Antioxidant and Antimicrobial Evaluation and Chemical Investigation of Rosa gallica var. aegyptiaca Leaf Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative Phytochemical Screening
2.2. Extraction Yields
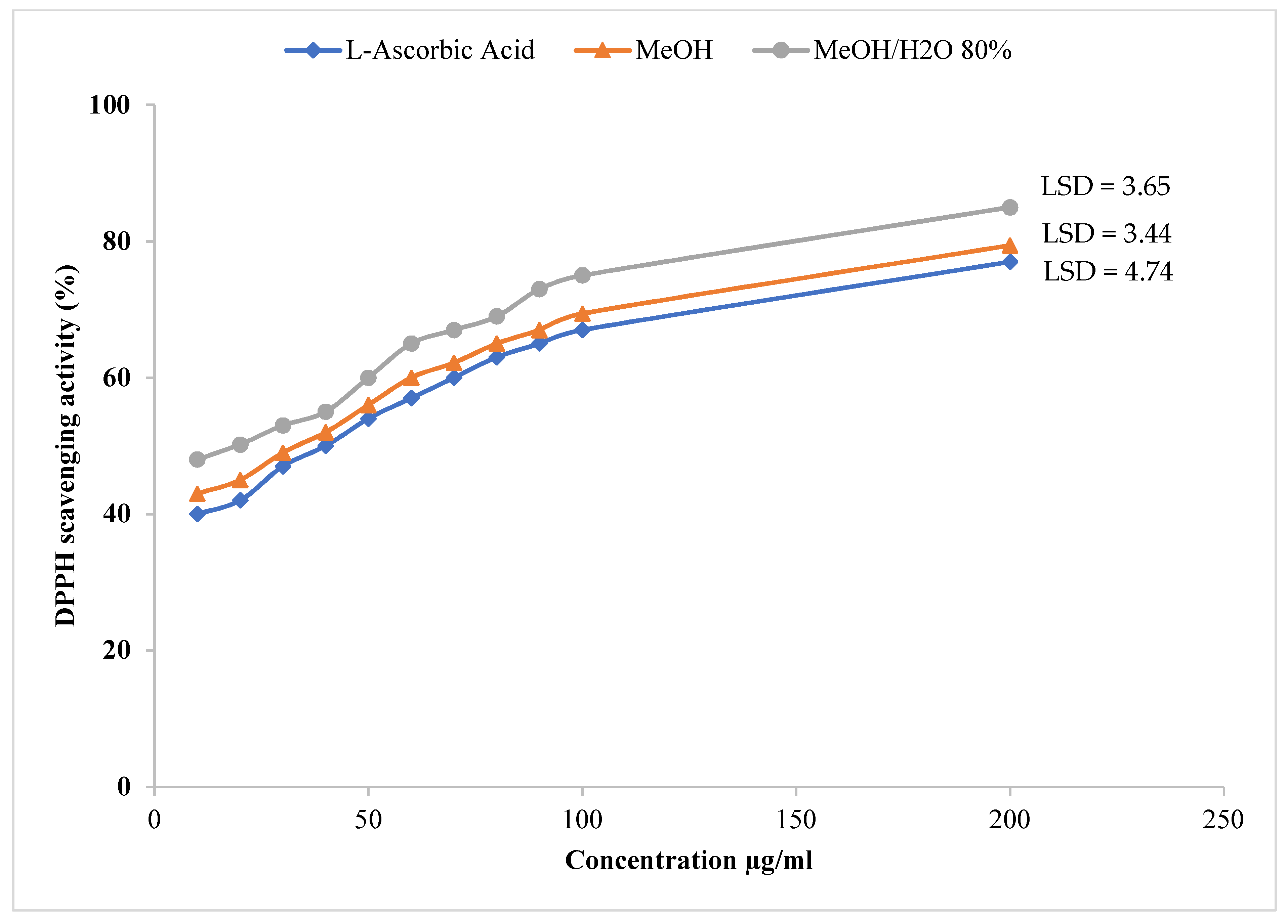
2.3. Antioxidant Activity via DPPH Free Radical Scavenging Assay
2.4. Total Phenolic and Flavonoid Contents
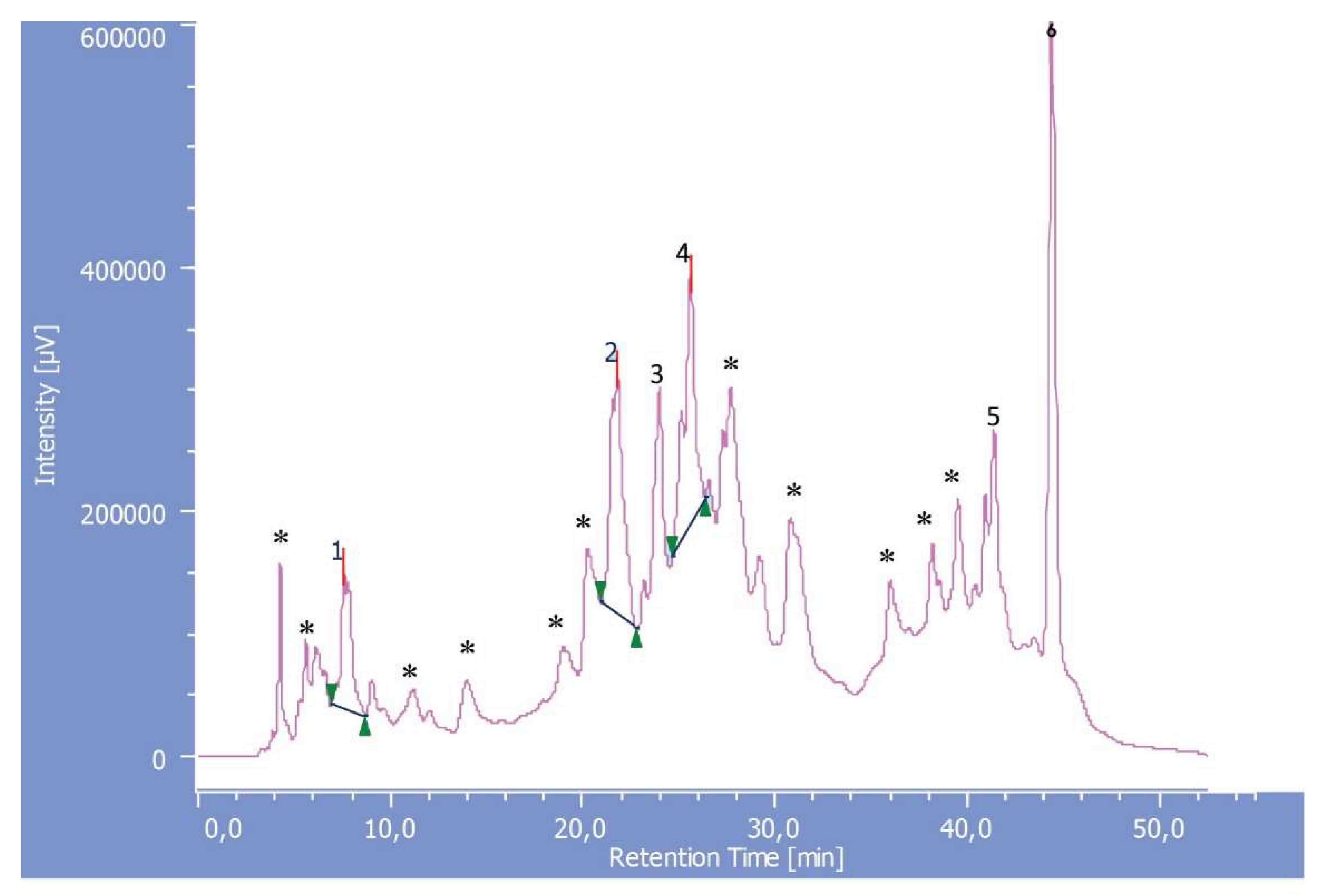
2.5. HPLC-DAD Analysis
2.6. Antimicrobial Activity
2.7. Antimicrobial Activity of the Identified Phenolic Compounds from MeOH/H2O 80% Extract of R. gallica var. aegyptiaca Leaves
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Qualitative Phytochemical Screening
3.5. DPPH Radical Scavenging Activity
3.6. Total Phenolic Content Determination (TPC)
3.7. Total Flavonoid Content Determination (TFC)
3.8. HPLC-DAD Analysis
3.9. Antimicrobial Activity
3.9.1. Microbial Strains
3.9.2. Inoculums Preparation
3.9.3. Antimicrobial Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stanković, N.; Mihajilov-Krstev, T.; Zlatković, B.; Stankov-Jovanović, V.; Mitić, V.; Jović, J.; Bernstein, N. Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS-Wagening J. Life Sci. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Tortosa, V.; Pietropaolo, V.; Brandi, V.; Macari, G.; Pasquadibisceglie, A.; Polticelli, F. Computational Methods for the Identification of Molecular Targets of Toxic Food Additives. Butylated Hydroxytoluene as a Case Study. Molecules 2020, 25, 2229. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Lim, W.; You, S.; Song, G. Butylated hydroxyanisole induces testicular dysfunction in mouse testis cells by dysregulating calcium homeostasis and stimulating endoplasmic reticulum stress. Sci. Total Environ. 2020, 702, 134775. [Google Scholar] [CrossRef]
- Ham, J.; Lim, W.; Whang, K.Y.; Song, G. Butylated hydroxytoluene induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in mouse Leydig cell death. Environ. Pollut. 2020, 256, 113421. [Google Scholar] [CrossRef] [PubMed]
- Eskandani, M.; Hamishehkar, H.; Ezzati Nazhad Dolatabadi, J. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014, 153, 315–320. [Google Scholar] [CrossRef]
- Sapkota, R.; Dasgupta, R.; Nancy, D.S. Antibacterial effects of plants extracts on human microbial pathogens & microbial limit tests. Int. J. Res. Pharm. Chem. 2012, 2, 926–936. [Google Scholar]
- Silva, M.M.; Lidon, F. Food preservatives. An overview on applications and side effects. Emirates J. Food Agric. 2016, 26, 366–373. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P. Antibacterial activity of Syzygium aromaticum (Clove) with metal ion effect against food borne pathogens. Asian J. Plant Sci. Res. 2011, 1, 69–80. [Google Scholar]
- Braga, L.C.; Shupp, J.W.; Cummings, C.; Jett, M.; Takahashi, J.A.; Carmo, L.S. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J. Ethnopharmacol. 2005, 96, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Celiktas, O.Y.; Kocabas, E.E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus oficinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull. Natl. Res. Cent. 2019, 43, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [Green Version]
- Brown, D. New Encyclopedia of Herbs & Their Uses, 1st ed.; Dorling Kindersley: London, UK, 2002; pp. 346–347. [Google Scholar]
- Bitis, L.; Sen, A.; Ozsoy, N.; Birteksoz-Tan, S.; Kultur, S.; Melikoglu, G. Flavonoids and biological activities of various extracts from Rosa sempervirens leaves. Biotechnol. Biotechnol. Equip. 2017, 31, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Caliskan, U.K.; Aka, C.; Oz, M.G. Plants used in anatolian traditional medicine for the treatment of hemorrhoid. Rec. Nat. Prod. 2017, 11, 235–250. [Google Scholar]
- Lattanzio, F.; Greco, E.; Carretta, D.; Cervellati, R.; Govoni, P.; Speroni, E. In vivo anti-inflammatory effect of Rosa canina L. extract. J. Ethnopharmacol. 2011, 137, 880–885. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Willich, S.N.; Rossnagel, K.; Roll, S.; Wagner, A.; Mune, O.; Erlendson, J.; Kharazmi, A.; Sörensen, H.; Winther, K. Rose hip herbal remedy in patients with rheumatoid arthritis—A randomized controlled trial. Phytomedicine 2010, 17, 87–93. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yold, M.J. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef] [PubMed]
- Knapp, H.; Straubinger, M.; Fornari, S.; Oka, N.; Watanabe, N.; Winterhalter, P. (S)-3,7-dimethyl-5-octene-1,7-diol and related oxygenated monoterpenoids from petals of Rosa damascena Mill. J. Agric. Food Chem. 1998, 46, 1966–1970. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Gupta, A.P.; Kaul, V.K. Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J. Sep. Sci. 2008, 31, 262–267. [Google Scholar] [CrossRef]
- Oka, N.; Ikegami, A.; Ohki, M.; Sakata, K.; Yagi, A.; Watanabe, N. Citronellyl disaccharide glycoside as an aroma precursor from rose flowers. Phytochemistry 1998, 47, 1527–1529. [Google Scholar] [CrossRef]
- Bitis, L.; Kultur, S.; Melikoglu, G. Flavonoids and antioxidant activity of Rosa agrestis leaves. Nat. Prod. Res. 2010, 24, 580–589. [Google Scholar] [CrossRef]
- Mileva, M.M.; Kusovski, V.K.; Krastev, D.S. Chemical composition, in vitro antiradical and antimicrobial activities of Bulgarian Rosa alba L. essential oil against some oral pathogens. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 11–20. [Google Scholar]
- Mileva, M.; Krumova, E.; Miteva-Staleva, J. Chemical compounds, in vitro antioxidant and antifungal activities of some plant essential oils belonging to Rosaceae family. C. R. Acad. Bulg. Sci. 2014, 67, 1363–1368. [Google Scholar]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa, L. leaves extracts and their radical scavenging activity. Z. Nat. 2007, 62c, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and antioxidant activity of extracts from rose fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef] [Green Version]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Sudjaroen, Y.; Haubner, R.; Würtele, G.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Changbumrunga, S.; Bartschb, H.; Owen, R.W. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. 2005, 43, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.M.; Emam, A.M.; Diab, Y.M.; Mahmoud, M.E.; Mahmoud, A.S. Evaluation of antioxidant potential of 124 Egyptian plants with emphasis on the action of Punica granatum leaf extract on rats. Int. Food Res. J. 2011, 18, 532–539. [Google Scholar]
- Dehghan, K.A.; Rasooli, I.; Rezaee, M.B.; Owlia, P. Antioxidative properties and toxicity of white rose extract. Iranian J. Toxicol. 2011, 5, 415–425. [Google Scholar]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Ozkan, G.; Sagdic, O.; Baydar, N.G.; Baydar, H.A.S.A.N. Antioxidant and antibacterial activities of Rosa damascena flower extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Peschel, W.; Sanchez-Rabaneda, F.; Dn, W.P.A.; Gartzia, I.; Jimenez, D.; Lamuela-Raventos, R.M.; Buxaderas, S.; Condina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Tatke, P.; Satyapal, U.S.; Mahajan, D.C.; Naharwar, V. Phytochemical Analysis, In-Vitro Antioxidant and Antimicrobial Activities of Flower Petals of Rosa damascena. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 246–250. [Google Scholar]
- Zieliński, H.; Achremowicz, B.; Przygodzka, M. Antioxidants of cereal grains. Żywność. Nauka Technol. Jakość. 2012, 1, 5–26. [Google Scholar]
- Giada, M.D.L.R. Food phenolic compounds: Main classes, sources and their antioxidant power. Oxid. Stress Chronic Degener. Dis. 2013, 2013, 87–112. [Google Scholar]
- Sang, S.; Lapsley, K.; Jeong, W.S.; Lachance, P.A.; Ho, C.T.; Rosen, R.T.J. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.M.; Gonzáles-Aguilar, G.A. Identification and quantification of major phenolic compounds (Mangifera indica, cv. Ataulfo) fruit by HPLC-DAD-MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharm. Rev. 2016, 10, 84. [Google Scholar]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Rezaee, R. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Cho, W.C. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Halawani, E.M. Antimicrobial activity of Rosa damascena petals extracts and chemical composition by gas chromatography-mass spectrometry (GC/MS) analysis. Afr. J. Microbiol. Res. 2014, 8, 2359–2367. [Google Scholar]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253. [Google Scholar]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Harbone, J.B. Phytochemical Methods; Chapman and Hall: London, UK, 1998; pp. 117–119. [Google Scholar]
- Farnsworth, N.R. Biological and phytochemical screening of plants. J. Pharm. Sci. 1966, 55, 225–276. [Google Scholar] [CrossRef] [PubMed]
- Rangari, V.D. Pharmacognosy and Phytochemistry; Carrier Publication: Nashik, Indian, 2002; p. 132. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wissenchaft und Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Lamaison, J.L.C.; Carnet, A. Teneurs en principaux flavonoides des fleurs de Crataegus monogyna Jacq et de Crataegus Laevigata (Poiret, D.C) en fonction de la vegetation pharmaceut. Acta Helve. 1990, 65, 315–320. [Google Scholar]
- Bauer, A.W.; Kirby, M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Amr. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
| Constituent | Detection Test | Result |
|---|---|---|
| Saponins | Foam test | + |
| Steroids | Liebermann–Burchard test | – |
| Triterpenoids | Salkowski reaction | + |
| Phenolic compounds and tannins | Ferric chloride test | + |
| Flavonoids | Lead acetate test | + |
| Alkaloids | Wagner’s tests | + |
| Glycosides | Keller–Kiliani test | + |
| Carbohydrates | Molisch’s test | + |
| Extract | Extract Yield (%) | Inhibition Percentage (50 µg/mL) | IC50 (µg/mL) | TPs (mg GAE g/g Extract) | TFs (mg RE r/g Extract) |
|---|---|---|---|---|---|
| C6H14 | 1.6 | 20 ± 1.0 c | 20.00 ± 2.46 e | 5.300 ± 1.25 d | |
| CHCl3 | 6.5 | 15 ± 85 d | 25.00 ± 3.10 d | 6.500 ± 1.50 c | |
| MeOH | 9.2 | 95.08 ± 0.33 b | 20.28 ± 0.97 b | 181.6 ± 0.83 b | 54.48 ± 1.79 a |
| MeOH/H2O 80% | 9.9 | 97.20 ± 0.25 a | 19.38 ± 0.85 a | 253.8 ± 1.26 a | 41.02 ± 1.55 b |
| H2O | 4.1 | 10 ± 1.1 e | 50.83 ± 1.25 c | 1.700 ± 0.22 e | |
| L-Ascorbic Acid | 21.30 ± 0.55 c |
| Peak | Rt (min) | Compound | mg/g |
|---|---|---|---|
| 1 | 7.56 | Gallic acid | 1.7 |
| 2 | 21.77 | Catechin | 2.9 |
| 3 | 23.68 | Chlorogenic acid | 1.8 |
| 4 | 25.56 | Epicatechin | 2.6 |
| 5 | 41.30 | quercetin-3-O-α-d-(glucopyranoside) | 0.5 |
| 6 | 44.33 | Quercetin | 19.8 |
| Extract | Antimicrobial Activity | |||||
|---|---|---|---|---|---|---|
| Diameter of Inhibition Zones (mm) | ||||||
| Gram (+) Pathogenic Bacteria | Gram (–) Pathogenic Bacteria | Fungi | ||||
| L. monocytogenes | B. subtilis | S. aureus | E. coli | S. enteritidis | C. albicans | |
| C6H14 | - | - | - | 13 ± 1.53 f | - | - |
| CHCl3 | - | - | - | 16 ± 1.00 d | - | - |
| MeOH | 16 ± 2.00 ab | 19 ± 1.00 b | 17 ± 1.15 b | 24 ± 1.15 ab | 19 ± 1.00 a | 10 ± 1.10 c |
| MeOH/H2O 80% | 17 ± 0.58 a | 20 ± 0.58 a | 17 ± 1.53 b | 25 ± 2.52 a | 19 ± 1.00 a | 11 ± 1.50 b |
| H2O | 12 ± 0.76 c | 12 ± 1.73 c | 19 ± 0.58 a | 20 ± 2.00 c | 18 ± 1.15 b | - |
| Gentamycin (10 mg) | 15 ± 1.00 b | 12 ± 1.15 c | 16 ± 0.58 c | 15 ± 2.08 e | 13 ± 1.00 c | n.d. |
| Fluconazole (10 mg) | n.d. | n.d. | n.d. | n.d. | n.d. | 14 ± 1.05 a |
| Compounds | Antimicrobial Activity | |||||
|---|---|---|---|---|---|---|
| Diameter of Inhibition Zones (mm) | ||||||
| Gram (+) Pathogenic Bacteria | Gram (–) Pathogenic Bacteria | Fungi | ||||
| L. monocytogenes | B. subtilis | S. aureus | E. coli | S. enteritidis | C. albicans | |
| Gallic acid | 17 ± 1.10 b | 16 ± 1.13 c | 18 ± 1.57 c | 20 ± 0.45 b | 18 ± 1.30 b | 11 ± 1.10 e |
| Catechin | 16 ± 0.95 c | 18 ± 1.50 b | 16 ± 0.20 e | 17 ± 1.16 c | 15 ± 0.56 c | 10 ± 1.35 ef |
| Chlorogenic acid | 15 ± 0.35 d | 15 ± 0.50 cd | 17 ± 0.13 d | 16 ± 0.95 d | 14 ± 1.65 d | 11 ± 1.00 e |
| Epicatechin | 16 ± 0.55 c | 14 ± 1.02 d | 17 ± 0.50 d | 15 ± 1.40 e | 14 ± 0.33 d | 12 ± 0.85 d |
| Quercetin-3-glucoside | 17 ± 1.02 b | 18 ± 0.40 b | 20 ± 1.16 b | 19 ± 1.22 bc | 17 ± 0.57 bc | 15 ± 1.15 b |
| Quercetin | 28 ± 0.57 a | 25 ± 0.87 a | 24 ± 1.12 a | 30 ± 1.18 a | 26 ± 0.56 a | 17 ± 0.46 a |
| Gentamycin (10 µg) | 15 ± 1.00 d | 12 ± 1.15 e | 16 ± 0.58 e | 15 ± 2.08 e | 13 ± 1.00 e | n.d. |
| Fluconazole (10 µg) | n.d. | n.d. | n.d. | n.d. | n.d. | 14 ± 1.05 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelbaky, A.S.; Mohamed, A.M.H.A.; Alharthi, S.S. Antioxidant and Antimicrobial Evaluation and Chemical Investigation of Rosa gallica var. aegyptiaca Leaf Extracts. Molecules 2021, 26, 6498. https://doi.org/10.3390/molecules26216498
Abdelbaky AS, Mohamed AMHA, Alharthi SS. Antioxidant and Antimicrobial Evaluation and Chemical Investigation of Rosa gallica var. aegyptiaca Leaf Extracts. Molecules. 2021; 26(21):6498. https://doi.org/10.3390/molecules26216498
Chicago/Turabian StyleAbdelbaky, Ahmed S., Abir M. H. A. Mohamed, and Salman S. Alharthi. 2021. "Antioxidant and Antimicrobial Evaluation and Chemical Investigation of Rosa gallica var. aegyptiaca Leaf Extracts" Molecules 26, no. 21: 6498. https://doi.org/10.3390/molecules26216498
APA StyleAbdelbaky, A. S., Mohamed, A. M. H. A., & Alharthi, S. S. (2021). Antioxidant and Antimicrobial Evaluation and Chemical Investigation of Rosa gallica var. aegyptiaca Leaf Extracts. Molecules, 26(21), 6498. https://doi.org/10.3390/molecules26216498







