Trial Proteomic Qualitative and Quantitative Analysis of the Protein Matrix of Submandibular Sialoliths
Abstract
1. Introduction
2. Results
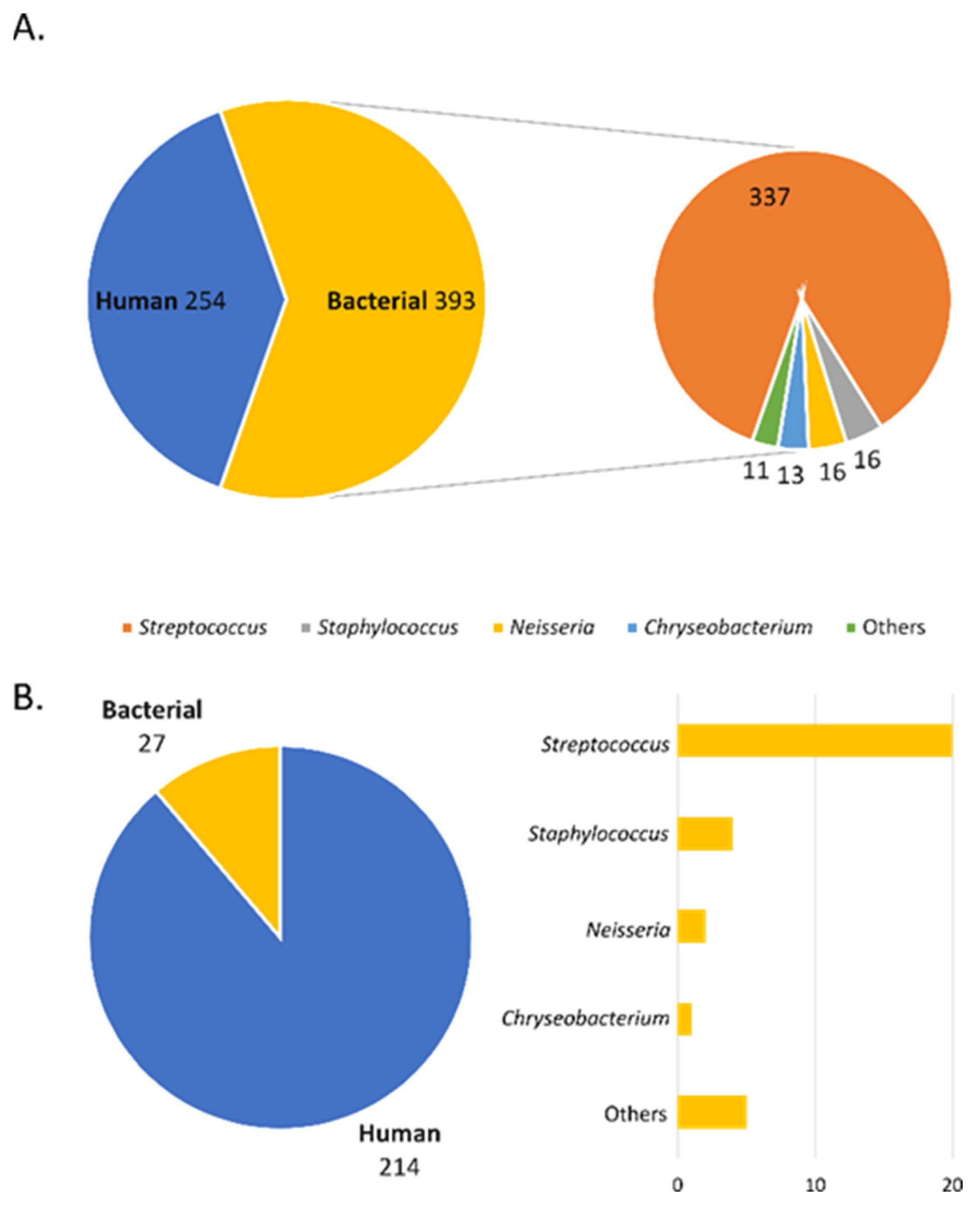
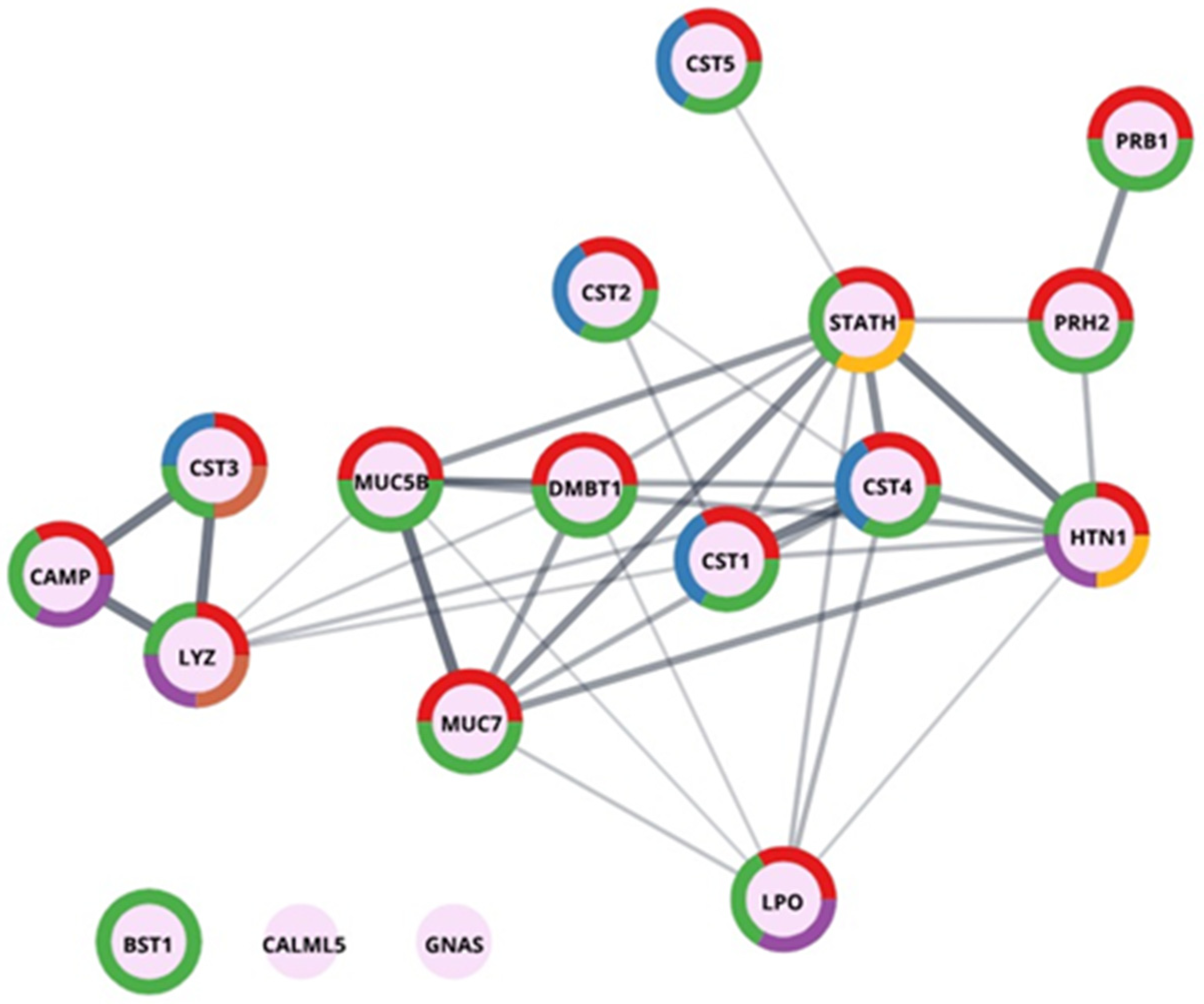
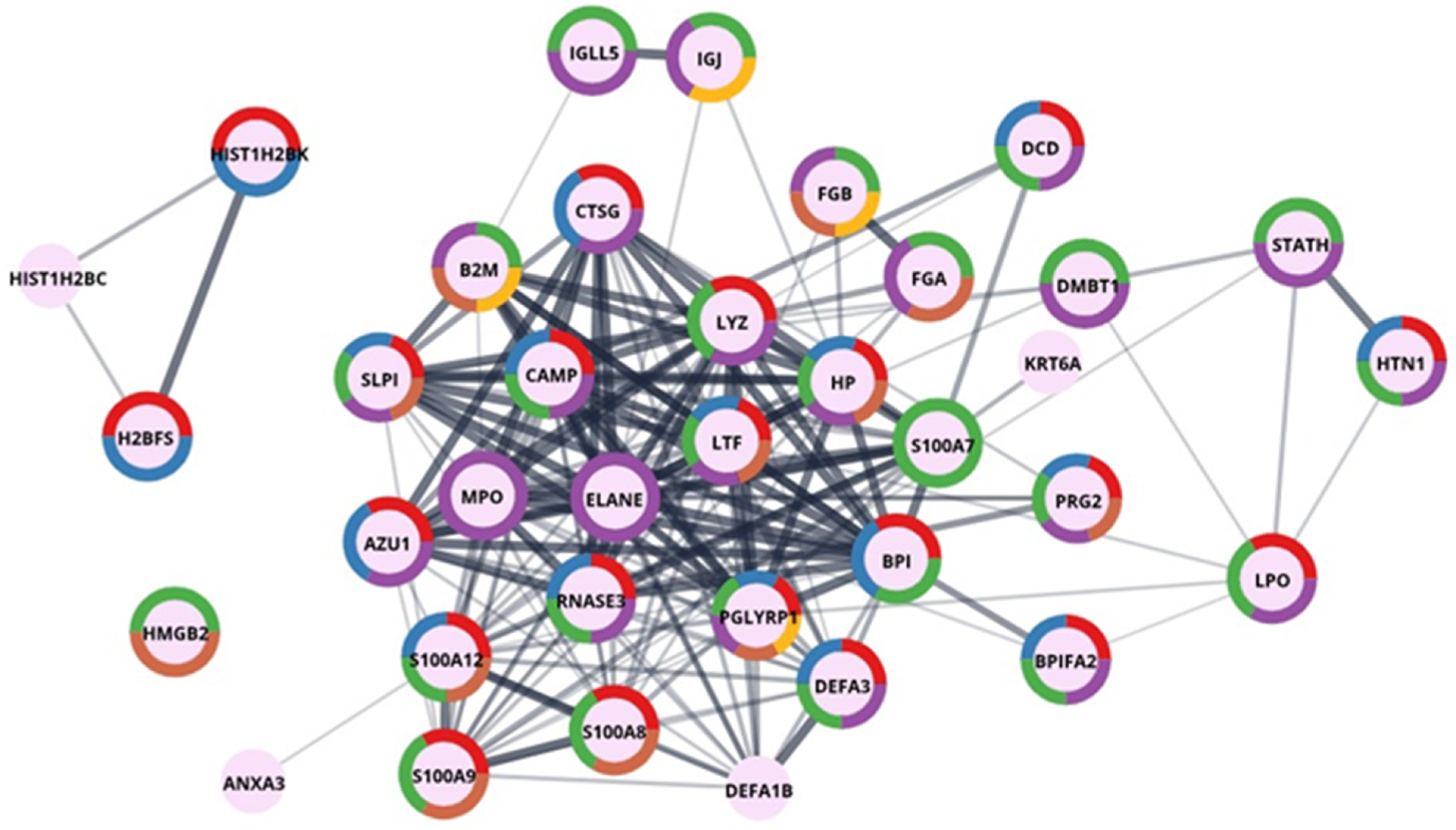
2.1. Classification and Qualitative Analysis of Sialoliths Protein Matrix
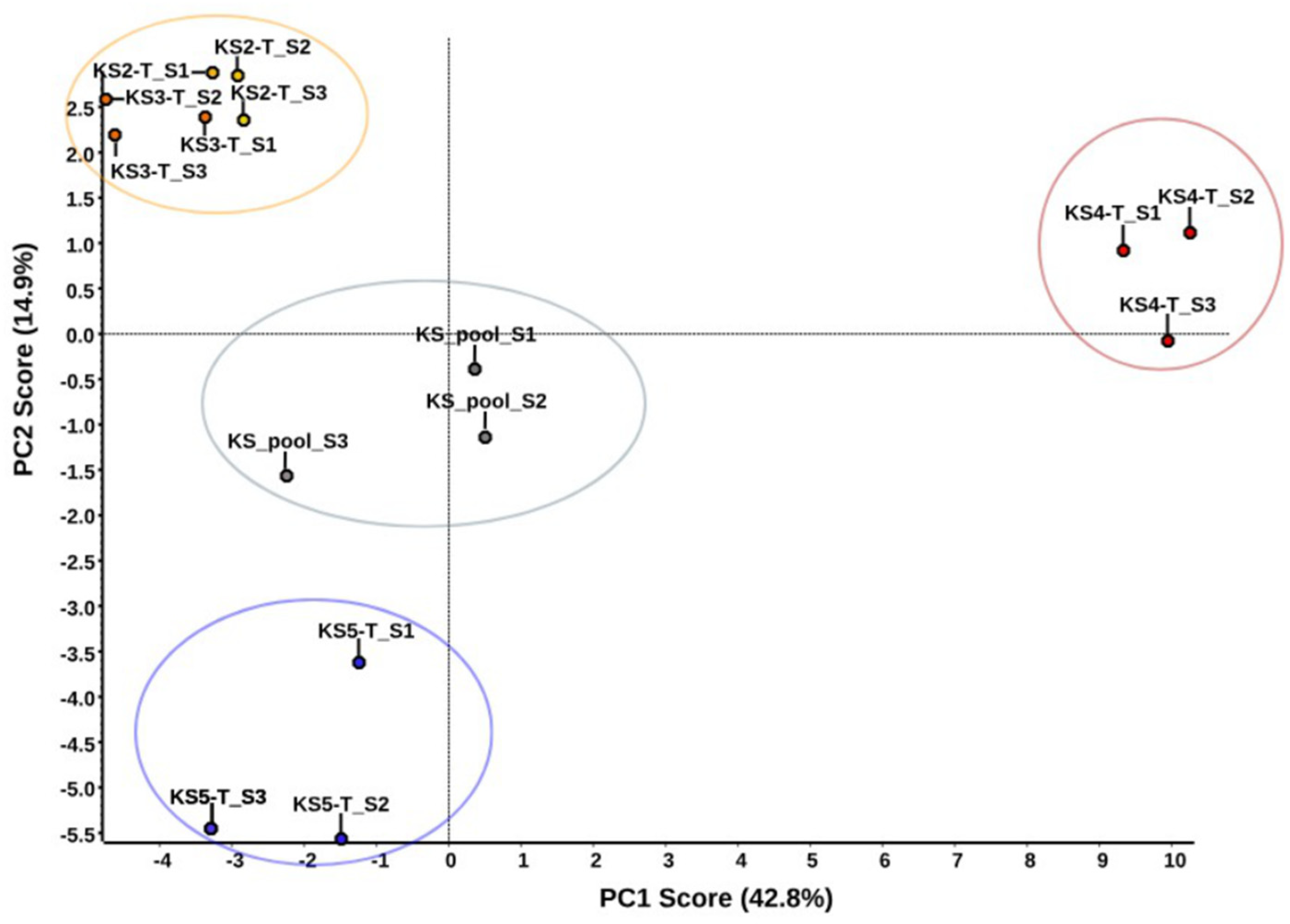
2.2. Trial of Quantitative Analysis of Proteins of Sialoliths S2–S5
3. Discussion
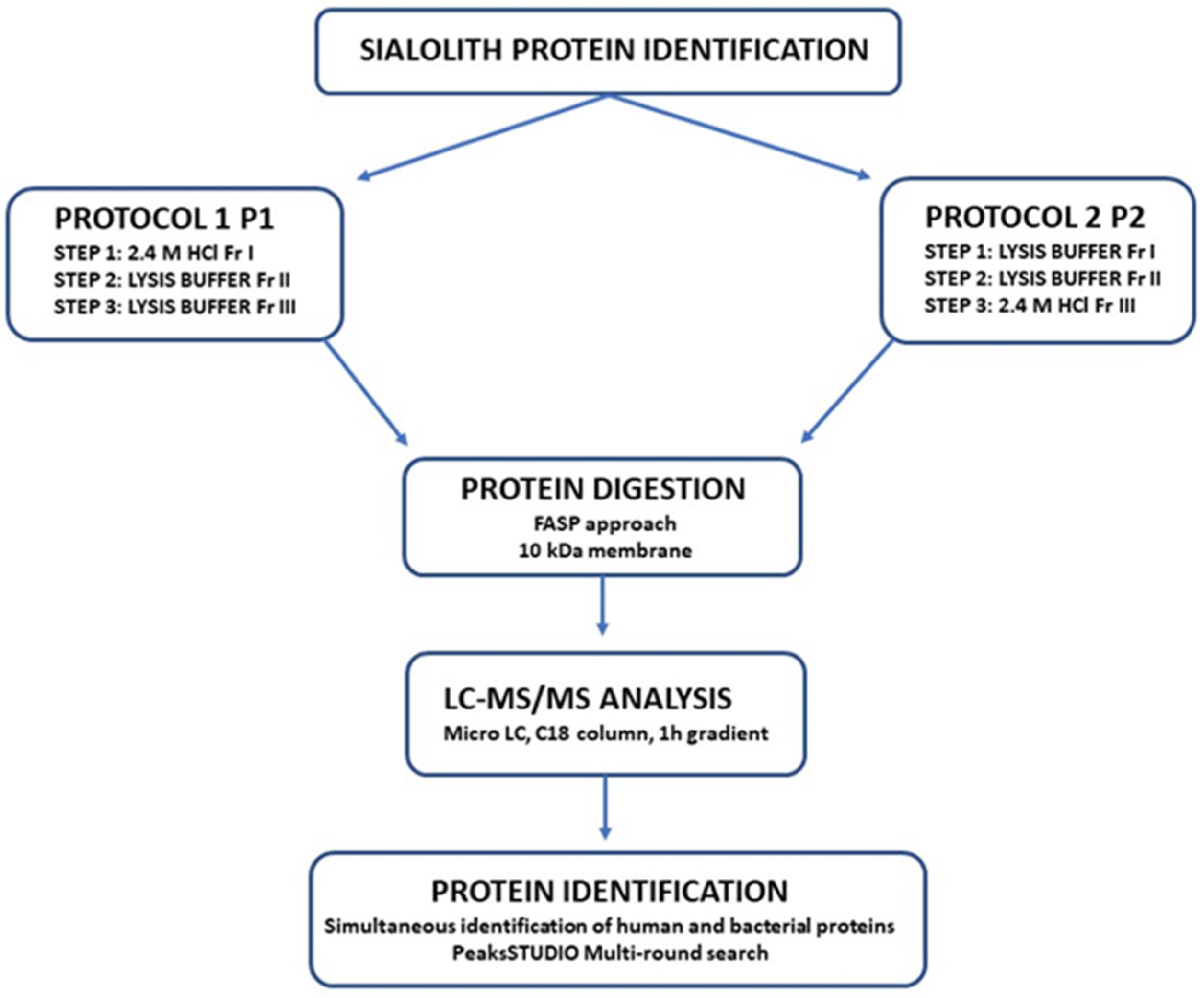
4. Materials and Methods
4.1. Protein Extraction
4.2. Protein Digestion and Final Clean-Up
4.3. Mass Spectrometry Analysis
4.4. Protein Identification
4.5. Relative Quantitative SWATH MS Analysis
4.6. Quantitative Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dev, B.; Sood, S.; DeWind, S.; Slattery, C. Kappa-Casein and beta-Caseins in Human-Milk Micelles: Structural Studies. Arch. Biochem. Biophys. 1994, 314, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.H.; Hay, D.I.; Levine, M.J. Complete primary structure of statherin, a potent inhibitor of calcium phosphate precipitation, from the saliva of the monkey, Macaca arctoides. Int. J. Pept. Protein Res. 2009, 34, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Marchal, F.; Dulguerov, P. Sialolithiasis Management: The state of the art. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, P.E.; Zenk, J.; Koch, M.; Schapher, M.; Rudes, M.; Iro, H. Nearly 3000 salivary stones: Some clinical and epidemiologic aspects. Laryngoscope 2015, 125, 1879–1882. [Google Scholar] [CrossRef]
- Kraaij, S.; Karagozoglu, K.H.; Forouzanfar, T.; Veerman, E.C.I.; Brand, H.S. Salivary stones: Symptoms, aetiology, biochemical composition and treatment. Br. Dent. J. 2014, 217, E23. [Google Scholar] [CrossRef]
- Kopeć, T.; Wierzbicka, M.; Szyfter, W.; Leszczyńska, M. Algorithm changes in treatment of submandibular gland sialolithiasis. Eur. Arch. Otorhinolaryngol. 2013, 270, 2089–2093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, W.; Wei, L.; Wang, F.; Peng, S.; Cheng, Y.; Li, B. An experimental chronic obstructive sialadenitis model by partial ligation of the submandibular duct characterised by sialography, histology, and transmission electron microscopy. J. Oral Rehabil. 2018, 45, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, E.; Berczi, I. Immunoregulation by the salivary glands. Biomed. Rev. 1998, 9, 79–91. [Google Scholar] [CrossRef]
- Yiu, A.J.; Kalejaiye, A.; Amdur, R.L.; Hesham, H.N.T.; Bandyopadhyay, B.C. Association of serum electrolytes and smoking with salivary gland stone formation. Int. J. Oral Maxillofac. Surg. 2016, 45, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, A.; Harrison, J.; Donath, K. Microlithiasis in parotid sialadenosis and chronic submandibular sialadenitis is related to the microenvironment: An ultrastructural and microanalytical investigation. Histopathology 1998, 32, 530–535. [Google Scholar] [CrossRef]
- Huoh, K.C.; Eisele, D.W. Etiologic Factors in Sialolithiasis. Otolaryngol. Neck Surg. 2011, 145, 935–939. [Google Scholar] [CrossRef]
- Sabot, J.-F.; Gustin, M.-P.; Delahougue, K.; Faure, F.; Machon, C.; Hartmann, D.-J. Analytical investigation of salivary calculi, by mid-infrared spectroscopy. Analyst 2012, 137, 2095–2100. [Google Scholar] [CrossRef]
- Szalma, J.; Böddi, K.; Lempel, E.; Sieroslawska, A.F.; Szabó, Z.; Harfouche, R.; Olasz, L.; Takátsy, A.; Guttman, A. Proteomic and scanning electron microscopic analysis of submandibular sialoliths. Clin. Oral Investig. 2012, 17, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, R.; Pawłowski, M.; Klimek, L.; Janas, A. Biochemical structure, symptoms, location and treatment of sialoliths. J. Dent. Sci. 2016, 11, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Tretiakow, D.; Skorek, A.; Ryl, J.; Wysocka, J.; Darowicki, K. Ultrastructural analysis of the submandibular sialoliths: Raman spectroscopy and electron back-scatter studies. Ultrastruct. Pathol. 2020, 44, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tretiakow, D.; Skorek, A.; Wysocka, J.; Darowicki, K.; Ryl, J. Classification of submandibular salivary stones based on ultrastructural studies. Oral Dis. 2020, 27, 1711–1719. [Google Scholar] [CrossRef]
- De Grandi, R.; Capaccio, P.; Bidossi, A.; Bottagisio, M.; Drago, L.; Torretta, S.; Pignataro, L.; De Vecchi, E. Salivary calculi microbiota: New insights into microbial networks and pathogens reservoir. Microbes Infect. 2018, 21, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Chaudhuri, G. Cystatin Superfamily. J. Health Care Poor Underserved 2010, 21, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.; Decarlo, A.; Featherstone, J. Functional aspects of the human salivary cystatins in the oral environment. Oral Dis. 2008, 5, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, A.; Jankowska, E.; Orlikowska, M.; Behrendt, I.; Czaplewska, P.; Rodziewicz-Motowidło, S. Influence of point mutations on the stability, dimerization, and oligomerization of human cystatin C and its L68Q variant. Front. Mol. Neurosci. 2012, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Spodzieja, M.; Szymańska, A.; Kołodziejczyk, A.; Prądzińska, M.; Maszota, M.; Stefanowicz, P.; Szewczuk, Z.; Grubb, A.; Czaplewska, P. Interaction of serum amyloid A with human cystatin C-identification of binding sites. J. Mol. Recognit. 2012, 25, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Spodzieja, M.; Rafalik, M.; Szymańska, A.; Kołodziejczyk, A.S.; Czaplewska, P. Interaction of serum amyloid A with human cystatin C-assessment of amino acid residues crucial for hCC-SAA formation (part II). J. Mol. Recognit. 2013, 26, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Spodzieja, M.; Kalejta, K.; Kołodziejczyk, A.S.; Maszota-Zieleniak, M.; Rodziewicz-Motowidło, S.; Żmudzińska, W.; Czaplewska, P. Characteristics of C-terminal, β-amyloid peptide binding fragment of neuroprotective protease inhibitor, cystatin C. J. Mol. Recognit. 2016, 30, e2581. [Google Scholar] [CrossRef] [PubMed]
- Gau, J.; Arnhold, J.; Flemmig, J. Reactivation of peroxidase activity in human saliva samples by polyphenols. Arch. Oral Biol. 2018, 85, 70–78. [Google Scholar] [CrossRef]
- Heo, S.-M.; Choi, K.-S.; Kazim, L.A.; Reddy, M.S.; Haase, E.; Scannapieco, F.A.; Ruhl, S. Host Defense Proteins Derived from Human Saliva Bind to Staphylococcus aureus. Infect. Immun. 2013, 81, 1364–1373. [Google Scholar] [CrossRef]
- Ellias, M.F.; Ariffin, S.H.Z.; Karsani, S.A.; Rahman, M.A.; Senafi, S.; Wahab, R.M.A. Proteomic Analysis of Saliva Identifies Potential Biomarkers for Orthodontic Tooth Movement. Sci. World J. 2012, 2012, 647240. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Nöbauer, K.; Soler, L.; Razzazi-Fazeli, E.; Gemeiner, M.; Cerón, J.; Miller, I. Detection of potential markers for systemic disease in saliva of pigs by proteomics: A pilot study. Vet. Immunol. Immunopathol. 2012, 151, 73–82. [Google Scholar] [CrossRef]
- Hay, D.; Smith, D.; Schluckebier, S.; Moreno, E. Basic Biological Sciences Relationship between Concentration of Human Salivary Statherin and Inhibition of Calcium Phosphate Precipitation in Stimulated Human Parotid Saliva. J. Dent. Res. 1984, 63, 857–863. [Google Scholar] [CrossRef]
- McDonald, E.E.; Goldberg, H.A.; Tabbara, N.; Mendes, F.; Siqueira, W.L. Histatin 1 Resists Proteolytic Degradation when Adsorbed to Hydroxyapatite. J. Dent. Res. 2010, 90, 268–272. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Kobayashi, H.; Tokimitsu, I.; Matsukubo, T.; Takaesu, Y. Statherin and Histatin 1 Reduce Parotid Saliva-Promoted Streptococcus mutans Strain MT8148 Adhesion to Hydroxyapatite Surfaces. Caries Res. 2006, 40, 403–411. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Øvstebø, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. 2017, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Inzitari, R.; Vento, G.; Capoluongo, E.; Boccacci, S.; Fanali, C.; Cabras, T.; Romagnoli, C.; Giardina, B.; Messana, A.I.; Castagnola, M. Proteomic Analysis of Salivary Acidic Proline-Rich Proteins in Human Preterm and At-Term Newborns. J. Proteome Res. 2007, 6, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Bennick, A. Salivary proline-rich proteins. Mol. Cell. Biochem. 1982, 45, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441868/ (accessed on 6 May 2021).
- Seixas, R.; Varanda, D.; Bexiga, R.; Tavares, L.; Oliveira, M. Biofilm-formation by Staphylococcus aureus and Staphylococcus epidermidis isolates from subclinical mastitis in conditions mimicking the udder environment. Pol. J. Vet. Sci. 2015, 18, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Staphylococcus aureus and Staphylococcus epidermidis Virulence Strains as Causative Agents of Persistent Infections in Breast Implants. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4727902/ (accessed on 6 May 2021).
- Duarte, R.S.; Barros, R.R.; Facklam, R.R.; Teixeira, L.M. Phenotypic and Genotypic Characteristics of Streptococcus porcinus Isolated from Human Sources. J. Clin. Microbiol. 2005, 43, 4592–4601. [Google Scholar] [CrossRef][Green Version]
- Khan, S.; Wong, T.T.; Prasad, N.; Lee, B.; Urban, C.; Segal-Maurer, S.; Turett, G. Streptococcus pseudoporcinus: Case Reports and Review of the Literature. Case Rep. Infect. Dis. 2020, 2020, 4135246. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, G.; Chen, H.; Li, H.; Li, S.; Zhang, C.; Pang, X.; Wang, L.; Zhao, L.; Shen, J. Quantification of Human Oral and Fecal Streptococcus parasanguinis by Use of Quantitative Real-Time PCR Targeting the groEL Gene. Front. Microbiol. 2019, 10, 2910. [Google Scholar] [CrossRef]
- Pimenta, F.; Gertz, R.E.J.; Park, S.H.; Kim, E.; Moura, I.; Milucky, J.; Rouphael, N.; Farley, M.M.; Harrison, L.H.; Bennett, N.M.; et al. Streptococcus infantis, Streptococcus mitis, and Streptococcus oralis Strains with Highly Similar cps5 Loci and Antigenic Relatedness to Serotype 5 Pneumococci. Front. Microbiol. 2019, 9, 3199. [Google Scholar] [CrossRef]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar] [CrossRef]
- Zheng, W.; Tan, M.F.; Old, L.A.; Paterson, I.C.; Jakubovics, N.S.; Choo, S.W. Distinct Biological Potential of Streptococcus gordonii and Streptococcus sanguinis Revealed by Comparative Genome Analysis. Sci. Rep. 2017, 7, 2949. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Genet. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Clinical, Phenotypic, and Genotypic Evidence for Streptococcus sinensis as the Common Ancestor of Anginosus and Mitis Groups of Streptococci—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0306987705004433?via%3Dihub (accessed on 6 May 2021).
- Shewmaker, P.L.; Steigerwalt, A.G.; Whitney, A.M.; Morey, R.E.; Graziano, J.C.; Facklam, R.R.; Musser, K.A.; Merquior, V.L.C.; Teixeira, L.M. Evaluation of Methods for Identification and Determination of the Taxonomic Status of Strains Belonging to the Streptococcus porcinus-Streptococcus pseudoporcinus Complex Isolated from Animal, Human, and Dairy Sources. J. Clin. Microbiol. 2012, 50, 3591–3597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahlen, S.D.; Clarridge, J.E. Thumb Infection Caused by Streptococcus pseudoporcinus. J. Clin. Microbiol. 2009, 47, 3041–3042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sawamura, S.; Niimori, D.; Ihn, H. A case of leg cellulitis caused by multidrug-resistant Streptococcus pseudoporcinus. Intractable Rare Dis. Res. 2018, 7, 280–282. [Google Scholar] [CrossRef]
- Kilian, M.; Tettelin, H. Identification of Virulence-Associated Properties by Comparative Genome Analysis of Streptococcus pneumoniae, S. pseudopneumoniae, S. mitis, Three, S. oralis Subspecies, and S. infantis. mBio 2019, 10, e01985-19. [Google Scholar] [CrossRef] [PubMed]
- Keith, E.R.; Podmore, R.G.; Anderson, T.P.; Murdoch, D.R. Characteristics of Streptococcus pseudopneumoniae Isolated from Purulent Sputum Samples. J. Clin. Microbiol. 2006, 44, 923–927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennett, J.S.; Jolley, K.; Earle, S.G.; Corton, C.; Bentley, S.D.; Parkhill, J.; Maiden, M. A genomic approach to bacterial taxonomy: An examination and proposed reclassification of species within the genus Neisseria. Microbiology 2012, 158, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Siddaramappa, S.; Narjala, A.; Viswanathan, V.; Maliye, C.; Lakshminarayanan, R. Phylogenetic insights into the diversity of Chryseobacterium species. Access Microbiol. 2019, 1, e000019. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, X.; Feng, C.; Li, A.; Dong, H.; Wu, S.; Zheng, B. Whole genome sequencing uncovers a novel IND-16 metallo-β-lactamase from an extensively drug-resistant Chryseobacterium indologenes strain J31. Gut Pathog. 2016, 8, 47. [Google Scholar] [CrossRef]
- Busso, C.S.; Guidry, J.; Gonzalez, J.J.; Zorba, V.; Son, L.S.; Winsauer, P.J.; Walvekar, R.R. A comprehensive analysis of sialolith proteins and the clinical implications. Clin. Proteom. 2020, 17, 12–14. [Google Scholar] [CrossRef]
- AZGP1 Is Androgen Responsive and Involved in AR-Induced Prostate Cancer Cell Proliferation and Metastasis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30820960/ (accessed on 6 November 2020).
- Wiśniewski, J.R.; Zougman, A.; Mann, M. Combination of FASP and StageTip-Based Fractionation Allows In-Depth Analysis of the Hippocampal Membrane Proteome. J. Proteome Res. 2009, 8, 5674–5678. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Lewandowska, A.; Fel, A.; Thiel, M.; Czaplewska, P.; Łukaszuk, K.; Wiśniewski, J.; Ołdziej, S. Compatibility of Distinct Label-Free Proteomic Workflows in Absolute Quantification of Proteins Linked to the Oocyte Quality in Human Follicular Fluid. Int. J. Mol. Sci. 2021, 22, 7415. [Google Scholar] [CrossRef] [PubMed]
- PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data|Nucleic Acids Research|Oxford Academic. Available online: https://academic.oup.com/nar/article/47/D1/D442/5160986 (accessed on 30 April 2021).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2018, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]


| Sialolith 2 | Sialolith 3 | Sialolith 4 | Sialolith 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein ID | Group | p-Value | Fold Change | Log (Fold Change) | p-Value | Fold Change | Log (Fold Change) | p-Value | Fold Change | Log (Fold Change) | p-Value | Fold Change | Log (Fold Change) |
| P63261 | Actin. cytoplasmic 2 | 0.0000556 | 7.284207094 | 0.862382285 | |||||||||
| P59998 | Actin-related protein 2/3 complex subunit 4 | 0.04393 | 8.260782456 | 0.917021185 | |||||||||
| P02768 | Albumin | 0.03581 | 1.541531139 | 0.187952302 | |||||||||
| P04745 | Alpha-amylase 1 | 0.00116 | 0.418105336 | −0.378714289 | 0.00073 | 0.212342882 | −0.672962293 | 0.0000533 | 6.938583149 | 0.841270797 | 0.00174 | 0.276827038 | −0.557791494 |
| P01008 | Antithrombin-III | 0.03109 | 1.7503964 | 0.243136411 | 0.03258 | 2.069788471 | 0.315925964 | ||||||
| P08311 | Cathepsin G | 0.03194 | 0.215401717 | −0.66675084 | |||||||||
| P28325 | Cystatin-D | 0.00014 | 12.34453279 | 1.091474658 | |||||||||
| P01036 | Cystatin-S | 0.04307 | 3.873435826 | 0.588096365 | |||||||||
| P01037 | Cystatin-SN | 0.04697 | 2.411624112 | 0.382309617 | |||||||||
| P12724 | Eosinophil cationic protein | 0.02326 | 0.727721066 | −0.138035053 | 0.0031 | 0.486055211 | −0.313314396 | 0.0000154 | 4.412958749 | 0.644729868 | |||
| P11678 | Eosinophil peroxidase | 0.04349 | 0.419790017 | −0.376967894 | |||||||||
| P00738 | Haptoglobin | 0.02436 | 0.454821305 | −0.3421592 | 0.00809 | 0.228983086 | −0.640196596 | 0.00067 | 3.059357569 | 0.485630239 | |||
| P69905 | Hemoglobin subunit alpha | 0.01515 | 0.30617954 | −0.514023834 | 0.00391 | 0.015164453 | −1.819173248 | 0.01928 | 1.689412748 | 0.227735767 | |||
| P68871 | Hemoglobin subunit beta | 0.00904 | 0.542480283 | −0.265616042 | 0.0000434 | 0.132372198 | −0.878203219 | ||||||
| P02042 | Hemoglobin subunit delta | 0.02124 | 2.668392602 | 0.426249728 | |||||||||
| Q99880 | Histone H2B type 1-L | 0.04376 | 0.382441371 | −0.417435133 | |||||||||
| P62805 | Histone H4 | 0.00074 | 0.615579981 | −0.210715512 | 0.00077 | 0.569335782 | −0.24463152 | 0.00023 | 3.057790931 | 0.485407788 | 0.03587 | 1.337430686 | 0.126271284 |
| P02788 | Lactotransferrin | 0.03229 | 2.969429146 | 0.472672967 | 0.02921 | 0.215013951 | −0.66753336 | ||||||
| P30740 | Leukocyte elastase inhibitor | 0.00072 | 0.295559993 | −0.529354352 | 0.00044 | 0.270358218 | −0.568060425 | 0.0000901 | 8.758657111 | 0.942437525 | 0.0134 | 0.44069474 | −0.355862133 |
| P08493 | Matrix Gla protein | 0.00909 | 5.121921883 | 0.709432951 | |||||||||
| P98088 | Mucin-5AC | 0.02361 | 0.35644789 | −0.448003952 | 0.0198 | 0.357020126 | −0.447307301 | 0.00386 | 3.117583194 | 0.493818052 | |||
| Q8TAX7 | Mucin-7 | 0.00921 | 1.683950908 | 0.226329426 | 0.01667 | 0.435705277 | −0.36080718 | 0.0271 | 0.517439675 | −0.286140275 | |||
| P24158 | Myeloblastin | 0.04279 | 0.440744162 | −0.355813431 | 0.012 | 0.300535469 | −0.522104266 | 0.00643 | 4.525451976 | 0.65566196 | 0.02779 | 1.55929247 | 0.192927582 |
| P05164 | Myeloperoxidase | 0.00425 | 6.591029535 | 0.818953258 | |||||||||
| P80188 | Neutrophil gelatinase-associated lipocalin | 0.00821 | 8.288015407 | 0.91845055 | |||||||||
| O75594 | Peptidoglycan recognition protein 1 | 0.00758 | 0.184102407 | −0.734940533 | 0.01342 | 1.71067952 | 0.233168656 | ||||||
| P12273 | Prolactin-inducible protein | 0.04007 | 3.075452718 | 0.487909055 | |||||||||
| Q6MZM9 | Proline-rich protein 27 | 0.04956 | 5.728810271 | 0.758064439 | 0.01618 | 0.473683666 | −0.324511591 | ||||||
| P80511 | Protein S100-A12 | 0.04541 | 0.426860545 | −0.369713985 | 0.04279 | 1.814077348 | 0.2586558 | ||||||
| P06702 | Protein S100-A9 | 0.00073 | 2.087914099 | 0.319712627 | |||||||||
| P02808 | Statherin | 0.02952 | 4.175986159 | 0.620759051 | |||||||||
| P02814 | Submaxillary gland androgen-regulated protein 3B | 0.00181 | 2.030083123 | 0.307513821 | 0.00293 | 0.135815988 | −0.867049102 | 0.0031 | 0.104953618 | −0.979002587 | |||
| P25311 | Zinc-alpha-2-glycoprotein | 0.03592 | 3.065769997 | 0.48653957 | |||||||||
| Q96DA0 | Zymogen granule protein 16 homolog B | 0.02283 | 0.793156904 | −0.100640891 | 0.04406 | 0.747544585 | −0.1263629 | 0.00000717 | 3.008601253 | 0.478364632 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaplewska, P.; Bogucka, A.E.; Musiał, N.; Tretiakow, D.; Skorek, A.; Stodulski, D. Trial Proteomic Qualitative and Quantitative Analysis of the Protein Matrix of Submandibular Sialoliths. Molecules 2021, 26, 6725. https://doi.org/10.3390/molecules26216725
Czaplewska P, Bogucka AE, Musiał N, Tretiakow D, Skorek A, Stodulski D. Trial Proteomic Qualitative and Quantitative Analysis of the Protein Matrix of Submandibular Sialoliths. Molecules. 2021; 26(21):6725. https://doi.org/10.3390/molecules26216725
Chicago/Turabian StyleCzaplewska, Paulina, Aleksandra E. Bogucka, Natalia Musiał, Dmitry Tretiakow, Andrzej Skorek, and Dominik Stodulski. 2021. "Trial Proteomic Qualitative and Quantitative Analysis of the Protein Matrix of Submandibular Sialoliths" Molecules 26, no. 21: 6725. https://doi.org/10.3390/molecules26216725
APA StyleCzaplewska, P., Bogucka, A. E., Musiał, N., Tretiakow, D., Skorek, A., & Stodulski, D. (2021). Trial Proteomic Qualitative and Quantitative Analysis of the Protein Matrix of Submandibular Sialoliths. Molecules, 26(21), 6725. https://doi.org/10.3390/molecules26216725






