Abstract
The aim of the study was to determine the effectiveness of selected seven commercial essential oils (EsO) (grapefruit, lemongrass, tea tree (TTO), thyme, verbena, cajeput, and Litsea cubeba) on isolates of common Central European parasitic fungal species of Fusarium obtained from infected wheat kernels, and to evaluate the oils as potential natural fungicides. The study was conducted in 2 stages. At each stage, the fungicidal activity of EsO (with concentrations of 0.025; 0.05; 0.125; 0.25; 0.50; 1.0, and 2.0%) against Fusarium spp. was evaluated using the disc plate method and zones of growth inhibition were measured. At the first stage, the fungistatic activity of EsO was evaluated against four species of Fusarium from the Polish population (F. avenaceum FAPL, F. culmorum FCPL, F. graminearum FGPL and F. oxysporum FOPL). The correlation coefficient between the mycelial growth rate index (T) and the fungistatic activity (FA) was calculated. At the second stage, on the basis of the mycelium growth rate index, the effectiveness of the EsO in limiting the development of Fusarium isolates from the German population (F. culmorum FC1D, F. culmorum FC2D, F. graminearum FG1D, F. graminearum FG2D and F. poae FP0D) was assessed. The first and second stage results presented as a growth rate index were then used to indicate essential oils (as potential natural fungicides) effectively limiting the development of various common Central European parasitic species Fusarium spp. Finally, the sensitivity of four Fusarium isolates from the Polish population and five Fusarium isolates from the German population was compared. The data were compiled in STATISTICA 13.0 (StatSoft, Inc, CA, USA) at the significance level of 0.05. Fusarium isolates from the German population were generally more sensitive than those from the Polish population. The sensitivity of individual Fusarium species varied. Their vulnerability, regardless of the isolate origin, in order from the most to the least sensitive, is as follows: F. culmorum, F. graminearum, F. poae, F. avenaceum and F. oxysporum. The strongest fungicidal activity, similar to Funaben T, showed thyme oil (regardless of the concentration). Performance of citral oils (lemongrass and Litsea cubeba) was similar but at a concentration above 0.025%.
1. Introduction
According to the latest systematics (MycoBank—http://www.mycobank.org (accessed date: 10 June 2021), Fusarium fungi belong to the following taxa: domain Eucaryota, kingdom Fungi, phylum Ascomycota, subclass Pezizomycotina, class Sordariomycetes, subclass Hypocreomycetidae, order Hypocreales, and family Nectriaceae. Fusarium includes species with anamorphic development (imperfect fungi).
Species of Fusarium fungi are among the most diverse and widespread saprotrophs and pathogenic species in the environment. Due to their ability to produce various metabolites, mainly mycotoxins, they not only pose a serious threat to humans and animals, but also adversely affect soil fertility, biological productivity of agroecosystems, grassland, and forest ecosystems, and reduce the value of agricultural crops. They occur in many ecological niches, including cereal growing environments [1,2,3]. The Fusarium ear blight caused by them is considered increasingly important in many parts of Europe, including Germany, Poland, France, Denmark, Italy, and Hungary. These toxigenic polyphagous pathogens occur in varying degrees on plants every growing season in all climate zones [4] as they spread easily and attack plants at all stages of development. In central Europe, the most dominant Fusarium ear blight-causing species are F. graminearum, F. poae, F. avenaceum, F. culmorum, F. langsethiae, and F. cerealis [5,6,7].
The attempt to reduce losses caused by these phytopathogens and the increasingly evident drawbacks of synthetic fungicides [8,9] encourage a constant search for natural fungicidal substances. This is especially since the spreading phytopathogens are more likely to acquire resistance to the fungicides [10].
In recent years, many scientific centres have focused on the study of the fungicidal activity of natural plant components. Plant extracts, especially essential oils (EsO), significantly reduce the growth of fungi and can be used to eradicate Fusarium [1,11,12,13,14,15,16,17,18]. Currently, a research stream assuming the selection of high activity essential oils (EsO) at low concentrations and a broad spectrum of action on phytopathogens is increasingly common. The properties of EsO are particularly beneficial due to the minimal risk of pathogen resistance, relatively low toxicity to humans and the environment [19,20], as well as biodegradability and lack of bioaccumulation in the environment [21].
The chemical composition of EsO, the type and concentration of active substances and the synergistic relations between them determine the fungicidal or fungistatic mode of action [1,13,18,22]. Therefore, only some EsO are able to completely inhibit Fusarium growth and therefore are fungicidal. Given the varying sensitivity of fungi, it is very difficult to choose the right oil at the right concentration, especially if the essential oil is expected to effectively limit the growth of different species within Fusarium. Our previous study [1], whose aim was to determine the fungistatic activity of oils in relation to phytopathogens of Fusarium from the Polish population (F. avenaceum FAPL, F. culmorum FCPL, F. graminearum FGPL and F. oxysporum FOPL) showed that the activity of oils with a high content of thymol (thyme) and citral (Litsea cubeba, lemongrass and verbena) is most similar to the activity of Funaben T.
Natural populations harbour a stunning diversity of phenotypic variation for morphology, physiology, behaviour and disease susceptibility [23], and consequently sensitivity to EsO.
The aim of the study was to determine the effectiveness of selected seven commercial essential oils on isolates of common Central European parasitic fungal species of Fusarium obtained from infected wheat kernels and to evaluate the oils as potential natural fungicides.
2. Results
2.1. Variation in Sensitivity of Fusarium Isolates to Essential Oils
The coefficients of fungistatic activity (FA) and mycelial growth rate index (T) were found to be highly correlated (correlation coefficient FA/T was 0.99), which means that an increase in mycelial growth rate index causes a decrease in fungistatic activity. Formula (1) describes 99.15% of the variation in fungistatic activity. Therefore, the T index was used to describe the relationship between the sensitivity of the isolates and the fungicidal activity of the EsO.
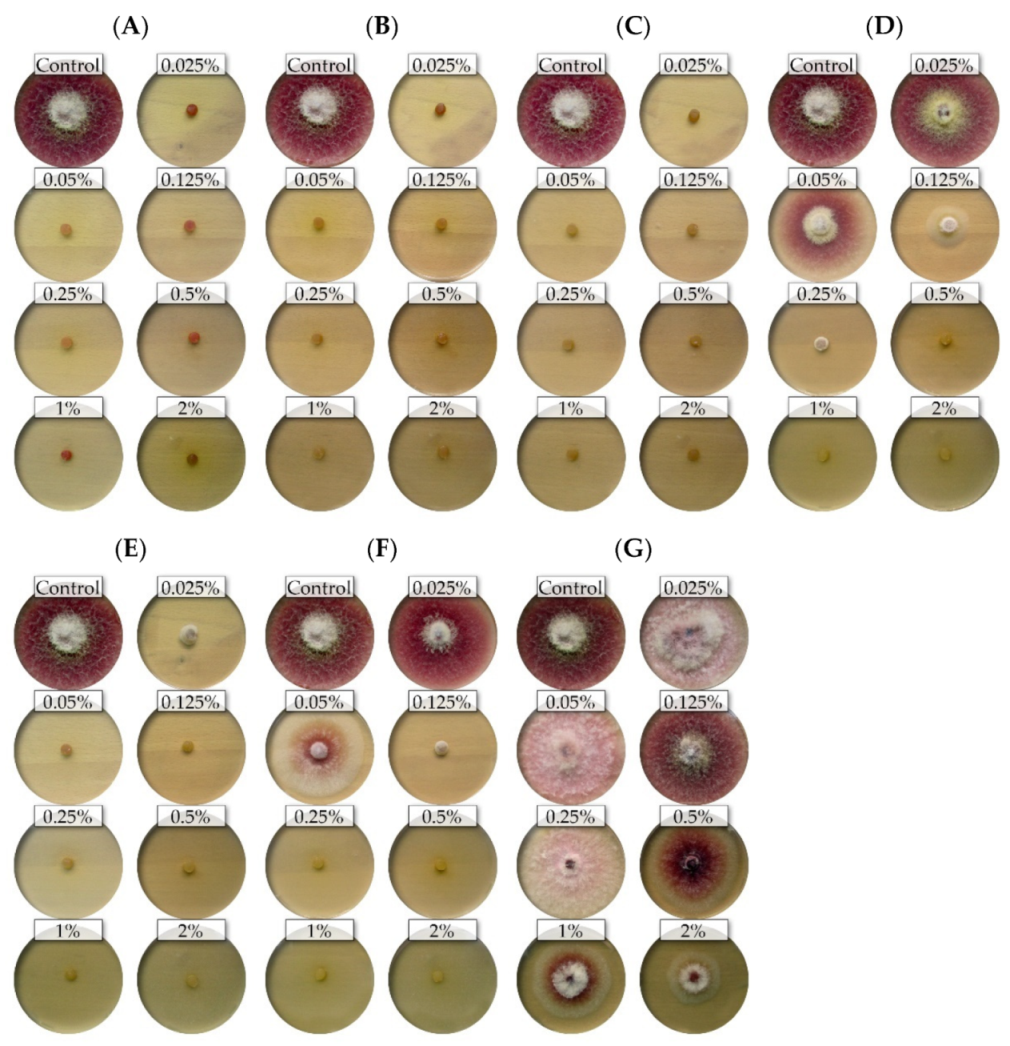
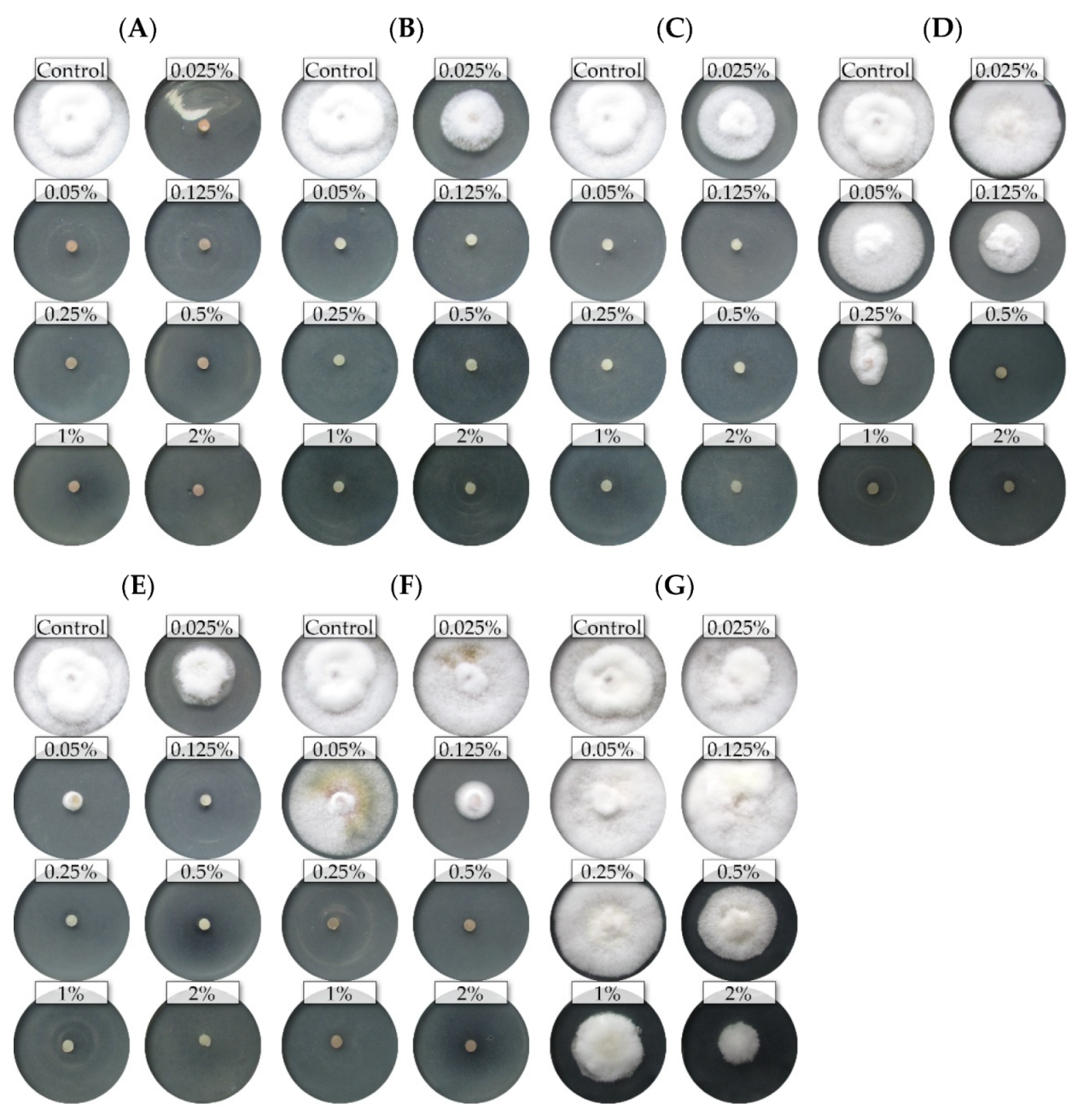
The nine isolates (representing five Fusarium species) showed differential sensitivity to seven commercial EsO; the fungicidal and fungistatic effect is shown on the example of F. culmorum FC2D and F. poae FP0D (Figure 1 and Figure 2).
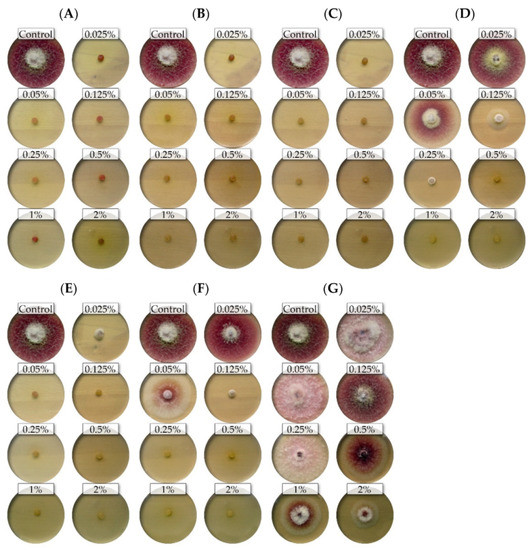
Figure 1.
The effects of the different concentrations of the essential oils on the mycelial growth of the Fusarium culmorum FC2D: (A)—thyme (T); (B)—lemongrass (L); (C)—Litsea cubeba (LC); (D)—cajeput (C); (E)—verbena (V), (F)—TTO; (G)—grapefruit (G) (light background).
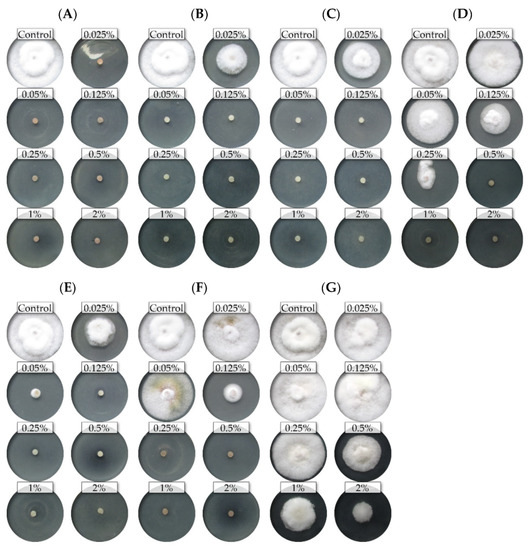
Figure 2.
The effects of the different concentrations of the essential oils on the mycelial growth of the Fusarium poae FP0D: (A)—thyme (T); (B)—lemongrass (L); (C)—Litsea cubeba (LC); (D)—cajeput (C); (E)—verbena (V), (F)—TTO; (G)—grapefruit (G) (dark background).
The Kruskal–Wallis test showed that the T index differences obtained for individual isolates were statistically significant. In terms of the mean T index, the individual isolates in the presence of individual EsO can be described using numbers shown in Table 1.

Table 1.
Results of Kruskal–Wallis test. Comparison of the index of linear growth of mycelial isolate in presence of used essential oils.
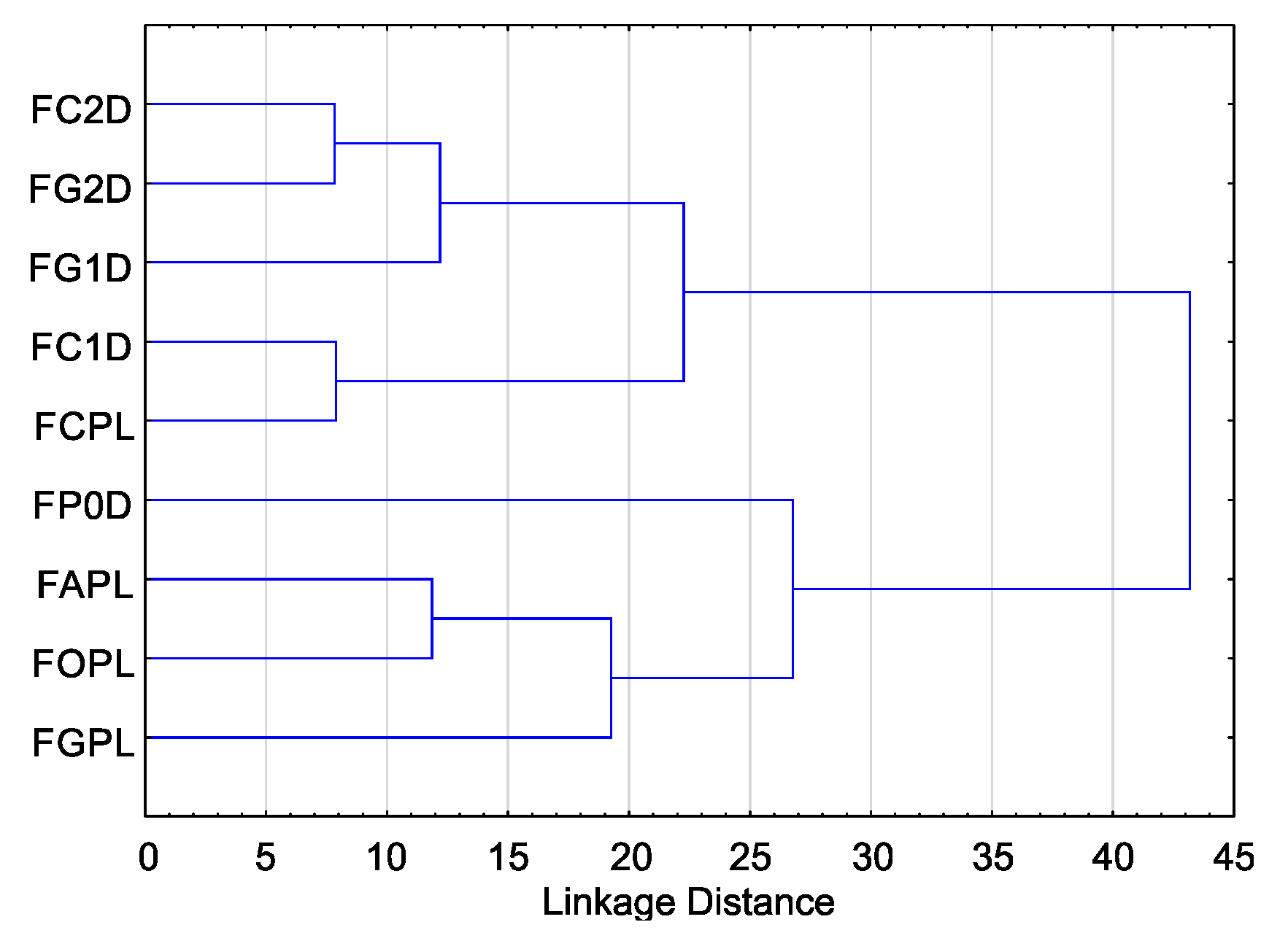
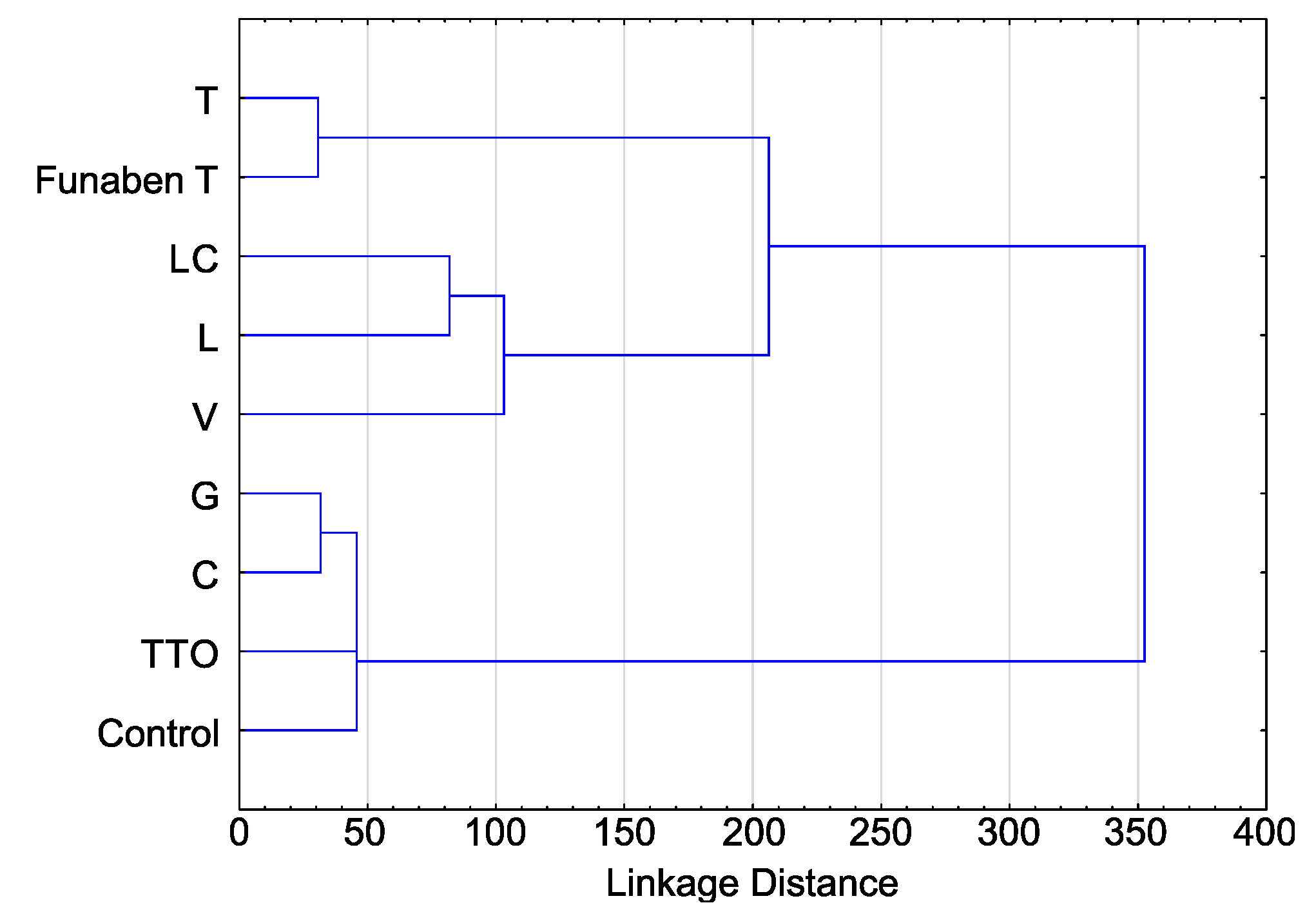
Cluster analysis for T index values showed that they can be divided into two groups due to their sensitivity to EsO. The highest sensitivity was observed in five isolates, including four isolates from the German population (F. culmorum FC1D, F. culmorum FC2D, F. graminearum FG1D, F. graminearum FG2D), and one isolate from the Polish population (F. culmorum FCPL) (Figure 3).
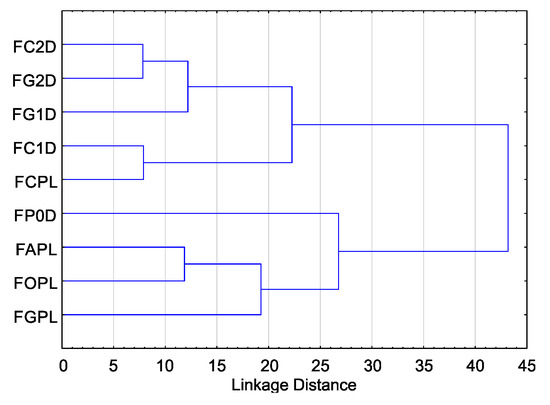
Figure 3.
Variability of sensitivity of Fusarium spp. isolates—ordered after cluster analysis (D-Germany, PL-Poland.): FC2D—F. culmorum, FG2D—F. graminearum, FG1D—F. graminearum, FC1D—F. culmorum, FCPL—F. culmorum, FP0D—F. poae, FAPL—F. avenaceum, FOPL—F. oxysporum, FGPL—F. graminearum.
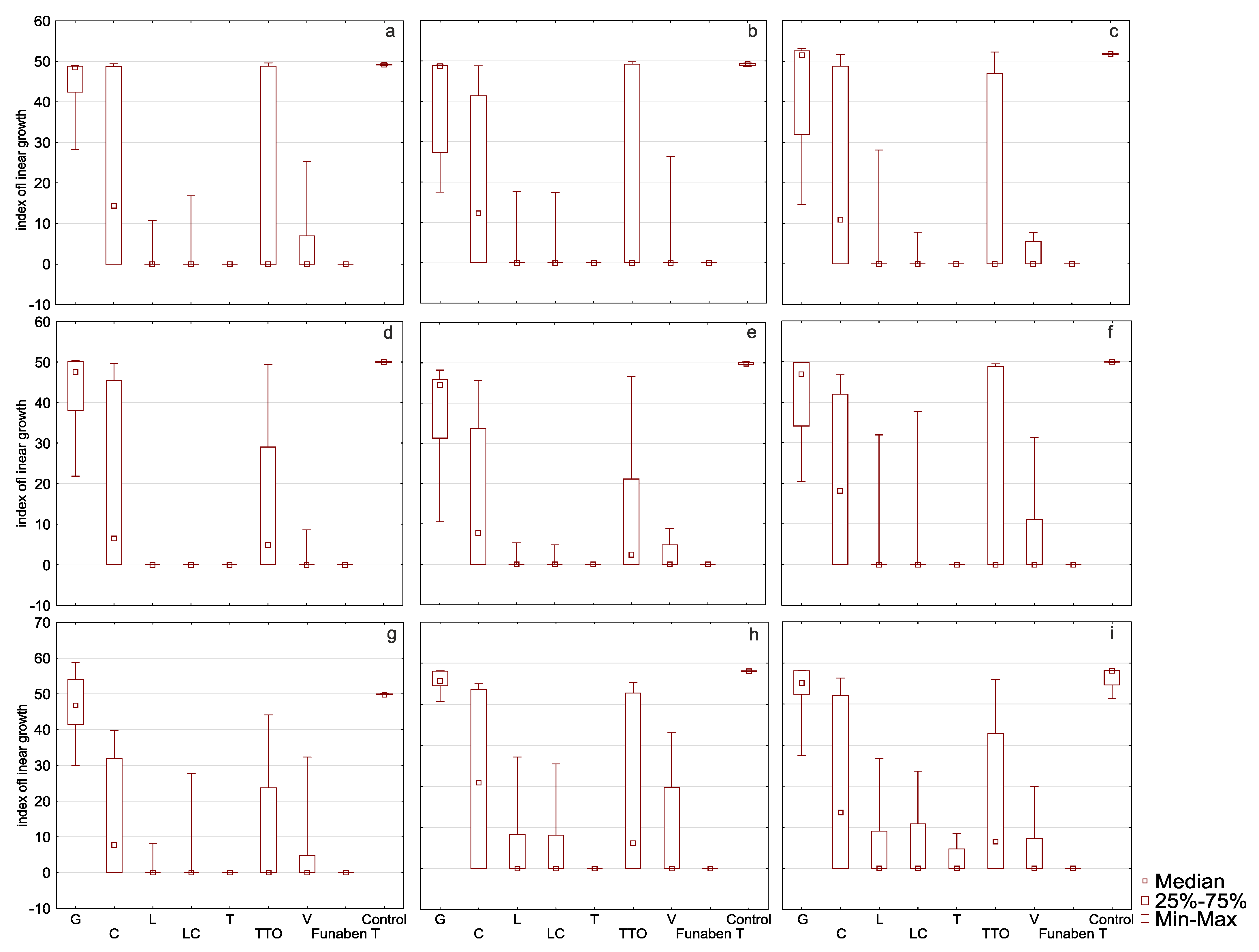
In the presence of grapefruit, cajeput, and tea tree oils, F. culmorum FC2D had a high T index mean, ranging from 44.57 to 16.17 (control 49.18) (Table 2, Figure 4a). The Supplementary Materials contains a Table with the minimum growth rate index of the analysed oil at a minimum concentration of each considered isolates of Fusarium.

Table 2.
Descriptive statistics for the growth rate index of all tested Fusarium isolates; for all concentrations of essential oils together Fusarium: FC2D—F. culmorum, FG2D—F. graminearum, FG1D—F. graminearum, FC1D—F. culmorum, FCPL—F. culmorum, FP0D—F. poae, FGPL—F. graminearum, FAPL—F. avenaceum, FOPL—F. oxysporum. control–isolates without EsO or Funaben T. Notation: N—test volume, SD—standard deviation.
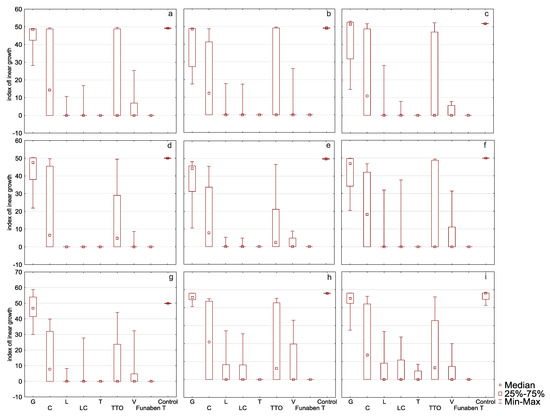
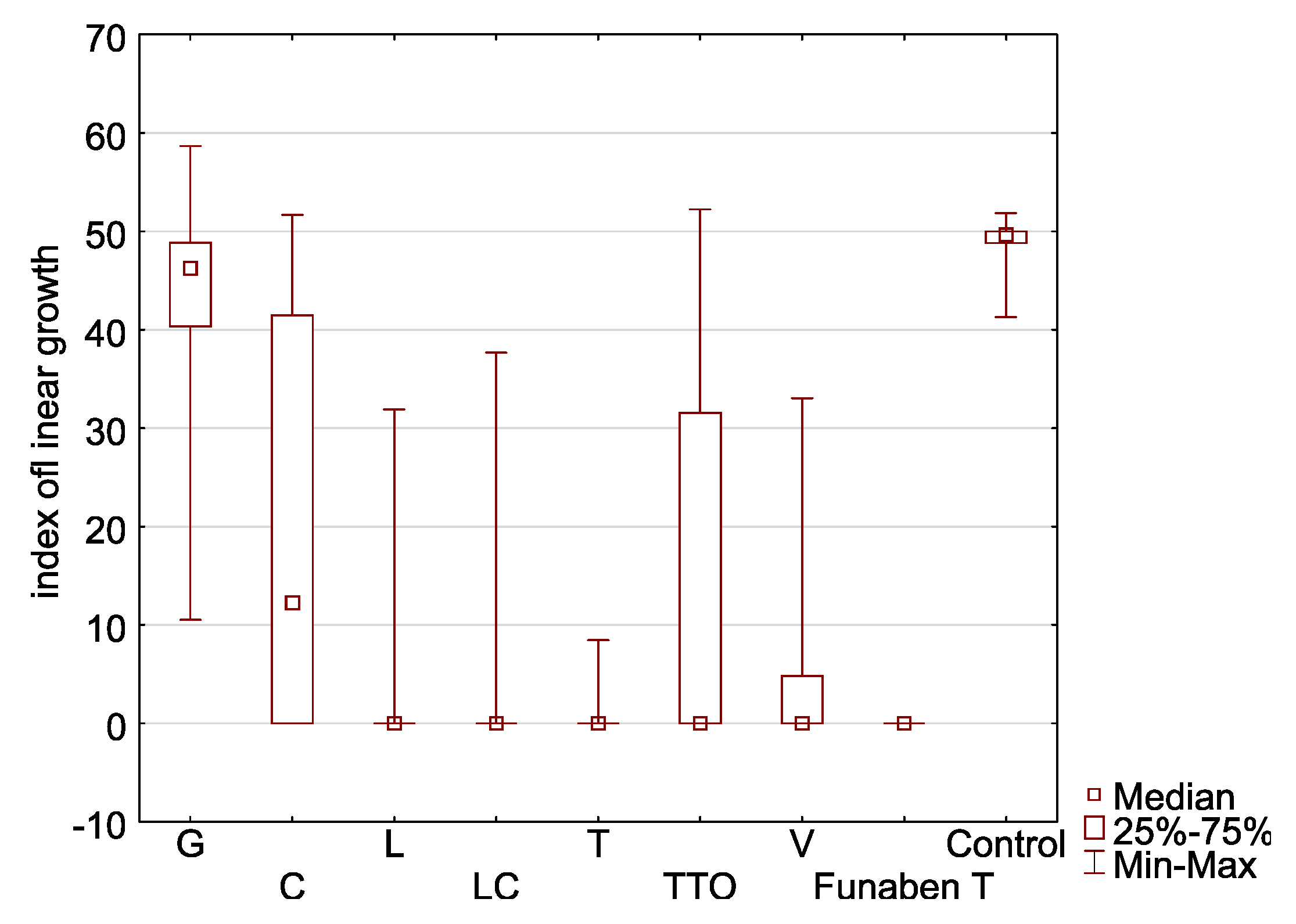
Figure 4.
Indexes of linear growth (T) of Fusarium ssp. isolates for all concentration of EsO together (D-Germany, PL-Poland): (a) FC2D—F. culmorum, (b) FG2D—F. graminearum, (c) FG1D—F. graminearum, (d) FC1D—F. culmorum, (e) FCPL—F. culmorum, (f) FP0D—F. poae, (g) FGPL—F. graminearum, (h) FAPL—F. avenaceum, (i) FOPL—F. oxysporum; index T calculated after treated of isolates with essential oils and Funaben T: G—grapefruit, C—cajeput, L—lemongrass, LC—Litsea cubeba, T—thyme, TTO—tea tree, V—verbena. Control-isolates without EsO or Funaben T.
This indicates the low sensitivity of the isolate to the EsO. In the presence of thyme, lemongrass, Litsea cubeba, and verbena oils, the T index of this isolate was significantly lower, ranging from 0.00 to 3.62, which shows that the oils are highly effective. The post hoc analysis showed that no significant T index differences occurred in the presence of thyme, lemongrass, Litsea cubeba, and verbena oils as compared to the results obtained in the presence of Funaben T.
EsO sensitivity of F. graminearum FG2D (Table 2, Figure 4b) was similar to the previous isolate as the ranges of T index mean values were similar. Post hoc analysis showed that the T index in the presence of all EsO, except grapefruit, was similar to the results obtained in the presence of Funaben T, which makes this isolate essentially different from FC2D.
EsO sensitivity of F. graminearum FG1D (Table 2, Figure 4c) was similar to the previous isolates: FC2D and FG2D. Post hoc analysis revealed that the results are identical to FG2D.
The response of F. culmorum FC1D to three oils with poor activity (grapefruit, cajeput, and tea tree) was similar to the previously discussed isolates (43.10 to 13.31) (control 50.02) (Table 2, Figure 4d). Very low T index mean values in the presence of the remaining four oils (0.00 to 0.98) reveal complete inhibition of mycelial growth (0.00) in the presence of thyme, Litsea cubeba, and lemongrass oils, similarly to Funaben T. The activity of verbena oil was slightly weaker (0.98). This isolate showed the greatest sensitivity to EsO out of the isolates discussed so far. Post hoc analysis showed that, in the presence of all oils, except grapefruit oil, the T index did not differ from the results obtained in the presence of Funaben T for this isolate.
The response of F. culmorum FCPL to three oils with poor activity (grapefruit, cajeput and tea tree) was similar to the previously discussed isolates (38.19 to 11.32) (control 49.83) (Table 2, Figure 4e). Low T index values against thyme, lemongrass, Litsea cubeba, and verbena oil (0.00 to 1.79) prove their high effectiveness. The sensitivity of this isolate was similar to F. culmorum FC1D. Post hoc analysis for this isolate showed that only the activity of grapefruit and cajeput oils was significantly different from Funaben T.
The cluster analysis showed that the four remaining isolates, including one isolate from the German population (Fusarium poae FP0D), and three multi-species isolates from the Polish population (F. avenaceum FAPL, F. graminearum FGPL and F. oxysporum FOPL) had higher resistance to EsO (Figure 3).
In the presence of grapefruit, cajeput, and tea tree oils, F. poae FP0D had a high T index, ranging from 40.69 to 16.79 (control 49.93) (Table 2, Figure 4f). In the presence of the other four EsO (thyme, lemongrass, Litsea cubeba, and verbena), lower T index values were observed (0.00 to 6.02). However, these values were higher as compared to the results of the five previously discussed (more sensitive) isolates. Post hoc analysis showed that only the activity of grapefruit oil was significantly different from Funaben T.
EsO sensitivity of F. graminearum FGPL (Table 2, Figure 4g) was similar to F. poae FP0D in the presence of individual oils (the range of T index values was similar). Post hoc analysis showed that only the activity of grapefruit and cajeput oils was significantly different from Funaben T.
EsO sensitivity of F. avenaceum FAPL (Table 2, Figure 4h) was similar to the previous isolates (FP0D and FGPL) in the presence of individual oils (the range of T index mean values was similar). The post hoc analysis showed that no significant T index differences occurred in the presence of thyme, lemongrass, Litsea cubeba, verbena oils, and Funaben T.
In the presence of grapefruit, cajeput, and tea tree oils, F. oxysporum FOPL had a high T index, ranging from 43.52 to 13.90 (control 46.39) (Table 2, Figure 4i). In the presence of the other EsO (thyme, lemongrass, Litsea cubeba, and verbena), lower T index mean values were observed (1.77 to 5.09). This indicates the relatively high effectiveness. The post hoc analysis showed that no significant T index differences occurred in the presence of thyme, lemongrass, Litsea cubeba, verbena oils, and Funaben T. However, it was the only isolate that grew when treated with the lowest concentration of thyme oil.
The Kruskal–Wallis test (H (8, 1877) = 1061.18, p = 0.00) showed that the T index values for all isolates treated with essential oils were significantly different. The post hoc analysis showed that only thyme, Litsea cubeba and lemongrass oils produced results that were similar to Funaben T.
Based on the cluster analysis of the sensitivity of individual isolates (Figure 3) and the T index mean values (Table 2), it can be concluded that isolates from the German population are generally more sensitive than isolates from the Polish population. There are exceptions to this rule: F. poae FP0D (Germany) is more resistant than F. culmorum FCPL (Poland). Furthermore, the isolates of F. culmorum and F. graminearum were found to be more sensitive than the other three species. Similarly, there are exceptions to this rule: F. graminearum FGPL (Poland) is more resistant than F. poae FP0D (Germany). Moreover, it seems that the two-species group consisting of F. culmorum and F. graminearum is more sensitive than the two-species group consisting of F. avenaceum and F. oxysporum. F. poae shows intermediate sensitivity.
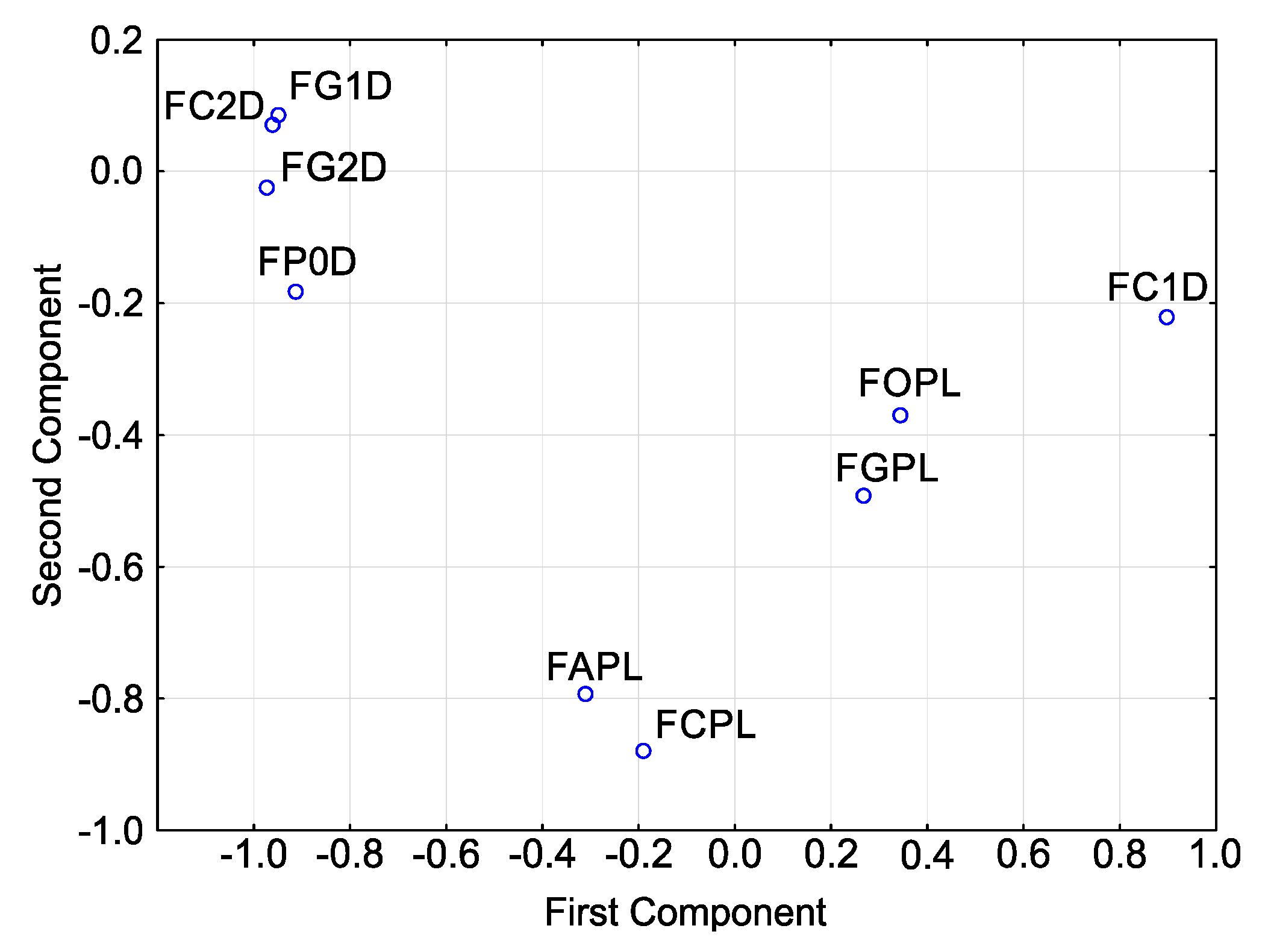
Principal component analysis (PCA) for sensitivities (Figure 5) showed that, in terms of T index, the isolates cluster into one relatively large group with three isolates: F. culmorum FC2D, F. graminearum FG1D and F. graminearum FG2D.
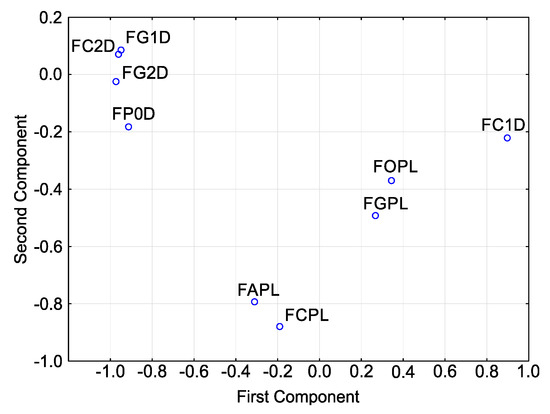
Figure 5.
Variability of sensitivity of Fusarium spp. isolates—ordered after cluster analysis (D-Germany, PL-Poland.): FC2D—F. culmorum, FG2D—F. graminearum, FG1D—F. graminearum, FC1D—F. culmorum, FCPL—F. culmorum, FP0D—F. poae, FAPL—F. avenaceum, FOPL—F. oxysporum, FGPL—F. graminearum.
They have high values for the second principal component and low values for the first principal component. F. poae FP0D can also be included in this group. It reveals slight differences as compared to the group, but its close proximity to the group shows that it is highly similar to the isolates in this group. The remaining isolates are scattered to a greater or lesser extent, reaching different values of both principal components. Note that three F. culmorum isolates do not lie next to each other. They occur in three different and distant locations. The distribution of points representing the three isolates of F. graminearum is different: two isolates from the German population are grouped together while the third representative (the Polish isolate) is distant from the other two.
2.2. Assessment of the Effectiveness of Individual Essential Oils
The combined analysis of the T index values of nine Fusarium isolates showed that the mycelium growth is most effectively inhibited by thyme oil (Figure 6).
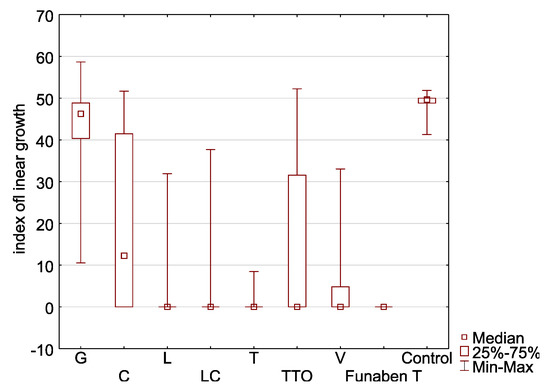
Figure 6.
Characteristic of the indexes of linear growth (T) of Fusarium spp. isolates (aggregate numbers of all tested isolates) in relation to the used essential oils and Funaben T: G—grapefruit, C—cajeput, L—lemongrass, LC—Litsea cubeba, T—thyme, TTO—tea tree, V—verbena.
The activity of thyme oil representing the so-called thymol oils is almost identical to Funaben T and thus can be described as fungicidal. Three other EsO (lemongrass, Litsea cubeba, and verbena) clearly exhibit a fungistatic activity, the latter being slightly weaker than the first two. The other three EsO (tea tree, cajeput and grapefruit) have the weakest antifungal activity, of which the latter is weakest. A similar relationship is shown in the cluster analysis diagram (Figure 7).
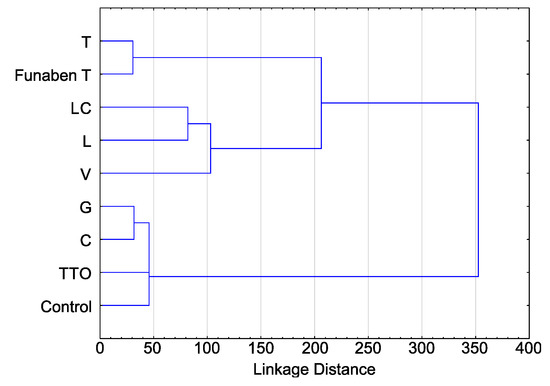
Figure 7.
The clustering of the indexes of linear growth (T) of Fusarium spp. isolates (aggregate numbers of all tested isolates) in relation to the used essential oils and Funaben T: C—cajeput, G—grapefruit, L—lemongrass, LC—Litsea cubeba, T—thyme, TTO—tea tree, V—verbena.
The groups of bioactive substances with fungicidal and fungistatic activity have not yet been identified. To this end, the correlation matrix of the T index against main groups of compounds was determined (Table 3).

Table 3.
Growth rate index correlation matrix against the main groups of compounds in essential oils; the obtained results showed no statistical significance.
A linear relationship was particularly found between the T index and the presence of monoterpenoids. This is a strong correlation whose sign shows that the T index decreases with the increasing content of monoterpenoids. Monoterpenes and sesquiterpenoids exhibit the average correlation with the T index, with a sign indicating that the T index increases with the increasing content of these groups of compounds. Sesquiterpenes are the only group that does not show any significant effect on the increase of the T index. Due to the high linear dependencies, a multiple regression analysis was performed (Table 4).

Table 4.
The model of multiple regression: b—regression coefficient; b1—standardized coefficient; SE standard error; t—Student’s t-test value.
The model describing the influence of the main groups of compounds contained in essential oils on the T index clearly shows that all the main groups except sesquiterpenes have a significant effect on the T index. The model accounts for 59% of T index variability. In this model, the T index can be predicted with the error of +/−11.9. Identical data were obtained for the fungistatic activity.
3. Discussion
Currently, research is largely focused on selecting oils with high activity and a broad spectrum of activity against microorganisms. Our research focused on the identification of EsO, which at low concentrations exhibit a broad spectrum of fungicidal activity (comparable to fungicides) against parasitic Fusarium spp. isolates, regardless of their origin. This is also justified in the light of the more and more frequently observed resistance of Fusarium isolates to fungicides and diversified sensitivity to essential oils.
Contemporary publications on Fusarium indicate that this morphologically poorly differentiated group of fungi is strongly diverse in genetic terms. The number of chromosomes varies (n = 4–20), and their karyotype has core (CCs) and accessory (ACs) chromosomes [24]. Cytological karyotyping shows a variation in chromosome number, also within species. For example, the number of F. oxysporum chromosomes varies from n = 9–10 to n = 19–20 [25].
Within Fusarium, a process called horizontal gene transfer (HGT) was identified. HGT is an important mechanism of eukaryotic genome evolution, particularly in unicellular organisms [26]. For example, it was experimentally demonstrated that two LS chromosomes between strains of F. oxysporum can be transferred. The transfer leads to the transformation of a non-pathogenic strain into a pathogenic one [27].
Fusarium fungi with anamorphic stages (imperfect fungi) do not reproduce sexually. However, they undergo genetic recombination. Recombination occurs during the parasexual cycle. The parasexual cycle involves crossing over [28,29]. Genetic diversity was found in mitochondrial populations present in Fusarium cells [30]. The mitochondrial genetic recombination was also reported [31]. Other detected phenomena resulting from the genetic structure of Fusarium include the interspecies transfer of chromosomal markers of microsatellites (in Fusarium oxysporum) [32] and interspecies variation of ribosomes [33]. The genetic diversity of organisms is significantly increased by mutations. It was found that Fusarium is also subject to mutagenesis [34,35].
For Fusarium ssp., genetic differentiation of the population structure of races was reported. In particular, the occurrence of specific pathotypes was found [34,35,36,37]. Another paper reported the biological, physiological, and pathogenic differentiation of a genetically homogeneous population of F. oxysporum f.sp. cubense. In particular, the differential growth of isolates was observed [38].
Given the occurrence of so many complex genetic mechanisms in Fusarium fungi, it is hard to argue with what Professor Shay Covo (Hebrew University, Rechowot, Israel), said about the state of knowledge on fungal pathogens: “Research into the genomic dynamics of fungal plant pathogens is in its infancy” [39]. It is also difficult to argue with the statement of Professor Sephra N. Rampersad: “There is an urgency to supplant the heavy reliance on chemical control of Fusarium diseases in different economically important, staple food crops due to development of resistance in the pathogen population, the high cost of production to the risk-averse grower, and the concomitant environmental impacts” [40].
These works indicate the existence of multiple sources of genetic and epigenetic diversity in Fusarium spp. In the experiment discussed in this paper, the differences in the mycelial growth rate index of individual isolates were expected and demonstrated. This proves the differential sensitivity of the nine tested isolates to seven commercial EsO. The experiment tested isolates of five species: F. avenaceum, F. culmorum, F. graminearum, F. oxysporum and F. poae. It was shown that F. culmorum and F. graminearum isolates, irrespective of origin, were more sensitive to the essential oils than isolates of the other three species. Phylogenetically, F. culmorum is similar to F. graminearum, both belonging to section Discolour [41].
F. oxysporum was most resistant. Fusarium poae and F. avenaceum showed intermediate properties. F. avenaceum was more similar to F. oxysporum (the most resistant species). The literature shows that detailed comparative analysis of the mitogenome may offer new insights into the biology of the studied organism and will allow an understanding of the mechanism of sensitivity to essential oils. Mitochondrial genomes are highly informative for resolving phylogenetic relationships even between closely related species and populations. Complete mitochondrial genome sequences offer a stable basis and reference point for phylogenetic and population genetic studies [31].
Our study showed that the right EsO used at low concentrations (up to 2.0%) gives a sound fungicidal effect. Both thyme oil (thymol) and three citral oils (Litsea cubeba, lemongrass, and verbena) revealed a strong fungicidal activity, similar to the fungicidal activity of the synthetic fungicide Funaben T confirmed in our previous study [1]. Many researchers emphasise that compounds with phenolic structure (thymol and carvacrol) and terpenoids (citral) are definitely the most effective active ingredients against most fungal species [1,42,43,44,45,46,47]. Due to their lipophilic nature and low molecular weight, these compounds can cause structural and functional damage in the cells of organisms by disrupting the membrane permeability and osmotic balance of the cell, inhibiting the activity of certain enzymes and interfering with ergosterol biosynthesis [48,49,50,51].
Thymol and its isomer carvacrol are definitely the most effective active ingredients against most species of Fusarium [52,53,54]. Oils of this type are found not only in Thymus vulgaris but also in Elsholtzia polystachya, Origanum vulgare, Origanum majorana, Citrus limon, Coriandrum sativum, Trachyspermum ammi, Monarda punctata, Satureja montana, Lavandula multifida, Anabasis setifera, Zataria multiflora, Oliveria decumbens Vent. [22,43,54,55,56,57,58]. Oils rich in other compounds with phenolic structure—eugenol (Pimenta dioica L. clove, cinnamon, allspice, and basil) also show strong fungicidal activity against Fusarium [11,18,56,59,60,61].
The citral chemotype includes oil-producing plants whose chemical composition shows a predominance of the monoterpenoid citral (i.e., isomeric mixture of geranial and neral and citronellol), with an admixture of monoterpene hydrocarbons, e.g., myrcene [62]. Citral oils are found in a wide variety of plants, such as Cymbopogon citratus, Verbena officinalis, Litsea cubeba, Melissa officinalis, Aloysia citrodora, Vepris macrophylla, Citrus bergamia, Zingiber officinale, Eucalyptus citriodora, Salvia officinalis, Ocimum gratissimum, Lindera citriodora, Calypranthes parriculata, Tagetes patula, bitter orange leaves (petitgrain), and lemon peel [20,63,64].
The total inhibition of Fusarium mycelial growth is also caused by oils other than thymol and citral, e.g., geranium oil (citronellol and geraniol) [18,60,61]. Similar, strong fungicidal activity, despite differences in chemical composition, is shown in rose oil (linalool) [18,65]. Linalool is also found in coriander, clary sage, lavender oil, and lavandin oil.
These EsO, if used at low concentrations, show the best fungicidal activity against Fusarium fungi. This means that they can be used in the development of biodegradable and non-accumulating chemicals (the so-called “green chemicals”).
Three other EsO (cajeput, TTO, and grapefruit) containing active substances other than thymol and citral had a weaker activity on the Fusarium isolates. In the presence of the latter, the mycelial growth rate index was similar to the control. Grapefruit oil, with monoterpene (limonene) as the main ingredient, exhibited the weakest activity against the Fusarium fungi. This was also confirmed by Thielmann and Muranyi [66]. Other oils with limonene as the main ingredient (lemon oil, tangerine oil, orange oil, and pepper oil) also show low effectiveness at low concentrations [56,67].
EsO with 1,8-cyneol (eucalyptol) as the main ingredient (eucalyptus oil, rosemary oil, laurel oil, turmeric oil, and lavender oil) or α-terpineol (cajeput oil), or its isomer 1-terpinen-4-ol (TTO) also show low effectiveness against the Fusarium fungi [67]. In our study, cajeput oil and TTO at low concentrations showed weak activity on the Fusarium fungi. However, high fungicidal activity of TTO was also reported [68]. Although some of the results are debatable and the researchers disagree on the issue, biopreparations based on TTO and grapefruit extract essential oils are produced and used. The preparations show long-lasting inhibitory activity against many species of Fusarium: F. avenaceum, F. culmorum, F. graminearum, F. oxysporum, and F. poae [69].
Adequate use of the allelopathic potential of EsO against polyphagous fungi of the Fusarium would be safe for humans and the environment [70] and would result in the reduced use of chemical pesticides, contributing to the development of integrated agricultural production [71,72,73]. However, for the selection of oils to be used in green chemicals, the criteria to be taken into account are as follows: the varying sensitivity of Fusarium fungi (both fungi within species and isolates belonging to the same species) and the chemical composition of EsO depending on the plant chemotype [11,18,74,75,76].
4. Materials and Methods
The study was conducted in two stages. At each stage the fungicidal activity of seven commercial EsO of varying chemical composition (Table 5) against Fusarium spp. was evaluated using the disc plate method (method of poisoned substrates) [77,78]:

Table 5.
Chemical composition of the tested essential oils in [%]: T—thyme; L—lemongrass; LC—Litsea cubeba; V—verbena; TTO—tea tree; C—cajeput; G—grapefruit [1].
- Cultures of fungi were grown in PDA medium for 14 days at 25 °C
- Inoculum. The spore suspension of Fusarium spp. in 0.01% sterile Tween 80 were obtained from 14 days old culture. The haemocytometer Thoma was used to obtain a spore suspension of 2 × 106 CFU·cm3. Petri dishes (9 cm diameter) containing 20 × cm3 PDA medium were inoculating this spore suspension and stored at 25 °C for 14 days. Inoculum—rings with a diameter of 10 mm overgrown by mycelium.
- Inoculum was placed on the surface of the oil-modified PDA medium.
- The samples were incubated at 25 °C. Every 2 days, the diameter of developing colonies was measured until the surface of the medium in the control plates was overgrown. Tests were performed in four repetitions (n = 4). One petri dish with inoculum (disc overgrown with pathogen mycelium) was treated as a repetition
- PDA medium with the Funaben T (at concentrations of 0.125; 0.25 and 0.50%) was used as a positive control. Unmodified PDA medium (without oils) with a ring was used as a negative control
The tests were performed in four repetitions (n = 4), taking as a repetition one Petri dish from the inoculum in the form of a disc overgrown with pathogen mycelium.
In the study were used EsO, i.e., thyme (T), Thymus vulgaris (produced by MELASAN, Eugendorf, Austria); lemongrass (L), Cymbopogon citratus (Lemongrass), Litsea cubeba (LC), Litsea cubeba, and grapefruit (G), Citrus paradisi (produced by TAOASIS GmbH, Berlin, Germany); verbena (V), Lippia javanica (produced by Piping Rock Health Products, LLC, Ronkonkoma, NY 11,779 USA); tea tree (TTO), Melaleuca alternifolia (produced by MEDESIGN IC GmbH Dietramszell—Linden, Germany); cajeput (C), Melaleuca leucadendron var. cajaputi (produced by PRIMAVERA LIFE GmbH, Oy-Mittelberg, Germany. Based on the chemical composition, the following EsO groups were distinguished: three citral oils (lemongrass, Litsea cubeba, and verbena), one thymol oil (thyme oil), two oils containing mainly monocyclic monoterpenoids, i.e., 1-terpinen-4-ol (tea tree oil (TTO) and α-terpineol (cajeput oil), and one limonene (grapefruit oil). The following concentrations of EsO were used: 0.025; 0.05; 0.125; 0.25; 0.50; 1.0; and 2.0%.
The oil colloid solutions were prepared in water with 0.05% Tween 80 (produced by BTL, Poland) and fed into a liquefied PDA medium (Potato Dextrose Agar (BIOCORP, Warszawa, Poland). A relative control of the effectiveness of EsO was chemical seed treatment Funaben T (containing 20% carbendazim and 45% thiocarbamate), produced by Zakłady Chemiczne “Organika Azot” S.A., Jaworzno, Poland), applied in concentrations lower, higher and recommended by the manufacturer (0.125, 0.25, and 0.5%).
At the first stage, the fungistatic activity of EsO was evaluated against four species of Fusarium from the Polish population (F. avenaceum FAPL, F. culmorum FCPL, F. graminearum FGPL and F. oxysporum FOPL) isolated from infected wheat kernels in south-west Poland in 2012–2014 [1]. The fungistatic activity of the tested oils was evaluated on the basis of the percentage of inhibition of fungal colony growth calculated from the Abbott formula [1]. The correlation coefficient between the mycelial growth rate index (T) and the fungistatic activity (FA) was determined. This relationship was expressed by the formula:
FA = 99.74 − 2.00 ∗ T
At the second stage, on the basis of the mycelium growth rate index, the effectiveness of the EsO in limiting the rise of Fusarium isolates from the German population (F. culmorum FC1D, F. culmorum FC2D, F. graminearum FG1D, F. graminearum FG2D and F. poae FP0D) was assessed. The isolates were separated in 2012–2014 from infected wheat kernels obtained from Leibniz Zentrum für Agrarlandschaftsforschung e.V., Institut für Landschaftsbiogeochemie (ZALF, Müncheberg, Germany) collection.
The fungicidal activity of seven EsO (with concentrations of 0.025, 0.05, 0.125, 0.25, 0.50, 1.0 and 2.0%) against five Fusarium isolates from the German population was evaluated too, using the disc plate method.
The growth rate index (T) of the isolates was determined based on measurements of mycelial colony growth using the formula:
where T—growth rate index; A—average measurement value of diameter colonies [mm]; D—duration of the experiment; b1 (…) bx—increase in colonies diameter [mm]; d1 (…) dx—number of days since last measurement.
The results of the first and second stage presented as a growth rate index were then used to indicate the EsO (as potential natural fungicides) effectively limiting the development of various common Central European parasitic species Fusarium spp. Finally, the oils sensitivity of four Fusarium isolates from the Polish population and five Fusarium isolates from the German population were compared. In order to standardize the description of isolates, the relevant markings were introduced. Polish population (symbols used in the cited publication are given in brackets): F. avenaceum FAPL (GM2), F. culmorum FCPL (KP17), F. graminearum FGPL (L22) and F. oxysporum FOPL (P6). German population (symbols used in ZALF are given in brackets): F. culmorum FC1D (ZALF 186), F. culmorum FC2D (ZALF 187), F. graminearum FG1D (ZALF 24), F. graminearum FG2D (ZALF 339) and F. poae FP0D (ZALF 338). All tested isolates were stored on PDA slants at 4 °C and subcultured every two months.
Statistical Data Analysis
Statistical analysis of the mycelial growth rate index (T) in the presence of each of the seven EsO at different concentrations was performed. Each experimental variant was repeated four times for nine isolates. For each experimental variant, the values of descriptive statistics (mean, median, minimum value, and maximum value) were determined. The Shapiro–Wilk test was used to check whether the mycelial growth rate index (in the presence of a certain amount of EsO) is a variable of normal distribution. Next, a non-parametric Kruskal–Wallis test was applied to assess whether the mycelial growth rate index (T) of Fusarium isolates in the presence of individual EsO and the activity of Funaben T differ from the control test for each of the mycelium isolates separately. If the test results were significant, multiple comparison of mean ranks for all groups (the post hoc analysis) was used to determine which pairs of essential oils differ from each other.
Additionally, the relationship between the percentage share of a given group of compounds in the EsO and the growth rate index of a given isolate in the presence of a certain amount of essential oil was examined. For this purpose, the chemical compounds contained in the EsO were divided into monoterpenes, terpenoids, sesquiterpenes, sesquiterpenoids, and other compounds, and a distinction was made between the main groups of compounds. Next, the correlation coefficients between the growth rate index of a given isolate in the presence of a certain amount of EsO and the percentage of a given group of compounds in the oil were determined.
The correlation coefficient between the growth rate index (T) and the fungistatic activity (FA) was determined. Since the correlation coefficient was high, the linear regression equation describing the relationship between FA and T was determined.
Cluster analysis and principal component analysis (PCA) were used to group the oils in terms of their effectiveness on individual isolates and to find similarities in the response of individual isolates to the oils. All statistical analyses were performed using STATISTICA 13.0 (StatSoft, Inc. TIBCO Software Inc., Carlsbad, CA, USA) at the significance level of 0.05.
5. Conclusions
A growing body of evidence shows that essential oils significantly reduce the growth of Fusarium spp. and minimize the risk of pathogens acquiring resistance. Moreover, the oils are characterized by low toxicity to humans and the environment, as they are biodegradable. Our research focused on the identification of EsO, which at low concentrations exhibit a broad spectrum of fungicidal activity (comparable to fungicides) against parasitic Fusarium spp. isolates, regardless of their origin. Given the diverse sensitivity of Fusarium spp., it is difficult to choose the type and concentration of such an oil. The sensitivity of individual Fusarium species varied. Their sensitivity, regardless of the isolates origin, in order from the most to the least sensitive, is as follows: F. culmorum, F. graminearum, F. poae, F. avenaceum and F. oxysporum. Fusarium isolates from the German population (F. culmorum FC1D, F. culmorum FC2D, F. graminearum FG1D, F. graminearum FG2D and F. poae FP0D) were generally more sensitive than those from the Polish population (F. avenaceum FAPL, F. culmorum FCPL, F. graminearum FGPL and F. oxysporum FOPL). Thyme oil has also shown a concentration independent fungicidal effect (similar to Funaben T). Citral oils (lemongrass and Litsea cubeba) acted in a similar way, but in a concentration above 0.025%. On the other hand, the fungicidal activity of the remaining oils (cajeput, verbene, TTO and grapefruit) depended on the concentration and sensitivity of the tested Fusarium isolate. Our observations demonstrate that the construction of “green chemicals” should focus on thymol and citral oils. Therefore, the presented research results may contribute to the effective protection of plants in agro-ecosystems. On the other hand, a detailed comparative analysis of the mitogenome of Fusarium spp. may offer new insights into the biology of the studied organism and will allow an understanding of the mechanism of sensitivity to essential oils.
Supplementary Materials
The following are available online. Table S1: Minimum of growth rate index of the analyzed oil at minimum concentration of the each considered isolates of Fusarium.
Author Contributions
Conceptualization, T.K.-Ł. and S.S.; methodology, T.K.-Ł.; software, A.P.-S.; formal analysis, T.K.-Ł. and M.S.; resources, T.K.-Ł. and W.W.-L.; writing—original draft preparation, T.K.-Ł., S.S. and A.P.-S.; writing—review and editing, T.K.-Ł., M.S. and A.S.; visualization, A.S.; supervision, T.K.-Ł. and M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Krzyśko-Łupicka, T.; Sokół, S.; Piekarska-Stachowiak, A. Evaluation of Fungistatic Activity of Eight Selected Essential Oils on Four Heterogeneous Fusarium Isolates Obtained from Cereal Grains in Southern Poland. Molecules 2020, 25, 292. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; de Souza Rodrigues dos Santos, P.A.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski, M. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2016, 73, 806–813. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 163–164. [Google Scholar]
- Somma, S.; Petruzzella, A.L.; Logrieco, A.F.; Meca, G.; Cacciola, O.S.; Moretti, A. Phylogenetic analyses of Fusarium graminearum strains from cereals in Italy, and characterisation of their molecular and chemical chemotypes. Crop Pasture Sci. 2014, 65, 52–60. [Google Scholar] [CrossRef]
- Bottalico, G. Perrone Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Da Cruz Cabral, L.; Pinto, V.F.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Danielewicz, B.; Gwiazdowski, R.; Bednarek-Bartsch, A. Influence of some selected fungicides on Fusarium genus cultures growth limitation. Prog. Plant Prot. 2013, 53, 759–761. [Google Scholar]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crops Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Kumar, A.; Sharma, A. Antifungal efficacy of plant essential oils against stored grain fungi of Fusarium spp. J. Food Sci. Technol. 2016, 53, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh against selected Fusarium spp. Int. J. Microbiol. 2017, 7, 8761610. [Google Scholar]
- Hara, P.; Szparaga, A.; Czerwińska, E. Ecological Methods Used to Control Fungi that Cause Diseases of the Crop Plant. Annu. Set Environ. Protect. 2018, 20, 1764–1775. [Google Scholar]
- Perczak, A.; Gwiazdowska, D.; Marchwińska, K.; Juś, K.; Gwiazdowski, R.; Waśkiewicz, A. Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch. Microbiol. 2019, 201, 1085–1097. [Google Scholar] [CrossRef]
- Krzyśko-Łupicka, T.; Walkowiak, W. Evaluation of susceptibility of phytopathogenic Fusarium culmorum strain on selected essential oils. Ecol. Chem. Eng. A 2014, 21, 355–366. [Google Scholar] [CrossRef]
- Krzyśko-Łupicka, T.; Walkowiak, W.; Białoń, M. Comparison of the fungistatic activity of selected essential oils relative to Fusarium graminearum isolates. Molecules 2019, 24, 311. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.M.D.; Hirooka, E.Y.; Ferreira, F.D.; Silva, M.V.; Mossini, S.A.G.; Machinski, M.J. Effect of Zingiber officinale Roscoe essential oil in fungus control and deoxynivalenol production of Fusarium graminearum Schwabe in vitro. Food Addit. Contam Part A 2018, 35, 2168–2174. [Google Scholar] [CrossRef]
- Giamperi, L.; Bucchini, A.E.A.; Ricci, D.; Tirillini, B.; Nicoletti, M.; Rakotosaona, R.; Maggi, F. Vepris macrophylla (Baker) I. Verd Essential Oil: An Antifungal Agent against Phytopathogenic Fungi. Int. J. Mol. Sci. 2020, 21, 2776. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes. World. Annu Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Naeini, A.; Ziglarib, T.; Shokrib, H.; Khosravi, A.R. Assessment of growth-inhibiting effect of some plant essential oils on different Fusarium isolates. J. Med. Mycol. 2010, 20, 174–178. [Google Scholar] [CrossRef]
- Mackay, T.F.C.; Stone, E.A.; Ayroles, J.F. The genetics of quantitative traits: Challenges and prospects. Nat. Rev. Genet. 2009, 10, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Waalwijk, C.; Taga, M.; Zheng, S.L.; Proctor, R.H.; Vaughan, M.M.; O’Donnell, K. Karyotype evolution in Fusarium. IMA Fungus 2018, 9, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Komatsu, K.; Taga, M.; Arie, T. Cytological karyotyping of Fusarium oxysporum by the germ tube burst method (GTBM). J. Gen. Plant Pathol. 2018, 84, 254–261. [Google Scholar] [CrossRef]
- Fitzpatrick, D.A. Horizontal gene transfer in fungi. FEMS Microbiol. Lett. 2012, 329, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef]
- Tuveson, R.W.; Garber, E.D. Genetics of phytopathogenic fungi. IV. Experimentally induced alterations in nuclear ratios of heterocaryons of Fusarium oxysporium F. Pisi. Genetics 1961, 46, 485–492. [Google Scholar] [CrossRef]
- Teunissen, H.A.S.; Verkooijen, J.; Cornelissen, B.J.C.; Haring, M.A. Genetic exchange of avirulence determinants and extensive karyotype rearrangements in parasexual recombinants of Fusarium oxysporum. Mol. Gen. Genom. 2002, 268, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Láday, M.; Mulè, G.; Moretti, A.; Hamari, Z.; Juhász, Á.; Szécsi, Á.; Logrieco, A. Mitochondrial DNA variability in Fusarium proliferatum (Gibberella intermedia). Eur. J. Plant Pathol. 2004, 110, 563–571. [Google Scholar] [CrossRef]
- Brankovics, B.; van Dam, P.; Rep, M.; Sybren de Hoog, G.; van der Lee, T.A.J.; Waalwijk, C.; van Diepeningen, A.D. Mitochondrial genomes reveal recombination in the presumed asexual Fusarium oxysporum species complex. BMC Genom. 2017, 18, 735. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, S.; Maurya, D.K.; Kashyap, P.L.; Srivastava, A.K.; Anandaraj, M. Cross-species transferability of microsatellite markers from Fusarium oxysporum for the assessment of genetic diversity in Fusarium udum. Phytoparasitica 2013, 41, 615–622. [Google Scholar] [CrossRef]
- Duggal, A.; Dumas, M.T.; Jeng, R.S.; Hubbes, M. Ribosomal variation in six species of Fusarium. Mycopathologia 1997, 140, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Kageyama, K.; Shimizu, M.; Hyakumachi, M. A natural mutation involving both pathogenicity and perithecium formation in the Fusarium graminearum species complex. G3 Genes Genomes Genet. 2016, 6, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zehraoui, E.; Atanasoff-Kardjalieff, A.K.; Strauss, J.; Studt, L.; Ponts, N. Effect of H2A.Z deletion is rescued by compensatory mutations in Fusarium graminearum. PLoS Genet. 2020, 16, e1009125. [Google Scholar] [CrossRef] [PubMed]
- Magdama, F.; Monserrate-Maggi, L.; Serrano, L.; Onofre, J.G.; del Mar Jiménez-Gasco, M. Genetic diversity of Fusarium oxysporum f. sp. cubense, the Fusarium wilt pathogen of banana, in Ecuador. Plants 2020, 9, 1133. [Google Scholar] [CrossRef]
- Halpern, H.C.; Qi, P.; Kemerait, R.C.; Brewer, M.T. Genetic diversity and population structure of races of Fusarium oxysporum causing cotton wilt. G3 Genes Genomes Genet. 2020, 10, 3261–3269. [Google Scholar] [CrossRef]
- Groenewald, S.; van den Berg, N.; Marasas, W.F.O.; Viljoen, A. Biological, physiological and pathogenic variation in a genetically homogenous population of Fusarium oxysporum f. sp. cubense. Australas. Plant Pathol. 2006, 35, 401–409. [Google Scholar] [CrossRef]
- Covo, S. Genomic Instability in Fungal Plant Pathogens. Genes 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Scientific Publications: Hoboken, NJ, USA, 2006; 400p. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Numpaque, M.A.; Oviedo, L.A.; Gil, J.H.; García, C.M.; Durango, D.L. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop. Plant Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef]
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1359–1366. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Vera-López, O.; Palou, E.; Avila-Sosa, R. Growth modelling to control (in vitro) Fusarium verticillioides and Rhizopus stolonifer with thymol and carvacrol. Rev. Argent. Microbiol. 2018, 50, 70–74. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboodyb, M.S.; Vijayakumarb, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Dhamodaran, N.; Siddaiah, C.; Mudili, V.; Sreepathi, M.H. Antifungal and Zearalenone Inhibitory Activity of Ocimum sanctum L. Essential Oil on Fusarium graminearum Determined by UHPLC and RT-qPCR. Bio-Protocol 2016, 6, e1893. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds. Pathogens 2020, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Bermejo, D.; Angelov, I.; Vicente, G.; Stateva, R.P.; Rodriguez García-Risco, M.; Reglero, G.; Ibañez, E.; Fornari, T. Extraction of thymol from different varieties of thyme plantsusing green solvents. J. Sci. Food Agric. 2015, 95, 2901–2907. [Google Scholar] [CrossRef]
- Gavarić, N.; Smole Možina, S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 6, 630–656. [Google Scholar] [CrossRef]
- Barrera-Necha, L.L.; Garduno-Pizana, C.; Garcia-Barrera, L.J. In vitro Antifungal Activity of Essential Oils and Their Compounds on Mycelial Growth of Fusarium oxysporum f. sp. gladioli (Massey) Snyder and Hansen. Plant Pathol. J. 2009, 8, 17–21. [Google Scholar] [CrossRef]
- Seseni, L.; Regnier, T.; Roux van der Merwe, M.P.; Mogale, E.; Badenhorst, J. Control of Fusarium spp. causing damping off of pine seedlings by means of selected essential oils. Ind. Crops Prod. 2015, 76, 329–332. [Google Scholar] [CrossRef]
- Ćosić, J.; Vrandečić, K.; Postić, J.; Jurković, D.; Ravlić, M. In vitro antifungal activity of essential oils on growth of phytopathogenic fungi. Poljoprivreda 2010, 16, 25–28. [Google Scholar]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiol. Open 2017, 6, e00459. [Google Scholar] [CrossRef]
- Manganyi, M.C.; Regnier, T.; Olivier, E.I. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S. Afr. J. Bot. 2015, 99, 115–121. [Google Scholar] [CrossRef]
- Simić, A.; Soković, M.D.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; Marin, P.D. The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother. Res. 2004, 18, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, V.M.; Conti, R.; Araújo, J.M.; Souza-Motta, C.M. Endophytic fungi from the medicinal plant Lippia sidoides Cham. and their antimicrobial activity. Symbiosis 2011, 53, 89–95. [Google Scholar] [CrossRef]
- Maggi, F.; Randriana, R.F.; Rasoanaivo, P.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; et al. Chemical composition and in vitro biological activities of the essential oil of Veprys macrophylla (Baker) I. Verd. Endemic to Madagascar. Chem. Biodivers. 2013, 10, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Dudai, N.; Weinstein, Y.; Krup, M.; Rabinski, T.; Ofir, R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005, 71, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Tajidin, N.E.; Ahmad, S.H.; Rosenani, A.B.; Azimah, H.; Munirah, M. Chemical composition and citral content in lemongrass (Cymbopogon citratus) essential oil at three maturity stages. Afr. J. Biotechnol. 2012, 11, 2685–2693. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, J.; Muranyi, P. Review on the chemical composition of Litsea cubeba essential oils and the bioactivity of its major constituents citral and limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Sadowska, K.; Łukaszewska-Skrzypniak, N.; Wojczyńska, J.; Stępniewska-Jarosz, S.; Tyrakowska, M.; Rataj-Guranowska, M. Evaluation of susceptibility of potential rape pathogens to selected essential oils. Prog. Plant Prot. 2017, 57, 201–205. [Google Scholar] [CrossRef]
- Morcia, C.; Malanati, M.; Terzi, V. In vitro activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 415–422. [Google Scholar] [CrossRef][Green Version]
- Jamiołkowska, A. Laboratory effect of azoxystrobin (Amistar 250 SC) and grapefruit extract (Biosept 33 SL) on growth of fungi colonizing zucchini plants. Acta Sci. Pol. Hortorum Cultus 2011, 10, 245–257. [Google Scholar]
- Hashem, M.; Moharam, A.M.; Zaied, A.A.; Saleh, F.E.M. Efficacy of essential oils in the control of cumin root rot disease caused by Fusarium spp. Crop Prot. 2010, 29, 1111–1117. [Google Scholar] [CrossRef]
- Macias, F.A.; Marin, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Feng, W.; Zheng, X. Essential oils to control Alternaria alternata in vitro and in vivo. Food Control 2007, 18, 1126–1130. [Google Scholar] [CrossRef]
- Riccioni, L.; Orzeli, L. Activity of tea tree (Melaleuca alternifolia, Cheel) and thyme (Thymus vulgaris, Linnaeus.) essential oil against some pathogenic seed borne fungi. J. Essent. Oil Res. 2011, 23, 43–47. [Google Scholar] [CrossRef]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P. Chemical composition of two different lavender essential oils and their effect on facial skin microbiota. Molecules 2019, 18, 3270. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chen, J.; Zheng, X.; Liu, Q. Thyme oil to control Alternaria alternata in vitro and in vivo as fumigant and contact treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Wagle, B.; Budathoki, U. Antifungal Activities of Essential Oils and Crude Extracts of Some Aromatic Plants against Fusarium Rot of Trichosanthes dioica. Nepal J. Sci. Technol. 2013, 13, 97–102. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).