60-nt DNA Direct Detection without Pretreatment by Surface-Enhanced Raman Scattering with Polycationic Modified Ag Microcrystal Derived from AgCl Cube
Abstract
:1. Introduction
2. Results
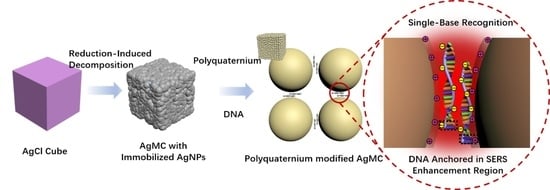
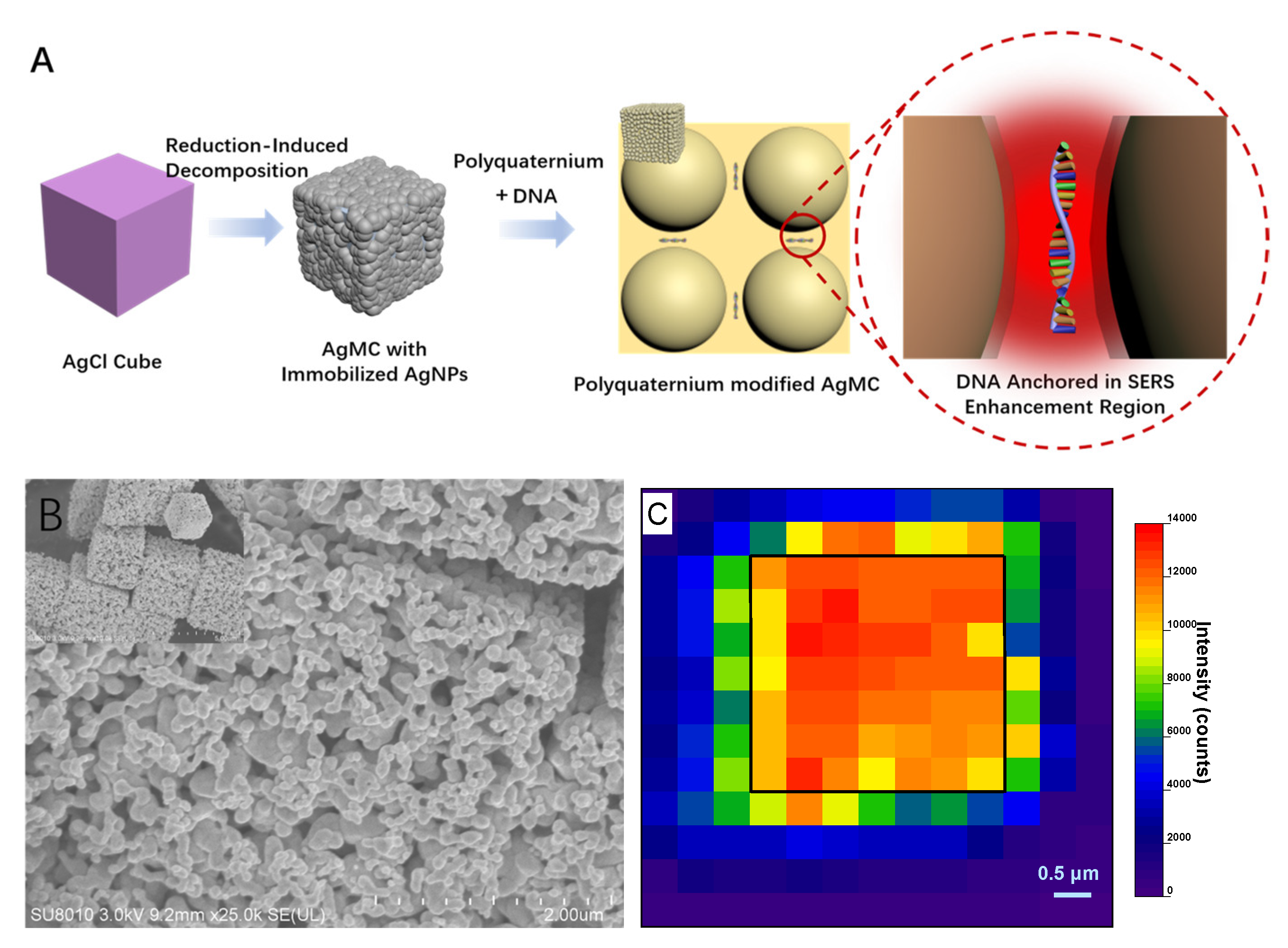
2.1. Synthesis of the Ag Cube with Immobilized AgNPs
2.2. Performance as SERS Substrate
2.3. Performance in DNA Detection
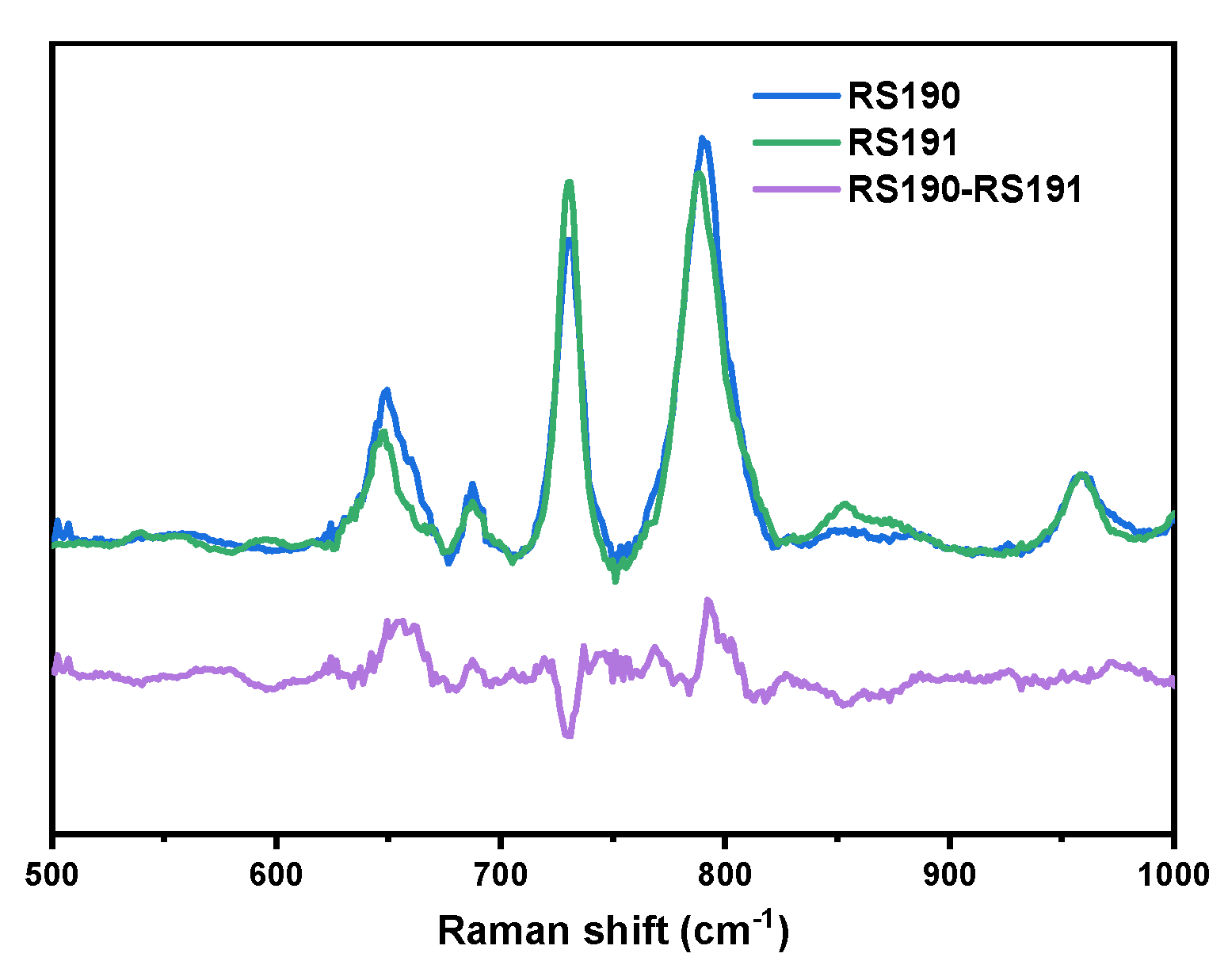
2.4. Beta-Thalassemia Mutation Detection
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of AgCl Microcubes
3.2.2. Reduction of Cubic AgCl
3.2.3. Modification of Polyquaternium
3.3. The SERS Detection
3.4. Equipment and Parameter Settings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taher, A.T.; Weatherall, D.J.; Cappellini, M.D. Thalassaemia. Lancet 2018, 391, 155–167. [Google Scholar] [CrossRef]
- Noth, S.; Brysbaert, G.; Pellay, F.; Benecke, A. High-Sensitivity transcriptome data structure and implications for analysis and biologic interpretation. Genom. Proteom. Bioinform. 2006, 4, 212–229. [Google Scholar] [CrossRef] [Green Version]
- Barhoumi, A.; Zhang, D.; Tam, F.; Halas, N.J. Surface-Enhanced raman spectroscopy of DNA. J. Am. Chem. Soc. 2008, 130, 5523–5529. [Google Scholar] [CrossRef] [PubMed]
- Petrovykh, D.Y.; Pérez-Dieste, V.; Opdahl, A.; Kimura-Suda, H.; Sullivan, J.M.; Tarlov, M.J.; Himpsel, F.J.; Whitman, L.J. Nucleobase orientation and ordering in films of Single-Stranded DNA on gold. J. Am. Chem. Soc. 2006, 128, 2–3. [Google Scholar] [CrossRef]
- Chen, X.; Xun, D.; Zheng, R.; Zhao, L.; Lu, Y.; Huang, J.; Wang, R.; Wang, Y. Deep-Learning-Assisted assessment of DNA damage based on foci images and its application in High-Content screening of lead compounds. Anal. Chem. 2020, 92, 14267–14277. [Google Scholar] [CrossRef]
- Kwon, H.; Jeong, J.; Bae, Y.; Choi, K.; Yang, I. Stable isotope labeled DNA: A new strategy for the quantification of total DNA using liquid chromatography–mass spectrometry. Anal. Chem. 2019, 91, 3936–3943. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by Surface-Enhanced raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Chikkaraddy, R.; de Nijs, B.; Benz, F.; Barrow, S.J.; Scherman, O.A.; Rosta, E.; Demetriadou, A.; Fox, P.; Hess, O.; Baumberg, J.J. Single-molecule strong coupling at room temperature in plasmonic nanocavities. Nature 2016, 535, 127–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.; Ahn, H.; Won, J.; Son, J.G.; Shon, H.K.; Lee, T.G.; Han, S.W.; Baik, M. Electro-inductive effect: Electrodes as functional groups with tunable electronic properties. Science 2020, 370, 214–219. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez De Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of Surface-Enhanced raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Man, T.; Lai, W.; Xiao, M.; Wang, X.; Chandrasekaran, A.R.; Pei, H.; Li, L. A versatile biomolecular detection platform based on photo-induced enhanced Raman spectroscopy. Biosens. Bioelectron. 2020, 147, 111742. [Google Scholar] [CrossRef]
- Shaik, M.; Albalawi, G.; Khan, S.; Khan, M.; Adil, S.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.; Alkhathlan, H.; Khan, M. “miswak” based green synthesis of silver nanoparticles: Evaluation and comparison of their microbicidal activities with the chemical synthesis. Molecules 2016, 21, 1478. [Google Scholar] [CrossRef] [Green Version]
- Jun, B.; Kang, H.; Lee, Y.; Jeong, D.H. Fluorescence-Based multiplex protein detection using optically encoded microbeads. Molecules 2012, 17, 2474–2490. [Google Scholar] [CrossRef] [PubMed]
- Altunbek, M.; Kuku, G.; Culha, M. Gold nanoparticles in Single-Cell analysis for surface enhanced raman scattering. Molecules 2016, 21, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrak, E.; Krajczewski, J.; Kowalik, A.; Kudelski, A.; Jaworska, A. Surface enhanced raman spectroscopy for DNA biosensors—how far are we? Molecules 2019, 24, 4423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farquharson, S.; Gift, A.; Shende, C.; Inscore, F.; Ordway, B.; Farquharson, C.; Murren, J. Surface-enhanced raman spectral measurements of 5-Fluorouracil in saliva. Molecules 2008, 13, 2608–2627. [Google Scholar] [CrossRef]
- Lee, T.; Mohammadniaei, M.; Zhang, H.; Yoon, J.; Choi, H.K.; Guo, S.; Guo, P.; Choi, J.W. Single functionalized pRNA/Gold nanoparticle for ultrasensitive MicroRNA detection using electrochemical surface-enhanced raman spectroscopy. Adv. Sci. 2019, 7, 1902477. [Google Scholar] [CrossRef]
- Ding, S.; Yi, J.; Li, J.; Ren, B.; Wu, D.; Panneerselvam, R.; Tian, Z. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Garcia-Rico, E.; Alvarez-Puebla, R.A.; Guerrini, L. Direct surface-enhanced Raman scattering (SERS) spectroscopy of nucleic acids: From fundamental studies to real-life applications. Chem. Soc. Rev. 2018, 47, 4909–4923. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Ding, S.; Panneerselvam, R.; Tian, Z. Core-shell Nanoparticle-Enhanced raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Bell, S.E.J. Label-Free detection of Single-Base mismatches in DNA by Surface-Enhanced raman spectroscopy. Angew. Chem. Int. Ed. 2011, 50, 9058–9061. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Lei, Z.; Li, J.; Zong, C.; Yang, C.J.; Ren, B. Label-Free Surface-Enhanced raman spectroscopy detection of DNA with Single-Base sensitivity. J. Am. Chem. Soc. 2015, 137, 5149–5154. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, A.; Halas, N.J. Label-Free detection of DNA hybridization using surface enhanced raman spectroscopy. J. Am. Chem. Soc. 2010, 132, 12792–12793. [Google Scholar] [CrossRef] [PubMed]
- Masetti, M.; Xie, H.; Krpetić, Z.; Recanatini, M.; Alvarez-Puebla, R.A.; Guerrini, L. Revealing DNA interactions with exogenous agents by Surface-Enhanced raman scattering. J. Am. Chem. Soc. 2015, 137, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Wang, D.; Li, C.; Ma, K.; Zhang, Y.; Jin, Y. Plasmonic SERS au nanosunflowers for sensitive and Label-Free diagnosis of DNA base damage in Stimulus-Induced cell apoptosis. Anal. Chem. 2020, 92, 11755–11762. [Google Scholar] [CrossRef]
- Wang, J.; Koo, K.M.; Wee, E.J.; Wang, Y.; Trau, M. A nanoplasmonic label-free surface-enhanced Raman scattering strategy for non-invasive cancer genetic subtyping in patient samples. Nanoscale 2017, 9, 3496–3503. [Google Scholar] [CrossRef]
- Guerrini, L.; Krpetić, Z.; van Lierop, D.; Alvarez-Puebla, R.A.; Graham, D. Direct Surface-Enhanced raman scattering analysis of DNA duplexes. Angew. Chem. Int. Ed. 2015, 54, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lyu, N.; Rajendran, V.K.; Piper, J.; Rodger, A.; Wang, Y. Sensitive and direct DNA mutation detection by Surface-Enhanced raman spectroscopy using rational designed and tunable plasmonic nanostructures. Anal. Chem. 2020, 92, 5708–5716. [Google Scholar] [CrossRef] [PubMed]
- Morla-Folch, J.; Xie, H.; Gisbert-Quilis, P.; Pedro, S.G.; Pazos-Perez, N.; Alvarez-Puebla, R.A.; Guerrini, L. Ultrasensitive direct quantification of nucleobase modifications in DNA by Surface-Enhanced raman scattering: The case of cytosine. Angew. Chem. 2015, 127, 13854–13858. [Google Scholar] [CrossRef]
- Pazos-Perez, N.; Wagner, C.S.; Romo-Herrera, J.M.; Liz-Marzán, L.M.; García De Abajo, F.J.; Wittemann, A.; Fery, A.; Alvarez-Puebla, R.A. Organized plasmonic clusters with high coordination number and extraordinary enhancement in Surface-Enhanced raman scattering (SERS). Angew. Chem. Int. Ed. 2012, 51, 12688–12693. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, Q. Reduction-Induced decomposition: Spontaneous formation of monolithic nanoporous metals of tunable structural hierarchy and porosity. Chem. Mater. 2018, 30, 3894–3900. [Google Scholar] [CrossRef]
- Saiki, R.K.; Walsh, P.S.; Levenson, C.H.; Erlich, H.A. Genetic analysis of amplified DNA with immobilized Sequence-Specific oligonucleotide probes. Proc. Natl. Acad. Sci. USA 1989, 86, 6230–6234. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Name | Sequence |
|---|---|
| A60 | AAAAAAAAAA AAAAAAAAAA AAAAAAAAAA AAAAAAAAAA AAAAAAAAAA AAAAAAAAAA |
| C60 | CCCCCCCCCC CCCCCCCCCC CCCCCCCCCC CCCCCCCCCC CCCCCCCCCC CCCCCCCCCC |
| G10 | GGGGGGGGGG |
| T60 | TTTTTTTTTT TTTTTTTTTT TTTTTTTTTT TTTTTTTTTT TTTTTTTTTT TTTTTTTTTT |
| SS1 | AGATCAGGTCAGTTCAGCTCAGATCAGGTCAGTTCAGCTCAGATCAGGTCAGTTCAGCTC |
| 20norm | AGATCAGGTCAGTTCAGCTC |
| 20mutA | AGATCAGGTCAGTTCAGATC |
| 40norm | AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGCTC |
| 40mutA | AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGATC |
| 60norm | AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGCTC |
| 60mutA | AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGATC |
| 60mutA5′ | AGATCAGGTCAGTTCAGATC AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGCTC |
| 60mutAmid | AGATCAGGTCAGTTCAGCTC AGATCAGGTCAGTTCAGATC AGATCAGGTCAGTTCAGCTC |
| RS190 | TGGGCAGGTTGGCATCAAGCCCACAGGGCAGTAACGGCAGACTTCTCCTCAGGAGTCAG |
| RS191 | TGGGCAGATTGGTATCAAGCCCACAGGGCAGTAACGGCAGACTTCTCCTCAGGAGTCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Huang, L.; Fan, L.; Chen, F.; Lou, J.; Shan, X.; Yu, D.; Zhou, J. 60-nt DNA Direct Detection without Pretreatment by Surface-Enhanced Raman Scattering with Polycationic Modified Ag Microcrystal Derived from AgCl Cube. Molecules 2021, 26, 6790. https://doi.org/10.3390/molecules26226790
Mao J, Huang L, Fan L, Chen F, Lou J, Shan X, Yu D, Zhou J. 60-nt DNA Direct Detection without Pretreatment by Surface-Enhanced Raman Scattering with Polycationic Modified Ag Microcrystal Derived from AgCl Cube. Molecules. 2021; 26(22):6790. https://doi.org/10.3390/molecules26226790
Chicago/Turabian StyleMao, Jikai, Lvtao Huang, Li Fan, Fang Chen, Jingan Lou, Xuliang Shan, Dongdong Yu, and Jianguang Zhou. 2021. "60-nt DNA Direct Detection without Pretreatment by Surface-Enhanced Raman Scattering with Polycationic Modified Ag Microcrystal Derived from AgCl Cube" Molecules 26, no. 22: 6790. https://doi.org/10.3390/molecules26226790
APA StyleMao, J., Huang, L., Fan, L., Chen, F., Lou, J., Shan, X., Yu, D., & Zhou, J. (2021). 60-nt DNA Direct Detection without Pretreatment by Surface-Enhanced Raman Scattering with Polycationic Modified Ag Microcrystal Derived from AgCl Cube. Molecules, 26(22), 6790. https://doi.org/10.3390/molecules26226790