Abstract
The addition of functionalized organolithium compounds derived from 5-chloro-2-methoxy-1-pentene and 6-chloro-2-methoxy-1-hexene to N-tert-butanesulfinyl aldimines imines, and a subsequent hydrolysis of the enol ether moiety, yielded different δ- and ε-amino ketone derivatives, respectively, in moderate yields and diastereoselectivities. The application of these compounds in organic synthesis was demonstrated by the preparation of 2-substituted 6-methylpiperidines in a stereoselective manner, among them natural alkaloids (+)- and (−)-isosolenopsin A.
1. Introduction
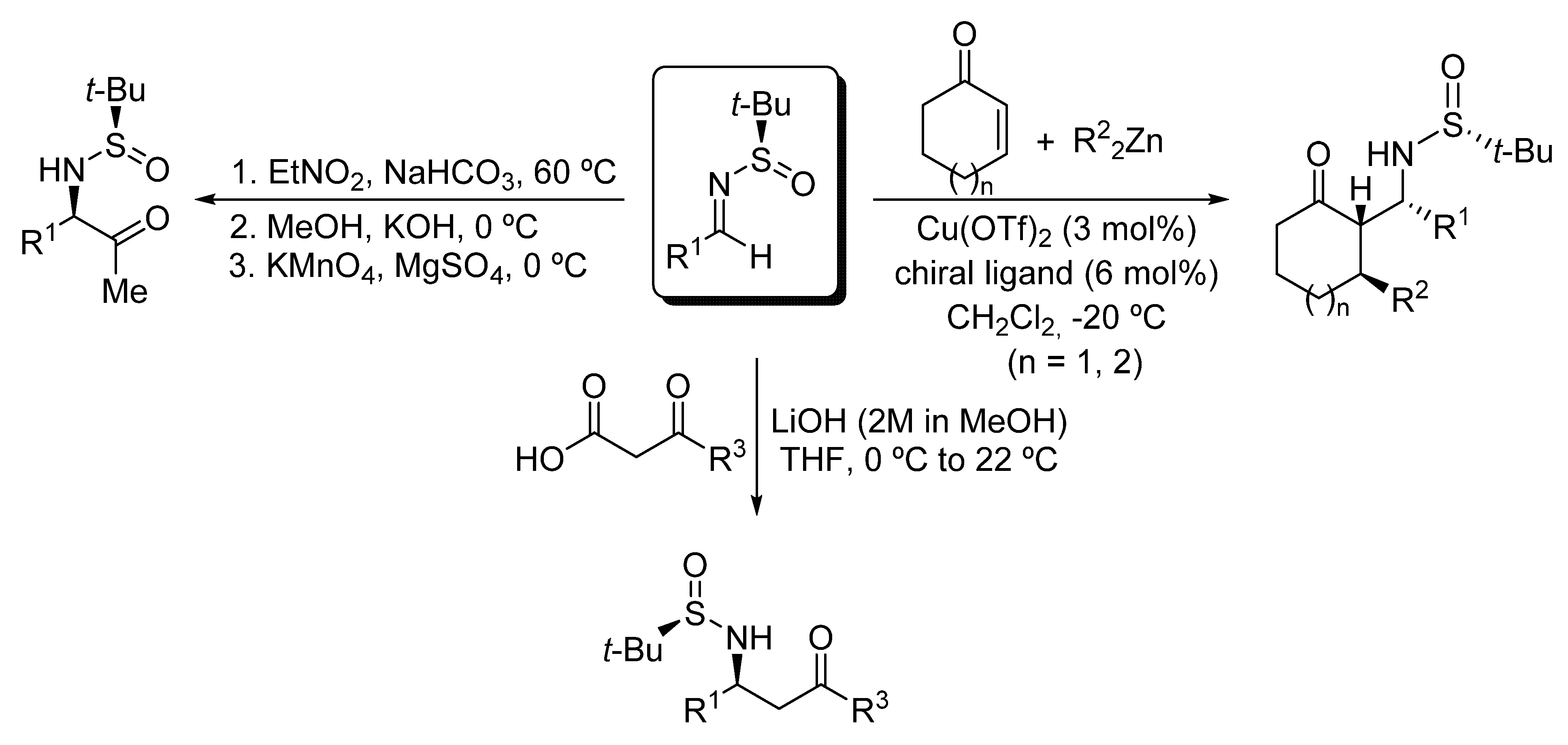
Amino carbonyl compounds are versatile intermediates in organic synthesis to access complex nitrogen-containing molecules. Their usefulness derives from the broad catalogue of synthetic transformations that can be carried out on both the amino and carbonyl moieties. Additionally, some amino carbonyl compounds display biological activity by themselves and have multiple applications in medicinal chemistry. For these reasons, the development of methodologies to prepare amino carbonyl compounds is a topic of great interest in organic synthesis [1]. These compounds usually require the presence of protecting groups of the amino or the carbonyl functionalities to avoid inter- and intramolecular undesired condensations. The preparation of α- [2] and β-amino [3,4,5,6,7,8,9,10,11,12,13] ketones have been extensively studied, and in many of the methodologies leading to these compounds, nucleophilic additions to chiral imines are involved. Among chiral imines, those derived from tert-butanesulfinamide are of special relevance and have been recurrently used in organic synthesis [14,15,16]. For instance, our research group have also accomplished the stereoselective synthesis of α-amino ketone derivatives in moderate yields from β-nitro amine derivatives that were obtained by a coupling reaction of N-tert-butanesulfinyl imines and nitroethane under basic conditions [17] (Scheme 1). On the other hand, we reported the synthesis of β-amino ketones through the nucleophilic addition to these chiral imines of enolates obtained by a copper-catalyzed addition of dialkylzinc reagents to α,β-unsaturated cyclic enones [18,19,20], and more recently by a decarboxylative Mannich coupling with β-keto acids under mild basic conditions [21,22], proceeding these reactions in excellent yields and diastereoselectivities (Scheme 1).
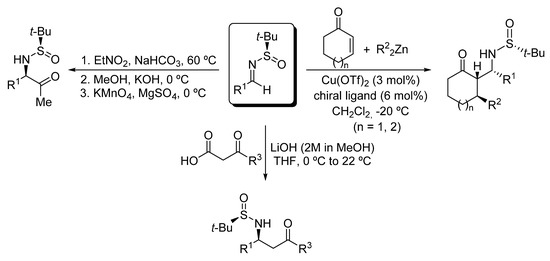
Scheme 1.
Synthesis of α- and β-amino ketone derivatives from N-tert-butanosulfinyl imines.
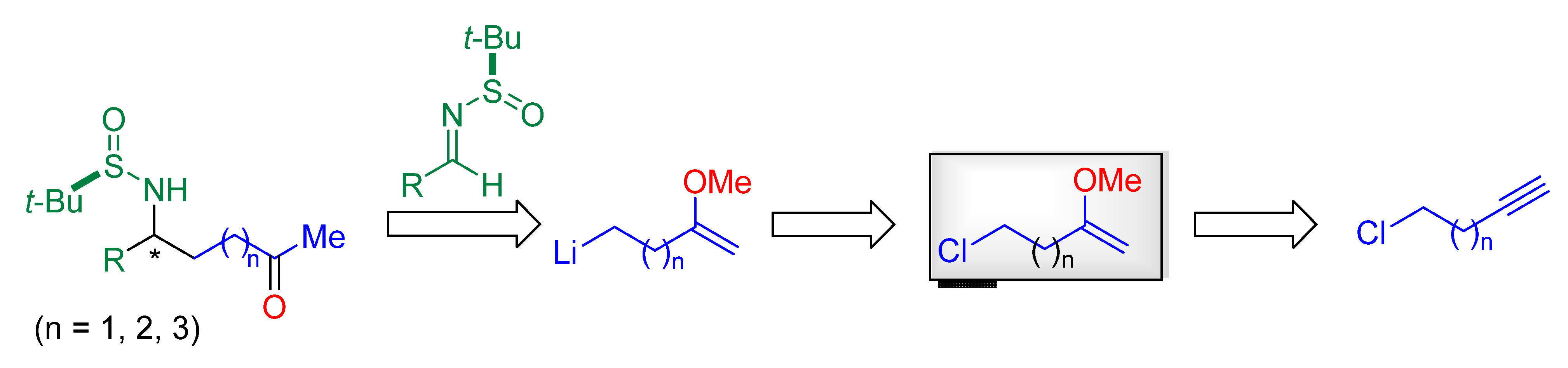
Continuing our interest in the stereoselective synthesis of amino ketone derivatives from chiral N-tert-butanesulfinyl imines, and being aware of the potential interest as synthetic intermediates of these compounds, we decide to explore a new synthetic pathway to access to γ-, δ- and ε-amino ketone derivatives in an enantioenriched form, by performing a diastereoselective addition of a functionalized organolithium compound to these imines. Functionalized organolithium compounds are valuable reagents, because upon reaction with electrophiles, polyfunctionalized molecules are directly produced [23,24]. These organolithium compounds can be prepared from appropriate precursors following classical procedures, the most commonly used are halogen-lithium exchange [25]. Our retrosynthetic analysis for the preparation of these amino ketone derivatives is depicted on Scheme 2, involving lithiation of the corresponding chloroenol ether, which could be prepared from the corresponding chloroalkyne, subsequent reaction with the imine, and final hydrolysis.
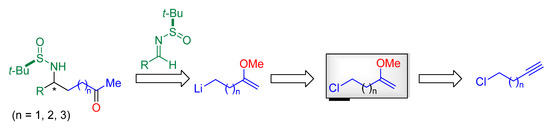
Scheme 2.
Proposed retrosynthetic pathway for the construction of γ-, δ- and ε-amino ketone derivatives.
2. Results and Discussion
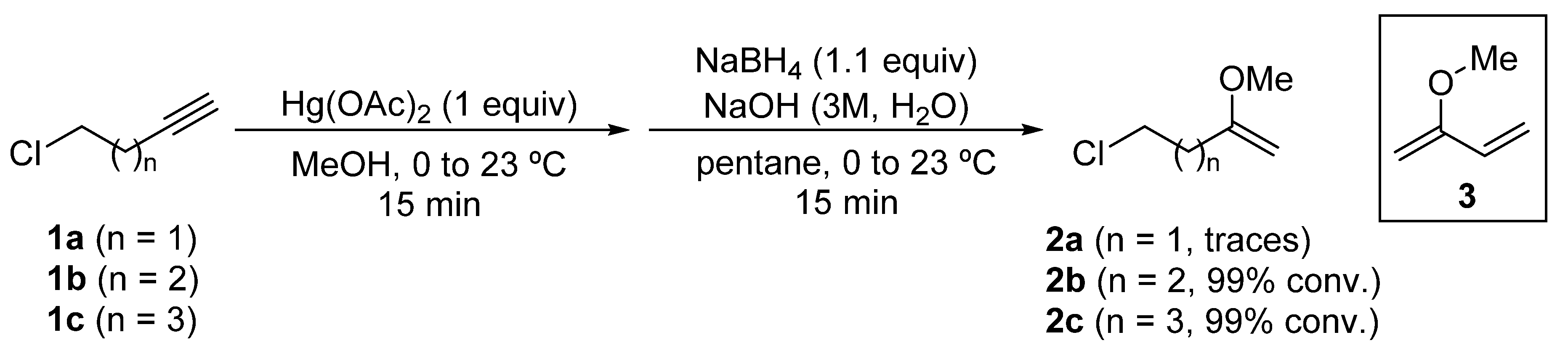
2.1. Synthesis of 2-Methoxy-1-alkenyl Chlorides 2
2-Methoxy-1-alkenyl chlorides 2 were prepared from the corresponding alkynyl chlorides 1, through an oxymercuration reaction using mercury(II) acetate in methanol as solvent, and a subsequent reduction of the organomercury species with sodium borohydride [26]. In the case of 5-chloropent-1-yne (1b) and 6-chlorohex-1-yne (1c), 5-chloro-2-methoxypent-1-ene (2b) and 6-chloro-2-methoxyhex-1-ene (2c) were obtained quantitatively as determined by GC-MS analysis of the reaction crude. However, in the oxymercuration and reduction of 4-chlorobut-1-yne (1a), only traces of the desired enol ether 2a were detected by GC-MS analysis, being the major product diene 3 or dimers derived from it (Scheme 3).
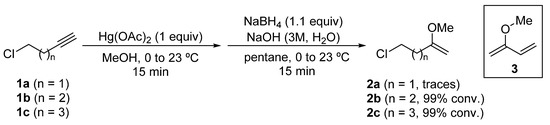
Scheme 3.
Synthesis of 2-methoxy-1-alkenyl chlorides 2 from chloroalkynes 1.
As 4-chloro-2-methoxybut-1-ene (2a) was not produced in significant amounts, it was not possible to access N-tert-butanesulfinyl γ-amino ketone derivatives using this methodology.
2.2. Synthesis of N-tert-Butanesulfinyl δ-Amino Ketone Derivatives 7
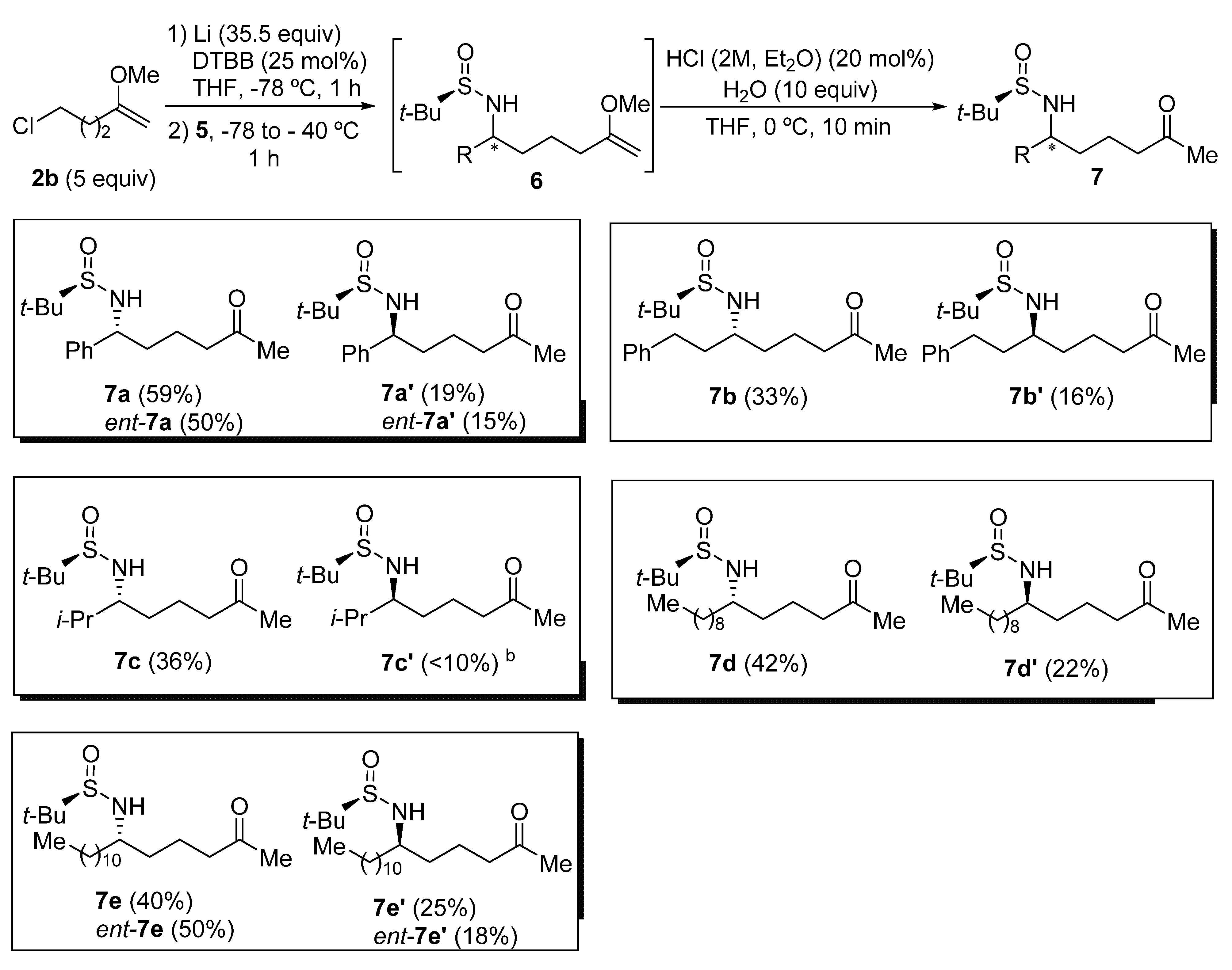
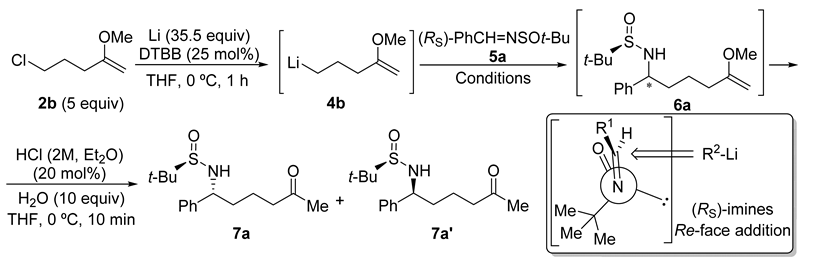
Chiral sulfinyl imine 5a, derived from benzaldehyde and (R)-tert-butanesulfinamide, was chosen as the model substrate to optimize the reaction conditions to perform the addition to sulfinyl imines 5 of the organolithium compound 4b, generated from 5-chloro-2-methoxypent-1-ene (2b) in the presence of excess of lithium metal, and a substoichiometric amount of 4,4′-di-tert-butylbiphenyl (DTBB). The product that results from this addition after hydrolysis with water (6a) was found to be slightly unstable, so it was directly transformed into the corresponding diastereomeric N-tert-butanesulfinyl δ-amino ketone derivatives 7a or 7a′ under slightly acidic conditions. These compounds were easier to handle and were separated by means of column chromatography (Table 1). Firstly, it was studied the effect of the solvent, in which imine 5a was dissolved, on the diastereoselectivity of the addition of a solution of organolithium compound 4b in THF at −78 °C. The best result in terms of stereoselectivity (70:30 dr) was obtained when imine 5a was dissolved in toluene (Table 1, entry 3). At this point, we assumed that the major diastereoisomer (7a) was the one that is formed by addition of the organolithium reagent 4b to the Re-face of imine 5a with (RS)-configuration via an open transition state (Table 1) [27], in accordance with previous results of our research group [28,29]. Moreover, we found that the addition of the imine 5a to the solution of the organolithium compound 4b in THF resulted in an enhanced diastereoselectivity (Table 1, entry 4). The formation of the organolithium compound 4b was also accomplished using Et2O as solvent, and without the presence of DTBB. However, after adding imine 5a to the resulting ethereal solution containing functionalized organolithium compound 4b, the stereochemical outcome of the reaction was slightly worse than in the previous case, when THF was employed as solvent (Table 1, entry 5). It is known that the addition of Grignard reagents to these chiral sulfinyl imines proceeds with higher levels of diastereocontrol than in the case of organolithium derivatives [16]. However, all the attempts to prepare the corresponding organomagnesium compound from chloromethoxyalknes 2 failed, due probably to their instability and the demanding reaction conditions (sonication, higher temperatures, bases as additives) [30]. On the other hand, bromoalkynes of type 1 are not commercially available.

Table 1.
Optimization of the reaction conditions.
The addition of the organolithium compound 4b was then studied on a variety of tert-butanesulfinyl imines 5, employing the optimal conditions found for imine 5a (Table 1, entry 4). As in the optimization of the reaction conditions, the products of the addition 6 were directly transformed into the corresponding N-tert-butanesulfinyl δ-amino ketone derivatives 7, which are more stable and easier to separate by means of column chromatography. Products 7 were isolated in moderate to good yields, obtaining the best result in the case of the aromatic sulfinyl imine 5a (Figure 1). Additionally, the ratio of diastereoisomers 7 and 7′ was always ranging approximately between 2:1 and 3:1 (7:7′ ratio), except in the case of the highly hindered sulfinyl imine 5c, derived from isobutyraldehyde, in which the minor diastereoisomer 7c′ was isolated in low yield and was pure enough to be properly characterized (Table 1, compounds 7c and 7c′). This methodology allowed access to the corresponding enantiomers ent-7 and ent-7′ of these N-tert-butanesulfinyl δ-amino ketone derivatives by using as starting materials (SS)-N-tert-butanesulfinyl imines ent-5, at it was exemplified for imines ent-5a and ent-5e derived from benzaldehyde and dodecanal, respectively. As a limitation, halogen atoms, ester, nitrile and carbonyl groups will not be tolerated in these transformations, due to the extremely reductive reaction medium (Figure 1).
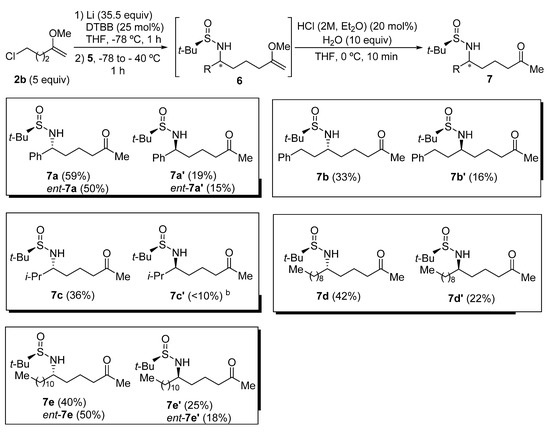
Figure 1.
Synthesis of N-tert-butanesulfinyl δ-amino ketone derivatives 7 a. a Isolated yields after column chromatography purification are given in parentheses. b This compound was not pure enough after column chromatography for full unambiguous characterization.
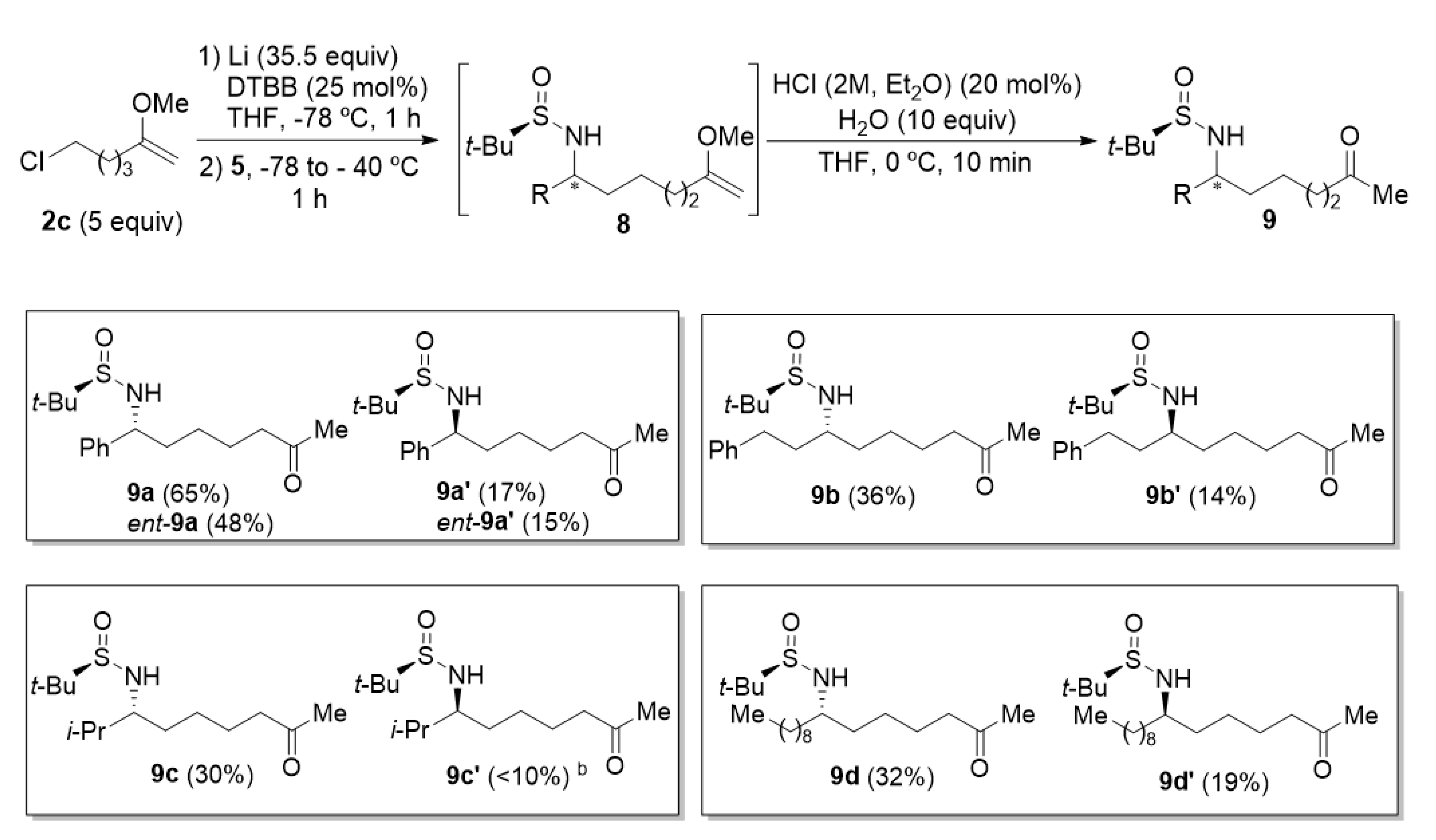
2.3. Synthesis of N-tert-Butanesulfinyl ε-Amino Ketone Derivatives 9
The reaction of 6-chloro-2-methoxyhex-1-ene (2c) with N-tert-butanesulfinyl imines 5 under the same reaction conditions described in the previous section for the synthesis of N-tert-butanesulfinyl δ-amino ketone derivatives 7, led to the homologous ε-amino ketone derivatives 9 (Figure 2). The highest yield was also obtained working with aromatic aldimine 5a, diastereomeric amino ketone derivatives 9a and 9a′ being isolated in a combined 82% yield in an almost enantiopure form. Concerning the diastereoselectivity of these transformations, better diastereoselectivities were observed for aromatic and α-disubstituted aldimines 5a and 5c, with near 3:1 diastereomeric ratios. On the other hand, for aliphatic not hindered aldimines, those values of diastereomeric ratio were closer to 2:1 (Figure 2).
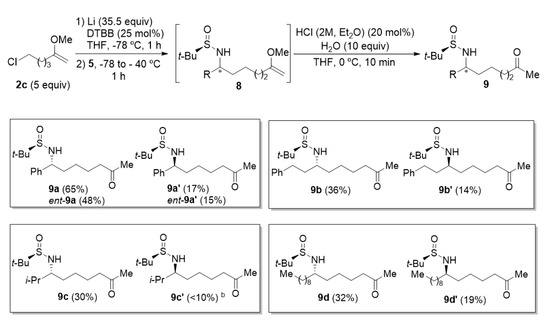
Figure 2.
Synthesis of N-tert-butanesulfinyl ε-amino ketone derivatives 9 a. a Isolated yields after column chromatography purification are given in parentheses. b This compound was not pure enough after column chromatography for full unambiguous characterization.
2.4. Synthesis of Piperidines 11 and Azepanes 13 from N-tert-Butanesulfinyl Amino Ketone Derivatives 7 and 9
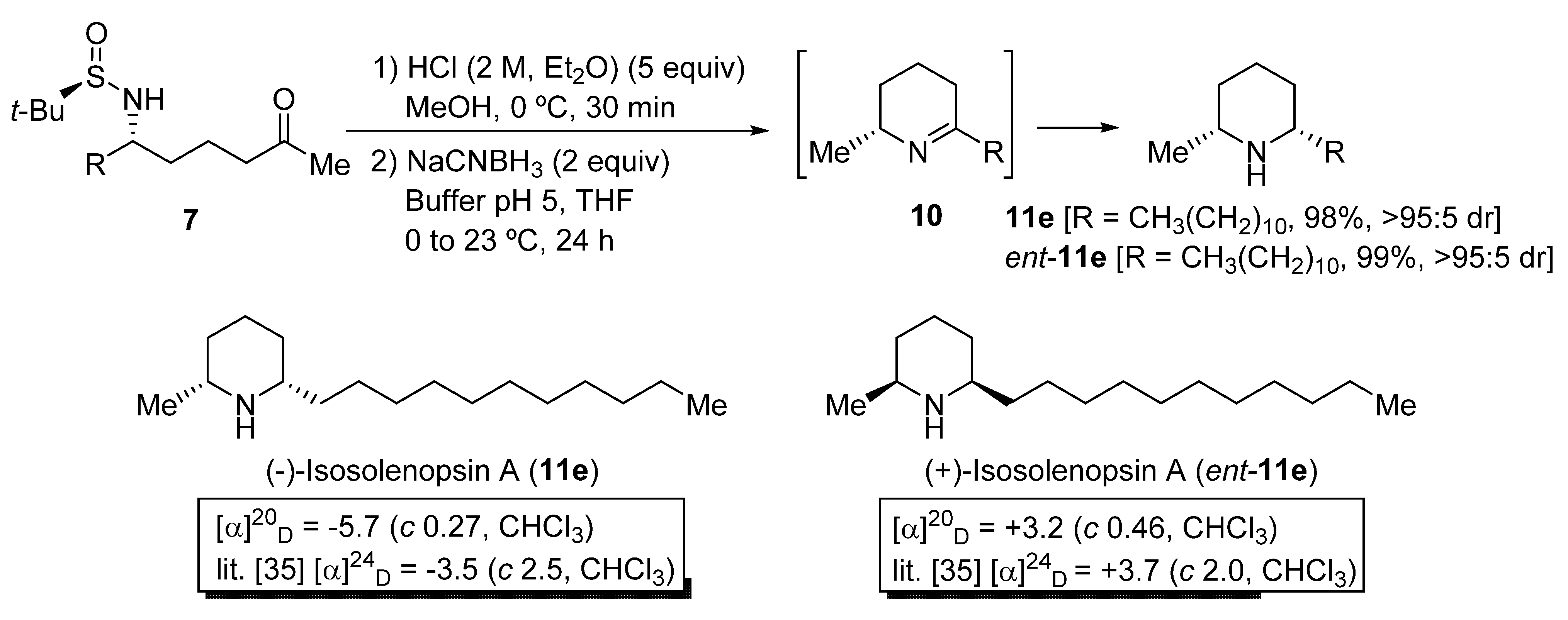
To demonstrate the synthetic utility of N-tert-butanesulfinyl δ-amino ketone derivatives 7, the stereoselective preparation of piperidine ring systems was accomplished in two steps. This methodology consists in an initial desulfinylation under acidic conditions, and a subsequent reduction of the resulting imine intermediate 10 with NaCNBH3 [31,32]. As a result, 2,6-cis-disubstituted piperidines 11 were isolated in good to excellent yields (Scheme 4). The cis:trans diastereomeric ratio of compounds 11 was excellent in all cases, as determined by GC-MS analysis. It was not possible to determine by HPLC, or GC using chromatographic columns with a chiral packing, the enantiomeric purities of compounds 11e and ent-11e, but we consider that these values should be similar to the diastereomeric ratios of their precursors aminoketone derivatives 7e (>95:5 dr after column chromatography purification). Piperidines 11e and ent-11e [33] are, respectively, the alkaloids (−)-isosolenopsin A and (+)-isosolenopsin A, that have been isolated from the venom of the fire ants of the genus Solenopsis and display cytotoxic, antibacterial, insecticidal and antifungal activity (Scheme 4) [34,35]. It is worth noting that, by comparison of the optical rotation values of these natural products with those reported in the literature, we were able to confirm that the attack of the organolithium compound 4b occurred through the Re-face of chiral sulfinyl imine 5e, to give N-tert-butanesulfinyl δ-amino ketone derivative 7e as the major diastereoisomer, with S configuration at the stereocenter that is formed after this addition, as in piperidine 11e. The opposite configuration of piperidine ent-11e can be explained then by a selective attack of compound 4b through the Si-face of the (SS)-tert-butanesulfinyl imine ent-5e, which is the precursor in this case.
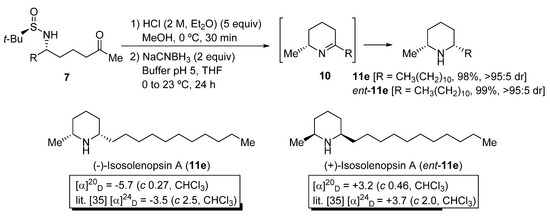
Scheme 4.
Synthesis of piperidines 11 from N-tert-butanesulfinyl amino ketone derivatives 7.
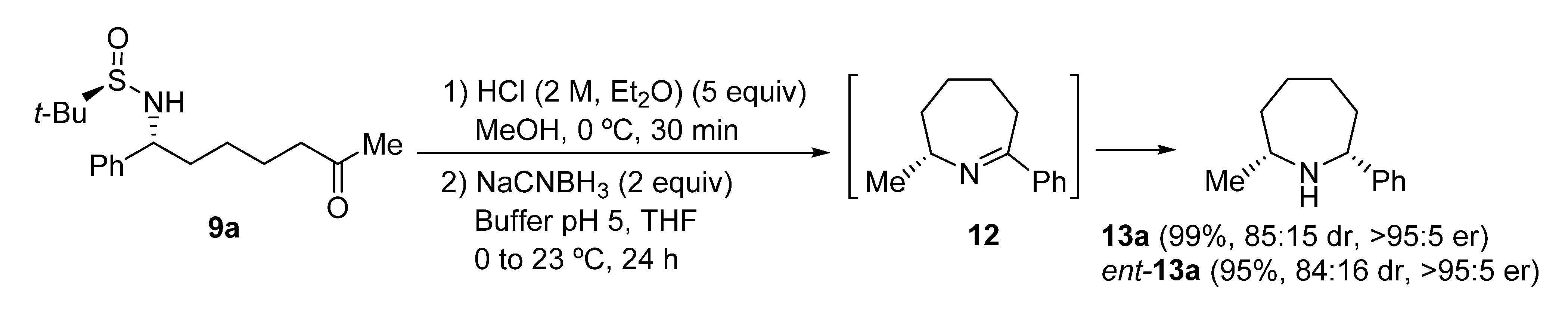
Azepane ring systems are also easily accessible from N-tert-butanesulfinyl ε-amino ketone derivatives 9 employing the same methodology that was used for the synthesis of piperidines 11, starting from N-tert-butanesulfinyl δ-amino ketone derivatives 9. In this case, azepanes 13a and ent-13a were obtained in excellent yields and enantiopurities. Reduction of cyclic imine intermediate 12 took place with poorer diastereoselectivity than for six-membered cyclic imines 10, leading in this case to a 85:15 cis:trans diastereomeric ratio. However, the enantiopurity of compounds 13a and ent-13a was analyzed by GC (see in Supplementary Materials) using a column containing a chiral stationary phase and both showed excellent enantiomeric ratios (Scheme 5).
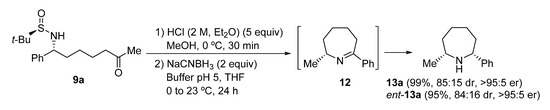
Scheme 5.
Synthesis of azepanes 13a from N-tert-butanesulfinyl amino ketone derivative 9a.
3. Materials and Methods
3.1. General Information
Reagents and solvents were purchased from commercial suppliers and used as received. (R)- and (S)-tert-Butanesulfinamide were a gift from Medalchemy (>99% ee by chiral HPLC on a Chiracel AS column, 90:10 n-hexane/i-PrOH, 1.2 mL/min, λ = 222 nm).
Infrared spectra (IR) were obtained with an ATR Jasco FT/IR-4100, without previous preparation of the sample. The frequencies are given in cm−1. Optical rotations were measured using a Jasco P-1030 polarimeter with a thermally jacketed 5 cm cell at approximately 23 °C and concentrations (c) are given in g/100 mL. Low-resolution mass spectra (LRMS) were obtained in the electron impact mode (EI) with an Agilent MS5973N spectrometer with a SIS (Scientific Instrument Services) direct insertion probe (73DIP-1) at 70 eV, and with an Agilent GC/MS5973N spectrometer in the electron impact mode (EI) at 70 eV. In both cases, fragment ions are given in m/z with relative intensities (%) in parentheses. High-resolution mass spectra (HRMS) were also carried out in the electron impact mode (EI) at 70 eV on an Agilent 7200 spectrometer equipped with a time of flight (TOF) analyzer and the samples were introduced through a direct insertion probe, or through an Agilent GC7890B. NMR spectra were recorded at 300 or 400 MHz for 1H NMR and at 75 or 100 MHz for 13C NMR with a Bruker AV300 Oxford or a Bruker AV400 spectrometers, respectively, using CDCl3 as solvent, and TMS as internal standard (0.00 ppm). The data are reported as: (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet or unresolved, br s = broad signal, coupling constant(s) in Hz, integration). 13C NMR spectra were recorded with 1H-decoupling at 100 MHz and referenced to CDCl3 at 77.16 ppm. DEPT-135 experiments were performed to assign CH, CH2 and CH3.
TLCs were performed on prefabricated Merck aluminum plates with silica gel 60 coated with fluorescent indicator F254 and were visualized with phosphomolybdic acid (PMA) stain. The Rf values were calculated under these conditions. Flash chromatography was carried out on handpacked columns of silica gel 60 (230–400 mesh). GC-MS analysis were carried out in an Agilent 6890N spectrometer with FID detector, helium gas transportation (2 mL/min), injection pressure: 12 psi, temperature in detection an injection blocks: 270 °C, column type HP-1 (12 m long, 0.22 mm internal diameter, 0.25 μm thickness methylsilicone rubber and OV-101 stationary phase). Temperature programs: (A) initial temperature (60 °C) for 3 min, heating 15 °C/min until final temperature (270 °C), final temperature (270 °C) for 10 min or (B) initial temperature (80 °C) for 5 min, heating 15 °C/min until final temperature (270 °C), final temperature (270 °C) for 10 min.
Known chiral N-tert-butanesulfinyl imines 5a [36], 5b [37], 5c [36], 5d [38], and 5e [39] were prepared according to the reported procedures, and spectroscopic data are in accordance with the literature.
3.2. Preparation and Characterization of Compounds
3.2.1. Synthesis of 2-Methoxy-1-alkenyl Chlorides 2
General Procedure. A solution of Hg(OAc)2 (0.640 g, 2.0 mmol) in dry methanol (5 mL) was stirred under argon at 23 °C for 5 min. Then, the corresponding alkynyl chloride 1 (2.0 mmol) was slowly added to this solution at 0 °C, and the reaction was stirred at 23 °C for 15 min. After this time, petroleum ether (5 mL) was added to the reaction mixture, and it was cooled to 0 °C, and a solution of NaBH4 (0.082 g, 2.2 mmol, 1.1 equiv) in NaOH 3M (2 mL) was slowly added. The reaction was stirred at 23 °C for 15 min, and then the layers were separated, and the aqueous layer was extracted with petroleum ether (3 × 10 mL). The combined organic layers were dried over anhydrous magnesium sulfate, and the solvent was evaporated under vacuum (15 Torr, <30 °C), leading to the expected compounds 2, which were pure enough to be used for the next step.
5-Chloro-2-methoxypent-1-ene (2b). Following the general procedure, compound 2b was obtained from 5-chloropent-1-yne (1b) as a colorless liquid (99% conversion of 1b into 2b by GC-MS analysis): C6H11OCl; 1H NMR (400 MHz, CDCl3) δ 1.92–1.99 (m, 2H), 2.26 (dd, J = 7.8, 6.7 Hz, 2H), 3.53 (s, 3H), 3.52–3.57 (m, 2H), 3.90–3.93 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 30.3 (CH2), 32.3 (CH2), 44.5 (CH2), 54.9 (CH3), 81.4 (CH2), 162.6 (C); GC-MS (temperature program A in Section 3.1) Rt 5.3 min; LRMS (EI) m/z 136 (M+ + 2, 1.5%), 134 (M+, 4.2%), 72 (100), 43 (11), 42 (36), 41 (19), 39 (13); HRMS (EI-TOF) Calcd for C6H11OCl35 [M+] 134.0498, found 134.0493.
6-Chloro-2-methoxyhex-1-ene (2c). Following the general procedure, compound 2c was obtained from 6-chlorohex-1-yne (1c) as a colorless liquid (99% conversion of 1c into 2c by GC-MS analysis): C7H13OCl; 1H NMR (400 MHz, CDCl3) δ 1.59–1.67 (m, 2H), 1.73–1.82 (m, 2H), 2.13 (t, J = 7.4 Hz, 2H), 3.53 (s, 3H), 3.52–3.56 (m, 2H) 3.86–3.89 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 24.7 (CH2), 31.1 (CH2), 34.2 (CH2), 45.0 (CH2), 54.8 (CH3), 80.8 (CH2), 163.6 (C); GC-MS (temperature program A in Section 3.1) Rt 7.0 min; LRMS (EI) m/z 150 (M+ + 2, 4.8%), 148 (M+, 14.7%), 85 (100), 72 (23), 55 (40), 43 (16), 41 (11); HRMS (EI-TOF) Calcd for C7H13OCl35 [M+] 148.0655, found 148.0652.
3.2.2. Synthesis of N-tert-Butanesulfinyl Amino Ketone Derivatives 7 and 9
General Procedure. To a blue suspension of lithium powder (50 mg, 7.1 mmol) and DTBB (15 mg, 0.05 mmol, 25 mol%) in dry THF (3 mL) was added the corresponding 2-methoxy-1-alkenyl chloride 2 (1.0 mmol) at −78 °C, and the reaction mixture was stirred at this temperature for 1 h. Then the corresponding chiral sulfinyl imine 5 (0.2 mmol) was added dropwise, and the resulting reaction mixture was stirred for 1 h, and allowed to warm up until the temperature reached −40 °C. After that, it was hydrolyzed with water (3 mL), and allowed to warm up to reach the room temperature. Then it was extracted with ethyl acetate (3 × 10 mL), dried over magnesium sulfate and the solvent was evaporated under vacuum (15 Torr). The reaction crude was then dissolved in THF (8 mL) and distilled water was added (36 μL, 36 mg, 2 mmol, 10 equiv) and HCl (2M in Et2O, 20 μL, 0.04 mmol, 20 mol%) were successively added at 0 °C, and the reaction mixture was stirred at this temperature for 10 min. After that, it was hydrolyzed with a saturated aqueous solution of NaHCO3 (10 mL), extracted with ethyl acetate (3 × 10 mL), dried over magnesium sulfate, and the solvent was evaporated (15 Torr). The residue was purified by column chromatography (silica gel, hexane/EtOAc) to yield pure products 7 and 9.
(1R,RS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylhexan-5-one (7a). Following the general procedure, compound 7a was obtained as the major diastereoisomer from sulfinyl imine 5a and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (35 mg, 0.12 mmol, 59%): C16H25NO2S; Rf 0.28 (hexane/EtOAc 1:2); [α]20D −35.4 (c 0.93, CH2Cl2); IR (film) ν 2950, 2923, 2869, 1708, 1361, 1052, 728, 701 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.22 (s, 9H), 1.36–1.60 (m, 2H), 1.70–1.80 (m, 1H), 1.93–2.04 (m, 1H), 2.07 (s, 3H), 2.37–2.42 (m, 2H), 3.47 (d, J = 3.7 Hz, 1H), 4.31–4.37 (m, 1H), 7.25–7.37 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 19.9 (CH2), 22.7 (CH3), 29.9 (CH3), 35.9 (CH2), 43.3 (CH2), 55.9 (C), 58.9 (CH), 127.3 (CH), 128.1 (CH), 128.9 (CH), 142.2 (C), 208.5 (C); LRMS (EI) m/z 295 (M+, 0.03%), 239 (2), 175 (62), 118 (11), 117 (100), 104 (11), 57 (20), 43 (21); HRMS (EI-TOF) Calcd for C16H25NO2S [M+] 295.1606, found 295.1604.
(1S,RS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylhexan-5-one (7a′). Following the general procedure, compound 7a′ was obtained as the minor diastereoisomer from sulfinyl imine 5a and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (11.2 mg, 0.038 mmol, 19%): C16H25NO2S; Rf 0.18 (hexane/EtOAc 1:2); [α]20D −72.9 (c 0.28, CH2Cl2); IR (film) ν 2923, 2865, 1708, 1361, 1049, 763, 701 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.19 (s, 9H), 1.40–1.52 (m, 1H), 1.51–1.65 (m, 1H), 1.75–1.84 (m, 2H), 2.08 (s, 3H), 2.41 (t, J = 7.0 Hz, 2H), 3.63 (d, J = 3.0 Hz, 1H), 4.36 (td, J = 7.0, 2.9 Hz, 1H), 7.24–7.36 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 20.0 (CH2), 22.7 (CH3), 30.0 (CH3), 38.2 (CH2), 43.1 (CH2), 55.7 (C), 59.2 (CH), 127.7 (CH), 127.8 (CH), 128.6 (CH), 141.9 (C), 208.5 (C); LRMS (EI) m/z 295 (M+, 0.05%), 239 (2), 175 (62), 118 (11), 117 (100), 104 (11), 57 (23), 43 (26); HRMS (EI-TOF) Calcd for C12H17NO2S [M+-C4H8] 239.0980, found 239.0956.
(1S,SS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylhexan-5-one (ent-7a). Following the general procedure, compound ent-7a was obtained as the major diastereoisomer from sulfinyl imine ent-5a and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (29.5 mg, 0.10 mmol, 50%). Physical and spectroscopic data were found to be the same as for 7a. [α]20D +47.5 (c 1.25, CH2Cl2).
(1R,SS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylhexan-5-one (ent-7a′). Following the general procedure, compound ent-7a′ was obtained as the minor diastereoisomer from sulfinyl imine ent-5a and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (8.8 mg, 0.03 mmol, 15%). Physical and spectroscopic data were found to be the same as for 7a′. [α]20D +78.2 (c 0.28, CH2Cl2).
(3R,RS)-3-Amino-N-(tert-butanesulfinyl)-1-phenyloctan-7-one (7b). Following the general procedure, compound 7b was obtained as the major diastereoisomer from chiral imine 5b and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (21 mg, 0.065 mmol, 33%): C18H29NO2S; Rf 0.33 (hexane/EtOAc 1:2); [α]20D −44.8 (c 0.45, CH2Cl2); IR (film) ν 3050, 2950, 2869, 1708, 1265, 1056, 732 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.21 (s, 9H), 1.41–1.64 (m, 4H), 1.83–1.97 (m, 2H), 2.12 (s, 3H), 2.42 (t, J = 6.9 Hz, 2H), 2.67–2.76 (m, 2H), 3.14 (d, J = 6.6 Hz, 1H), 3.18–3.27 (m, 1H), 7.15–7.24 (m, 3H), 7.24–7.31 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 19.6 (CH2), 22.8 (CH3), 30.1 (CH3), 31.2 (CH2), 35.3 (CH2), 37.8 (CH2), 43.3 (CH2), 55.9 (C), 56.1 (CH), 126.1 (CH), 128.6 (CH), 128.6 (CH), 141.6 (C), 208.7 (C); LRMS (EI) m/z 323 (M+, 0.07%), 267 (43), 203 (11), 185 (60), 145 (11), 143 (36), 129 (29), 117 (17), 107 (14), 91 (100), 57 (46), 56 (11), 43 (40), 41 (16); HRMS (EI-TOF) Calcd for C14H21NO2S [M+-C4H8] 267.1293, found 267.1289.
(3S,RS)-3-Amino-N-(tert-butanesulfinyl)-1-phenyloctan-7-one (7b′). Following the general procedure, compound 7b′ was obtained as the minor diastereoisomer from sulfinyl imine 5b and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (10 mg, 0.031 mmol, 16%): C18H29NO2S; Rf 0.21 (hexane/EtOAc 1:2); [α]20D −15.7 (c 0.43, CH2Cl2); IR (film) ν 3027, 2931, 2865, 1708, 1361, 1052, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.25 (s, 9H), 1.53–1.86 (m, 6H), 1.74–1.86 (m, 2H), 2.13 (s, 3H), 2.38–2.50 (m, 2H), 2.55–2.65 (m, 1H), 2.69–2.75 (m, 1H), 3.19–3.29 (m, 1H), 7.13–7.21 (m, 3H), 7.25–7.31 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 19.6 (CH2), 22.9 (CH3), 30.1 (CH3), 32.0 (CH2), 35.7 (CH2), 37.7 (CH2), 43.2 (CH2), 56.1 (C), 56.3 (CH), 126.1 (CH), 128.5 (CH), 128.6 (CH), 141.9 (C), 208.7 (C); LRMS (EI) m/z 323 (M+, 0.06%), 267 (41), 203 (11), 185 (58), 145 (11), 143 (36), 129 (27), 117 (18), 107 (13), 91 (100), 57 (47), 56 (11), 43 (38), 41 (16); HRMS (EI-TOF) Calcd for C14H21NO2S [M+-C4H8] 267.1293, found 267.1290.
(3R,RS)-3-Amino-N-(tert-butanesulfinyl)-2-methyloctan-7-one (7c). Following the general procedure, compound 7c was obtained from sulfinyl imine 5c and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (19 mg, 0.073 mmol, 36%): C13H27NO2S; Rf 0.32 (hexane/EtOAc 1:2); [α]20D −28.5 (c 0.44, CH2Cl2); IR (film) ν 2954, 2877, 1708, 1365, 1052, 732 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.92 (d, J = 6.9 Hz, 3H), 0.94 (d, J = 6.8 Hz, 3H), 1.23 (s, 9H), 1.35–1.48 (m, 2H), 1.50–1.79 (m, 2H), 1.91–2.06 (m, 1H), 2.14 (s, 3H), 2.41–2.47 (m, 2H), 3.03–3.07 (m, 1H), 3.16 (d, J = 7.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 18.0 (CH3), 18.5 (CH3), 20.4 (CH2), 22.9 (CH3), 30.1 (CH3), 31.5 (CH2), 32.4 (CH3), 43.5 (CH2), 56.2 (C), 61.9 (CH), 208.7 (C); LRMS (EI) m/z 261 (M+, 0.58%), 205 (25), 144 (11), 141 (11), 123 (62), 120 (14), 111 (11), 104 (13), 97 (17), 95 (12), 85 (15), 83 (46), 81 (19), 72 (17), 71 (33), 70 (14), 69 (16), 59 (28), 57 (76), 56 (21), 55 (33), 43 (100), 41 (37); HRMS (EI-TOF) Calcd for C9H19NO2S [M+-C4H8] 205.1136, found 205.1137.
(6S,RS)-6-Amino-N-(tert-butanesulfinyl)pentadecan-2-one (7d). Following the general procedure, compound 7d was obtained as the major diastereoisomer from sulfinyl imine 5d and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (29 mg, 0.084 mmol, 42%): C19H39NO2S; Rf 0.50 (hexane/EtOAc 1:2); [α]20D −34.6 (c 0.52, CH2Cl2); IR (film) ν 2923, 2857, 1712, 1457, 1361, 1052, 728 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 3H), 1.21 (s, 9H), 1.24–1.33 (br s, 16H), 1.50–1.63 (m, 4H), 2.13 (s, 3H), 2.44 (t, J = 7.2 Hz, 2H), 3.03 (d, J = 6.4 Hz, 1H), 3.14–3.23 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.2 (CH3), 19.2 (CH2), 22.7 (CH2), 22.8 (CH3), 25.8 (CH2), 29.4 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 30.1 (CH3), 31.9 (CH2), 35.1 (CH2), 36.4 (CH2), 43.5 (CH2), 55.8 (C), 56.5 (CH), 208.8 (C); LRMS (EI) m/z 345 (M+, 0.10%), 289 (40), 225 (51), 207 (16), 123 (20), 113 (12), 111 (16), 109 (36), 97 (26), 95 (41), 85 (11), 83 (44), 81 (23), 71 (30), 70 (14), 69 (31), 67 (12), 57 (86), 56 (20), 55 (28), 43 (100), 41 (37); HRMS (EI-TOF) Calcd for C15H31NO2S [M+-C4H8] 289.2075, found 289.2068.
(6R,RS)-6-Amino-N-(tert-butanesulfinyl)pentadecan-2-one (7d′). Following the general procedure, compound 7d′ was obtained as the minor diastereoisomer from sulfinyl imine 5d and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (15 mg, 0.043 mmol, 22%): C19H39NO2S; Rf 0.26 (hexane/EtOAc 1:2); [α]20D −20.1 (c 0.50, CH2Cl2); IR (film) ν 2923, 2857, 1712, 1457, 1365, 1052, 732 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 3H), 1.22 (s, 9H), 1.25–1.32 (br s, 16H), 1.52–1.61 (m, 2H), 1.62–1.71 (m, 2H), 2.13 (s, 3H), 2.44–2.49 (m, 2H), 3.09 (d, J = 6.4 Hz, 1H), 3.15–3.24 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.2 (CH3), 19.6 (CH2), 22.8 (CH2), 22.9 (CH3), 25.6 (CH2), 29.4 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 30.1 (CH3), 32.0 (CH2), 35.8 (CH2), 35.8 (CH2), 43.4 (CH2), 55.9 (C), 56.7 (CH), 208.9 (C); LRMS (EI) m/z 345 (M+, 0.05%), 289 (34), 225 (43), 207 (13), 123 (15), 113 (11), 111 (14), 109 (30), 97 (25), 95 (35), 85 (12), 83 (37), 81 (20), 71 (28), 70 (15), 69 (28), 57 (73), 56 (16), 55 (23), 43 (100), 41 (31); HRMS (EI-TOF) Calcd for C15H31NO2S [M+-C4H8] 289.2075, found 289.2067.
(6S,RS)-6-Amino-N-(tert-butanesulfinyl)heptadecan-2-one (7e). Following the general procedure, compound 7e was obtained as the major diastereoisomer from sulfinyl imine 5e and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (29.8 mg, 0.08 mmol, 40%): C21H43NO2S; Rf 0.51 (hexane/EtOAc 1:2); [α]20D −33.9 (c 0.57, CH2Cl2); IR (film) ν 2923, 2854, 1712, 1457, 1361, 1052, 728 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 3H), 1.21 (s, 9H), 1.24–1.33 (br s, 20H), 1.49–1.75 (m, 4H), 2.14 (s, 3H), 2.44 (t, J = 7.2 Hz, 2H), 3.02 (d, J = 6.4 Hz, 1H), 3.15–3.25 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.2 (CH3), 19.7 (CH2), 22.8 (CH3), 25.9 (CH2), 29.5 (CH2), 29.7 (CH2), 29.7 (CH2), 29.7 (CH2), 29.8 (CH2), 30.1 (CH3), 32.0 (CH2), 35.1 (CH2), 36.4 (CH2), 43.6 (CH2), 55.9 (C), 56.5 (CH), 208.8 (C); LRMS (EI) m/z 373 (M+, 0.04%), 317 (40), 254 (11), 253 (57), 235 (15), 123 (18), 113 (14), 111 (13), 109 (36), 97 (34), 95 (40), 85 (12), 83 (47), 81 (22), 71 (32), 70 (14), 69 (24), 67 (11), 57 (90), 56 (21), 55 (26), 43 (100), 41 (36); HRMS (EI-TOF) Calcd for C17H35NO2S [M+-C4H8] 317.2389, found 317.2372.
(6R,RS)-6-Amino-N-(tert-butanesulfinyl)heptadecan-2-one (7e′). Following the general procedure, compound 7e′ was obtained as the minor diastereoisomer from sulfinyl imine 5e and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (18.5 mg, 0.05 mmol, 25%): C21H43NO2S; Rf 0.31 (hexane/EtOAc 1:2); [α]20D −19.4 (c 0.61, CH2Cl2); IR (film) ν 2923, 2854, 1712, 1457, 1365, 1052, 728 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.7 Hz, 3H), 1.22 (s, 9H), 1.24–1.32 (br s, 20H), 1.48–1.75 (m, 4H), 2.14 (s, 3H), 2.43–2.51 (m, 2H), 3.09 (d, J = 7.0 Hz, 1H), 3.15–3.23 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.3 (CH3), 19.6 (CH2), 22.8 (CH3), 22.8 (CH2), 25.6 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 29.7 (CH2), 29.8 (CH2), 30.1 (CH3), 32.0 (CH2), 35.8 (CH2), 35.8 (CH2), 43.4 (CH2), 55.9 (C), 56.7 (CH), 208.9 (C); LRMS (EI) m/z 373 (M+, 0.04%), 317 (48), 254 (13), 253 (65), 235 (18), 123 (21), 113 (16), 111 (16), 109 (41), 97 (43), 95 (48), 85 (14), 83 (57), 81 (25), 71 (37), 70 (15), 69 (29), 67 (13), 57 (99), 56 (22), 55 (31), 43 (100), 41 (38); HRMS (EI-TOF) Calcd for C17H35NO2S [M+-C4H8] 317.2389, found 317.2378.
(6R,SS)-6-Amino-N-(tert-butanesulfinyl)heptadecan-2-one (ent-7e). Following the general procedure, compound ent-7e was obtained as the major diastereoisomer from sulfinyl imine ent-5e and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (37.3 mg, 0.10 mmol, 50%). Physical and spectroscopic data were found to be the same as for 7e. [α]20D +33.8 (c 0.45, CH2Cl2).
(6S,SS)-6-Amino-N-(tert-butanesulfinyl)heptadecan-2-one (ent-7e′). Following the general procedure, compound ent-7e′ was obtained as the minor diastereoisomer from sulfinyl imine ent-5e and 5-chloro-2-methoxypent-1-ene (2b) as a yellow oil (13.4 mg, 0.036 mmol, 18%). Physical and spectroscopic data were found to be the same as for 7e′. [α]20D +28.9 (c 0.48, CH2Cl2).
(1R,RS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylheptan-6-one (9a). Following the general procedure, compound 9a was obtained as the major diastereoisomer from sulfinyl imine 5a and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (40.2 mg, 0.13 mmol, 65%): C17H27NO2S; Rf 0.48 (hexane/EtOAc 1:2); [α]20D −54.8 (c 0.64, CH2Cl2); IR (film) ν 3062, 2938, 2865, 1708, 1361, 1052, 759, 701 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.22 (s, 9H), 1.24–1.30 (m, 2H), 1.48–1.61 (m, 2H), 1.66–1.78 (m, 2H), 2.09 (s, 3H), 2.36 (t, J = 7.4 Hz, 2H), 3.38 (d, J = 3.5 Hz, 1H), 4.28–4.36 (m, 1H), 7.23–7.38 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 22.7 (CH3), 23.7 (CH2), 25.3 (CH2), 30.0 (CH3), 36.4 (CH2), 43.6 (CH2), 55.8 (C), 59.0 (CH), 127.3 (CH), 128.0 (CH), 128.9 (CH), 142.4 (C), 208.9 (C); LRMS (EI) m/z 309 (M+, 0.03%), 191 (17), 189 (42), 171 (21), 154 (20), 131 (47), 129 (17), 117 (28), 107 (100), 106 (20), 105 (20), 104 (26), 91 (82), 57 (52), 43 (45), 41 (19); HRMS (EI-TOF) Calcd for C13H19NO2S [M+-C4H8] 253.1136, found 253.1131.
(1S,RS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylheptan-6-one (9a′). Following the general procedure, compound 9a′ was obtained as the minor diastereoisomer from sulfinyl imine 5a and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow wax (10.3 mg, 0.034 mmol, 17%): C17H27NO2S; Rf 0.26 (hexane/EtOAc 1:2); [α]20D −69.8 (c 0.77, CH2Cl2); IR (film) ν 2935, 2865, 1708, 1361, 1049, 767, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.17 (s, 9H), 1.21–1.25 (m, 2H), 1.48–1.60 (m, 2H), 1.73–1.86 (m, 2H), 2.09 (s, 3H), 2.37 (t, J = 7.4 Hz, 2H), 3.40 (d, J = 3.1 Hz, 1H), 4.30–4.38 (m, 1H), 7.22–7.36 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 22.6 (CH3), 23.5 (CH2), 25.6 (CH2), 30.0 (CH3), 38.7 (CH2), 43.5 (CH2), 55.6 (C), 59.3 (CH), 127.7 (CH), 127.7 (CH), 128.6 (CH), 142.0 (C), 208.8 (C); LRMS (EI) m/z 309 (M+, 0.08%), 189 (42), 171 (22), 131 (44), 129 (16), 107 (100), 104 (20), 91 (60), 57 (37), 43 (41), 41 (14); HRMS (EI-TOF) Calcd for C17H27NO2S [M+] 309.1762, found 309.1756.
(1S,SS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylheptan-6-one (ent-9a). Following the general procedure, compound ent-9a was obtained as the major diastereoisomer from sulfinyl imine ent-5a and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (30 mg, 0.097 mmol, 48%). Physical and spectroscopic data were found to be the same as for 9a. [α]20D +40.7 (c 0.20, CH2Cl2).
(1R,SS)-1-Amino-N-(tert-butanesulfinyl)-1-phenylheptan-6-one (ent-9a′). Following the general procedure, compound ent-9a′ was obtained as the minor diastereoisomer from t-BS imine ent-5a and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (9.3 mg, 0.03 mmol, 15%). Physical and spectroscopic data were found to be the same as for 9a′. [α]20D +50.9 (c 0.28, CH2Cl2).
(3R,RS)-3-Amino-N-(tert-butanesulfinyl)-1-phenylnonan-8-one (9b). Following the general procedure, compound 9b was obtained as the major diastereoisomer from sulfinyl imine 5b and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (23.9 mg, 0.071 mmol, 36%): C19H31NO2S; Rf 0.34 (hexane/EtOAc 1:2); [α]20D −48.6 (c 0.20, CH2Cl2); IR (film) ν 3050, 2950, 2869, 1708, 1265, 1056, 732 cm−1; 1H NMR (300 MHz, CDCl3) δ 1.20 (s, 9H), 1.25–1.36 (m, 2H), 1.48–1.64 (m, 4H), 1.84–1.95 (m, 2H), 2.13 (s, 3H), 2.42 (t, J = 7.2 Hz, 2H), 2.62–2.78 (m, 2H), 3.08 (d, J = 6.6 Hz, 1H), 3.21–3.29 (m, 1H), 7.12–7.31 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 22.8 (CH3), 23.7 (CH2), 25.1 (CH2), 30.0 (CH3), 32.0 (CH2), 35.8 (CH2), 37.9 (CH2), 43.6 (CH2), 55.9 (C), 56.2 (CH), 126.1 (CH), 128.6 (CH), 128.6 (CH), 141.7 (C), 208.9 (C); LRMS (EI) m/z 337 (M+, 0.07%), 281 (46), 199 (25), 157 (27), 143 (45), 131 (15), 129 (18), 117 (61), 107 (14), 91 (100), 57 (43), 56 (11), 43 (43), 41 (16); HRMS (EI-TOF) Calcd for C15H23NO2S [M+-C4H8] 281.1449, found 281.1443.
(3S,RS)-3-Amino-N-(tert-butanesulfinyl)-1-phenylnonan-8-one (9b′). Following the general procedure, compound 9b′ was obtained as the minor diastereoisomer from sulfinyl imine 5b and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (9.4 mg, 0.028 mmol, 14%): C19H31NO2S; Rf 0.26 (hexane/EtOAc 1:2); [α]20D −22.0 (c 0.34, CH2Cl2); IR (film) ν 3027, 2927, 2861, 1708, 1454, 1049, 736, 698 cm−1; 1H NMR (300 MHz, CDCl3) δ 1.24 (s, 9H), 1.33–1.48 (m, 2H), 1.51–1.68 (m, 4H), 1.73–1.94 (m, 2H), 2.12 (s, 3H), 2.44 (t, J = 7.2 Hz, 2H), 2.55–2.79 (m, 2H), 3.03 (d, J = 7.2 Hz, 1H), 3.18–3.29 (m, 1H), 7.11–7.24 (m, 3H), 7.25–7.31 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 22.9 (CH3), 23.7 (CH2), 25.2 (CH2), 30.0 (CH3), 32.1 (CH2), 36.4 (CH2), 37.9 (CH2), 43.6 (CH2), 56.1 (C), 56.6 (CH), 126.1 (CH), 128.5 (CH), 128.6 (CH), 142.0 (C), 209.0 (C); LRMS (EI) m/z 337 (M+, 0.04%), 281 (39), 199 (21), 157 (22), 143 (39), 131 (17), 129 (15), 117 (60), 107 (14), 91 (100), 57 (42), 43 (30), 41 (14); HRMS (EI-TOF) Calcd for C15H23NO2S [M+-C4H8] 281.1449, found 281.1449.
(3R,RS)-3-Amino-N-(tert-butanesulfinyl)-2-methylnonan-8-one (9c). Following the general procedure, compound 9c was obtained from sulfinyl imine 5c and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (16.6 mg, 0.060 mmol, 30%): C14H29NO2S; Rf 0.35 (hexane/EtOAc 1:2); [α]20D −27.1 (c 0.38, CH2Cl2); IR (film) ν 2950, 2869, 1712, 1365, 1052, 732 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.92 (d, J = 6.9 Hz, 3H), 0.94 (d, J = 6.8 Hz, 3H), 1.22 (s, 9H), 1.25–1.62 (m, 6H), 1.92–2.02 (m, 1H), 2.13 (s, 3H), 2.43 (t, J = 7.3 Hz, 2H), 3.01–3.06 (m, 1H), 3.10 (d, J = 7.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 18.1 (CH3), 18.4 (CH3), 20.9 (CH3), 23.8 (CH2), 25.8 (CH2), 30.0 (CH3), 31.9 (CH2), 32.4 (CH3), 43.7 (CH2), 56.1 (C), 61.9 (CH), 209.0 (C); LRMS (EI) m/z 275 (M+, 0.20%), 219 (19), 137 (29), 97 (27), 95 (20), 83 (12), 81 (19), 73 (12), 71 (16), 70 (12), 69 (16), 59 (12), 57 (45), 56 (10), 55 (21), 43 (100), 41 (22); HRMS (EI-TOF) Calcd for C10H21NO2S [M+-C4H8] 219.1293, found 219.1294.
(7S,RS)-7-Amino-N-(tert-butanesulfinyl)hexadecan-2-one (9d). Following the general procedure, compound 9d was obtained as the major diastereoisomer from sulfinyl imine 5d and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (23 mg, 0.064 mmol, 32%): C20H42NO2S; Rf 0.54 (hexane/EtOAc 1:2); [α]20D −31.9 (c 0.58, CH2Cl2); IR (film) ν 2923, 2854, 1712, 1461, 1361, 1164, 1052, 721 cm−1; 1H NMR (300 MHz, CDCl3) δ 0.86 (t, J = 6.8 Hz, 3H), 1.20 (s, 9H), 1.23–1.31 (br s, 18H), 1.41–1.62 (m, 4H), 2.13 (s, 3H), 2.43 (t, J = 7.23 Hz, 2H), 2.99 (d, J = 6.4 Hz, 1H), 3.14–3.24 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.2 (CH3), 22.8 (CH3), 23.8 (CH2), 25.1 (CH2), 25.8 (CH2), 29.4 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 30.0 (CH3), 32.0 (CH2), 35.5 (CH2), 36.4 (CH2), 43.7 (CH2), 55.8 (C), 56.5 (CH), 209.0 (C); LRMS (EI) m/z 359 (M+, 0.28%), 303 (46), 239 (42), 221 (20), 156 (14), 123 (21), 111 (14), 109 (40), 97 (30), 95 (45), 83 (56), 81 (25), 71 (28), 69 (41), 57 (88), 55 (27), 43 (100), 41 (36); HRMS (EI-TOF) Calcd for C16H33NO2S [M+-C4H9] 303.2232, found 303.2229.
(7R,RS)-7-Amino-N-(tert-butanesulfinyl)hexadecan-2-one (9d′). Following the general procedure, compound 9d′ was obtained as the minor diastereoisomer from sulfinyl imine 5d and 6-chloro-2-methoxyhex-1-ene (2c) as a yellow oil (14 mg, 0.039 mmol, 19%): C20H42NO2S; Rf 0.35 (hexane/EtOAc 1:2); [α]20D −22.4 (c 0.33, CH2Cl2); IR (film) ν 2923, 2857, 1712, 1457, 1226, 1052, 725 cm−1; 1H NMR (300 MHz, CDCl3) δ 0.84–0.91 (m, 3H), 1.21 (s, 9H), 1.24–1.30 (br s, 18H), 1.50–1.62 (m, 4H), 2.13 (s, 3H), 2.45 (t, J = 2.5 Hz, 2H), 2.93 (d, J = 7.1 Hz, 1H), 3.11–3.27 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.2 (CH3), 22.8 (CH3), 23.7 (CH2), 25.3 (CH2), 25.6 (CH2), 29.4 (CH2), 29.6 (CH2), 29.7 (CH2), 29.7 (CH2), 30.0 (CH3), 32.0 (CH2), 36.0 (CH2), 36.4 (CH2), 43.6 (CH2), 55.9 (C), 56.9 (CH), 209.2 (C); LRMS (EI) m/z 359 (M+, 0.06%), 303 (36), 239 (32), 196 (16), 156 (13), 123 (18), 112 (20), 111 (19), 109 (33), 97 (35), 95 (39), 85 (15), 83 (55), 81 (23), 71 (30), 70 (14), 69 (44), 57 (91), 56 (15), 55 (32), 43 (100), 41 (38); HRMS (EI-TOF) Calcd for C16H33NO2S [M+-C4H9] 303.2232, found 303.2238.
3.2.3. Synthesis of Piperidines 11 and Azepanes 13 from N-tert-Butanesulfinyl Amino Ketone Derivatives 7 and 9
General Procedure. To a solution of the corresponding N-tert-butanesulfinyl amino ketone derivative 7 or 9 (0.1 mmol) in methanol (0.42 mL) was added a 2M solution of HCl in Et2O (0.25 mL, 0.5 mmol, 5 equiv) at 0 °C. The reaction mixture was stirred at this temperature for 30 min. After that, solvents were evaporated (15 Torr), and the resulting residue was dissolved in THF (0.17 mL) and citrate-phosphate buffer (0.17 mL). To the resulting mixture, NaCNBH3 (8.2 mg, 0.2 mmol, 2 equiv) was then added in portions at 0 °C, and it was stirred for 15 h at room temperature. After that, the reaction mixture was basified with a 2M aqueous solution of NaOH (0.5 mL), and extracted with CH2Cl2 (5 × 5 mL). The combined organic layers were dried over magnesium sulfate and concentrated under vacuum (15 Torr). The residue was purified by column chromatography (silica gel, hexane/EtOAc) to yield pure products 11 and 13.
(−)-Isosolenopsin A [(2R,6S)-6-Undecyl-2-methylpiperidine (11e)] [33]. Following the general procedure, compound 11e was obtained from N-tert-butanesulfinyl amino ketone derivative 7e as a yellow oil (24.8 mg, 0.098 mmol, 98%): C17H35N; 97:3 dr [GC-MS (temperature program B in Section 3.1): tcis = 12.54 min, ttrans = 12.80 min]; Rf 0.28 (CH2Cl2/MeOH 19:1); [α]20D −5.7 (c 0.27, CH2Cl2) [lit. [33] −3.6 (c 2.5, CHCl3)]; IR (film) ν 2923, 2854, 1319, 1099, 725 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.7 Hz, 3H), 1.09 (d, J = 6.3 Hz, 3H), 1.26 (br s, 20H), 1.32–1.40 (m, 4H), 1.56–1.69 (m, 2H), 1.73–1.79 (m, 1H), 2.45–2.54 (m, 1H), 2.60–2.70 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.3 (CH3), 22.8 (CH2), 23.0 (CH3), 24.9 (CH2), 26.1 (CH2), 29.5 (CH2), 29.7 (CH2), 29.8 (CH2), 29.8 (CH2), 30.0 (CH2), 32.1 (CH2), 32.1 (CH2), 34.3 (CH2), 37.3 (CH2), 58.7 (CH), 57.4 (CH); LRMS (EI) m/z 292 (M+, 2.27%), 238 (4), 99 (7), 98 (100).
(+)-Isosolenopsin A [(2S,6R)-6-Undecyl-2-methylpiperidine (ent-11e)] [33]. Following the general procedure, compound ent-11e was obtained from N-tert-butanesulfinyl amino ketone derivative ent-7e as a yellow oil (25.0 mg, 0.099 mmol, 99%): Physical and spectroscopic data were found to be the same as for 11e. 96:4 dr (GC-MS, tcis and ttrans were found to be the same as for 11e); [α]20D +3.2 (c 0.46, CH2Cl2) [lit. [33] +3.7 (c 2.0, CHCl3)].
(2R,7R)-2-Methyl-7-phenylazepane (13a). Following the general procedure, compound 13a was obtained from N-tert-butanesulfinyl amino ketone derivative 9a as a yellow oil (28.6 mg, 0.099 mmol, 99%): C13H19N; 85:15 dr [GC-MS (temperature program B in Section 3.1): tcis = 10.13 min, ttrans = 10.34 min]; 1:99 er [GC (CP-Chirasil-Dex CB column, Tinlet = 275 °C, Tdetector = 250 °C, Tcolumn = 70 °C and 70–200 °C (4 °C/min), P = 101 kPa): tminor = 30.84 min, tmajor = 30.98 min]; Rf 0.17 (CH2Cl2/MeOH 19:1); [α]20D +20.8 (c 0.54, CH2Cl2); IR (film) ν 3031, 2927, 2861, 1454, 1122, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.11 (d, J = 6.4 Hz, 3H), 1.32–1.41 (m, 1H), 1.63–1.92 (m, 8H), 2.90–3.02 (m, 1H), 3.77 (dd, J = 9.9, 3.7 Hz, 1H), 7.20–7.36 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 24.2 (CH3), 25.3 (CH2), 26.1 (CH2), 38.8 (CH2), 39.6 (CH2), 55.4 (CH), 65.1 (CH), 126.7 (CH), 127.0 (CH), 128.6 (CH), 147.2 (C); LRMS (EI) m/z 189 (M+, 96.8%), 188 (38), 174 (26), 160 (71), 147 (44), 146 (100), 133 (32), 132 (99), 118 (20), 117 (40), 115 (18), 106 (25), 105 (33), 104 (53), 91 (52), 77 (22); HRMS (EI-TOF) Calcd for C13H19N [M+] 189.1517, found 189.1513.
(2S,7S)-2-Methyl-7-phenylazepane (ent-13a). Following the general procedure, compound ent-13a was obtained from N-tert-butanesulfinyl amino ketone derivative ent-9a as a yellow oil (27.4 mg, 0.095 mmol, 95%): Physical and spectroscopic data were found to be the same as for 13a. 84:16 dr (GC-MS, tcis and ttrans were found to be the same as for 13a); 95:5 er [GC (CP-Chirasil-Dex CB column, Tinlet = 275 °C, Tdetector = 250 °C, Tcolumn = 70 °C and 70–200 °C (4 °C/min), P = 101 kPa): tmajor = 30.82 min, tminor = 30.99 min]; [α]20D −25.5 (c 0.45, CH2Cl2).
4. Conclusions
N-tert-Butanesulfinyl δ- and ε-amino ketone derivatives can be accessed in moderate yields and diastereoselectivities from chiral N-tert-butanesulfinyl imines, upon reaction of organolithium compounds derived from 2-methoxy-1-alkenyl chlorides, and final selective acidic hydrolysis of enol ether functionality. These amino ketone derivatives can be in turn used as direct precursors of 2-substituted 6-methylpiperidines, including natural alkaloids (−)-, and (+)-isosolenopsin A, and 2-substituted 7-methylazepanes, in a stereoselective manner.
Supplementary Materials
The following are available online. Copies of 1H-NMR, 13C-NMR, DEPT spectra of compounds 2, 7, 9, 11 and 13. Chiral GC chromatograms of compounds 13a and ent-13a.
Author Contributions
A.S. performed chemical synthesis experiments, analyzed results, and wrote the manuscript. F.F. and M.Y. designed chemical synthesis, analyzed results, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Spanish Ministerio de Economía y Competitividad (MINECO; projects Funding: CTQ2014–53695-P, CTQ2014-51912-REDC, CTQ2016-81797-REDC, CTQ2017-85093-P), Ministerio de Ciencia, Innovación y Universidades (RED2018-102387-T, PID2019-107268GB-100), FEDER, the Generalitat Valenciana (PROMETEOII/2014/017), and the University of Alicante (VIGROB-068).
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Baktharaman, S.; Hili, R.; Yudin, A.K. Amino carbonyl compounds in organic synthesis. Aldrichim. Acta 2008, 41, 109–119. [Google Scholar]
- Allen, L.A.T.; Raclea, R.-C.; Natho, P.; Parsons, P.J. Recent advances in the synthesis of α-amino ketones. Org. Biomol. Chem. 2021, 19, 498–513. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; da Silva, E.N., Jr.; Neto, B.A.D. What do we know about multicomponent reactions? Mechanisms and trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv. 2014, 4, 54282–54299. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhu, B.; Zhu, D.; Han, J.; Wzorek, A.; Sato, A.; Soloshonok, V.A.; Zhou, J.; Pan, Y. Diastereoselective regiodivergent Mannich versus tandem Mannich-cyclization reactions. Adv. Synth. Catal. 2017, 359, 4267–4273. [Google Scholar] [CrossRef]
- Khandare, S.P.; Reddy, P.O.; Prasad, K.R. Addition of lithium anion of (acetylmethylene)triphenylphosphorane to nonracemic sulfinimines: Total synthesis of (+)-241D and formal total synthesis of (+)-preussin. Org. Lett. 2020, 22, 7273–7277. [Google Scholar] [CrossRef]
- Mazzeo, G.; Longhi, G.; Abbate, S.; Mangiavacchi, F.; Santi, C.; Han, J.; Soloshonok, V.A.; Melensi, L.; Ruzziconi, R. Mannich-type addition of 1,3-dicarbonyl compounds to chiral tert-butanesulfinyltrifluoro-acetaldimines. Mechanistic aspects and chiroptical studies. Org. Biomol. Chem. 2018, 16, 8742–8750. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Xu, D. Asymmetric synthesis of β-amino ketones by using cinchona alkaloid-based chiral phase transfer catalysts. Org. Biomol. Chem. 2018, 16, 8704–8709. [Google Scholar] [CrossRef]
- Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constantieux, T. Metal-free multicomponent syntheses of pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef] [PubMed]
- Cayuelas, A.; Serrano, L.; Najera, C.; Sansano, J.M. Synthesis of α,β-diamino acid derivatives via asymmetric Mannich reactions of glycine imino esters catalyzed by a chiral phosphoramidite·silver complex. Tetrahedron Asymmetry 2014, 25, 1647–1653. [Google Scholar] [CrossRef]
- Ishitani, H.; Suzuki, H.; Saito, Y.; Yamashita, Y.; Kobayashi, S. Hafnium trifluoromethanesulfonate [Hf(OTf)4] as a unique Lewis acid in organic synthesis. Eur. J. Org. Chem. 2015, 5485–5499. [Google Scholar] [CrossRef]
- Decostanzi, M.; Campagne, J.-M.; Leclerc, E. Fluorinated enol ethers: Their synthesis and reactivity. Org. Biomol. Chem. 2015, 13, 7351–7380. [Google Scholar] [CrossRef]
- Ji, X.; Huang, H. Synthetic methods for 1,3-diamines. Org. Biomol. Chem. 2016, 14, 10557–10566. [Google Scholar] [CrossRef]
- Vargas-Caporali, J.; Juaristi, E. The diamino analogues of privileged Corey–Bakshi–Shibata and Jørgensen–Hayashi catalysts: A comparison of their performance. Synthesis 2016, 48, 3890–3906. [Google Scholar] [CrossRef]
- Lin, G.-Q.; Xu, M.-H.; Zhong, Y.-W.; Sun, X.-W. An advance on exploring N-tert-butanesulfinyl imines in asymmetric synthesis of chiral amines. Acc. Chem. Res. 2008, 41, 831–840. [Google Scholar] [CrossRef]
- Ferreira, F.; Botuha, C.; Chemla, F.; Pérez-Luna, A. tert-Butanesulfinimines: Structure, synthesis and synthetic applications. Chem. Soc. Rev. 2009, 38, 1162–1186. [Google Scholar] [CrossRef]
- Robak, M.A.T.; Herbage, M.A.; Ellman, J.A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, M.J.; Dema, H.K.; Foubelo, F.; Yus, M. Base-promoted diastereoselective addition of nitromethane and nitroethane to N-tert-butylsulfinyl imines: Synthesis of N-protected α-amino acids and amino ketones. Tetrahedron Asymmetry 2014, 25, 362–372. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Foubelo, F.; Yus, M. Tandem enantioselective conjugate addition-Mannich reactions: Efficient multicomponent assembly of dialkylzincs, cyclic enones and chiral N-sulfinimines. Tetrahedron Lett. 2008, 49, 2343–2347. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Foubelo, F.; Yus, M. Stereocontrolled synthesis of 1,3-amino alcohols by reduction of substituted 2-1-[tert-butylsulfinyl)amino]alkylcyclohexanones. Synthesis 2009, 12, 2083–2088. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Foubelo, F.; Yus, M. Modular stereocontrolled assembly of R2Zn, cyclic enones and N-tert-butylsulfinyl imines. J. Org. Chem. 2009, 74, 2547–2553. [Google Scholar] [CrossRef]
- Lahosa, A.; Soler, T.; Arrieta, A.; Cossio, F.P.; Foubelo, F.; Yus, M. Stereoselective coupling of N-tert-butanesulfinyl aldimines and β-keto acids: Access to β-amino ketones. J. Org. Chem. 2017, 82, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Lahosa, A.; Yus, M.; Foubelo, F. Enantiodivergent approach to the synthesis of cis-2,6-disubstituted piperidin-4-ones. J. Org. Chem. 2019, 84, 7331–7341. [Google Scholar] [CrossRef] [PubMed]
- Nájera, C.; Yus, M. Functionalized organolithium compounds: New synthetic adventures. Curr. Org. Chem. 2003, 7, 867–926. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C.; Yus, M. Metalated heterocycles and their applications in synthetic organic chemistry. Chem. Rev. 2004, 104, 2667–2722. [Google Scholar] [CrossRef] [PubMed]
- Foubelo, F.; Abou, A.; Yus, M. Chemoselective lithiation of 1-bromo-n-chloroalkanes. Eur. J. Org. Chem. 2005, 5089–5093. [Google Scholar] [CrossRef]
- Hudrlik, P.F.; Hudrlik, A.M. Enol acetates, enol ethers, and amines by mercuration of acetylenes. J. Org. Chem. 1973, 38, 4254–4258. [Google Scholar] [CrossRef]
- Plobeck, N.; Powell, D. Asymmetric synthesis of diarylmethylamines by diastereoselective addition of organometallic reagents to chiral N-tert-butanesulfinimines: Switchover of diastereofacial selectivity. Tetrahedron Asymmetry 2002, 13, 303–310. [Google Scholar] [CrossRef]
- García, D.; Moreno, B.; Soler, T.; Foubelo, F.; Yus, M. Stereoselective synthesis of 3-substituted tetrahydroisoquinolines from phthalan and chiral N-sulfinylimines. Tetrahedron Lett. 2009, 50, 4710–4713. [Google Scholar] [CrossRef]
- García, D.; Foubelo, F.; Yus, M. Reductive ring-opening of phthalan and isochroman: Application to the stereoselective synthesis of tetrahydroisoquinolines and tetrahydrobenzazepines. Eur. J. Org. Chem. 2010, 2893–2903. [Google Scholar] [CrossRef]
- Sassian, M.; Panov, D.; Tuulmets, A. Grignard reagents in toluene solutions. Appl. Organomet. Chem. 2002, 16, 525–529. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Foubelo, F.; Yus, M. Cross-metathesis of chiral N-tert-butylsulfinyl homoallylamines: Application to the enantioselective synthesis of naturally occurring 2,6-cis-disubstituted piperidines. Synlett 2008, 18, 2777–2780. [Google Scholar] [CrossRef]
- Medjahdi, M.; González-Gómez, J.C.; Foubelo, F.; Yus, M. Enantioselective synthesis of cis- and trans-2-methyl-6-nonylpiperidines: Alkaloids solenopsin and isosolenopsin. Heterocycles 2012, 86, 727–734. [Google Scholar]
- Bandara Herath, H.M.T.; Dhammika Nanayakkara, N.P. Synthesis of enantiomerically pure fire ant venom alkaloids: Solenopsins and isosolenopsins A, B and C. J. Heterocycl. Chem. 2008, 45, 129–136. [Google Scholar] [CrossRef]
- Jones, T.H.; Blum, M.S.; Fales, H.M. Ant venom alkaloids from Solenopsis and Monorium species. Tetrahedron 1982, 38, 1949–1958. [Google Scholar] [CrossRef]
- Leclercq, S.; Thirionet, I.; Broeders, F.; Daloze, D.; Vander Meer, R.; Braekman, J.C. Absolute configuration of the solenopsins, venom alkaloids of the fire ants. Tetrahedron 1994, 50, 8465–8478. [Google Scholar] [CrossRef]
- Liu, G.; Cogan, D.A.; Owens, T.D.; Tang, T.P.; Ellman, J.A. Synthesis of enantiomerically pure N-tert-butanesulfinyl imines (tert-butanesulfinimines) by the direct condensation of tert-butanesulfinamide with aldehydes and ketones. J. Org. Chem. 1999, 64, 1278–1284. [Google Scholar] [CrossRef]
- Schenkel, L.B.; Ellman, J.A. Self-condensation of N-tert-butanesulfinyl aldimines: Application to the rapid asymmetric synthesis of biologically important amine-containing compounds. Org. Lett. 2004, 6, 3621–3624. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.B.; Wolfe, J.P. A concise stereoselective synthesis of preussin, 3-epi-preussin, and analogues. Org. Lett. 2006, 8, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, C.Q.; Conroy, M.; Light, M.; Poisson, T.; Pannecoucke, X.; Linclau, B. Stereoselectivity of the Honda–Reformatsky reaction in reactions with ethyl bromodifluoroacetate with α-oxygenated sulfinylimines. J. Org. Chem. 2014, 79, 4186–4195. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).