Catrileo, A., Ed.; Instituto de Investigaciones Agropecuarias: Temuco, Chile, 2005; pp. 513–550. [Google Scholar]
- Espinoza, J.; Chacón-Fuentes, M.; Quiroz, A.; Bardehle, L.; Escobar-Bahamondes, P.; Ungerfeld, E. Antifeedant Effects and Repellent Activity of Loline Alkaloids from Endophyte-Infected Tall Fescue against Horn Flies, Haematobia irritans (Diptera: Muscidae). Molecules 2021, 26, 817. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Castelli, M.E.; Idiart, J.; Fisher, W.F.; Volpogni, M.M.; Quaino, O.; Anziani, O.S.; Flores, S.G.; Warnke, O. Skin lesions and cattle hide damage from Haematobia irritans in cattle. Med. Vet. Entomol. 1999, 13, 323–328. [Google Scholar] [CrossRef]
- Barros, A.T.M.; Ottea, J.; Sanson, D.; Foil, L.D. Horn fly (Diptera: Muscidae) resistance to organophosphate insecticides. Vet. Parasitol. 2001, 96, 243–256. [Google Scholar] [CrossRef]
- Oyarzún, M.P.; Li, Y.; Figueroa, C.C. High levels of insecticide resistance in introduced horn fly (Diptera: Muscidae) populations and implications for management. J. Econ. Entomol. 2011, 104, 258–265. [Google Scholar] [CrossRef]
- Steelman, C.D.; Brown, M.A.; Gbur, E.E.; Tolley, G. The effects of hair density of beef cattle on Haematobia irritans horn fly populations. Med. Vet. Entomol. 1997, 11, 257–264. [Google Scholar] [CrossRef]
- Steelman, C.D.; McNew, R.W.; Simpson, R.B.; Rorie, R.W.; Phillips, J.M.; Rosenkrans, C.F. Evaluation of alternative tactics for management of insecticide resistant horn flies (Diptera: Muscidae). J. Econ. Entomol. 2003, 96, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Cicchino, A.; Abrahamovich, A.; Nuñez, J.; Prieto, O. Los enemigos naturales de Haematobia irritans irritans (Díptera: Muscidae) en dos áreas ganaderas de la Argentina. Rev. Med. Vet. 1994, 75, 6–16. [Google Scholar]
- Geden, C.J.; Moon, R.D.; Butler, J.F. Host ranges of six solitary filth fly parasitoids (Hymenoptera: Pteromalidae, Chalcididae) from Florida, Eurasia, Morocco, and Brazil. Environ. Entomol. 2006, 35, 405–412. [Google Scholar] [CrossRef]
- Markin, G.P.; Yoshioka, E.R. Biological Control of the Horn Fly, Haematobia irritans L., in Hawai’i (Diptera: Muscidae). Proc. Hawaii Entomol. Soc. 1998, 33, 43–50. [Google Scholar]
- Hu, G.Y.; Frank, J.H. Effect of the red imported fire ant (Hymenoptera: Formicidae) on dung-inhabiting arthropods in Florida. Environ. Entomol. 1996, 25, 1290–1296. [Google Scholar] [CrossRef]
- Hu, G.Y.; Frank, J.H. Effect of the arthropod community on survivorship of immature Haematobia irritans (Diptera: Muscidae) in north central Florida. J. Fla. Entomol. 1996, 79, 497–503. [Google Scholar] [CrossRef]
- Mochi, D.; Monteiro, A.; Simi, L.; Moraes, A. Susceptibility of adult and larval stages of the horn fly, Haematobia irritans, to the entomopathogenic fungus Metarhizium anisopliae under field conditions. Vet. Parasitol. 2009, 166, 136–143. [Google Scholar] [CrossRef]
- Mochi, D.; Monteiro, A.; Ribeiro, A.; Yoshida, L. Efficiency of entomopathogenic fungi in the control of eggs and larvae of the horn fly Haematobia irritans (Diptera: Muscidae). Vet. Parasitol. 2010, 168, 105–110. [Google Scholar] [CrossRef]
- Denning, S.; Washburn, S.; Watson, D.J. Development of a novel walk-through fly trap for the control of horn flies and other pests on pastured dairy cows. Dairy Sci. 2014, 97, 1–8. [Google Scholar] [CrossRef]
- Galindo, E.; Lezama, R.; Cruz, C.; Pescador, A.; Angel, C.; Ojeda, M.; Rodríguez, R.; Contreras, D. Efficacy of entomopathogenic fungi (Ascomycetes:Hypocreales) against adult Haematobia irritans (Diptera:Muscidae) under stable conditions in the Mexican dry tropics. Vet. Parasitol. 2015, 209, 173–178. [Google Scholar] [CrossRef]
- Velasco-Reyes, I.; Cruz-Vázquez, C.; Ángel-Sahagún, C.; Medina-Esparza, L.; Ramos-Parra, M. Control de Haematobia irritans y Stomoxys calcitrans con Metarhizium anisopliae en ganado naturalmente infestado. Rev. MVZ Cordoba 2019, 24, 7091–7096. [Google Scholar] [CrossRef]
- Parra, L.; Rojas, C.; Catrileo, A.; Galdames, R.; Mutis, A.; Birkett, M.A.; Quiroz, A. Differences in the fly-load of Haematobia irritans (Diptera: Muscidae) on cattle is modified by endophyte infection of pastures. Electron. J. Biotechnol. 2013, 16, 1–16. [Google Scholar] [CrossRef]
- Parra, L.; Mutis, A.; Chacón, M.; Lizama, M.; Rojas, C.; Catrileo, A.; Rubilar, O.; Tortella, G.; Birkett, M.A.; Quiroz, A. Horn fly larval survival in cattle dung is reduced by endophyte infection of tall fescue pasture. Pest Manag. Sci. 2016, 72, 1328–1334. [Google Scholar] [CrossRef]
- Dougherty, C.T.; Knapp, F.W.; Bush, L.P.; Maul, J.E.; van Willigen, J. Mortality of horn fly (Diptera: Muscidae) larvae in bovine dung supplemented with loline alkaloids from tall fescue. J. Med. Entomol. 1998, 35, 798–803. [Google Scholar] [CrossRef]
- Dougherty, C.T.; Knapp, F.W.; Bush, L.P. Mortality of horn fly larvae (Diptera: Muscidae) in bovine dung supplemented with ergotamine and N-formyl loline. J. Med. Entomol. 1999, 36, 73–77. [Google Scholar] [CrossRef]
- Zhu, J.J.; Brewer, G.J.; Boxler, D.J.; Friesen, K.; Taylor, D.B. Comparisons of antifeedancy and spatial repellency of three natural product repellents against horn flies, Haematobia irritans (Diptera:Muscidae). Pest Manag. Sci. 2015, 71, 1553–1560. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Lucia, A.; Guzmán, E. Emulsions containing essential oils, their components or volatile semiochemicals as promising tools for insect pest and pathogen management. Adv. Colloid Interface Sci. 2021, 287, 102330. [Google Scholar] [CrossRef]
- Showler, A.T. Botanically based repellent and insecticidal effects against horn flies and stable flies (Diptera: Muscidae). J. Integr. Pest Manag. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Beyond mosquitoes—Essential oil toxicity and repellency against bloodsucking insects. Ind. Crops Prod. 2018, 117, 382–392. [Google Scholar] [CrossRef]
- Klauck, V.; Pazinato, R.; Stefani, L.M.; Santos, R.C.; Vaucher, R.A.; Baldissera, M.D.; Raffin, R.; Boligon, A.; Athayde, M.; Baretta, D.; et al. Insecticidal and repellent effects of tea tree and andiroba oils on flies associated with livestock. Med. Vet. Entomol. 2014, 28, 33–39. [Google Scholar] [CrossRef]
- Lachance, S.; Grange, F. Repellency effectiveness of seven plant essential oils, sunflower oil and natural insecticides against horn flies on pastured dairy cows and heifers. Med. Vet. Entomol. 2014, 28, 193–200. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, M.J.; Ures, X.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal activity of microencapsulated Schinus molle essential oil. Ind. Crops Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Boito, J.P.; Da Silva, A.S.; dos Reis, J.H.; Santos, D.S.; Gebert, R.R.; Biazus, A.H.; Santos, R.C.V.; Quatrin, P.M.; Ourique, A.F.; Boligon, A.A.; et al. Insecticidal and repellent effect of cinnamon oil on flies associated with livestock. Rev. MVZ Córdoba 2018, 23, 6628–6636. [Google Scholar] [CrossRef]
- Juan, L.W.; Lucia, A.; Zerba, E.N.; Harrand, L.; Marco, M.; Masuh, H.M. Chemical composition and fumigant toxicity of the essential oils from 16 species of Eucalyptus against Haematobia irritans (Diptera: Muscidae) adults. J. Econ. Entomol. 2011, 104, 1087–1092. [Google Scholar] [CrossRef]
- Klauck, V.; Pazinato, R.; Radavelli, W.M.; Volpato, A.; Stefani, L.M.; Santos, R.C.; Vaucher, R.A.; Boligon, A.A.; Athayde, M.S.; da Silva, A.S. In vitro repellent effect of tea tree (Melaleuca alternifolia) and andiroba (Carapa guianensis) oils on Haematobia irritans and Chrysomya megacephala flies. Trop. Biomed. 2015, 32, 160–166. [Google Scholar]
- Miranda, O. Flora y fitosociología de un Relicto de Pilgerodendron uvifera (D. Don) Florin en el fundo San Pablo de Tregua (Valdivia-Chile). Bosque 1981, 4, 3–11. [Google Scholar] [CrossRef]
- Bannister, J.R.; Donoso, P.J.; Bauhus, J. Persistence of the slow growing conifer Pilgerodendron uviferum in old-growth and fire-disturbed southern bog forests. Ecosystems 2012, 15, 1158–1172. [Google Scholar] [CrossRef]
- Solis, C.; Becerra, J.; Fores, C.; Rebolledo, J.; Silva, M. Antibacterial and antifungal terpenes from Pilgerodendron uviferum (D. Don) Florin. J. Chil. Chem. Soc. 2004, 49, 157–161. [Google Scholar] [CrossRef]
- Donoso, C.A.; Becerra, J.; Bittner, M.; Elissetche, J.P.; Freer, J.; Mendoza, R.; Sterner, O.; Silva, M. Allelochemicals and natural durability in Chilean Cupressaceae heartwoods. Allelopathy J. 2008, 21, 119–132. [Google Scholar]
- Espinoza, J.; Urzúa, A.; Tampe, J.; Parra, L.; Quiroz, A. Repellent activity of the essential oil from the heartwood of Pilgerodendron uviferum (D. Don) Florin against Aegorhinus superciliosus (Coleoptera: Curculionidae). Molecules 2016, 21, 533. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Urzúa, A.; Bardehle, L.; Quiroz, A.; Echeverría, J.; González-Teuber, M. Antifeedant effects of essential oil, extracts, and isolated sesquiterpenes from Pilgerodendron uviferum (D. Don) florin heartwood on red clover borer Hylastinus obscurus (Coleoptera: Curculionidae). Molecules 2018, 23, 1282. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.J.; Alderman, S.C. Red Clover Seed Production. VI. Effect and economic of soil pH adjusted by lime application. Crop Sci. 2003, 43, 624–630. [Google Scholar] [CrossRef]
- Quiroz, A.; Ortega, F.; Ramírez, C.C.; Wadhams, L.J.; Pinilla, K. Response of the beetle Hylastinus obscurus Marsham (Coleoptera: Scolytidae) to red clover (Trifolium pratense L.) volatiles in a laboratory olfactometer. Environ. Entomol. 2005, 34, 690–695. [Google Scholar] [CrossRef]
- Espinoza, J.; Urzúa, A.; Sanhueza, L.; Walter, M.; Fincheira, P.; Muñoz, P.; Mendoza, L.; Wilkens, M. Essential Oil, Extracts, and Sesquiterpenes Obtained From the Heartwood of Pilgerodendron uviferum Act as Potential Inhibitors of the Staphylococcus aureus NorA Multidrug Efflux Pump. Front. Microbiol. 2019, 10, 337. [Google Scholar] [CrossRef]
- Latsague, M.; Sáez, P.; Hauenstein, E.; Peña-Cortés, F. Vegetative propagation of Myrceugenia exsucca and Blepharocalyx cruckshanksii, swamp forest dominant species of the central depression of Araucanía region, Chile. Bosque 2010, 31, 247–251. [Google Scholar] [CrossRef][Green Version]
- Baez, C.; Villena, J.; Montenegro, I.; Russo, A.; Said, B.; Madrid, A. Cytotoxic activity of crude extracts and fractions from Blepharocalyx cruckshanksii against selected human cancer cell lines. Bol. Latinoam. Caribe Plantas Med. Aromat. 2020, 19, 357–362. [Google Scholar] [CrossRef]
- González, M.; Hauenstein, E.; Peña-Cortés, F.; García, M.; Urrutia, O. Comentarios sobre bosques pantanosos, humedales importantes del centro-sur de Chile. Gestión Ambient. 2003, 9, 3–13. [Google Scholar]
- Gusinde, M. Plantas medicinales que los indios Araucanos recomiendan. Anthropos 1936, 31, 850–873. [Google Scholar]
- Massardo, F.; Rozzi, R. Valoración de la Biodiversidad: Usos medicinales de la flora nativa chilena. Ambiente Desarro. 1996, 12, 76–81. [Google Scholar]
- Furtado, F.B.; Borges, B.C.; Teixeira, T.L.; Garces, H.G.; Almeida Junior, L.D.d.; Alves, F.C.B.; Silva, C.V.d.; Fernandes Junior, A. Chemical Composition and Bioactivity of Essential Oil from Blepharocalyx salicifolius. Int. J. Mol. Sci. 2018, 19, 33. [Google Scholar] [CrossRef]
- Stefanello, M.E.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef]
- Tucker, A.O.; Maciarello, M.J.; Landrum, L.R. Volatile Leaf Oils of American Myrtaceae: I. Blepharocalyx cruckshanksii (Hook. & Arn.) Niedenzu of Chile and B. salicifolius (Humb., Bonpl. & Kunth) Berg of Argentina. J. Essent. Oil Res. 1993, 5, 333–335. [Google Scholar] [CrossRef]
- Oyarzún, M.L.; Garbarino, J.A. Sesquiterpenoids from Pilgerodendron uvífera. Phytochemistry 1988, 27, 1121–1123. [Google Scholar] [CrossRef]
- Malizia, R.A.; Cardell, D.A.; Molli, J.S.; González, S.; Guerra, P.E.; Grau, R.J. Volatile constituents of leaf oils from the Cupressacea family: Part II. Austrocedrus chilensis, Fitzroya cupressoides and Pilgerodendron uviferum species growing in Argentina. J. Essent. Oil Res. 2000, 12, 233–237. [Google Scholar] [CrossRef]
- Showler, A.T.; Harlien, J.L. Effects of the botanical compound p-anisaldehyde on horn fly (Diptera: Muscidae) repellency, mortality, and reproduction. J. Med. Entomol. 2018, 55, 183–192. [Google Scholar] [CrossRef]
- Showler, A.T.; Harlien, J.L.; Perez de Léon, A.A. Effects of Laboratory Grade Limonene and a Commercial Limonene-Based Insecticide on Haematobia irritans irritans (Muscidae: Diptera): Deterrence, Mortality, and Reproduction. J. Med. Entomol. 2019, 56, 1064–1070. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Kovats, E.; Keulemans, A.I.M. The Kováts Retention Index System. Anal. Chem. 1964, 36, 31A–41A. [Google Scholar]
- Tampe, J.; Espinoza, J.; Chacón-Fuentes, M.; Quiroz, A.; Rubilar, M. Evaluation of Drimys winteri (Canelo) Essential Oil as Insecticide against Acanthoscelides obtectus (Coleoptera: Bruchidae) and Aegorhinus superciliosus (Coleoptera: Curculionidae). Insects 2020, 11, 335. [Google Scholar] [CrossRef]
- Oyarzún, M.P.; Palma, R.; Alberti, E.; Hormazabal, H.; Pardo, F.; Birkett, M.A.; Quiroz, A. Olfactory Response of Haematobia irritans (Diptera: Muscidae) to Cattle-Derived Volatile Compounds. Med. Entomol. 2009, 46, 1320–1326. [Google Scholar] [CrossRef]

| RT | RI | Compound | % | Identification |
|---|---|---|---|---|
| 9.50 | 924 | α-Thujene | 0.37 | RI, MS |
| 9.65 | 930 | α-Pinene | 36.50 | RI, MS, Co-I |
| 10.87 | 969 | β-Pinene | 0.81 | RI, MS, Co-I |
| 12.66 | 1027 | Limonene | 20.50 | RI, MS, Co-I |
| 21.93 | 1350 | α-Cubebene | 1.16 | RI, MS |
| 22.49 | 1371 | Ylangene | 0.07 | RI, MS |
| 22.62 | 1376 | Copaene | 1.18 | RI, MS |
| 23.02 | 1390 | β-Elemene | 0.24 | RI, MS |
| 23.42 | 1406 | α-Gurjunene | 2.59 | RI, MS |
| 23.63 | 1414 | Caryophyllene | 4.63 | RI, MS |
| 23.96 | 1428 | β-Gurjunene | 0.20 | RI, MS |
| 24.17 | 1437 | Aromadendrene | 1.43 | RI, MS |
| 24.38 | 1445 | Bicyclosesquiphellandrene | 0.82 | RI, MS |
| 24.51 | 1450 | α-Caryophyllene | 0.86 | RI, MS |
| 24.65 | 1456 | Alloaromadendrene | 1.19 | RI, MS |
| 24.96 | 1468 | β-Cadinene | 1.23 | RI, MS |
| 25.31 | 1482 | α-Selinene | 1.48 | RI, MS |
| 25.52 | 1490 | Ledene | 2.04 | RI, MS |
| 25.71 | 1498 | δ-Cadinene | 3.63 | RI, MS |
| 25.89 | 1505 | γ-Cadinene | 0.21 | RI, MS |
| 26.06 | 1513 | Calamenene | 8.69 | RI, MS |
| 26.33 | 1525 | Cubenene | 5.65 | RI, MS |
| 26.62 | 1537 | α-Calacorene | 0.21 | RI, MS |
| 27.48 | 1574 | Spathulenol | 0.81 | RI, MS |
| 27.65 | 1581 | Caryophyllene oxide | 0.60 | RI, MS |
| 28.04 | 1597 | Ledol | 0.21 | RI, MS |
| 28.17 | 1603 | Humulene-1,2-epoxide | 1.10 | RI, MS |
| 28.59 | 1622 | Epicubenol | 0.40 | RI, MS, Co-I |
| 28.91 | 1637 | Cubenol | 1.18 | RI, MS, Co-I |
| Monoterpene hydrocarbons | 58.18 | |||
| Sesquiterpene hydrocarbons | 37.52 | |||
| Oxygenated sesquiterpenes | 4.30 | |||
| Total sesquiterpenes | 41.82 |
| EO | Time (h) | LC50 (95% FCI) (µL L−1 Air) | χ2 | p | Slope (±SE) |
|---|---|---|---|---|---|
| B. cruckchanksii | 1 | - | - | - | - |
| 4 | 3.583 (0.003–6.146) | 0.329 | 0.028 | 1.167 ± 0.531 | |
| P. uviferum | 1 | 9.414 (6.438–18.348) | 1.672 | <0.001 | 1.287 ± 0.241 |
| 4 | 1.016 (0.553–1.494) | 2.810 | <0.001 | 1.140 ± 0.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, J.; Medina, C.; Aniñir, W.; Escobar-Bahamondes, P.; Ungerfeld, E.; Urzúa, A.; Quiroz, A. Insecticidal, Repellent and Antifeedant Activity of Essential Oils from Blepharocalyx cruckshanksii (Hook. & Arn.) Nied. Leaves and Pilgerodendron uviferum (D. Don) Florin Heartwood against Horn Flies, Haematobia irritans (Diptera: Muscidae). Molecules 2021, 26, 6936. https://doi.org/10.3390/molecules26226936
Espinoza J, Medina C, Aniñir W, Escobar-Bahamondes P, Ungerfeld E, Urzúa A, Quiroz A. Insecticidal, Repellent and Antifeedant Activity of Essential Oils from Blepharocalyx cruckshanksii (Hook. & Arn.) Nied. Leaves and Pilgerodendron uviferum (D. Don) Florin Heartwood against Horn Flies, Haematobia irritans (Diptera: Muscidae). Molecules. 2021; 26(22):6936. https://doi.org/10.3390/molecules26226936
Chicago/Turabian StyleEspinoza, Javier, Cristian Medina, Washington Aniñir, Paul Escobar-Bahamondes, Emilio Ungerfeld, Alejandro Urzúa, and Andrés Quiroz. 2021. "Insecticidal, Repellent and Antifeedant Activity of Essential Oils from Blepharocalyx cruckshanksii (Hook. & Arn.) Nied. Leaves and Pilgerodendron uviferum (D. Don) Florin Heartwood against Horn Flies, Haematobia irritans (Diptera: Muscidae)" Molecules 26, no. 22: 6936. https://doi.org/10.3390/molecules26226936
APA StyleEspinoza, J., Medina, C., Aniñir, W., Escobar-Bahamondes, P., Ungerfeld, E., Urzúa, A., & Quiroz, A. (2021). Insecticidal, Repellent and Antifeedant Activity of Essential Oils from Blepharocalyx cruckshanksii (Hook. & Arn.) Nied. Leaves and Pilgerodendron uviferum (D. Don) Florin Heartwood against Horn Flies, Haematobia irritans (Diptera: Muscidae). Molecules, 26(22), 6936. https://doi.org/10.3390/molecules26226936







