Abstract
Nowadays, organic chemists are interested in the field of heterocyclic chemistry due to its use in the synthesis of a great variety of biologically active compounds. Heterocyclic compounds are widely found in nature and are essential for life. Among these, some natural nitrogen containing heterocyclic compounds have been used as chemotherapeutic agents. Their attachment to sugar molecules either as thioglycosides or as nucleosides analogues plays an important role in vital biological processes as well as in synthetic organic chemistry. Molecules containing benzothiazole (BT) nuclei are of this interesting class of compounds because some of them have been found to have a wide variety of biological activities. In this sense, we selected this topic to review and to then summarize the procedures related to the condensation reactions of o-aminothiophenoles (ATPs) as well as their disulfides with carboxylic acids, esters, orthoesters, acyl chlorides, amides, and nitriles. The condensation reactions with carbon dioxide (CO2) are included. Conventional methods with the use of acid and metal catalysts as well as recent green techniques, such as microwave irradiation, the use of ionic liquids, and ultrasound (US) chemistry, which have proven to have many advantages, were found in the review.
1. Introduction
Benzothiazole (BT) belongs to the family of benzazole compounds. The base structure of BT consists of a benzene ring fused with the 4 and 5 positions of a thiazole ring. These two rings together constitute the bases of the planar structure of the BT nucleus. BT is a colorless and slightly viscous liquid compound with a molecular formula C7H5NS. This is considered as a weak base with a melting point of 2.0 °C, a boiling point of 227–228 °C, a density of 1.24 g/mol, and a molecular mass of 135.19 g/mol. This privileged bicyclic ring system has been found in various natural compounds, which have a wide range of pharmaceutical applications [1,2,3]. Scientists have discovered the BT nucleus in natural products such as bisabolane-type sesquiterpenoid from the roots of Ligularia dentate (compositae) and diterpenes erythrazoles A and B of mangrove sediments (Figure 1) [4,5].
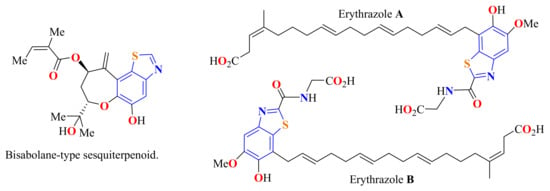
Figure 1.
Benzothiazole ring core in natural compounds with pharmaceutical applications.
BT heterocyclic compounds are rarely found in alkaloids, terrestrial, and marine natural products. However, the compounds found range from docile to a broad range of more complex structures created by the substitution of several groups at the carbon site between the nitrogen and sulfur atoms. These derivatives range in complexity from the well-known simple-structured firefly d-luciferin, which was isolated in the late of 1940s [6,7,8], to more complex molecules, such as rifamycins P and Q [9] and thiazinotrienomycin F and G (Figure 2) [10]. Some BT derivatives are also known to be aroma constituents of tea leaves and cranberries or flavor compounds such as the alkaloids nordercitine Ia, dercitamine Ib, dercitamide Ic, and cyclodercitin Id produced by the fungi Aspergillus clavatus and Polyporus frondosus (Figure 2) [1,11]. In addition, since 1950, medicinal chemists have been interested in BT derivative compounds, since according to its pharmacological profile, riluzole (6-trifluoromethoxy-2-benzothiazolamine) was found to be a clinically anticonvulsant drug (Figure 2) [12,13].
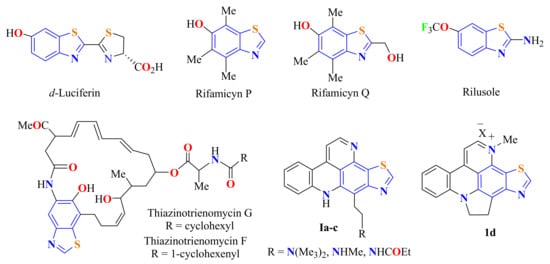
Figure 2.
Benzothiazole derivative compounds found in natural products.
BTs have been found to act as core nucleus in various synthesized drugs. For instance, the substituted ones are present in many pharmaceuticals that exhibit remarkable biological and therapeutic activities. For example, since 1991, Zopolrestat (Figure 3a) has been used to treat diabetes [14], while (GW610, NSC 721648) (Figure 3b and its derivatives show excellent antitumor activity [15,16], and Schiff bases (Figure 3c) are used as amyloid inhibitors for the treatment of Alzheimer’s disease [17]. Moreover, the BT moiety is also found in many functional molecules, such as ratiometric fluorescent pH indicators and ligands for catalytic reactions [18,19].
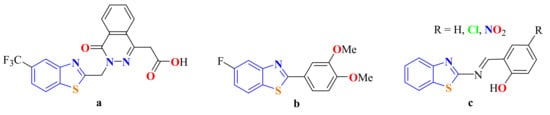
Figure 3.
Benzothiazole as core nucleous in synthesized drugs.
Since 1991, BT has been a moiety known to play an important role in chemistry, biochemistry, and medicinal chemistry, with a wide array of interesting biological activities and therapeutic functions, such as antimicrobial [20,21,22,23], anticancer [24,25,26,27,28,29], anthelmintic [30], antidiabetic [31,32], antituberculotic [33,34], antitumor [35,36,37,38,39,40,41], antitrypanosomal [42], antiviral [43,44,45], antibacterial [46,47,48,49], antioxidant [50], antiglutamate and antiparkinsonism [51,52], analgesic [53], anti-inflammatory [54,55], antifungal [56,57], antileishmanial [58], anticonvulsant [59], neuroprotective [60], muscle relaxant [61], vasodilator [62], and orexin receptor [63]. In addition, it acts as an inhibitor of several enzymes [14,64]; helps in atherosclerosis [65], insomnia [66], and epilepsy [67]; functions as an LTD4 receptor antagonist [68,69]; has antiparasitic and photosensitizer function [70]; and acts as a calcium channel antagonist [71,72], as an imaging agent for Aβ plaques in cerebral amyloid angiopathy [73], and as a falcipain inhibitor [74]. In addition, BT has other applications, e.g., as a radioactive β-amyloid imagining agent [75,76,77,78,79] and a schistosomicidal agent [80].
The studies of structure–activity relationship (SAR) of BTs have revealed that a change in the structure of the substituent group at the C2 position, in general, changes the bioactivity. Therefore, the biological profiles of this compound encourages researchers to design novel and efficient methods for the synthesis of BTs and their structural analogues. The development of these synthetic processes is undoubtedly one of the most significant challenges facing researchers.
Since the 2-substitutedBTs synthesized by A. W. Hofmann in 1887, organic chemists have developed synthetic processes to access BT compounds. Investigation into the synthesis of BT derivatives has been increasing over the years, and several reviews about this topic have been reported in the literature [81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,96,97,98,99,100,101,102,103,104]. It is worth mentioning that at least eight reviews, performed in 2020 alone, have been found about the synthetic strategies and biological activities of BT nucleus derivatives [97,98,99,100,101,102,103,104]. In these works, we found that many protocols have been developed for the synthesis of BTs. However, only a few methods for the condensation of o-aminotiphenoles (ATPs) with carboxylic acids and their derivatives were discussed. Accordingly, in our article, we present a literature review on the aspects of the research progress related to the condensation of ATPs with carboxylic acids and their derivatives, such as acid chlorides, amides, esters, orthoesters, nitriles, and thioesters, including carbon dioxide (CO2), as starting materials. This report includes protocols ranging from those for conventional methods, such as the use of heterogeneous solid acid catalysts and reactions performed under mild and solvent-free conditions, to those for green chemistry methods, such as the use of ionic liquids, reactions under microwave irradiation, as well as the use of ultrasound energy performed in the last 20 years.
2. Results and Discussion
2.1. Condensation with Carboxylic Acids
Considering a retro-synthetically point of view, a carbon-sulfur (C2–S) bond of 2-substitutedBTs 5 can be cleaved to represent an o-amidethiophenol 3, which can be obtained from the condensation of ATP 1 and a carboxylic acid 2 (Scheme 1). This approach proceeds with the intramolecular cyclization of the o-amidethiophenol 3 via the SH group by an o-aryl-S cross-coupling protocol to the hydroxybenzothiazolidine ring 4, which on heating or catalytic dehydration, produces the corresponding benzothiazole derivative 5 (Scheme 1).
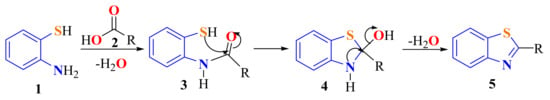
Scheme 1.
Mechanistic pathway in the condensation of ATPs with carboxylic acids.
2.1.1. Polyphosphoric Acid (PPA) as a Catalyst and a Dehydrating Agent
Polyphosphoric acid (PPA) has been extensively used as a good solvent for many organic compounds in organic synthesis. It has been used as one of the most effective reagents for acylation, alkylation, cyclization, and acid-catalyzed reactions. It is often the reagent of choice for a variety of synthetic transformations, such as dehydrations, rearrangements, and synthesis of nitrogen-containing heterocycles. PPA has also proved to be very useful in polymer synthesis.
Hein et al. used PPA as a solvent, a catalyst, and a dehydration agent in the condensation of ATP with carboxylic acid, ester, amide, or nitrile to generate 2-arylBTs and 2-alkylBTs in good yields. This procedure gives products not obtainable by other procedures [105].
The same authors used PPA in the condensation of substituted o-ATP with substituted 4-aminobenzoic acids at 220 °C to synthesize the 2-(p-aminophenyl)BTs 6a (Scheme 2) to be evaluated against breast cancer cell lines in vitro and in vivo [46]. ATP was condensed with substituted 4-aminobenzoic acids in PPA to give the 2-substitutedBTs 6b–d (Scheme 2). This route also afforded the 2-(2-aminopyridin-5-yl)BT 7 in a 59% yield from the reaction of 6-aminonicotinic acid and ATP in PPA (Scheme 2) [106].
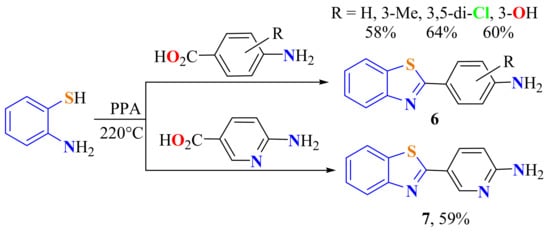
Scheme 2.
Synthesis of 2-(p-aminophenyl)benzothiazoles and 2-(2-aminopyridin-5-yl)benzothiazoles.
Rosenberg et al. used PPA to synthesize a polyBT 8 (Scheme 3) by the condensation reaction of terephthalic acid (TA) with 4,6-diamino-1,3-dithiohydroxybenzene [107].
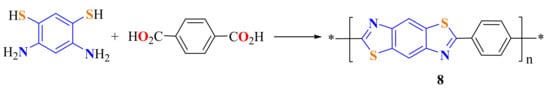
Scheme 3.
Synthesis of a polybenzothiazole.
A mechanistic pathway for this condensation was proposed. ATP and benzoic acid react with PPA to produce o-ammoniumthiophenol a in a mixture of benzoic-phosphoric/benzoic-polyphosphoric anhydrides b, which react to form o-ammoniumphenylbenzoate c as an intermediate. This undergoes acyl migration to generate o-benzanilidethiophenol d, which undergoes an acid-catalyzed ring closure and then dehydration to form 2-phenylBT (Scheme 4) [108].
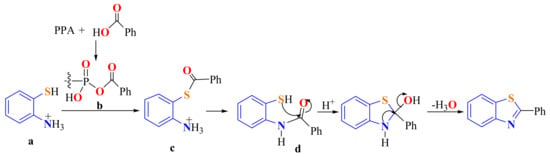
Scheme 4.
Proposed mechanistic path way in the condensation of o-aminothiophenol with carboxylic acid in PPA.
Condensation of ATP with 4-acetylamino-3-hydroxybenzoic acid in PPA as a catalyst on heating at 150 °C for 12 h gave a direct route to access 2-(3-hydroxy-4-acetylamine)phenylbenzothiazole 9 (Scheme 5) in a 61% yield [41].
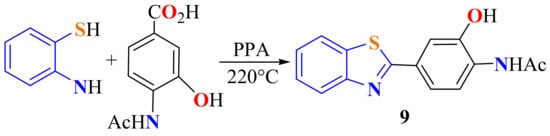
Scheme 5.
Condensation of ATP with 4-acetylamino-3-hydroxybenzoic acid to access 2-(3-hydroxy-4-acetylamine-phenyl)benzothiazole.
ATP was condensed with 5-aminosalicylic acid on heating PPA at 200 °C for 8–10 h via cyclization to produce the 2-(2-hydroxy-5-aminophenyl)BT 10 (Scheme 6) in good yield. 2-Azo-linked substituted BTs derived from 10 were synthesized to be evaluated for in vitro antibacterial activity against Staphylococcus aureus and Escherichia Coli strains by the Resazurin microtiter assay method (REMA) [109].
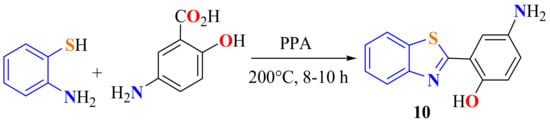
Scheme 6.
Synthesis of 2-(2-hydroxy-5-aminophenyl)benzothiazole.
ATP was heated with 2-hydroxy-aminobenzoic acids in PPA at 220 °C to synthesize 2-(2-hydroxy, 4-aminophenyl)BT 11 and 2-(2-hydroxy, 5-aminophenyl)BT 12, used as starting materials to synthesize positional isomers of BT-pyridone and BT-pyrazole containing disperse azo dyes, (Scheme 7) [110].
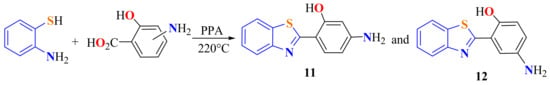
Scheme 7.
Synthesis of 2-(2-hydroxyaminophenyl)benzothiazoles.
Mathis et al. condensed 5-MeO-ATP with p-(methylamino)benzoic acid in PPA at 170 °C under N2 atmosphere for 1.5 h to generate the corresponding 2-(4-N-methylphenyl)-6-methoxyBT 13 in 21% yield, which was selectively O-demethylated at the 6-position to afford 6-OH-BT-1 14 (Scheme 8). Staining of the AD frontal cortex tissue sections with 6-OH-BT-1 indicated the selective binding of the compound to amyloid plaques and cerebrovascular amyloid [75].
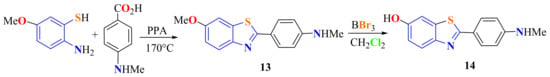
Scheme 8.
Synthesis of 2-(4-N-methylphenyl)benzothiazole.
Hutchinson et al. condensed ATP with first 4-amino-3-fluorobenzoic acid and then 4-amino-3-trifluoromethylbenzoic acid in PPA as a catalyst after heating at 110 °C for 0.5 h to generate 2-(4-amino-3-fluorophenyl)BT (Figure 4 (15) when R = F) and 2-(4-amino-3-trifluoromethylphenyl)BT (Figure 4 (15) when R = CF3) in 68% and 37% yields, respectively, to evaluate their in vitro antitumor biological properties [17b]. The lipophilic thioflavin-T analogue 2-(4-(methylamino)phenyl)BT 16 was obtained by heating ATP with p-N-methylaminobenzoic acid in PPA at 180 °C for 4 h in a 40% yield (Figure 4). This compound was reported to have a high affinity for amyloid plaque [111].
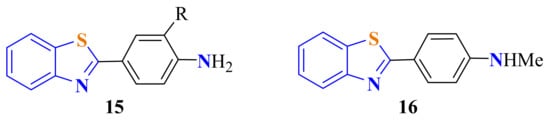
Figure 4.
2-(4-aminophenyl)benzothiazoles from the corresponding p-aminophenylbenzoic acid.
2-ChloromethylBT 17 (Scheme 9) was obtained in 62% yield by cyclocondensation of ATP with chloroacetic acid on heating in the presence of PPA at 180 °C for 8 h [112].
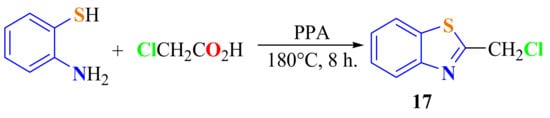
Scheme 9.
Condensation to access 2-chloromethylbenzothiazole.
Billeau et al. condensed ATP with several substituted benzoic acids by heating in the presence of PPA at 140 °C for 24 h to generate the corresponding 2-arylBTs 18a–v and 19a–d (Figure 5), which precipitated from water and were purified by recrystallization from CH2Cl2 to give the products in 80–90% yields [113].
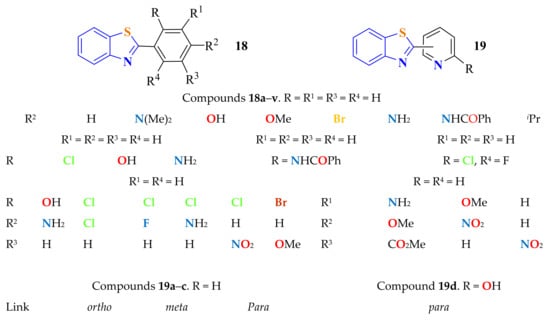
Figure 5.
2-substitutedphenylbenzothiazoles and 2-substitutedpyridinylbenzothiazoles.
ATP and 2-picolinic acid were condensed in the presence of PPA while stirring at 120 °C under N2 and then for 20 h at 160 °C. After separation and purification, the precipitate was recrystallized from methanol to afford 2-(o-pyridyl)BT 19a (Figure 5) as a pale-yellow solid in a 45% yield (the table in Figure 5) [114]. Compound 19a was used as a bidentate ligand to afford a palladium (II) complex. Antibacterial activity of the ligand and the corresponding complex against three Gram−negative and two Gram-positive microorganisms was performed, and the complex showed better microbial inhibition activity than the ligand and the palladium salt as reference.
Kini et al. refluxed ATP with substituted benzoic acids in the presence of PPA at a high temperature to get 2-arylBTs 20 (Scheme 10) in 74% yield. Compound 20 was evaluated against the human cervical cancer cell lines as an anticancer drug [115].
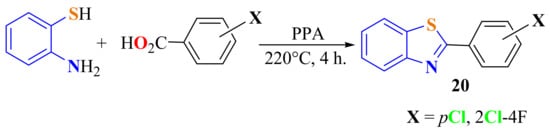
Scheme 10.
Condensation reaction to give 2-halophenylbenzothiazoles.
Serdons et al. reported the condensation of ATP with p-nitrobenzoic acid in the presence of PPA at 150 °C for 1 h to give 2-(p-nitrophenyl)BT 21 in a 35% yield as the starting material for the synthesis of F-labeled 2-(p-fluorophenyl)BT 22 (Scheme 11). The compounds were evaluated as amyloid imaging agents in Alzheimer’s disease [78].
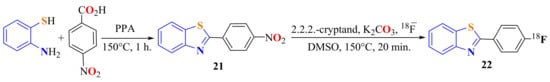
Scheme 11.
Synthesis of 2-(p-nitrophenyl)benzothiazole to access 2-(p-fluorophenyl)benzothiazole.
An interesting method for the synthesis of naphthyridineBT derivatives 23 has been described by Kou et al. In this method, ATP undergoes cyclization on treatment with naphthyridine-3-carboxylic acids in the presence of PPA at 170–250 °C to afford compounds 23 in 10–60% yields (Figure 6) [116].
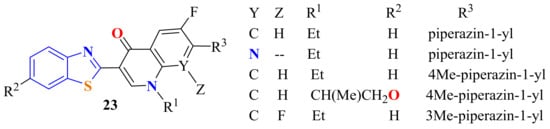
Figure 6.
Naphthyridinebenzothiazoles from condensation reaction of substituted ATPs.
The condensation reactions of 4-amidino-substituted ATPs with 2,5-furan- and 2,5-thiophene dicarboxylic acid were performed on heating in PPA at 180 °C for 2 h. The bisamidinodiBTyl compounds 24 and 25 were isolated as hydrochloride salts in 35–76% yields (Scheme 12) [117].
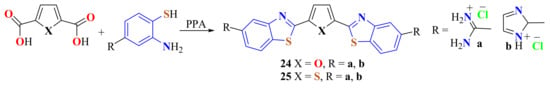
Scheme 12.
Condensation to access Bisamidinobenzothiazoles as hydrochlorides.
A single-step method to synthesize 2-arylBTs 26 in 50–60% yields has been reported from the condensation of ATP with substituted p-aminobenzoic acids in the presence of PPA on heating at 220 °C for 3 h (Scheme 13) [118]. To improve the yield, 4-nitrobenzoic acid was condensed on heating at 180 °C for 5 h to afford 2-(p-nitrophenyl)BT in an 81% yield, with subsequent reduction using Fe/NH4Cl in refluxing 75% ethanol for 2 h, which gave access to 2(p-aminophenyl)BT 26a (Scheme 13). The same reaction conditions were used in the condensation of ATP with p-nitrobenzoic acid and 4-aminobenzoic acid to produce the corresponding 2-(p-aminophenyl)BT in a 90% yield (Scheme 13) [119].
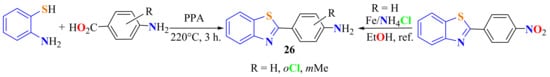
Scheme 13.
Two methods to access 2-(p-aminophenyl)benzothiazoles.
Isomeric-amidinoBTyl-disubstituted pyridines 27a–i and pyrazine 28 were synthesized in 46–79% yields by the condensation reaction of pyridine and pyrazine dicarboxylic acids with 5-amidino- and 5-imidazolinyl-substituted ATPs. To improve yields, the quantity of the byproducts was reduced on heating in PPA first at 120–140 °C for 1 h and then for 2 h at 160–180 °C (Figure 7) [120].
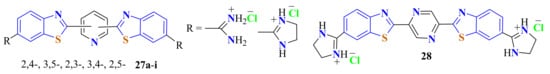
Figure 7.
Amidinobenzothiazolyl-disubstituted pyridines and pyrazines.
In a method developed by Santos et al., ATP was condensed with amino acids, such as glycine and d-valine, as hydrochlorides on heating at 220 °C in the presence of PPA for 4 h to give the 2-substitutedBTs 29a and 29b in low yields (Scheme 14). However, 53.2% and 41.0% yields of the respective compounds were obtained when the corresponding amino acid ethyl esters was used [121].
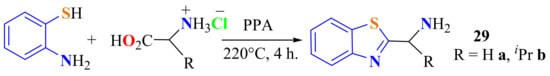
Scheme 14.
2-substitutedBTs.
Condensation of 5-amidino-substituted ATP with carboxylic acids by heating in the presence of PPA at 110–180 °C for 3 h as a method to prepare 6-amidino-2-aryl/heteroarylBTs 30a–u in 31–74% yields was also reported (Figure 8) [122].
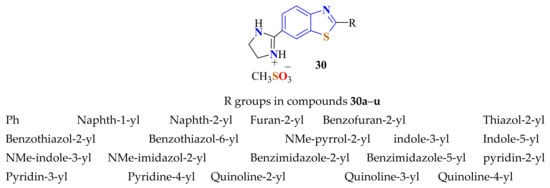
Figure 8.
6-imidazolium-2-substitutedbenzothiazoles.
2.1.2. Other Acids as Catalysts
The use of PPA requires high temperatures (110–220 °C) and long reaction times (1–12 h). These harsh conditions depend on the starting materials’ stability and are a limitant to generalizing this condensation. Therefore, as an alternative to PPA, other acids as catalysts have been designed to be used in this condensation.
2-SubstitutedBTs 31 were obtained from ATP and the corresponding carboxylic acid by treatment with P2O5/MeSO3H (1/10, w/w) while warming. The reaction was effective for a wide range of aliphatic and aromatic carboxylic acids. The general procedure involves treating a mixture of P2O5/MeSO3H (1/10, w/w) and o-ATP in the ratio 1.5 g/1.0 mmol with 1.0 equivalent of the corresponding carboxylic acid and warming for 10 h [123].
Yildiz et al. reported the condensation of ATP with several carboxylic acids in the presence of trimethylsilylpolyphosphate ester (PPSE) as the cyclodehydration reagent and on refluxing for 3–4 h (140 °C) to afford a series of 2-substitutedBTs 32 in 43–72% yields (Scheme 15). All 2-substitutedBTs were tested for their antibacterial activities against Gram-positive and Gram-negative bacteria and antifungal activity against the fungus Candida albicans [124].
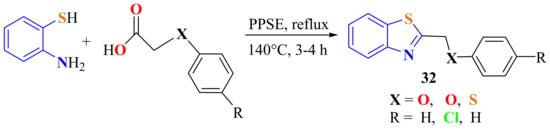
Scheme 15.
2-[β-methylphenol(thiophenol)]atesubstitutedbenzothiazoles.
Ge et al., in a one-pot synthesis, condensed ATP with trifluoroacetic acid or difluoroacetic acid in the presence of PPh3, excess of Et3N, and CCl4 on refluxing to produce 2-trifluoro- and 2-difluoromethylBTs 33 (Scheme 16) in 72% and 85% yields, respectively (Scheme 16). The proposed mechanistic procedure is based on the electrophilicity of the imino group of the fluorinated N-aryl imidoyl chloride intermediate b (Scheme 16). The (-SH) nucleophilic group at the ortho position on benzene produces an intramolecular nucleophilic substitution and leads to the formation of 2-fluoroalkyl BTs [125].
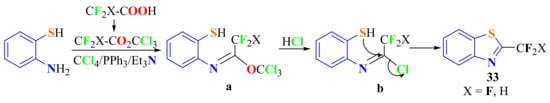
Scheme 16.
Proposed mechanistic pathway in the condensation of ATP with trifluoroacetic acid.
Jiang et al. used triphenylphosphine (Ph3P) as the catalyst in the condensation reaction of ATP with bromodifluoroacetic acid in the presence of 3 molar equivalents of carbon tetrabromide (CBr4) in refluxing toluene for 24 h to prepare 2-bromodifluoromethylBT 34 in a 65% yield to be used as a building block in the subsequent reactions. The reaction mechanism involves the formation of imidoyl bromide and intramolecular ring-closure reaction, similar to the mechanism represented in Scheme 16 [126].
Sharghi et al., in a one-pot procedure, condensed ATP with several aliphatic or aromatic carboxylic acids using a MeSO3H/SiO2 system as a dehydrating catalyst at and heating at 140 °C for 2–12 h to synthesize 2-substituted aliphatic and aromatic BTs 35 in 70–92% yields (Scheme 17). A simple work-up, using diverse carboxylic acids, and easy handling reaction conditions are the benefits of this method. The 2-(o-, m-, and p-NO2-phenyl)BTs were obtained in 35%, 11%, and 53% yields, respectively [127].
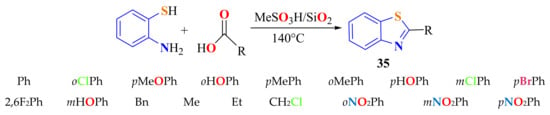
Scheme 17.
Aromatic and aliphatic 2-substitutedbenzothiazoles.
For instance, 2-chloromethylBT was obtained in a 92% yield on the condensation of ATP with chloroacetic acid and heating the product at 140 °C for 2.5 h, using MeSO3H/SiO2 as the catalyst, in the synthesis of BT derivatives [128].
The cyclocondensation of ATP and aromatic carboxylic acids to give 2-substitutedBTs 36 in 60–87% yields has been carried out under mild conditions using tetrabutylammonium bromide (TBAB) as the reaction medium and triphenyl phosphite (TPP) as the catalyst (Scheme 18). Shorter reaction times, rapid isolation of products, and good yields are advantages of this method. The reaction was found to be general and quite tolerant to the nature of the substituted carboxylic acids. The mechanism shown in Scheme 19 is assumed to operate. Triphenyl phosphite reacts with aromatic carboxylic acids to produce the corresponding ester a, which reacts with o-aminothiophenol to form the phosphoester intermediate b. The thiol group of phosphoester b attacks the C=O to give, after intramolecular cyclization, the title products [129].
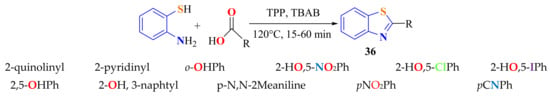
Scheme 18.
Condensation of ATP with aromatic carboxylic acids.
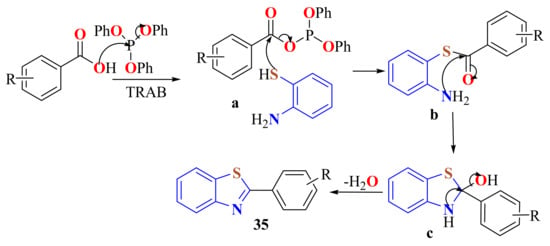
Scheme 19.
Mechanistic path way with the use of triphenyl phosphite in the condensation of ATP with carboxylic acids.
N-Boc-protected valine amino acid reacts with ATP in the presence of EDC, HOBT, and DCM, followed by the intramolecular Mitsunobu reactions in the presence of DIAD, PPh3, and TEA to afford 2-substitutedBT 37 as an intermediate (Scheme 20) [130].
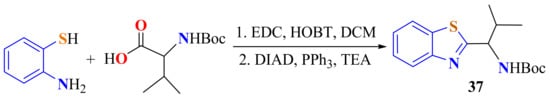
Scheme 20.
Condensation of ATP with N-Boc-protected valine.
Fifteen 5-substituted-2-(pyridyl)BTs 38 and 39 were synthesized in 34–42% yields by the condensation reaction of substituted ATPs with the corresponding pyridinylcarboxylic acids under the PPA/H3PO4 system (Scheme 21). Activities of these synthesized compounds were evaluated on Bcap-37, HCT-15, and HepG2 tumor cells in vitro by a standard MTT assay. 5-Fluorouracil (5-FU) was used as the positive control [131].
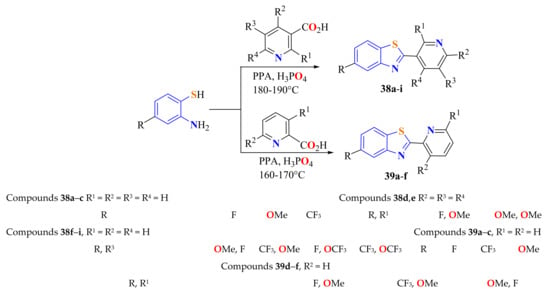
Scheme 21.
Condensation of substituted ATP with 2- and 3-pyridinylcarboxylic acids.
Recently, condensation of ATP with piperidine-4-carboxylic acid on heating PPA has been carried out to synthesize 2-(piperidin-4-yl)BT 40 as an intermediate to be used as a building block (Scheme 22) [132].
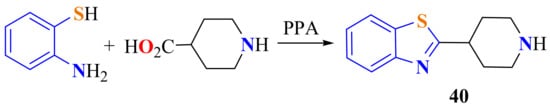
Scheme 22.
Condensatio of ATP with piperidine-4-carboxylic acid.
A series of 2-(3-butynoicamidophenyl)BTs 41 were synthesized starting from 4-fluoro-3-nitrobenzoic acid and ATP. Their antitumor activities against human tumor cell lines (HCT116, Mia-PaCa2, U87-MG, A549, NCI-H1975) were evaluated by an MTT assay [133].
2-SubstitutedBTs 42 were synthesized in 72–92% yields from the reaction of ATP with a set of substituted aromatic carboxylic acids using Samarium(III) triflate as a catalyst (Scheme 23). Short reaction times, aqueous reaction media, and easy work-up are the advantages of this method. The catalysts were reused without any appreciable loss of efficiency [134].
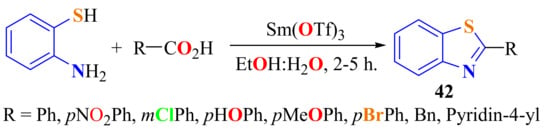
Scheme 23.
Condensation of ATP with aromatic carboxylic acids in presence of Samarium (III) triflate.
A simple trituration method for the synthesis of 2-substitutedBTs 43 from the reaction of N-protected amino acids and ATP using molecular iodine as a mild Lewis acid catalyst has been proposed (Scheme 24). The reaction occurs in one step that lasts for 20–25 min in solvent-free conditions to afford the products in 66–97% yields [135].

Scheme 24.
Condensation of ATP with amino acids in presence of iodine.
2.1.3. Condensation on Direct Heating
In addition to the harsh conditions, such as toxic solvents, strong acidic conditions, high temperatures, and long reaction times, employed in condensation reactions of ATPs with carboxylic acids, the use of PPA as a catalyst and the side reactions carried out lead to the lowering of selectivities and low yields. Therefore, there is a strong demand for a highly efficient and environmentally benign method for the synthesis of these heterocycles. In this sense, the use of ionic liquids (ILs) as green solvents in organic synthesis has gained considerable importance due to their solvating ability, negligible vapor pressure, and easy recyclability. In addition, they have been shown to promote and catalyze organic transformations due to their high polarity. ILs can also be recovered and recycled many times with marginal loss.
Substituted 2-arylBTs 44 can be rapidly (10 min) synthesized in 80–94% yields by the condensation of a set of carboxylic acids with ATP under atmospheric conditions using the ionic liquid 1-butyl-3-methyl-imidazoliumtetraflouroborate ((bmim)BF4) as a dissolvent and dehydrating agent at 100 °C (Scheme 25) [136].
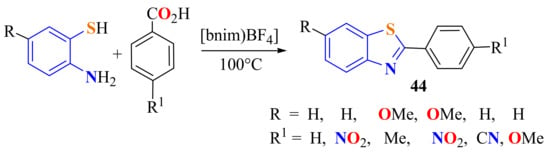
Scheme 25.
Condensation of ATP with p-substituted benzoic acids using the ionic liquid bnimBF4.
In an autocatalytic procedure, ATP and trifluoroacetic acid (TFA) were heated to 70 °C for 16 h to produce the corresponding 2-(trifluoromethyl)BTs 45a (Scheme 26). Evaporation of the excess TFA afforded the product in a 99% yield. However, the same reaction with the electron-deficient 4-trifluoromethyl-substituted o-aminothiophenol produced the corresponding 2-trifluoromethylBT 45b in only 58% yield [137].
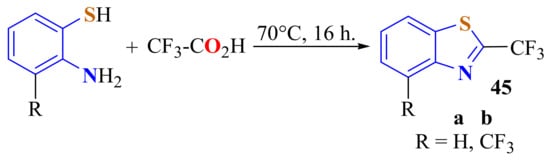
Scheme 26.
Condensation of 3-substituted ATP with trifluoroacetic acid on direct heating.
A metal-free methodology by the use of a base as an oxidant has been developed for the condensation of ATP with oxalic and malonic acids followed by a decarboxylation to afford BT 46a and 2-methylBT 46b in 80% and 82% yields, respectively (Scheme 27). Easy work-up procedure, high yield, and easy isolation of products are the advantages of this methodology [138].
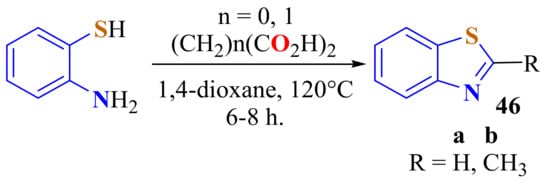
Scheme 27.
Condensation of ATP with oxalic and malonic acids.
2.2. Condensation with Esters
The condensation of esters with ATPs produces the corresponding thioester a (Scheme 4). The acyl group migrates to form the amide b (Scheme 4), which is cyclicized and then dehydrated with the aid of a Brönsted or Lewis acid to the corresponding 2-substitutedBTs, as depicted in Scheme 4.
Substituted ATPs have been condensed with methyl 4-amino-3-iodobenzoate ester under PPA on heating to 220 °C to produce 2-(4-aminophenyl)BT 47 in a 14% yield, instead of the expected iodinated compound (Scheme 28) [106]. In acid conditions, the iodo group is electrophylically substituted. The ATP amino group is cauterized and the nucleophile is blocked to directly form the corresponding amide and the thioester. Then the acyl group migration is carried out at a high temperature.
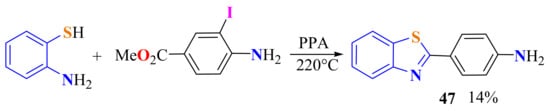
Scheme 28.
Condensation of ATP with 4-amino-3-iodobenzoate methyl ester to afford 2-(4-aminophenyl)benzotthiazole.
Khalil et al. condensed the amino ethyl ester 5-carbonyl-(3,4-diamino-2-ethylcarboxylate thieno[2,3-b]thiophene-5-yl)-8-hydroxyquinolin with ATP by heating in the presence of PPA at 160 °C for 3 h and then carried out neutralization with aqueous ammonia to afford the corresponding 2-substitutedBT 48 in an 80% yield (Scheme 29) [139]. In this case, the neutralization regenerates the ATP amino group to form the corresponding amide for the cyclization to be continued.
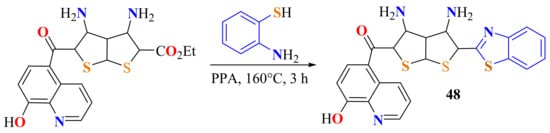
Scheme 29.
Condensation of ATP with the amino ethyl ester 5-carbonyl-(3,4-diamino-2-ethylcarboxylate thieno[2,3-b]thiophene-5-yl)-8-hydroxyquinolin.
Phenolic esters were efficiently converted to 2-substitutedBTs 49 in a one-pot reaction by treatment with ATP in the presence of a catalytic amount of K2CO3 and then converted to a thiolate salt on heating with N-methyl-2-pyrrolidone (NMP) at 100 °C (Scheme 30). The formation of a thioester b with the elimination of the corresponding phenol and migration of the acyl group to the nitrogen atom c can be proposed, followed by dehydration [79].
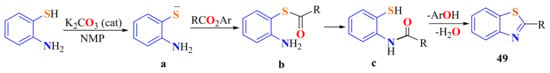
Scheme 30.
Condensation of the ATP thiolate with phenyl esters.
Polymer-bound esters were treated with ATP in the presence of AlMe3 as the Lewis acid dehydrating agent in refluxing toluene to afford the 2-substitutedBts 50 as the cleavage products in a 46–75% yields (Scheme 31) [140].
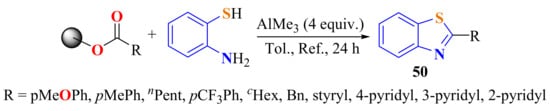
Scheme 31.
Condensation of ATP with plymer-bound esters.
Shantakumar et al. synthesized 2-(ethylcarboxilate)BT 51 in a 53% yield by the condensation of ATP and diethyl oxalate in a mild reflux for 4 h. During heating, the temperature decreased from 147 to 93 °C [63]. The same condensation has been carried out on refluxing for 8 h to afford compound 51 in a 92% yield (Scheme 32) [141].
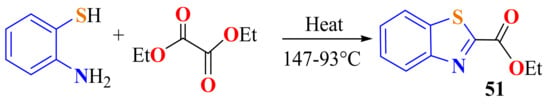
Scheme 32.
Condensation of ATP with oxalate ethyl ester.
Sheng et al. condensed ATP with ethyl chloroacetate in dichloromethane as a dissolvent on heating from 0 °C to room temperature to afford 2-chloromethylBT 17 in a 68.7% yield (Scheme 33) [142].
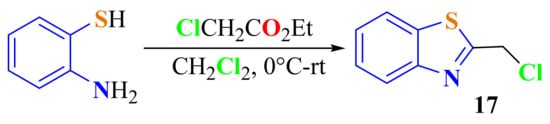
Scheme 33.
Condensation of ATP with chloroacetate ethyl ester.
Santos et al. condensed ATP with glycine and d-valine ethyl ester hydrochlorides in the presence of PPA, heating at 220 °C for 4 h, to give the corresponding 2-substitutedBTs 29a and 29b in 52.3% and 41% yields, respectively (Scheme 34) [121].
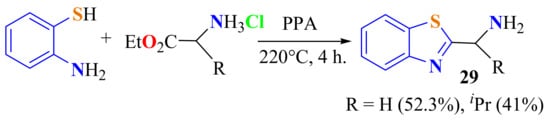
Scheme 34.
Condensation of ATP with hydrochlorides of aminoacid ethyl esters.
5-Fluoro ATP potassium salt was reacted with HCl, followed by the addition of (R)-4-methyl-oxazolidine-2,5-dione derived from d-alanine, to give the (1R)-1-(6-fluoro-1,3-BT-2-yl)ethanamine hydrochloride 52, which was transformed to its p-toluenesulfonate salt in an 81% yield (Scheme 35). This compound was used as the starting material in the synthesis of a series of diamides [143].
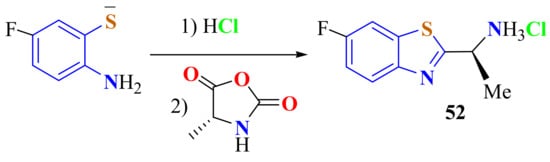
Scheme 35.
Condensation of 5-fluoro ATP thiolate with (R)-4-methyl-oxazolidine-2,5-dione derived from d-alanine.
2.3. Condensation with Orthoesters
The general mechanistic pathway of condensation reactions of ATPs with orthoesters has been proposed as represented in Scheme 36.
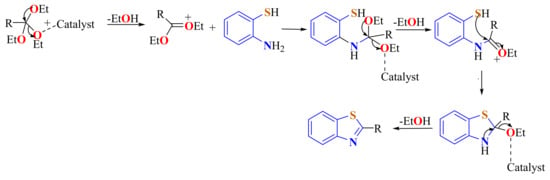
Scheme 36.
General mechanistic pathway proposed for the condensation of ATPs with orthoesters.
The condensation of ATP with three ethyl orthoesters was carried out in the presence of catalytic quantities of H2SO4 on refluxing to produce the corresponding 2-substitutedBTs 46 in a 75–85% yield (Scheme 37) [144].
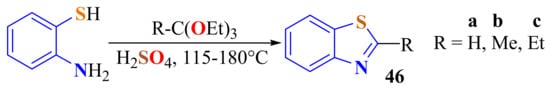
Scheme 37.
Condensation of ATP with alkyl ethylorthoesters in acid media.
In an efficient procedure, 2-chloro-1,1,1-triethoxyethane was condensed with ATP in a versatile synthesis of 2-chloromethylBT 17 [145].
A series of 2-alkylBTs (R = H, Me, Et) 46a–c were synthesized in 81–86% yields from the reactions of ATP with orthoesters in the presence of catalytic amounts of Bi(III) salts, such as Bi(TFA)3, Bi(OTf)3, and BiOClO4xH2O, under solvent-free conditions at room temperature. High conversion, very short reaction times, cleaner reaction profiles, solvent-free conditions, straightforward procedure, and use of low-toxic catalysts are the features of this protocol [146]. The same condensation was carried out in the presence of catalytic amounts of the eco-friendly and inexpensive ZrOCl2·8H2O under solvent-free conditions to afford the compounds 46a–c in 93–97% yields. The reusability of the catalyst, high yields, very short reaction times (4–6 min), solvent-free reaction conditions, and an easy experimental and work-up procedure are the advantages of this protocol [147].
Silica-supported fluoroboric acid HBF4-SiO2 was used as a catalyst in the condensation of ATP with orthoesters under solvent-free conditions at room temperature. A simple and environmentally benign synthesis of 2-aliphaticBTs 46a–e in 84–97% yields was carried out by this procedure in 45 min (Scheme 38) [148].
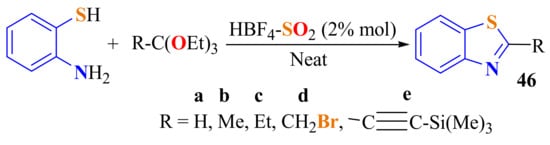
Scheme 38.
Condensation of ATP with alkyl and alquilil ethyl ortho esters using silica-supported fluoroboric acid.
Bastug et al. condensed substituted ATP with functionalized orthoesters under mild conditions to prepare various 2-substitutedBTs 53 in 90–95% yields. The condensations were carried out at room temperature in the presence of 1 equiv. of BF3 OEt2 and were complete within 3 h. Multifunctional heterocycles are the specialty of this protocol (Scheme 39) [149].
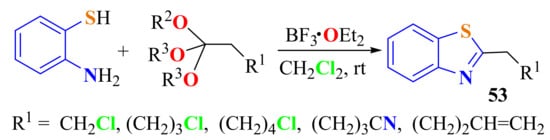
Scheme 39.
Condensation of ATP with substituted alkyl ortho esters in presence of BF3OEt2.
o-Benzenedisulfonimide was used as an efficient catalyst in the condensation of ATP with various orthoesters, giving the respective 2-substitutedBTs 54 in 1–2 h in 86–92% yields. The reaction conditions were very simple. The catalyst was easily recovered and reused (Scheme 40) [150].
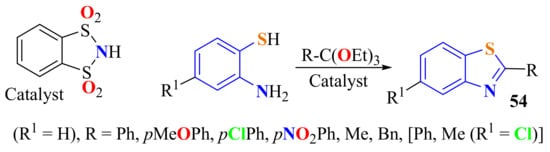
Scheme 40.
o-Benzenedisulfonimide as catalyst in the condensation of ATP with ortho esters.
An eco-friendly procedure for the condensation of ATP with orthoesters employed 15% mol of catalytic ammonium chloride (NH4Cl) in H2O or ethanol to produce 2-substitutedBTs 46 in a short reaction time (30 min), involving easy work-up, and in a high yield (87–93%) (Scheme 41) [151].
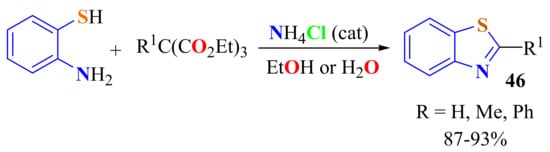
Scheme 41.
Ammonium chloride in the condensation of ATP with aliphatic and aromatic ethyl ortho esters.
A solvent-free method, with sulfonated rice husk ash (RHA) as the solid acid catalyst, was developed for the condensation of ATP with orthoesters in the preparation of BT 46a and 2-methylBT 46b in 92% and 95% yields in 1 min. The catalyst could be reused five times without any loss of its catalytic activity (Scheme 42) [152].
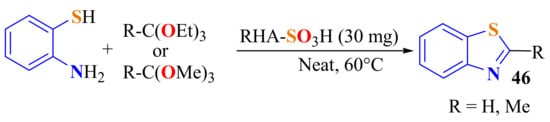
Scheme 42.
Sulfonated rice husk ash (RHA) as catalyst in the condensation of ATP with ortho esters.
2.4. Condensation with Acyl Chlorides
In this method, acid chlorides react with ATPs to produce directly the corresponding amides as intermediates. In general, tertiary amines are used to trap the chlorohydric acid produced in the reaction. After the cyclization, the generated ammonium chloride salt formed is used as a catalyst in the dehydration of the 2-hydroxy thiazolidine intermediate to produce 2-substitutedBTs. Sometimes, any base is used. In this case, hydrochloric acid is used as the dehydrating agent.
Via a general method, ATP with 1-methyl-pyrrolidinone as the solvent was condensed with a stoichiometric amount of the corresponding acid chloride, added slowly at room temperature under an inert atmosphere. The corresponding 2-arylBTs 55 were obtained in 82–95% yields (Scheme 43). The reaction mixture was heated at 100 °C for 1 h. The intermediate di-irido and the six-coordinated mononuclear iridium (III) dopants of the above ligands were synthesized and characterized [153,154].
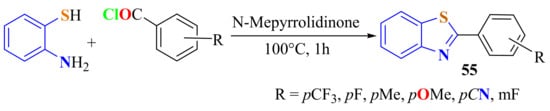
Scheme 43.
1-methyl-pyrrolidinone as solvent in the condensation of ATP with substituted benzoyl chlorides.
4-Methyl-ATP was condensed with chloroacetyl chloride in the presence of Et3N and EtOAc as the solvent on stirring from 0 °C to room temperature to produce in situ the corresponding 5-methyl-2-chloromethylBT 56, which was treated with thiourea to afford the more stable isothiouronium salt (Scheme 44) [155].
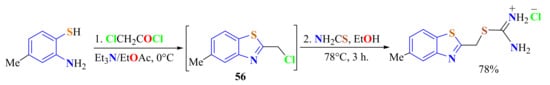
Scheme 44.
Synthesis of 5-methyl-2-(chloromethyl)benzothiazole as intermediate to afford the isothiouronium salt.
BTyl compounds 59a and 59b, synthesized from the corresponding dialdehydes 57a,b and 4-amino-3-mercaptobenzonitrile, were converted to the corresponding chlorocarbonyl derivatives 61a and 61b, which were condensed with 4-amino-3-mercaptobenzonitrile by Route A to produce the biscyanoBTyl compounds 61a and 61b in about 75% yields (Scheme 45). By Route B, 5-(4-carboxyphenyl)-2-furylcarboxylic acid 58a and 5-(4-carboxyphenyl)-2-thienylcarboxylic acid 58b were converted to the dichlorocarbonyl derivatives 60a and 60b and then were condensed with 4-amino-3-mercaptobenzonitrile to afford 62a and 62b in about 35% yields (Scheme 45). Route B has one step less than Route A. However, the overall yield of the reactions was considerably lower [156].
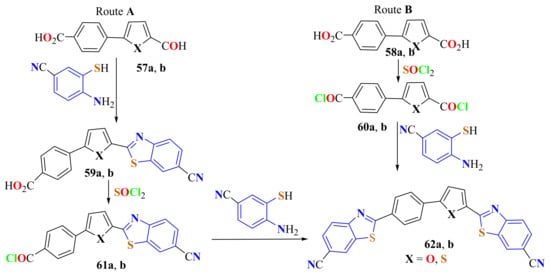
Scheme 45.
Two condensation ways to produce biscyanoBTyl compounds.
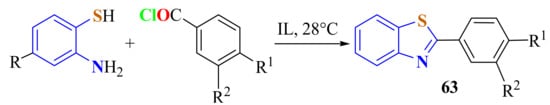
A regioselective one-pot synthesis of 2-arylBTs 63 was achieved in excellent isolated yields by the condensation of ATP and substituted benzoyl chlorides under ambient conditions using the ionic liquid mixtures 1-butylimidazolium tetraflouroborate ([Hbim]BF4) and 1,3-di-n-butylimidazolium tetrafluoroborate ([bbim]BF4) as the reaction medium and the reaction promoter, respectively (Scheme 46). The absence of a catalyst, room temperature conditions, and non-volatile ILs makes this protocol green and environment friendly [157].
Scheme 46.
Condensation of 4-substituted ATP with disubstituted benzoyl chlorides under (bbim)BF4.
Karlsson et al. carried out the same condensation reaction of ATP with 4-nitrobenzoyl chloride by applying N-methyl-2-pyrrolidone (NMP) as an oxidant and heating at 100 °C for 1 h to give 2-(p-nitrophenyl)BT [158].
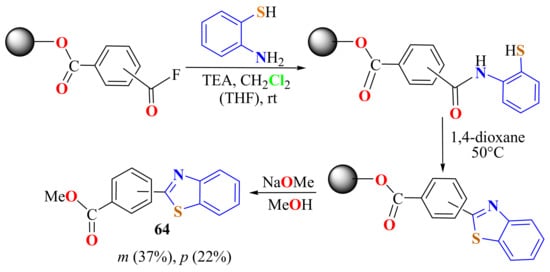
Chen et al. condensed ATP with isophthaloyl or terephthaloyl fluorides attached to a polyethylene glycol methyl ether PEG resin in a liquid-phase synthesis of two 2-aromaticBTs 64 (Scheme 47). The cleavage from PEG was achieved by treatment with sodium methoxide in methanol for 12 h. The compounds 64 were obtained in four steps, with poor isolated yields (22% and 37%) [159].
Scheme 47.
Condensation of o-ATP with isophthaloyl or terephthaloyl fluorides attached to a polyethylene glycol methyl ether PEG resin.
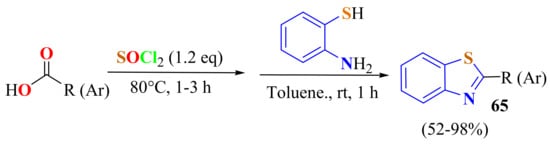
Kondaskar et al., in a one-pot reaction, generated acyl chlorides from carboxylic acids and condensed these with ATP under acid- and catalyst-free conditions to generate 2-alkyl and 2-arylBTs 65 (Scheme 48) [160].
Scheme 48.
In situ generation of aromatic acyl chlorides to be condensed with ATP.
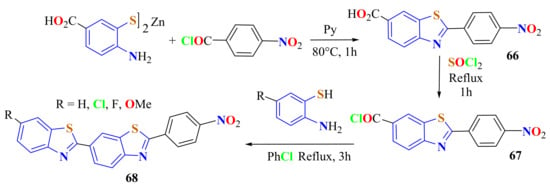
Wu et al. condensed the zinc salt of 4-amino-3-mercaptobenzoic acid with p-nitro benzoyl chloride by heating in the presence of pyridine at 80 °C for 1 h to give the intermediate 2-(4-nitro-phenyl)-BT-6-carboxylic acid 66 in an 83% yield (Scheme 49). This was treated with SOCl2 to be converted into the acyl chloride 67, followed by coupling with 5-substituted ATPs in refluxing chlorobenzene under heat treatment for 3 h to produce the corresponding 2-(4′-nitrophenyl)-(6-BTyl)BTs 68 in 73–79% yields [161].
Scheme 49.
Double condensation of substituted ATP derivatives with acid chlorides.
In 2010, Racané et al. reported the condensation of cyano- or nitro-substituted ATPate with 4-cyano- and 4-nitrobenzoylchloride under reflux in acetic acid (AcOH) for 4 h to prepare the corresponding bis-nitrile and nitro-nitrile 2-phenylBTs 69 in 71–84% yields (Scheme 50). Amidino- or imidazo-substituted 2-phenylBTs were prepared by the corresponding precursors in zwitterionic form with 4-nitrobenzoylchloride in a good yield (70%). 2-(p-Nitrophenyl)BTs were used as starting compounds for the preparation of 2(p-amidino-phenyl)BTs by the Pinner reaction. All compounds except diamidino-substituted 2-phenylBT showed exceptionally prominent tumor-cell-growth inhibitory activity [162].
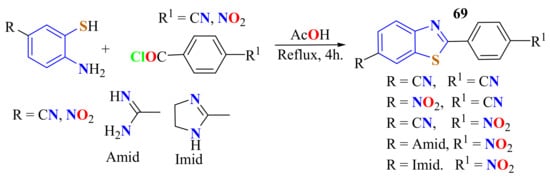
Scheme 50.
Condensation reaction to prepare bis-nitrile and nitro nitrile 2-phenylbenzothiazoles.
The condensation reaction of 2,5-furan and 2,5-thiophene di-acid chlorides with 5-amidino-substituted ATP was performed in refluxing glacial acetic acid for 4 h (Scheme 51). The bisamidino diBTyl compounds 70a,b and 71a,b were isolated as hydrochloride salts in good yields, of about 72–88% [117].
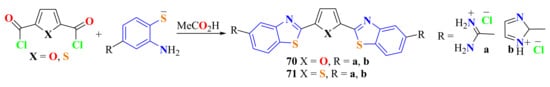
Scheme 51.
Condensation of di-acid chlorides with ATP to produce di-benzothiazolyl compounds.
In simple reaction conditions, the condensation of ATP with various acid chlorides in the presence of o-benzenedisulfonimide as the catalyst has been carried out in 2–3.5 h to give the respective 2-substitutedBTs 72 in 42–87% yields (Scheme 52) [150].
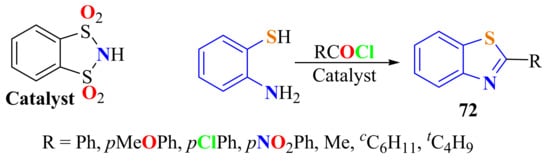
Scheme 52.
Condensation of ATP with aliphatic and aromatic acyl chlorides using o-benzenedisulfonimide as catalyst.
The intermediate BT alkylating agents 73 were prepared in 55–76% yields from the condensation reaction of ATP with chloroacyl chlorides while stirring in toluene for 15 min at room temperature (Scheme 53) [163].

Scheme 53.
Condensation of ATP with chloroacyl chlorides in stirring toluene.
Pyridine-2,6-dicarbonyl dichloride was condensed with the switter ionic amidino-substituted ATPs in refluxing acetic acid for 3 h to give the dihydrochloride salts 74a and 74b in 78% and 75% yields, respectively (Scheme 54) [120].
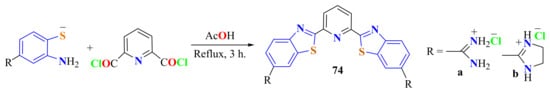
Scheme 54.
Condenastion of substituted ATP thiolates with pyridine-2,6-dicarbonyl chloride.
The same authors condensed isophthaloyl and terephthaloyl dichlorides with the corresponding 5-amidinium-ATPate and 5-(imidazolinium-2-yl)-o-ATPate switter ions in refluxing acetic acid for 3 h to give the corresponding m- and p-phenylene-bisBTs 75a–e, which were isolated as dihydrochloride salts in a 90% yield (Figure 9) [164].
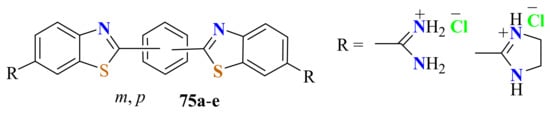
Figure 9.
Condensation of isophthaloyl and terephthaloyl dichlorides with 4-substitutes ATP thiolate to afford Phenylene-bisBTs.
Kumar et al. condensed ATP with benzoyl chloride and o-chlorobenzoyl chloride using an efficient and green catalyst NaHSO4-SiO2 to promote the solvent-free synthesis of 2-phenylBT 76a in 89% and 2-(o-chlorophenyl)BT 76b in 86% yields. The best results were obtained on refluxing at 100 °C for 12 h without a solvent. The catalyst could be easily recovered [165].
The synthesis of S-alkyl/arylBT-2-carbothioates 77 through a three-component reaction was reported from the condensation of substituted ATPs with thiols/oxalyl chloride system using 10 mol % of tetrabutylammonium iodide (TBAI) as the catalyst in acetonitrile as a solvent (Scheme 55). BTs and thioesters were formed via simultaneous C–N and C–S bond formation in 56–80% yields with several substrates. The synthesized derivatives were tested for their antimicrobial activity against the protozoan parasite Leishmania donovani, a causative agent of visceral leishmaniasis (VL) [166].
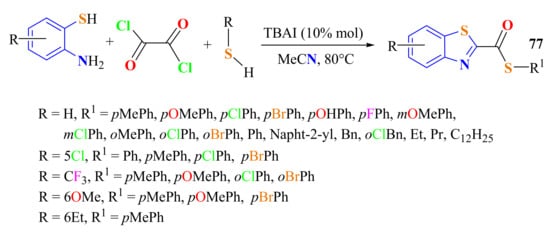
Scheme 55.
A three-component condensation reaction to produce S-alkyl/arylBT-2-carbothioates.
To understand the reaction mechanism, treatment of ATP with oxalyl chloride was carried out in the same conditions; however, the corresponding bis-BT 78 was obtained in only a 25% yield (Scheme 56).
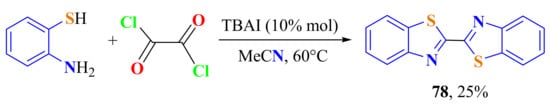
Scheme 56.
Condensation of ATP with oxalyl chloride in low yield.
The formation of the S-alkyl BT-2-carbothioates 77 can be proposed, as TBAI reacts with thiol to generate thiolate anion a, which attacks a side of oxalyl chloride to form a thioester c as an intermediate (Scheme 57). Finally, ATPate anion a, obtained from TBAI and o-ATP, undergoes condensation with the in situ formed thioester c to give the products (Scheme 57).
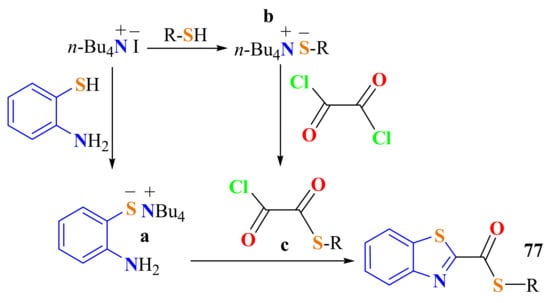
Scheme 57.
Explanation for the formation of the S-alkyl benzothiazol-2-carbothioates.
A useful protocol for the preparation of substituted 2-aminoBTs 78 was presented. Substituted ATPs were reacted with thiocarbamoyl chlorides under the catalysis of copper in a tandem manner to produce compounds 78 with 70–91% yields (Scheme 58). The broad substrate scope, a short reaction time, mild reaction conditions, easy performance, and excellent yields make this protocol attractive for the preparation of some biologically active compounds [167].
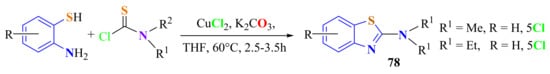
Scheme 58.
Condensation of substituted ATPs with carbamoyl chlorides under copper as cathalyst.
Various carboxylic acid chlorides were reacted with ATP in the presence of KF/Al2O3 (25% mol) as a heterogeneous base catalyst in refluxing dry acetonitrile at room temperature to form the corresponding 2-substitutedBTs 79 in 87–97% yields, with good recyclability of the catalyst (Scheme 59). However, the condensation of o-ATP with the mixture of acetic and benzoic anhydrides as carboxylic acid derivatives produced the BTs in 90% and 91% yields [168].
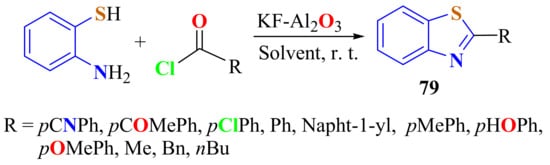
Scheme 59.
Condensation of ATP with carboxylic acid chlorides in the presence of KF/Al2O3.
2.5. Condensation with Nitriles
A proposed mechanism for this reaction is via the formation of an amidine a as an intermediate and subsequent cyclization followed by a deamination to afford the 2-substitutedBT (Scheme 60). This reaction can be catalyzed to accelerate the reaction.
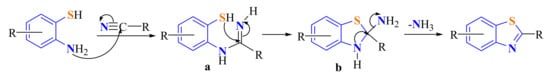
Scheme 60.
Proposed mechanism in the condensation of ATP with nitriles.
The 2-(4-amino-3-chloro)BT 80a and 2-(4-amino-3-chloro-5-methyl)BT 80b were prepared from the condensation of ATP with 4-amino-3-chlorobenzonitrile and 4-amino-3-chloro-5-methylbenzonitrile, respectively, on heating in PPA at 200 °C (Scheme 61) [106].
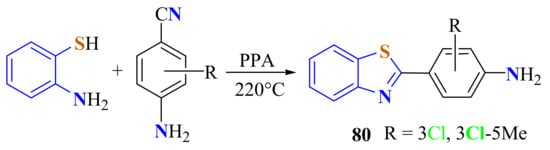
Scheme 61.
Condensation of ATP with 4-amino-3-chlorobenzonitriles.
Mokhir et al. condensed ATP and malonodinitrile in the presence of glacial acetic acid and ethanol as a solvent. After stirring for 1 h, 2-cyanomethylBT 81 precipitated as yellow crystals (Scheme 62) [169].
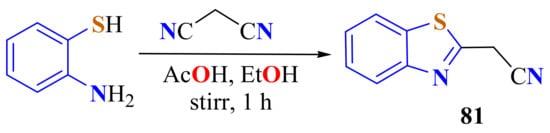
Scheme 62.
Condensation of ATP and malonodinitrile in the presence of glacial acetic acid.
Zandt et al. reported the synthesis of 4-fluoro-2-hydroxy-N(4,5,7,-trifluoroBT-2-yl-methyl)-benzamide 82 using N-cyanomethyl-4-fluoro-2-hydroxy-benzamide and o-amino-4,5,7-trifluorothiophenol hydrochloride in refluxing ethanol for 24 h (Scheme 63) [170].
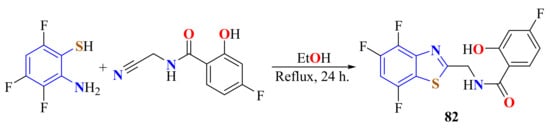
Scheme 63.
Condensation of a trifluorated ATP with N-cyanomethyl-4-fluoro-2-hydroxy-benzamide.
Manfroni et al. generated in situ substituted ATPs to be immediately condensed with ethyl cyanoacetate on heating at 120 °C under stirring and N2 flux. After 15 h, the 5-substituted ethyl (BT-2-yl) acetates 83 were isolated, 83a and 83b in 22% and 14% yields, respectively, 83c and 83d in 93% and 74% yields, respectively (Scheme 64) [171].
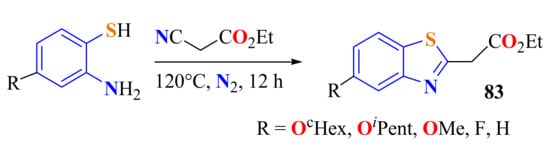
Scheme 64.
Condensation of substituted ATPs with ethyl cyanoacetate.
Sun et al. used 10 mol % of copper acetate Cu(OAc)2 as a catalyst in the presence of Et3N in the condensation of substituted ATPs with several nitriles on heating in ethanol at 70 °C for 6 h to afford 2-substitutedBTs 84 in 75–97% yields (Scheme 65). According to the proposed reaction mechanism, the formation of a sulfilimine b and intramolecular cyclization occur to afford compounds 84 [172].
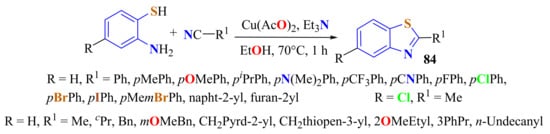
Scheme 65.
Copper cathalysed condensation reaction of substituted ATPs with aliphatic and aromatic nitriles.
A convenient and efficient method for the synthesis of 2-substitutedBTs 85 in 50–95% yields was carried out from the condensation of substituted ATPs with substituted benzonitriles by the use of trifluoromethanesulfonic acid on heating at 100 °C for 12 h (Figure 10). The Bronsted-acid-catalyzed cyclization reaction was performed under metal- and solvent-free conditions [173].
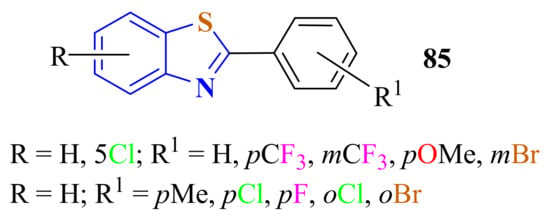
Figure 10.
2-(substitutedphenyl)benzothiazoles.
2.6. Condensation with Amides
In this case, deamination occurs to form the corresponding amide. Then, cyclization and dehydration produce 2-substitutedBTs. In some cases, this reaction requires to be catalyzed.
3-(BT-2-yl)coumarins 86 were prepared in 61–70% yields from the condensation of ATP with the corresponding 2-iminocoumarin-3-carboxamide in reflux with the minimum amount of n-butanol until the evolution of ammonia stops (1–1.5 h) (Scheme 66) [174].
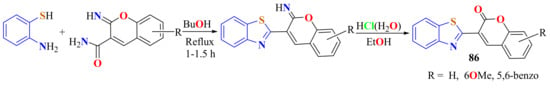
Scheme 66.
3-(benzothiazol-2-yl)coumarins from condensation of ATP with 2-iminocoumarin-3-carboxamides.
The synthesis of several substituted BTs 87 in 50–94% yields was carried out from the condensation of substituted ATPs with 2-acylpyridazin-3(2H)-ones as acyl transfer agents under transition-metal-free and eco-friendly conditions. The reaction was efficient, green, and economical, with several applications in organic synthesis and in medicinal and industrial chemistry (Scheme 67) [175].
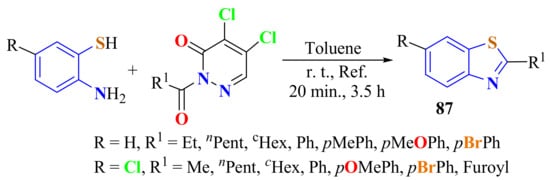
Scheme 67.
Condensation of substituted ATPs with 2-acylpyridazin-3(2H)-ones as acyl transfer agents under refluxing toluene.
An efficient and convenient one-pot procedure was developed for the synthesis of a series of substituted BTs 88 in up to 95% yields. This protocol uses various substituted ATPs and N-substituted formamides in zinc-catalyzed cyclization in the presence of poly(methylhydrosiloxane) PMHS (Scheme 68) [176].
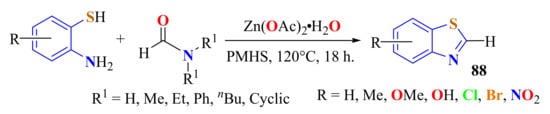
Scheme 68.
Condensation of substituted ATPs with formamides.
In an efficient and mild protocol, several substituted ATPs were condensed with thioamides to produce 2-substitutedBTs 89 in 62–93% yields using CBr4 as the catalyst under solvent- and metal-free conditions (Scheme 69). This condensation process involves the activation of a thioamide through halogen bond formation between the sulfur atom of the thioamide and the bromine atom of the CBr4 molecule. The presence of halogen-bonding interaction between N-methylthioamides and tetrabromomethane was demonstrated. Substituted 2-alkyl- and 2-arylBTs could be obtained from this methodology [177].
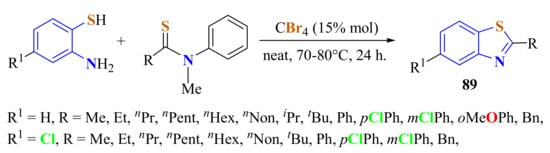
Scheme 69.
Condensation of substituted ATPs with thioamides.
A simple, economical, and metal-free approach for the synthesis of 2-substitutedBTs 90 was reported in 60–87% yields from the condensation of substituted ATP and DMF derivatives, using imidazolium chloride (50% mmol) as the only promoter, without any other additive (Scheme 70) [178]. The proposed mechanistic pathway is as described in Scheme 71.
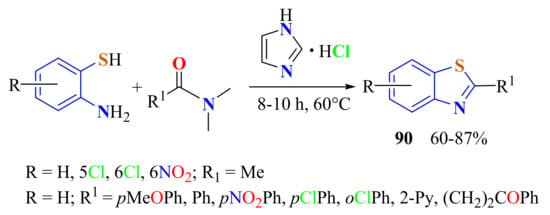
Scheme 70.
Cathalized condensation of substituted ATPs with N,N-dimethylamides.
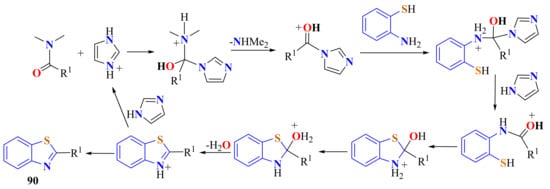
Scheme 71.
Proposed mechanism in the condensation of ATP with N,N-dimethylamides.
2.7. Condensation under Microwave Irradiation (MWI)
The Montmorillonite KSF Clay minerals act as strong Bronsted acids and have been used as solid, non-corrosive catalysts in the condensation of two orthoesters with ATP under toluene reflux or a solvent-free condition under MWI to produce BT and 2-methylBT 46 in 69% and 74% yields, respectively (Scheme 72) [179].

Scheme 72.
Minerals as strong Brönsted acids used in two ways for the condensation of ATP with ortho esters.
Reddy et al. synthesized 2-(1,1,1-trifluoroacetonyl)BT 91 in a 93% yield by condensation of ATP with trifluoroacetyl ketene diethyl acetal under MWI in toluene for 8 min (Scheme 73) [180].

Scheme 73.
Condensation of ATP with trifluoroacetyl ketene diethyl acetal in toluene under MWI.
Chakraborti et al., in a direct condensation of ATP with aromatic or aliphatic carboxylic acids under MWI in the absence of a solvent, produced the corresponding 2-substituedBTs 92 in 45–97% yields (Scheme 74) [181].

Scheme 74.
Condensation of ATP with aromatic or aliphatic carboxylic acids under direct MWI.
To improve yields, a MWI-assisted procedure to condense ATP with 2-chloroacetyl chloride in acetic acid to produce 2-chloromethylBT 17 was carried out for a 90% yield in 10 min [182].
Several aliphatic and aromatic carboxylic acids were condensed with ATP in the presence of 0.35 equivalents of Lawesson’s reagent as promoters under solvent-free MWI (300 W, 190 °C) for 0.5–4 min to afford 2-substitutedBTs 93 with good yields (Scheme 75). In this methodology, Lawesson’s reagent in situ converts carboxylic acids to thiocarboxylic acids, which are cyclocondensed with desulfhydration. The same protocol was used directly with thiobenzoic acid to afford 2-phenylBT in quantitative yields in 1 min [183].
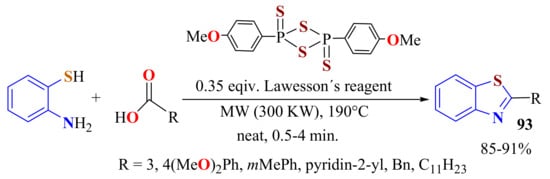
Scheme 75.
Condensation of o-ATP with aliphatic and aromatic carboxylic acids in presence of Lawesson’s reagent under solvent-free MWI.
Gupta et al. condensed ATP with various benzoic acid derivatives employing a catalytic quantity of molecular iodine (I2) as the dehydrating agent in a one-pot, solid-phase, solvent free, and MWI-assisted reaction to obtain 2-arylBTs 94 in 10 min with 60–70% yields (Scheme 76) [184]. Less time consumption and lower cost compared with the use of PPA and 1-pentyl-3-methyl imidizolium bromide [pmim]Br catalyst are the benefits of this procedure.
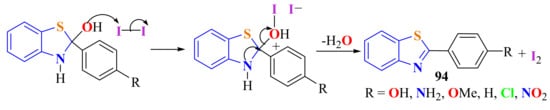
Scheme 76.
Condensation of ATP with benzoic acid derivatives employing molecular iodine aas cathalyst.
MWI-assisted condensation of four resin-bound esters with three substituted ATPs (4 equiv.) in 15% of methane sulfonic acid/1,2-dichlorobenzene system was carried out to give twelve 2-substitutedBTs 95 in 68–93% yields (Scheme 77) [185].
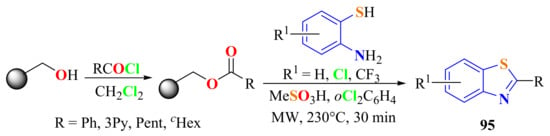
Scheme 77.
Condensation of substituted o-ATPs with resin-bound esters in methane sulfonic acid/1,2-dichlorobenzene.
Rauf et al., in an efficient and solvent-free one-pot method, condensed ATPs with various fatty acids with or without P4S10 as the catalyst under MWI (3–4 min) to afford 2-substitutedBTs 96 (Scheme 78) [147]. When P4S10 was used as the catalyst, the power and the reaction time were lowered from 100% to 60% and from 28–30 to 3–4 min, respectively, with an increase in the yields from 60–65% to 80–85% [186].
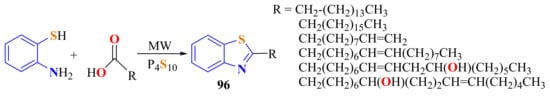
Scheme 78.
Condensation of o-ATPs with fatty acids in a solvent-free one-pot MWI.
Khoobi et al. prepared 3-(2-BTyl)coumarin derivatives 97 in a 88% yield via the reaction of 3-cyanocoumarin with ATP in the presence of a catalytic amount of acetic acid (AcOH) under reflux overnight (Scheme 79) [187]. The same reaction with a series of substituted o-aminothiophenoles and substituted 3-cyanocumarines was carried out by the use of acetic acid or catalytic amounts of H6[PMo9V3O40] (HPMo) in a MWI-assisted protocol in 15 min to synthesize compounds 97 with 62–88% yields [188].

Scheme 79.
MWI-assisted condensation of substituted ATPs with 3-cyanocoumarines.
In a one-pot procedure, propylphosphonic anhydride (®T3P) promoted the cyclo-condensation of ATP with aromatic and aliphatic carboxylic acids for the quick preparation (10 min) of 2-substitutedBTs 98 in 78–97% yields, under MWI, (Scheme 80) [189].

Scheme 80.
Cathalysed condensation of ATP with aromatic and aliphatic carboxylic acids under MWI.
The same procedure was used to prepare 2-(para-pyridyl)BT (p-PBT) and 2-(para-pyridyl)BT N-oxide (2-(N-O p-PBT). The synthesized PBTs were used as ligands to react with metal ions such as Co(II), UO2(II), Sm(III), Eu(III), and Zn(II)) to afford a series of metallic complexes [190]. The photoluminescence properties of Samarium and Europium complexes were studied.
Panda et al. discovered that an excess of ATP (10 equiv.) and benzoyl benzotriazolide (1 equiv.) was coupled under microwave heating at 70 °C for 1 h in 91–95% yields without any catalyst or solvent used to produce 2-substituteBTs 99 (Scheme 81). The liquid o-aminothiophenol acts as both a solvent and a reagent. The same reaction under conventional heating was less efficient [191].
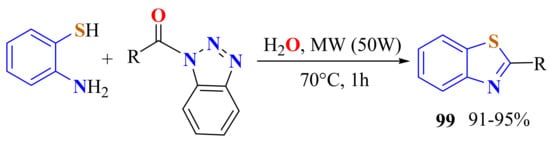
Scheme 81.
Condensation of ATP with benzoyl benzotriazolide in H2O under MWI.
The same “suspension-in-water” protocol was used in the coupling of ATP with peptidylbenzotriazolides to afford 2-peptidylBTs 100a–o in 70–89% yields (Scheme 82).
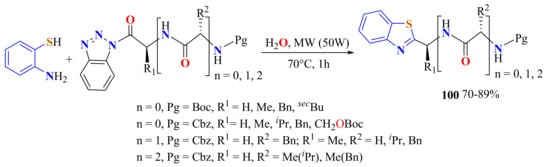
Scheme 82.
Condensation of ATP with peptidylbenzotriazolides in H2O under MWI.
Condensation of ATP with (un)substituted p-aminobenzoic acids under the action of melamine formaldehyde resin (MFR)-supported sulfuric acid under microwave irradiation (900 W) and solvent-free conditions was performed (Scheme 83). The simple method afforded 2-(4-aminophenyl)BTs 101 in 81–88% yields in 6–8 min. The catalyst could be recycled three times, with the yield lowering from 87% to 80% [192].
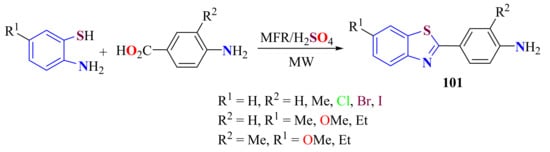
Scheme 83.
Condensation of substituted ATPs with substituted-p-aminobenzoic acids under MFR-supported sulfuric acid system.
A metal-free and catalytic method for the synthesis of 2-substitutedBTs 102 and 103 in 815–92% yields was carried out involving the condensation of ATP with N-protected amino acids and carboxylic acids under MW irradiation (Scheme 84). The reactions proceeded through a two-step mechanism. The coupling step was carried out with ethyl 2-cyano-2-(2-nitrobenzenesulfonyloxyimino)acetate (I) (ortho-No-sylOXY), and the cyclization step took place with the use of para-toluenesulfonic acid as the catalyst [193].
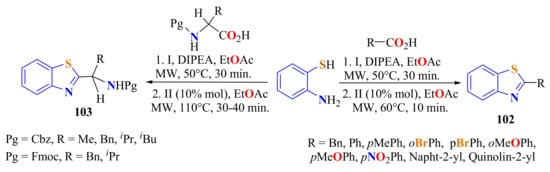
Scheme 84.
Two ways to condense ATP with protected aminoacids or carboxylic acids under MWI.
Luo et al. found that 2-chloromethylBT 17 could be obtained in an 87% yield from the condensation of ATP with chloroacetyl chloride in acetic acid under MW irradiation for 10 min. The MW-assisted procedures were efficient and environmentally friendly, and used less time, compared with traditional methods [194].
A method for the synthesis of a series of 2-substitutedBTs 104 in 82–92% yields was developed involving the condensation of ATP with a series of carboxylic acids using Amberlyst-15 as a recyclable catalyst under ultrasound irradiation in water (Scheme 85). This methodology does not use hazardous organic solvents and is carried out in an inert or anhydrous atmosphere [195].
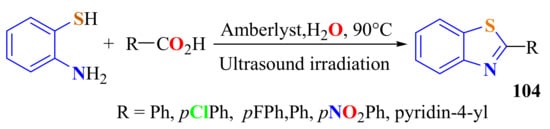
Scheme 85.
Amberlyst-15 as catalyst in the condensation of ATP with carboxylic acids under MWI.
2.8. Condensation of o-Aminothiophenol Disulfides (ATPDs)
In this condensation reaction, a reducing agent was required before or after the amide formation to afford the corresponding amidethiophenoles, which were cyclized to the 2-substitutedBTs.
Interaction of the bis(2-amino-4-fluorophenyl)disulfide with either 3-methyl-4-nitrobenzoyl chloride or 4-nitrobenzoyl chloride gave the corresponding amides, which were cyclized and reduced with tin(II) chloride dihydrate and HCl in ethanol, under reflux for 2 h, to afford the pure 5-fluoro-2-(4-aminophenyl)BTs 105 (Scheme 86). 2-(4-Amino-3-methylphenyl)-5-fluoroBT was resistant to metabolic C-hydroxylation and because of its potency and broad spectrum in vitro, this compound was proposed as a clinical candidate [25].
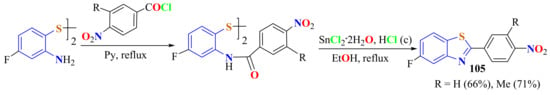
Scheme 86.
Condensation of 4-fluoro ATPD with substituted p-aminobenzoyl chloride in presence of SnCl2.
2-PhenylBTs 106 were cleanly and efficiently synthesized from reductive cyclization of the disulfide of ATP with a set of acyl chlorides, employing different reducing agents [196].
Shi et al., in short reaction times, synthesized 2-arylBTs 107 in 70–92% yields via the reductive cyclization of bis-(2-benzalaminophenyl)disulfide promoted by a titanium tetrachloride (TiCl4)/Samarium (Sm) system using tetrahydrofuran (THF) as a solvent (Scheme 87) [197].
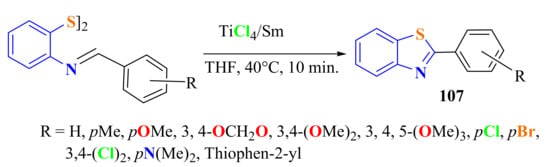
Scheme 87.
Reaction of bis-(2-benzalaminophenyl)disulfide in the presence of TiCl4/Sm system.
2-ArylBTs 108 were synthesized using a PCl3 and DBU as the organic base to promote the cleavage/acylation/cyclization tandem reaction of the disulfides of ATP and aromatic carboxylic acids. PCl3 acted as both acylating and disulfide cleaving reagents, which prompted the disulfides of ATP to react with the aromatic carboxylic acid (Scheme 88) [198].
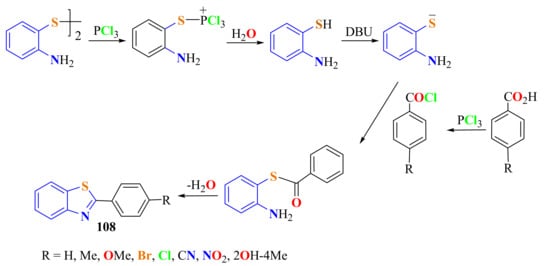
Scheme 88.
Mechanistic path way in the condensation of ATPD with p-substituted benzoyl chlorides in presence of PCl3/DBU.
Coelho et al. described a methodology to condensate ATP disulfide with 4-substituted benzoic acids in the presence of tributylphosphine as both the promoter of disulfide bond cleavage and the activating agent for coupling with carboxylic acids containing donor/withdrawing substituents for preparing 2-arylBTs 109 in 47–92% yields (Scheme 89) [199].
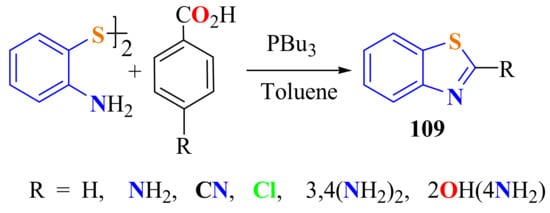
Scheme 89.
Condensation of ATPD with p-substituted benzoic acids in presence of PBu3.
This method was used to prepare the amyloid probe 2-(4-aminophenyl)-6-methoxyBT. A plausible mechanism was proposed as depicted in Scheme 90. A nucleophilic attack of tributylphosphine promotes the cleavage of the S–S bond of disulfide to produce a thiolate phosphonium salt a. Thiolate deprotonates the carboxylic acid, and the generated carboxylate reacts with the phosphonium salt to form a pentacoordinate acyloxyphosphonium intermediate b. Intramolecular nucleophilic attack of the amino group on the carbonyl produces amide c and tributylphosphoxide. Finally, a nucleophilic attack of the thiol group on the carbonyl of the amide c followed by dehydration to leads to the BT.
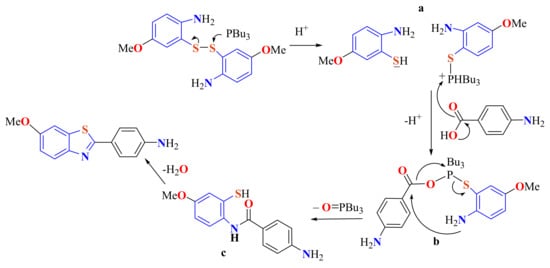
Scheme 90.
Mechanistic path way in the condensation of 5-methoxy ATPD with p-aminobenzoic acid in presence of PBu3.
2.9. Condensation by Cyclization of CO2 as the Raw Material
In this reaction, the CO2 must be activated to form a formiate ester or formamide as an intermediate to be condensed with ATPs to produce the corresponding BTs.
A series of substituted o-ATPs were cyclized with CO2 in the presence of diethylsilane and 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) as the catalyst at 5 MPa to synthesize substituted BTs 110 in 35–82% yields (Scheme 91) [200]. The proposed mechanism shows that hydrosilane played an important role in activating CO2 in the formation of compounds 110.
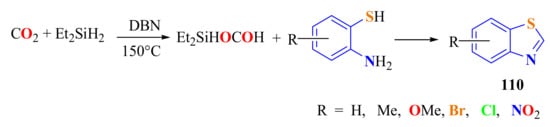
Scheme 91.
CO2 activated with Et2SiH in the condensation of substituted ATPs.
The same authors cyclized substituted ATPs with CO2 and hydrosilane to produce a series of substituted BTs 111a–j in up to 99% yields under metal-free and mild conditions using the acetate-based ionic liquid ([Bmim][OAc]) as a catalyst at 60 °C and 0.5 Mpa (Scheme 92) [201]. The IL was reused five times without change. A possible mechanism was proposed as shown in Scheme 88. CO2 was activated by the IL to produce intermediate a. The Si–H bond of (EtO)3SiH b was activated by the IL to facilitate the insertion of a to form formoxysilane intermediate c; meanwhile, o-ATP was activated via the hydrogen bond by the anion [−OAc] of IL and the nucleophilic N atom attacked the carbon atom of intermediate c to form intermediate e, which suffered intramolecular nucleophilic cyclization to produce f. Finally, the dehydration of f yielded substituted BT products 111.
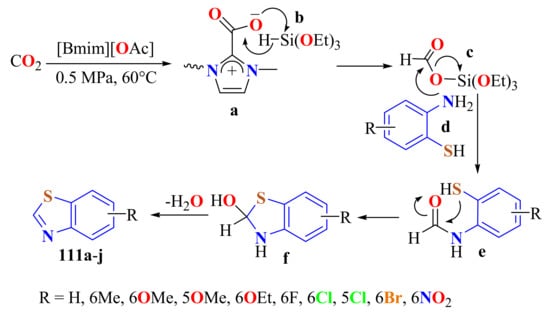
Scheme 92.
Mechanistic path way in the condensation of CO2 with substituted ATPs in presence of ionic liquid [Bmim][OAc].
The same series of BTs 111a–j were obtained in 42–99% yields by an effective cyclization of substituted ATPs with DMF in the presence of B(C6F5)3 combined with atmospheric CO2 (Scheme 93). The outgoing intermediate dimethylamine in b further reacted with CO2 and silane catalyzed by B(C6F5)3, forming trimethylamine, which drove the cyclization reaction to the right, promoting the reaction [202].
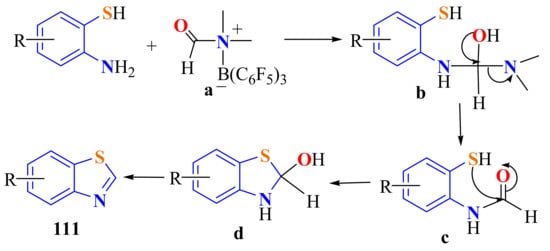
Scheme 93.
Mechanistic path way in the condensation of substituted ATPs with CO2/DMF/B(C6F5)3 activation system.
A scalable CO2-mediated synthesis of BT 111a in 86% yields was developed. ATP was cyclized with DMF as the carbon source in water as the solvent in the presence of CO2. The CO2 used could be recycled (Scheme 94) [203].
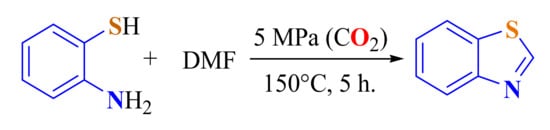
Scheme 94.
Condensation of ATP with activated CO2 under high pressure.
Chun et al. reported the synthesis of an extended series of substituted BTs 112a–n in 33–84% yields from substituted ATPs and CO2 using poly(3,4-dimethyl-5-vinylthiazolium) iodide as a precatalyst, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as a base, and phenylsilane as the reductant to in situ generate N-heterocyclic carbenes a (NHCs) by deprotonation (Scheme 95), which capture CO2 to be transferred to phenylsilane as the formiate group in intermediate c. Then o-ATP forms the formyltioester d. The reaction was successfully carried out under mild conditions (1 atm of CO2 and 60–70 °C), with a broad substrate scope and functional group tolerance. The precatalyst salt was recovered and reused several times without any loss of activity [204].
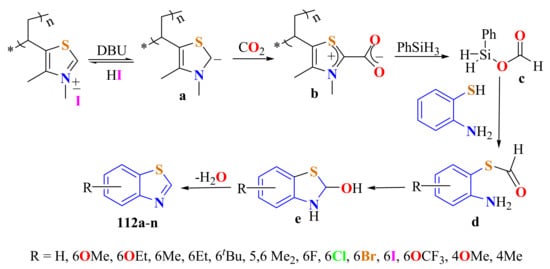
Scheme 95.
Mechanistic path way in the condensation of substituted ATPs and activated CO2 using poly(3,4-dimethyl-5-vinylthiazolium) iodide as a precatalyst.
3. Conclusions
Investigation into the synthesis of BT derivatives has increased in recent years. For example, in the past 5 years, we found 19 article reviews related to the strategies of synthesis and biological activities of BTs, of which 8 were reported in 2020. It shows the importance of the BT core in the pharmaceutical chemistry field. These works included only a few methods on the condensation of ATPs with carboxylic acids and their derivatives. Accordingly, in our article review, we have reported an investigation of about 20 years on the aspects of the research progress related to the condensation of ATPs with carboxylic acids and their derivatives, such as acid chlorides, amides, esters, orthoesters, nitriles, and thioesters, including carbon dioxide (CO2), as starting materials.
In this review, we found that the condensation of ATPs with carboxylic acids in the presence of PPA as the catalyst is still popular, although high temperatures, from 110 to 220 °C, and reaction times from 0.5 to 18 h are required. The best yields, of 80% to 90%, were obtained on heating at 140 °C for 24 h. These condensations have been carried out using other catalyst, such as P2O5/MeSO3H, PPA/H3PO4, trimethylsilylpolyphosphate ester (PPSE), MeSO3H/SiO2, triphenylphosphine (Ph3P), triphenyl phosphite (TPP), Samarium(III) triflate, trifluoroacetic acid (TFA), and iodo (I2). In these cases, the yields were from 60% to 99% but less reaction times were required. Few examples were found in which 80% to 90% yields resulted on heating in ionic liquids (10 min), on direct heating (16 h), as well as on refluxing in high-boiling-point solvents (8 h).
The condensation of ATPs with esters on heating under acid catalysts such as PPA, K2CO3/NMP, and AlMe3; under stirring; under direct heating; or in refluxing solvents depending on the ester has been carried out leading to low (14%) to high (92%) yields.
Condensation with orthoesters uses catalysts such as H2SO4, Bi(III) salts, supported fluoroboric acid HBF4-SiO2, BF3.OEt2, o-benzenedisulfonamide, and NH4Cl, with good yields, from 75% to 97%.
Acid chlorides and a nitrile-substituted ATP were condensed in refluxing chlorobenzene for 70 h under a stream of nitrogen to give the product in a 35% yield. The same condensation with unsubstituted ATP in refluxing chlorobenzene for 3 h gives the condensation products in 73–79% yields. On stirring in toluene for 15 min or 1 h at room temperature, the condensation yields were 50–98%. On refluxing in acetic acid, the yields increased to 70–88%. However, using n-methyl-pyrrolidone as a solvent on heating at 100 °C for 1 h gave the best results (82–95%). Ionic liquids such as 1-butylimidazolium tetra-fluoroborate ([Hbim]BF4) and 1,3-di-n-butylimidazolium tetra-fluoroborate ([bbim]BF4) as reaction media were used with excellent isolated yields. Pyridine as a base to trap the liberated HCl in the presence of o-benzene-di-sulfonamide as a catalyst has been used to produce BTs in 42–87% yields. On heating for 12 h at 100 °C with NaHSO4-SiO2 as a catalyst under solvent-free conditions, the condensation yields were 86–89%. The CuCl2/K2CO3 and KF/Al2O3 systems were excellent heterogeneous catalysts on refluxing in an adequate solvent to give 70% to 97% yields in this condensation. Acid fluorides attached to PEG resins were used, but poor isolated yields were obtained in the condensation (22–37%).
Condensation with nitriles produced moderate yields on direct heating with solvents or with the use of catalysts such as PPA. On using a heterogeneous catalyst such as Cu(OAc)2 on refluxing in ethanol or trifluoromethanesulfonic acid, BTs in best yields (50–97%) were produced.
Amides were condensed with ATPs on refluxing in solvents such as butanol or toluene by short reaction times (20 min–3 h) to obtain 50% to 94% yields. The use of imidazolium chloride or tetrabromo-methane as catalysts produced BTs in 60–90% yields, but 8–24 h were required. Condensation with substituted formamides in the presence of Zn(OAc)2∙H2O as a catalyst gives BTs in up to 95% yield.
Condensation reactions using microwave irradiation is the green method of choice because this method reduces the reaction time (less than 1 h), with or without the use of solvents, with good to excellent yields (50–97%).
Only one example on the use of ultrasound irradiation in the condensation of aromatic and aliphatic carboxylic acids was found with very good yields (82–92%).
Author Contributions
Writing—original draft preparation, A.C.; writing—review and editing, I.I.P.-M.; bibliographic research, E.V.G.-B. and F.T.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.C. thanks the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-IPN) for financial support, Grant no. 20210756.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Gunawardana, G.P.; Kohmoto, S.; Gunasekera, S.P.; McConnell, O.J.; Koehn, F.E. Dercitine, a new biologically active acridine alkaloid from a deep water marine sponge, Dercitus sp. J. Am. Chem. Soc. 1988, 110, 4856–4858. [Google Scholar] [CrossRef]
- Gunawardana, G.P.; Koehn, F.E.; Lee, A.Y.; Clardy, J.; He, H.Y.; Faulkner, D.J. Pyridoacridine alkaloids from deep-water marine sponges of the family Pachastrellidae: Structure revision of dercitin and related compounds and correlation with the kuanoniamines. J. Org. Chem. 1992, 57, 1523–1526. [Google Scholar] [CrossRef]
- Carroll, A.R.; Scheuer, P.J. Kuanoniamines A, B, C, and D: Pentacyclic alkaloids from a tunicate and its prosobranch mollusk predator Chelynotus semperi. J. Org. Chem. 1990, 55, 4426–4431. [Google Scholar] [CrossRef]
- Baba, H.; Yaoita, Y.; Kikuchi, M. Sesquiterpenoids and Lactone Derivatives from Ligularia dentata. Helv. Chim. Acta 2007, 90, 1028–1037. [Google Scholar] [CrossRef]
- Hu, Y.; MacMillan, J.B. Erythrazoles A–B, Cytotoxic Benzothiazoles from a Marine-Derived Erythrobacter sp. Org. Lett. 2011, 13, 6580–6583. [Google Scholar] [CrossRef]
- Bozec, L.L.; Moody, C. Naturally Occurring Nitrogen–Sulfur Compounds. The Benzothiazole Alkaloids. J. Aust. J. Chem. 2009, 62, 639–647. [Google Scholar] [CrossRef]
- McElroy, W.D. The Energy Source for Bioluminescence in an Isolated System. Proc. Natl. Acad. Sci. USA 1947, 33, 342–345. [Google Scholar] [CrossRef]
- White, E.H.; McCapra, F.; Field, G.F. The Structure and Synthesis of Firefly Luciferin. J. Am. Chem. Soc. 1963, 85, 337–343. [Google Scholar] [CrossRef]
- Cricchio, R.; Antonini, P.; Sartori, G. Thiazorifamycins III. Biosynthesis of Rifamycins P, Q and Verde, Novel Metabolites from a mutant of Nocardia Mediterranea. J. Antibiot. 1980, 33, 842–846. [Google Scholar] [CrossRef]
- Hosokawa, N.; Naganawa, H.; Hamada, M.; Iinuma, H.; Takeuchi, T.; Tsuchiya, K.S.; Hori, M. New Triene-ansamycins, Thiazinotrienomycins F and G and a Diene-ansamycin, Benzoxazomycin. J. Antibiot. 2000, 53, 886–894. [Google Scholar] [CrossRef][Green Version]
- Gunawardana, G.P.; Kohmoto, S.; Burres, N.S. New cytotoxic acridine alkaloids from two deep water marine sponges of the family Pachastrellidae. Tetrahedron Lett. 1989, 30, 4359–4362. [Google Scholar] [CrossRef]
- Harriet, M.B.; Bret, F.; Paul, B. Riluzole. A review of its pharmacodynamics and Pharmacokinetic Properties and Therapeutic Potential in Amyotrophic Lateral Sclerosis. Drugs 1996, 52, 549–563. [Google Scholar]
- Pittenger, C.; Coric, V.; Banasr, M.; Bloch, M.; Krystal, J.H.; Sanacora, G. Rluzole in the Treatment of Mood and Anxiety Disorders. CNS Drugs 2008, 22, 761–786. [Google Scholar] [CrossRef]
- Aldinger, C.E.; Dee, M.F.; Siegel, T.W.; Singleton, D.H. Novel, potent aldose reductase inhibitors: 3,4-Dihydro-4-oxo-3-[[5-(trifluoromethyl)-2-benzothiazolyl]methyl]-1-phthalazineacetic acid (zopolrestat) and congeners. J. Med. Chem. 1991, 34, 108–122. [Google Scholar]
- Mortimer, C.G.; Wells, G.; Crochard, J.P.; Stone, E.L.; Bradshaw, T.D.; Stevens, M.F.G.; Westwell, A.D. Antitumor Benzothiazoles. 26. 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a Simple Fluorinated 2-Arylbenzothiazole, Shows Potent and Selective Inhibitory Activity against Lung, Colon, and Breast Cancer Cell Lines. J. Med. Chem. 2006, 49, 179–185. [Google Scholar] [CrossRef]
- Aiello, S.; Wells, G.; Stone, E.L.; Kadri, H.; Bazzi, R.; Bell, D.R.; Stevens, M.F.G.; Matthews, C.S.; Bradshaw, T.D.; Westwell, A.D. Synthesis and Biological Properties of Benzothiazole, Benzoxazole, and Chromen-4-one Analogues of the Potent Antitumor Agent 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648). J. Med. Chem. 2008, 51, 5135–5139. [Google Scholar] [CrossRef]
- Geng, J.; Li, M.; Wu, L.; Ren, J.; Qu, X. Liberation of Copper from Amyloid Plaques: Making a Risk Factor Useful for Alzheimer’s Disease Treatment. J. Med. Chem. 2012, 55, 9146–9155. [Google Scholar] [CrossRef]
- Yao, S.; Schafer-Hales, K.J.; Belfield, K.D. A New Water-Soluble Near-Neutral Ratiometric Fluorescent pH Indicator. Org. Lett. 2007, 9, 5645–5648. [Google Scholar] [CrossRef]
- Rodionov, V.O.; Presolski, S.I.; Gardinier, S.; Lim, Y.H.; Finn, M.G. Benzimidazole and Related Ligands for Cu-Catalyzed Azide−Alkyne Cycloaddition. J. Am. Chem. Soc. 2007, 129, 12696–12704. [Google Scholar] [CrossRef]
- Palmer, P.; Trigg, R.; Warrington, J. Antimicrobials. 3. Benzothiazolyl-phenylalkylamines. J. Med. Chem. 1971, 14, 1228–1230. [Google Scholar] [CrossRef]
- Gupta, S.; Ajmera, N.; Gautam, N.; Sharma, R.; Gautam, D. Novel synthesis and biological activity study of pyrimido[2,1-b]benzothiazoles. Indian, J. Chem. B 2009, 48, 853–857. [Google Scholar]
- Murthi, Y.; Pathak, D. Synthesis and Antimicrobial screening of Substituted 2-Mercaptobenzothiazoles. J. Pharm. Res. 2008, 7, 153–155. [Google Scholar]
- Mahran, M.A.; William, S.; Ramzy, F.; Sembel, A.M. Synthesis and in vitro Evaluation of New Benzothiazole Derivatives as Schistosomicidal Agents. Molecules 2007, 12, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Kok, S.H.L.; Gambari, R.; Chui, C.H.; Yuen, M.C.W.; Lin, E.; Wong, R.S.M.; Lau, F.Y.; Cheng, G.Y.M.; Lam, W.S.; Chan, S.H. Synthesis and anti-cancer activity of benzothiazole containing phthalimide on human carcinoma cell lines. Bioorg. Med. Chem. 2008, 16, 3626–3631. [Google Scholar] [CrossRef]
- Hutchinson, I.; Chua, M.-S.; Browne, H.L.; Trapani, V.; Bradshaw, T.D.; Westwell, A.D.; Stevens, M.F. Antitumor Benzothiazoles. 14.1 Synthesis and in Vitro Biological Properties of Fluorinated 2-(4-Aminophenyl)benzothiazoles. J. Med. Chem. 2001, 44, 1446–1455. [Google Scholar] [CrossRef]
- Ćaleta, I.; Grdiša, M.; Mrvoš-Sermek, D.; Cetina, M.; Tralić-Kulenović, V.; Pavelić, K.; Karminski-Zamola, G. Synthesis, crystal structure and antiproliferative evaluation of some new substituted benzothiazoles and styrylbenzothiazoles. Il Farm. 2004, 59, 297–305. [Google Scholar] [CrossRef]
- Wells, G.; Bradshaw, T.D.; Diana, P.; Seaton, A.; Shi, D.-F.; Westwell, A.D.; Stevens, M.F. Antitumour benzothiazoles. Part 10: The synthesis and antitumour activity of benzothiazole substituted quinol derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 513–515. [Google Scholar] [CrossRef]
- Heo, Y.; Song, Y.S.; Kim, B.T.; Heo, J.-N. A highly regioselective synthesis of 2-aryl-6-chlorobenzothiazoles employing microwave-promoted Suzuki–Miyaura coupling reaction. Tetrahedron Lett. 2006, 47, 3091–3094. [Google Scholar] [CrossRef]
- Huang, S.T.; Hsei, I.J.; Chen, C. Synthesis and anticancer evaluation of bis(benzimidazoles), bis(benzoxazoles), and benzothiazoles. Bioorg. Med. Chem. 2006, 14, 6106–6119. [Google Scholar] [CrossRef]
- Sreenivasa, G.; Jayachandran, E.; Shivakumar, B.; Jayaraj, K.; Vijay, M.K. Synthesis of bioactive molecule fluoro benzothiazole comprising potent heterocyclic moieties for anthelmintic activity. Arch. Pharm. Sci. Res. 2009, 1, 150–157. [Google Scholar]
- Su, X.; Vicker, N.; Ganeshapillai, D.; Smith, A.; Purohit, A.; Reed, M.J.; Potter, B.V. Benzothiazole derivatives as novel inhibitors of human 11β-hydroxysteroid dehydrogenase type 1. Mol. Cell. Endocrinol. 2006, 248, 214–217. [Google Scholar] [CrossRef]
- Moreno-Diaz, H.; Villalobos-Molina, R.; Ortiz-Andrade, R.; Diaz-Coutino, D.; Medina-Franco, J.L.; Webster, S.P.; Binnie, M.; Estrada-Soto, S.; Ibarra-Barajas, M.; Leon-Rivera, I. Antidiabetic activity of N-(6-substituted-1,3-benzothiazol-2-yl)benzenesulfonamides. Bioorg. Med. Chem. Lett. 2008, 18, 2871–2877. [Google Scholar] [CrossRef]
- Palmer, P.; Trigg, R.; Warrington, J. Benzothiazolines as antituberculous agents. J. Med. Chem. 1971, 14, 248–251. [Google Scholar] [CrossRef]
- Das, J.; Moquin, R.V.; Lin, J.; Liu, C.; Doweyko, A.M.; DeFex, H.F.; Fang, Q.; Pang, S.; Pitt, S.; Shen, D.R. Discovery of 2-amino-heteroaryl-benzothiazole-6-anilides as potent p56lck inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 2587–2590. [Google Scholar] [CrossRef]
- Bradshaw, T.; Westwell, A. The Development of the Antitumour Benzothiazole Prodrug, Phortress, as a Clinical Candidate. Curr. Med. Chem. 2004, 11, 1009–1021. [Google Scholar] [CrossRef]
- Yoshida, M.; Hayakawa, I.; Hayashi, N.; Agatsuma, T.; Oda, Y.; Tanzawa, F.; Iwasaki, S.; Koyama, K.; Furukawa, H.; Kurakata, S. Synthesis and biological evaluation of benzothiazole derivatives as potent antitumor agents. Bioorg. Med. Chem. Lett. 2005, 15, 3328–3332. [Google Scholar] [CrossRef]
- Lion, C.J.; Matthews, C.S.; Wells, G.; Bradshaw, T.D.; Stevens, M.F.; Westwell, A.D. Antitumour properties of fluorinated benzothiazole-substituted hydroxycyclohexa-2,5-dienones (‘quinols’). Bioorg. Med. Chem. Lett. 2006, 16, 5005–5008. [Google Scholar] [CrossRef]
- Beneteau, V.; Besson, T.; Guillard, J.; Leonce, S.; Pfeiffer, B. Synthesis and in vitro antitumour evaluationof benzothiazole-2-carbonitrile derivatives. Eur. J. Med. Chem. 1999, 34, 1053–1060. [Google Scholar] [CrossRef]
- Hutchinson, I.; Bradshaw, T.D.; Matthews, C.S.; Stevens, M.F.; Westwell, A.D. Antitumour benzothiazoles. Part 20:† 3′-Cyano and 3′-Alkynyl-Substituted 2-(4′-Aminophenyl)benzothiazoles as new potent and selective analogues. Bioorg. Med. Chem. Lett. 2003, 13, 471–474. [Google Scholar] [CrossRef]
- Hall, I.H.; Peaty, N.J.; Henry, J.R.; Easmon, J.; Heinisch, G.; Purstinger, G. Investigations on the Mechanism of Action of the Novel Antitumor Agents 2-Benzothiazolyl, 2-Benzoxazolyl, and 2-Benzimidazolyl Hydrazones Derived from 2-Acetylpyridine. Arch. Pharm. 1999, 332, 115–123. [Google Scholar] [CrossRef]
- Kashiyama, E.; Hutchinson, I.; Chua, M.-S.; Stinson, S.F.; Phillips, L.R.; Kaur, G.; Sausville, E.A.; Bradshaw, T.D.; Westwell, A.D.; Stevens, M.F. Antitumor benzothiazoles. 8. Synthesis, metabolic formation, and biological properties of the C- and N-oxidation products of antitumor 2-(4-aminophenyl)benzothiazoles. J. Med. Chem. 1999, 42, 4172–4184. [Google Scholar] [CrossRef]
- Neres, J.; Brewer, M.L.; Ratier, L.; Botti, H.; Buschiazzo, A.; Edwards, P.N.; Mortenson, P.N.; Charlton, M.H.; Alzari, P.M.; Frasch, A.C.; et al. Discovery of novel inhibitors of Trypanosoma cruzi trans-sialidase from in silico screening. Bioorg. Med. Chem. Lett. 2009, 19, 589–596. [Google Scholar] [CrossRef]
- Paget, C.J.; Kisner, K.; Stone, R.L.; DeLong, D.C. Heterocyclic substituted ureas. II. Immunosuppressive and antiviral activity of benzothiazolyl- and benzoxazolylureas. J. Med. Chem. 1969, 12, 1016–1018. [Google Scholar] [CrossRef]
- Vicini, P.; Geronikaki, A.; Incerti, M.; Busonera, B.; Poni, G.; Cabras, C.A.; La Colla, P. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg. Med. Chem. 2003, 11, 4785–4789. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; De Crescenzo, G.A.; Getman, D.P.; Lu, H.-F.; Sikorski, J.A.; Walker, J.L.; McDonald, J.J.; Houseman, K.A.; Kocan, G.P.; Kishore, N. Discovery of novel benzothiazolesulfonamides as potent inhibitors of HIV-1 protease. Bioorg. Med. Chem. 2003, 11, 4769–4777. [Google Scholar] [CrossRef]
- Alaimo, R.J.; Pelosi, S.S.; Freedman, R. Synthesis and Antibacterial Evaluation of 2- (Substituted Phenylureido) -4-thiocyanatobenzothiazoles. J. Pharm. Sci. 1978, 67, 281–282. [Google Scholar] [CrossRef]
- Sogn, D.D.; Evans, R.; Shepherd, G.M.; Casale, T.B.; Condemi, J.; Greenberger, P.A.; Kohler, P.F.; Saxon, A.; Summers, R.J.; VanArsdel, P.P. Results of the National Institute of Allergy and Infectious Diseases Collaborative Clinical Trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch. Intern. Med. 1992, 152, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Mistry, K.; Desai, K.R. Microwave assisted rapid and efficient synthesis of nitrogen and sulphur containing heterocyclic compounds and their pharmacological evaluation. Indian J. Chem. Sect. B 2006, 45, 1762–1766. [Google Scholar]
- Singh, M.; Singh, S.K.; Gangwar, M.; Nath, G.; Singh, S.K. Design, synthesis and mode of action of some benzothiazole derivatives bearing an amide moiety as antibacterial agents. RSC Adv. 2014, 4, 19013–19023. [Google Scholar] [CrossRef]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Benazzouz, A.; Boraud, T.; Dubedat, P.; Boireau, A.; Stutzmann, J.-M.; Gross, C. Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: A pilot study. Eur. J. Pharmacol. 1995, 284, 299–307. [Google Scholar] [CrossRef]
- Jimonet, P.; Audiau, F.; Barreau, M.; Blanchard, J.-C.; Boireau, A.; Bour, Y.; Coleno, M.-A.; Doble, A.; Doerflinger, G.; Do Huu, C. Riluzole Series. Synthesis and In Vivo “Antiglutamate” Activity of 6-Substituted-2-benzothiazolamines and 3-Substituted-2-imino-benzothiazolines. J. Med. Chem. 1999, 42, 2828–2843. [Google Scholar] [CrossRef]
- Hutchinson, I.; Jennings, S.A.; Vishnuvajjala, B.R.; Westwell, A.D.; Stevens, M.F. Antitumor Benzothiazoles. 16. Synthesis and Pharmaceutical Properties of Antitumor 2-(4-Aminophenyl)benzothiazole Amino Acid Prodrugs. J. Med. Chem. 2002, 45, 744–747. [Google Scholar] [CrossRef]
- El-Sherbeny, M.A. Synthesis of Certain Pyrimido[2,1-b]benzo-thiazole and Benzothiazolo[2,3-b]quinazoline Derivatives for in vitro Antitumor and Antiviral Activities. Arzneim. Forsch. 2000, 50, 848–853. [Google Scholar] [CrossRef]
- Naik, P.R.; Pandeya, S.N.; Pandey, A. Anti-inflammatory and analgesic activities of 1-[2-(substituted benzothiazole)]-1,3-diethyl-4-aryl guanidines. Indian J. Physiol. Pharmacol. 1996, 40, 189–190. [Google Scholar]
- Dogruer, D.S.; Ünlü, S.; Sahin, M.F.; Yqilada, E. Anti-nociceptive and anti-inflammatory activity of some (2-benzoxazolone-3-yl and 2-benzothiazolone-3-yl) acetic acid derivatives. Il Farm. 1998, 53, 80–84. [Google Scholar] [CrossRef]
- Chavan, A.A.; Pai, N.R. Synthesis and Biological Activity of N-Substituted-3-chloro-2-azetidinones. Molecules 2007, 12, 2467–2477. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Dong, G.; Zhang, Y.; Wu, S.; Miao, Z.; Yao, J.; Zhang, W.; Sheng, C. Novel benzothiazole derivatives with a broad antifungal spectrum: Design, synthesis and structure–activity relationships. MedChemComm 2013, 4, 1551–1561. [Google Scholar] [CrossRef]
- Tapia, R.A.; Prieto, Y.; Pautet, F.; Domard, M.; Sarciron, M.E.; Walchshofer, N.; Fillion, H. Synthesis and Antileishmanial Activity of Indoloquinones Containing a Fused Benzothiazole Ring. Eur. J. Org. Chem. 2002, 2002, 4005–4010. [Google Scholar] [CrossRef]
- Nadeem, S.; Rana, A.; Khan, S.A.; Haque, S.E.; Alam, M.S.; Ahsana, W. Anticonvulsant and Toxicity Evaluation of Newly Synthesized 1-[2-(3,4-disubstituted phenyl)-3-chloro-4-oxoazetidin-1-yl]-3-(6-substituted-1,3-benzothiazol-2-yl)ureas. Acta Chim. Slov. 2009, 56, 462–469. [Google Scholar]
- Danzeisen, R.; Schwalenstoecker, B.; Gillardon, F.; Buerger, E.; Krzykalla, V.; Klinder, K.; Schild, L.; Hengerer, B.; Ludolph, A.C.; Dorner-Ciossek, C. Targeted Antioxidative and Neuroprotective Properties of the Dopamine Agonist Pramipexole and Its Nondopaminergic Enantiomer SND919CL2x [(+)2-Amino-4,5,6,7-tetrahydro-6-L-propyl-amino-benzathiazole Dihydrochloride]. J. Pharmacol. Exp. Ther. 2006, 316, 189–199. [Google Scholar] [CrossRef]
- Rajeeva, B.; Srinivasulu, N.; Shantakumar, S. Synthesis and Antimicrobial Activity of Some New 2-Substituted Benzothiazole Derivatives. J. Chem. 2009, 6, 775–779. [Google Scholar]
- Gurupadayya, B.; Manohara, Y.; Gopal, M. Synthesis and Biological Activities of 2H-3-(6’-fluoro-7’-substituted-2’-aminobenzothiazolyl)-3,4-dihydro-1,3-Benzoxazines. Indian J. Heterocycl. Chem. 2006, 15, 113–116. [Google Scholar]
- Bergman, J.M.; Coleman, P.J.; Cox, C.D.; Hartman, G.D.; Lindsley, C.; Mercer, S.P.; Roecker, A.J.; Whitman, D.B. Proline Bis-amide Orexin Receptor Antagonists. Available online: https://portal.unifiedpatents.com/patents/patent/WO-2006127550-A1 (accessed on 22 May 2006).
- Sato, G.; Chimoto, T.; Aoki, T.; Hosokawa, S.; Sumigama, S.; Tsukidate, K.; Sagami, F.J. Toxicological response of rats to a novel monoamine oxidase type-A inhibitor,(5R)-3-[2-((1S)-3-cyano-1-hydrox-ypropyl) benzothiazol-6-yl]-5-methoxymethyl-2-oxazolidi-none (E2011), orally administered for 13 weeks. Toxicol. Sci. 1999, 24, 165–175. [Google Scholar] [CrossRef][Green Version]
- Yoshino, K.; Kohno, T.; Uno, T.; Morita, T.; Tsukamoto, G. Organic phosphorus compounds. 1. 4-(Benzothiazol-2-yl)benzylphosphonate as potent calcium antagonistic vasodilator. J. Med. Chem. 1986, 29, 820–825. [Google Scholar] [CrossRef]
- Hays, S.J.; Rice, M.J.; Ortwine, D.F.; Johnson, G.; Schwarz, R.D.; Boyd, D.K.; Copeland, M.L.; Vartanian, F.G.; Boxer, P.A. Substituted 2-benzothiazolamines as sodium flux inhibitors: Quantitative structure–activity relationships and anticonvulsant activity. J. Pharm. Sci. 1994, 83, 1425–1432. [Google Scholar] [CrossRef]
- Lau, C.; Dufresne, C.; Gareau, Y.; Zamboni, R.; Labelle, M.; Young, R.; Metters, K.; Rochette, C.; Sawyer, N.; Slipetz, D. Evolution of a series of non-quinoline leukotriene D4 receptor antagonist; synthesis and sar of benzothiazoles and thiazoles substituted benzyl alcohols as potent LTD4 antagonists. Bioorg. Med. Chem. Lett. 1995, 5, 1615–1620. [Google Scholar] [CrossRef]
- Itoh, T.; Mase, T. A Novel Practical Synthesis of Benzothiazoles via Pd-Catalyzed Thiol Cross-Coupling. Organic Lett. 2007, 9, 3687–3689. [Google Scholar] [CrossRef]
- Petkov, I.; Deligeorgiev, T.; Markov, P.; Evstatiev, M.; Fakirov, S. Effect of some 2-arylbenzothiazoles on the photo-degradation of poly(vinyl chloride). Polym. Degrad. Stab. 1991, 33, 53–66. [Google Scholar] [CrossRef]
- Yamamoto, K.; Fujita, M.; Tabashi, K.; Kawashima, Y.; Kato, E.; Oya, M.; Iso, T.; Iwao, J. Novel calcium antagonists. Synthesis and structure-activity relationship studies of benzothiazoline derivatives. J. Med. Chem. 1988, 31, 919–930. [Google Scholar] [CrossRef]
- Lara, B.; Gandía, L.; Torres, A.; Olivares, R.; Martínez-Sierra, R.; García, A.G.; Lopez, M.G. Wide-spectrum Ca2+ channel antagonists’: Lipophilicity, inhibition, and recovery of secretion in chromaffin cells. Eur. J. Pharmacol. 1997, 325, 109–119. [Google Scholar] [CrossRef]
- Jia, J.; Cui, M.; Dai, J.; Wang, X.; Ding, Y.S.; Jia, H.; Liu, B. 99m Tc-labeled benzothiazole and stilbene derivatives as imaging agents for Aβ plaques in cerebral amyloid angiopathy. MedChemComm 2014, 5, 153–158. [Google Scholar] [CrossRef]
- Shah, F.; Wu, Y.; Gut, J.; Pedduri, Y.; Legac, J.; Rosenthal, P.J.; Avery, M.A. Design, synthesis and biological evaluation of novel benzothiazole and triazole analogs as falcipain inhibitors. MedChemComm 2011, 2, 1201–1207. [Google Scholar] [CrossRef]
- Mathis, C.A.; Wang, Y.; Holt, D.P.; Huang, G.-F.; Debnath, M.L.; Klunk, W.E. Synthesis and Evaluation of 11C-Labeled 6-Substituted 2-Arylbenzothiazoles as Amyloid Imaging Agents. J. Med. Chem. 2003, 46, 2740–2754. [Google Scholar] [CrossRef] [PubMed]
- Alagille, D.; Baldwin, R.M.; Tamagnan, G.D. One-step synthesis of 2-arylbenzothiazole (‘BTA’) and -benzoxazole precursors for in vivo imaging of β-amyloid plaques. Tetrahedron Lett. 2005, 46, 1349–1351. [Google Scholar] [CrossRef]
- Zheng, M.-Q.; Yin, D.-Z.; Qiao, J.-P.; Zhang, L.; Wang, Y.-X. Syntheses and evaluation of fluorinated benzothiazole anilines as potential tracers for β-amyloid plaques in Alzheimer’s disease. J. Fluorine Chem. 2008, 129, 210–216. [Google Scholar] [CrossRef]
- Serdons, K.; Verduyckt, T.; Vanderghinste, D.; Cleynhens, J.; Borghgraef, P.; Vermaelen, P.; Terwinghe, C.; Leuven, F.V.; Laere, K.V.; Kung, H. Synthesis of 18F-labelled 2-(4′-fluorophenyl)-1,3-benzothiazole and evaluation as amyloid imaging agent in comparison with [11C]PIB. Bioorg. Med. Chem. Lett. 2009, 19, 602–605. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Rudrawar, S.; Kaur, G.; Sharma, L. An Efficient Conversion of Phenolic Esters to Benzothiazoles under Mild and Virtually Neutral Conditions. Synlett 2004, 1533–1536. [Google Scholar] [CrossRef]
- Yoshino, K.; Goto, K.; Yoshiizumi, K.; Morita, T.; Tsukamoto, G. Organic phosphorus compounds. 5. (4-Benzothiazol-2-ylbenzyl)amidophosphonate as potent calcium antagonistic vasodilators. J. Med. Chem. 1990, 33, 2192–2196. [Google Scholar] [CrossRef]
- Prajapati, N.P.; Vekariya, R.H.; Mayuri, A.; Borad, M.A.; Patel, H.D. Recent advances in the synthesis of 2-substituted Benzothiazoles: A Review. RSC Adv. 2004, 4, 60176–60208. [Google Scholar] [CrossRef]
- Yadav, P.S.; Devprakash, D.; Senthilkumar, G.P. Benzothiazole: Different Methods of Synthesis and Diverse Biological Activities. Int. J. Pharm. Sci. Drug Res. 2011, 3, 01–07. [Google Scholar]
- Henary, M.; Paranjpe, S.; Owens, E.A. Substituted benzothiazoles: Synthesis and medicinal characteristics. Heterocycl. Comm. 2013, 19, 89–99. [Google Scholar] [CrossRef]
- Sompalle, R.; Roopan, S.M. Review on Benzothiazoles: Synthesis and Diverse Biological Activities. Chem. Sci. Rev. Lett. 2014, 2, 408–414. [Google Scholar]
- Xie, X.; Yan, Y.; Zhu, N.; Liu, G. Benzothiazoles exhibit broad-spectrum antitumor activity: Their potency, structure-activity and structure-metabolism relationship. Eur. J. Med. Chem. 2014, 76, 67–78. [Google Scholar] [CrossRef]
- Kamal, A.; Syed, M.-A.H.; Mohammed, S.M. Therapeutic potential of benzothiazoles: A patent review (2010–2014). Expert. Opin. Ther. Pat. 2015, 25, 335–349. [Google Scholar] [CrossRef]
- Raju, G.N.; Karumudi, B.S.; Rao, N.R. Benzothiazole-Versatile heterocyclic nucleus in medicinal chemistry: A review. Int. J. Pharm. Chem. 2015, 05, 104–114. [Google Scholar]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Seth, S. A Comprehensive Review on Recent advances in Synthesis & Pharmacotherapeutic potential of Benzothiazoles. Antiinflamm. Antiallergy Agents Med. Chem. 2015, 14, 98–112. [Google Scholar]
- Gill, R.K.; Rawal, R.K.; Bariwal, J. Recent advances in the chemistry and biology of benzothiazoles. Arch. Pharm. (Weinh.) 2015, 348, 155–178. [Google Scholar] [CrossRef]
- Mene, D.; Kale, M. Exploration of Different Methodologies for Synthesizing Biologically Important Benzothiazoles: An Overview. Curr. Org. Synth. 2016, 13, 41–57. [Google Scholar] [CrossRef]
- Achaiah, G.; Sridhar Goud, S.N.; Praveen Kumar, K.; Mayuri, P. Review on 2-substituted benzothiazoles: Diversity of synthetic methods and biological activities. Int. J. Pharm. Sci. Res. 2016, 7, 1375–1382. [Google Scholar]
- Gulati, S.; Wakode, S.; Kaur, A.; Anand, K. Synthesis, biological activity and recent advancement of benzothiazoles: A classical review. World J. Pharm. Pharm. Sci. 2017, 6, 1842–1869. [Google Scholar]
- Banerjee, S.; Payra, S.; Saha, A. A Review on Synthesis of Benzothiazole Derivatives. Curr. Organocatal. 2017, 4, 164–181. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, A.P.; Chandra, S.; Bajpai, A. Benzothiazole derivative: A review on its pharmacological importance towards synthesis of lead. Int. J. Pharm. Sci. Res. 2019, 10, 1553–1566. [Google Scholar]
- Tariq, S.; Kanboj, P.; Amir, M. Therapeutic advancement of benzothiazole derivatives in the last decennial period. Arch. Pharm. 2019, 352, e1800170. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Z.-B. A Review on Domino Condensation/Cyclization Reactions for the Synthesis of 2-Substituted 1,3-Benzothiazole Derivatives. Eur. J. Org. Chem. 2020, 2020, 408–419. [Google Scholar] [CrossRef]
- Xiang Gao, X.; Liu, J.; Zuo, X.; Feng, X.; Gao, Y. Recent Advances in Synthesis of Benzothiazole Compounds Related to Green Chemistry. Molecules 2020, 25, 1675. [Google Scholar]
- Pathak, N.; Rathi, E.; Kumar, N.; Kini, S.G.; Rao, C.M. A Review on Anticancer Potentials of Benzothiazole Derivatives. Mini-Rev. Med. Chem. 2020, 20, 12–23. [Google Scholar] [CrossRef]
- Elgemeire, G.H.; Azzam, R.A.; Osman, R.R. Recent advances in synthesis, metal complexes and biological evaluation of 2-aryl, 2-pyridyl and 2-pyrimidylbenzothiazoles as potential chemotherapeutics. Inorg. Chim. Acta. 2020, 502, 119302. [Google Scholar] [CrossRef]
- Irfana, A.; Batoola, F.; Naqvia, S.A.Z.; Islamb, A.; Osmanc, S.M.; Nocentinid, A.; Alissae, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef]
- Djuidje, E.N.; Sciabica, S.; Buzzi, R.; Dissette, V.; Balzarini, J.; Liekens, S.; Serra, E.; Andreotti, E.; Manfredini, S.; Vertuani, S.; et al. Design, synthesis and evaluation of benzothiazole derivatives as multifunctional agents. Bioorg. Chem. 2020, 101, 103960. [Google Scholar] [CrossRef] [PubMed]
- Chander Sharma, P.; Sharma, D.; Sharma, A.; Bansal, K.K.; Rajak, H.; Sharma, S.; Thakur, V.K. New horizons in benzothiazole scaffold for cancer therapy: Advances in bioactivity, functionality, and chemistry. Appl. Mater. Today 2020, 20, 100783. [Google Scholar] [CrossRef]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef]
- Hein, D.W.; Alheim, R.J.; Leavitt, J.J. The Use of Polyphosphoric Acid in the Synthesis of 2-Aryl- and 2-Alkyl-substituted Benzimidazoles, Benzoxazoles and Benzothiazoles. J. Am. Chem. Soc. 1957, 79, 427–429. [Google Scholar] [CrossRef]
- Shi, D.-F.; Bradshaw, T.D.; Wrigley, S.; McCall, C.J.; Lelieveld, P.; Fichtner, I.; Stevens, M.F. Antitumor Benzothiazoles. 3. Synthesis of 2-(4-Aminophenyl)benzothia-zoles and Evaluation of Their Activities against Breast Cancer Cell Lines In Vitro and In Vivo. J. Med. Chem. 1996, 39, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.; Quarderer, G.J., Jr.; Sen, A.; Nakagawa, M.; Faley, T.L.; Serrano, M.; Teramoto, Y.; Chau, C.C. Method for Rapid Spinning of a Polybenzazole Fiber. U.S. Patent 5,294,390, 15 March 1994. [Google Scholar]
- So, Y.-H.; Heeschen, J.P. Mechanism of Polyphosphoric Acid and Phosphorus Pentoxide-Methanesulfonic Acid as Synthetic Reagents for Benzoxazole Formation. J. Org. Chem. 1997, 62, 3552–3561. [Google Scholar] [CrossRef]
- Mishra, V.R.; Ghanavatkar, C.W.; Mali, S.N.; Qureshia, S.I.; Chaudharib, H.K.; Sekar, N. Design, synthesis, antimicrobial activity and computational studies of novel azo linked substituted benzimidazole, benzoxazole and benzothiazole derivatives. Comp. Biol. Chem. 2019, 78, 330–337. [Google Scholar] [CrossRef]
- Ghanavatkar, C.W.; Mishra, V.R.; Sekar, N. Benzothiazole-pyridone and benzothiazole-pyrazole clubbed emissive azo dyes and dyeing application on polyester fabric: UPF, biological, photophysical and fastness properties with correlative computational assessments. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2020, 230, 118064. [Google Scholar] [CrossRef]
- Mathis, C.A.; Bacskai, B.J.; Kajdasz, S.T.; McLellan, M.E.; Frosch, M.P.; Hyman, B.T.; Holt, D.P.; Wang, Y.; Huang, G.-F.; Debnathd, M.L.; et al. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg. Med. Chem. Lett. 2002, 12, 295–298. [Google Scholar] [CrossRef]
- Njoya, Y.; Gellis, A.; Crozet, M.P.; Vanelle, P. Efficient synthesis of new 6-nitrobenzothiazoles using microwave irradiation. Sulfur Lett. 2003, 26, 67–75. [Google Scholar] [CrossRef]
- Billeau, S.; Chatel, F.; Robin, M.; Faure, R.; Galy, J.-P. 1H and 13C Chemical shifts for 2-aryl and 2-N-arylaminobenzothiazole derivatives. Magn. Reson. Chem. 2006, 44, 102–105. [Google Scholar] [CrossRef]
- Patel, M.N.; Dosi, P.A.; Bhatt, B.S. Nucleic acid interaction and antibacterial behaviours of a ternary palladium(II) complexes. Spectrochim. Acta Part A 2012, 86, 508–514. [Google Scholar] [CrossRef]
- Kini, S.; Swain, S.; Gandhi, A. Synthesis and Evaluation of novel Benzothiazole Derivates against Human Cervical Cancer cell lines. Ind. J. Pharm. Sci. 2007, 69, 46–50. [Google Scholar] [CrossRef]
- You, Q.D.; Li, Z.Y.; Huang, C.H.; Yang, Q.; Wang, X.J.; Guo, Q.L.; Chen, X.G.; He, X.G.; Li, T.K.; Cherm, J.W. Discovery of a Novel Series of Quinolone and Naphthyridine Derivatives as Potential Topoisomerase I Inhibitors by Scaffold Modification. J. Med. Chem. 2009, 52, 5649–5661. [Google Scholar] [CrossRef]
- Racané, L.; Trali-Kulenovi, V.; Sandra Paveli, S.K.; Ratkaj, I.; Peixoto, P.; Nhili, R.; Depauw, S.; Hildebrand, M.P.; David-Cordonnier, M.-E.; Paveli, K.; et al. Novel Diamidino-Substituted Derivatives of Phenyl Benzothiazolyl and Dibenzothiazolyl Furans and Thiophenes: Synthesis, Antiproliferative and DNA Binding Properties. J. Med. Chem. 2010, 53, 2418–2432. [Google Scholar] [CrossRef]
- Kamal, B.A.; Kumar, P.; Suresh, N.; Shankaraiah, M.S. Kumar an efficient one-pot synthesis of benzothiazolo-4b-anilino-podophyllotoxincongeners: DNA topoisomerase-II inhibition and anticancer activity. Bioorg. Med. Chem. Lett. 2011, 21, 350–353. [Google Scholar] [CrossRef]
- Lin, G.W.; Wang, Y.; Jin, Q.M.; Yang, T.T.; Song, J.M.; Lu, Y.; Huang, Q.J.; Song, K.; Zhou, J.; Lu, T. Synthesis, structures and the biological activity study on the metal complexes of2-(4-aminophenyl)benzothiazole derivative. Inorg. Chim. Acta 2012, 382, 35–42. [Google Scholar] [CrossRef]
- Racané, L.; Paveli, S.K.; Ratkaj, I.; Stepani, V.; Paveli, K.; Trali-Kulenovi, V.; Karminski-Zamola, G. Synthesis and antiproliferative evaluation of some new amidino-substituted bis-benzothiazolyl-pyridines and pyrazine. Eur. J. Med. Chem. 2012, 55, 108–116. [Google Scholar] [CrossRef]
- Marques, S.M.; Abate, C.C.; Chaves, S.; Marques, F.; Santos, I.; Nuti, E.; Rossello, A.; Santos, M.A. New bifunctional metalloproteinase inhibitors: An integrated approach towards biological improvements and cancer therapy. J. Inorg. Biochem. 2013, 127, 188–202. [Google Scholar] [CrossRef]
- Racané, L.; Pticek, L.; Sedic, M.; Grbcic, P.; Pavelic, S.K.; Bertoša, B.; Sovic, I.; Karminski-Zamola, G. Eco-friendly synthesis, in vitro anti-proliferative evaluation, and 3D-QSAR analysis of a novel series of monocationic 2-aryl/heteroaryl-substituted 6-(2-imidazolinyl)-benzothiazole mesylates. Mol. Divers. 2018, 22, 723–741. [Google Scholar] [CrossRef]
- Boger, D.L. A convenient preparation of 2-substituted benzothiazoles. J. Org. Chem. 1978, 43, 2296–2297. [Google Scholar] [CrossRef]
- Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Synthesis and structure–activity relationships of new antimicrobial active multisubstituted benzazole derivatives. Eur. J. Med. Chem. 2004, 39, 291–298. [Google Scholar] [CrossRef]
- Ge, F.; Wang, Z.; Wan, W.; Lu, W.; Hao, J. One-pot synthesis of 2-trifluoromethyl and 2-difluoromethyl substituted benzo-1,3-diazoles. Tetrahedron Lett. 2007, 48, 3251–3254. [Google Scholar] [CrossRef]
- Jiang, H.; Yuan, S.; Cai, Y.; Wan, W.; Zhu, S.; Hao, J. A facile preparation of 2-bromodifluoromethyl benzo-1,3-diazoles and its application in the synthesis of gem-difluoromethylene linked aryl ether compounds. J. Fluorine Chem. 2012, 133, 167–170. [Google Scholar] [CrossRef]
- Sharghi, H.; Asemani, O. MeSO3H/SiO2 as an Efficient Combination for the Synthesis of 2-Substituted Aromatic and Aliphatic Benzothiazoles from Carboxylic Acids. Synth. Commun. 2009, 39, 860–867. [Google Scholar] [CrossRef]
- Morais, G.R.; Miranda, H.V.; Santos, I.C.; Santos, I.; Outeiro, T.F.; Paulo, A. Synthesis and in vitro evaluation of fluorinated styryl benzazoles as amyloid-probes. Bioorg. Med. Chem. 2011, 19, 7698–7710. [Google Scholar] [CrossRef]
- Meghdadi, S.; Amirnasr, M.; Ford, P.C. A robust one-pot synthesis of benzothiazoles from carboxylic acids including examples with hydroxyl and amino substituents. Tetrahedron Lett. 2012, 53, 6950–6953. [Google Scholar] [CrossRef]
- Hou, J.; Li, Z.; Fang, Q.; Feng, C.; Zhang, H.; Guo, W.; Wang, H.; Gu, G.; Tian, Y.; Liu, P.; et al. Discovery and extensive in vitro evaluations of NK-HDAC-1: A chiral histone deacetylase inhibitor as a promising lead. J. Med. Chem. 2012, 55, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Chang, J.X.; Liu, Y. Synthesis and antitumor activity of 5-substituted-2-(pyridyl)benzothiazole compounds. Acta Pharm. Sin. 2013, 48, 83–88. [Google Scholar]
- Wang, S.; Chen, Y.; Zhao, S.; Xu, X.; Liu, X.; Bi-Feng Zhang, G. Synthesis and biological evaluation of a series of benzoxazole/benzothiazole-containing 2,3-dihydrobenzo[b][1,4] dioxine derivatives as potential antidepressants. Bioorg. Med. Chem. Lett. 2014, 24, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.L.; Tang, K.; Xin, X.F.; Chen, X.G.; Li, L.; Feng, Z.Q. Synthesis and biological evaluation of 2-(3-butynoicamidophenyl) benzothiazole derivatives as antitumor agents. Acta Pharm. Sinica 2014, 49, 888–895. [Google Scholar]
- Gorepatil, P.B.; Mane, Y.D.; Gorepatil, A.B.; Gaikwad, M.V.; Ingle, V.S. Samarium(III) triflate: A new catalyst for facile synthesis of benzothiazoles and benzoxazoles. from carboxylic acids in aqueous media. Res. Chem. Intermed. 2015, 41, 8355–8362. [Google Scholar] [CrossRef]
- Arfana, M.; Tahiraa, A.; Mannanb, A.; Fatima, T. A Facile Approach to the Synthesis of Benzothiazoles from N-Protected Amino Acids. Russ. J. Org. Chem. 2020, 56, 292–297. [Google Scholar] [CrossRef]
- Maradolla, M.B.; Allam, S.K.; Mandha, A.; Ghandramouli, G.V.P. One pot synthesis of benzoxazoles, benzthiazoles and benzimidazoles from carboxylic acids using ionic liquids. Arkivoc 2008, xv, 42–46. [Google Scholar] [CrossRef]
- René, O.; Souverneva, A.; Magnuson, S.R.; Fauber, P.B. Efficient syntheses of 2-fluoroalkylbenzimidazoles and benzothiazoles. Tetrahedron Lett. 2013, 54, 201–204. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacherjee, D.; Das, P. Oxalic/malonic acids as carbon building blocks for benzazole, quinazoline and quinazolinone synthesis. Org. Biomol. Chem. 2018, 16, 1337–1342. [Google Scholar] [CrossRef]
- Khalil, Z.H.; Yanni, A.S.; Gaber, A.M.; Abdel-Mohsen, S.A. Synthesis and reactions of some new 5-carbonyl(4-amino-3-cyano-2-substituted thiophene-5-yl)-8-hydroxyquinoline (part ii). Synthesis of thiazole; isoxazole; pyrazole, pyrimidine and pyridazine derivatives as possible antimicrobial agents. Phosphorus Sulfur Silicon Relat. Elem. 2000, 166, 57–69. [Google Scholar] [CrossRef]
- Matsushita, H.; Lee, S.-H.; Joung, M.; Clapham, B.; Janda, K.D. Smart cleavage reactions: Synthesis of benzimidazoles and benzothiazoles from polymer-bound esters. Tetrahedron Lett. 2004, 45, 313–316. [Google Scholar] [CrossRef]
- Bhat, M.; Belagali, S.L.; Hemanth Kumar, N.K.; Kumar, S.M. Synthesis and characterization of novel benzothiazole amide derivatives and screening as. possible antimitotic and antimicrobial agents. Res. Chem. Intermed. 2017, 43, 361–378. [Google Scholar] [CrossRef]
- Sheng, C.; Che, X.; Wang, W.; Wang, S.; Cao, Y.; Yao, Y.; Miao, Z.; Zhang, W. Design and synthesis of antifungal benzoheterocyclic derivatives by scaffold hopping. Eur. J. Med. Chem. 2011, 46, 1706. [Google Scholar] [CrossRef]
- Pejchalová, M.; Havelek, R.; Královec, K.; Růžičková, Z.; Pejchal, V. Novel derivatives of substituted 6-fluorobenzothiazole diamides: Synthesis, antifungal activity and cytotoxicity. Med. Chem. Res. 2017, 26, 1847–1862. [Google Scholar] [CrossRef]
- Jenkins, G.L.; Knevel, A.M.; Davis, C.S. A new synthesis of the benzothiazole and benzoxazole rings. J. Org. Chem. 1961, 26, 274. [Google Scholar] [CrossRef]
- Mylari, B.L.; Scott, P.J.; Zembrowski, W.J. 2-Chloro-1,1,1-Triethoxyethane and its Use in a Versatile Synthesis of Substituted, 2-Chloromethyl Heterocycles Including Benzothiazole and Benzoxazole. Synth. Commun. 1989, 19, 2921–2924. [Google Scholar] [CrossRef]
- Mohammadpoor-Baltork, I.; Khosropour, A.R.; Hojati, S.F. Mild and Efficient Synthesis of Benzoxazoles, Benzothiazoles, Benzimidazoles, and Oxazolo[4,5-b]pyridines Catalyzed by Bi(III) Salts Under Solvent-Free Conditions. Monatsh. Chem. 2007, 138, 663–667. [Google Scholar] [CrossRef]
- Mohammadpoor-Baltork, I.; Khosropour, A.R.; Hojati, S.F. ZrOCl2·8H2O as an efficient, environmentally friendly and reusable catalyst for synthesis of benzoxazoles, benzothiazoles, benzimidazoles and oxazolo[4,5-b]pyridines under solvent-free conditions. Catal. Commun. 2007, 8, 1865–1870. [Google Scholar] [CrossRef]
- Patil, A.V.; Lee, S.H.; Bandgar, B.P. Silica Supported Fluoroboric Acid: An Efficient and Reusable Heterogeneous Catalyst for Facile Synthesis of 2-Aliphatic Benzothiazoles, Benzoxazoles, Benzimidazoles and Imidazo[4,5-b]pyridines. Bull. Korean Chem. Soc. 2010, 31, 1719–1722. [Google Scholar] [CrossRef]
- Bastug, G.; Eviolitte, C.; Marko, I.E. Functionalized Orthoesters as Powerful Building Blocks for the Efficient Preparation of Heteroaromatic Bicycles. Org. Lett. 2012, 14, 3502–3505. [Google Scholar] [CrossRef]
- Barbero, M.; Cadamuro, S.; Dughera, S. The efficient o-benzenedisulfonimide catalysed synthesis of benzothiazoles, benzoxazoles and benzimidazoles. Arkivoc 2012, ix, 262–279. [Google Scholar] [CrossRef]
- Fortenberry, C.; Nammalwar, B.; Bunce, R.A. Ammonium Chloride-catalyzed Synthesis of Benzofused Heterocycles from o-Substituted Anilines and Orthoesters. Org. Prep. Proc. Int. 2013, 45, 57–65. [Google Scholar] [CrossRef]
- Shirini, F.; Mamaghani, M.; Seddighi, M. Facile synthesis of benzimidazole, benzoxazole, and benzothiazole derivatives catalyzed by sulfonated rice husk ash (RHA-SO3H) as an efficient solid acid catalyst. Res. Chem. Intermediates 2015, 41, 5611–5619. [Google Scholar] [CrossRef]
- Brembilla, A.; Roizard, D.; Lochon, P. 1-Methyl-2-Pyrrolidinone: A Well-Adapted Solvent in the Benzothiazoles Synthesis. Synth. Commun. 1990, 20, 3379–3384. [Google Scholar] [CrossRef]
- Laskar, I.R.; Chen, M.T. Tuning of Wavelengths: Synthesis and Photophysical Studies of Iridium Complexes and Their Applications in Organic Light Emitting Devices. Chem. Mater. 2004, 16, 111–117. [Google Scholar] [CrossRef]
- Kálai, T.; Jekő, J.; Ősz, E.; Hideg, K. Synthesis and Study of 2-(2-Thienyl)benzazole Type Fluorophores. Synth. Commun. 2003, 33, 1433–1442. [Google Scholar] [CrossRef]
- Racanè, L.; Tralić-Kulenović, V.; Boykin, D.W.; Karminski-Zamol, G. Synthesis of New Cyano-Substituted bis-Benzothiazolyl Arylfurans and Arylthiophenes. Molecules 2003, 8, 342–349. [Google Scholar] [CrossRef]
- Nadaf, R.N.; Siddiqui, S.A.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Room temperature ionic liquid promoted regioselective synthesis of 2-aryl benzimidazoles, benzoxazoles and benzthiazoles under ambient conditions. J. Mol. Catal. A Chem. 2004, 214, 155–160. [Google Scholar] [CrossRef]
- Karlsson, H.J.; Bergqvist, M.H.; Lincoln, P.; Westman, G. Syntheses and DNA-binding studies of a series of unsymmetrical cyanine dyes: Structural influence on the degree of minor groove binding to natural DNA. Bioorg. Med. Chem. 2004, 12, 2369–2384. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.-J. “Liquid-phase synthesis of 2-substituted benzimidazoles, benzoxazoles and benzothiazoles. Tetrahedron Lett. 2004, 45, 113–115. [Google Scholar] [CrossRef]
- Rudrawar, S.; Kondaskar, A.; Chakraborti, A.K. An Efficient Acid- and Metal-Free One-Pot Synthesis of Benzothiazoles from Carboxylic Acids. Synthesis 2005, 2521–2526. [Google Scholar] [CrossRef]
- Wu, C.; Wei, J.; Gao, K.; Wang, Y. Dibenzothiazoles as novel amyloid-imaging agents. Bioorg. Med. Chem. 2007, 15, 2789–2796. [Google Scholar] [CrossRef]
- Racané, L.; Kralj, M.; Šuman, L.; Stojkovi’cc, R.; Trali’c-Kulenovi’cb, V.; Karminski-Zamola, G. Novel amidino substituted 2-phenylbenzothiazoles: Synthesis, antitumor evaluation in vitro and acute toxicity testing in vivo. Bioorg. Med. Chem. 2010, 18, 1038–1044. [Google Scholar] [CrossRef]
- Peprah, K.; Zhu, X.Y.; Eyunni, S.V.; Etukala, J.R.; Setola, V.; Roth, B.L.; Ablordeppey, S.Y. Structure–activity relationship studies of SYA 013, a homopiperazine analog of haloperidol. Bioorg. Med. Chem. 2012, 20, 1671–1678. [Google Scholar] [CrossRef]
- Racané, L.; Pavelić, S.K.; Nhili, R.; Depauw, S.; Paul-Constant, C.; Ratkaj, I.; David-Cordonnier, M.-E.; Pavelić, K.; Tralić-Kulenović, V.; Karminski-Zamola, G. New anticancer active and selective phenylene-bisbenzothiazoles: Synthesis, antiproliferative evaluation and DNA binding. Eur. Med. Chem. 2013, 63, 882–891. [Google Scholar] [CrossRef]
- Kumar, K.R.; Satyanarayana, P.V.V.; Reddy, B.S. NaHSO4-SiO2-Promoted Solvent-Free Synthesis of Benzoxazoles, Benzimidazoles, and Benzothiazole Derivatives. J. Chem. 2012, 2013, 151273. [Google Scholar] [CrossRef]
- Dar, A.A.; Shadab, M.; Khan, S.; Ali, N.; Khan, A.T. One-pot synthesis and evaluation of antileishmanial activities of functionalized S-alkyl/aryl benzothiazole-2-carbothioate scaffold. J. Org. Chem. 2016, 81, 3149–3160. [Google Scholar] [CrossRef]
- Xu, W.; Zeng, M.-T.; Liu, S.-S.; Li, Y.-S.; Dong, Z.-B. Copper catalyzed synthesis of benzoxazoles and benzothiazoles via tandem manner. Tetrahedron Lett. 2017, 58, 4289–4292. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Sardarian, A.R. KF-Al2O3 as a Base Heterogeneous Catalyst for the Synthesis of 2-Substituted Benzoxazoles and Benzothiazoles under Mild Reaction Conditions at Room Temperature. Polycycl. Aromat. Comp. 2020, 40, 990–997. [Google Scholar] [CrossRef]
- Mokhir, A.; Domasevich, K.; Kent, D.N.; Kou, X.; Gerasimchuk, N.; Gerasimchuk, O. Syntheses, crystal structures and coordination compounds of some 2-hetarylcyanoximes. Inorg. Chim. Acta 1999, 284, 85–98. [Google Scholar] [CrossRef]
- Van Zandt, M.C.; Sibley, E.O.; McCann, E.E.; Combs, K.J.; Flam, B.; Sawicki, D.R.; Sabetta, A.; Carrington, A.; Sredy, J.; Howard, E.; et al. Design and synthesis of highly potent and selective (2-arylcarbamoyl-phenoxy)-acetic acid inhibitors of aldose reductase for treatment of chronic diabetic complications. Bioorg. Med. Chem. 2004, 12, 5661–5675. [Google Scholar] [CrossRef]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole derivatives as new chemotype targeting the HCV NS5B polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef]
- Yadong, S.; Huanfeng, J.; Wanqing, W.; Wei, Z.; Xia, W.; Nagasawa, H. Copper-Catalyzed Synthesis of Substituted benzothiazoles via Condensation of o-aminothiophenol with Nitriles. Org. Lett. 2013, 15, 1598–1601. [Google Scholar]
- Yu, X.; Yin, Q.; Zhang, Z.; Huang, T.; Pu, Z.; Bao, M. Synthesis of 2-substituted benzothiazoles via the Brønsted acid catalyzed cyclization of 2-amino thiophenols with nitriles. Tetrahedron Lett. 2019, 60, 1964–1966. [Google Scholar] [CrossRef]
- Kovalenko, S.N.; Vasil’ev, M.V.; Sorokina, I.V.; Chernykh, V.P.; Turov, A.V.; Rudnev, S.A. Recyclization of 2-imino-2H-1-benzopyrans using nucleophilic reagents 3. Reaction of 2-iminocoumarin-3-carboxamides with o-phenylenediamines and o-amino(thio)phenols. Chem. Heterocycl. Compds. 1998, 34, 1412–1415. [Google Scholar] [CrossRef]
- Sung, G.H.; Lee, I.-H.; Kim, B.-R.; Shin, D.-S.; Kim, J.-J.; Lee, S.-G.; Yoon, Y.-J. Eco-friendly atom-economical synthesis of 2-substituted-benzo[d]thiazoles and 2-substituted-benzo[d]oxazoles using 2-acylpyridazin-3(2H)-ones. Tetrahedron 2013, 69, 3530–3539. [Google Scholar] [CrossRef]
- Nale, D.B.; Bhanage, B.M. N-Substituted Formamides as C1-Sources for the Synthesis of Benzimidazole and Benzothiazole Derivatives by Using Zinc Catalysts. Synlett 2015, 26, 2835–2842. [Google Scholar] [CrossRef]
- Kazi, I.; Sekar, G. An efficient synthesis of benzothiazole using tetrabromomethane as a halogen bond donor catalyst. Org. Biomol. Chem. 2019, 17, 9743–9756. [Google Scholar] [CrossRef]
- Tian, Q.; Luo, W.; Gan, Z.; Li, D.; Dai, Z.; Wang, H.; Wang, X.; Yuan, J. Eco-Friendly Syntheses of 2-Substituted Benzoxazoles and 2-Substituted Benzothiazoles from 2-Aminophenols, 2-Aminothiophenols and DMF Derivatives in the Presence of Imidazolium Chloride. Molecules 2019, 24, 174. [Google Scholar] [CrossRef]
- Villemin, D.; Hammadi, M.; Martin, B. Clay Catalysis: Condensation of Orthoesters with O-Substituted Aminoaromatics into Heterocycles. Synth. Commun. 1996, 26, 2895–2899. [Google Scholar] [CrossRef]
- Reddy, A.C.; Rao, P.S.; Venkataratnam, R.V. Fluoro organics: Facile syntheses of novel 2- or 4-trifluoromethyl-1H-arylo-1,5-diazepines, oxazepines, thiazepines, 2-(1,1,1-trifluoroacetonyl)imidazoles, oxazoles and thiazoles. Tetrahedron 1997, 53, 5847–5854. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Selvam, C.; Kaur, G.; Bhagat, S. An Efficient Synthesis of Benzothiazoles by Direct Condensation of Carboxylic Acids with 2-Aminothiophenol under Microwave Irradiation. Synlett 2004, 851–855. [Google Scholar] [CrossRef]
- Gellis, A.; Boufatah, N.; Vanelle, P. Rapid microwave-promoted synthesis of new sulfonylmethylbenzothiazoles in water. Green Chem. 2006, 8, 483–487. [Google Scholar] [CrossRef]
- Seijas, J.A.; Vazquez-Tato, M.P.; Carballido-Reboredo, M.R.; Crecente-Campo, J.; Romar-Lopez, L. Lawesson’s reagent and microwaves: A new efficient access to benzoxazoles and benzothiazoles from carboxylic acids under solvent-free conditions. Synlett 2007, 313–317. [Google Scholar] [CrossRef]
- Gupta, S.D.; Singh, H.P.; Moorthy, N. Iodine-Catalyzed, One-Pot, Solid-Phase Synthesis of Benzothiazole Derivatives. Synth. Commun. 2007, 37, 4327–4329. [Google Scholar] [CrossRef]
- Lim, H.-J.; Myung, D.; Lee, I.Y.C.; Jung, M.H. Microwave-Assisted Synthesis of Benzimidazoles, Benzoxazoles, and Benzothiazoles from Resin-Bound Esters. J. Comb. Chem. 2008, 10, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Abdul, R.; Saloni, G.; Shweta, S. Solvent-free synthesis of 2-alkyl and 2-alkenylbenzothiazoles from fatty acids under microwave irradiation. Indian J. Chem. 2008, 47B, 601–605. [Google Scholar]
- Khoobi, M.; Emami, S.; Dehghan, G.; Foroumadi, A.; Ramazani, A.; Shafiee, A. Synthesis and Free Radical Scavenging Activity of Coumarin Derivatives Containing a 2-Methylbenzothiazoline Motif. Arch. Pharm. 2011, 344, 588–594. [Google Scholar] [CrossRef]
- Khoobi, M.; Ramazani, A.; Foroumadi, A.; Hamadi, H.; Hojjati, Z.; Shafiee, A. Efficient Microwave-Assisted Synthesis of 3-Benzothiazolo and 3-Benzothiazolino Coumarin Derivatives Catalyzed by Heteropoly Acids. J. Iran. Chem. Soc. 2011, 8, 1036–1042. [Google Scholar] [CrossRef]
- Wen, X.; El Bakali, J.; Deprez-Poulain, R.; Deprez, B. Efficient propylphosphonic anhydride (®T3P) mediated synthesis of benzothiazoles, benzoxazoles and benzimidazoles. Tetrahedron Lett. 2012, 53, 2440–2443. [Google Scholar] [CrossRef]
- Kurzajewska, M.; Kwiatek, D.; Kubicki, M.; Brzezinski, B.; Hnatejko, Z. New complexes of 2-(p-pyridyl)-1,3-benzothiazole with metal ions; synthesis, structural and spectral studies. Polyhedron 2018, 148, 1–8. [Google Scholar] [CrossRef]
- Panda, S.S.; Ibrahim, M.A.; Oliferenko, A.A.; Asiri, A.M.; Katritzky, A.R. Catalyst-free facile synthesis of 2-substituted benzothiazoles. Green Chem. 2013, 15, 2709–2712. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, X.; Zhang, G.; Russ, C.A. Synthesis of 2-(4-Aminophenyl)benzothiazoles Using MF Resin Supported H+ under Solvent Free Conditions. Russ. J. Gen. Chem. 2015, 85, 679–682. [Google Scholar] [CrossRef]
- Dev, D.; Chandra, J.; Palakurthy, N.B.; Thalluri, K.; Kalita, T.; Mandal, B. Benzoxazole and Benzothiazole Synthesis from Carboxylic Acids in Solution and on Resin by Using Ethyl 2-Cyano-2-(2-nitrobenzenesulfonyloxyimino)acetate and para-Toluenesulfonic Acid. Asian J. Org. Chem. 2016, 5, 663–675. [Google Scholar] [CrossRef]
- Luo, B.; Li, D.; Zhang, A.L.; Gao, J. Synthesis, antifungal activities and molecular docking studies of benzoxazole and benzothiazole derivatives. Molecules 2018, 23, 2457. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, D.; Murthi, P.R.K.; Dulla, B.; Rao, M.V.B.; Pal, M. Amberlyst-15–catalyzed synthesis of 2-substituted 1,3-benzazoles in water under ultrasound. Synth. Commun. 2013, 43, 3083–3092. [Google Scholar] [CrossRef]
- Chang, Y.H.; Peak, J.D.; Wierschke, S.W.; Feld, W.A. A General and Efficient Synthesis of 2-Phenylbenzothiazoles from Diphenyl Disulfides. Synth. Commun. 1993, 23, 663–670. [Google Scholar] [CrossRef]
- Shi, D.-Q.; Rong, S.-F.; Dou, G.-L. Efficient synthesis of 2-arylbenzothiazole derivatives with the aid of a low-valent titanium reagent. Synth. Commun. 2010, 40, 2302–2310. [Google Scholar] [CrossRef]
- Du, G.; Zhu, N.; Hong, H.; Hana, L.; Suo, Q. Synthesis of benzothiazoles by the reaction of disulfide and aromatic carboxylic acid promoted by PCl3 and DBU. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 1154–1159. [Google Scholar] [CrossRef]
- Coelho, F.L.; Campo, L.F. Synthesis of 2-arylbenzothiazoles via direct condensation between in situ generated 2-aminothiophenol from disulfide cleavage and carboxylic acids. Tetrahedron Lett. 2017, 58, 2330–2333. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Zhao, Y.; Hao, L.; Liu, C. Hydrosilane-promoted cyclization of 2-aminothiophenols by CO2 to benzothiazoles. RSC Adv. 2014, 4, 56957–56960. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Yang, Z.; Zhao, Y.; Zhang, H.; Hao, L.; Han, B.; Liu, Z. Ionic liquid-catalyzed C–S bond construction using CO2 as a C1 building block under mild conditions: A metal-free route to synthesis of benzothiazoles. ACS Catal. 2015, 5, 6648–6652. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Mei, Q.; Yang, Z.; Zhao, Y.; Zhang, H.; Hao, L.; Liu, Z. Atmospheric CO2 promoted synthesis of N-containing heterocycles over B(C6F5)3 catalyst. New J. Chem. 2016, 40, 8282–8287. [Google Scholar] [CrossRef]
- Rasal, K.B.; Yadav, G.D. Carbon dioxide mediated novel synthesis of 2, 4(1H, 3H)- quinazolinedione in water. Org. Process Res. Dev. 2016, 20, 2067–2073. [Google Scholar] [CrossRef]
- Chun, S.; Yang, S.; Chung, Y.K. Synthesis of benzothiazoles from 2-aminobenzenethiols in the presence of a reusable polythiazolium precatalyst under atmospheric pressure of carbon dioxide. Tetrahedron 2017, 73, 3438–3442. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).