The Phosphorylated Form of the Histone H2AX (γH2AX) in the Brain from Embryonic Life to Old Age
Abstract
1. Introduction
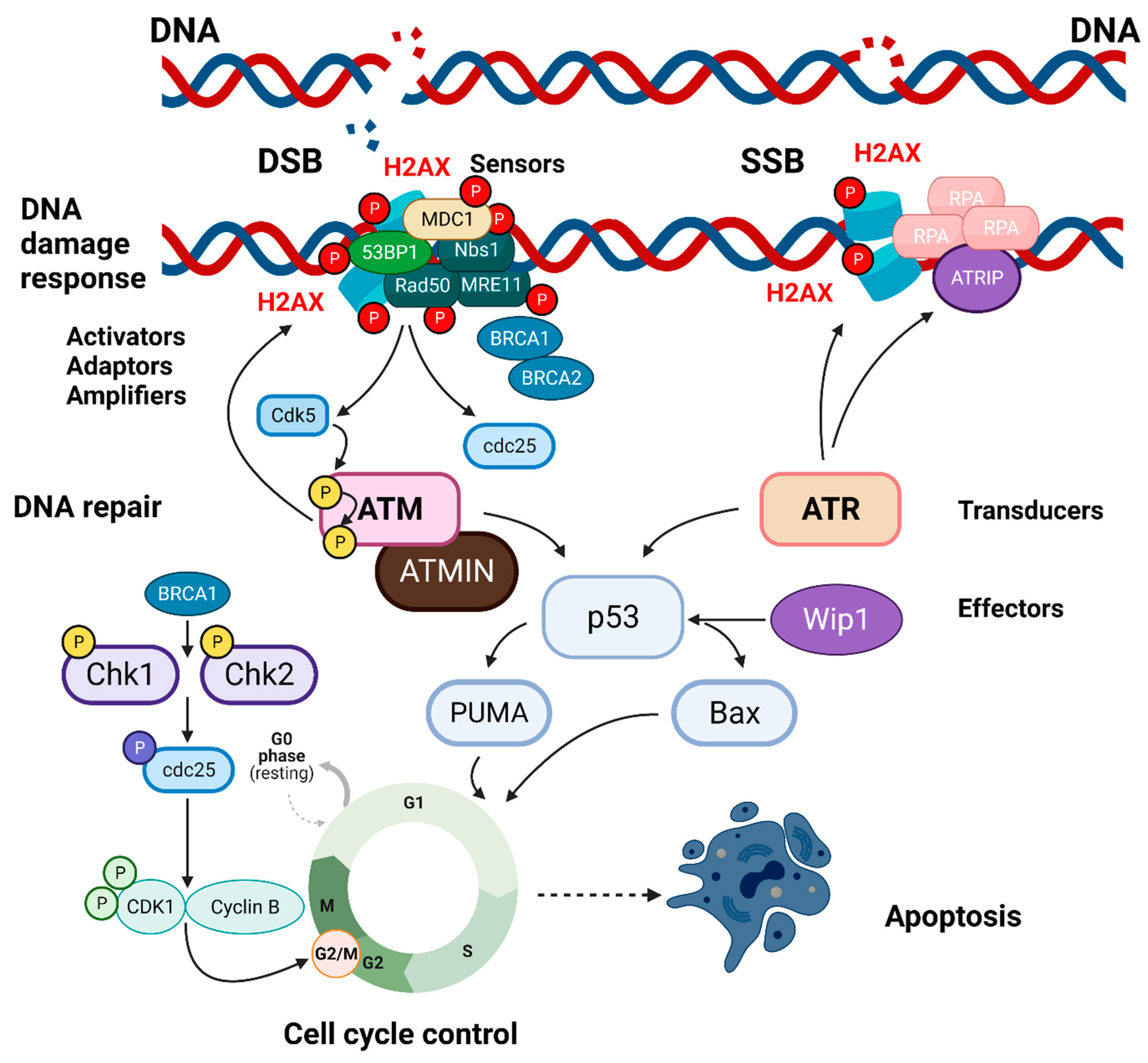
1.1. The Histone Families, H2A, and the DNA Damage Response
1.2. H2AX, Cell Cycle Checkpoints, and Genomic Instability
1.3. H2AX and Cell Growth
1.4. H2AX and Mitosis
1.5. H2AX and Embryogenesis
1.6. H2AX and Naturally Occurring DSBs in Gametes and Immune Cells
1.6.1. H2AX and Gametogenesis
1.6.2. H2AX and Lymphocyte Development
1.7. H2AX and Mitochondrial Homeostasis
1.8. H2AX and Apoptosis
1.9. H2AX and Hereditary Syndromes
1.10. H2AX and Tumors
2. H2AX in the Normal Brain
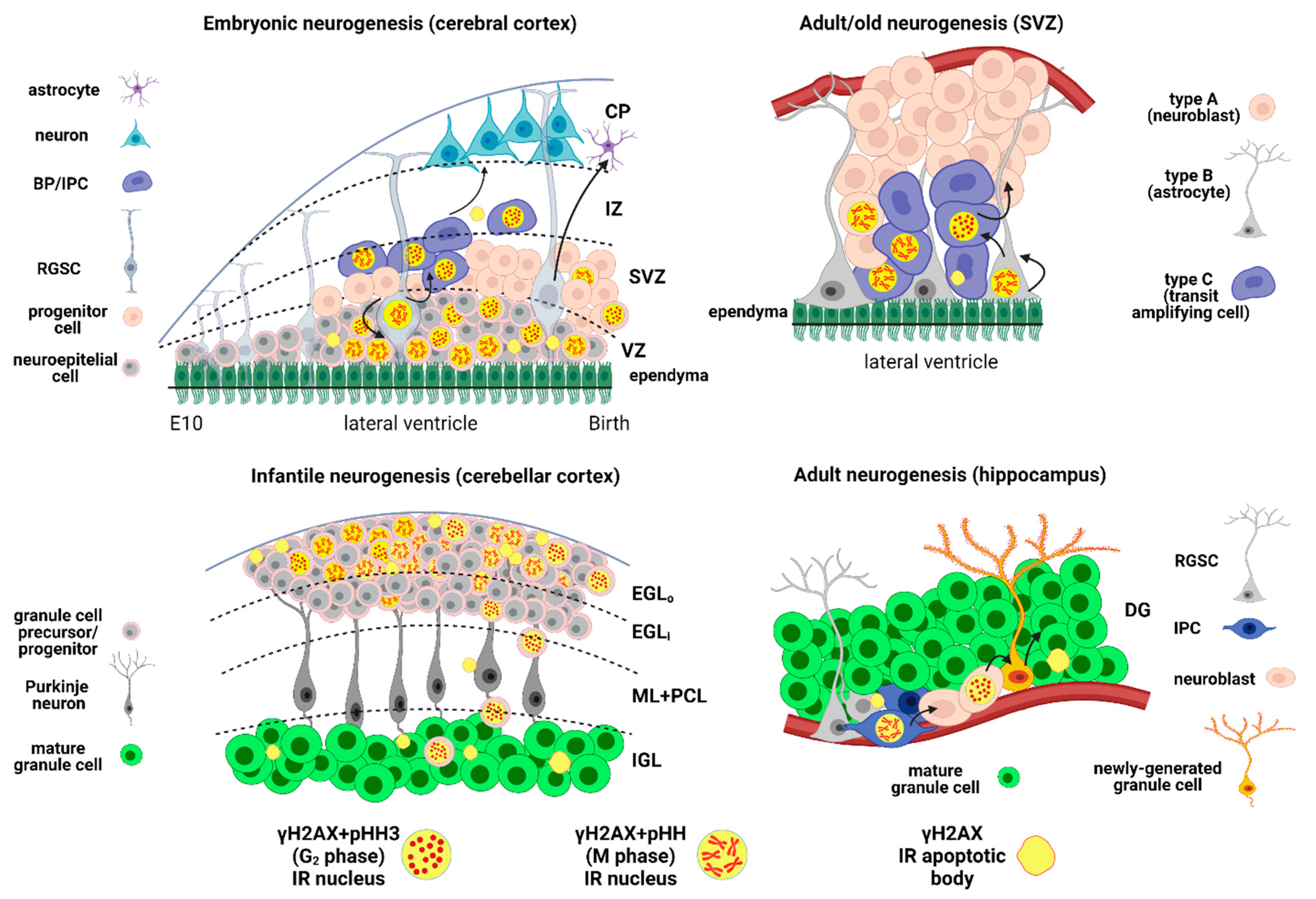
2.1. H2AX in the Developing Brain
2.1.1. γH2AX in Embryonic Neurogenesis
2.1.2. γH2AX in Infantile Neurogenesis
2.1.2.1. SVZ/RMS/OB
2.1.2.2. Cerebellum
2.2. γH2AX in the Adult Brain
2.2.1. γH2AX in Adult Neurogenesis
Classical Neurogenetic Areas
Others
2.2.2. Cerebral Cortex
2.3. γH2AX in the Aging Brain
2.3.1. Neurogenic Areas
SVZ/RMS/OB
Hippocampus
2.3.2. Cerebral Cortex
3. γH2AX in the Experimentally Damaged Brain and Neuropathology
3.1. γH2AX and DNA Damaging Agents
3.1.1. Ionizing Radiations
3.1.2. Neurotoxic Substances
3.1.3. Oxidative Stress
3.1.4. Telomere Dysfunction
3.1.5. Injury
3.1.6. Neurologic Disorders
Alzheimer’s Disease (AD)
Huntington’s Disease (HD)
Parkinson’s Disease (PD)
Fragile X Syndrome
Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD)
Others
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- West, M.H.; Bonner, W.M. Histone 2A, a heteromorphous family of eight protein species. Biochemistry 1980, 19, 3238–3245. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Fernandez-Capetillo, O.; Lee, A.; Nussenzweig, M.; Nussenzweig, A. H2AX: The histone guardian of the genome. DNA Repair 2004, 3, 959–967. [Google Scholar] [CrossRef]
- Turinetto, V.; Giachino, C. Multiple facets of histone variant H2AX: A DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015, 43, 2489–2498. [Google Scholar] [CrossRef]
- Zhou, K.; Gaullier, G. Nucleosome structure and dynamics are coming of age. Nat. Struct. Mol. Biol. 2019, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Ausió, J.; Abbott, D.W. The many tales of a tail: Carboxyl-terminal tail heterogeneity specializes histone H2A variants for defined chromatin function. Biochemistry 2002, 41, 5945–5949. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.-X. γ-H2AX—A Novel Biomarker for DNA Double-strand Breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Bártová, E.; Krejcí, J.; Harnicarová, A.; Galiová, G.; Kozubek, S. Histone modifications and nuclear architecture: A review. J. Histochem. Cytochem. 2008, 56, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Filomeni, E.; François, M.; Collins, S.R.; Cooper, T.; Glatz, R.V.; Taylor, P.W.; Fenech, M.; Leifert, W.R. Exposure of insect cells to ionising radiation in vivo induces persistent phosphorylation of a H2AX homologue (H2AvB). Mutagenesis 2013, 28, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.A.; Lowndes, N.F.; Jackson, S.P. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 2000, 408, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Casellas, R.; Reina-San-Martin, B.; Chen, H.T.; Difilippantonio, M.J.; Wilson, P.C.; Hanitsch, L.; Celeste, A.; Muramatsuk, M.; Pilch, D.R.; et al. AID is required to initiate Nbs1/gamma-H2AX focus formation and mutations at sites of class switching. Nature 2001, 414, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.; Petersen, S.; Romanienko, P.J.; Fernandez-Capetillo, O.; Chen, H.T.; Sedelnikova, O.A.; Reina-San-Martin, B.; Coppola, V.; Meffre, E.; Difilippantonio, M.J.; et al. Genomic instability in mice lacking histone H2AX. Science 2002, 296, 922–927. [Google Scholar] [CrossRef]
- Bassing, C.H.; Chua, K.F.; Sekiguchi, J.; Suh, H.; Whitlow, S.R.; Fleming, J.C.; Monroe, B.C.; Ciccone, D.N.; Yan, C.; Vlasakova, K.; et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 2002, 99, 8173–8178. [Google Scholar] [CrossRef] [PubMed]
- Reina-San-Martin, B.; Difilippantonio, S.; Hanitsch, L.; Masilamani, R.F.; Nussenzweig, A.; Nussenzweig, M.C. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J. Exp. Med. 2003, 197, 1767–1778. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. GammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef]
- Niida, H.; Nakanishi, M. DNA damage checkpoints in mammals. Mutagenesis 2006, 21, 3–9. [Google Scholar] [CrossRef]
- Lamarche, B.J.; Orazio, N.I.; Weitzman, M.D. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 2010, 584, 3682–3695. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Shao, Z.; Li, F.; Niu, L.; Shi, Y.; Teng, M.; Li, X. Structural basis of γH2AX recognition by human PTIP BRCT5-BRCT6 domains in the DNA damage response pathway. FEBS Lett. 2011, 585, 3874–3879. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, J.; Tauchi, H.; Sakamoto, S.; Nakamura, A.; Morishima, K.; Matsuura, S.; Kobayashi, T.; Tamai, K.; Tanimoto, K.; Komatsu, K. NBS1 localizes to gamma-H2AX foci through interaction with the FHA/BRCT domain. Curr. Biol. 2002, 12, 1846–1851. [Google Scholar] [CrossRef]
- Zhang, P.; Furukawa, K.; Opresko, P.L.; Xu, X.; Bohr, V.A.; Mattson, M.P. TRF2 dysfunction elicits DNA damage responses associated with senescence in proliferating neural cells and differentiation of neurons. J. Neurochem. 2006, 97, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Yang, Q.; Mao, Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat. Cell Biol. 2009, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Kessinger, C.; Kobayashi, J.; Chen, B.P.; Chen, D.J.; Chatterjee, A.; Burma, S. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair 2006, 5, 575–590. [Google Scholar] [CrossRef]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 920161. [Google Scholar] [CrossRef]
- Ward, I.M.; Wu, X.; Chen, J. Threonine 68 of Chk2 is phosphorylated at sites of DNA strand breaks. J. Biol. Chem. 2001, 276, 47755–47758. [Google Scholar] [CrossRef]
- Barnum, K.J.; O’Connell, M.J. Cell cycle regulation by checkpoints. Meth. Mol. Biol. 2014, 1170, 29–40. [Google Scholar]
- Killander, D.; Zetterberg, A. A quantitative cytochemical investigation of the relationship between cell mass and initiation of DNA synthesis in mouse fibroblasts in vitro. Exp. Cell. Res. 1965, 40, 12–20. [Google Scholar] [CrossRef]
- Killander, D.; Zetterberg, A. Quantitative cytochemical studies on interphase growth. i. determination of DNA, RNA, and mass content of age determined mouse fibroblasts in vitro and of intercellular variation in generation time. Exp. Cell Res. 1965, 38, 272–284. [Google Scholar] [CrossRef]
- Redon, C.; Pilch, D.R.; Rogakou, E.P.; Orr, A.H.; Lowndes, N.F.; Bonner, W.M. Yeast histone 2A serine 129 is essential for the efficient repair of checkpoint-blind DNA damage. EMBO Rep. 2003, 4, 678–684. [Google Scholar] [CrossRef]
- Fernandez-Capetillo, O.; Chen, H.T.; Celeste, A.; Ward, I.; Romanienko, P.J.; Morales, J.C.; Naka, K.; Xia, Z.; Camerini-Otero, R.D.; Motoyama, N.; et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 2002, 4, 993–997. [Google Scholar] [CrossRef] [PubMed]
- DiTullio, R.A., Jr.; Mochan, T.A.; Venere, M.; Bartkova, J.; Sehested, M.; Bartek, J.; Halazonetis, T.D. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat. Cell Biol. 2002, 4, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Matsuoka, S.; Carpenter, P.B.; Elledge, S.J. 53BP1, a mediator of the DNA damage checkpoint. Science 2002, 298, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Van Attikum, H.; Gasser, S.M. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009, 19, 207–217. [Google Scholar] [CrossRef]
- Martínez, P.; Blasco, M.A. Telomere-driven diseases and telomere-targeting therapies. J. Cell Biol. 2017, 216, 875–887. [Google Scholar] [CrossRef]
- D’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef]
- Fernandez-Capetillo, O.; Liebe, B.; Scherthan, H.; Nussenzweig, A. H2AX regulates meiotic telomere clustering. J. Cell Biol. 2003, 163, 15–20. [Google Scholar] [CrossRef]
- McManus, K.J.; Hendzel, M.J. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol. Biol. Cell 2005, 16, 5013–5025. [Google Scholar] [CrossRef]
- Ichijima, Y.; Sakasai, R.; Okita, N.; Asahina, K.; Mizutani, S.; Teraoka, H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem. Biophys. Res. Commun. 2005, 336, 807–812. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Huang, Y.-C.; Xu, Q.-Z.; Zhou, L.-J.; Shang, Z.-F.; Huang, B.; Wang, Y.; Liu, X.-D.; Wu, D.-C.; Zhou, P.-K. DNA-PKcs plays a dominant role in the regulation of H2AX phosphorylation in response to DNA damage and cell cycle progression. BMC Mol. Biol. 2010, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.Z.; Li, B.; Huang, B.; Wang, Y.; Liu, X.D.; Guan, H.; Zhang, S.M.; Tang, Y.; Rang, W.Q.; Zhou, P.K. γH2AX foci formation in the absence of DNA damage: Mitotic H2AX phosphorylation is mediated by the DNA-PKcs/CHK2 pathway. FEBS Lett. 2013, 587, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, Y.; Argaman, L.; Kornowski, M.; Roniger, M.; Goldberg, M. Interplay between the DNA damage proteins MDC1 and ATM in the regulation of the spindle assembly checkpoint. J. Biol. Chem. 2014, 289, 8182–8193. [Google Scholar] [CrossRef] [PubMed]
- Zineldeen, D.H.; Shafik, N.M.; Li, S.F. Alternative Chk1-independent S/M checkpoint in somatic cells that prevents premature mitotic entry. Med. Oncol. 2017, 34, 70. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Goshima, T.; Matsuo, H.; Johmura, Y.; Haruta, M.; Murata, K.; Tanaka, H.; Ikawa, M.; Nakanishi, K.; Nakanishi, M. Essential role of autoactivation circuitry on Aurora B-mediated H2AX-pS121 in mitosis. Nat. Commun. 2016, 7, 12059. [Google Scholar] [CrossRef] [PubMed]
- Hamatani, T.; Carter, M.G.; Sharov, A.A.; Ko, M.S. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell 2004, 6, 117–131. [Google Scholar] [CrossRef]
- Kafer, G.R.; Lehnert, S.A.; Pantaleon, M.; Kaye, P.L.; Moser, R.J. Expression of genes coding for histone variants and histone-associated proteins in pluripotent stem cells and mouse preimplantation embryos. Gene Expr. Patterns 2010, 10, 299–305. [Google Scholar] [CrossRef]
- Nashun, B.; Yukawa, M.; Liu, H.; Akiyama, T.; Aoki, F. Changes in the nuclear deposition of histone H2A variants during pre-implantation development in mice. Development 2010, 137, 3785–3794. [Google Scholar] [CrossRef]
- Ziegler-Birling, C.; Helmrich, A.; Tora, L.; Torres-Padilla, M.E. Distribution of p53 binding protein 1 (53BP1) and phosphorylated H2A.X during mouse preimplantation development in the absence of DNA damage. Int. J. Dev. Biol. 2009, 53, 1003–1011. [Google Scholar] [CrossRef]
- Banáth, J.P.; Bañuelos, C.A.; Klokov, D.; MacPhail, S.M.; Lansdorp, P.M.; Olive, P.L. Explanation for excessive DNA single-strand breaks and endogenous repair foci in pluripotent mouse embryonic stem cells. Exp. Cell Res. 2009, 315, 1505–1520. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Orlando, L.; Sanchez-Ripoll, Y.; Kumpfmueller, B.; Storm, M.P.; Porcedda, P.; Minieri, V.; Saviozzi, S.; Accomasso, L.; Cibrario Rocchietti, E.; et al. High basal γH2AX levels sustain self-renewal of mouse embryonic and induced pluripotent stem cells. Stem Cells 2012, 30, 1414–1423. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Wen, D.; Tseng, Z.; Tahmasian, M.; Zhong, M.; Rafii, S.; Stadtfeld, M.; Hochedlinger, K.; Xiao, A. Histone variant H2A.X deposition pattern serves as a functional epigenetic mark for distinguishing the developmental potentials of iPSCs. Cell Stem Cell 2014, 15, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Shechter, D.; Chitta, R.K.; Xiao, A.; Shabanowitz, J.; Hunt, D.F.; Allis, C.D. A distinct H2A.X isoform is enriched in Xenopus laevis eggs and early embryos and is phosphorylated in the absence of a checkpoint. Proc. Natl. Acad. Sci. USA 2009, 106, 749–754. [Google Scholar] [CrossRef]
- Bohrer, R.C.; Che, L.; Gonçalves, P.B.; Duggavathi, R.; Bordignon, V. Phosphorylated histone H2A.x in porcine embryos produced by IVF and somatic cell nuclear transfer. Reproduction 2013, 146, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.L.; Williams, E.J.; Hawes, S.; Saffery, R. Characterisation of histone variant distribution in human embryonic stem cells by transfection of in vitro transcribed mRNA. Mol. Reprod. Dev. 2009, 76, 1128–1142. [Google Scholar] [CrossRef]
- Chuykin, I.A.; Lianguzova, M.S.; Pospelova, T.V.; Pospelov, V.A. Activation of DNA damage response signaling in mouse embryonic stem cells. Cell Cycle 2008, 7, 2922–2928. [Google Scholar] [CrossRef]
- Scully, R.; Xie, A. Double-strand break repair functions of histone H2AX. Mutat. Res. 2013, 750, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Keeney, S. Spo11 and the Formation of DNA Double-Strand Breaks in Meiosis. Genome Dyn. Stab. 2008, 2, 81–123. [Google Scholar]
- Mahadevaiah, S.K.; Turner, J.M.; Baudat, F.; Rogakou, E.P.; de Boer, P.; Blanco-Rodríguez, J.; Jasin, M.; Keeney, S.; Bonner, W.M.; Burgoyne, P.S. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 2001, 27, 271–276. [Google Scholar] [CrossRef]
- Fernandez-Capetillo, O.; Mahadevaiah, S.K.; Celeste, A.; Romanienko, P.J.; Camerini-Otero, R.D.; Bonner, W.M.; Manova, K.; Burgoyne, P.; Nussenzweig, A. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev. Cell 2003, 4, 497–508. [Google Scholar] [CrossRef]
- Roig, I.; Liebe, B.; Egozcue, J.; Cabero, L.; Garcia, M.; Scherthan, H. Female-specific features of recombinational double-stranded DNA repair in relation to synapsis and telomere dynamics in human oocytes. Chromosoma 2004, 113, 22–33. [Google Scholar] [CrossRef]
- Yermanos, A.; Dounas, A.; Greiff, V.; Stadler, T.; Oxenius, A.; Reddy, S.T. Inter- and intraspecies comparison of phylogenetic fingerprints and sequence diversity of immunoglobulin variable genes. Immunogenetics 2020, 72, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Fugmann, S.D.; Lee, A.I.; Shockett, P.E.; Villey, I.J.; Schatz, D.G. The RAG proteins and V(D)J recombination: Complexes, ends, and transposition. Annu. Rev. Immunol. 2000, 18, 495–527. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.; Difilippantonio, S.; Difilippantonio, M.J.; Fernandez-Capetillo, O.; Pilch, D.R.; Sedelnikova, O.A.; Eckhaus, M.; Ried, T.; Bonner, W.M.; Nussenzweig, A. H2AX Haploinsufficiency modifies genomic stability and tumor susceptibility. Cell 2003, 114, 371–383. [Google Scholar] [CrossRef]
- Bassing, C.H.; Suh, H.; Ferguson, D.O.; Chua, K.F.; Manis, J.; Eckersdorff, M.; Gleason, M.; Bronson, R.; Lee, C.; Alt, F.W. Histone H2AX: A dosage-dependent suppressor of oncogenic translocations and tumors. Cell 2003, 114, 359–370. [Google Scholar] [CrossRef]
- Weyemi, U.; Paul, B.D.; Bhattacharya, D.; Malla, A.P.; Boufraqech, M.; Harraz, M.M.; Bonner, W.M.; Snyder, S.H. Histone H2AX promotes neuronal health by controlling mitochondrial homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 7471–7476. [Google Scholar] [CrossRef]
- Wyllie, A.H.; Kerr, J.F.; Currie, A.R. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar]
- Lossi, L.; Castagna, C.; Merighi, A. Neuronal Cell Death: An Overview of Its Different Forms in Central and Peripheral Neurons. Methods Mol. Biol. 2015, 1254, 1–18. [Google Scholar] [PubMed]
- Jellinger, K.A. Cell death mechanisms in neurodegeneration. J. Cell. Mol. Med. 2001, 5, 1–17. [Google Scholar] [CrossRef]
- Roth, K.A. Caspases, apoptosis, and Alzheimer disease: Causation, correlation, and confusion. J. Neuropathol. Exp. Neurol. 2001, 60, 829–838. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Nieves-Neira, W.; Boon, C.; Pommier, Y.; Bonner, W.M. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J. Biol. Chem. 2000, 275, 9390–9395. [Google Scholar] [CrossRef]
- Solier, S.; Pommier, Y. The apoptotic ring: A novel entity with phosphorylated histones H2AX and H2B, and activated DNA damage response kinases. Cell Cycle 2009, 8, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Solier, S.; Sordet, O.; Kohn, K.W.; Pommier, Y. Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol. Cell. Biol. 2009, 29, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Solier, S.; Pommier, Y. The nuclear γ-H2AX apoptotic ring: Implications for cancers and autoimmune diseases. Cell. Mol. Life Sci. 2014, 71, 2289–2297. [Google Scholar] [CrossRef]
- Kaseb, H.; Rayi, A.; Hozayen, S. Chromosome Instability Syndromes. In StatPearls; StatPearls Publishing Copyright © 2021; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- De Winter, J.P.; Joenje, H. The genetic and molecular basis of Fanconi anemia. Mutat. Res. 2009, 668, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Becker-Catania, S.; Gatti, R.A. Ataxia-telangiectasia: Diagnosis and treatment. Semin. Pediatr. Neurol. 2003, 10, 173–182. [Google Scholar] [CrossRef]
- Digweed, M.; Sperling, K. Nijmegen breakage syndrome: Clinical manifestation of defective response to DNA double-strand breaks. DNA Repair 2004, 3, 1207–1217. [Google Scholar] [CrossRef]
- German, J.; Sanz, M.M.; Ciocci, S.; Ye, T.Z.; Ellis, N.A. Syndrome-causing mutations of the BLM gene in persons in the Bloom’s Syndrome Registry. Hum. Mutat. 2007, 28, 743–753. [Google Scholar] [CrossRef]
- di Masi, A.; Viganotti, M.; Polticelli, F.; Ascenzi, P.; Tanzarella, C.; Antoccia, A. The R215W mutation in NBS1 impairs gamma-H2AX binding and affects DNA repair: Molecular bases for the severe phenotype of 657del5/R215W Nijmegen breakage syndrome patients. Biochem. Biophys. Res. Commun. 2008, 369, 835–840. [Google Scholar] [CrossRef]
- Matsuura, S.; Kobayashi, J.; Tauchi, H.; Komatsu, K. Nijmegen breakage syndrome and DNA double-strand break repair by NBS1 complex. Adv. Biophys. 2004, 38, 65–80. [Google Scholar] [CrossRef]
- Rao, V.A.; Conti, C.; Guirouilh-Barbat, J.; Nakamura, A.; Miao, Z.H.; Davies, S.L.; Saccá, B.; Hickson, I.D.; Bensimon, A.; Pommier, Y. Endogenous gamma-H2AX-ATM-Chk2 checkpoint activation in Bloom’s syndrome helicase deficient cells is related to DNA replication arrested forks. Mol. Cancer Res. 2007, 5, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Bogliolo, M.; Lyakhovich, A.; Callén, E.; Castellà, M.; Cappelli, E.; Ramírez, M.J.; Creus, A.; Marcos, R.; Kalb, R.; Neveling, K.; et al. Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. EMBO J. 2007, 26, 1340–1351. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Meira, L.B. Database of mouse strains carrying targeted mutations in genes affecting biological responses to DNA damage Version 7. DNA Repair 2006, 5, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Span, P.N. Staining Against Phospho-H2AX (γ-H2AX) as a Marker for DNA Damage and Genomic Instability in Cancer Tissues and Cells. Adv. Exp. Med. Biol. 2016, 899, 1–10. [Google Scholar] [PubMed]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair, and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Palla, V.V.; Karaolanis, G.; Katafigiotis, I.; Anastasiou, I.; Patapis, P.; Dimitroulis, D.; Perrea, D. γH2AX: Can it be established as a classical cancer prognostic factor? Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. GammaH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.; Ju, B.; Telese, F.; Wang, X.; Glass, C.K.; Rosenfeld, M.G. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 2009, 458, 591–596. [Google Scholar] [CrossRef]
- Clancy, B.; Finlay, B.L.; Darlington, R.B.; Anand, K.J. Extrapolating brain development from experimental species to humans. NeuroToxicology 2007, 28, 931–937. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef]
- Martynoga, B.; Drechsel, D.; Guillemot, F. Molecular control of neurogenesis: A view from the mammalian cerebral cortex. Cold Spring Harb. Perspect. Biol. 2012, 4, a008359. [Google Scholar] [CrossRef] [PubMed]
- Barral, S.; Beltramo, R.; Salio, C.; Aimar, P.; Lossi, L.; Merighi, A. Phosphorylation of histone H2AX in the mouse brain from development to senescence. Int. J. Mol. Sci. 2014, 15, 1554–1573. [Google Scholar] [CrossRef]
- Van Hooser, A.; Goodrich, D.W.; Allis, C.D.; Brinkley, B.R.; Mancini, M.A. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J. Cell. Sci. 1998, 111, 3497–3506. [Google Scholar] [CrossRef]
- LoTurco, J.J.; Owens, D.F.; Heath, M.J.S.; Davis, M.B.E.; Kriegstein, A.R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 1995, 15, 1287–1298. [Google Scholar] [CrossRef]
- Andang, M.; Hjerling-Leffler, J.; Moliner, A.; Lundgren, T.K.; Castelo-Branco, G.; Nanou, E.; Pozas, E.; Bryja, V.; Halliez, S.; Nishimaru, H.; et al. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature 2008, 451, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.N.; Eleuteri, B.; Abdelhady, S.; Nussenzweig, A.; Andäng, M.; Ernfors, P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5837–5842. [Google Scholar] [CrossRef]
- Frappart, P.-O.; Lee, Y.; Lamont, J.; McKinnon, P.J. BRCA2 is required for neurogenesis and suppression of medulloblastoma. EMBO J. 2007, 26, 2732–2742. [Google Scholar] [CrossRef]
- Michel, N.; Majumdar, U.B.; Lannigan, J.; McConnell, M.J. Imaging Flow Cytometry Quantifies Neural Genome Dynamics. Cytom. Part A 2019, 95, 825–835. [Google Scholar] [CrossRef]
- Álvarez-Lindo, N.; Baleriola, J.; de Los Ríos, V.; Suárez, T.; de la Rosa, E.J. RAG-2 deficiency results in fewer phosphorylated histone H2AX foci, but increased retinal ganglion cell death and altered axonal growth. Sci. Rep. 2019, 9, 18486. [Google Scholar] [CrossRef]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.-H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.-M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, Z.G.; Lu, M.; Wang, Y.; Yang, J.J.; Chopp, M. Reduction of the cell cycle length by decreasing G1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J. Cereb. Blood Flow Metab. 2006, 26, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Daniels, S.B.; Lennington, J.B.; Notti, R.Q.; Conover, J.C. The aging neurogenic subventricular zone. Aging Cell 2006, 5, 139–152. [Google Scholar] [CrossRef]
- Marzban, H.; Del Bigio, M.R.; Alizadeh, J.; Ghavami, S.; Zachariah, R.M.; Rastegar, M. Cellular commitment in the developing cerebellum. Front. Cell. Neurosci. 2015, 8, 450. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. Caspase-3 Mediated Cell Death in the Normal Development of the Mammalian Cerebellum. Int. J. Mol. Sci. 2018, 19, 3999. [Google Scholar] [CrossRef] [PubMed]
- Swahari, V.; Nakamura, A.; Baran-Gale, J.; Garcia, I.; Crowther, A.J.; Sons, R.; Gershon, T.R.; Hammond, S.; Sethupathy, P.; Deshmukh, M. Essential Function of Dicer in Resolving DNA Damage in the Rapidly Dividing Cells of the Developing and Malignant Cerebellum. Cell. Rep. 2016, 14, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Tzur-Gilat, A.; Ziv, Y.; Mittelman, L.; Barzilai, A.; Shiloh, Y. Studying the cerebellar DNA damage response in the tissue culture dish. Mech. Ageing Dev. 2013, 134, 496–505. [Google Scholar] [CrossRef]
- Dar, I.; Yosha, G.; Elfassy, R.; Galron, R.; Wang, Z.Q.; Shiloh, Y.; Barzilai, A. Investigation of the functional link between ATM and NBS1 in the DNA damage response in the mouse cerebellum. J. Biol. Chem. 2011, 286, 15361–15376. [Google Scholar] [CrossRef]
- Chun, H.H.; Gatti, R.A. Ataxia-telangiectasia, an evolving phenotype. DNA Repair 2004, 3, 1187–1196. [Google Scholar] [CrossRef]
- Biton, S.; Barzilai, A.; Shiloh, Y. The neurological phenotype of ataxia-telangiectasia: Solving a persistent puzzle. DNA Repair 2008, 7, 1028–1038. [Google Scholar] [CrossRef]
- Miale, I.L.; Sidman, R.L. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp. Neurol. 1961, 4, 277–296. [Google Scholar] [CrossRef]
- Castagna, C.; Merighi, A.; Lossi, L. Cell death and neurodegeneration in the postnatal development of cerebellar vermis in normal and Reeler mice. Ann. Anat. 2016, 207, 76–90. [Google Scholar] [CrossRef]
- Cheng, X.S.; Li, M.S.; Du, J.; Jiang, Q.Y.; Wang, L.; Yan, S.Y.; Yu, D.M.; Deng, J.B. Neuronal apoptosis in the developing cerebellum. Anat. Histol. Embryol. 2011, 40, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Marti-Clua, J. Natural apoptosis in developing mice dopamine midbrain neurons and vermal Purkinje cells. Folia Neuropathol. 2016, 54, 180–189. [Google Scholar] [CrossRef] [PubMed]
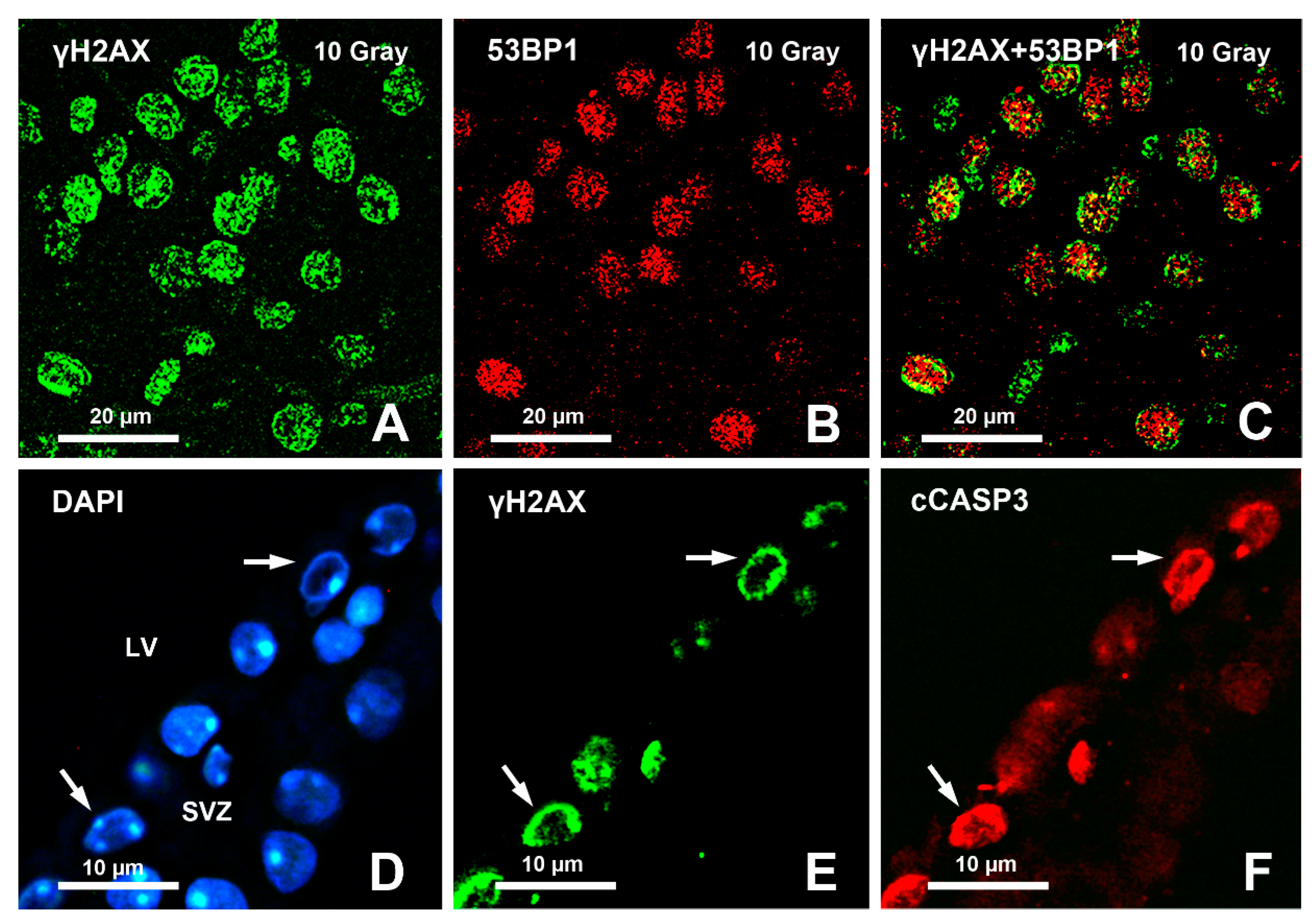
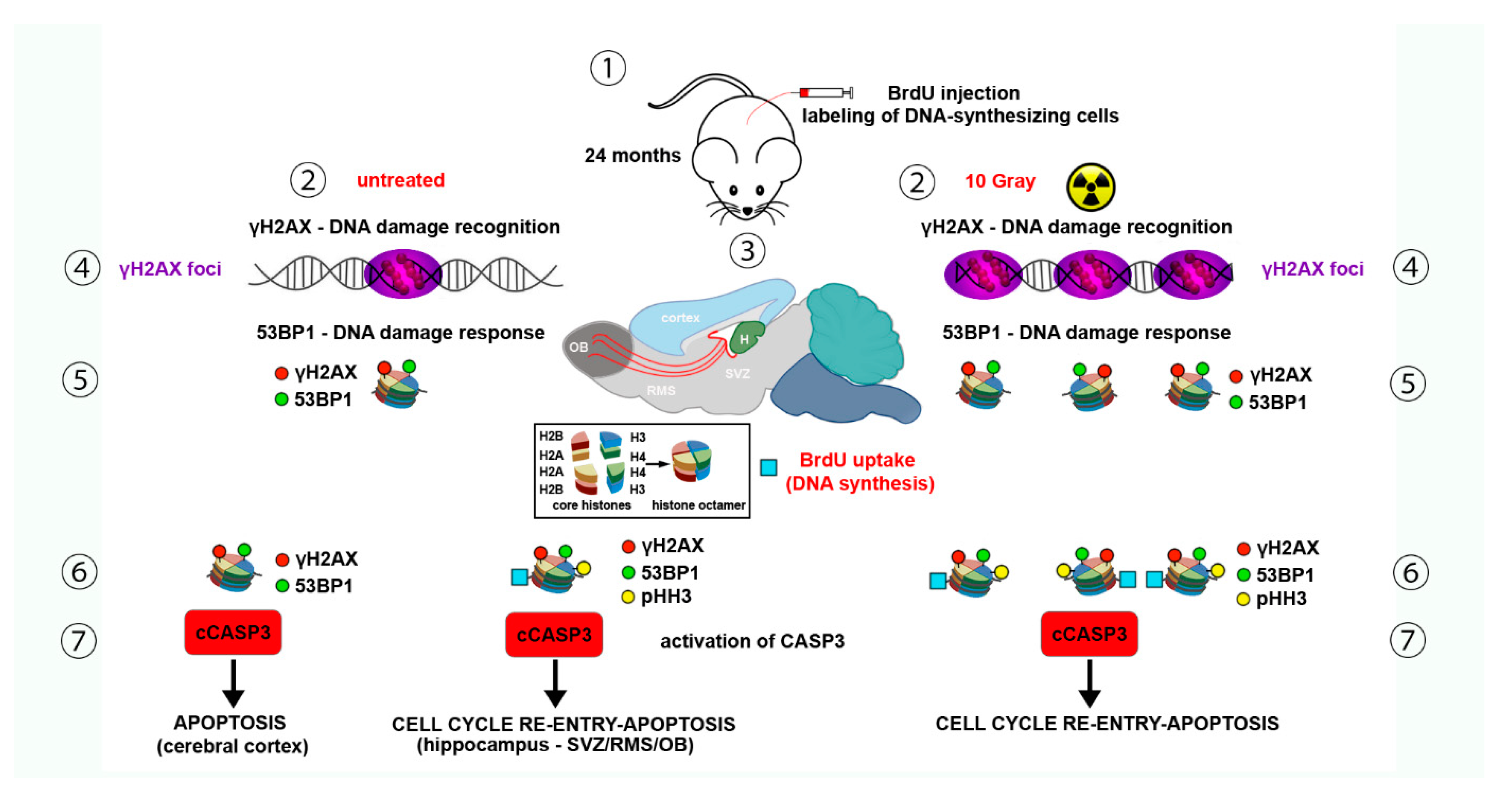
- Gionchiglia, N.; Granato, A.; Merighi, A.; Lossi, L. Association of Caspase 3 Activation and H2AX γ phosphorylation in the aging brain: Studies on untreated and irradiated mice. Biomedicines 2021, 9, 1166. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the hippocampus and the SVZ: Adult neurogenesis throughout the brain. Front. Cell. Neurosci. 2020, 14, 293. [Google Scholar] [CrossRef]
- Hudson, D.; Kovalchuk, I.; Koturbash, I.; Kolb, B.; Martin, O.A.; Kovalchuk, O. Induction and persistence of radiation-induced DNA damage is more pronounced in young animals than in old animals. Aging 2011, 3, 609–620. [Google Scholar] [CrossRef]
- Watson, C.; Puelles, L. Developmental gene expression in the mouse clarifies the organization of the claustrum and related endopiriform nuclei. J. Comp. Neurol. 2017, 525, 1499–1508. [Google Scholar] [CrossRef]
- Puelles, L.; Medina, L.; Borello, U.; Legaz, I.; Teissier, A.; Pierani, A.; Rubenstein, J.L.R. Radial derivatives of the mouse ventral pallium traced with Dbx1-LacZ reporters. J. Chem. Neuroanat. 2016, 75, 2–19. [Google Scholar] [CrossRef]
- Farzanehfar, P. Comparative review of adult midbrain and striatum neurogenesis with classical neurogenesis. Neurosci. Res. 2018, 134, 1–9. [Google Scholar] [CrossRef]
- Tight, B.; Chabbert, C. Adult neurogenesis promotes balance recovery after vestibular loss. Prog. Neurobiol. 2019, 174, 28–35. [Google Scholar]
- Crowe, S.L.; Movsesyan, V.A.; Jorgensen, T.J.; Kondratyev, A. Rapid phosphorylation of histone H2A.X following ionotropic glutamate receptor activation. Eur. J. Neurosci. 2006, 23, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Blain, S.W. Chk1 has an essential role in the survival of differentiated cortical neurons in the absence of DNA damage. Apoptosis 2011, 16, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Lorat, Y.; Schanz, S.; Schuler, N.; Wennemuth, G.; Rübe, C.; Rübe, C.E. Beyond repair foci: DNA double-strand break repair in euchromatic and heterochromatic compartments analyzed by transmission electron microscopy. PLoS ONE 2012, 7, e38165. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, N.M.; Evans, M.D.; Mao, W.; Nana, A.L.; Seeley, W.W.; Adame, A.; Rissman, R.A.; Masliah, E.; Mucke, L. Early neuronal accumulation of DNA double-strand breaks in Alzheimer’s disease. Acta Neuropathol. Commun. 2019, 7, 77. [Google Scholar] [CrossRef]
- Garwood, C.J.; Simpson, J.E.; Al Mashhadi, S.; Axe, C.; Wilson, S.; Heath, P.R.; Shaw, P.J.; Matthews, F.E.; Brayne, C.; Ince, P.G.; et al. DNA damage response and senescence in endothelial cells of human cerebral cortex and relation to Alzheimer’s neuropathology progression: A population-based study in the Medical Research Council Cognitive Function and Ageing Study (MRC-CFAS) cohort. Neuropathol. Appl. Neurobiol. 2014, 40, 802–814. [Google Scholar] [CrossRef]
- Vazquez-Villaseñor, I. Expression of p16 and p21 in the frontal association cortex of ALS/MND brains suggests neuronal cell cycle dysregulation and astrocyte senescence in early stages of the disease. Acta Neuropathol. Commun. 2020, 46, 171–185. [Google Scholar] [CrossRef]
- Lledo, P.-M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Jin, K.; Sun, Y.; Xie, L.; Batteur, S.; Mao, X.O.; Smelick, C.; Logvinova, A.; Greenberg, D.A. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2003, 2, 175–183. [Google Scholar] [CrossRef]
- Maslov, A.Y.; Barone, T.A.; Plunkett, R.J.; Pruitt, S.C. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 2004, 24, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, A.; Beauséjour, C.; d’Adda di Fagagna, F. A novel single-cell method provides direct evidence of persistent DNA damage in senescent cells and aged mammalian tissues. Aging Cell 2017, 16, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; DePinho, R.A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010, 464, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Ferrón, S.; Mira, H.; Franco, S.; Cano-Jaimez, M.; Bellmunt, E.; Ramírez, C.; Fariñas, I.; Blasco, M.A. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development 2004, 131, 4059–4070. [Google Scholar] [CrossRef]
- Conover, J.C.; Todd, K.L. Development and aging of a brain neural stem cell niche. Exp. Gerontol. 2017, 94, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ahlenius, H.; Visan, V.; Kokaia, M.; Lindvall, O.; Kokaia, Z. Neural Stem and Progenitor Cells Retain Their Potential for Proliferation and Differentiation into Functional Neurons Despite Lower Number in Aged Brain. J. Neurosci. 2009, 29, 4408–4419. [Google Scholar] [CrossRef]
- Mobley, A.S.; Rodriguez-Gil, D.J.; Imamura, F.; Greer, C.A. Aging in the olfactory system. Trends Neurosci. 2014, 37, 77–84. [Google Scholar] [CrossRef]
- Omais, S.; Jaafar, C.; Ghanem, N. “Till Death Do Us Part”: A potential irreversible link between aberrant cell cycle control and neurodegeneration in the adult olfactory bulb. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Tropepe, V.; Craig, C.G.; Morshead, C.M.; van der Kooy, D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J. Neurosci. 1997, 17, 7850–7859. [Google Scholar] [CrossRef]
- Daynac, M.; Morizur, L.; Chicheportiche, A.; Mouthon, M.-A.; Boussin, F.D. Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci. Rep. 2016, 6, 21505. [Google Scholar] [CrossRef]
- Conover, J.C.; Shook, B.A. Aging of the subventricular zone neural stem cell niche. Aging Dis 2011, 2, 49–63. [Google Scholar] [PubMed]
- Shook, B.A.; Manz, D.H.; Peters, J.J.; Kang, S.; Conover, J.C. Spatiotemporal Changes to the Subventricular Zone Stem Cell Pool through Aging. J. Neurosci. 2012, 32, 6947–6956. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Horikawa, I.; Redon, C.; Nakamura, A.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell 2008, 7, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Horikawa, I.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M.; Barrett, J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004, 6, 168–170. [Google Scholar] [CrossRef]
- Chow, H.-m.; Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef]
- Fielder, E.; von Zglinicki, T.; Jurk, D. The DNA damage response in neurons: Die by apoptosis or survive in a senescence-like state? J. Alzheimers Dis. 2017, 60, S107–S131. [Google Scholar] [CrossRef]
- Suberbielle, E.; Sanchez, P.E.; Kravitz, A.V.; Wang, X.; Ho, K.; Eilertson, K.; Devidze, N.; Kreitzer, A.C.; Mucke, L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci. 2013, 16, 613–621. [Google Scholar] [CrossRef]
- Fiscella, M.; Zhang, H.; Fan, S.; Sakaguchi, K.; Shen, S.; Mercer, W.E.; Vande Woude, G.F.; O’Connor, P.M.; Appella, E. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc. Natl. Acad. Sci. USA 1997, 94, 6048–6053. [Google Scholar] [CrossRef]
- He, Z.Y.; Wang, W.Y.; Hu, W.Y.; Yang, L.; Li, Y.; Zhang, W.Y.; Yang, Y.S.; Liu, S.C.; Zhang, F.L.; Mei, R.; et al. Gamma-H2AX upregulation caused by Wip1 deficiency increases depression-related cellular senescence in hippocampus. Sci. Rep. 2016, 6, 34558. [Google Scholar] [CrossRef]
- Silva, A.R.; Santos, A.C.; Farfel, J.M.; Grinberg, L.T.; Ferretti, R.E.; Campos, A.H.; Cunha, I.W.; Begnami, M.D.; Rocha, R.M.; Carraro, D.M.; et al. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer’s disease. PLoS ONE 2014, 9, e99897. [Google Scholar] [CrossRef]
- Ma, A.; Dai, X. The relationship between DNA single-stranded damage response and double-stranded damage response. Cell Cycle 2018, 17, 73–79. [Google Scholar] [CrossRef]
- Pospelova, T.V.; Demidenko, Z.N.; Bukreeva, E.I.; Pospelov, V.A.; Gudkov, A.V.; Blagosklonny, M.V. Pseudo-DNA damage response in senescent cells. Cell Cycle 2009, 8, 4112–4118. [Google Scholar] [CrossRef] [PubMed]
- Riches, L.C.; Lynch, A.M.; Gooderham, N.J. Early events in the mammalian response to DNA double-strand breaks. Mutagenesis 2008, 23, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J. DNA repair in neural cells: Basic science and clinical implications. Mutat. Res. 2002, 509, 93–108. [Google Scholar] [CrossRef]
- Kruman, I.I. Why do neurons enter the cell cycle? Cell Cycle 2004, 3, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; Vasko, M.R.; Kelley, M.R. DNA repair in neurons: So if they don’t divide what’s to repair? Mutat. Res. 2007, 614, 24–36. [Google Scholar] [CrossRef]
- Barzilai, A. DNA damage, neuronal and glial cell death and neurodegeneration. Apoptosis 2010, 15, 1371–1381. [Google Scholar] [CrossRef]
- Hou, Y.; Song, H.; Croteau, D.L.; Akbari, M.; Bohr, V.A. Genome instability in Alzheimer disease. Mech. Ageing Dev. 2017, 161, 83–94. [Google Scholar] [CrossRef]
- Saez-Atienzar, S.; Masliah, E. Cellular senescence and Alzheimer disease: The egg and the chicken scenario. Nat. Rev. Neurosci. 2020, 21, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Palanca, A.; Ruiz-Soto, M.; Llorca, J.; Marín, M.P.; Renau-Piqueras, J.; Berciano, M.T.; Lafarga, M. Chronic alcohol exposure decreases 53BP1 protein levels leading to a defective DNA repair in cultured primary cortical neurons. Neurotox. Res. 2016, 29, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mata-Garrido, J.; Tapia, O.; Casafont, I.; Berciano, M.T.; Cuadrado, A.; Lafarga, M. Persistent accumulation of unrepaired DNA damage in rat cortical neurons: Nuclear organization and ChIP-seq analysis of damaged DNA. Acta Neuropathol. Commun. 2018, 6, 68. [Google Scholar] [CrossRef]
- Tang, F.R.; Liu, L.; Wang, H.; Ho, K.J.N.; Sethi, G. Spatiotemporal dynamics of γH2AX in the mouse brain after acute irradiation at different postnatal days with special reference to the dentate gyrus of the hippocampus. Aging 2021, 13, 15815–15832. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; François, M.; Fenech, M.F.; Leifert, W.R. Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat. Res. Rev. Mutat. Res. 2015, 766, 1–19. [Google Scholar] [CrossRef]
- Kanu, N.; Behrens, A. ATMIN defines an NBS1-independent pathway of ATM signalling. EMBO J. 2007, 26, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Kanu, N.; Penicud, K.; Hristova, M.; Wong, B.; Irvine, E.; Plattner, F.; Raivich, G.; Behrens, A. The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain. J. Biol. Chem. 2010, 285, 38534–38542. [Google Scholar] [CrossRef] [PubMed]
- Gobbel, G.T.; Bellinzona, M.; Vogt, A.R.; Gupta, N.; Fike, J.R.; Chan, P.H. Response of postmitotic neurons to X-irradiation: Implications for the role of DNA damage in neuronal apoptosis. J. Neurosci. 1998, 18, 147–155. [Google Scholar] [CrossRef]
- Gobbel, G.T.; Chan, P.H. Neuronal death is an active, caspase-dependent process after moderate but not severe DNA damage. J. Neurochem. 2001, 76, 520–531. [Google Scholar] [CrossRef]
- Adams, B.R.; Golding, S.E.; Rao, R.R.; Valerie, K. Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants. PLoS ONE 2010, 5, e10001. [Google Scholar]
- Mills, C.N.; Nowsheen, S.; Bonner, J.A.; Yang, E.S. Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front. Mol. Neurosci. 2011, 4, 47. [Google Scholar] [CrossRef]
- Thotala, D.K.; Hallahan, D.E.; Yazlovitskaya, E.M. Inhibition of glycogen synthase kinase 3 beta attenuates neurocognitive dysfunction resulting from cranial irradiation. Cancer Res. 2008, 68, 5859–5868. [Google Scholar] [CrossRef]
- Yao, X.; Zhai, M.; Zhou, L.; Yang, L. Protective effects of SND1 in retinal photoreceptor cell damage induced by ionizing radiation. Biochem. Biophys. Res. Commun. 2019, 514, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, B.; Chico, Y.; Martínez, M.J. Insights Into SND1 Oncogene Promoter Regulation. Front. Oncol. 2018, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Hughes, L.; Di Tomaso, M.V.; Liddle, P.; Toledo, A.; Reyes-Ábalos, A.L.; Folle, G.A. Preferential localization of γH2AX foci in euchromatin of retina rod cells after DNA damage induction. Chromosome Res. 2013, 21, 789–803. [Google Scholar] [CrossRef]
- Sims, R.J.; Nishioka, K.; Reinberg, D. Histone lysine methylation: A signature for chromatin function. Trends Genet. 2003, 19, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Mergui, X.; Leteurtre, F.; Lipinski, M.; Bénard, J.; Amor-Guéret, M. Two distinctly altered cellular responses to DNA double-strand breaks in human neuroblastoma. Biochimie 2008, 90, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Bylicky, M.A.; Mueller, G.P.; Day, R.M. Radiation resistance of normal human astrocytes: The role of non-homologous end-joining DNA repair activity. J. Radiat. Res. 2019, 60, 37–50. [Google Scholar] [CrossRef]
- Nowak, E.; Etienne, O.; Millet, P.; Lages, C.S.; Mathieu, C.; Mouthon, M.A.; Boussin, F.D. Radiation-induced H2AX phosphorylation and neural precursor apoptosis in the developing brain of mice. Radiat. Res. 2006, 165, 155–164. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, H.; Shao, Y.; Chang, X.; Zhang, Y.; Zhou, Z. Paraquat increases Interleukin-1β in hippocampal dentate gyrus to impair hippocampal neurogenesis in adult mice. Ecotoxicol. Environ. Saf. 2020, 200, 110733. [Google Scholar] [CrossRef]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef]
- Cook, A.R.; Botham, P.A.; Breckenridge, C.B.; Minnema, D.J.; Sturgess, N.C.; Travis, K.Z. Neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab. Invest. 2016, 96, 1028–1029. [Google Scholar] [CrossRef]
- Ossowska, K.; Smiałowska, M.; Kuter, K.; Wierońska, J.; Zieba, B.; Wardas, J.; Nowak, P.; Dabrowska, J.; Bortel, A.; Biedka, I.; et al. Degeneration of dopaminergic mesocortical neurons and activation of compensatory processes induced by a long-term paraquat administration in rats: Implications for Parkinson’s disease. Neuroscience 2006, 141, 2155–2165. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, J.E.; Moon, I.S. Paraquat induces apoptosis of cultured rat cortical cells. Mol. Cells 2004, 17, 102–107. [Google Scholar]
- Casafont, I.; Navascués, J.; Pena, E.; Lafarga, M.; Berciano, M.T. Nuclear organization and dynamics of transcription sites in rat sensory ganglia neurons detected by incorporation of 5’-fluorouridine into nascent RNA. Neuroscience 2006, 140, 453–462. [Google Scholar] [CrossRef]
- Rulten, S.L.; Hodder, E.; Ripley, T.L.; Stephens, D.N.; Mayne, L.V. Alcohol induces DNA damage and the Fanconi anemia D2 protein implicating FANCD2 in the DNA damage response pathways in brain. Alcohol Clin. Exp. Res. 2008, 32, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Sordet, O.; Redon, C.E.; Guirouilh-Barbat, J.; Smith, S.; Solier, S.; Douarre, C.; Conti, C.; Nakamura, A.J.; Das, B.B.; Nicolas, E.; et al. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 2009, 10, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Bobola, M.S.; Kolstoe, D.D.; Blank, A.; Silber, J.R. Minimally cytotoxic doses of temozolomide produce radiosensitization in human glioblastoma cells regardless of MGMT expression. Mol. Cancer Ther. 2010, 9, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Cubilla, M.A.; Bermúdez, V.; Marquioni Ramella, M.D.; Bachor, T.P.; Suburo, A.M. Mifepristone, a blocker of glucocorticoid receptors, promotes photoreceptor death. Invest. Ophthalmol. Vis. Sci. 2013, 54, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Musich, P.R.; Serrano, M.A.; Zou, Y.; Zhang, J.; Zhu, M.Y. Effects of DSP4 on the noradrenergic phenotypes and its potential molecular mechanisms in SH-SY5Y cells. Neurotox. Res. 2014, 25, 193–207. [Google Scholar] [CrossRef][Green Version]
- Wan, C.; Liu, J.; Nie, X.; Zhao, J.; Zhou, S.; Duan, Z.; Tang, C.; Liang, L.; Xu, G. 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS ONE 2014, 9, e89811. [Google Scholar] [CrossRef]
- Kelley, M.R.; Jiang, Y.; Guo, C.; Reed, A.; Meng, H.; Vasko, M.R. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS ONE 2014, 9, e106485. [Google Scholar]
- Nakayama, S.; Adachi, M.; Hatano, M.; Inahata, N.; Nagao, T.; Fukushima, N. Cytosine arabinoside induces phosphorylation of histone H2AX in hippocampal neurons via a noncanonical pathway. Neurochem. Int. 2021, 142, 104933. [Google Scholar] [CrossRef] [PubMed]
- Paravani, E.V.; Simoniello, M.F.; Poletta, G.L.; Zolessi, F.R.; Casco, V.H. Cypermethrin: Oxidative stress and genotoxicity in retinal cells of the adult zebrafish. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 826, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Costa, C.; Kiliç, G.; Costa, S.; Pásaro, E.; Laffon, B.; Teixeira, J.P. Neuronal cytotoxicity and genotoxicity induced by zinc oxide nanoparticles. Environ. Int. 2013, 55, 92–100. [Google Scholar] [CrossRef]
- Crowe, S.L.; Tsukerman, S.; Gale, K.; Jorgensen, T.J.; Kondratyev, A.D. Phosphorylation of histone H2A.X as an early marker of neuronal endangerment following seizures in the adult rat brain. J. Neurosci. 2011, 31, 7648–7656. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Yoshida, T.; Miyake, S.; Tsubota, K.; Ozawa, Y. Neuroprotective role of superoxide dismutase 1 in retinal ganglion cells and inner nuclear layer cells against N-methyl-d-aspartate-induced cytotoxicity. Exp. Eye Res. 2013, 115, 230–238. [Google Scholar] [CrossRef]
- Giorgi, G.; Marcantonio, P.; Del Re, B. LINE-1 retrotransposition in human neuroblastoma cells is affected by oxidative stress. Cell. Tissue Res. 2011, 346, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Loniewska, M.M.; Gupta, A.; Bhatia, S.; MacKay-Clackett, I.; Jia, Z.; Wells, P.G. DNA damage and synaptic and behavioural disorders in glucose-6-phosphate dehydrogenase-deficient mice. Redox Biol. 2020, 28, 101332. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.; Wang, Y.; Liu, N.; Wang, X.; Chen, R.; Qin, J.; Ge, P.; Feng, C. Sevoflurane exposure induces neuronal cell parthanatos initiated by DNA damage in the developing brain via an increase of intracellular reactive oxygen species. Front. Cell. Neurosci. 2020, 14, 583782. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, Y.; Xu, H.; Huang, H.; Tang, S.; Song, F.; Zhou, J. TRESK Regulates Gm11874 to Induce Apoptosis of Spinal Cord Neurons via ATP5i Mediated Oxidative Stress and DNA Damage. Neurochem. Res. 2021, 46, 1970–1980. [Google Scholar] [CrossRef]
- Takashima, K.; Nakajima, K.; Shimizu, S.; Ojiro, R.; Tang, Q.; Okano, H.; Takahashi, Y.; Ozawa, S.; Jin, M.; Yoshinari, T.; et al. Disruption of postnatal neurogenesis and adult-stage suppression of synaptic plasticity in the hippocampal dentate gyrus after developmental exposure to sterigmatocystin in rats. Toxicol. Lett. 2021, 349, 69–83. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Z.; Xiang, C.; Molczan, A.; Baubet, V.; Conejo-Garcia, J.; Xu, X.; Lieberman, P.M.; Dahmane, N. Formation of telomeric repeat-containing RNA (TERRA) foci in highly proliferating mouse cerebellar neuronal progenitors and medulloblastoma. J. Cell Sci. 2012, 125, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Kotipatruni, R.R.; Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H.; Fassett, D.; Rao, J.S. p53- and Bax-mediated apoptosis in injured rat spinal cord. Neurochem. Res. 2011, 36, 2063–2074. [Google Scholar] [CrossRef]
- Kim, G.S.; Jung, J.E.; Narasimhan, P.; Sakata, H.; Yoshioka, H.; Song, Y.S.; Okami, N.; Chan, P.H. Release of mitochondrial apoptogenic factors and cell death are mediated by CK2 and NADPH oxidase. J. Cereb. Blood Flow Metab. 2012, 32, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Harrison, F.E.; Xia, F. Altered DNA repair; an early pathogenic pathway in Alzheimer’s disease and obesity. Sci. Rep. 2018, 8, 5600. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Francois, M.; Hecker, J.; Faunt, J.; Fenech, M.F.; Leifert, W.R. γH2AX is increased in peripheral blood lymphocytes of Alzheimer’s disease patients in the South Australian Neurodegeneration, Nutrition and DNA Damage (SAND) study of aging. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 829–830, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Violet, M.; Delattre, L.; Tardivel, M.; Sultan, A.; Chauderlier, A.; Caillierez, R.; Talahari, S.; Nesslany, F.; Lefebvre, B.; Bonnefoy, E.; et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front. Cell. Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef]
- Jeon, G.S.; Kim, K.Y.; Hwang, Y.J.; Jung, M.K.; An, S.; Ouchi, M.; Ouchi, T.; Kowall, N.; Lee, J.; Ryu, H. Deregulation of BRCA1 leads to impaired spatiotemporal dynamics of gamma-H2AX and DNA damage responses in Huntington’s disease. Mol. Neurobiol. 2012, 45, 550–563. [Google Scholar] [CrossRef]
- Tidball, A.M.; Bryan, M.R.; Uhouse, M.A.; Kumar, K.K.; Aboud, A.A.; Feist, J.E.; Ess, K.C.; Neely, M.D.; Aschner, M.; Bowman, A.B. A novel manganese-dependent ATM-p53 signaling pathway is selectively impaired in patient-based neuroprogenitor and murine striatal models of Huntington’s disease. Hum. Mol. Genet. 2015, 24, 1929–1944. [Google Scholar] [CrossRef]
- Zhan, Y.; Raza, M.U.; Yuan, L.; Zhu, M.Y. Critical Role of Oxidatively Damaged DNA in Selective Noradrenergic Vulnerability. Neuroscience 2019, 422, 184–201. [Google Scholar] [CrossRef]
- Milanese, C.; Cerri, S.; Ulusoy, A.; Gornati, S.V.; Plat, A.; Gabriels, S.; Blandini, F.; Di Monte, D.A.; Hoeijmakers, J.H.; Mastroberardino, P.G. Activation of the DNA damage response in vivo in synucleinopathy models of Parkinson’s disease. Cell Death Dis. 2018, 9, 818. [Google Scholar] [CrossRef]
- Alpatov, R.; Bluma, J.L.; Nakamoto-Kinoshita, M.; Blanco, A.; Chen, S.; Stützer, A.; Armache, K.J.; Simon, M.D.; Xu, C.; Ali, M.; et al. A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response. Cell 2014, 157, 869–881. [Google Scholar] [CrossRef]
- Farg, M.A.; Konopka, A.; Soo, K.Y.; Ito, D.; Atkin, J.D. The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2017, 26, 2882–2896. [Google Scholar] [CrossRef]
- Dormann, D.; Haass, C. Fused in sarcoma (FUS): An oncogene goes awry in neurodegeneration. Mol. Cell Neurosci. 2013, 56, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Svetoni, F.; Frisone, P.; Paronetto, M.P. Role of FET proteins in neurodegenerative disorders. RNA Biol. 2016, 13, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Holler, C.J.; Taylor, G.; Hudson, K.F.; Watkins, W.; Gearing, M.; Ito, D.; Murray, M.E.; Dickson, D.W.; Seyfried, N.T.; et al. FUS is phosphorylated by DNA-PK and accumulates in the cytoplasm after DNA damage. J. Neurosci. 2014, 34, 7802–7813. [Google Scholar] [CrossRef] [PubMed]
- Martín-Doncel, E.; Rojas, A.M.; Cantarero, L.; Lazo, P.A. VRK1 functional insufficiency due to alterations in protein stability or kinase activity of human VRK1 pathogenic variants implicated in neuromotor syndromes. Sci. Rep. 2019, 9, 13381. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.; Albulescu, L.O.; Cristini, A.; Gromak, N. Senataxin: Genome Guardian at the Interface of Transcription and Neurodegeneration. J. Mol. Biol. 2017, 429, 3181–3195. [Google Scholar] [CrossRef]
- Bennett, C.L.; La Spada, A.R. SUMOylated Senataxin functions in genome stability, RNA degradation, and stress granule disassembly, and is linked with inherited ataxia and motor neuron disease. Mol. Genet. Genom. Med. 2021, e1745. [Google Scholar] [CrossRef]
| Chemical/Drug | Experimental Target | Mechanism of Action | Other Effects besides Induction of γH2AX | References |
|---|---|---|---|---|
| Actinomycin D | Rat sensory ganglion neurons | Inhibition RNA synthesis | Heterochromatin silencing | [184] |
| Ethanol | Mouse brain and human neuronal cells | Induction of apoptosis | Induction of FANCD2 | [185] |
| Camptothecin | Rat cortical neurons | Inhibition of topoisomerase I with apoptosis | Activation of ATM, CHK2, MDC1, and 53BP1 | [186] |
| Temozolomide | Human glioblastoma cell lines | Methylation of DNA guanine bases with apoptosis | N/A | [187] |
| Mifepristone | Mouse photoreceptors | Glucocorticoid receptors antagonism | Induction of pro-apoptotic factors | [188] |
| DSP4 | SHSY5Y cells | Block of noradrenaline uptake | Degeneration of noradrenergic terminals | [189] |
| TCDD | SHSY5Y and PC12 cells | Activation of the aryl hydrocarbon receptor | Premature senescence | [190] |
| Cisplatin, oxaliplatin, carboplatin | Cultured rat sensory neurons | Crosslink with the DNA urine bases with apoptosis | Reduction of the capsaicin-evoked release of CGRP | [191] |
| Ara-C | Cultured mouse hippocampal neurons | Inhibition of DNA polymerases, block of cell mitosis | N/A | [192] |
| Cypermethrin | Adult zebrafish retinal cells | Disruption of voltage-gated Na+ channel function | Increase of cCASP3 | [193] |
| Zinc oxide nanoparticles | SHSY5Y cells | Viability decrease, apoptosis, cell cycle alterations DNA damage | Production of micronuclei | [194] |
| Oxidative Stress Inducer | Experimental Target | Mechanism of Action | Other Effects besides Induction of γH2AX | References |
|---|---|---|---|---|
| Fluorescent immunohistochemical techniques | Cultured rat cortical neurons | Supra threshold activation of ionotropic glutamate receptors | Induction of MRE11 | [124] |
| KA | Rat hippocampus and entorhinal cortex in vivo | Activation of KA receptors and induction of seizures | Induction of MRE11 | [195] |
| NMDA | Mouse retinal ganglion cells and inner nuclear layer cells | Activation of NMDA receptors | Increase in 8-OHdG and TUNEL positive cells | [196] |
| Hydrogen peroxide | BE(2)C neuroblastoma cells | Induction of oxidative stress | Change in cellular levels of MRE11, RAD50, nibrin, and ERCC1 | [197] |
| Genetic mutation | Glucose-6-phosphate dehydrogenase-deficient mice | Reduction in NADPH levels | Synaptic and behavioral disorders | [198] |
| Sevoflurane | In vitro and in vivo rat neurons | Decrease of gap junction mediated cell-cell coupling and alteration of the action potential | Increase of intracellular ROSNeuronal cell parthanatos | [199] |
| TRESK silencing | Cultured mouse spinal cord dorsal horn neurons | Regulation of primary sensory neurons excitability | Induction of apoptosis | [200] |
| Sterigmatocystin | Rat hippocampal DG | Induction of oxidative stress, mitochondrial dysfunction, apoptosis, cell cycle arrest | Disruption of postnatal neurogenesis and adult-stage suppression of synaptic plasticity | [201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merighi, A.; Gionchiglia, N.; Granato, A.; Lossi, L. The Phosphorylated Form of the Histone H2AX (γH2AX) in the Brain from Embryonic Life to Old Age. Molecules 2021, 26, 7198. https://doi.org/10.3390/molecules26237198
Merighi A, Gionchiglia N, Granato A, Lossi L. The Phosphorylated Form of the Histone H2AX (γH2AX) in the Brain from Embryonic Life to Old Age. Molecules. 2021; 26(23):7198. https://doi.org/10.3390/molecules26237198
Chicago/Turabian StyleMerighi, Adalberto, Nadia Gionchiglia, Alberto Granato, and Laura Lossi. 2021. "The Phosphorylated Form of the Histone H2AX (γH2AX) in the Brain from Embryonic Life to Old Age" Molecules 26, no. 23: 7198. https://doi.org/10.3390/molecules26237198
APA StyleMerighi, A., Gionchiglia, N., Granato, A., & Lossi, L. (2021). The Phosphorylated Form of the Histone H2AX (γH2AX) in the Brain from Embryonic Life to Old Age. Molecules, 26(23), 7198. https://doi.org/10.3390/molecules26237198






