Discovery of Guanidine Derivatives from Buthus martensii Karsch with Metal-Binding and Cholinesterase Inhibition Properties
Abstract
:1. Introduction
2. Results
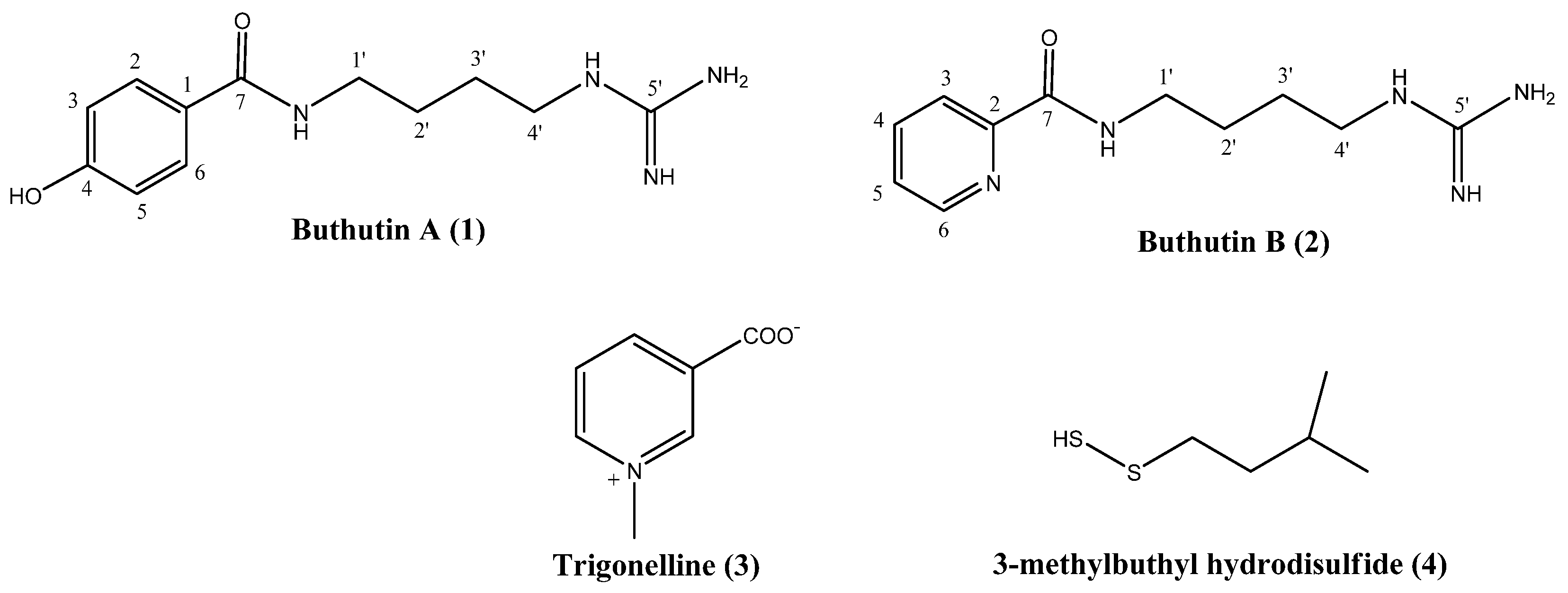
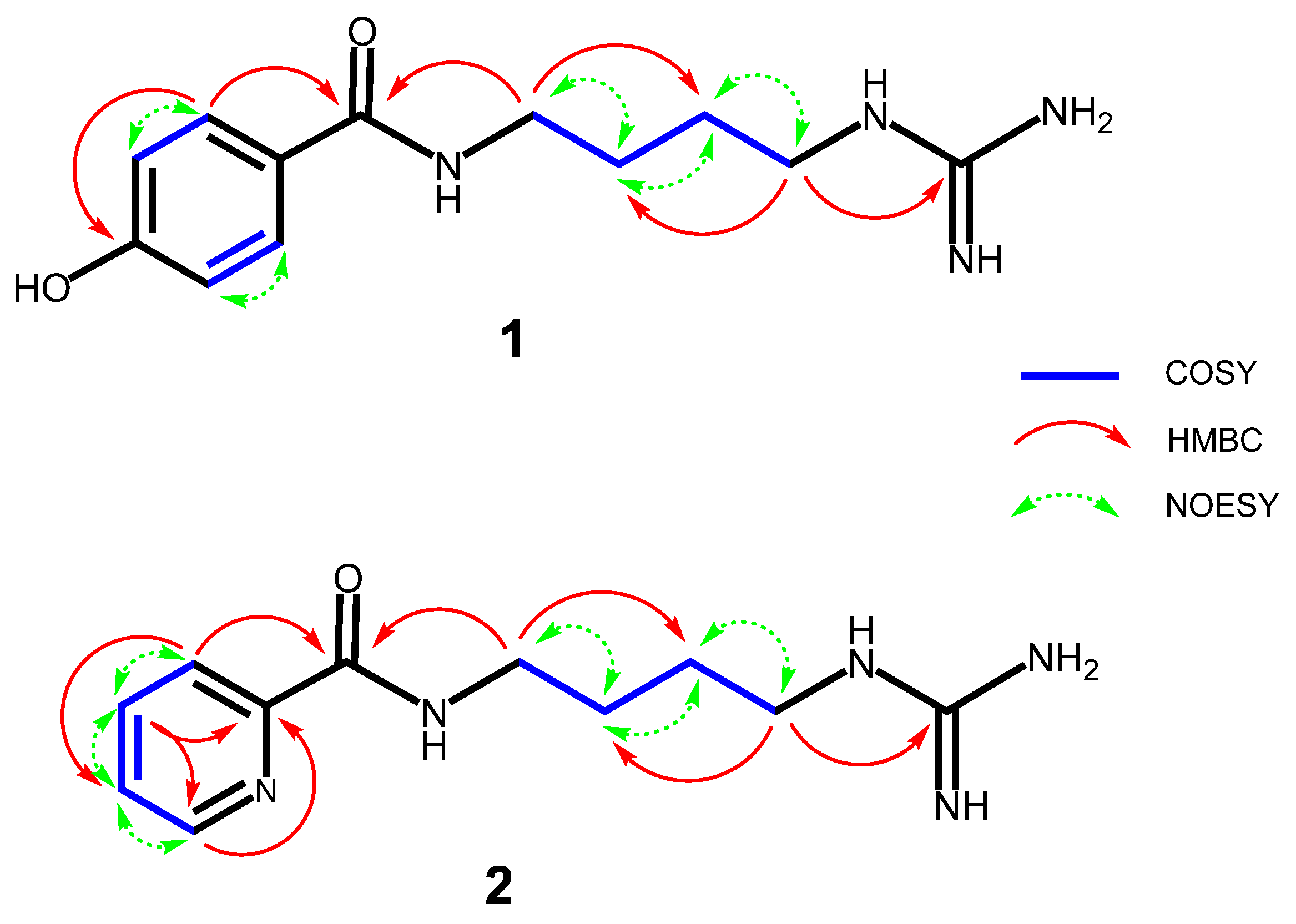
2.1. Structural Elucidation of Obtained Compounds
2.2. Cholinesterase Inhibition Activities
2.3. Propidium Iodide Displacement Assay
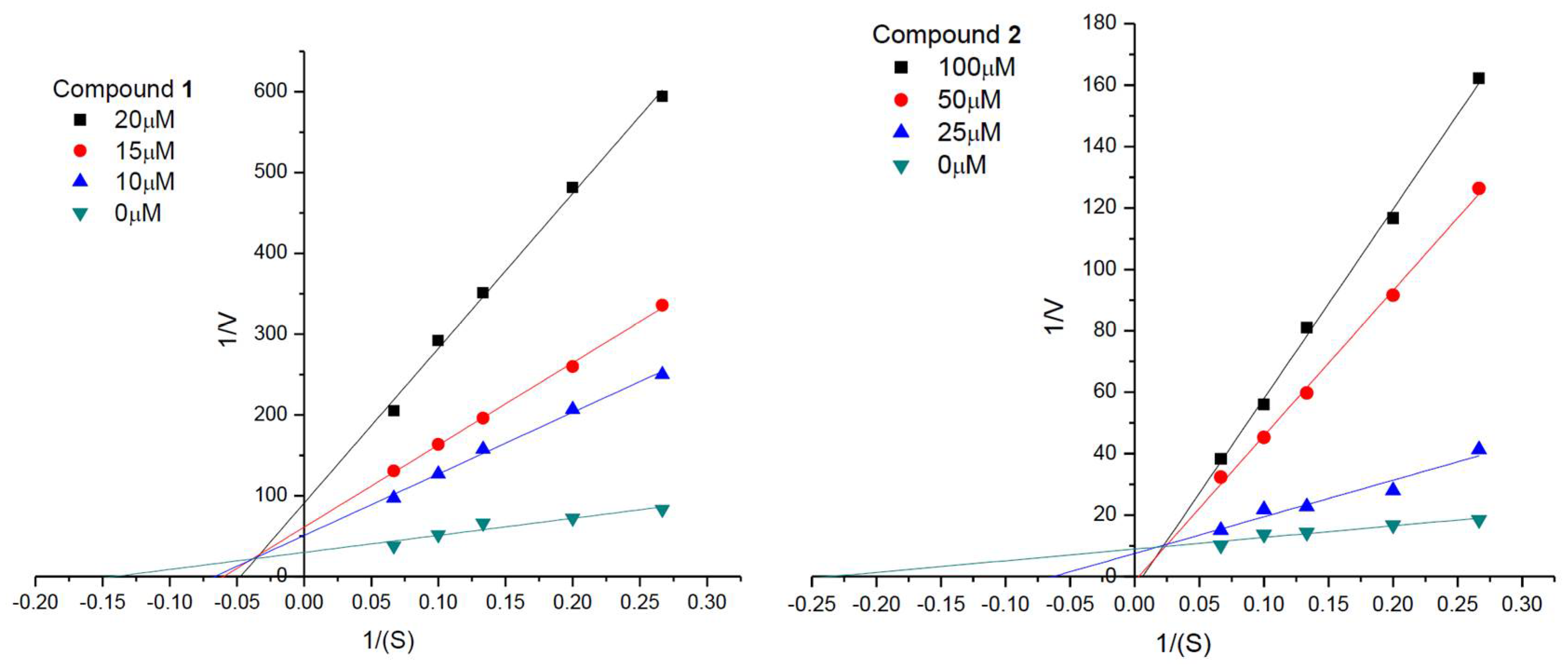
2.4. Enzyme Kinetic Analyses against AChE
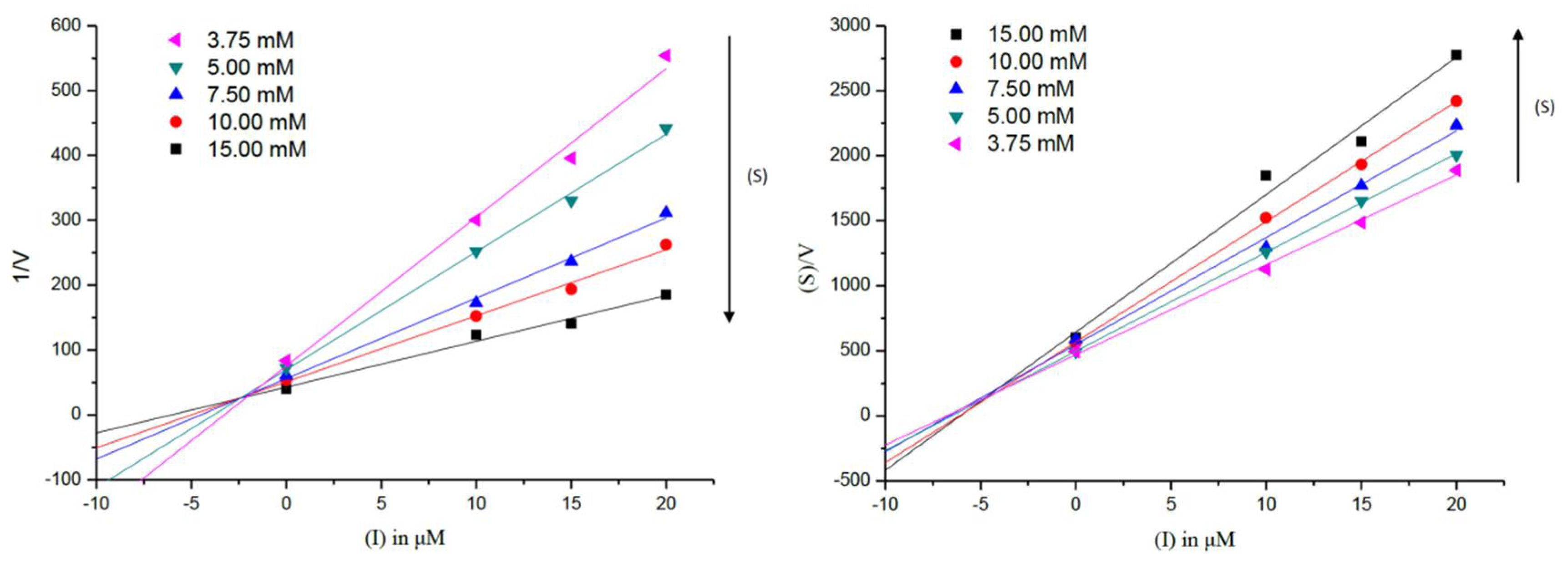
2.5. Metal-Binding Studies
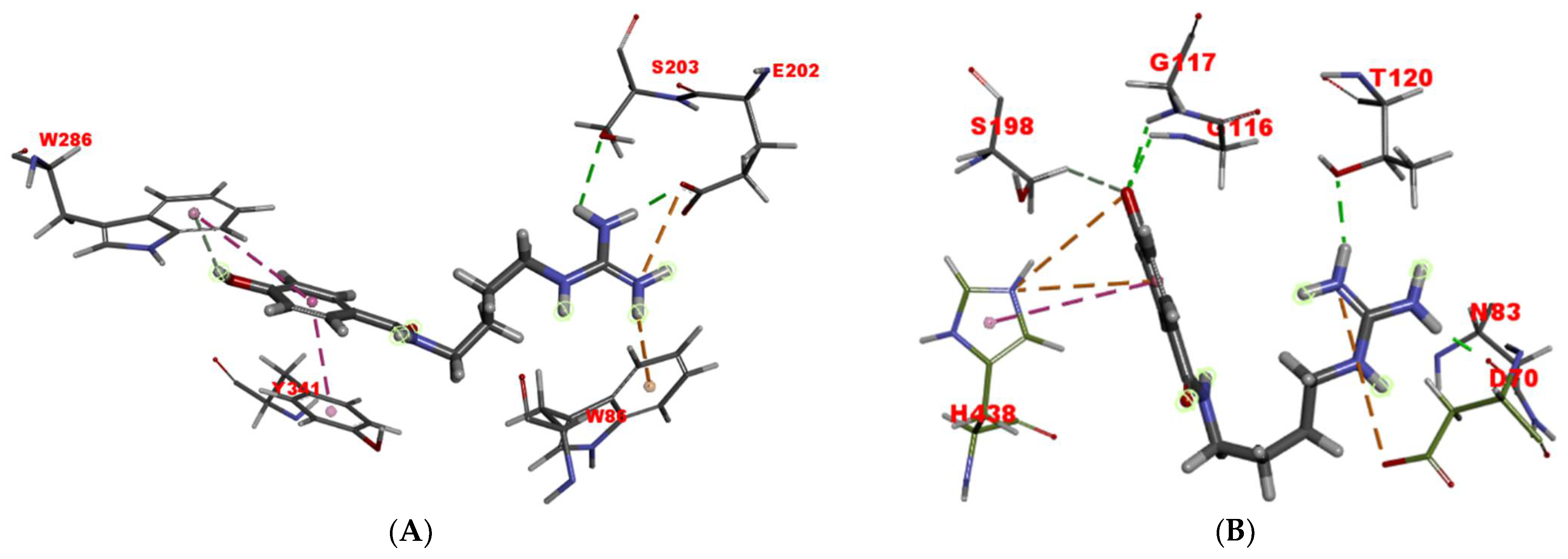
2.6. Molecular Docking Studies
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Arthropod Material
3.3. Extraction and Isolation
3.3.1. N-(4-Guanidinobutyl)-4-hydroxybenzamide (1)
3.3.2. N-(4-Guanidinobutyl)picolinamide (2)
3.3.3. N-Methylnicotinic Acid (3)
3.3.4. 3-Methylbuthyl Hydrodisulfide (4)
3.4. Cholinesterase Inhibitory Assay
3.5. Propidium Iodide Displacement Assay
3.6. Enzyme Kinetic Analysis against AChE
3.7. Metal-Binding Studies
3.8. Molecular Docking Studies
3.8.1. Molecular Docking of Compound 1 into AChE
3.8.2. Molecular Docking of Compound 1 into BchE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kumawat, A.; Raheem, S.; Ali, F.; Dar, T.A.; Chakrabarty, S.; Rizvi, M.A. Organoselenium compounds as acetylcholinesterase inhibitors: Evidence and mechanism of mixed inhibition. J. Phys. Chem. B 2021, 125, 1531–1541. [Google Scholar] [CrossRef]
- Samadi, A.; Estrada, M.; Pérez, C.; Rodríguez-Franco, M.I.; Iriepa, I.; Moraleda, I.; Chioua, M.; Marco-Contelles, J. Pyridonepezils, new dual AChE inhibitors as potential drugs for the treatment of Alzheimer’s disease: Synthesis, biological assessment, and molecular modeling. Eur. J. Med. Chem. 2012, 57, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.E. Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: Are irreversible inhibitors the future? Int. J. Mol. Sci. 2020, 21, 3438. [Google Scholar] [CrossRef]
- Jing, L.; Wu, G.; Kang, D.; Zhou, Z.; Song, Y.; Liu, X.; Zhan, P. Contemporary medicinal-chemistry strategies for the discovery of selective butyrylcholinesterase inhibitors. Drug Discov. Today 2019, 24, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.A.; Ross, B.P. Recent advances in virtual screening for cholinesterase inhibitors. ACS Chem. Neurosci. 2021, 12, 30–41. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Rodríguez, C.; Telpoukhovskaia, M.; Orvig, C. The art of building multifunctional metal-binding agents from basic molecular scaffolds for the potential application in neurodegenerative diseases. Coord. Chem. Rev. 2012, 256, 2308–2332. [Google Scholar] [CrossRef]
- Bharate, S.S.; Mignani, S.; Vishwakarma, R. Why are the majority of active Compounds in the CNS domain natural products? A critical analysis. J. Med. Chem. 2018, 61, 10345–10374. [Google Scholar] [CrossRef]
- Martins, M.; Silva, R.; Pinto, M.M.M.; Sousa, E. Marine natural products, multitarget therapy and repurposed agents in Alzheimer’s disease. Pharmaceuticals 2020, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- El-Asmar, M.F.; Ismail, M.; Ghoneim, K.; Osman, O.H. Scorpion (Buthus minax, L. Koch) venom fractions with anticholinesterase activity. Toxicon 1977, 15, 63–69. [Google Scholar] [CrossRef]
- Venkateswarlu, D.; Babu, K.S. Inhibitory effect of venom of the scorpion Heterometrus fulvipes (C.L. Koch) on acetylcholinesterase activity in brain, muscle & liver of the frog Rana hexadactyla lesson. Indian J. Exp. Biol. 1977, 15, 1056–1058. [Google Scholar]
- Banerjee, S.; Gnanamani, E.; Lynch, S.R.; Zuñiga, F.Z.; Jiménez-Vargas, J.M.; Possani, L.D.; Zare, R.N. An alkaloid from scorpion venom: Chemical structure and synthesis. J. Nat. Prod. 2018, 81, 1899–1904. [Google Scholar] [CrossRef]
- Gao, J.Y.; Yin, W.; Gao, T.; Deng, R.; Li, X. Two bioactive compounds from the Chinese scorpion Buthus martensii Karsch. Nat. Prod. Res. 2014, 28, 698–703. [Google Scholar] [CrossRef]
- Kim, S.D. α-Glucosidase inhibitor from Buthus martensi Karsch. Food Chem. 2013, 136, 297–300. [Google Scholar] [CrossRef]
- Song, Z.B. Studies on the production process of scorpion cans. Shandong Food Ferment 1994, 1, 8–10. [Google Scholar]
- Wali, A.; Wubulikasimu, A.; Mirzaakhmedov, S.; Gao, Y.; Omar, A.; Arken, A.; Yili, A.; Aisa, H.A. Optimization of scorpion protein extraction and characterization of the proteins’ functional properties. Molecules 2019, 24, 4103. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.F.; Hu, J.; Zhang, J.H.; Wang, S.L.; Wu, C.F. Isolation, purification, and N-terminal partial sequence of an antitumor peptide from the venom of the Chinese scorpion Buthus martensii Karsch. Prep. Biochem. Biotech. 2002, 32, 317–327. [Google Scholar] [CrossRef]
- Shao, J.H.; Cui, Y.; Zhao, M.Y.; Wu, C.F.; Liu, Y.F.; Zhang, J.H. Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides 2014, 53, 89–96. [Google Scholar] [CrossRef]
- Lv, B.; Yin, W.; Gao, J.; Liu, H.; Liu, K.; Bai, J.; Yang, Q. Neo-5,22E-cholestadienol derivatives from Buthus martensii Karsch and targeted bactericidal action mechanisms. Molecules 2019, 24, 72. [Google Scholar] [CrossRef] [Green Version]
- Tapiolas, D.M.; Bowden, B.F.; Abou-Mansour, E.; Willis, R.H.; Doyle, J.R.; Muirhead, A.N.; Liptrot, C.; Llewellyn, L.E.; Wolff, C.W.W.; Wright, A.; et al. Eusynstyelamides A, B, and C, nNOS Inhibitors, from the Ascidian Eusynstyela latericius. J. Nat. Prod. 2009, 72, 1115–1120. [Google Scholar] [CrossRef]
- Zhong, G.C.; Peng, J.Z.; Li, J.M.; Xu, Y.G.; Zhou, P.; Sheng, R.Z. Design, synthesis and sodium hydrogen exchanger isoform-1 (NHE-1) inhibitory activity of leonurine analogues. Chin. J. Org. Chem. 2011, 31, 1445–1451. [Google Scholar]
- Wang, X.Z.; Wu, F.H.; Qu, W.; Liang, J.Y. A new β-carboline alkaloid from the seeds of Griffonia simplicifolia. Chin. J. Nat. Med. 2013, 11, 401–405. [Google Scholar] [CrossRef]
- Kim, C.S.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R.; Kim, K.H. Cytotoxic and anti-inflammatory disulfide compounds from the fruiting bodies of Boletus pseudocalopus. J. Antibiot. 2015, 68, 414–416. [Google Scholar] [CrossRef]
- Almeida, J.; Figueiro, M.; Almeida, W.P.; Silva, C.H.T.P. Discovery of novel dual acetylcholinesterase inhibitors with antifibrillogenic activity related to Alzheimer’s disease. Future Med. Chem. 2018, 10, 1037–1053. [Google Scholar] [CrossRef]
- Zondagh, L.S.; Malan, S.F.; Joubert, J. Design, synthesis and biological evaluation of edaravone derivatives bearing the N-benzyl pyridinium moiety as multifunctional anti-Alzheimer’s agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 1596–1605. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cai, R.; Wang, L.N.; Fan, J.J.; Geng, S.Q.; Liu, Y.M. New 4-N-phenylaminoquinoline derivatives as antioxidant, metal chelating and cholinesterase inhibitors for Alzheimer’s disease. Bioorg. Chem. 2019, 93, 103328. [Google Scholar] [CrossRef]
- Taylor, P.; Lappi, S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry 1975, 14, 1989–1997. [Google Scholar] [CrossRef]
- Loesche, A.; Köwitsch, A.; Lucas, S.D.; Al-Halabi, Z.; Sippl, W.; Al-Harrasi, A.; Csuk, R. Ursolic and oleanolic acid derivatives with cholinesterase inhibiting potential. Bioorg. Chem. 2019, 85, 23–32. [Google Scholar] [CrossRef]
- Rastegari, A.; Nadri, H.; Mahdavi, M.; Moradi, A.; Mirfazli, S.S.; Edraki, N.; Moghadam, F.H.; Larijani, B.; Akbarzadeh, T.; Saeedi, M. Design, synthesis and anti-Alzheimer’s activity of novel 1,2,3-triazolechromenone carboxamide derivatives. Bioorg. Chem. 2019, 83, 391–401. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Mo, J.; Yang, H.; Jiang, X.; Lin, H.; Gu, K.; Pei, Y.; Wu, L.; Tan, R.; et al. Synthesis and bioevaluation of new tacrinecinnamic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2018, 33, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Leong, S.W.; Abas, F.; Lam, K.W.; Shaari, K.; Lajis, N.H. 2-Benzoyl-6-benzylidenecyclohexanone analogs as potent dual inhibitors of acetylcholinesterase and butyrylcholinesterase. Bioorgan. Med. Chem. 2016, 24, 3742–3751. [Google Scholar] [CrossRef]
- Ashihara, H. Trigonelline (N-methylnicotinic acid) biosynthesis and its biological role in plants. Nat. Prod. Commun. 2008, 3, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Ebada, S.S.; Proksch, P. Chemical and pharmacological significance of natural guanidines from marine invertebrates. Mini-Rev. Med. Chem. 2011, 11, 225–246. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Romminger, S. The chemistry and biology of guanidine natural products. Nat. Prod. Rep. 2016, 33, 456–490. [Google Scholar] [CrossRef]
- Olsen, E.K.; Hansen, E.; Moodie, L.W.K.; Isaksson, J.; Sepčić, K.; Cergolj, M.; Svenson, J.; Andersen, J.H. Marine AChE inhibitors isolated from Geodia barretti: Natural compounds and their synthetic analogs. Org. Biomol. Chem. 2016, 14, 1629–1640. [Google Scholar] [CrossRef]
- Loesche, A.; Wiese, J.; Sommerwerk, S.; Simon, V.; Brandt, W.; Csuk, R. Repurposing N,N’-bis-(arylamidino)-1,4-piperazinedicarboxamidines: An unexpectedet class of potent inhibitors of cholinesterases. Eur. J. Med. Chem. 2017, 125, 430–434. [Google Scholar] [CrossRef]
- Neto, D.C.F.; Ferreira, M.S.; Petronilho, E.C.; Lima, J.A.; Azeredo, S.O.F.; Brum, J.O.C.; Nascimento, C.J.; Villar, D.F. A new guanylhydrazone derivative as a potential acetylcholinesterase inhibitor for Alzheimer’s disease: Synthesis, molecular docking, biological evaluation and kinetic studies by nuclear magnetic resonance. RSC Adv. 2017, 7, 33944–33952. [Google Scholar] [CrossRef] [Green Version]

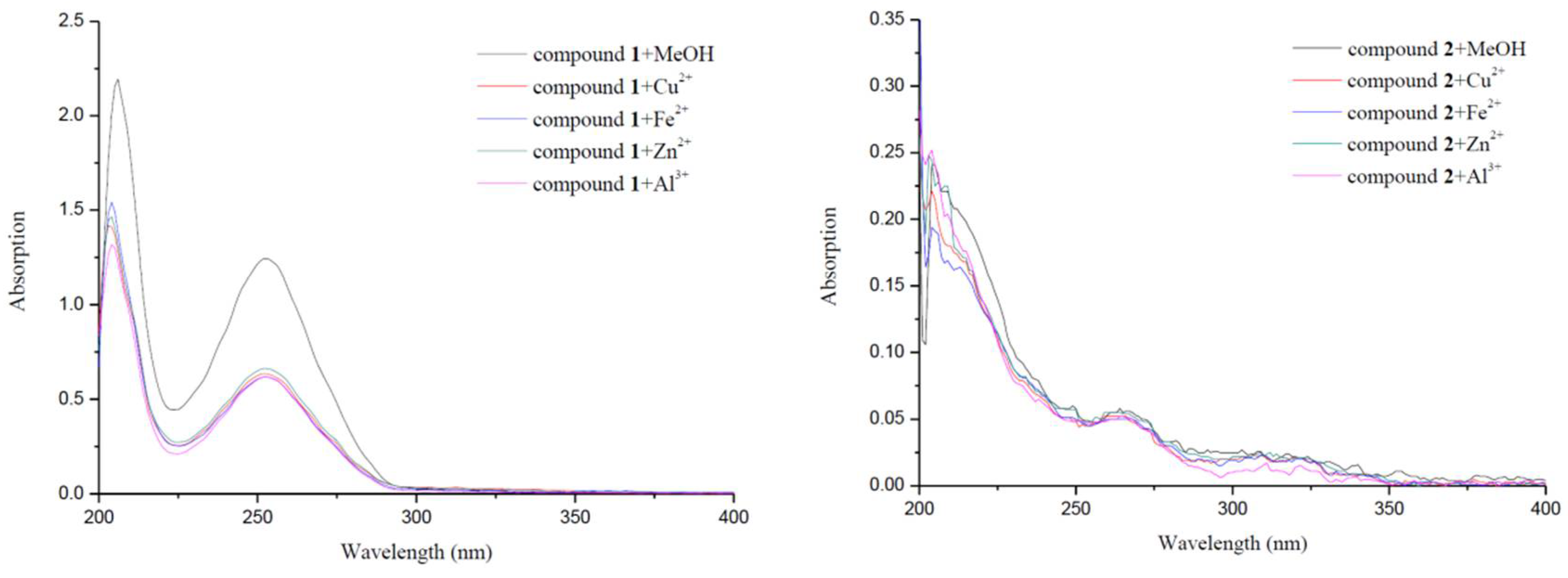
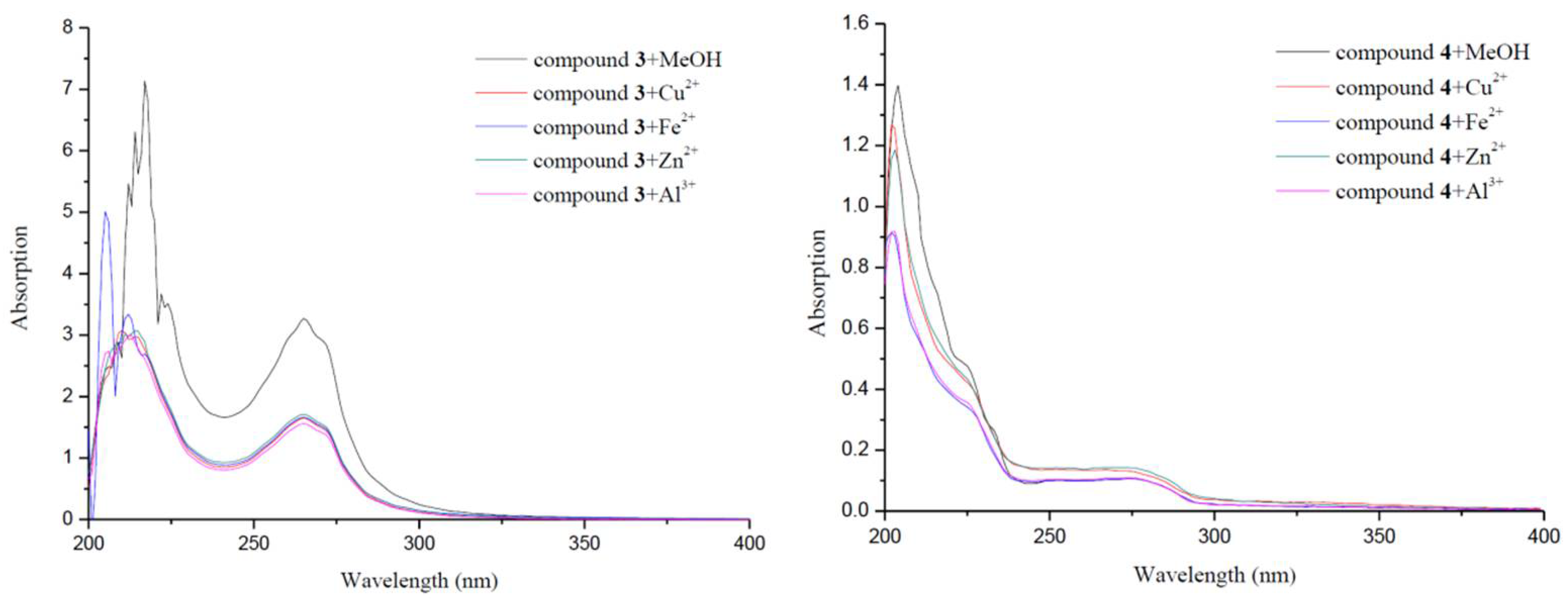
| Compounds | IC50 a (μM) | SI b | Propidium Iodide Displacement | |
|---|---|---|---|---|
| AChE | BChE | (%) | ||
| 1 | 7.83 ± 0.06 | 47.44 ± 0.95 | 6.05 | 18.29 ± 0.53 |
| 2 | 61.45 ± 2.34 | 122.64 ± 5.21 | 1.99 | 17.95 ± 0.98 |
| 3 | 97.30 ± 4.18 | 441.87 ± 7.99 | 4.54 | 3.52 ± 0.21 |
| 4 | 40.93 ± 3.21 | 152.84 ± 7.22 | 3.73 | 4.37 ± 0.16 |
| Galanthamine | 1.17 ± 0.01 | 18.78 ± 1.81 | 16.05 | |
| Donepezil | 0.049 ± 0.004 | 5.536 ± 0.018 | 112.98 | 18.50 ± 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-M.; Fan, J.-J.; Wang, L.-N. Discovery of Guanidine Derivatives from Buthus martensii Karsch with Metal-Binding and Cholinesterase Inhibition Properties. Molecules 2021, 26, 6737. https://doi.org/10.3390/molecules26216737
Liu Y-M, Fan J-J, Wang L-N. Discovery of Guanidine Derivatives from Buthus martensii Karsch with Metal-Binding and Cholinesterase Inhibition Properties. Molecules. 2021; 26(21):6737. https://doi.org/10.3390/molecules26216737
Chicago/Turabian StyleLiu, Yu-Ming, Jing-Jing Fan, and Li-Ning Wang. 2021. "Discovery of Guanidine Derivatives from Buthus martensii Karsch with Metal-Binding and Cholinesterase Inhibition Properties" Molecules 26, no. 21: 6737. https://doi.org/10.3390/molecules26216737
APA StyleLiu, Y.-M., Fan, J.-J., & Wang, L.-N. (2021). Discovery of Guanidine Derivatives from Buthus martensii Karsch with Metal-Binding and Cholinesterase Inhibition Properties. Molecules, 26(21), 6737. https://doi.org/10.3390/molecules26216737






