Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review
Abstract
:1. Introduction
2. Starch Modification Processes
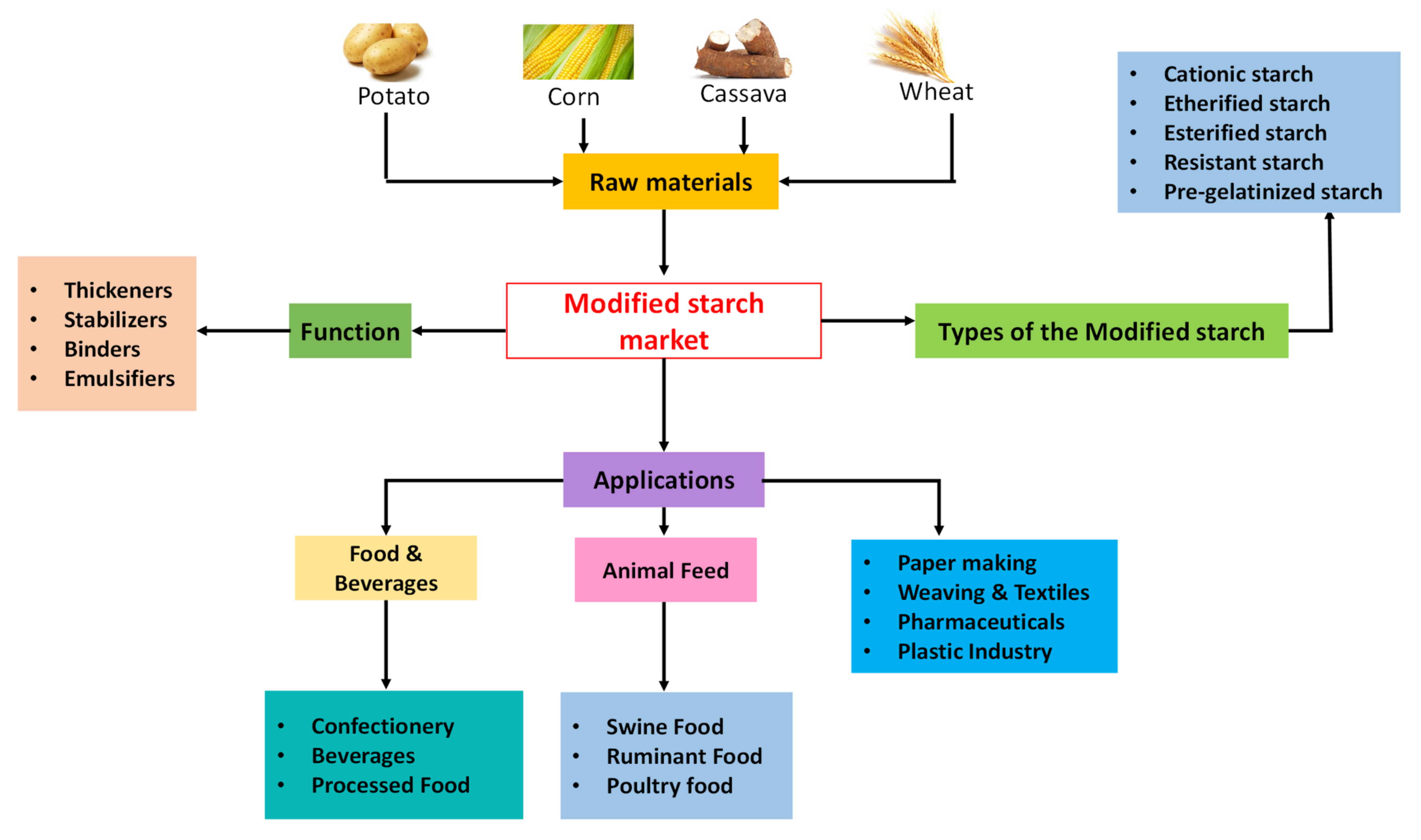
2.1. Genetic Modifications
2.2. Enzymatic Process
2.3. Physical Modifications
| Physical Modification | Description | References | |
|---|---|---|---|
| Thermal treatments | 1. Pre-gelatinization |
| [53] |
| 2. Heat Moisture Treatment (HMT) |
| [54] | |
| 3. Annealing |
| [53] | |
| 4. Microwave (MV) heating |
| [55] | |
| Non-thermal treatments | 1. Milling |
| [56] |
| 2. High-Pressure Treatment (HPT) |
| [56] | |
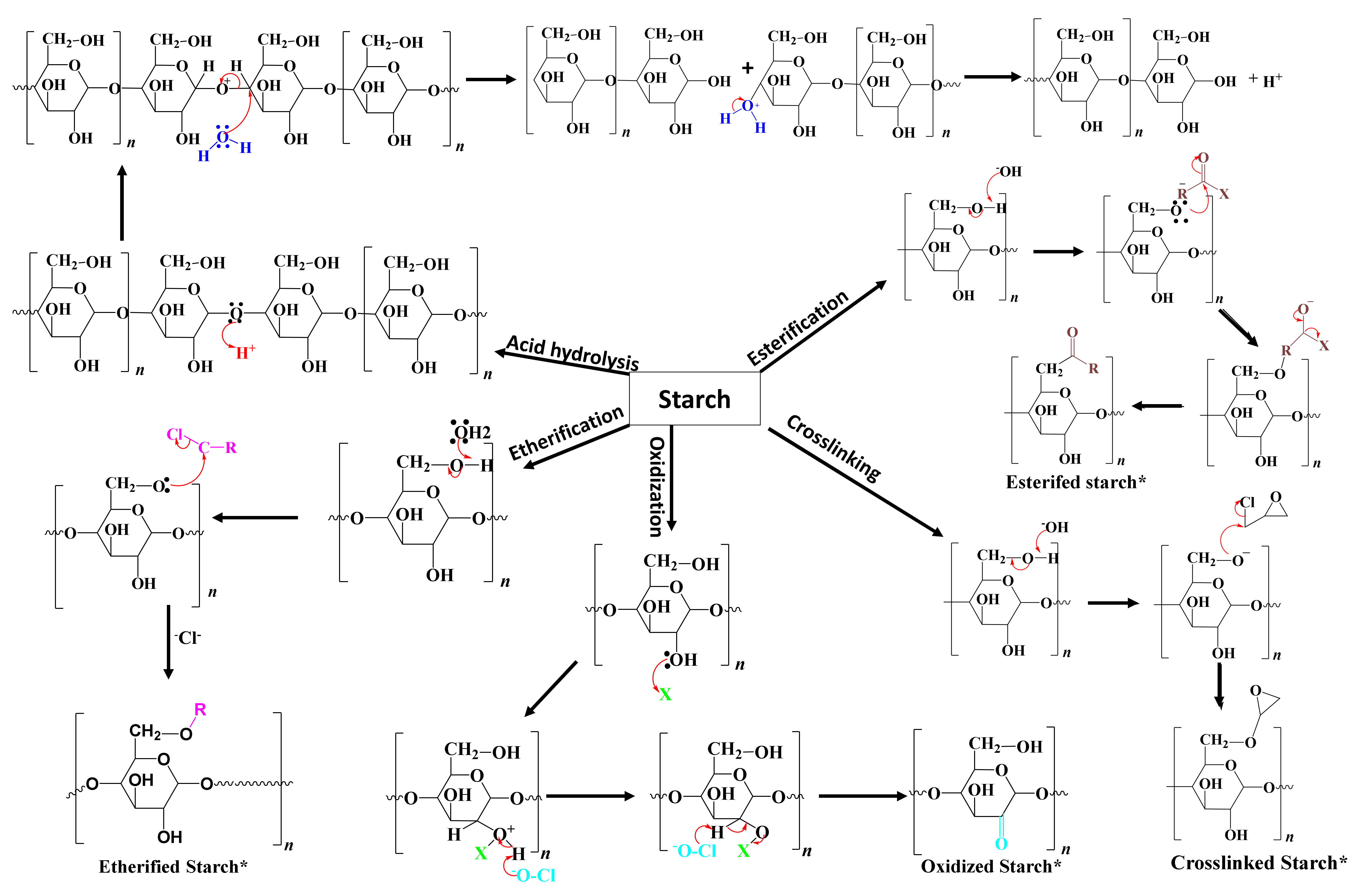
2.4. Chemical Modifications
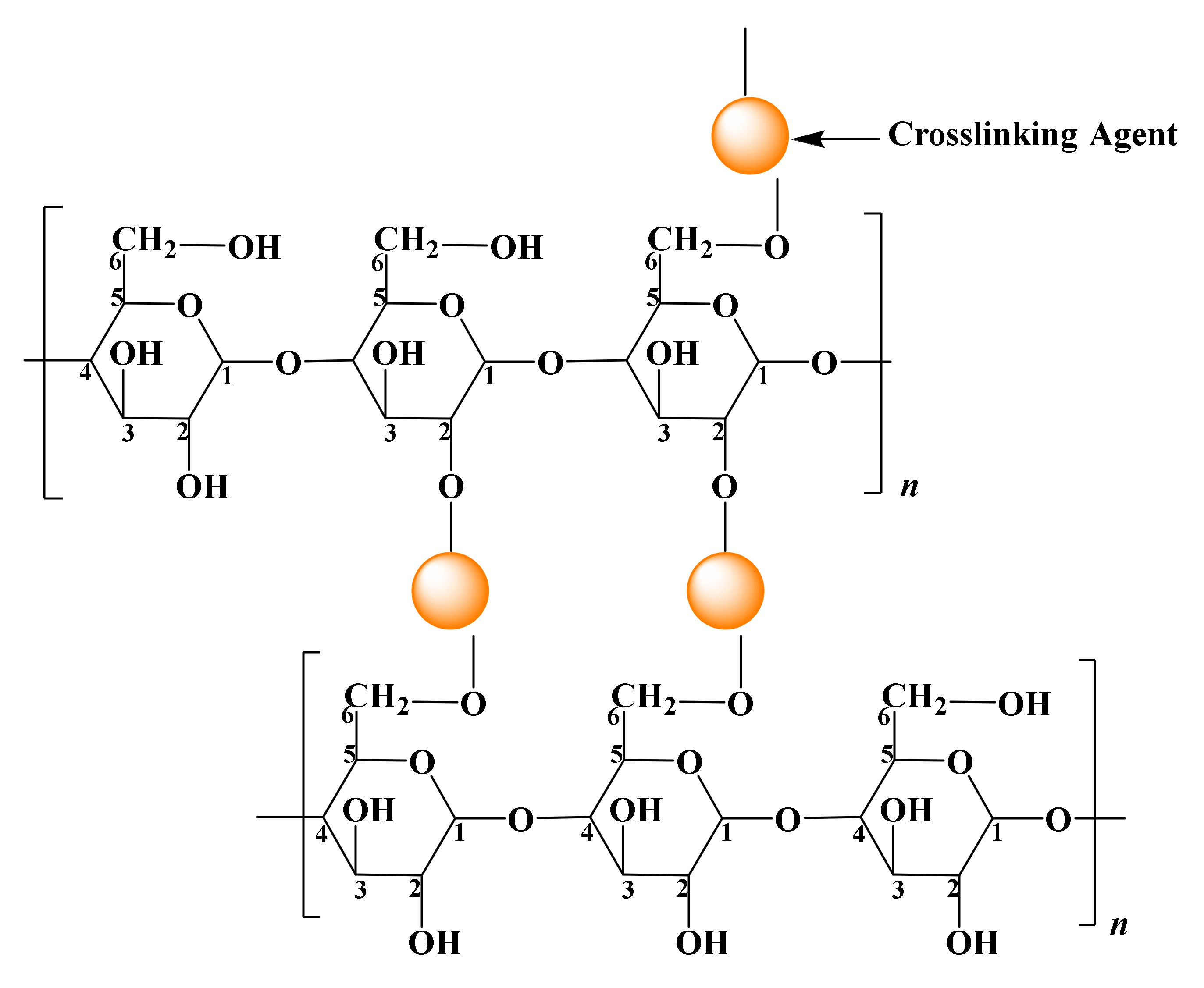
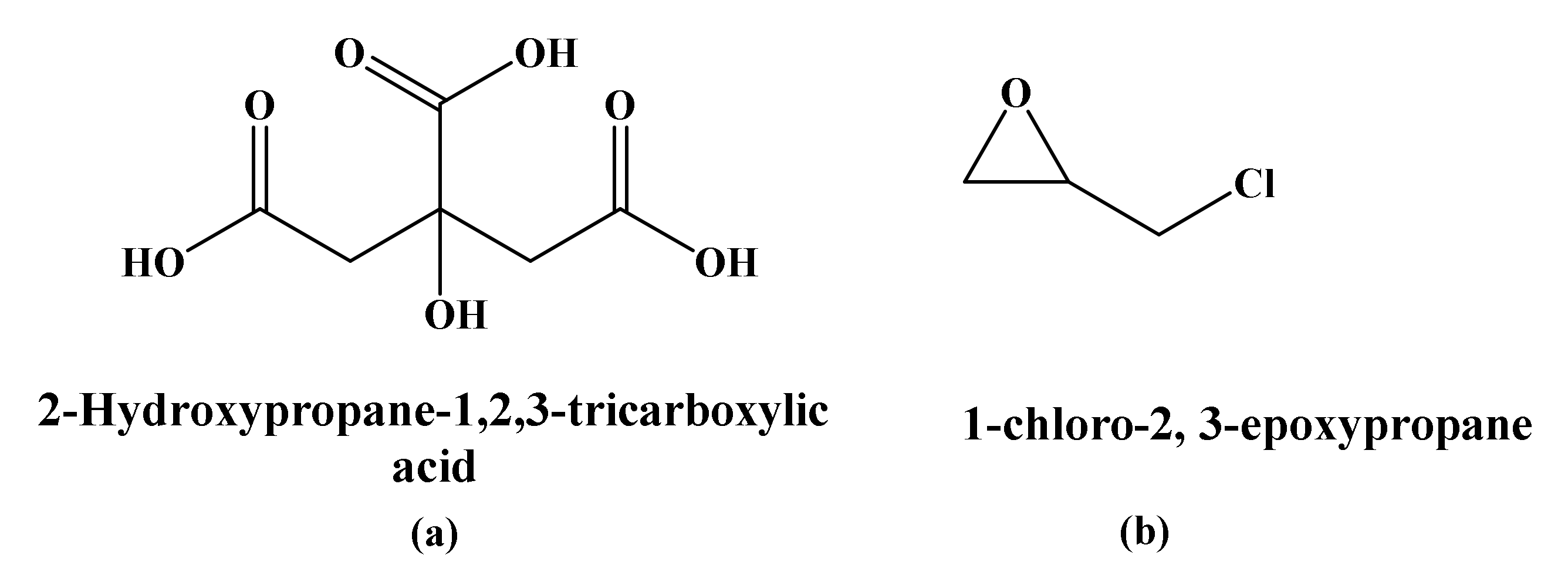
2.4.1. Crosslinking
2.4.2. Acid Hydrolysis
2.4.3. Alkali Treatment
| Property of Starch | Chemical Modification | Values | References | ||
|---|---|---|---|---|---|
| Reaction Type | Reagent | Before | After | ||
| Viscosity | Oxidation | Sodium hypochlorite | 173.8 mPa·s | 151.8 mPa·s | [75] |
| Grafting | Cassava starch grafted with Poly(acrylamide) (CS-g-PAM) | 1717 mPa·s | 4178 mPa·s | [76] | |
| Tensile strength | Crosslinking | Epichlorohydrin (EPI) | 10.04 MPa | 15.51 MPa | [66] |
| Crosslinking | Epichlorohydrin (EPI) | 5.01 MPa | 7.99 MPa | [67] | |
| Crosslinking | Sodium Trimetaphosphate (STMP) | 5.01 MPa | 8.23 MPa | [67] | |
| Crosslinking | Sodium Trimetaphosphate (STMP)/Sodium Tripolyphosphat (STPP) | 5.01 MPa | 7.57 MPa | [67] | |
| Alkali treatment | Sodium Hydroxide | 9.51 MPa | 10.03 MPa | [74] | |
| Etherification | Carboxymethyl starch | 1.1 MPa | 0.2 MPa | [77] | |
| Oxidation | Sodium hypochlorite | 3.8 MPa | 1.8 MPa | [75] | |
| Oxidation | Sodium hypochlorite | 3.53 MPa | 6.07 MPa | [78] | |
| Grafting | Thermoplastic Starch Grafted with Polylactic acid | 2.40 MPa | 0.06 MPa | [79] | |
| Oxidation | Sodium hypochlorite | 3.88 MPa | 5.05 MPa | [80] | |
| Oxidation | Sodium hypochlorite | 4.66 MPa | 8.39 MPa | [81] | |
| Oxidation | Hydrogen peroxide | 18.8 MPa | 24.7 MPa | [82] | |
| Etherification | Hydroxypropylated starch | 3.1 MPa | 4.9 MPa | [83] | |
| Grafting | Starch grafted with polystyrene | 43 MPa | 25 MPa | [84] | |
| Etherification | Carboxymethyl starch | 31 MPa | 14 MPa | [85] | |
| Water vapour permeability | Crosslinking | Citric acid | 33 g h−1·m−2 | 31 g·h−1·m−2 | [61] |
| Crosslinking | Citric acid | 2.8 × 10−10 g/ms·Pa | 1.8 × 10−10 g/ms·Pa | [63] | |
| Crosslinking | Epichlorohydrin (EPI) | 6.19 g·mm/m2·day·KPa | 1.89 g·mm/m2·day·KPa | [67] | |
| Crosslinking | Sodium Trimetaphosphate (STMP) | 6.19 g·mm/m2·day·KPa | 2.28 g·mm/m2·day·KPa | [67] | |
| Crosslinking | STMP/STPP | 6.19 g·mm/m2·day·KPa | 2.72 g·mm/m2·day·KPa | [67] | |
| Acid hydrolysis | Hydrochloric acid | 3.16 × 10−12 g·cm·cm−2·s−1·Pa−1 | 5.74 × 10−12 g·cm·cm−2·s−1· Pa−1 | [72] | |
| Acid hydrolysis | HCl (36%, w/w) at 45 °C for 24 h under stirring (225 rpm) | 0.30 g·mm·day−1·m−2·mm·Hg−1 | 0.10 g·mm·day−1·m−2·mmHg−1 | [73] | |
| Oxidation | Sodium hypochlorite | 9.3 g·mm/m2·day·kPa | 4.4 g·mm/m2·day·kPa | [75] | |
| Oxidation | Sodium hypochlorite | 16.23 × 10−11 g·Pa−1· s−1·m−1 | 21.54 × 10−11 g·Pa−1·s−1·m−1 | [80] | |
| Elongation at break | Crosslinking | Epichlorohydrin (EPI) | 200.42 | 217.11 | [66] |
| Etherification | Carboxymethyl starch | 40% | 140% | [77] | |
| Oxidation | Sodium hypochlorite | 85.20% | 84.90% | [78] | |
| Grafting | Thermoplastic Starch Grafted with Poly(lactic acid) (TPS-g-PLA) | 68.00% | 150.00% | [79] | |
| Oxidation | Sodium hypochlorite | 17.91% | 27.2% | [80] | |
| Oxidation | Hydrogen peroxide | 650% | 575% | [82] | |
| Oxidation | Hydroxypropylated starch | 46.4% | 43.5% | [83] | |
| Grafting | Poly(methyl methacrylate) grafted with modified Starch and styrene-butadiene rubber bio composites (PMMA-g-TPS/NR) | 564% | 888% | [86] | |
| Thermal stability (Decomposition Temperature) | Esterification | Acetic anhydride | 297 °C | 352 °C | [87] |
| Grafting | Corn starch grafted with Poly(methyl methacrylate) (CS-g-PMMA) | 310 °C | 332 °C | [88] | |
| Grafting | Starch grafted with N-tert-butylacrylamide | 316 °C | 343 °C | [89] | |
| Moisture absorbance | Crosslinking | Citric acid | 30% | 20% | [65] |
| Crosslinking | Phosphoryl chloride (POCl3) | 6.72% | 1.24% | [64] | |
| Crosslinking | Sodium Trimetaphosphate (STMP) | 6.72% | 3.29% | [64] | |
| Crosslinking | Epichlorohydrin (EPI) | 6.72% | 3.22% | [64] | |
| Crystallinity | Crosslinking | Epichlorohydrin (EPI) | 39.59% | 38.11% | [66] |
| Crosslinking | Epichlorohydrin (EPI) | 16.10% | 15.67% | [67] | |
| Crosslinking | Sodium Trimetaphosphate (STMP) | 16.10% | 15.13% | [67] | |
| Crosslinking | Sodium Trimetaphosphate (STMP)/Sodium Tripolyphosphat (STPP) | 16.10% | 14.79% | [67] | |
2.4.4. Esterification
2.4.5. Etherification
2.4.6. Oxidation
2.4.7. Grafting
2.4.8. Dual Modification
2.5. Applications of Modified Starch
2.5.1. Applications of Enzymatically Modified Starch
2.5.2. Applications of Physically Modified Starch
2.5.3. Applications of Chemically Modified Starch
| Applications | Modification Techniques | Examples | References |
|---|---|---|---|
| Packaging | Crosslinking | Cross-linked- TPS | [116] |
| Cross-linked starch | [117] | ||
| Cross-linked films of quaternary ammonium modified starch and Polyvinyl alcohol | [137] | ||
| Grafting | PVA (Polyvinyl alcohol)/starch and cellulosic material barley husk (BH) | [117] | |
| Grafting | Polyvinyl alcohol/modifiedstarch-based biodegradablenanocomposite films | [138] | |
| Oxidation | 7-Hydroxy-4-methylcoumarin doped Polyvinyl alcohol/oxidized maize starch | [119] | |
| Corn starch oxidation was performed using sodium periodate | [118] | ||
| Dual Modification | Etherified–oxidized cassavastarch/Polyvinyl alcohol blends | [139] | |
| Bio-based adhesives | Crosslinking | Cassava starch, citric acid and Polycarboxylic acid. | [120] |
| Grafting | Corn starch-g-poly (vinyl acetate-co-butyl acrylate) | [121] | |
| Starch-g-polyvinyl alcohol | [122] | ||
| Pharmaceutical Industries | Etherification | Anionic carboxymethyl and cationic carboxymethyl | [140] |
| Carboxymethyl starch | [141] | ||
| Carboxymethyl starch | [123] | ||
| Carboxymethyl starch | [124] | ||
| Carboxymethyl corn starch | [126] | ||
| Acetylation | Acetylated starch nano-crystals | [142] | |
| Crosslinking | Starch coated iron oxide nanoparticles | [143] | |
| Grafting | Fe3O4/starch-g-polyester nanocomposite | [144] | |
| Cellulose nanofiber-assisted starch graft-Polyacrylic acid | [145] | ||
| Esterification | Octenyl succinic anhydride modified starch | [125] | |
| Agriculture Industry | Crosslinking | Controlled-release nitrogen fertilizer | [127] |
| Oxidization | Starch-Based Antibacterial Nanocomposites | [129] | |
| Grafting | Polyacrylic acid graft- starch | [130] | |
| Cassava starch-graft-polyacrylonitrile-coated urea fertilizer | [128] | ||
| Superabsorbents | Crosslinking | Starch crosslinked with acrylic monomers | [131] |
| Crosslinked starch xanthate | [146] | ||
| Dithiocarbamate-modified starch | [147] | ||
| Fe2O3 nanoparticles-Starch nanocomposite | [148] | ||
| Grafting | Modified starch with zinc oxide and tetraethyl orthosilicate and graft copolymerized with potassium acrylate monomer | [132] | |
| Low-density polyethylene-g-poly (acrylic 2 acid)-co-starch/organo-montmorillonite hydrogel | [149] | ||
| Crown ether modification of starch | [150] | ||
| Grafted amylose and amylopectin using poly(sodium acrylate) | [136] | ||
| Esterification | Succinylated starch | [133] | |
| Facile esterification | [135] | ||
| Modified corn starch with maleic acid (MA) and itaconic acid (IA) | [151] | ||
| Dual modification | Modified cassava starch using tetraethylorthosilicate (TEOS) as the chemical modifying agent and Pluronic 123 as the structure directing agent | [134] | |
| Oxidations | Oxidized starch nanoparticles (SNPs) | [152] |
3. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, A.K.; Halley, P.J. Starch modification to develop novel starch-biopolymer blends: State of art and perspectives. In Starch Polymers; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.B.L.R.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef]
- Bertolini, A. (Ed.) Starches: Characterization, Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Vilaplana, F.; Hasjim, J.; Gilbert, R.G. Amylose content in starches: Toward optimal definition and validating experimental methods. Carbohydr. Polym. 2012, 88, 103–111. [Google Scholar] [CrossRef]
- European Bioplastic. 2019. Available online: https://docs.european-bioplastics.org/PR/2019/EUBP_PR_Marketdata_20191204.pdf (accessed on 11 February 2021).
- BeMiller, J.N.; Whistler, R.L. (Eds.) Starch: Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Parker, R.; Ring, S.G. Aspects of the physical chemistry of starch. J. Cereal Sci. 2001, 34, 1–17. [Google Scholar] [CrossRef]
- Canisag, H. Bio-Crosslinking of Starch Films with Oxidized Sucrose. Ph.D. Thesis, Faculty of the Graduate College, The University of Nebraska, Lincoln, NE, USA, 2015. [Google Scholar]
- Li, L.; Jiang, H.; Campbell, M.; Blanco, M.; Jane, J. Characterization of maize amylose-extender (ae) mutant starches. Part I: Relationship between resistant starch contents and molecular structures. Carbohydr. Polym. 2008, 74, 396–404. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Corcuera, V.; Salmoral, E.M.; Salerno, J.C.; Krisman, C.R. Starch molecular fractionation of bread wheat varieties. Agriscientia 2007, 24, 11–18. [Google Scholar]
- Sasaki, T.; Yasu, T.; Matsuki, J. Effect of amylose content on gelatinization, retrogradation, and pasting properties of starches from waxy and nonwaxy wheat and their F1 seeds. Cereal Chem. 2000, 77, 58–63. [Google Scholar] [CrossRef]
- Duan, D.X.; Donner, E.; Liu, Q.; Smith, D.C.; Ravenelle, F. Potentiometric titration for determination of amylose content of starch—A comparison with colorimetric method. Food Chem. 2012, 130, 1142–1145. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Zhuang, X.; Chen, X.; Jing, X. Compatibilizing effect of starch-grafted-poly (l-lactide) on the poly (ε-caprolactone)/starch composites. J. Appl. Polym. Sci. 2010, 117, 2724–2731. [Google Scholar] [CrossRef]
- Ahmed, J.; Tiwari, B.K.; Iman, S.H.; Rao, M.A. Starch-Based Polymeric Materials and Nanocomposites. In Chemistry, Processing, and Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kalambur, S.; Rizvi, S.S.H. An overview of starch-based plastic blends from reactive extrusion. J. Plast. Film Sheeting 2006, 22, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Jantanasakulwons, K.; Leksawasdi, N.; Seesuriyachan, P.; Wongsuriyasak, S.; Techapun, C.; Ougizawa, T. Reactive blending of thermoplastic starch and polyethylene-graft- maleic anhydride with chitosan as compatibilizer. Carbohydr. Polym. 2016, 153, 89–95. [Google Scholar] [CrossRef]
- Ma, P.; Xu, P.; Chen, M.; Dong, W.; Cai, X.; Schmit, P.; Spolstra, A.B.; Lemistic, P.J. Structure-properties relationship of reactively compatibilized PHB/EVA/Starch blends. J. Carbohydr. Polym. 2014, 108, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Wojtowicz, A.; Janssen, L.P.B.M.; Moscicki, L. Blends of Natural and Synthetic Polymers. In Thermoplastic Starch: A Green Material for Various Industries; Janssen, L.P.B.M., Moscicki, L., Eds.; Wiley: Weinheim, Germany, 2009. [Google Scholar]
- Fishman, M.L.; Cooke, P.; White, B.; Damert, W. Size distributions of amylose and amylopectin solubilized from corn starch granules. Carbohydr. Polym. 1995, 26, 245–253. [Google Scholar] [CrossRef]
- Aberle, T.; Burchard, W.; Vorwerg, W.; Radosta, S. Conformational contributions of amylose and amylopectin to the structural properties of starches from various sources. Starch 1994, 46, 329–335. [Google Scholar] [CrossRef]
- Hanselmann, R.; Burchard, W.; Ehrat, M.; Widmer, H.M. Structural properties of fractionated starch polymers and their dependence on the dissolution process. Macromolecules 1996, 29, 3277–3282. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Paredes-Lopez, O. Starch and Amylopectin-Effects of solutes on clarity of pastes. Starch 1996, 48, 205–207. [Google Scholar] [CrossRef]
- Han, J.A.; Lim, H.; Lim, S.T. Comparison between size exclusion chromatography and micro-batch analyses of corn starches in DMSO using light scattering detector. Starch 2005, 57, 262–267. [Google Scholar] [CrossRef]
- Tang, H.; Mitsunaga, T.; Kawamura, Y. Molecular arrangement in blocklets and starch granule architecture. Carbohydr. Polym. 2006, 63, 555–560. [Google Scholar] [CrossRef]
- Grand View Research, Inc. Modified Starch Market Size, by Product 2014–2025 (USD Billion). Global Modified Starch Market Size, Share Report, 2019–2025. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/modified-starch-market (accessed on 11 February 2021).
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef]
- Guida, C.; Aguiar, A.C.; Cunha, R.L. Green techniques for starch modification to stabilize Pickering emulsions: A current review and future perspectives. Curr. Opin. Food Sci. 2021, 38, 52–61. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Hj. Latip, D.N.; Samsudin, H.; Utra, U.; Alias, A.K. Modification methods toward the production of porous starch: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2841–2862. [Google Scholar] [CrossRef]
- Neelam, K.; Vijay, S.; Lalit, S. Various techniques for the modification of Starch and the applications of its derivatives. Int. Res. J. Pharm. 2012, 3, 25–31. [Google Scholar]
- Johnson, L.A.; Hardy, C.L.; Baumel, C.P.; White, P.J. Identifying valuable corn quality traits for starch production. Cereal Foods World 2001, 46, 417–423. [Google Scholar]
- Schwall, G.P.; Safford, R.; Westcott, R.J.; Jeffcoat, R.; Tayal, A.; Shi, Y.C.; Gidley, M.J.; Jobling, S.A. Production of very-high-amylose potato starch by inhibition of SBE A and B. Nat. Biotechnol. 2000, 18, 551–554. [Google Scholar] [CrossRef]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W.; et al. Accelerated ex situ breeding of GBSS-and PTST1-edited cassava for modified starch. Sci. Adv. 2018, 4, 6086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Andersson, M.; Andersson, R. Resistant starch and other dietary fiber components in tubers from a high-amylose potato. Food Chem. 2018, 251, 58–63. [Google Scholar] [CrossRef]
- Li, K.T.; Moulin, M.; Mangel, N.; Albersen, M.; Verhoeven-Duif, N.M.; Ma, Q.; Zhang, P.; Fitzpatrick, T.B.; Gruissem, W.; Vanderschuren, H. Increased bioavailable vitamin B 6 in field-grown transgenic cassava for dietary sufficiency. Nat. Biotechnol. 2015, 33, 1029–1032. [Google Scholar] [CrossRef]
- Hii, S.L.; Tan, J.S.; Ling, T.C.; Ariff, A.B. Pullulanase: Role in starch hydrolysis and potential industrial applications. Enzyme Res. 2012, 2012, 921362. [Google Scholar] [CrossRef] [Green Version]
- Van der Maarel, M.J.; Van der Veen, B.; Uitdehaag, J.C.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef] [Green Version]
- Haki, G.D.; Rakshit, S.K. Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef]
- BeMiller, J.N. Physical modification of starch. In Starch in Food; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Zhang, H.; Wang, J.K.; Liu, W.J.; Li, F.Y. Microwave-assisted synthesis, characterization, and textile sizing property of carboxymethyl corn starch. Fibers Polym. 2015, 16, 2308–2317. [Google Scholar] [CrossRef]
- Eltaboni, F.; Alabidi, A. Physical and chemical modifications of starches. In Proceedings of the 2nd Libya Conference on Chemistry and Its Applications (LCCA-2), Benghazi, Libya, 9–11 May 2017; pp. 9–11. [Google Scholar]
- Alissandratos, A.; Halling, P.J. Enzymatic acylation of starch. Bioresour. Technol. 2012, 115, 41–47. [Google Scholar] [CrossRef]
- Golachowski, A.; Zięba, T.; Kapelko-Żeberska, M.; Drożdż, W.; Gryszkin, A.; Grzechac, M. Current research addressing starch acetylation. Food Chem. 2015, 176, 350–356. [Google Scholar] [CrossRef]
- Gill, A.N.; Iftikhar, A.; Rashid, A.; Amin, M.; Khan, R.R.M.; Rafique, H.M.; Jelani, S.; Adnan, A. Lipase-catalyzed green synthesis of starch–maleate monoesters and its characterization. J. Iran. Chem. Soc. 2018, 15, 1939–1945. [Google Scholar] [CrossRef]
- Adak, S.; Banerjee, R. A green approach for starch modification: Esterification by lipase and novel imidazolium surfactant. Carbohydr. Polym. 2016, 150, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible, and resistant starch levels in native and gelatinized corn, pea, and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Bertolini, A.C.; Mestres, C.; Colonna, P.; Raffi, J. Free radical formation in UV-and gamma-irradiated cassava starch. Carbohydr. Polym. 2001, 44, 269–271. [Google Scholar] [CrossRef]
- Xing, G.X.; Zhang, S.F.; Ju, B.Z.; Yang, J.Z. Microwave-assisted synthesis of starch maleate by dry method. Starch 2006, 58, 464–467. [Google Scholar] [CrossRef]
- Souza, A.C.; Benze, R.F.E.S.; Ferrão, E.S.; Ditchfield, C.; Coelho, A.C.V.; Tadini, C.C. Cassava starch biodegradable films: Influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT Food Sci. Technol. 2012, 46, 110–117. [Google Scholar] [CrossRef]
- Zhao, K.; Li, B.; Xu, M.; Jing, L.; Gou, M.; Yu, Z.; Zheng, J.; Li, W. Microwave pretreated esterification improved the substitution degree, structural and physicochemical properties of potato starch esters. LWT Food Sci. Technol. 2018, 90, 116–123. [Google Scholar] [CrossRef]
- Zia-ud-Din; Xiong, H.; Fei, P. Physical and chemical modification of starches: A review. Crit. Rev. Food Sci. 2017, 57, 2691–2705. [Google Scholar] [CrossRef]
- Hoover, R. The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit. Rev. Food. 2010, 50, 835–847. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, M.; Yuan, S.; Sun, S.; Huo, Q. Effect of microwave treatment on the physicochemical properties of potato starch granules. Chem Cent. J. 2013, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BeMiller, J.N.; Huber, K.C. Physical modification of food starch functionalities. Annu. Rev. Food Sci. Technol. 2015, 6, 19–69. [Google Scholar] [CrossRef] [PubMed]
- Oyeyinka, S.A.; Afunso, T.M.; Adeloye, A.A.; Diarra, S.S. Morphology, pasting and thermal properties of microwave-assisted cassava starch-stearic acid complex. Ceylon J. Sci. 2020, 49, 275–281. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Sajeev, M.S.; Moorthy, S.N.; Sreekumar, J.; Rajasekharan, K.N. Microwave-assisted Synthesis of Cassava Starch Phosphates and their Characterization. J. Root Crop. 2008, 34, 34–42. [Google Scholar]
- Lewandowicz, G.; Błaszczak, W.; Voelkel, E. Ionic starch derivatives obtained in microwave assisted reactions-structure and functionality. ZYWNOSC 2000, 2, 126. [Google Scholar]
- Chen, Y.F.; Kaur, L.; Singh, J. Chemical modification of starch. In Starch in Food; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Reddy, N.; Yang, Y. Citric acid crosslinking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yu, H.; Wang, L.; Abdin, Z.U.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R.U.; Zhang, H.; et al. Recent progress in chemical modification of Starch and its applications. RSC Adv. 2015, 5, 67459–67474. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Garcia, R.; Sanchez-Rivera, M.M.; Méndez-Montealvo, G.; Garza-Montoya, B.; Bello-Pérez, L.A. Effect of the crosslinked reagent type on some morphological, physicochemical, and functional characteristics of banana starch (Musa paradisiaca). Carbohydr. Polym. 2009, 76, 117–122. [Google Scholar] [CrossRef]
- Rioux, B.; Ispas-Szabo, P.; Aït-Kadi, A.; Mateescu, M.A.; Juhász, J. Structure–properties relationship in crosslinked high amylose starch cast films. Carbohydr. Polym. 2002, 50, 371–378. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.J. Characteristics of crosslinked potato starch and starch-filled linear low-density polyethylene films. Carbohydr. Polym. 2002, 50, 331–337. [Google Scholar] [CrossRef]
- Detduangchan, N.; Sridach, W.; Wittaya, T. Enhancement of the properties of biodegradable rice starch films by using chemical crosslinking agents. Int. Food. Res. J. 2014, 21, 1225–1235. [Google Scholar]
- Hoover, R. Acid-treated starches. Food Rev. Int. 2000, 16, 69–392. [Google Scholar] [CrossRef]
- Kramer, M.E. Structure and function of starch-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 113–134. [Google Scholar]
- Singh, J.; Colussi, R.; McCarthy, O.J.; Kaur, L. Potato starch and its modification. In Advances in Potato Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Sakkara, S.; Nataraj, D.; Venkatesh, K.; Xu, Y.; Patil, J.H.; Reddy, N. Effect of pH on the physicochemical properties of starch films. J. Appl. Polym. Sci. 2020, 137, 48563. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, H.; Liu, P.; Wang, W.; Dong, H. Effects of acid hydrolysis on the physicochemical properties of pea starch and its film forming capacity. Food Hydrocolloid. 2019, 87, 173–179. [Google Scholar] [CrossRef]
- Luchese, C.L.; Frick, J.M.; Patzer, V.L.; Spada, J.C.; Tessaro, I.C. Synthesis and characterization of biofilms using native and modified pinhão Starch. Food Hydrocoll. 2015, 45, 203–210. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, H.; Dai, Y.; Hou, H.; Dong, H. Effect of alkali treatment on structure and properties of high amylose corn starch film. Materials 2019, 12, 1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, L.M.; Gonçalves, J.R.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; Da Rosa Zavareze, E. Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT Food Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, A.N.; Sajeev, M.S.; Moorthy, S.N.; Sreekumar, J. Effect of graft-copolymerization with poly (acrylamide) on rheological and thermal properties of cassava starch. J. Appl. Polym. Sci. 2010, 116, 337–346. [Google Scholar] [CrossRef]
- Wilpiszewska, K. Hydrophilic films based on starch and carboxymethyl starch. Pol. J. ChemTechnol. 2019, 21, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Zavareze, E.; Pinto, V.Z.; Klein, B.; El Halal, S.L.M.; Elias, M.C.; Prentice-Hernández, C.; Dias, A.R.G. Development of oxidised and Heat–moisture treated potato starch film. Food Chem. 2012, 132, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas-Carballo, Z.B.; Duarte-Aranda, S.; Canché-Escamilla, G. Properties and biodegradation of thermoplastic starch obtained from grafted starches with poly (lactic acid). J. Polym. Environ. 2019, 27, 2607–2617. [Google Scholar] [CrossRef]
- García, A.E.; Solorza-Feria, J.; Rendón-Villalobos, J.R.; Rodríguez-González, F.; Jiménez-Pérez, A.; Flores-Huicochea, E. Properties of edible films based on oxidized starch and zein. Int. J. Polym. Sci. 2014, 2014, 292404. [Google Scholar] [CrossRef] [Green Version]
- Zamudio-Flores, P.B.; Torres, A.V.; Salgado-Delgado, R.; Bello-Pérez, L.A. Influence of the oxidation and acetylation of banana starch on the mechanical and water barrier properties of modified starch and modified starch/chitosan blend films. J. Appl. Polym. Sci. 2010, 115, 991–998. [Google Scholar] [CrossRef]
- Wang, Z.F.; Peng, Z.; Li, S.D.; Lin, H.; Zhang, K.X.; She, X.D.; Fu, X. The impact of esterification on the properties of starch/natural rubber composite. Compos. Sci. Technol. 2009, 69, 1797–1803. [Google Scholar] [CrossRef]
- Vorwerg, W.; Dijksterhuis, J.; Borghuis, J.; Radosta, S.; Kröger, A. Film properties of hydroxypropyl starch. Starch 2004, 56, 297–306. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Sonsuk, M.; Wittayapichet, S.; Prasassarakich, P.; Vejjanukroh, P.C. Degradation of styrene-g-cassava starch filled polystyrene plastics. Polym. Degrad. Stab. 1999, 66, 323–335. [Google Scholar] [CrossRef]
- Rachtanapun, P. Blended films of carboxymethyl cellulose from papaya peel (CMCp) and corn starch. Agric. Nat. Resour. 2009, 43, 259–266. [Google Scholar]
- Li, M.C.; Ge, X.; Cho, U.R. Mechanical performance, water absorption behavior and biodegradability of poly (methyl methacrylate)-modified starch/SBR biocomposites. Macromol. Res. 2013, 21, 793–800. [Google Scholar] [CrossRef]
- Xu, Y.; Miladinov, V.; Hanna, M.A. Synthesis and characterization of starch acetates with high substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Li, M.C.; Lee, J.K.; Cho, U.R. Synthesis, characterization, and enzymatic degradation of starch-grafted poly (methyl methacrylate) copolymer films. J. Appl. Polym. Sci. 2012, 125, 405–414. [Google Scholar] [CrossRef]
- Fares, M.M.; El-Faqeeh, A.S.; Osman, M.E. Graft copolymerization onto starch—I. Synthesis and optimization of starch grafted with N-tert-butylacrylamide copolymer and its hydrogels. J. Polym. Res. 2003, 10, 119–125. [Google Scholar] [CrossRef]
- Abdul Hadi, N.; Wiege, B.; Stabenau, S.; Marefati, A.; Rayner, M. Comparison of three methods to determine the degree of substitution of quinoa and rice starch acetates, propionates, and butyrates: Direct stoichiometry, FTIR, and 1H-NMR. Foods 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fringant, C.; Rinaudo, M.; Foray, M.F.; Bardet, M. Preparation of mixed esters of Starch or use of an external plasticizer: Two different ways to change the properties of starch acetate films. Carbohydr. Polym. 1998, 35, 97–106. [Google Scholar] [CrossRef]
- Bonacucina, G.; Di Martino, P.; Piombetti, M.; Colombo, A.; Roversi, F.; Palmieri, G.F. Effect of plasticizers on properties of pregelatinised starch acetate (Amprac 01) free films. Int. J. Pharm. 2006, 313, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical modification of Starch and its application as an adsorbent material. RSC Adv. 2016, 6, 78264–78285. [Google Scholar] [CrossRef]
- Isotton, F.S.; Bernardo, G.L.; Baldasso, C.; Rosa, L.M.; Zeni, M. The plasticizer effect on preparation and properties of etherified corn starchs films. Ind. Crop. Prod. 2015, 76, 717–724. [Google Scholar] [CrossRef]
- Schmitz, C.S.; De Simas, K.N.; Santos, K.; Joao, J.J.; de Mello Castanho Amboni, R.D.; Amante, E.R. Cassava starch functional properties by etherification–hydroxypropylation. Int. J. Food Sci. Techonl. 2006, 41, 681–687. [Google Scholar] [CrossRef]
- Ganorkar, P.M.; Kulkarni, A. Studies on preparation and functional properties of carboxymethyl starch from sorghum. Int. Food Res. J. 2013, 20, 26–30. [Google Scholar]
- Garrido, L.H.; Schnitzler, E.; Zortéa, M.E.B.; de Souza Rocha, T.; Demiate, I.M. Physicochemical properties of cassava starch oxidized by sodium hypochlorite. J. Food Sci. Technol. 2014, 51, 2640–2647. [Google Scholar] [CrossRef] [Green Version]
- Ludin, D.V.; Zaitsev, S.D.; Kuznetsova, Y.L.; Markin, A.V.; Mochalova, A.E.; Salomatina, E.V. Starch-graft-poly (methyl acrylate) copolymer: The new approach to synthesis and copolymer characterization. J. Polym. Res. 2017, 24, 117. [Google Scholar] [CrossRef]
- Liu, H.; Ramsden, L.; Corke, H. Physical properties of cross-linked and acetylated normal and waxy rice starch. Starch 1999, 51, 249–252. [Google Scholar] [CrossRef]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzyme Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Na, Y.; Kim, J.; Dal Kang, S.; Park, K.H. Properties and applications of starch modifying enzymes for use in the baking industry. Food Sci. Biotechnol. 2018, 27, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Le Loan, T.K.; Thuy, N.M.; Le Tri, Q.; Sunghoon, P. Characterization of gluten-free rice bread prepared using a combination of potato tuber and ramie leaf enzymes. Food Sci. Biotechnol. 2021, 30, 521–529. [Google Scholar] [CrossRef]
- Montoya, D.; Barbosa, L.O.; Méndez, J.; Murillo, W. Morphological, Structural, and Functional Evaluation of Rice Starch Acylated in a System Catalyzed by the B-Lipase of Candida antarctica. Starch 2020, 72, 2000010. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Q.; Fu, X.; Jane, J.L. Modification of starch octenylsuccinate by β-amylase hydrolysis in order to increase its emulsification properties. Food Hydrocoll. 2015, 48, 55–61. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, G.; Gu, Z. Recent advances of starch-based excipients used in extended-release tablets: A review. Drug Deliv. 2016, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Dura, A.; Rosell, C.M. Physico-chemical properties of corn starch modified with cyclodextrin glycosyltransferase. Int. J. Biol. Macromol. 2016, 87, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agama-Acevedo, E.; Pacheco-Vargas, G.; Bello-Pérez, L.A.; Alvarez-Ramirez, J. Effect of drying method and hydrothermal treatment of pregelatinized Hylon VII starch on resistant starch content. Food Hydrocoll. 2018, 77, 817–824. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Lu, P.; Miao, S.; Zhang, Y.; Chen, L. Dry heating and annealing treatment synergistically modulate starch structure and digestibility. Int. J. Biol. Macromol. 2019, 137, 554–561. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of physically modified oat starch with different sonication treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- Lei, N.; Chai, S.; Xu, M.; Ji, J.; Mao, H.; Yan, S.; Gao, Y.; Li, H.; Wang, J.; Sun, B. Effect of dry heating treatment on multi-levels of structure and physicochemical properties of maize starch: A thermodynamic study. Int. J. Biol. Macromol. 2020, 147, 109–116. [Google Scholar] [CrossRef]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars. LWT Food Sci. Technol. 2019, 110, 197–206. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Junior, M.D.M.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E. Preparation of cassava starch hydrogels for application in 3D printing using dry heating treatment (DHT): A prospective study on the effects of DHT and gelatinization conditions. Int. Food Res. J. 2020, 128, 108803. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7, 100028. [Google Scholar] [CrossRef]
- Peidayesh, H.; Heydari, A.; Mosnáčková, K.; Chodák, I. In situ dual crosslinking strategy to improve the physico-chemical properties of thermoplastic starch. Carbohydr. Polym. 2021, 269, 118250. [Google Scholar] [CrossRef]
- Sharma, V.; Kaur, M.; Sandhu, K.S.; Godara, S.K. Effect of cross-linking on physico-chemical, thermal, pasting, in vitro digestibility and film forming properties of Faba bean (Vicia faba L.) starch. Int. J. Biol. Macromol. 2020, 159, 243–249. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Fabrication and characteristics of poly (vinyl alcohol)-starch-cellulosic material based biodegradable composite film for packaging application. Mater. Today Proc. 2020, 21, 1577–1582. [Google Scholar] [CrossRef]
- Moreno, O.; Cárdenas, J.; Atarés, L.; Chiralt, A. Influence of starch oxidation on the functionality of starch-gelatin based active films. Carbohydr. Polym. 2017, 178, 147–158. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Sataraddi, S.; Bayannavar, P.K.; Gasti, T.; Masti, S.P.; Kamble, R.R.; Chougale, R.B. Mechanical, optical and antioxidant properties of 7-hydroxy-4-methyl coumarin doped polyvinyl alcohol/oxidized maize starch blend films. SN Appl. Sci. 2020, 2, 1877. [Google Scholar] [CrossRef]
- Monroy, Y.; Seré, P.; Rivero, S.; García, M.A. Sustainable panels based on starch bioadhesives: An insight into structural and tribological performance. Int. J. Biol. Macromol. 2020, 148, 898–907. [Google Scholar] [CrossRef]
- Chen, L.; Ullah, I.; Wang, P.K.; Javaid, A.B.; Hu, C.; Zhang, M.; Ahamd, I.; Xiong, H.; Wang, Z. Synthesis and characterization of starch-g-poly (vinyl acetate-co-butyl acrylate) bio-based adhesive for wood application. Int. J. Biol. Macromol. 2018, 114, 1186–1193. [Google Scholar]
- Chen, L.; Xiong, Z.; Li, Q.; Din, Z.U.; Xiong, H. Sodium dodecyl sulfate improves the properties of bio-based wood adhesive derived from micronized starch: Microstructure and rheological behaviors. Int. J. Biol. Macromol. 2019, 140, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Marcelino, H.R.; Cardoso, L.G.; de Souza, C.O.; Durian, J.I. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Quadrado, R.F.; Fajardo, A.R. Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arab J. Chem. 2020, 13, 2183–2194. [Google Scholar] [CrossRef]
- Mora, C.P.; Martinez-Alejo, J.M.; Roman, L.; Martinez, M.M.; Carbajal, T.; Pinal, R.; Mora-Huertas, C.E. Molecular and physical characterization of octenyl succinic anhydride-modified starches with potential applications in pharmaceutics. Int. J. Pharm. 2020, 579, 119163. [Google Scholar] [CrossRef]
- Jiang, M.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Preparation of a starch-based carrier for oral delivery of Vitamin E to the small intestine. Food Hydrocoll. 2019, 91, 26–33. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Naz, M.Y.; Shukrullah, S.; Sulaiman, S.A.; Ghaffar, A.; AbdEl-Salam, N.M. Nitrogen pollution impact and remediation through low cost starch based biodegradable polymers. Sci. Rep. 2020, 10, 5927. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, A.N.; Pillai, S.S.; Aravind, M.; Salim, S.A.; Kuzhivilayil, S.J. Cassava starch-graft-poly (acrylonitrile)-coated urea fertilizer with sustained release and water retention properties. Adv. Polym. Technol. 2018, 37, 2687–2694. [Google Scholar] [CrossRef]
- Merino, D.; Gutiérrez, T.J.; Mansilla, A.Y.; Casalongué, C.A.; Alvarez, V.A. Critical evaluation of starch-based antibacterial nanocomposites as agricultural mulch films: Study on their interactions with water and light. ACS Sustain. Chem. Eng. 2018, 6, 15662–15672. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Dai, R.; Shan, Z.; Chen, H. Fabrication and characterization of one high-hygroscopicity liquid starch-based mulching materials for facilitating the growth of plant. Carbohydr. Polym. 2020, 230, 115582. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural superabsorbents based on Starch-g-poly (acrylic acid): Modification, synthesis and application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef]
- Dispat, N.; Poompradub, S.; Kiatkamjornwong, S. Synthesis of ZnO/SiO2-modified starch-graft-polyacrylate superabsorbent polymer for agricultural application. Carbohydr. Polym. 2020, 249, 116862. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; León, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Succinylated Starches for Dye Removal. Starch 2021, 73, 2000043. [Google Scholar] [CrossRef]
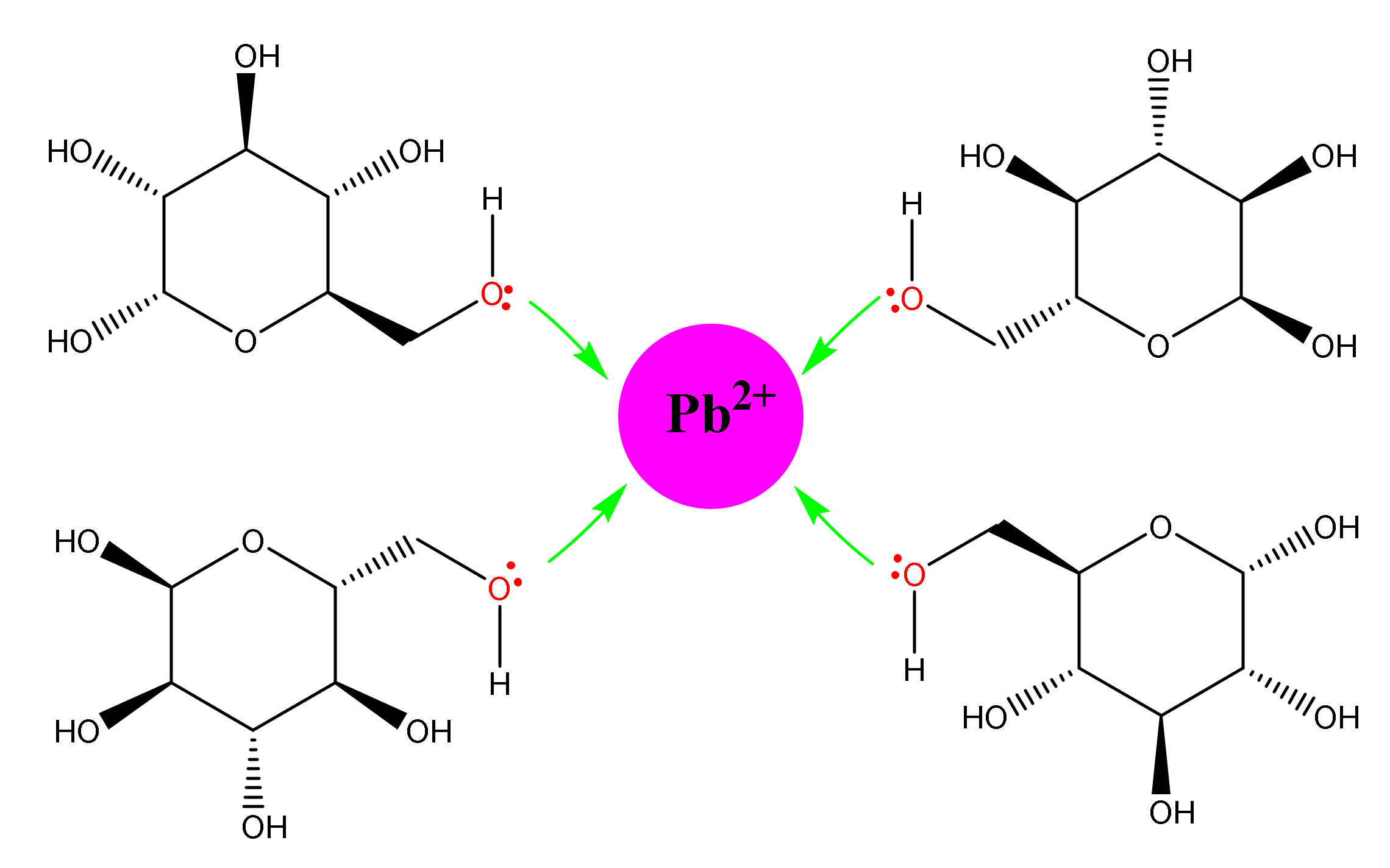
- Gunawardene, O.H.P.; Gunathilake, C.A.; Amaraweera, A.P.S.M.; Fernando, N.M.L.; Manipura, A.; Manamperi, W.A.; Kulatunga, K.M.A.K.; Rajapaksha, S.M.; Gamage, A.; Dassanayake, R.S.; et al. Removal of Pb (II) Ions from Aqueous Solution Using Modified Starch. J. Compos. Sci. 2021, 5, 46. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wang, Q.; Fan, X.; Yu, Y.; Wang, P.; Zhang, Y.; Yuan, J.; Cavaco-Paulo, A. Preparation and rheological properties of starch-g-poly (butyl acrylate) catalyzed by horseradish peroxidase. Process Biochem. 2017, 59, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Bai, W.; Fan, L.; Zhou, Y.; Zhang, Y.; Shi, J.; Lv, G.; Wu, Y.; Liu, Q.; Song, J. Removal of Cd2+ ions from aqueous solution using cassava starch–based superabsorbent polymers. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Pour, Z.S.; Makvandi, P.; Ghaemy, M. Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly (vinyl alcohol). Int. J. Biol. Macromol. 2015, 80, 596–604. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Premi, A.; Giri, A.S. Synthesis of polyvinyl alcohol/modified starch-based biodegradable nanocomposite films reinforced with starch nanocrystals for packaging applications. Polym. Polym. Compos. 2021, 29, 405–416. [Google Scholar]
- Li, W.; Wu, L.; Xu, Z.; Liu, Z. Adhesion-to-fibers and film properties of etherified–oxidized cassava starch/polyvinyl alcohol blends. Iran. Polym. J. 2020, 29, 331–339. [Google Scholar] [CrossRef]
- Sakeer, K.; Ispas-Szabo, P.; Benyerbah, N.; Mateescu, M.A. Ampholytic starch excipients for high loaded drug formulations: Mechanistic insights. Int. J. Pharm. 2018, 535, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Benyerbah, N.; Ispas-Szabo, P.; Sakeer, K.; Chapdelaine, D.; Mateescu, M.A. Ampholytic and polyelectrolytic starch as matrices for controlled drug delivery. Pharmaceutics 2019, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Yang, T.; Lin, Q.; Liu, G.Q.; Zhang, L.; Yu, F.; Chen, Y. Acetylated starch nanocrystals: Preparation and antitumor drug delivery study. Int. J. Biol. Macromol. 2016, 89, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Effect of crosslinker on drug delivery properties of curcumin loaded starch coated iron oxide nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, H.; Tavakoli, T. Preparation of a new Fe3O4/starch-g-polyester nanocomposite hydrogel and a study on swelling and drug delivery properties. Carbohydr. Polym. 2016, 144, 140–148. [Google Scholar] [CrossRef]
- Baghbadorani, N.B.; Behzad, T.; Darvanjooghi, M.H.K.; Etesami, N. Modelling of water absorption kinetics and biocompatibility study of synthesized cellulose nanofiber-assisted starch-graft-poly (acrylic acid) hydrogel nanocomposites. Cellulose 2020, 27, 9927–9945. [Google Scholar] [CrossRef]
- Feng, K.; Wen, G. Absorbed Pb2+ and Cd2+ ions in water by cross-linked starch xanthate. Int. J. Polym. Sci. 2017, 2017, 6470306. [Google Scholar] [CrossRef] [Green Version]
- Xiang, B.; Fan, W.; Yi, X.; Wang, Z.; Gao, F.; Li, Y.; Gu, H. Dithiocarbamate-modified starch derivatives with high heavy metal adsorption performance. Carbohydr. Polym. 2016, 136, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Nabil, G.M.; Zaki, M.M.; Saleh, M.M. Starch functionalization of iron oxide by-product from steel industry as a sustainable low cost nanocomposite for removal of divalent toxic metal ions from water. Int. J. Biol. Macromol. 2019, 137, 455–468. [Google Scholar] [CrossRef]
- Irani, M.; Ismail, H.; Ahmad, Z.; Fan, M. Synthesis of linear low-density polyethylene-g-poly (acrylic acid)-co-starch/organo-montmorillonite hydrogel composite as an adsorbent for removal of Pb(II) from aqueous solutions. J. Environ. Sci. 2015, 27, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, B.M.; Fakhre, N.A. Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int. J. Biol. Macromol. 2019, 123, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Urdaneta, J.; Pernía, K.; León, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Removal of heavy metal ions in water by starch esters. Starch 2016, 68, 37–46. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Lu, H.; Li, M.; Liu, J.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced dispersion stability and heavy metal ion adsorption capability of oxidized. Food Chem. 2018, 242, 256–263. [Google Scholar] [CrossRef]
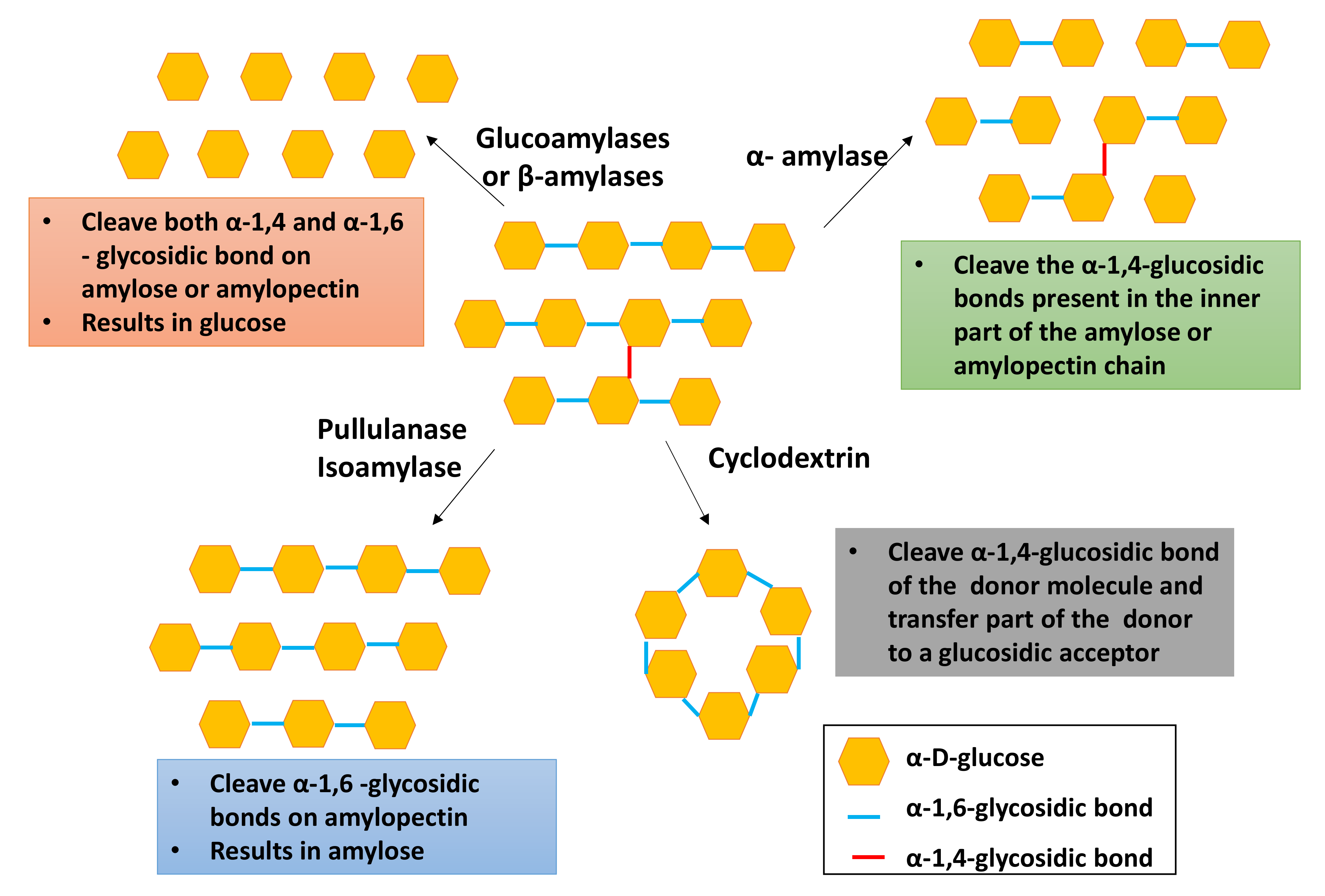
| Type of Starch Converting Enzymes | Biological Function | Example(s) | References |
|---|---|---|---|
| Endoamylses | Cleave the α-1,4-glucosidic bonds present in the inner part of the amylose or amylopectin chain | α-amylase | [39] |
| Exoamylases | Cleave both α-1,4 and α-1,6 bonds on amylose or amylopectin’s external glucose residues from the non-reducing end and produces only glucose (glucoamylase and α-glucosidases). | Glucoamylases α-glucosidases β-amylases | [40] |
| Debranching enzymes | Catalyze the hydrolysis of α-1,6-glucosidic bonds in amylopectin. | Amylo-1,6-glucosidase Pullulanase Isoamylase | [38] |
| Transferases | Cleave α-1,4-glucosidic bond of the donor molecule and transfer part of the donor to a glucosidic acceptor to form a new glucosidic bond. | Amylomaltase Cyclodextrin | [38] |
| Enzyme | Enzyme Activity (U) | Optimum Conditions | Substrate | Concentration of Enzyme (g) Required per 10 mL of Solution | References |
|---|---|---|---|---|---|
| Pullulanase EC 3.2.1.41 | ≥1000 | pH 6.5 at 50 °C | Oligosaccharides Polysaccharides Pullulan | 5 | [38] |
| Isoamyalse EC 3.2.1.68 | ≥10,000,000 | pH 6.5 at 40 °C | Polysaccharides | 0.00050 | [38] |
| Oligo-1,6-glucosidase EC 3.2.1.10 | ≥100 | pH 6.8 at 37 °C | Polysaccharides | 50 | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. https://doi.org/10.3390/molecules26226880
Amaraweera SM, Gunathilake C, Gunawardene OHP, Fernando NML, Wanninayaka DB, Dassanayake RS, Rajapaksha SM, Manamperi A, Fernando CAN, Kulatunga AK, et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules. 2021; 26(22):6880. https://doi.org/10.3390/molecules26226880
Chicago/Turabian StyleAmaraweera, Sumedha M., Chamila Gunathilake, Oneesha H. P. Gunawardene, Nimasha M. L. Fernando, Drashana B. Wanninayaka, Rohan S. Dassanayake, Suranga M. Rajapaksha, Asanga Manamperi, Chakrawarthige A. N. Fernando, Asela K. Kulatunga, and et al. 2021. "Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review" Molecules 26, no. 22: 6880. https://doi.org/10.3390/molecules26226880
APA StyleAmaraweera, S. M., Gunathilake, C., Gunawardene, O. H. P., Fernando, N. M. L., Wanninayaka, D. B., Dassanayake, R. S., Rajapaksha, S. M., Manamperi, A., Fernando, C. A. N., Kulatunga, A. K., & Manipura, A. (2021). Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules, 26(22), 6880. https://doi.org/10.3390/molecules26226880