In Situ Synthesis of β-Na1.5Y1.5F6: Er3+ Crystals in Oxyfluoride Silicate Glass for Temperature Sensors and Their Spectral Conversion and Optical Thermometry Analysis
Abstract
:1. Introduction
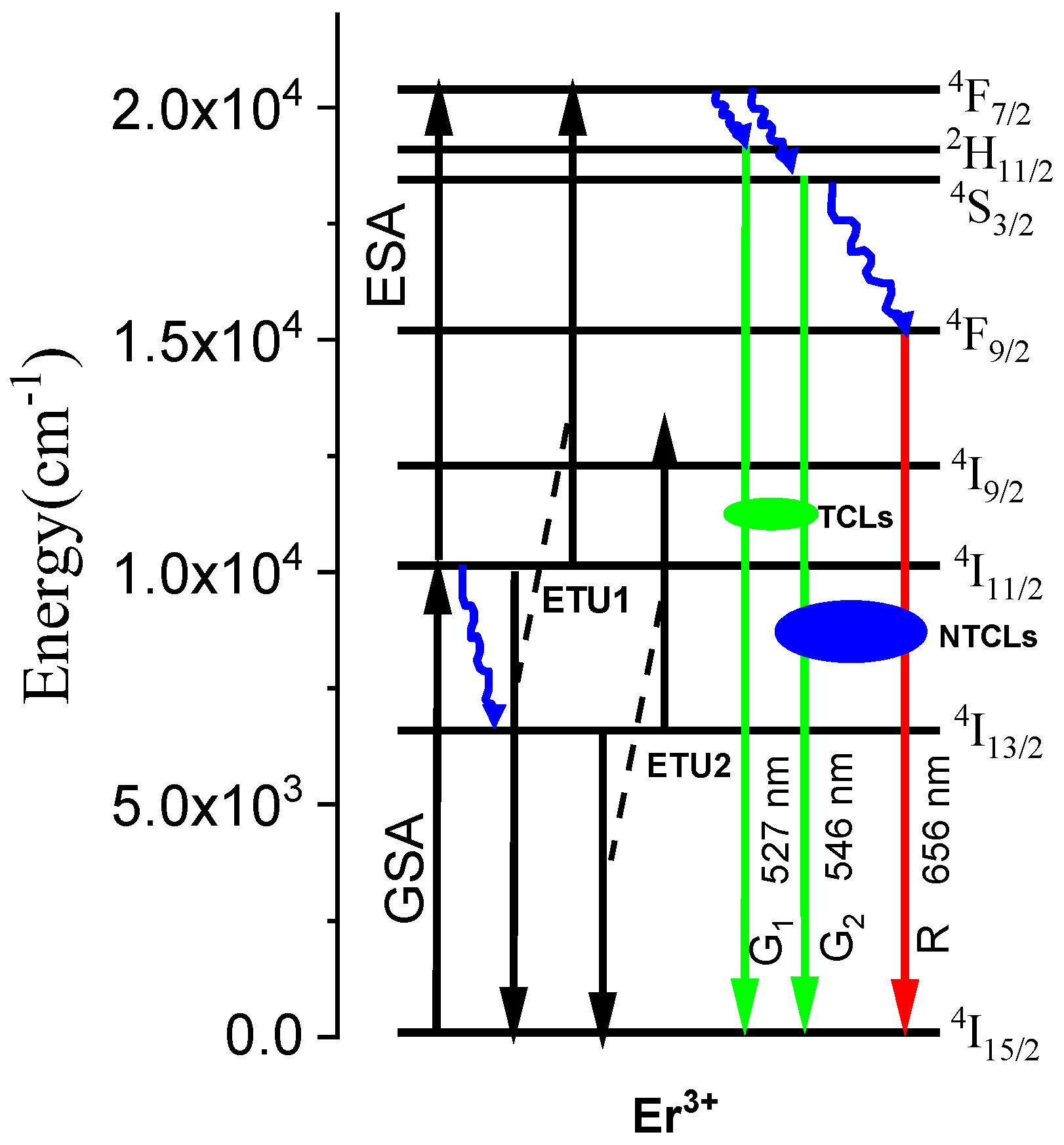
1.1. Fundamental Principle of FIR Sensing Thermometry Based on TCLs and NTCLs of Er3+ Ion
1.1.1. Thermally Coupled Levels (TCLs)
1.1.2. Non-Thermally Coupled Levels (NTCLs)
2. Results and Discussion
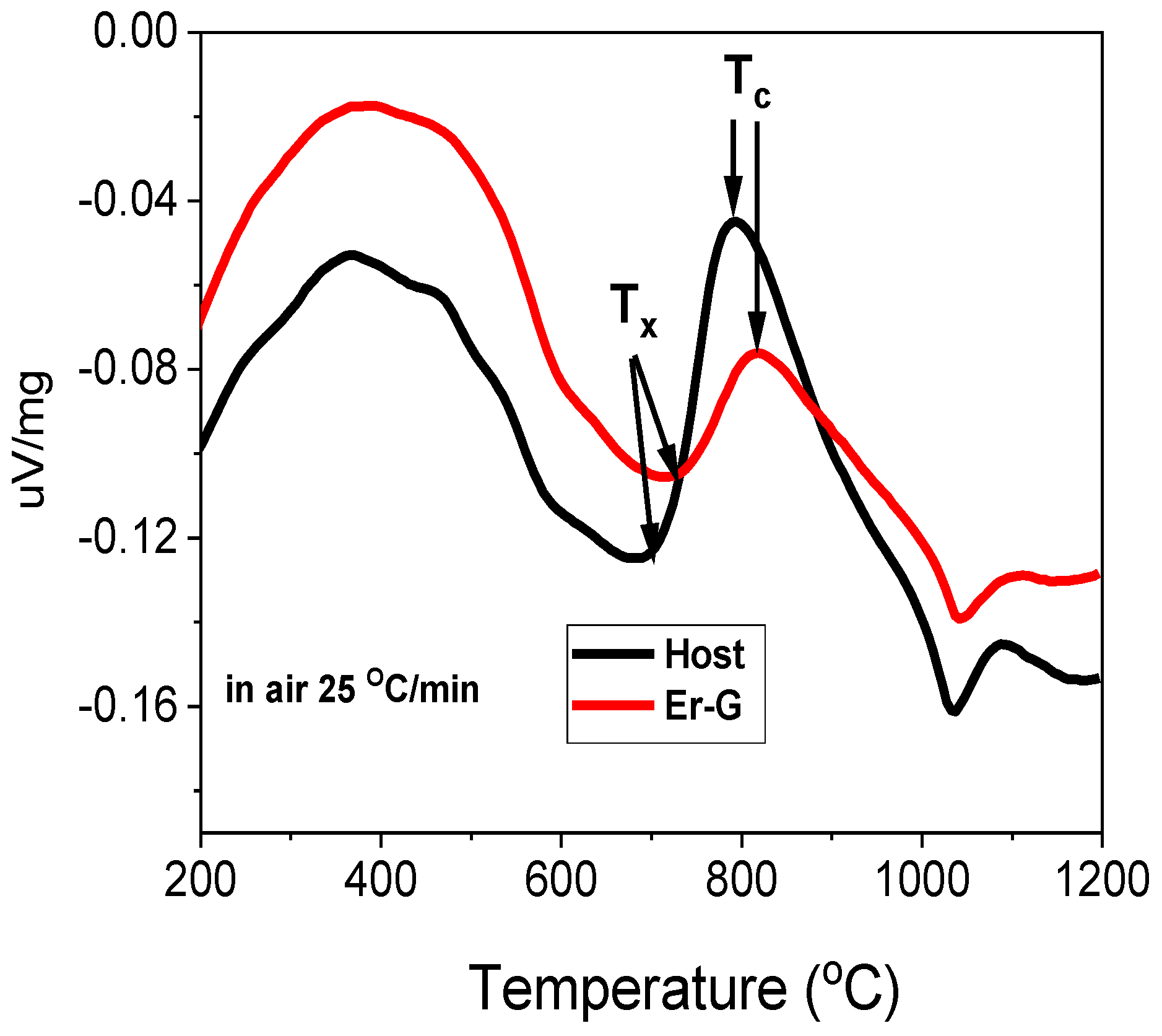
2.1. Thermal and Structural Characterization
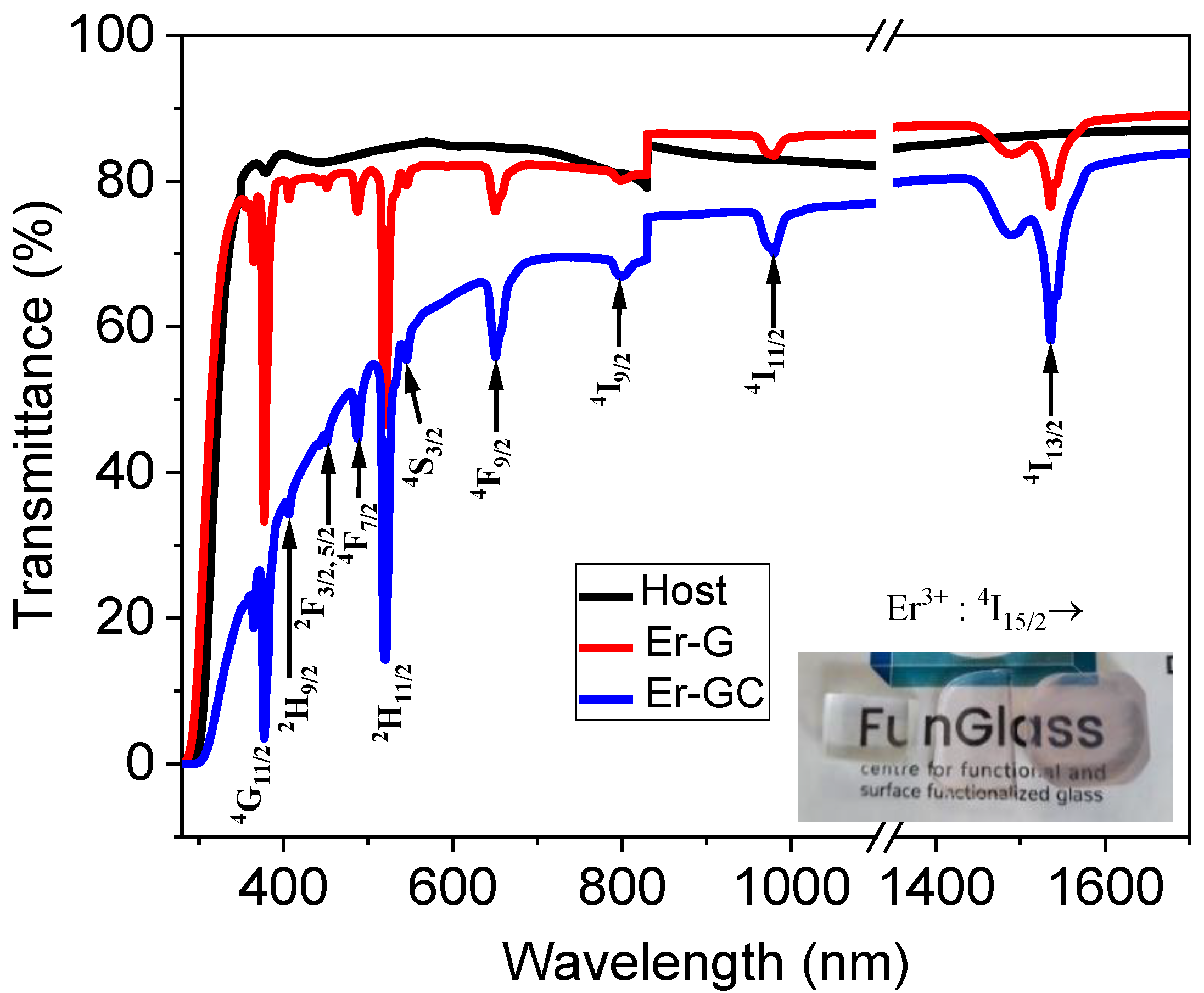
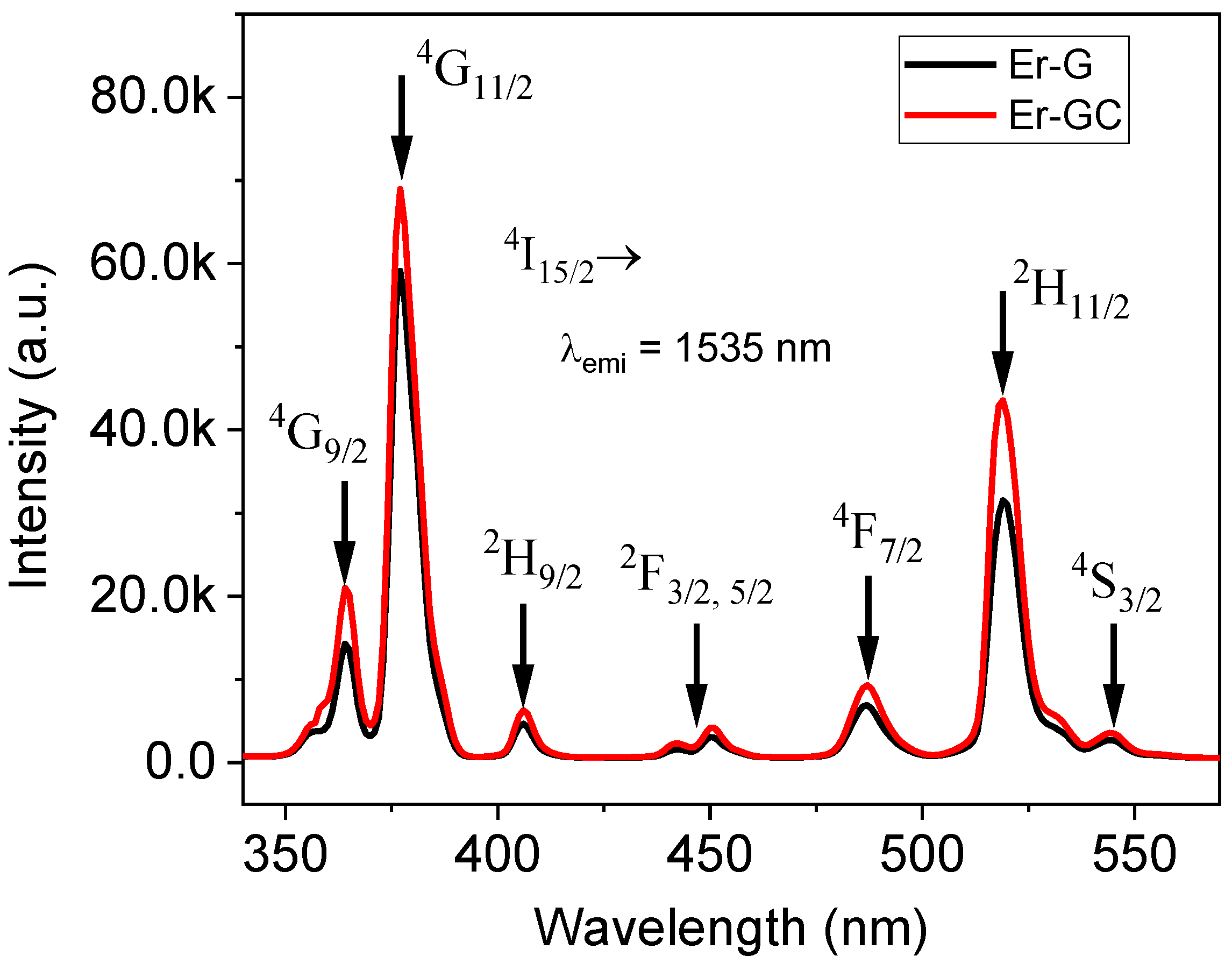
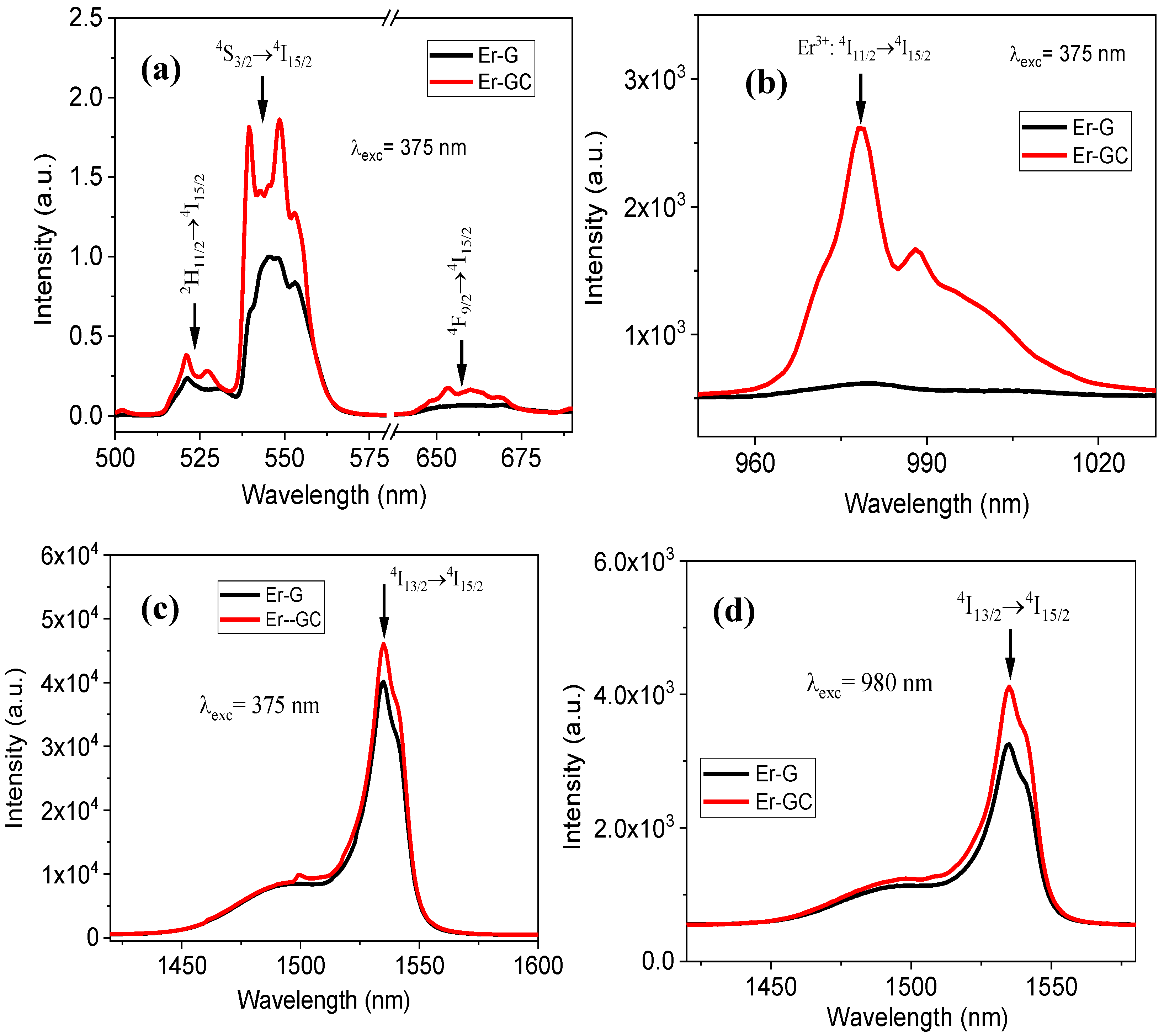
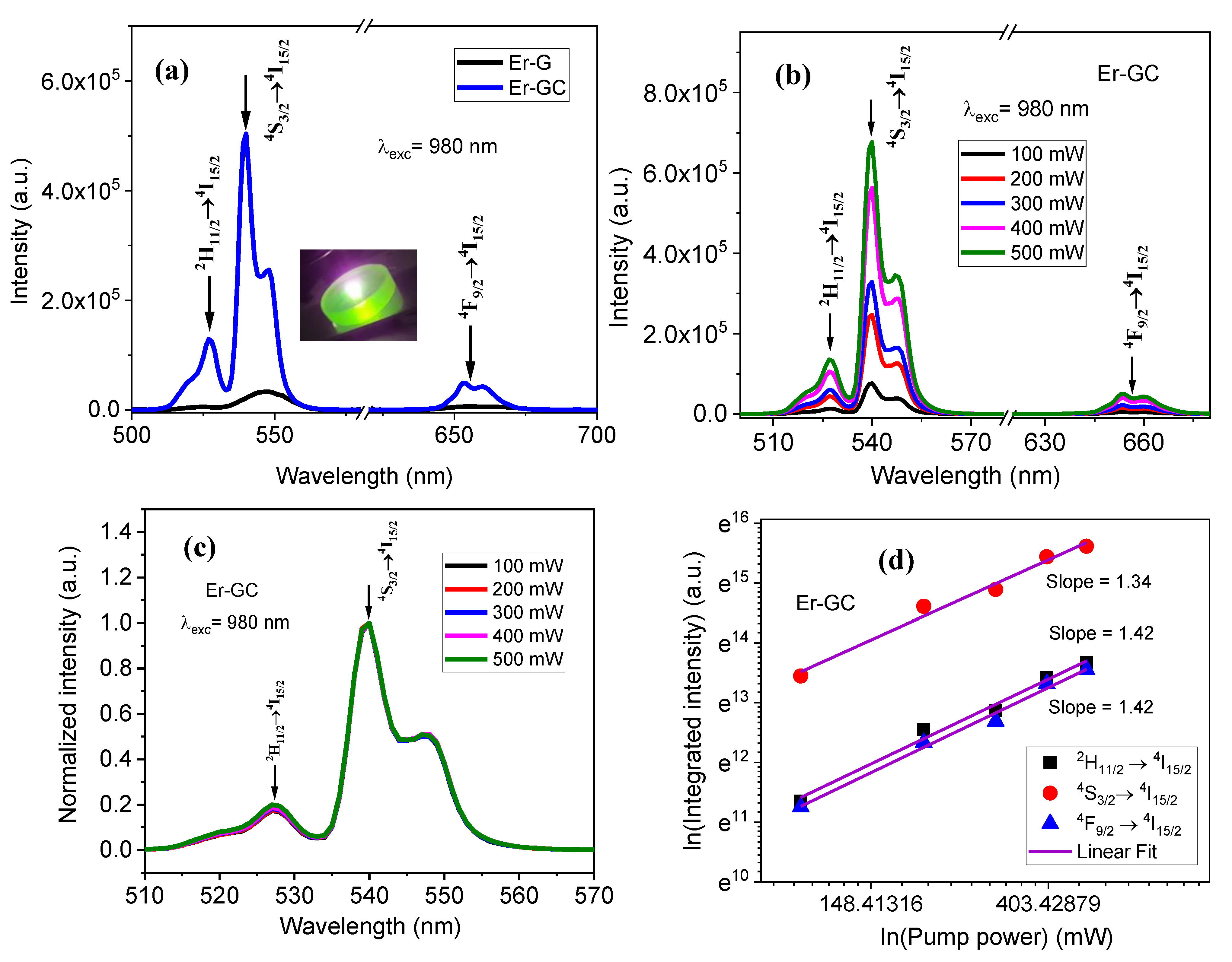
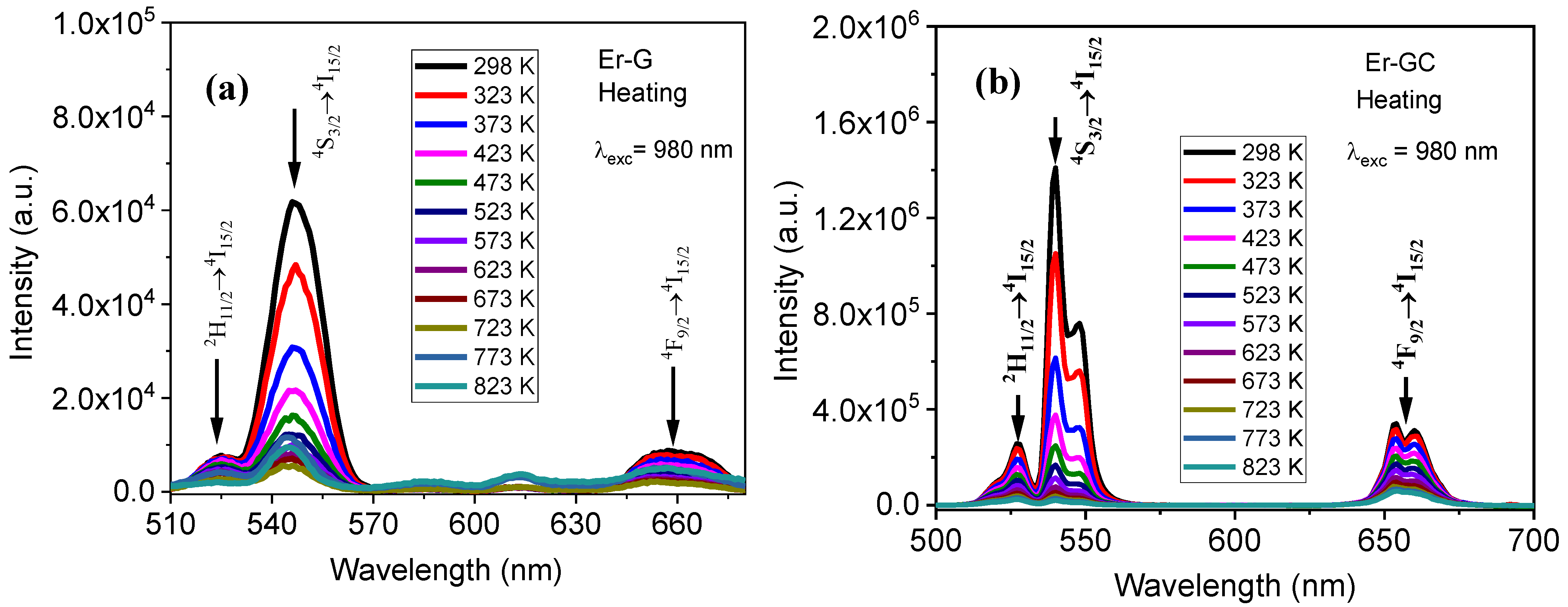
2.2. Optical Characterization
3. Materials and Methods
3.1. Glass and Glass-Ceramic Preparation
3.2. Structural and Optical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carlos, L.D.; Palacio, F. (Eds.) Thermometry at the Nanoscale: Techniques and Selected Applications; Royal Society of Chemistry: Oxfordshire, UK, 2016. [Google Scholar]
- Zohar, O.; Ikeda, M.; Shinagawa, H.; Inoue, H.; Nakamura, H.; Elbaum, D.; Daniel, L.; Yoshioka, T. Thermal imaging of receptor-activated heat production in single cells. Biophys. J. 1998, 74, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Gota, C.; Okabe, K.; Funatsu, T.; Harada, Y.; Uchiyama, S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J. Am. Chem. Soc. 2009, 131, 2766–2767. [Google Scholar] [CrossRef] [PubMed]
- Benayas, A.; del Rosal, B.; Pérez-Delgado, A.; Santacruz-Gómez, K.; Jaque, D.; Alonso Hirata, G.; Vetrone, F. Nd:YAG nearinfrared luminescent nanothermometers. Adv. Opt. Mater. 2015, 3, 687–694. [Google Scholar] [CrossRef]
- Antić, Ž.; Dramićanin, M.D.; Prashanthi, K.; Jovanović, D.; Kuzman, S.; Thundat, T. Pulsed laser deposited dysprosium-doped gadolinium-vanadate thin films for noncontact, self-referencing luminescence thermometry. Adv. Mater. 2016, 28, 7745–7752. [Google Scholar] [CrossRef]
- Puddu, M.; Mikutis, G.; Stark, W.J.; Grass, R.N. Submicrometer-sized thermometer particles exploiting selective nucleic acid stability. Small 2016, 12, 452–456. [Google Scholar] [CrossRef]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback up-conversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef]
- Rocha, U.; Da Silva, C.J.; Silva, W.F.; De Guedes, I.; Benayas, A.; Maestro, L.M.; Elias, M.A.; Bovero, E.; Van Veggel, C.J.M.; García, S.; et al. Subtissue Thermal sensing based on neodymium-doped LaF3 Nanoparticles. ACS Nano 2012, 7, 1188–1199. [Google Scholar] [CrossRef]
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef]
- León-Luis, S.F.; Rodríguez-Mendoza, U.R.; Haro-González, P.; Martín, I.R.; Lavín, V. Role of the host matrix on the thermal sensitivity of the Er3+ luminescence in optical temperature sensors. Sens. Actuators B. 2012, 174, 176–186. [Google Scholar] [CrossRef]
- Kusama, H.; Sovers, O.J.; Oshioka, T.Y. Line shift method for phosphor temperature measurements. Japan J. Appl. Phys. 1976, 15, 2349–2358. [Google Scholar] [CrossRef]
- Shinn, M.D.; Sibley, W.A.; Drexhage, M.G.; Brown, R.N. Optical transitions of Er3+ ions in fluorozirconate glass. Phys. Rev. B 1983, 27, 6635–6648. [Google Scholar] [CrossRef]
- Shalav, A.; Richards, B.S.; Green, M.A. Luminescent layers for enhanced silicon solar cell performance: Up-conversion. Sol. Energy Mater. Sol. Cells 2007, 91, 829–842. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Zamarro´n, A.; de la Fuente, A.J.; Sanz-Rodrıguez, F.; Maestro, L.M.; Rodriguez, E.M.; Jaque, D.; Garcia Sole, J.; Capobianco, J.A. Temperature sensing using fluorescent nanothermometers. ACS Nano 2010, 4, 3254–3258. [Google Scholar] [CrossRef]
- Balabhadra, S.; Debasu, M.L.; Brites, C.D.; Nunes, L.A.; Malta, O.L.; Rocha, J.; Bettinelli, M.; Carlos, L.D. Boosting the Sensitivity of Nd3+ based luminescent nanothermometers. Nanoscale 2015, 7, 17261–17267. [Google Scholar] [CrossRef]
- Mc Laurin, E.J.; Bradshaw, L.R.; Gamelin, D.R. Dual-emitting nanoscale temperature sensors. Chem. Mater. 2013, 25, 1283–1292. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, F.; Lin, H.; Zhou, J.; Xu, J.; Wang, Y. A novel optical thermometry strategy based on diverse thermal response from two intervalence charge transfer States. Adv. Funct. Mater. 2016, 26, 3139–3145. [Google Scholar] [CrossRef]
- Carrasco, E.; del Rosal, B.; Sanz-Rodríguez, F.; de la Fuente, Á.J.; Gonzalez, P.H.; Rocha, U.; Kumar, K.U.; Jacinto, C.; Solé, J.G.; Jaque, D. Intratumoral thermal reading during photo-thermal therapy by multifunctional fluorescent nanoparticles. Adv. Funct. Mater. 2015, 25, 615–626. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Lei, P.; Yu, Y.; Yao, S.; Song, S.; Liu, X.; Su, Y.; Dong, L.; Feng, J.; et al. α-NaYb(Mn)F4:Er3+/Tm3+@NaYF4 UCNPs as “Band-Shape” luminescent nano-thermometers over a wide temperature range. ACS Appl. Mater. Inter. 2015, 7, 20813–20819. [Google Scholar] [CrossRef]
- Cheng, Y.; Gao, Y.; Lin, H.; Huang, F.; Wang, Y. Strategy design for ratiometric luminescence thermometry: Circumventing the limitation of thermally coupled levels. J. Mater. Chem. C 2018, 6, 7462–7478. [Google Scholar] [CrossRef]
- Lu, H.; Hao, H.; Shi, G.; Gao, Y.; Wang, R.; Song, Y.; Wang, Y.; Zhang, X. Optical temperature sensing in b-NaLuF4:Yb3+/Er3+/Tm3+ based on thermal, quasi-thermal and non-thermal coupling levels. RSC Adv. 2016, 6, 55307–55311. [Google Scholar] [CrossRef]
- Min, Q.; Bian, W.; Qi, Y.; Lu, W.; Yu, X.; Xu, X.; Zhou, D.; Qiu, J. Temperature sensing based on the up-conversion emission of Tm3+ in a single KLuF4 microcrystal. J. Alloys Compd. 2017, 728, 1037–1042. [Google Scholar] [CrossRef]
- Liu, S.; Ming, H.; Cui, J.; Liu, S.; You, W.; Xinyu, Y.; Yang, Y.; Nie, H.; Wang, R. Color-tunable up-conversion luminescence and multiple temperature sensing and optical heating properties of Ba3Y4O9:Er3+/Yb3+ phosphors. J. Phys. Chem. C 2018, 122, 16289–16303. [Google Scholar] [CrossRef]
- Rai, V.K.; Rai, D.; Rai, S. Pr3+ doped lithium tellurite glass as a temperature sensor. Sens. Actuators A. 2006, 128, 14–17. [Google Scholar] [CrossRef]
- Rai, V.K.; Rai, A. Temperature sensing behaviour of Eu3+ doped tellurite and calibo glasses. Appl. Phys. B. 2007, 86, 333–335. [Google Scholar] [CrossRef]
- Du, P.; Luo, L.; Su Yu, J. Low-temperature thermometry based on up-conversion emission of Ho/Yb co-doped Ba0.77Ca0.23TiO3 ceramics. J. Alloys Compd. 2015, 632, 73–77. [Google Scholar] [CrossRef]
- Sarakovskis, A.; Krieke, G. Up-conversion luminescence in erbium doped transparent oxyfluoride glass ceramics containing hexagonal NaYF4 nanocrystals. J. Eur. Ceram. Soc. 2015, 35, 3665–3671. [Google Scholar] [CrossRef]
- Herrmann, A.; Tylkowski, M.; Bocker, C.; Rüssel, C. Cubic and hexagonal NaGdF4 crystals precipitated from an aluminosilicate glass: Preparation and luminescence properties. Chem. Mater. 2013, 25, 2878–2884. [Google Scholar] [CrossRef]
- Mai, H.X.; Zhang, Y.W.; Si, R.; Yan, Z.G.; Sun, L.D.; You, L.P.; Yan, C.H. High-quality sodium rare-earth fluoride nanocrystals: controlled synthesis and optical properties. J. Am. Chem. Soc. 2006, 128, 6426–6436. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.H.; Wang, J.; Xu, J.; Chen, H.Y.; Zhang, C.; Hong, M.H.; Liu, X.G. Simultaneous phase and size control of up-conversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Zhu, Y.; Zhong, J.; Chen, D. Up-conversion of transparent glass ceramics containing β-NaYF4:Yb3+, Er3+ nanocrystals for optical thermometry. RSC Adv. 2019, 9, 7948–7954. [Google Scholar] [CrossRef] [Green Version]
- Kochanowicz, M.; Dorosz, D.; Zmojda, J.; Dorosz, J.; Miluski, P. Influence of temperature on up-conversion luminescence in tellurite glass co-doped with Yb3+/Er3+ and Yb3+/Tm3+. J. Lumin. 2014, 151, 155–160. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, K.; Liu, C.; Yin, Q.; Han, J.; Heo, J. Intense up-conversion emission from Er3+/Yb3+ ion co-doped transparent oxyfluoride glass ceramics containing Y5O4F7 nanorods for optical thermometry. J. Mater. Chem. C. 2019, 7, 6134–6143. [Google Scholar] [CrossRef]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal Sodium Yttrium Fluoride Based Green and Blue Emitting Up-conversion Phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Grzechnik, A.; Bouvier, P.; Mezouar, M.; Mathews, M.D.; Tyagi, A.K.; Köhler, J. Hexagonal Na1.5Y1.5F6 at High Pressures. J. Solid State Chem. 2002, 165, 159–164. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Up-conversion Luminescence Properties of NaYF4:Yb:Er Nanoparticles Co-doped with Gd3+. J. Phys. Chem. C 2015, 119, 3363–3373. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, V.K. Pr3+–Yb3+ co-doped Y2O3 phosphor for display devices. Mater. Res. Bull. 2014, 57, 156–161. [Google Scholar] [CrossRef]
- Pollnau, M.; Gamelin, D.R.; Lüthi, S.R.; Güdel, H.U. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Feng, L.; Lai, B.; Wang, J.; Du, G.; Su, Q. Spectroscopic properties of Er3+ in a oxyfluoride glass and upconversion and temperature sensor behaviour of Er3+/Yb3+-codoped oxyfluoride glass. J. Lumin. 2010, 130, 2418–2423. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Lisiecki, R.; Romanowski, W.R. Sensitive optical temperature sensor based on up-conversion luminescence spectra of Er3+ ions in PbO-Ga2O3-XO2 (X = Ge, Si) glasses. Opt Mater. 2016, 59, 87–90. [Google Scholar] [CrossRef]
- Hua, F.; Caob, J.; Weia, X.; Lia, X.; Caia, J.; Guob, H.; Chena, Y.; Duan, C.; Yin, M. Luminescent properties of Er3+-doped transparent NaYb2F7 glass ceramics for optical thermometry and spectral conversion. J. Mater. Chem. C 2016, 4, 9976–9985. [Google Scholar] [CrossRef]
- Cao, J.; Chen, W.; Xu, D.; Hu, F.; Chen, L.; Guo, H. Wide-range thermometry based on green up-conversion of Yb3+/Er3+ co-doped KLu2F7 transparent bulk oxyfluoride glass ceramics. J. Lumin. 2018, 194, 219–224. [Google Scholar] [CrossRef]
- Jiang, S.; Zeng, P.; Liao, L.; Tian, S.; Guo, H.; Chen, Y.; Duan, C.; Yin, M. Optical thermometry based on upconverted luminescence in transparent glass ceramics containing NaYF4:Yb3+/Er3+ nanocrystals. J. Alloys Comp. 2014, 617, 538–541. [Google Scholar] [CrossRef]
- Lai, B.; Feng, L.; Wang, J.; Su, Q. Optical transition and up-conversion luminescence in Er3+ doped and Er3+-Yb3+ co-doped fluorophosphates glasses. Opt Mater. 2010, 32, 1154–1160. [Google Scholar] [CrossRef]

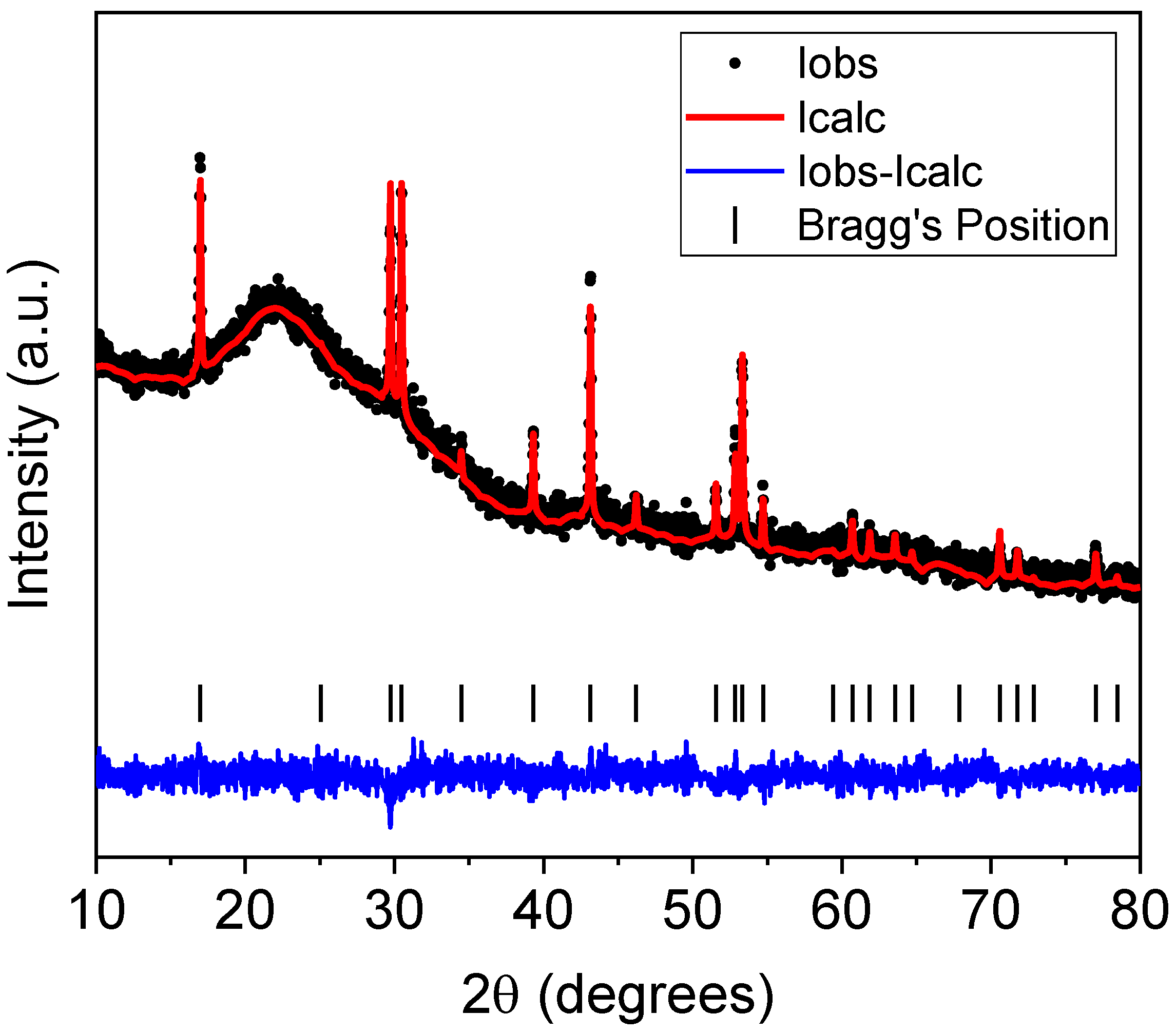
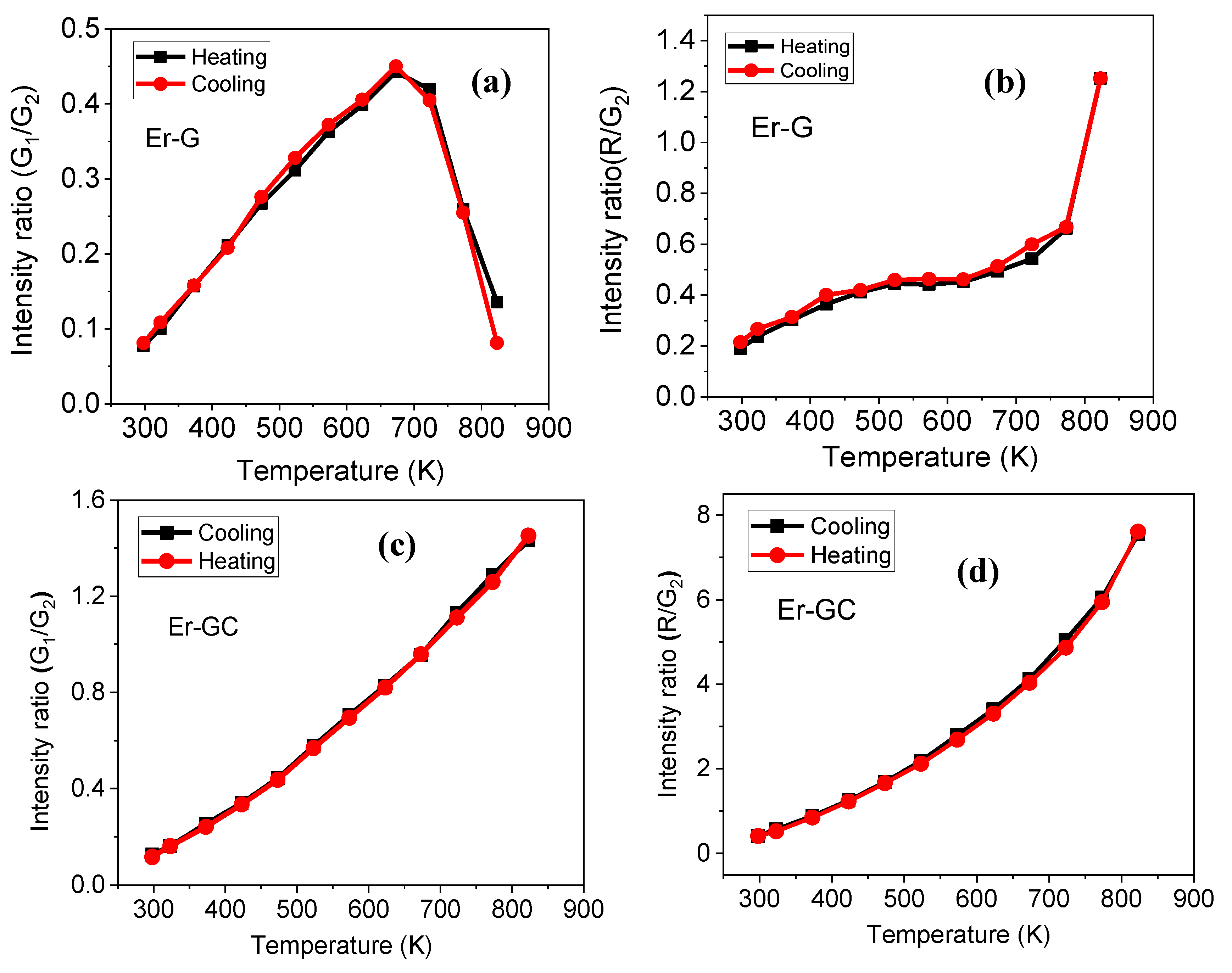
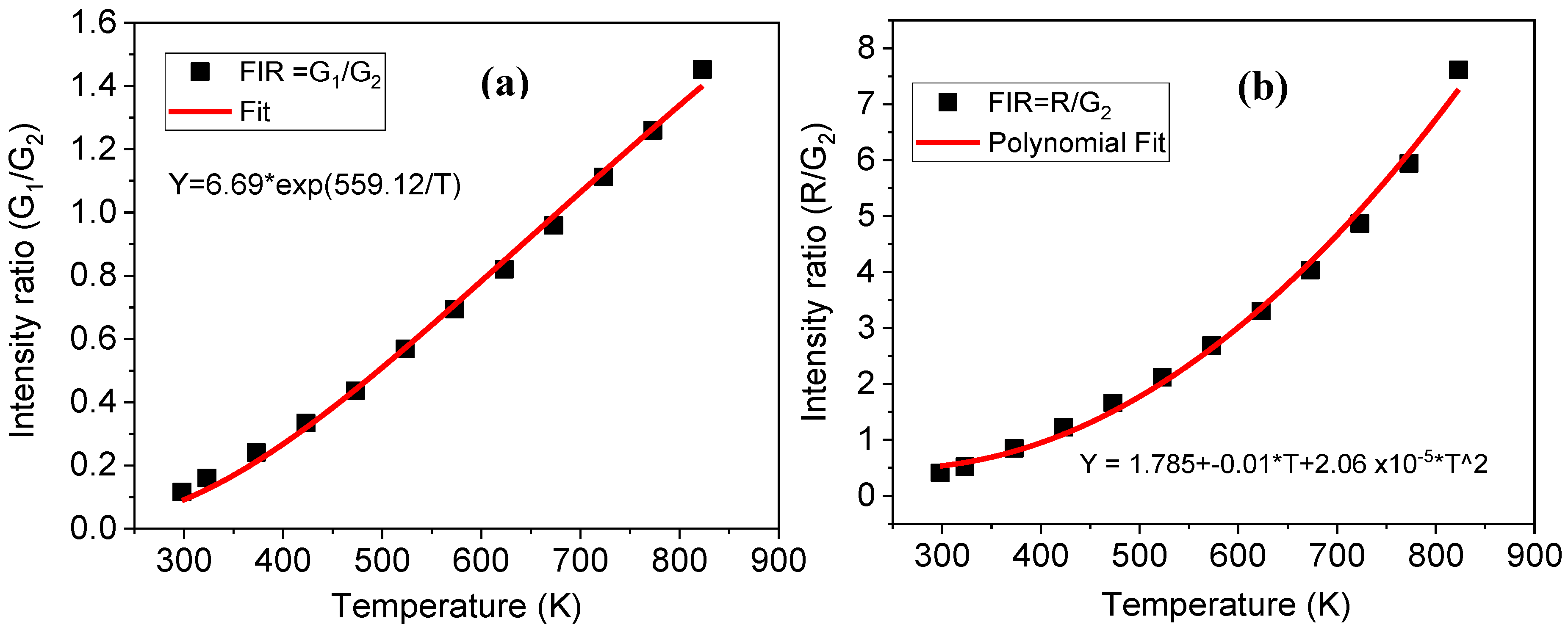
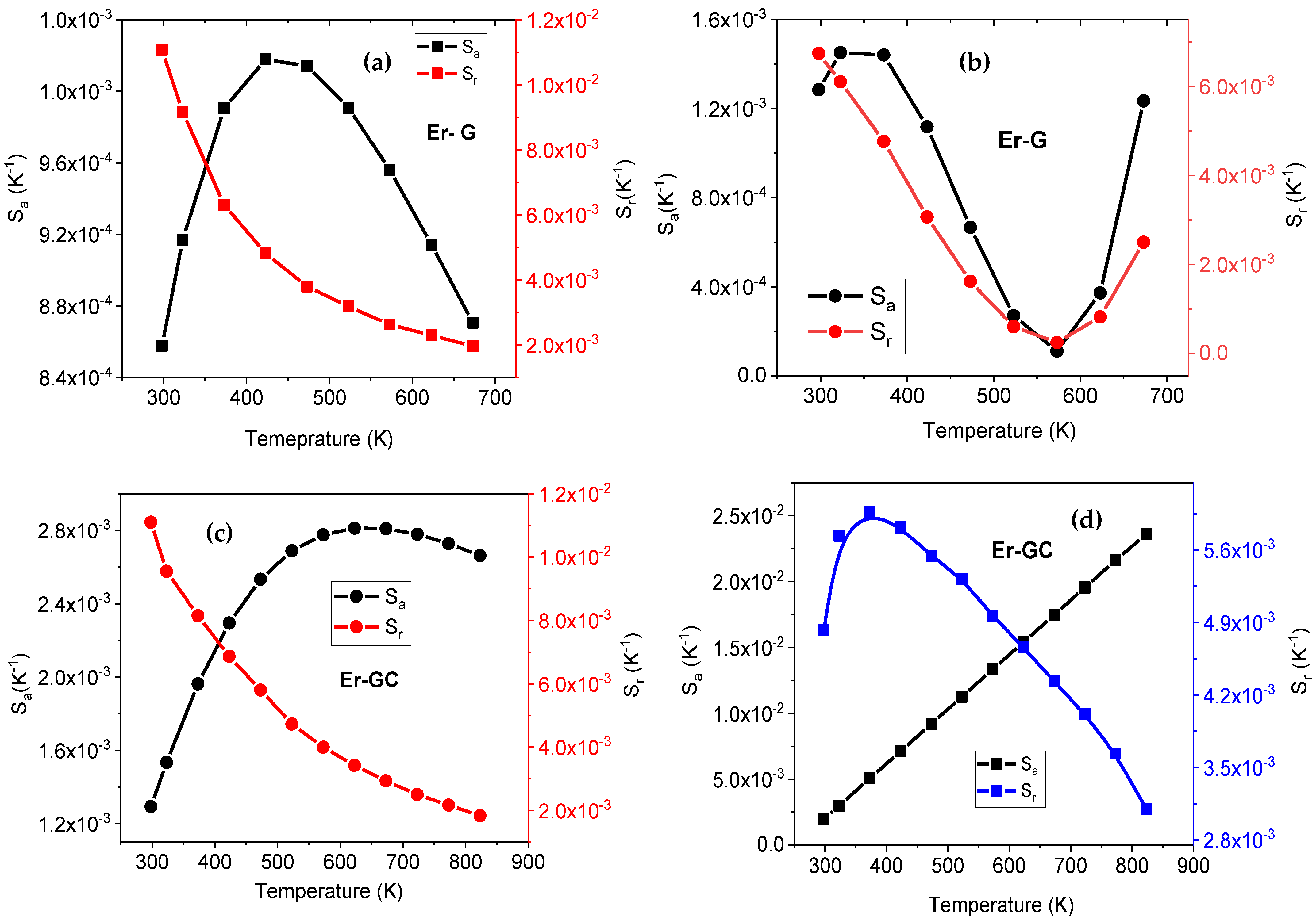
| Parameter | Er-GC |
|---|---|
| Phase | Na1.5Y1.45Er0.05F6 |
| Space group | P-6 (174) |
| a (Å) | 5.9831 |
| b (Å) | 5.9831 |
| c (Å) | 3.5350 |
| α (degrees) | 90 |
| β (degrees) | 90 |
| γ (degrees) | 120 |
| V (Å3) | 109.6094 |
| Re (%) | 3.42695 |
| Rp (%) | 2.52878 |
| Rwp (%) | 3.23458 |
| Sensing Materials | λexc (nm) | TCLs | NTCLs | Experimental Temperature Range (K) | Ref | |||
|---|---|---|---|---|---|---|---|---|
| ∆E (cm−1) | Maximum of Sa (×10−3 K−1) | Maximum of Sr (% K−1) | Maximum of Sa (× 10−3 K−1) | Maximum of Sr (% K−1) | ||||
| Er3+:Na1.5Y1.5F6-GC | 980 | 388 | 2.81 @ 623 | 1.1 | 23.64 @ 823 | 0.6 | 298–823 | Present work |
| Er3+/Yb3+: SiO2-BaF2-ZnF2 glass | 980 | 719 | 2.70 @ 513 | 1.2 | --- | --- | 291–450 | Feng et al. [40] |
| Er3+: PbO-Ga2O3-SiO2 glass | 980 | --- | 2.64 @ 590 | --- | --- | --- | 300–630 | Pisarski et al. [41] |
| Er3+/Yb3+: NaYb2F7-GC | 980 | 852 | 3.06 @ 600K | 1.3 | --- | --- | 300–773 | Hua et al. [42] |
| Er3+/Yb3+: NaLu2F7-GC | 980 | 861 | 4.73 @ 620 K | 1.3 | --- | --- | 300–773 | Cao et al. [43] |
| Er3+/Yb3+: NaYF4-GC | 980 | 774 | 2.30 @ 573 | 1.2 | --- | --- | 298–693 | Jiang et al. [44] |
| Er3+/Yb3+:fluoro phosphate glass | 980 | 390 | 1.5 @ 279 | --- | --- | --- | 130–490 | Lai et al. [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagupati, R.; Klement, R.; Rajavaram, R.; Velázquez, J.J.; Galusek, D. In Situ Synthesis of β-Na1.5Y1.5F6: Er3+ Crystals in Oxyfluoride Silicate Glass for Temperature Sensors and Their Spectral Conversion and Optical Thermometry Analysis. Molecules 2021, 26, 6901. https://doi.org/10.3390/molecules26226901
Dagupati R, Klement R, Rajavaram R, Velázquez JJ, Galusek D. In Situ Synthesis of β-Na1.5Y1.5F6: Er3+ Crystals in Oxyfluoride Silicate Glass for Temperature Sensors and Their Spectral Conversion and Optical Thermometry Analysis. Molecules. 2021; 26(22):6901. https://doi.org/10.3390/molecules26226901
Chicago/Turabian StyleDagupati, Rajesh, Robert Klement, Ramaraghavulu Rajavaram, José J. Velázquez, and Dušan Galusek. 2021. "In Situ Synthesis of β-Na1.5Y1.5F6: Er3+ Crystals in Oxyfluoride Silicate Glass for Temperature Sensors and Their Spectral Conversion and Optical Thermometry Analysis" Molecules 26, no. 22: 6901. https://doi.org/10.3390/molecules26226901
APA StyleDagupati, R., Klement, R., Rajavaram, R., Velázquez, J. J., & Galusek, D. (2021). In Situ Synthesis of β-Na1.5Y1.5F6: Er3+ Crystals in Oxyfluoride Silicate Glass for Temperature Sensors and Their Spectral Conversion and Optical Thermometry Analysis. Molecules, 26(22), 6901. https://doi.org/10.3390/molecules26226901










