Antineoplastics Encapsulated in Nanostructured Lipid Carriers
Abstract
:1. Introduction
1.1. Antineoplastics
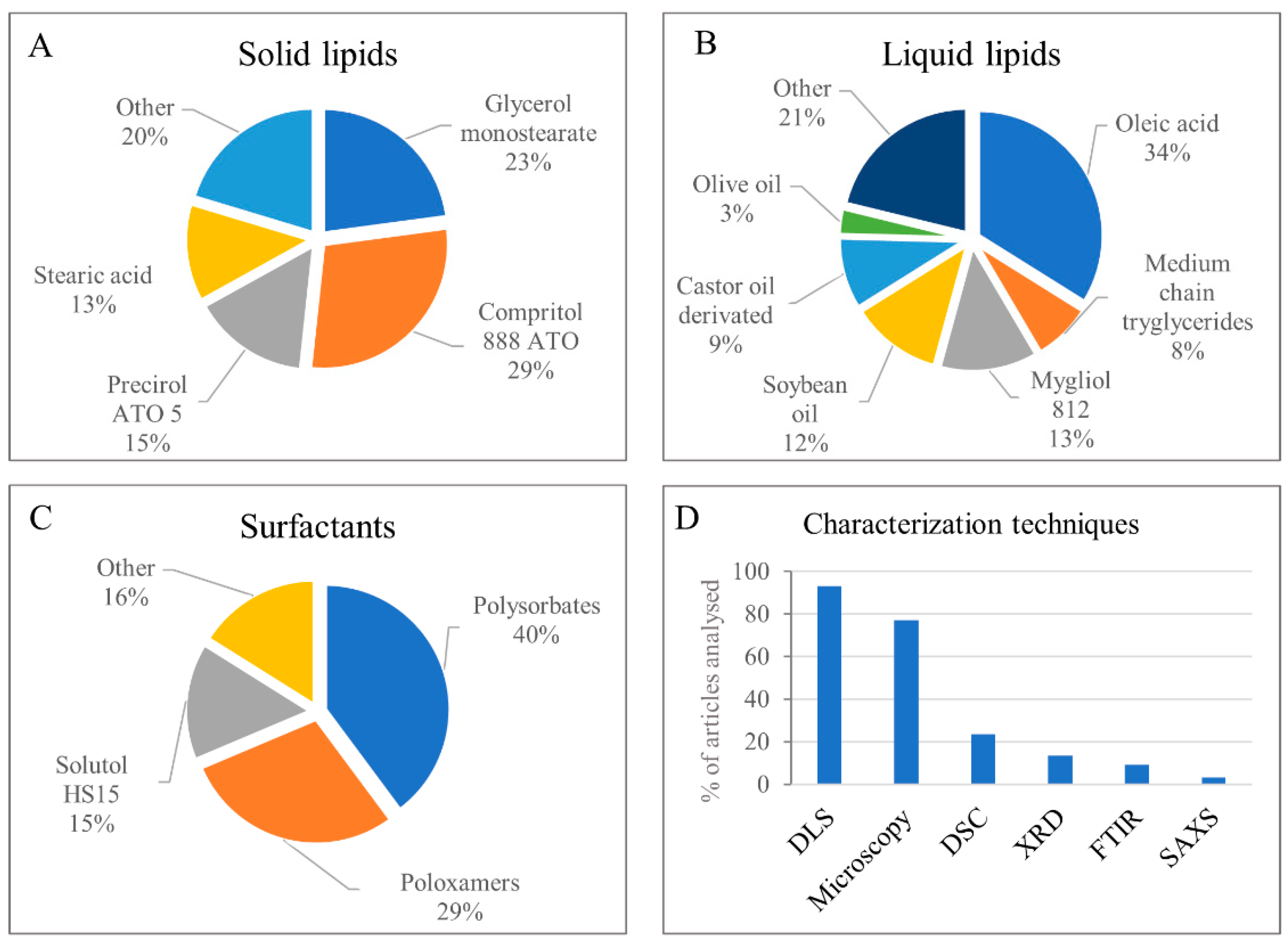
1.2. Nanostructured Lipid Carriers
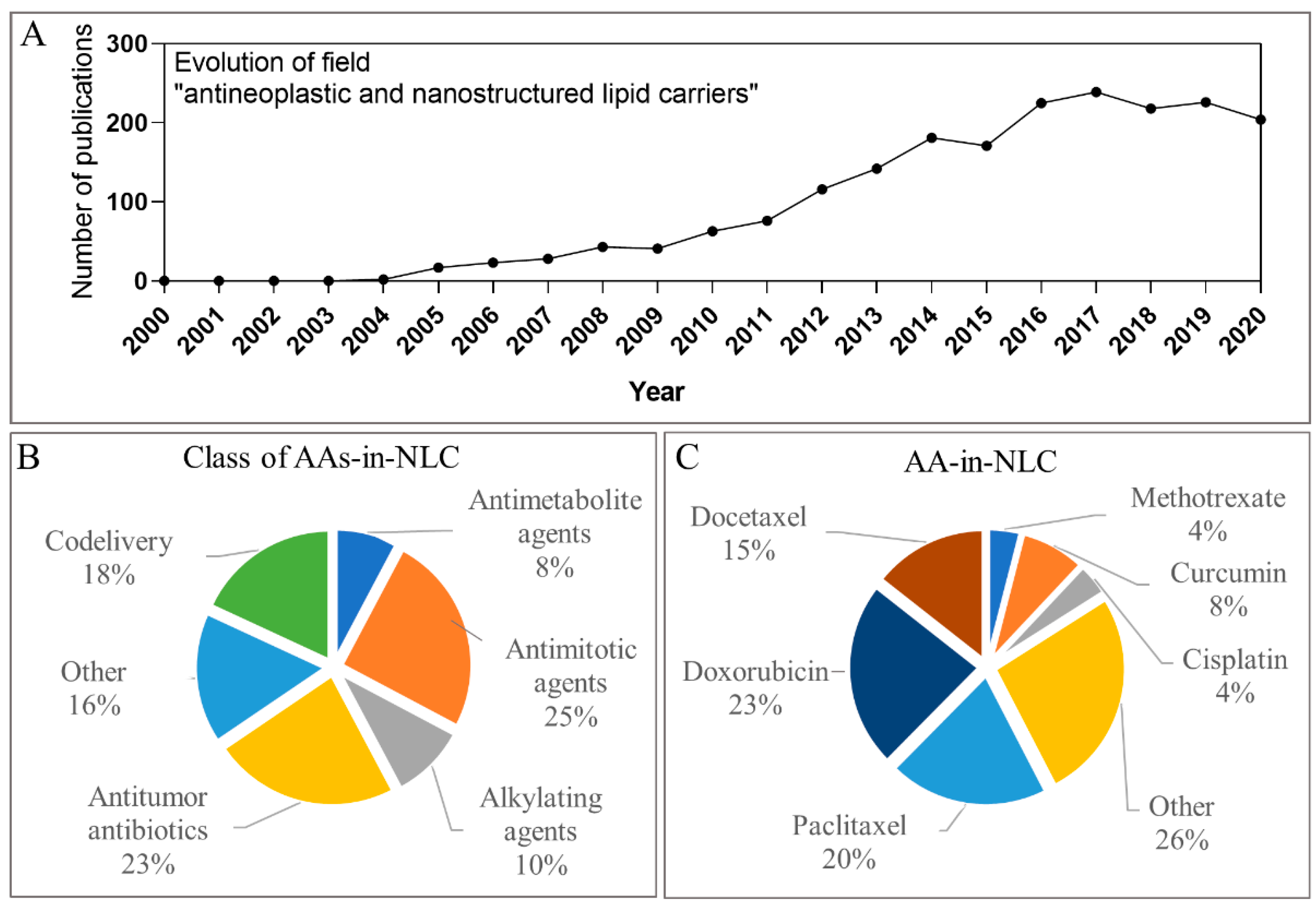
1.3. Metrics Regarding Articles with Antineoplastics Loaded in NLC
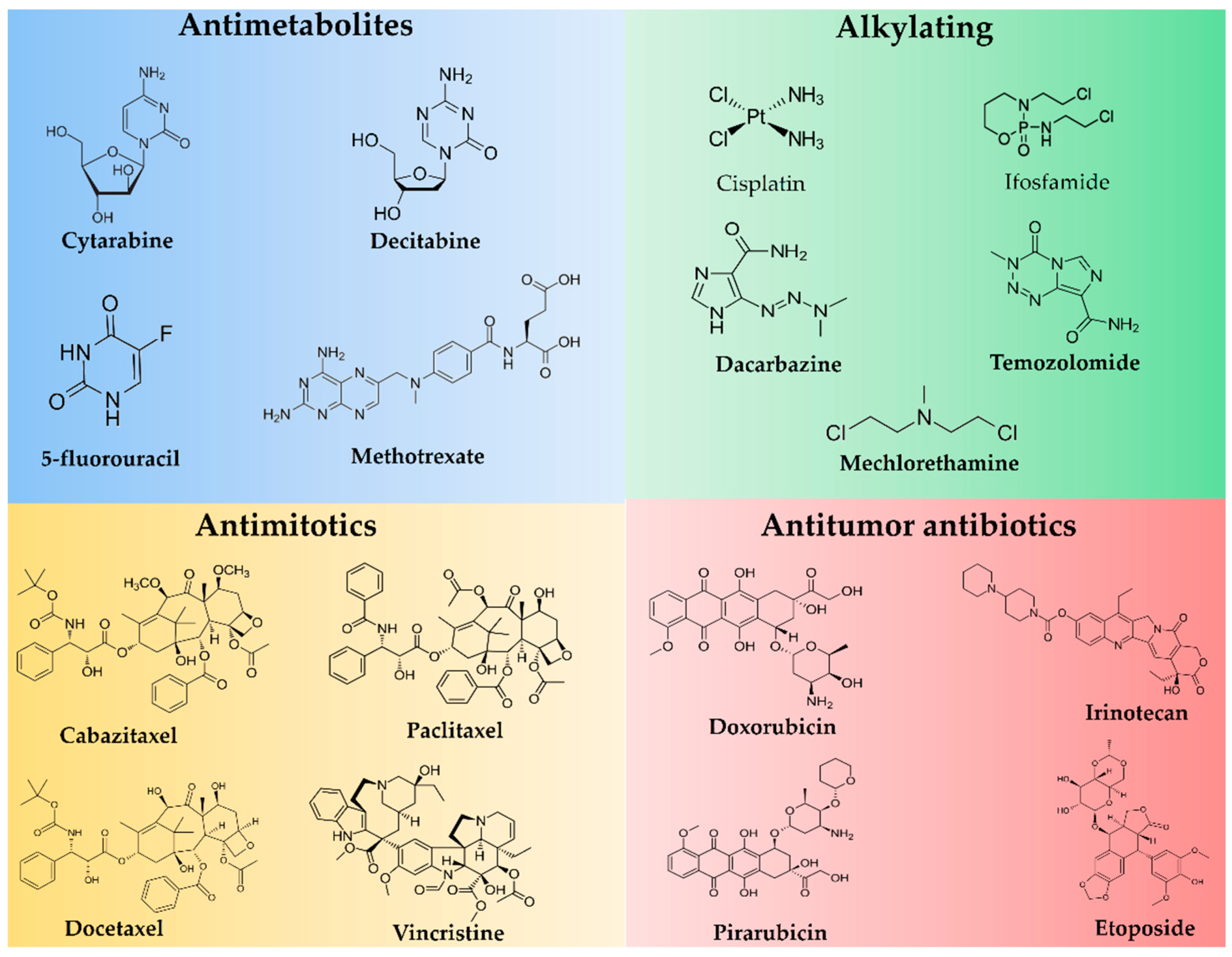
2. Conventional Antineoplastics Agents Uploaded in NLC
2.1. Antimetabolites
2.1.1. 5-Fluorouracil
2.1.2. Cytarabine
2.1.3. Decitabine
2.1.4. Methotrexate
2.2. Antimitotics
2.2.1. Taxanes
Cabazitaxel
Docetaxel
Paclitaxel
2.2.2. Vinca Alkaloids (Vincristine)
2.3. Alkylating Agents
2.3.1. Cisplatin
2.3.2. Dacarbazine
2.3.3. Ifosfamide
2.3.4. Mechlorethamine
2.3.5. Temozolomide
2.4. Antitumour Antibiotics
2.4.1. Anthracyclines
Doxorubicin
Pirarubicin
2.4.2. Irinotecan
2.4.3. Etoposide
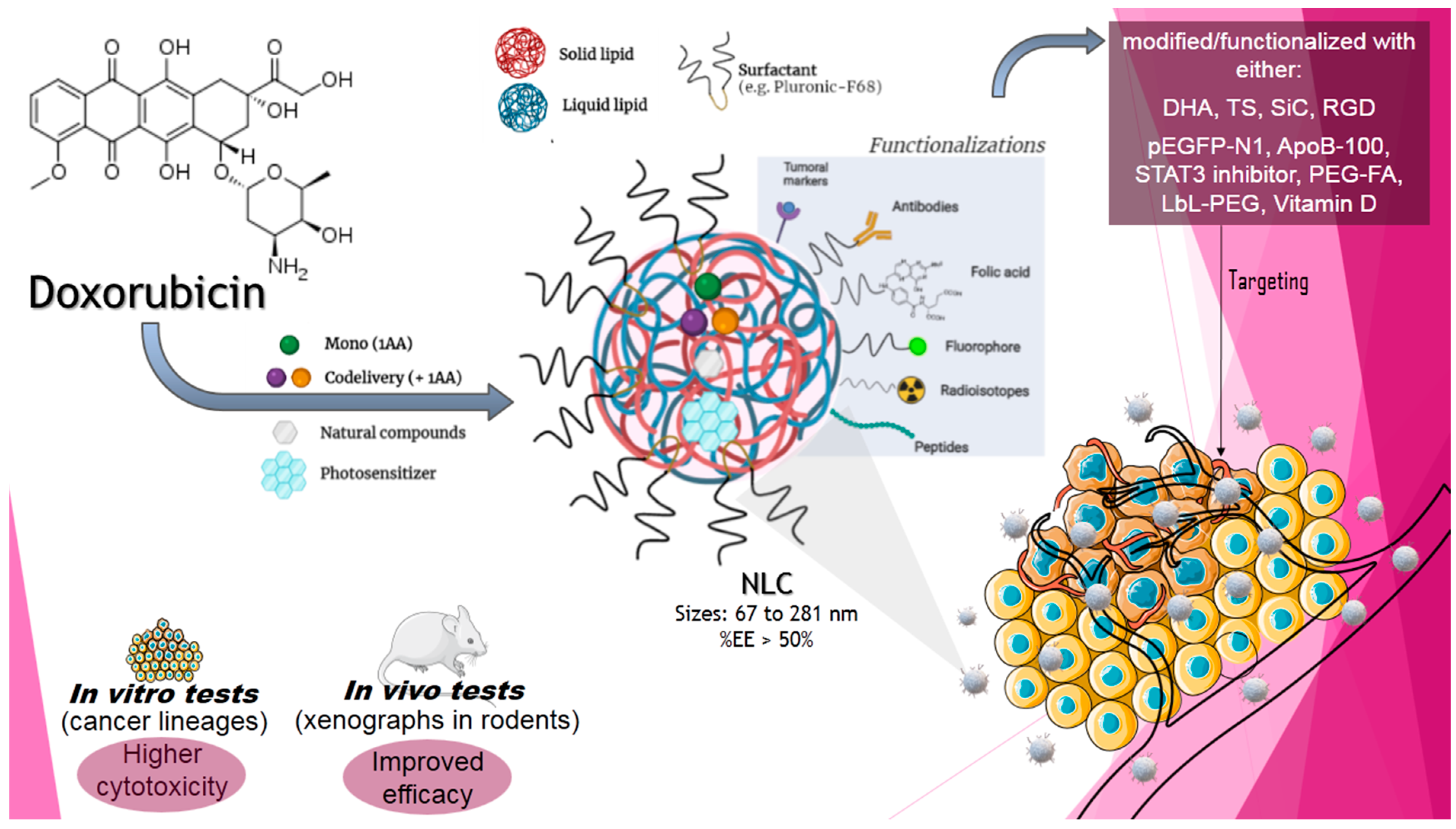
3. Codelivery of Conventional AAs by Nanostructured Lipid Nanoparticles
3.1. DOX Codelivery with NLC Formulations
3.1.1. DOX and PTX
3.1.2. DOX and CIS
3.1.3. DOX and VCR
3.1.4. DOX, GEM and VCR
3.1.5. DOX and Baicalein
3.1.6. DOX and Sclareol
3.1.7. DOX and β-Elemene
3.1.8. DOX and β-Lapachone
3.2. PTX Codelivery by NLC Formulations
3.2.1. PTX and CIS
3.2.2. PTX and Demethylnobitelin
3.3. CIS Codelivery by NLC Formulations
3.4. TMZ Codelivery by NLC Formulations
TMZ and VCR
4. New Approaches in Chemotherapy
4.1. Curcumin as a Non-Conventional AA Encapsulated in NLC Formulations
4.2. Photodynamic and Photothermal Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- What Is Cancer?—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer (accessed on 4 September 2021).
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- NIH. National Cancer Institute/USA. A to Z List of Cancer Drugs. Available online: https://www.cancer.gov/about-cancer/treatment/drugs (accessed on 25 May 2021).
- Feng, T.; Zhao, Y. Nanomaterial-Based Drug Delivery Carriers for Cancer Therapy. In SpringerBriefs in Applied Sciences and Technology: Nanotheranostics; Springer: Singapore, 2017; p. 55. ISBN 978-981-10-3297-4. [Google Scholar]
- Eldridge, S.; Davis, M. Antineoplastics agents. In Compreensive Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 219–232. ISBN 978-0-08-100601-6. [Google Scholar]
- Guichard, N.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Antineoplastic drugs and their analysis: A state of the art review. Analyst 2017, 142, 2273–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estanqueiro, M.; Amaral, M.H.; Conceição, J.; Sousa Lobo, J.M. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surf. B Biointerfaces 2015, 126, 631–648. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Keck, C.M.; Specht, D.; Brüßler, J. Influence of lipid matrix composition on biopharmaceutical properties of lipid nanoparticles. J. Control. Release 2021, 338, 149–163. [Google Scholar] [CrossRef]
- Müller, R.H.; Olechowski, F.; Köpke, D.; Pyo, S.M. SmartLipids—The Third Generation of Solid Submicron Lipid Particles for Dermal Delivery of Actives. In Nanocosmetics; Springer International Publishing: Cham, Switzerland, 2019; pp. 141–159. [Google Scholar]
- Neupane, Y.R.; Srivastava, M.; Ahmad, N.; Kumar, N.; Bhatnagar, A.; Kohli, K. Lipid based nanocarrier system for the potential oral delivery of decitabine: Formulation design, characterization, ex vivo, and in vivo assessment. Int. J. Pharm. 2014, 477, 601–612. [Google Scholar] [CrossRef]
- Harshita; Barkat, M.A.; Rizwanullah, M.; Beg, S.; Pottoo, F.H.; Siddiqui, S.; Ahmad, F.J. Paclitaxel-loaded Nanolipidic Carriers with Improved Oral Bioavailability and Anticancer Activity against Human Liver Carcinoma. AAPS PharmSciTech 2019, 20, 87. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Xu, Q.; Huang, Z.; Wang, Y.; Shen, Q. Hyaluronic acid-coated cationic nanostructured lipid carriers for oral vincristine sulfate delivery. Drug Dev. Ind. Pharm. 2017, 43, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A.; Kaur, C.D. Cytotoxicity and pharmacokinetics study of nanostructured lipid carriers of mechlorethamine: Preparation, optimization and characterization. Part. Sci. Technol. 2020, 38, 23–33. [Google Scholar] [CrossRef]
- Velmurugan, R.; Selvamuthukumar, S. In vivo antitumor activity of a novel orally bioavailable ifosfamide nanostructured lipid carrier against Dalton’s ascitic lymphoma. J. Pharm. Innov. 2014, 9, 203–211. [Google Scholar] [CrossRef]
- Zhang, H.-W.W.; Dang, Q.; Zhang, Z.-W.W.; Wu, F.-S.S. Development, characterization and evaluation of doxorubicin nanostructured lipid carriers for prostate cancer. J. BUON 2017, 22, 102–111. [Google Scholar]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.R.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural lipids-based NLC containing lidocaine: From pre-formulation to in vivo studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef] [PubMed]
- de Morais Ribeiro, L.N.; Couto, V.M.; Fraceto, L.F.; de Paula, E. Use of nanoparticle concentration as a tool to understand the structural properties of colloids. Sci. Rep. 2018, 8, 982. [Google Scholar] [CrossRef] [Green Version]
- Drugs.com List of Antimetabolites. Available online: https://www.drugs.com/drug-class/antimetabolites.html (accessed on 4 September 2021).
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Rider, B.J. 5: Fluorouracil. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1–5. [Google Scholar] [CrossRef]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi, H.; Khadem, M. Galactosylated nanostructured lipid carriers for delivery of 5-FU to hepatocellular carcinoma. J. Liposome Res. 2012, 22, 224–236. [Google Scholar] [CrossRef]
- El-Subbagh, H.I.; Al-Badr, A.A. Cytarabine. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 34, pp. 37–113. [Google Scholar]
- Sharma, P.; Dube, B.; Sawant, K. Development and evaluation of nanostructured lipid carriers of cytarabine for treatment of meningeal leukemia. J. Nanosci. Nanotechnol. 2011, 11, 6676–6682. [Google Scholar] [CrossRef]
- Mistry, B.; Jones, M.M.; Kubiak, P.; Garcia-Manero, G.; Litzow, M.R.; Mesa, R.A.; Rifkin, R.; Tarassoff, P.; Cortes, J.E. A Phase 1 Study to Assess the Absolute Bioavailability and Safety of An Oral Solution of Decitabine in Subjects with Myelodysplastic Syndromes (MDS). Blood 2011, 118, 3801. [Google Scholar] [CrossRef]
- Callen, J.P.; Kulp-Shorten, C.L. Methotrexate. In Comprehensive Dermatologic Drug Therapy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 156–168.e5. [Google Scholar]
- Inoue, K.; Yuasa, H. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab. Pharmacokinet. 2014, 29, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelbary, G.; Haider, M. In vitro characterization and growth inhibition effect of nanostructured lipid carriers for controlled delivery of methotrexate. Pharm. Dev. Technol. 2013, 18, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Chaves, L.L.; Lima, S.C.; Reis, S. Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. Int. J. Pharm. 2015, 492, 65–72. [Google Scholar] [CrossRef]
- Ong, Y.S.; Bañobre-López, M.; Costa Lima, S.A.; Reis, S. A multifunctional nanomedicine platform for co-delivery of methotrexate and mild hyperthermia towards breast cancer therapy. Mater. Sci. Eng. C 2020, 116, 111255. [Google Scholar] [CrossRef] [PubMed]
- Marzo, I.; Naval, J. Antimitotic drugs in cancer chemotherapy: Promises and pitfalls. Biochem. Pharmacol. 2013, 86, 703–710. [Google Scholar] [CrossRef]
- Zhao, P.; Astruc, D. Docetaxel Nanotechnology in Anticancer Therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef] [PubMed]
- Chand, P.; Kumar, H.; Badduri, N.; Gupta, N.V.; Bettada, V.G.; Madhunapantula, S.R.V.; Kesharwani, S.S.; Dey, S.; Jain, V. Design and evaluation of cabazitaxel loaded NLCs against breast cancer cell lines. Colloids Surf. B Biointerfaces 2021, 199, 111535. [Google Scholar] [CrossRef]
- De Weger, V.A.; Beijnenb, J.H.; Schellensa, J.H.M. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—A review. Anticancer Drugs 2014, 25, 488–494. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Khedekar, P.B.; Bharne, A.P.; Umekar, M.J. Development of self-assembled nanocarriers to enhance antitumor efficacy of docetaxel trihydrate in MDA-MB-231 cell line. Int. J. Biol. Macromol. 2019, 125, 1056–1068. [Google Scholar] [CrossRef]
- Zwain, T.; Alder, J.E.; Sabagh, B.; Shaw, A.; Burrow, A.J.; Singh, K.K. Tailoring functional nanostructured lipid carriers for glioblastoma treatment with enhanced permeability through in-vitro 3D BBB/BBTB models. Mater. Sci. Eng. C 2021, 121, 111774. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, F.; Liu, Z.; Wang, L.; Zhang, N. Tumor specific delivery and therapy by double-targeted nanostructured lipid carriers with anti-VEGFR-2 antibody. Mol. Pharm. 2011, 8, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Tang, B.; Chao, Y.; Xu, H.; Gou, J.; Zhang, Y.; Xu, H.; Tang, X. Cysteine-Functionalized Nanostructured Lipid Carriers for Oral Delivery of Docetaxel: A Permeability and Pharmacokinetic Study. Mol. Pharm. 2015, 12, 2384–2395. [Google Scholar] [CrossRef]
- Li, M.; Pei, J.; Ma, Z.; Fu, J.; Chen, F.; Du, S. Docetaxel-loaded ultrasmall nanostructured lipid carriers for cancer therapy: In vitro and in vivo evaluation. Cancer Chemother. Pharmacol. 2020, 85, 731–739. [Google Scholar] [CrossRef]
- Kim, C.H.; Kang, T.H.; Kim, B.D.; Lee, T.H.; Yoon, H.Y.; Goo, Y.T.; Choi, Y.S.; Kang, M.J.; Choi, Y.W. Enhanced docetaxel delivery using sterically stabilized RIPL peptide-conjugated nanostructured lipid carriers: In vitro and in vivo antitumor efficacy against SKOV3 ovarian cancer cells. Int. J. Pharm. 2020, 583, 119393. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef]
- Panchagnula, R. Pharmaceutical aspects of paclitaxel. Int. J. Pharm. 1998, 172, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Cui, S.; Xue, B.; Tian, J.; Achilefu, S.; Gu, Y. Glucosamine derivative modified nanostructured lipid carriers for targeted tumor delivery. J. Mater. Chem. 2012, 22, 5770–5783. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, M.; Zhang, L.; Feng, L.; Zhang, N. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013, 334, 338–345. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.R.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Sadeghi, H.; Khadivar, K. Development and optimization of transferrin-conjugated nanostructured lipid carriers for brain delivery of paclitaxel using Box–Behnken design. Pharm. Dev. Technol. 2017, 22, 370–382. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Y.; Zhu, Z.; Wang, H.; Han, C.; Yang, X.; Pan, W. A systematic in vitro investigation on poly-arginine modified nanostructured lipid carrier: Pharmaceutical characteristics, cellular uptake, mechanisms and cytotoxicity. Asian J. Pharm. Sci. 2017, 12, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ucar, E.; Teksoz, S.; Ichedef, C.; Kilcar, A.Y.; Medine, E.I.; Ari, K.; Parlak, Y.; Sayit Bilgin, B.E.; Unak, P. Synthesis, characterization and radiolabeling of folic acid modified nanostructured lipid carriers as a contrast agent and drug delivery system. Appl. Radiat. Isot. 2017, 119, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.H.; Na, Y.G.; Huh, H.W.; Hwang, S.J.; Kim, M.S.; Kim, M.; Lee, H.K.; Cho, C.W. The delivery strategy of paclitaxel nanostructured lipid carrier coated with platelet membrane. Cancers 2019, 11, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huitema, A.; Smits, K.D.; Matho, R.A.A.; Schellens, J.H.M. The clinical pharmacology of alkylating agents in high-dose chemotherapy. Anti-Cancer Drugs 2000, 11, 515–533. [Google Scholar] [CrossRef]
- Fu, D.; Calvo, J.A.; Samson, L.D. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat. Rev. Cancer 2012, 12, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Ralhan, R.; Kaur, J. Alkylating agents and cancer therapy. Expert Opin. Ther. Pat. 2007, 17, 1061–1075. [Google Scholar] [CrossRef]
- Siddik, Z.H. Mechanisms of action of cancer chemotherapeutic agents: DNA-interactive alkylating agents and antitumour platinum-based drugs. Cancer Handb. 2005. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Kitange, G.J.; James, C.D.; Plummer, R.; Calvert, H.; Weller, M.; Wick, W. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin. Cancer Res. 2008, 14, 2900–2908. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liu, F.; Jia, E.; Jia, L.; Zhang, Y. Folate-modified, cisplatin-loaded lipid carriers for cervical cancer chemotherapy. Drug Deliv. 2016, 23, 1393–1397. [Google Scholar] [CrossRef]
- Al-Badr, A.A.; Alodhaib, M.M. Dacarbazine. Profiles Drug Subst. Excipients Relat. Methodol. 2016, 41, 323–377. [Google Scholar] [CrossRef]
- Almoussalam, M.; Zhu, H. Encapsulation of cancer therapeutic agent dacarbazine using nanostructured lipid carrier. J. Vis. Exp. 2016, 2016, 53760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velmurugan, R.; Selvamuthukumar, S. Development and optimization of ifosfamide nanostructured lipid carriers for oral delivery using response surface methodology. Appl. Nanosci. 2016, 6, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, R.; Nair, K.G.S. Toxicity evaluation of ifosfamide nanostructured lipid carriers designed for oral delivery in Wistar albino rats. Drug Invent. Today 2018, 10, 192–196. [Google Scholar]
- Li, R.; Yin, X.; Zhang, J.; Tang, T.; Fang, X.; Zhang, L.; Xu, W.; Zhao, J.; Han, D. Improving the solubility of temozolomide by cosolvent and its correlation with the Jouyban-Acree and CNIBS/R-K models. J. Chem. Thermodyn. 2019, 139, 105875. [Google Scholar] [CrossRef]
- Khan, A.; Imam, S.S.; Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Brain Targeting of Temozolomide via the Intranasal Route Using Lipid-Based Nanoparticles: Brain Pharmacokinetic and Scintigraphic Analyses. Mol. Pharm. 2016, 13, 3773–3782. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lai, X.; Song, S.; Zhu, X.; Zhu, J. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv. 2016, 23, 1369–1373. [Google Scholar] [CrossRef]
- Song, S.; Mao, G.; Du, J.; Zhu, X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016, 23, 1404–1408. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Drugs from nature: Past achievements, future prospects. Adv. Phytomed. 2002, 1, 23–37. [Google Scholar] [CrossRef]
- Bachur, N.R. Anthracyclines. Encycl. Cancer 2002, 57–61. [Google Scholar] [CrossRef]
- Vigevani, A.; Williamson, M.J. Doxorubicin. Anal. Profiles Drug Subst. Excip. 1981, 9, 245–274. [Google Scholar] [CrossRef]
- Agrawal, K. Doxorubicin. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1–5. [Google Scholar] [CrossRef]
- Mussi, S.V.; Sawant, R.; Perche, F.; Oliveira, M.C.; Azevedo, R.B.; Ferreira, L.A.M.; Torchilin, V.P. Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm. Res. 2014, 31, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Mussi, S.V.; Parekh, G.; Pattekari, P.; Levchenko, T.; Lvov, Y.; Ferreira, L.A.M.; Torchilin, V.P. Improved pharmacokinetics and enhanced tumor growth inhibition using a nanostructured lipid carrier loaded with doxorubicin and modified with a layer-by-layer polyelectrolyte coating. Int. J. Pharm. 2015, 495, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, R.S.; Silva, J.O.; Monteiro, L.O.F.; Leite, E.A.; Cassali, G.D.; Rubello, D.; Cardoso, V.N.; Ferreira, L.A.M.; Oliveira, M.C.; de Barros, A.L.B. Doxorubicin-loaded nanocarriers: A comparative study of liposome and nanostructured lipid carrier as alternatives for cancer therapy. Biomed. Pharmacother. 2016, 84, 252–257. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Mussi, S.V.; Lopes, S.C.A.; Leite, E.A.; Cassali, G.D.; Cardoso, V.N.; Townsend, D.M.; Colletti, P.M.; Ferreira, L.A.M.; et al. Nanostructured Lipid Carrier Co-loaded with Doxorubicin and Docosahexaenoic Acid as a Theranostic Agent: Evaluation of Biodistribution and Antitumor Activity in Experimental Model. Mol. Imaging Biol. 2018, 20, 437–447. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Seabra, H.A.; Oliveira, M.S.; Carregal, V.M.; Vilela, J.M.C.; Andrade, M.S.; Townsend, D.M.; Colletti, P.M.; Leite, E.A.; et al. α- Tocopherol succinate loaded nano-structed lipid carriers improves antitumor activity of doxorubicin in breast cancer models in vivo. Biomed. Pharmacother. 2018, 103, 1348–1354. [Google Scholar] [CrossRef]
- Li, W.; Fu, J.; Ding, Y.; Liu, D.; Jia, N.; Chen, D.; Hu, H. Low density lipoprotein-inspired nanostructured lipid nanoparticles containing pro-doxorubicin to enhance tumor-targeted therapeutic efficiency. Acta Biomater. 2019, 96, 456–467. [Google Scholar] [CrossRef]
- Lages, E.B.; Fernandes, R.S.; Silva, J.D.O.; de Souza, Â.M.; Cassali, G.D.; de Barros, A.L.B.; Miranda Ferreira, L.A. Co-delivery of doxorubicin, docosahexaenoic acid, and α-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2020, 132, 110876. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Li, D.; Chen, Y.; Sun, J.; Kong, F. Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int. J. Nanomed. 2014, 9, 4107–4116. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Jia, M.; Wei, G.; Tan, T.; Fu, Y.; Gao, H.; Sun, X.; Zhang, Q.; Gong, T.; Zhang, Z. Inducing Optimal Antitumor Immune Response through Coadministering iRGD with Pirarubicin Loaded Nanostructured Lipid Carriers for Breast Cancer Therapy. Mol. Pharm. 2017, 14, 296–309. [Google Scholar] [CrossRef]
- Fujita, K.I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Negi, L.M.; Talegaonkar, S.; Jaggi, M.; Verma, A.K.; Verma, R.; Dobhal, S.; Kumar, V. Surface engineered nanostructured lipid carriers for targeting MDR tumor: Part II. In vivo biodistribution, pharmacodynamic and hematological toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Negi, L.M.; Talegaonkar, S.; Jaggi, M.; Verma, A.K.; Verma, R.; Dobhal, S.; Kumar, V. Surface engineered nanostructured lipid carriers for targeting MDR tumor: Part I. Synthesis, characterization and in vitro investigation. Colloids Surf. B Biointerfaces 2014, 123, 600–609. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, J.; Zhang, Y.; Shen, Q.; Pan, W. Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur. J. Pharm. Sci. 2011, 43, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Pei, L.; Liu, N.; Li, J.; Li, Z.; Zhang, S. Etoposide-loaded nanostructured lipid carriers for gastric cancer therapy. Drug Deliv. 2016, 23, 1379–1382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lu, C.; Zhang, X.; Li, J.; Jiang, H. Targeted delivery of etoposide to cancer cells by folate-modified nanostructured lipid drug delivery system. Drug Deliv. 2016, 23, 1838–1845. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, B.G.; Vit, F.F.; Carvalho, H.F.; Han, S.W.; Torre, L.G. de la Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J. Mater. Chem. B 2021, 9, 1208–1237. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef] [Green Version]
- Rawal, S.; Bora, V.; Patel, B.; Patel, M. Surface-engineered nanostructured lipid carrier systems for synergistic combination oncotherapy of non-small cell lung cancer. Drug Deliv. Transl. Res. 2020, 11, 2030–2051. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hao, J.; Li, B.; Li, M.; Xiuwen, W. Lung cancer combination therapy: Co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2016, 23, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, Y.; Li, M.; Zhao, X.; Wang, D.; Liu, J.; Zhou, F.; Zhang, J. Nanostructured lipid carrier co-delivering paclitaxel and doxorubicin restrains the proliferation and promotes apoptosis of glioma stem cells via regulating PI3K/Akt/mTOR signaling. Nanotechnology 2021, 32, 225101. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Wu, H.; Gao, Y.; Li, W.; Zou, D.; Dong, C. Doxorubicin- and cisplatin-loaded nanostructured lipid carriers for breast cancer combination chemotherapy. Drug Dev. Ind. Pharm. 2016, 42, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, W.; Qu, H.; Han, D.; Zheng, J.; Sun, G. Targeted delivery of doxorubicin and vincristine to lymph cancer: Evaluation of novel nanostructured lipid carriers in vitro and in vivo. Drug Deliv. 2016, 23, 1374–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, S.; Qiu, L.; Zhang, G.; Zhou, H.; Han, Y. Lymph cancer chemotherapy: Delivery of doxorubicin-gemcitabine prodrug and vincristine by nanostructured lipid carriers. Int. J. Nanomed. 2017, 12, 1565–1576. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2016, 23, 1364–1368. [Google Scholar] [CrossRef] [Green Version]
- Borges, G.S.M.; de Oliveira Silva, J.; Fernandes, R.S.; de Souza, Â.M.; Cassali, G.D.; Yoshida, M.I.; Leite, E.A.; de Barros, A.L.B.; Ferreira, L.A.M. Sclareol is a potent enhancer of doxorubicin: Evaluation of the free combination and co-loaded nanostructured lipid carriers against breast cancer. Life Sci. 2019, 232, 116678. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Q.; Liu, Y. Lung cancer combination therapy: Doxorubicin and β-elemene co-loaded, pH-sensitive nanostructured lipid carriers. Drug Des. Devel. Ther. 2019, 13, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jia, X.; Niu, H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018, 13, 4107–4119. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ju, Z.; Dong, S. Cisplatin and paclitaxel co-delivered by folate-decorated lipid carriers for the treatment of head and neck cancer. Drug Deliv. 2017, 24, 792–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Zhang, Y.; Wu, Z.; Zhang, L.; He, D.; Li, X.; Wang, Z. Synergistic combination therapy of lung cancer: Cetuximab functionalized nanostructured lipid carriers for the co-delivery of paclitaxel and 5-Demethylnobiletin. Biomed. Pharmacother. 2019, 118, 109225. [Google Scholar] [CrossRef]
- Zheng, J.; Song, M.; Dong, P.; Qiu, P.; Guo, S.; Zhong, Z.; Li, S.; Ho, C.T.; Xiao, H. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol. Nutr. Food Res. 2013, 57, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.-Y.Y.; Zhou, M.; Chen, Y.W.; Chen, M.M.; Shen, F.; Xu, L.-M.M. Engineering of lipid prodrug-based, hyaluronic acid-decorated nanostructured lipid carriers platform for 5-fluorouracil and cisplatin combination gastric cancer therapy. Int. J. Nanomed. 2015, 10, 3911–3920. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Fan, Y.; Lv, S.; Xiao, B.; Ye, M.; Zhu, X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: A comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016, 23, 2720–2725. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, L.; Jiang, M.; Li, D.; Jin, L. Nanostructured lipid carriers enhance the bioavailability and brain cancer inhibitory efficacy of curcumin both in vitro and in vivo. Drug Deliv. 2016, 23, 1383–1392. [Google Scholar] [CrossRef]
- Varshosaz, J.; Jandaghian, S.; Mirian, M.; Sajjadi, S.E. Co-delivery of rituximab targeted curcumin and imatinib nanostructured lipid carriers in non-Hodgkin lymphoma cells. J. Liposome Res. 2021, 31, 64–78. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, B.; Patel, M.M. Fabrication, optimisation and in vitro evaluation of docetaxel and curcumin Co-loaded nanostructured lipid carriers for improved antitumor activity against non-small cell lung carcinoma. J. Microencapsul. 2020, 37, 543–556. [Google Scholar] [CrossRef]
- Xu, M.; Li, G.; Zhang, H.; Chen, X.; Li, Y.; Yao, Q.; Xie, M. Sequential delivery of dual drugs with nanostructured lipid carriers for improving synergistic tumor treatment effect. Drug Deliv. 2020, 27, 983–995. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.S.C.; Gouvêa, A.L.; de Moura, L.D.; Paterno, L.G.; de Souza, P.E.N.; Bastos, A.P.; Damasceno, E.A.M.; Veiga-Souza, F.H.; de Azevedo, R.B.; Báo, S.N. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J. Nanobiotechnol. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginato, E.; Mroz, P.; Chung, H.; Kawakubo, M.; Wolf, P.; Hamblin, M.R. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen. Br. J. Cancer 2013, 109, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, Sinoporphyrin sodium. Theranostics 2015, 5, 772–786. [Google Scholar] [CrossRef] [Green Version]
- Eskiizmir, G.; Ermertcan, A.T.; Yapici, K. Chapter 17—Nanomaterials: Promising structures for the management of oral cancer. Nanostruct. Oral Med. 2017, 511–544. [Google Scholar] [CrossRef]
- Spikes, J.D. The Historical Development of Ideas on Applications of Photosensitized Reactions in the Health Sciences. In Primary Photo-Processes in Biology and Medicine; Springer US: Boston, MA, USA, 1985; pp. 209–227. [Google Scholar]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation Therapy for the Treatment of Malignant Tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- Qidwai, A.; Khan, S.; Md, S.; Fazil, M.; Baboota, S.; Narang, J.K.; Ali, J. Nanostructured lipid carrier in photodynamic therapy for the treatment of basal-cell carcinoma. Drug Deliv. 2016, 23, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhang, J.; de Sousa Júnior, W.T.; da Silva, V.C.M.; Rodrigues, M.C.; Morais, J.A.V.; Jiang, C.; Longo, J.P.F.; Azevedo, R.B.; Muehlmann, L.A. A xanthene derivative, free or associated to nanoparticles, as a new potential agent for anticancer photodynamic therapy. J. Biomater. Sci. Polym. Ed. 2020, 31, 1977–1993. [Google Scholar] [CrossRef]
- Oshiro-Junior, J.A.; Sato, M.R.; Boni, F.I.; Santos, K.L.M.; de Oliveira, K.T.; de Freitas, L.M.; Fontana, C.R.; Nicholas, D.; McHale, A.; Callan, J.F.; et al. Phthalocyanine-loaded nanostructured lipid carriers functionalized with folic acid for photodynamic therapy. Mater. Sci. Eng. C 2020, 108, 110462. [Google Scholar] [CrossRef]
- Chen, G.; Wang, K.; Zhou, Y.; Ding, L.; Ullah, A.; Hu, Q.; Sun, M.; Oupický, D. Oral Nanostructured Lipid Carriers Loaded with Near-Infrared Dye for Image-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 25087–25095. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.D.P.; Dipieri, L.V.; Rossetti, F.C.; Marchetti, J.M.; Bentley, M.V.L.B.; de S. Nunes, R.; Sarmento, V.H.V..; Valerio, M.E.G.; Júnior, R.J.J.; Montalvã, M.M.; et al. Skin permeation, biocompatibility and antitumor effect of chloroaluminum phthalocyanine associated to oleic acid in lipid nanoparticles. Photodiagn. Photodyn. Ther. 2018, 24, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Michy, T.; Massias, T.; Bernard, C.; Vanwonterghem, L.; Henry, M.; Guidetti, M.; Royal, G.; Coll, J.-L.; Texier, I.; Josserand, V.; et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers 2019, 11, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Zhao, J.; Hu, H.; Yan, Y.; Hu, X.; Zhou, K.; Xiao, S.; Zhang, Y.; Feng, N. Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6. Int. J. Pharm. 2019, 569, 118595. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues da Silva, G.H.; Moura, L.D.d.; Carvalho, F.V.d.; Geronimo, G.; Mendonça, T.C.; Lima, F.F.d.; de Paula, E. Antineoplastics Encapsulated in Nanostructured Lipid Carriers. Molecules 2021, 26, 6929. https://doi.org/10.3390/molecules26226929
Rodrigues da Silva GH, Moura LDd, Carvalho FVd, Geronimo G, Mendonça TC, Lima FFd, de Paula E. Antineoplastics Encapsulated in Nanostructured Lipid Carriers. Molecules. 2021; 26(22):6929. https://doi.org/10.3390/molecules26226929
Chicago/Turabian StyleRodrigues da Silva, Gustavo Henrique, Ludmilla David de Moura, Fabíola Vieira de Carvalho, Gabriela Geronimo, Talita Cesarim Mendonça, Fernando Freitas de Lima, and Eneida de Paula. 2021. "Antineoplastics Encapsulated in Nanostructured Lipid Carriers" Molecules 26, no. 22: 6929. https://doi.org/10.3390/molecules26226929
APA StyleRodrigues da Silva, G. H., Moura, L. D. d., Carvalho, F. V. d., Geronimo, G., Mendonça, T. C., Lima, F. F. d., & de Paula, E. (2021). Antineoplastics Encapsulated in Nanostructured Lipid Carriers. Molecules, 26(22), 6929. https://doi.org/10.3390/molecules26226929







