1. Introduction
Over the past few decades, a variety of functional nanomaterials with intrinsic enzyme mimetic activities have been explored, owing to the high stability, low cost, convenient storage, and easy synthesis [
1,
2,
3,
4,
5,
6,
7,
8]. One of the extensive applications and promising developments for nanozymes is in the biocomputing system, which makes noteworthy progress in the logic gate operations that follow the laws of Boolean algebra [
9,
10,
11,
12,
13,
14]. Recently, Boolean algebra has been widely used in the fields of engineering technology and occupies an irreplaceable position. Additionally, according to the presence of input and output signals generated from biological molecules, the Boolean logic gates are mainly classified into AND, OR, XOR, NAND, NOR, INHIBIT, half-adder, and half-subtractor [
9,
15,
16,
17,
18]. Owing to the demand for computer miniaturization, Boolean logic systems have been implemented at the molecular levels. In principle, logic gates are realized based on molecular switching or chemical reaction. Currently, the merging of nanotechnology with biology has ignited extensive research efforts for the design of logic gates and computer components based on nanozymes. Despite the tremendous opportunities and advantages, the nanozyme-based logic systems often suffer from the shortcomings of the difficulties in constructing complex logic systems, the reset of logic systems to the original state, and the limitation of the variety of nanozymes, etc. Therefore, a better understanding of the mechanism and design of the next-generation molecular logic systems has become urgent for further development.
On the other hand, great progress has been made in the construction of logic gates and biochemical computation based on DNA [
19,
20,
21], enzymes [
16,
22,
23,
24,
25], or complex biochemical systems [
26]. For example, like computer devices, the use of natural enzymes for the construction of different Boolean logic systems has aroused people’s wide concern due to a huge number of enzyme-based biocatalytic processes in living organisms (plants, animals, microorganisms) [
25]. Though tremendous accomplishments have been made, many of them rely on sophisticated instruments to monitor the output signals, which resulted in complicated test steps and high costs during detection. In this paper, by engineering the stability of a nanozyme-catalyzed product oxTMB through enzymatic reactions, colorimetric logic gate operations can be easily realized and output signals can be readily observed by the naked eye. In our logic systems, glucose oxidase (GOx), β-galactosidase (β-Gal), and xanthine oxidase (XO) were used as inputs, and the state of oxTMB solution was used as an output. The blue-colored solution of oxTMB obtained by the catalysis of nanozymes could become colorless by reductants, including the reduced transition state of natural enzymes (
Figure 1). For instance, GOx, with flavin adenine dinucleotide (FAD) as a redox prosthetic group, is one of the typical flavin enzymes. Its biological function is to catalyze glucose to form gluconolactone while the enzyme itself is turned from GOx(FAD) to GOx(FADH2) [
27,
28,
29]. The transition state of GOx(FADH2) can recover to GOx(FAD) again in the presence of O
2 as indicated in Equation (1).
The transition state of GOx(FADH2) can also result in the reduction of oxTMB into colorless TMB (
Figure 1). The obvious color change of TMB and oxTMB can be used as visual output signals. By the combination of enzymatic reactions, different colorimetric logic gate operations are easily obtained. Moreover, by taking advantage of the attractive properties of natural enzymes, simultaneous operation of several concatenated logic gates is allowed in the same chemical environment without any interference and “cross-talk” between them [
25]. Furthermore, the reducibility of FADH2 is firstly introduced as a molecular switching and may provide an example for designing more complex logic systems. In light of that, we expect that this biomolecular logic system with evident and fast output signals is conducive to the area of biosensors, environmental chemistry, and therapeutics sensing.
2. Results
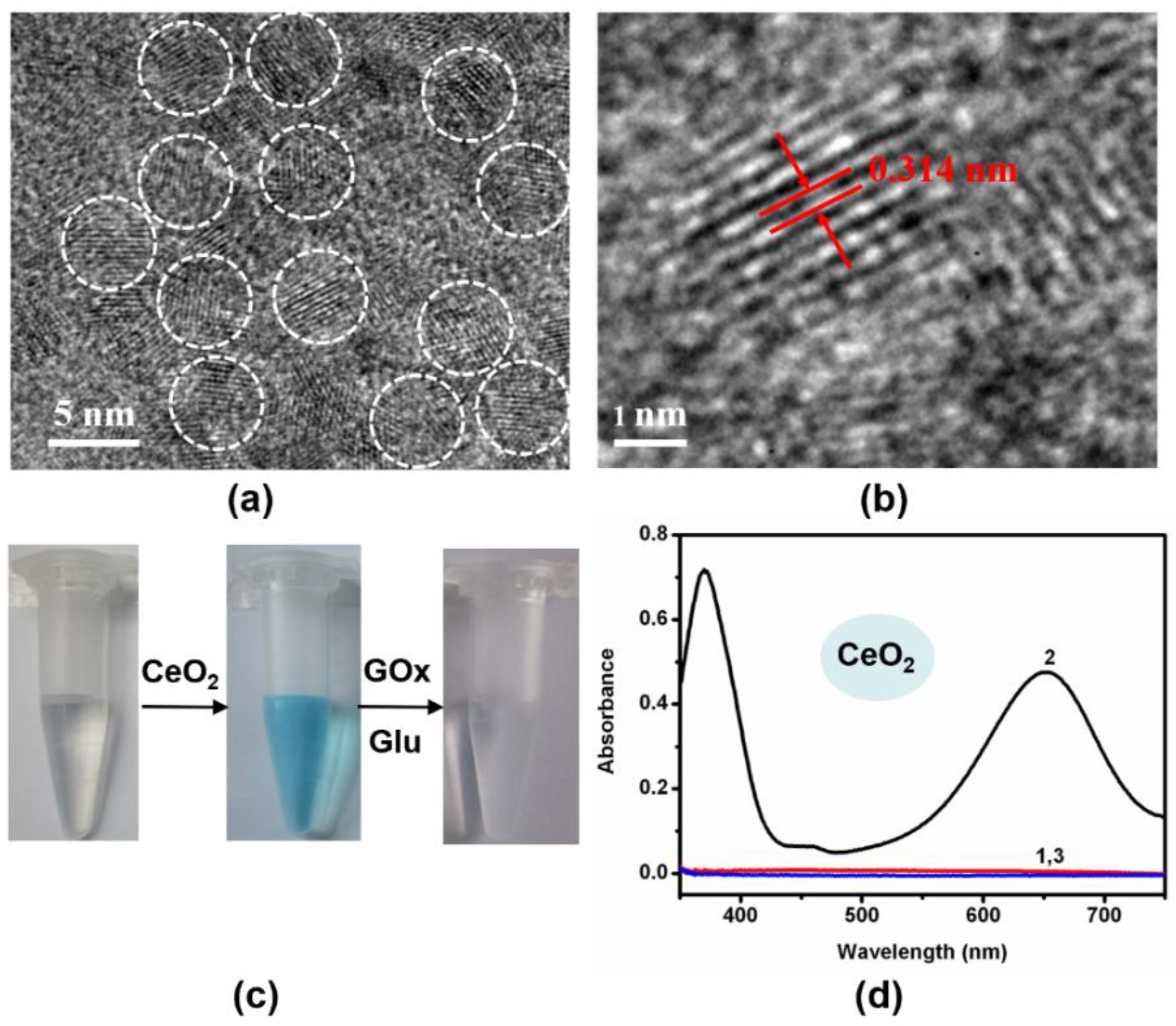
To implement the logic gates, CeO
2 and Fe
3O
4 nanoparticles (NPs) were firstly synthesized and studied by transmission electron microscopy (TEM) imaging. The representative TEM images showed the well-dispersed CeO
2 NPs and Fe
3O
4 NPs with spherical shapes were prepared (
Figure 2a,b and
Figure S1, Supplementary Materials). Additionally, CeO
2 NPs with a small size and preferred plane (111) on the surface were observed (
Figure S2). Additionally, the as-prepared materials were also characterized by TEM (
Figure S2). The solution of the TMB substrate was almost colorless. The colorless TMB can be oxidized to a blue-colored product (oxTMB) by both CeO
2- and Fe
3O
4-based nanozymes. Due to the oxidase-like activity of CeO
2 NPs, the addition of CeO
2 NPs into the above solution resulted in a color change from colorless to blue (
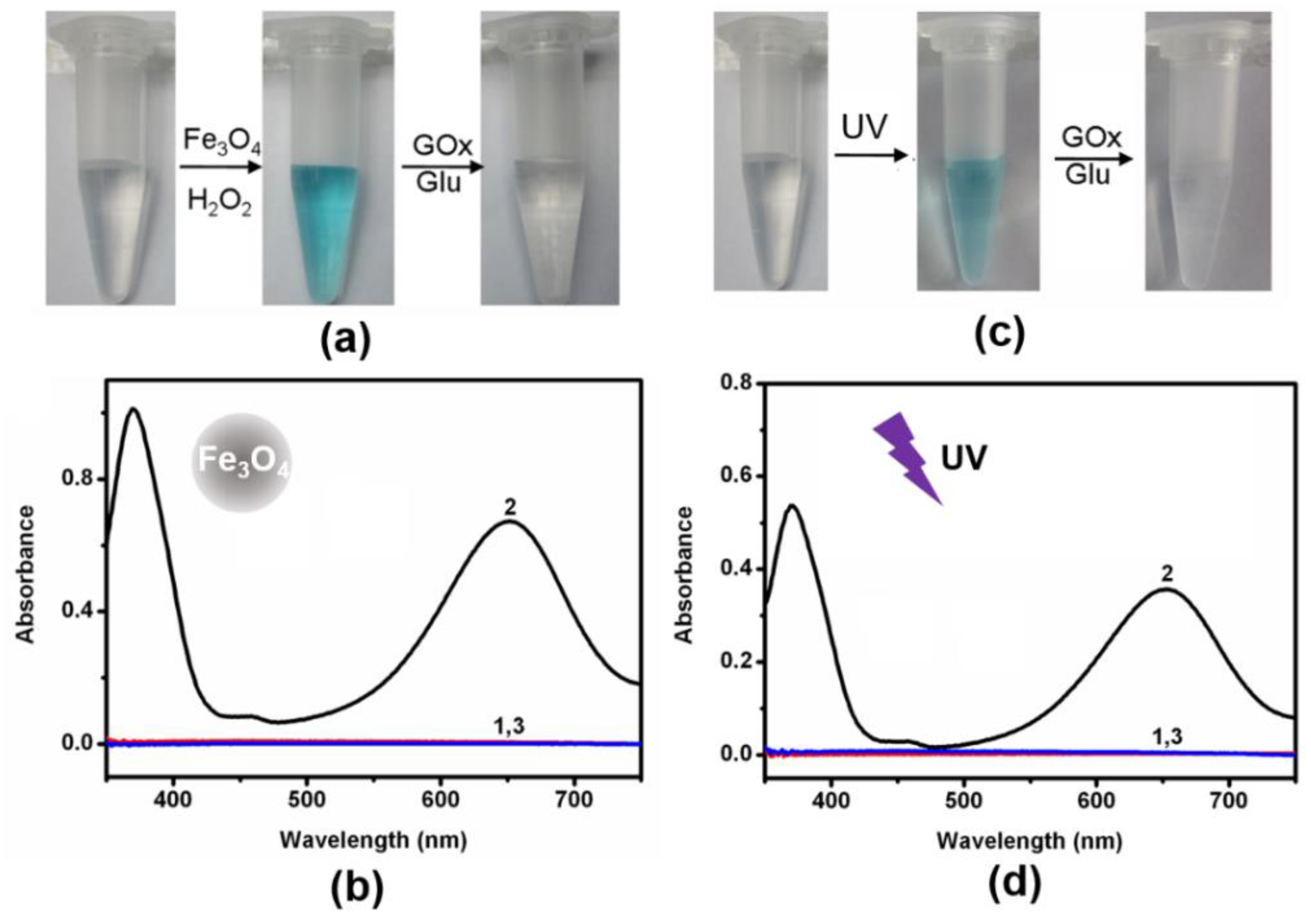
Figure 2c,d). According to previous reports, Fe
3O
4 is a typical peroxidase mimic, which can catalyze the oxidation of TMB in the presence of H
2O
2 (
Figure 3a,b)
. In addition, ultraviolet light can also convert TMB to oxTMB (
Figure 3c,d). It should be noted that the pH value was crucial to the oxidation reaction ofTMB. Both nanozymes showed the best catalytic performance at about pH 4.0. Since the optimal pH of natural enzymes is under physiological conditions, our logic operations for enzyme/nanozyme hybrid systems were carried out under the weak acid condition (pH 6.0). Interestingly, all the produced oxTMB solutions obtained in different ways were turned back to colorless TMB by adding glucose oxidase (GOx) and glucose (
Figure 2c and
Figure 3a,c). In contrast, the system containing only glucose or GOx did not show an apparent absorbance reduction toward oxTMB (
Figure 4a). Furthermore, other saccharides (lactose, sucrose, maltose, and fructose) were also used to examine whether they could replace glucose.
Figure 4b and
Figure S4 showed, upon introduction of different saccharides into a solution containing oxTMB and GOx, no obvious color changes. We guessed that the co-existence of glucose and GOx would produce the reductive transition state (FADH2), which could reduce oxTMB to TMB. To further verify the above assumption, we added reductive substances (NaBH
4 and cysteine) to the solutions containing oxTMB, and the same results were obtained (
Figure 4c). Meanwhile, the absorption spectra of TMB reaction solutions under different conditions was obtained (
Figure 2d and
Figure 3b,d). Moreover, we also used another enzyme substrate 2, 2’-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS). As a result, both CeO
2 NPs and Fe
3O
4 NPs/H
2O
2 systems with ultraviolet light showed a powerful ability to catalyze ABTS to produce green color, and could be further reset to their initial conditions by adding glucose/GOx (
Figure S5). Taking the reversible optical response of TMB into account, the colorless solution and blue solution could be defined as OFF and ON states, respectively.
The above results certified that the state of oxTMB was dependent on the participation of O
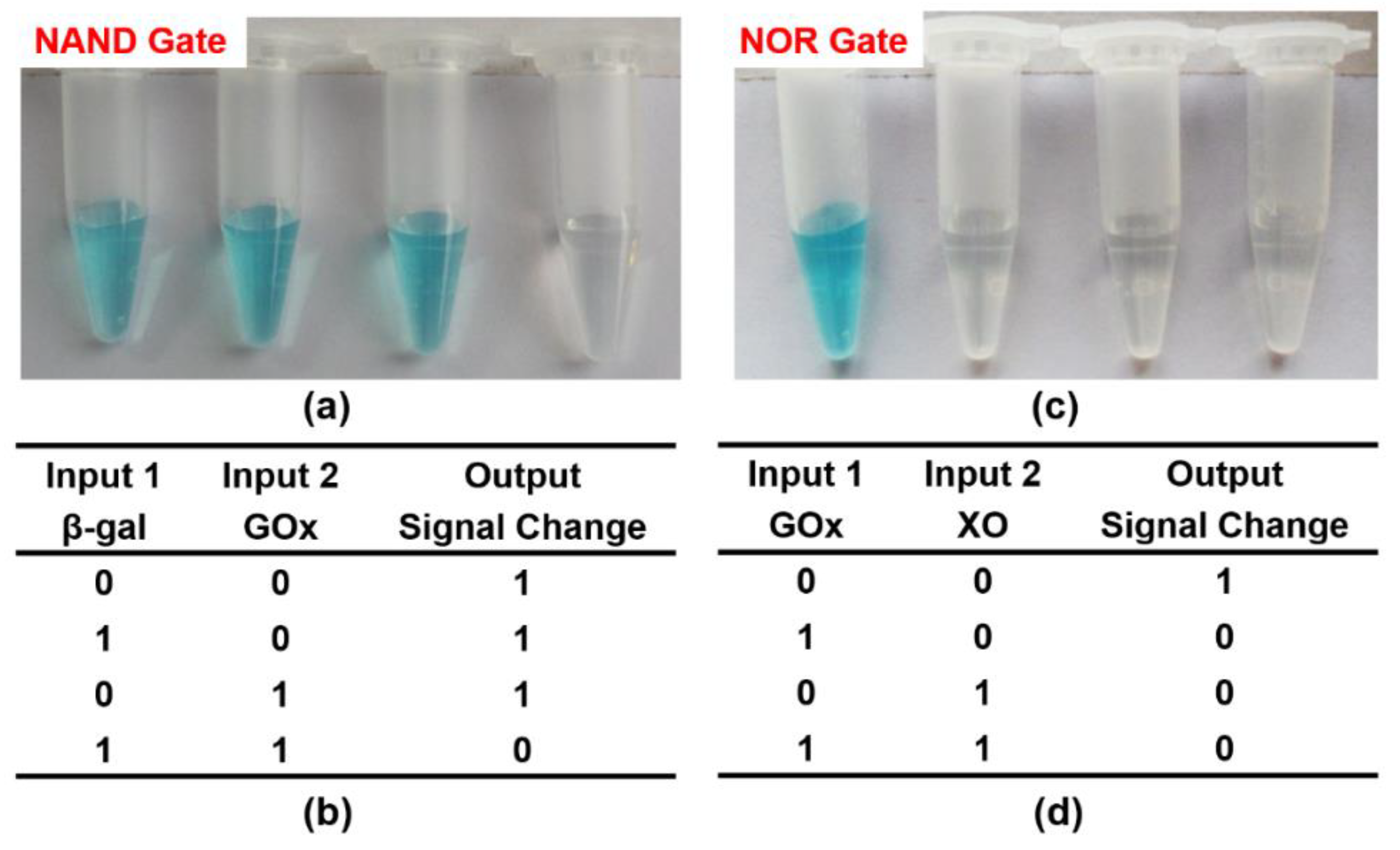
2− and FADH2. Inspired by the above phenomena, we firstly constructed an NAND gate by employing two enzymes: β-gal and GOx as inputs, and the final color of the reaction products as outputs (
Figure 5). The initial system contained lactose, CeO
2 NPs, TMB, and oxygen in citrate buffer (pH 6.0). β-gal could hydrolyze lactose to generate galactose and glucose. GOx presented glucose-oxidase activity, through which glucose could be catalyzed to produce gluconic acid, H
2O
2, and FADH2. Only when both enzymes participated (input = 1/1) could FADH2 be generated by the cascade reaction, thus simulating the logic operation NAND.
Figure 6a,b displayed the corresponding output results with different combinations of the input signals. Obviously, the formation of FADH2 could not be achieved when either or both of the inputs were absent (input = 0/0, 1/0, 0/1). As a result, CeO
2 NPs successfully catalyzed the oxidation of TMB to generate a blue color product (output = 1). Besides, in the presence of both β-gal and GOx (input = 1/1), the enzymatic cascade reactions could take place smoothly, successfully producing FADH2. Thus, the system displayed a color change from blue to colorless (output = 0). This result correlated well with the proper execution of the NAND logic operation.
Analogously, an NOR logic gate was constructed that used GOx and XO as inputs and an intense blue solution as the output signal (
Figure 5). The starting solutions containing glucose, xanthine, CeO
2 NPs, TMB, and oxygen in citrate buffer (pH 6.0) were first added to four tubes. Then, input-1 (GOx) and input-2 (XO) were added according to their corresponding states for each logic operation (0/0, 1/0, 0/1, 1/1). FADH2 can be generated in two parallel catalytic reactions, thus achieving the NOR logic gate. In the absence of any input, the logic operation produced a visual signal (oxTMB with blue color) that is recognized by the naked eye (input = 1). For another, FADH2 was generated when either or both inputs were added. As a result, the formation of oxTMB could be effectively prevented due to the reduction properties of FADH2, and the mixture became colorless (output = 0) (
Figure 6c,d). This response from the solution corresponds to the NOR logic gate.
Except for the simple logic circuit, the multi-enzyme-based complex system was also explored. Based on the above NAND and NOR gates, we further demonstrated the operation of a Boolean logic system to calculate (A NAND B) AND NOT C by employing three enzyme inputs (β-gal, GOx, and XO). The starting solutions contained lactose, xanthine, oxygen, TMB, and CeO
2 NPs in citrate buffer (pH 6.0). FADH2 could be generated when both β-gal and GOx co-existed. Specifically, the reaction process was as follows: β-gal could firstly hydrolyze lactose to generate galactose and glucose. GOx presented glucose-oxidase activity, through which the intermediated state of GOx(FADH2) could be produced during the oxidation of glucose. In addition, FADH2 could also be produced by introducing XO, since xanthine could be catalyzed by XO to generate FADH2. As a result, the formation of oxTMB could be effectively prevented due to the reduction properties of FADH2, and the system displayed a color change from blue to colorless (output = 0). As shown in
Figure 7, when input 1 and input 2 did not exist together or input 3 was absent, FADH2 could not be produced to prevent the formation of oxTMB (output = 1). This result correlated well with the (A NAND B) AND NOT C logic gate.
3. Discussion
It is well-known that a bi-enzymatic (horseradish peroxidase, HRP and GOx) cascade is used for the oxidation of TMB into the blue product oxTMB, which has been widely used in sensing applications [
27]. Amazingly, we found that oxTMB was not stable and the blue color can fade easily in the presence of reductants, including the reduced transition state of glucose oxidase and xanthine oxidase. It means that a false-negative test result might occur with the use of HPR-GOx for sensing applications. During the sensing process, the oxygen should be sufficient, which ensures that the transition state of GOx(FADH2) can quickly recover to Gox(FAD). In addition, the study of such a decolorization process allows us to construct different logic gates by combining enzymatic reactions. More importantly, since the output signal is 1 (the color of solution is blue) in the initial state, such a decolorization process can transform previously designed AND and OR logic gates into NAND and NOR gates.
In this work, we found that oxTMB can be reduced by FADH2. By taking advantage of this phenomenon, we combine the reducibility of FADH2 with the catalytic activity of nanozymes to achieve logic functions, which has not been reported before. Furthermore, since catalytic reactions can work together smoothly, this system can produce a multi-input network without any interference. More importantly, this process can be easily monitored by UV-vis spectroscopy and even by the naked eye, which might dramatically improve the stability of logical operations. Combined with these distinct advantages, we expect that our work will be beneficial in future biomedical and biological applications.
4. Materials and Methods
4.1. Materials
Cerium nitrate hexahydrate (Ce(NO3)3·6H2O) and ammonium hydroxide (25–28 wt%, NH3·H2O) were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). Sodium hydroxide, 3,3,5,5-Tetramethylbenzidine, 1,3,5-trimethylbenzene, 2,2’-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt, glucose oxidase (EC 232-601-0), xanthine (EC 200-718-6), β-galactosidase (EC 232-864-1), and xanthine oxidase (EC 32-657-6) were purchased from Sigma-Aldrich. Glucose and lactose were obtained from Aladdin Co. (Shanghai, China). H2O2 was obtained from Beijing Chemicals (Beijing, China). All other reagents were of analytical reagent grade and used as received. Ultrapure water (18.2 MU; Millipore Co., Burlington, MA, USA) was used throughout the experiment.
4.2. Measurements and Characterizations
Transmission electron microscope images were collected by a high-resolution TECNAI F30 HRTEM operated at 200 kV. The crystalline structure of the as-prepared CeO2 NPs was measured by a Bruker AXS D8 advance X-ray diffractometer with Cu-Kα radiation. The particle sizes of CeO2 NPs were measured by using a NanoBrook Omni instrument. The UV-Vis absorption spectra were recorded using a JASCO V-550 UV/Visible spectrophotometer (JASCO International Co., LTD., Tokyo, Japan).
4.3. Synthesis of CeO2 NPs and Fe3O4 NPs
CeO
2 NPs were prepared based on a previously reported procedure [
30]. Briefly, 2.17 g of cerium (III) nitrate were first dissolved in 5.0 mL of pure water, followed by mixing with 1.0 M dextran. Next, the above mixture was dropped into 30.0 mL of ammonium hydroxide solution (25%) and stirring was continued. The mixture was continually stirred at room temperature for 24 h. Moreover, the suspension was centrifuged. Finally, the resultant precipitate was washed with distilled water three times and then dissolved in 40 mL of distilled water. Fe
3O
4 NPs were synthesized as reported previously [
31]. In brief, 4.0 mmol of FeCl
3 and 0.68 mmol of trisodium citrate were mixed in 20.0 mL of ethylene glycol, followed by the addition of 1.20 g of sodium acetate and stirred for 30 min. Then, the mixture was transferred to a Teflon-lined stainless steel autoclave tube, which was heated at 200 °C and maintained for 10 h. The black products indicated the formation of Fe
3O
4 nanoparticles, which were washed with ethanol and deionized water several times before further use.
4.4. CeO2 and Fe3O4 NPs-Based Logic Systems in Solution
Firstly, the oxidase-like activities of CeO2 NPs were studied by incubating 1 mL of solution of the 40 mM citrate buffer (pH 6.0), 3 μL of 40 mM TMB, and 1.5 μL of 4 mg/mL CeO2 NPs suspension at 35 °C for 15 min. Similarly, the peroxidase-like activities of Fe3O4 NPs were investigated by incubating 1 mL of solution of the 40 mM citrate buffer (pH 6.0), 5 μL of 40 mM TMB, 10 μL of 2 mg/mL Fe3O4 NPs suspension, and 20 μL of 500 mM H2O2 at 35 °C for 15 min. Moreover, the effects of 254 nm ultraviolet light on oxidation of TMB were observed for 1 mL of solution of the 40 mM citrate buffer (pH 6.0) and 5 μL of 40 mM TMB under the radiation of the UV lamp at 35 °C for 20 min. Afterwards, both the 10 μL of 500 mM glucose and 50 μL of 20 units/mL GOx dissolved oxygen in equilibrium with air were added in the previous solutions, and the color turned from blue to colorless. Additionally, no color fading was observed in the solutions with the addition of either glucose or GOx. The influences of pH from 4.0 to 6.0 in the 1-mL solutions on the oxidase-like activities of ultraviolet light (40 mM citrate buffer, 3 μL of 40 mM TMB), CeO2 NPs (40 mM citrate buffer, 3 μL of 40 mM TMB, and 0.5 μL of 4 mg/mL CeO2 NPs suspension), and peroxidase-like activities Fe3O4 NPs (40 mM citrate buffer, 3 μL of 40 mM TMB, 2 μL of 2 mg/mL Fe3O4 NPs suspension, and 10 μL of 500 mM H2O2) were also discussed. In addition, the various saccharides including lactose, sucrose, maltose, and fructose were evaluated on the appearance properties of oxTMB/GOx reaction solutions. The UV-vis absorption spectra and corresponding optical photographs of each input were recorded.
Next, the oxidase-like activities of ultraviolet light, CeO2 NPs, and oxidase-like activities of Fe3O4 NPs were investigated on another enzyme-substrate ABTS (40 mM) in the citrate buffer (pH 6.0). All the colors of 1-mL solutions oxidized by UV-light (40 mM citrate buffer and 200 μL ABTS), with CeO2 NPs (40 mM citrate buffer, 200 μL ABTS, and 8 μL of 4 mg/mL CeO2 NPs suspension), and Fe3O4 NPs (40 mM citrate buffer, 500 μL ABTS, 20 μL of 2 mg/mL Fe3O4 NPs suspension, and 100 μL of 500 mM H2O2) incubated at 35 °C for 15 min turned to green. Additionally, the ABTS•+ reaction solutions were reduced with the addition of 200 μL of 20 units/mL GOx and 40 μL of 500 mM glucose solutions.
According to the phenomenon, the NAND logic gate could be constructed. Different input combinations of 100 μL of β-gal (input-1, 100 units/mL) and 50 μL of GOx (input-2, 20 units/mL) (0/0, 1/0, 0/1, 1/1) were added into four Eppendorf tubes with the 1-mL starting solutions (3 μL of 40 mM TMB, 100 μL of 250 mM lactose, 1 μL of 4 mg/mL CeO2 NPs, and oxygen dissolved in the 40 mM citrate buffer, pH 6.0). Each mixture was incubated at 35 °C for 15 min. To perform the NOR logic gate, 50 μL of GOx (input-1, 20 units/mL) and 4 μL of XO (input-2, 10 units/mL) were combined in all possible ways and added to four starting 1-mL solutions (3 μL of 40 mM TMB, 1 μL of 4 mg/mL CeO2 NPs, 10 μL of 500 mM glucose, 2 μL of 50 mM xanthine, and oxygen dissolved in 40 mM citrate buffer, pH 6.0). Each mixture was incubated at 35 °C for 15 min. Above all, to perform a complex [(A NAND B) AND NOT C] system, 100 μL of β-gal (input-1, 100 units/mL), 50 μL of GOx (input-2, 20 units/mL), and 4 μL of XO (input-3, 10 units/mL) were combined in all possible ways and added to eight equal starting solutions (3 μL of 40 mM TMB and 1 μL of 4 mg/mLCeO2 NPs, 100 μL of 250 mM lactose, 10 μL of 500 mM glucose, 2 μL of 50 mM xanthine, oxygen, dissolved in the 40 mM citrate buffer, pH 6.0). Finally, the UV-vis absorption spectra and corresponding optical photographs of each input were recorded.