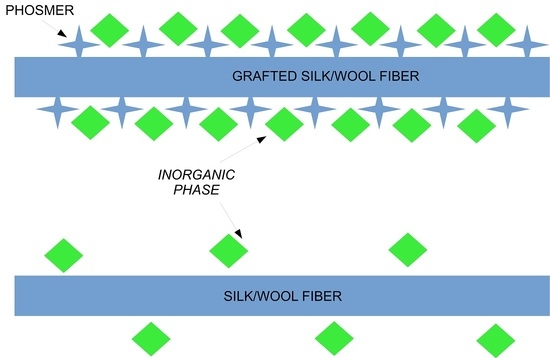
Vibrational Study on Structure and Bioactivity of Protein Fibers Grafted with Phosphorylated Methacrylates
Abstract
1. Introduction
2. Results
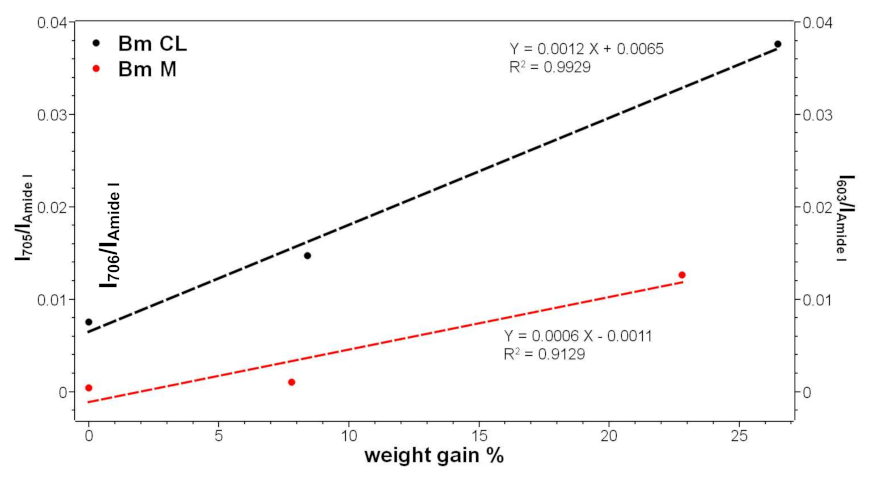
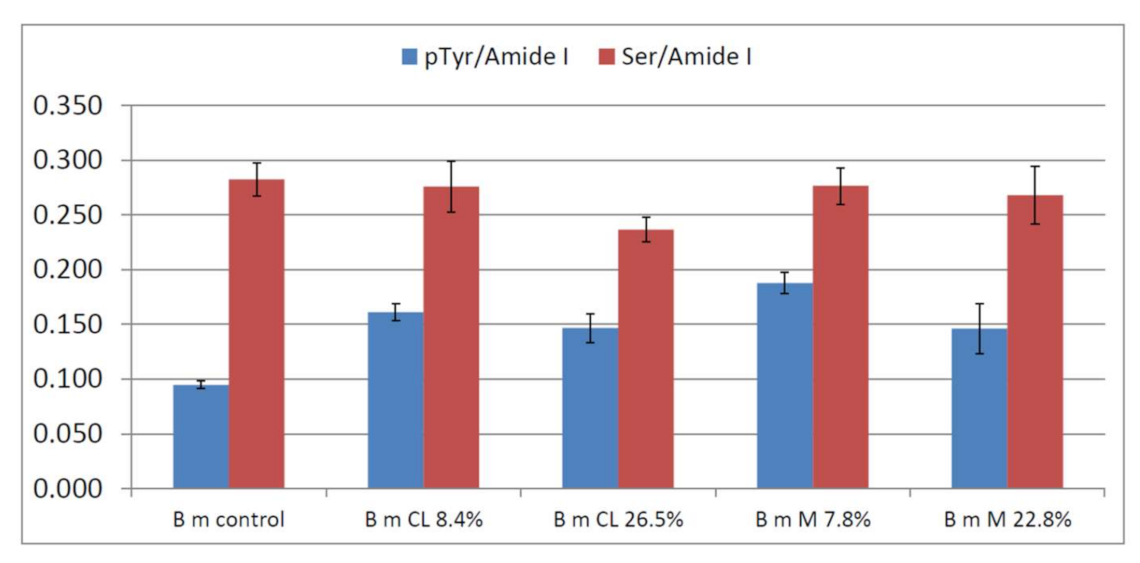
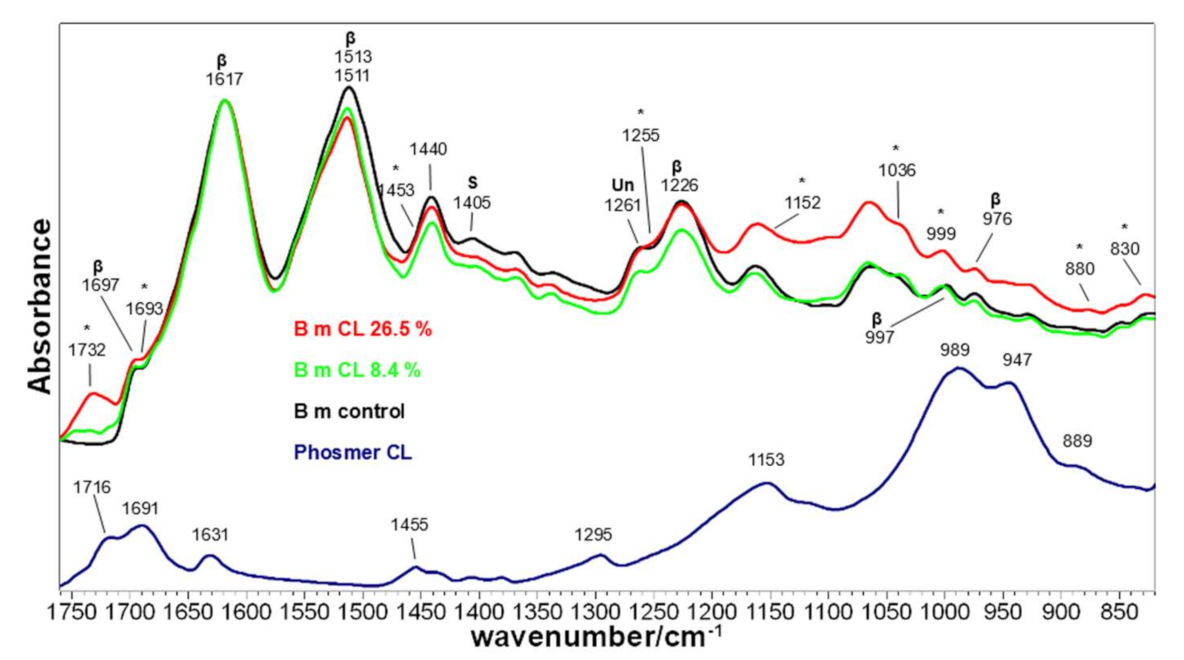
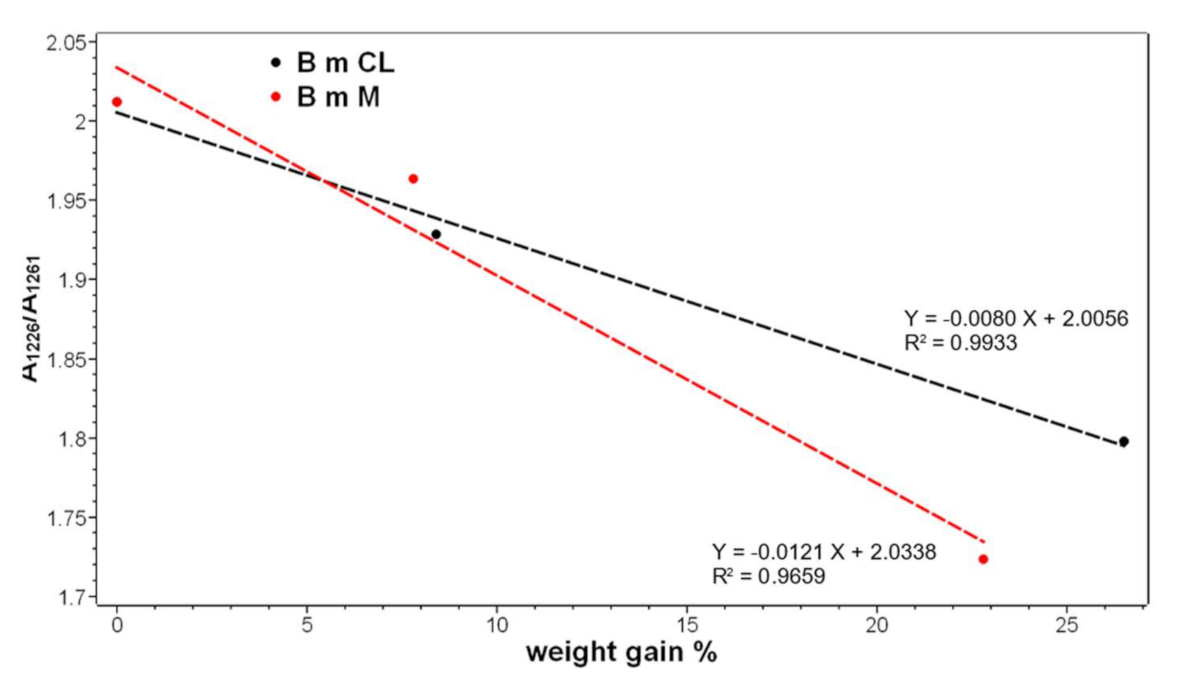
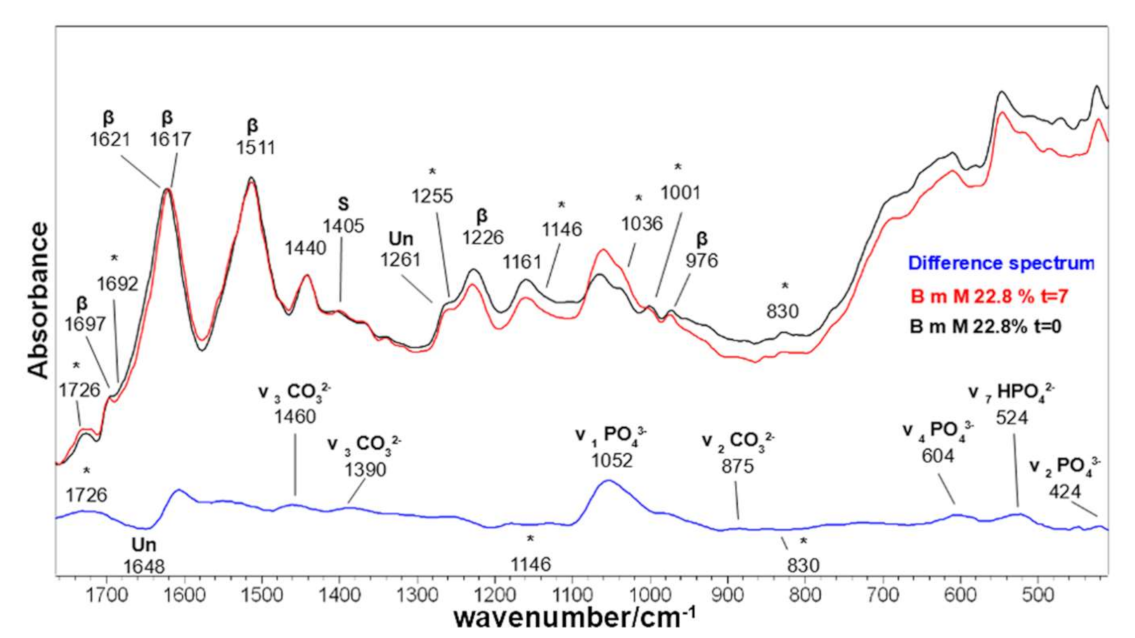
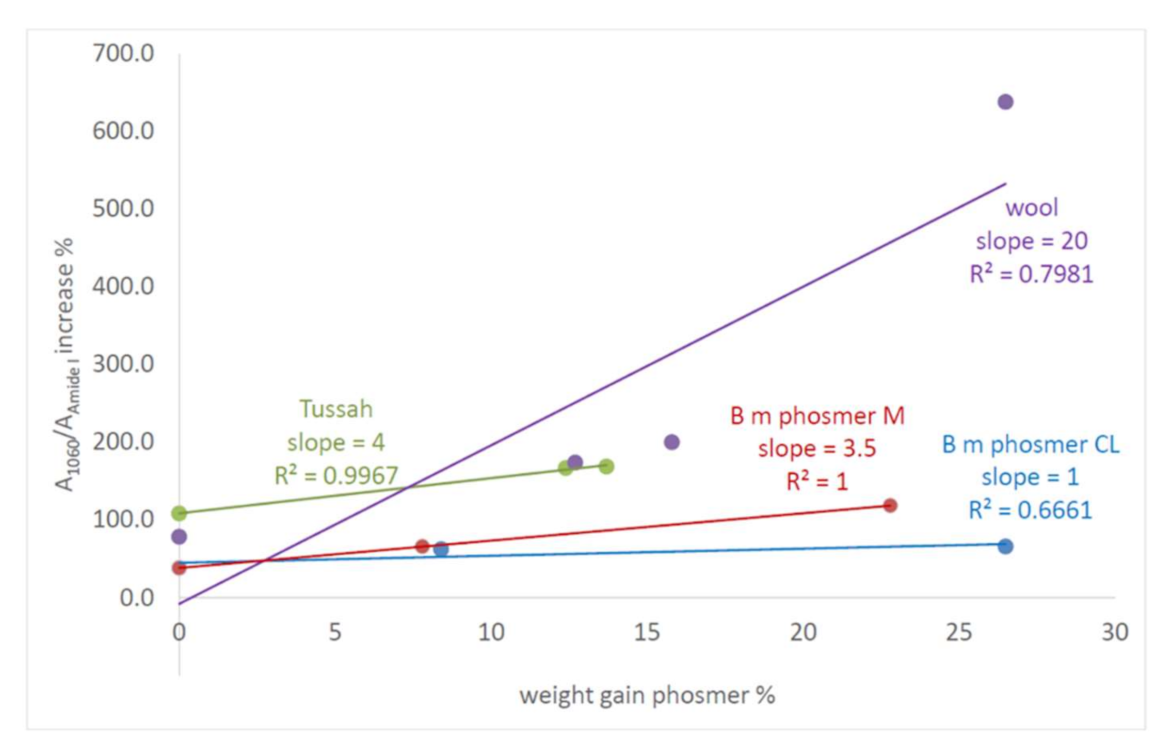
2.1. Vibrational Analyses of Grafted B. mori Silk Fibroin Fabrics
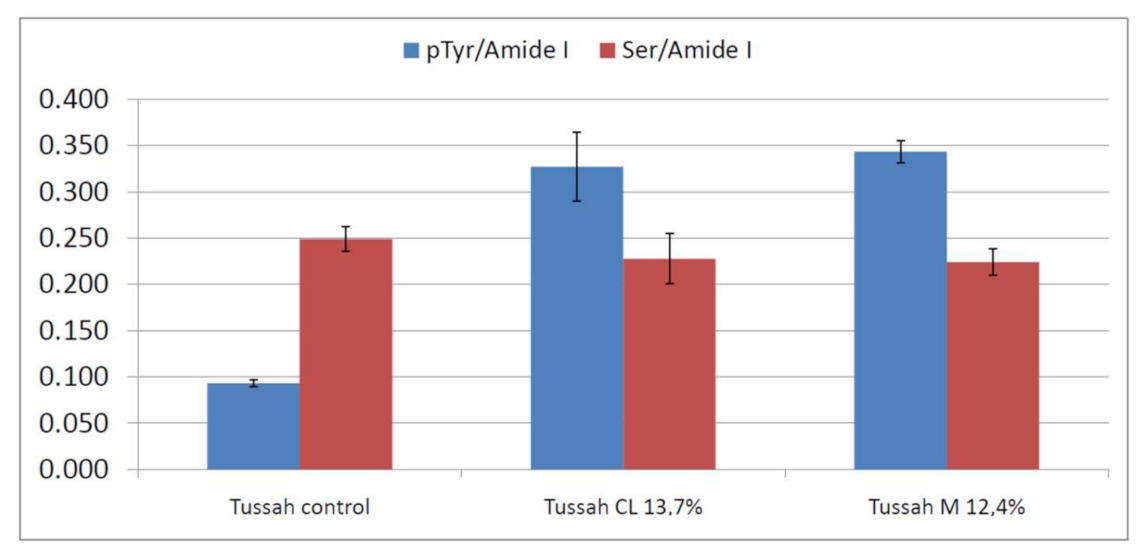
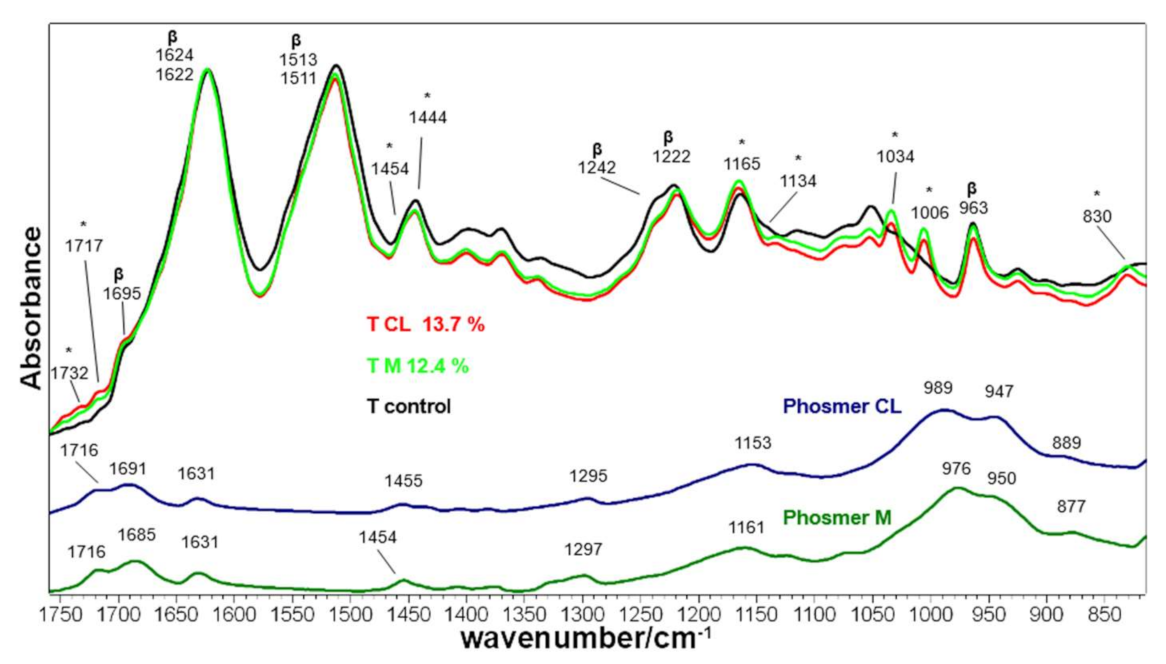
2.2. Vibrational Analyses of Grafted Tussah Silk Fibroin Fabrics
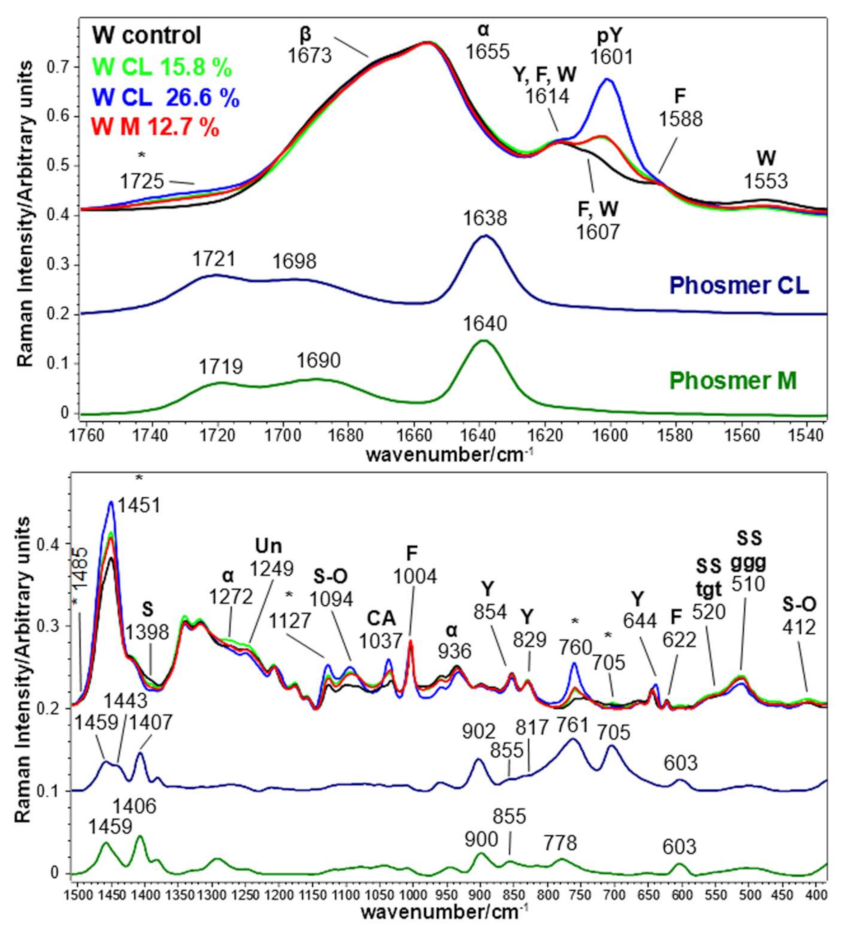
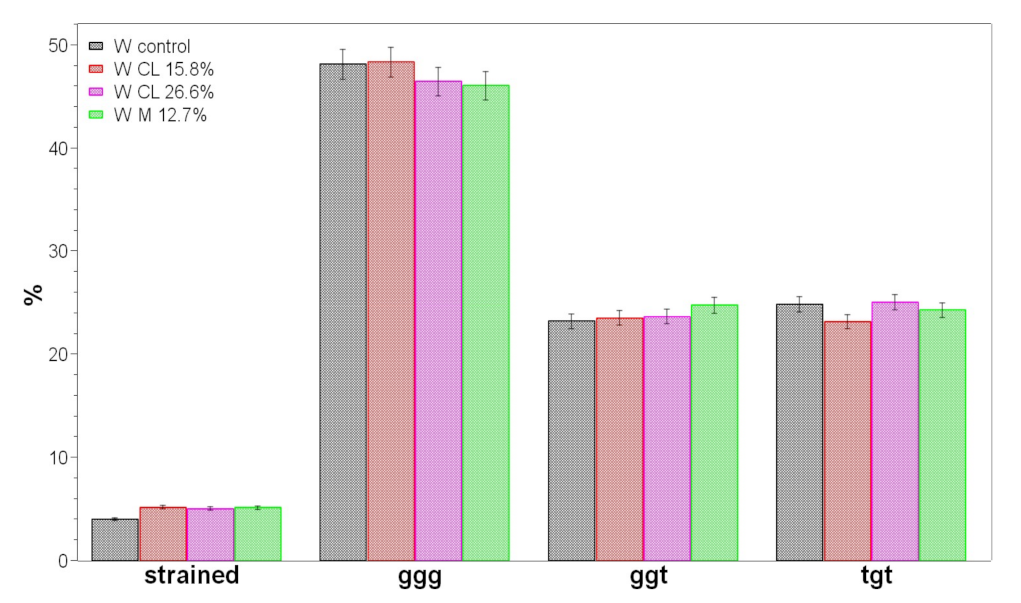
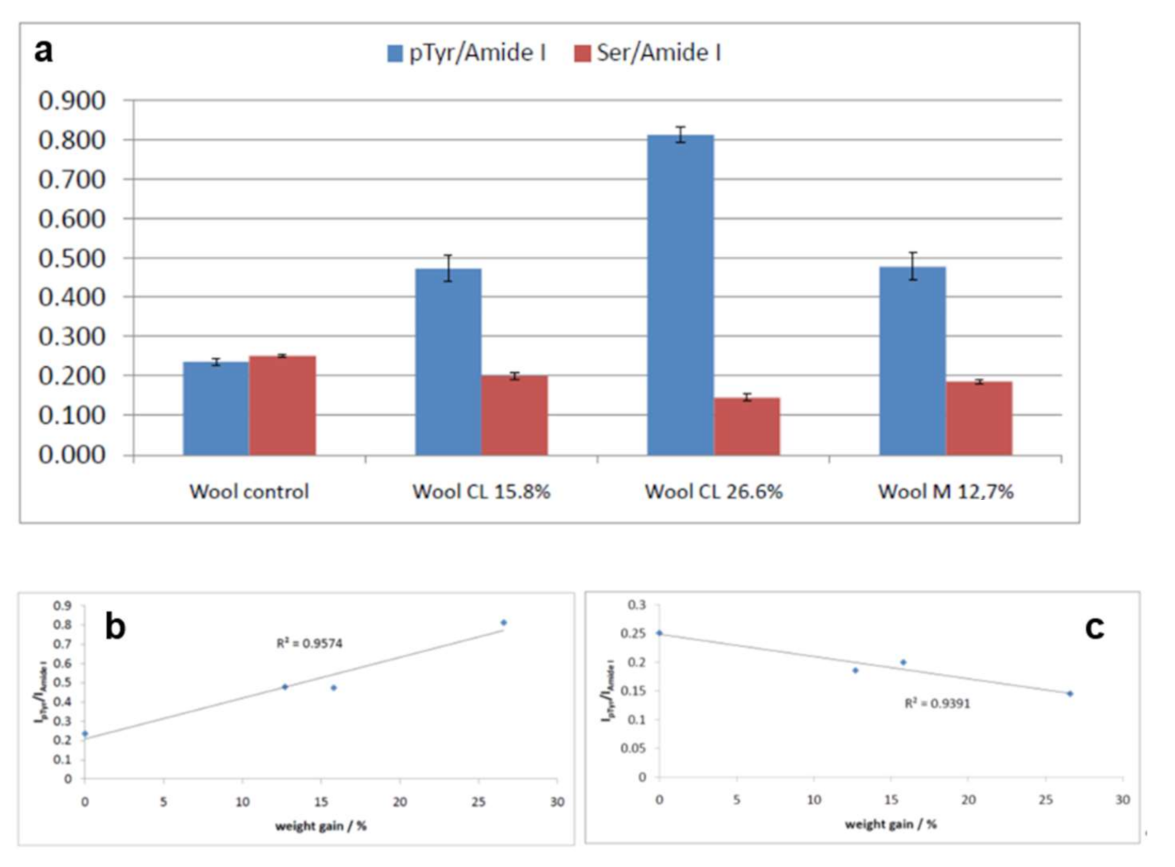
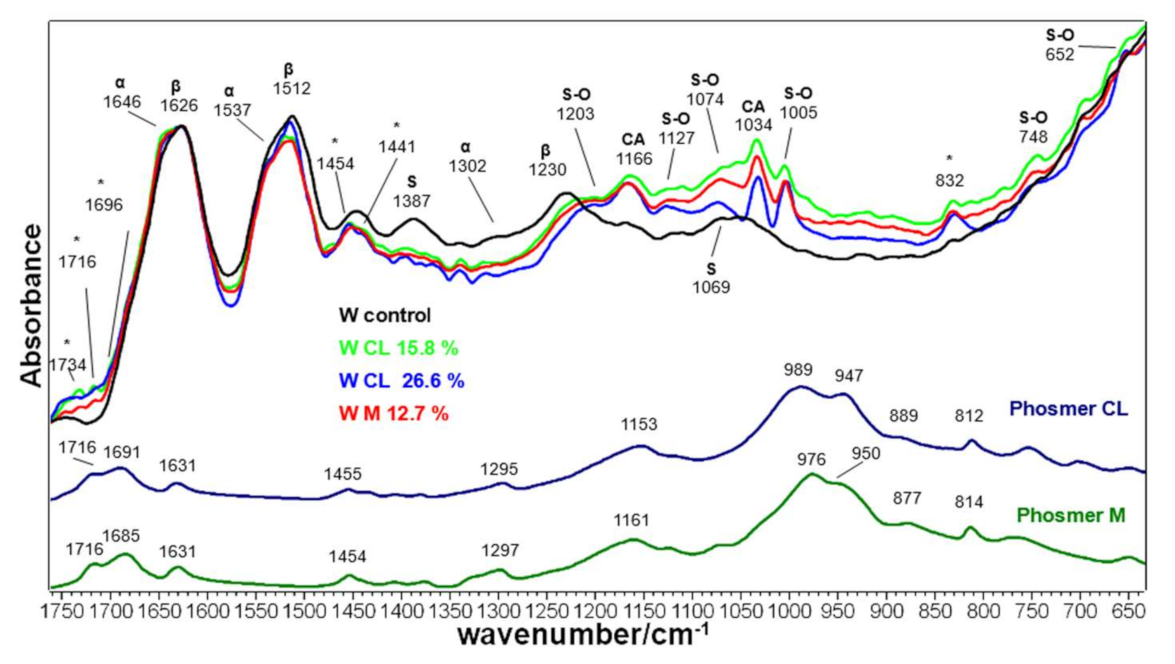
2.3. Vibrational Analyses of Grafted Wool Keratin Fabrics
2.4. Bioactivity Tests
3. Materials
3.1. Materials
3.2. Grafting
3.3. Bioactivity Tests
3.4. Vibrational Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guru, J.; Manishekhar, K.; Dimple, C.J.; Christakiran, M.; Ankit, G.; Sohenii, B.; Biman, B.M. Insight into silk-based biomaterials: From physicochemical attributes to recent biomedical applications. ACS Appl. Bio Mater. 2019, 2, 5460–5491. [Google Scholar]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zeng, W.; Meng, Z.; Luo, W.; Ma, L.; Li, Y.; Lin, C.; Huang, Q.; Lin, Y.; Liu, X.Y. Using wool keratin as a basic resist material to fabricate precise protein patterns. Adv. Mater. 2019, 31, 1900870. [Google Scholar] [CrossRef]
- Kaplan, D.L.; Adams, W.W.; Farmer, B.; Viney, C.; Kaplan, D.L.; Adams, W.W. Silk: Biology, structure, properties, and genetics. ACS Symp. Ser. 1994, 544, 2–16. [Google Scholar]
- Lucas, F.; Shaw, J.T.B.; Smith, S.G. The silk fibroins. Adv. Protein Chem. 1958, 13, 107. [Google Scholar] [PubMed]
- Nakazawa, Y.; Asakura, T. High-resolution 13C CP/MAS NMR study on structure and structural transition of Antheraea pernyi silk fibroin containing poly(l-alanine) and gly-rich regions. Macromolecules 2002, 35, 2393–2400. [Google Scholar] [CrossRef]
- Fraser, R.D.D.; MacRae, T.P. Conformation in Fibrous Proteins and Related Synthetic Polypeptides; Academic Press: Cambridge, MA, USA, 1973. [Google Scholar]
- Zahn, H.; Wortmann, F.J.; Wortmann, G.; Schäfer, K.; Hoffmann, R.; Finch, R. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH GmbH: Weinheim, Germany, 2006; Volume 39, p. 525. [Google Scholar]
- Bradbury, J.H.; Ley, K.F. The chemical composition of wool. XI. Separation and analysis of exocuticle and endocuticle. Aust. J. Biol. Sci. 1972, 25, 1235–1247. [Google Scholar] [CrossRef]
- Freddi, G.; Tsukada, M. Silk fibers (grafting). In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1996; Volume 10, p. 7734. [Google Scholar]
- Kameda, T.; Tsukada, M. Structure and thermal analyses of MAA-grafted silk fiber using DSC and solid-state NMR. Macromol. Mater. Eng. 2006, 291, 877–882. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Monti, P.; Bertoluzza, A.; Shiozaki, H. Physical Properties of 2-hydroxyethyl Methacrylate-grafted Silk Fibers. J. Appl. Polym. Sci. 1993, 49, 1835–1844. [Google Scholar] [CrossRef]
- Freddi, G.; Massafra, M.R.; Beretta, S.; Shibata, S.; Gotoh, Y.; Yasui, H.; Tsukada, M. Structure and properties of Bombyx mori silk fibers grafted with MAA and HEMA. J. Appl. Polym. Sci. 1996, 60, 1867–1876. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Rosaria, M.; Bretta, S. Structure and properties of tussah silk fibers Graft-copolymerised with MAA and HEMA. J. Appl. Polym. Sci. 1998, 67, 1393–1403. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Ishiguro, Y.; Shiozaki, H. Structural analysis of methacrylamide-grafted silk fibers. J. Appl. Polym. Sci. 1993, 50, 1519–1527. [Google Scholar] [CrossRef]
- Tsukada, M.; Gotoh, Y.; Freddi, G.; Yamamoto, T.; Nakabayashi, N. Molecular weight distribution of the methyl methacrylate (MMA) polymer separated from the MMA-grafted silk fiber. J. Appl. Polym. Sci. 1992, 44, 2197–2202. [Google Scholar] [CrossRef]
- Guan, J.; Chen, G. Flame resistant modification of silk fabric with vinyl phosphate. Fibers Polym. 2008, 9, 438–443. [Google Scholar] [CrossRef]
- Tsukada, M.; Khan, M.R.; Tanaka, T.; Morikawa, H. Thermal characteristics and physical properties of silk fabrics grafted with phosphorous flame retardant agents. Text. Res. J. 2011, 81, 1541–1548. [Google Scholar] [CrossRef]
- Sato, K.; Kumagai, Y.; Tanaka, J. Apatite formation on organic monolayers in simulated body environment. J. Biomed. Mater. Res. 2000, 50, 16–20. [Google Scholar] [CrossRef]
- Tanahashi, M.; Matsuda, T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 1997, 34, 305–315. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Tamada, Y. Influence of casting temperature on the near-surface structure and wettability of cast silk fibroin films. Langmuir 2001, 17, 7406–7413. [Google Scholar] [CrossRef]
- Kawai, T.; Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T.; Tanihara, M.; Sakaguchi, Y.; Konagaya, S. Coating of an apatite layer on polyamide films containing sulfonic groups by a biomimetic process. Biomaterials 2004, 25, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Griffanti, G.; James-Bhasin, M.; Donelli, I.; Freddi, G.; Nazhat, S.N. Functionalisation of silk fibroin through anionic fibroin derived polypeptides. Biomed. Mater. 2019, 14, 015006. [Google Scholar] [CrossRef]
- Beniash, E. Biominerals–hierarchical nanocomposites: The example of bone. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 47–69. [Google Scholar] [CrossRef]
- He, G.; Dahl, T.; Veis, A.; George, A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003, 2, 552–558. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Dollish, F.R.; Fateley, W.G.; Bentley, F.F. Characteristic Raman Frequencies of Organic Compounds; Wiley: Hoboken, NJ, USA, 1973. [Google Scholar]
- Edwards, H.G.M.; Farwell, D.W. Raman spectroscopic studies of silk. J. Raman Spectrosc. 1995, 26, 901–909. [Google Scholar] [CrossRef]
- Monti, P.; Taddei, P.; Freddi, G.; Asakura, T.; Tsukada, M. Raman spectroscopic characterization of Bombyx mori silk fibroin: Raman spectrum of silk I. J. Raman Spectrosc. 2001, 32, 103–107. [Google Scholar] [CrossRef]
- Lipschitz, I. The vibrational spectrum of poly(methyl methacrylate): A Review. Polym. Plast. Technol. Eng. 1982, 19, 53–106. [Google Scholar] [CrossRef]
- Taddei, P.; Balducci, F.; Simoni, R.; Monti, P. Raman, IR and thermal study of a new highly biocompatible phosphorylcholine-based contact lens. J. Mol. Struct. 2005, 744, 507–514. [Google Scholar] [CrossRef]
- Pavoni, E.; Tsukada, M.; Taddei, P. Influence of grafting with acrylate compounds on the conformational rearrangements of silk fibroin upon electrospinning and treatment with aqueous methanol. J. Raman Spectrosc. 2016, 47, 1367–1374. [Google Scholar] [CrossRef]
- Koyama, Y.; Toda, S.; Kyogoku, Y. Raman spectra and conformation of the glycerophosphorylcholine headgroup. Chem. Phys. Lipids 1977, 19, 74–92. [Google Scholar] [CrossRef]
- Spiker, R.C., Jr.; Levin, I.W. Raman spectra and vibrational assignments for dipalmitoyl phosphatidylcholine and structurally related molecules. Biochim. Biophys. Acta 1975, 383, 361–373. [Google Scholar] [CrossRef]
- Zhang, D.; Ortiz, C.; Xie, Y.; Davisson, V.J.; Ben-Amotz, D. Detection of the site of phosphorylation in a peptide using raman spectroscopy and partial least squares discriminant analysis. Spectrochim. Acta Part A 2006, 61, 471–475. [Google Scholar] [CrossRef]
- Monti, P.; Freddi, G.; Bertoluzza, A.; Kasai, N.; Tsukada, M. Raman spectroscopic studies of silk fibroin from Bombyx mori. J. Raman Spectrosc. 1998, 29, 297. [Google Scholar] [CrossRef]
- Taddei, P.; Di Foggia, M.; Martinotti, S.; Ranzato, E.; Carmagnola, I.; Chiono, V.; Tsukada, M. Silk fibers grafted with 2-hydroxyethyl methacrylate (HEMA) and 4-hydroxybutyl acrylate (HBA) for biomedical applications. Int. J. Biol. Macromol. 2018, 107, 537. [Google Scholar] [CrossRef] [PubMed]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation of Bombyx mori silk fibroin fibers and film. J. Appl. Polym. Sci. 2004, 91, 2383–2390. [Google Scholar] [CrossRef]
- Ashton, L.; Johannessen, C.; Goodacre, R. The importance of protonation in the investigation of protein phosphorylation using raman spectroscopy and raman optical activity. Anal. Chem. 2011, 83, 7978–7983. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.T. Raman Spectroscopy in Biology: Principles and Applications; John Wiley: Hoboken, NJ, USA, 1982. [Google Scholar]
- Tsafack, M.J.; Levalois-Grützmacher, J. Plasma induced graft-polymerisation of flame retardant monomers onto PAN fabrics. Surf. Coat. Technol. 2006, 200, 3503–3510. [Google Scholar] [CrossRef]
- Magoshi, J.; Magoshi, Y. Physical properties and structure of silk. VI. Conformational changes in silk fibroin induced by immersione in water at 2 to 130 °C. J. Polym. Sci. 1975, 13, 1347–1351. [Google Scholar]
- Magoshi, J.; Mizuide, M.; Magoshi, Y.; Takahashi, K.; Kubo, M.; Nakamura, S. Physical properties and structure of silk. II. Dynamic mechanical and dielectric properties of silk fibroin. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 515–520. [Google Scholar] [CrossRef]
- Monti, P.; Freddi, G.; Arosio, C.; Tsukada, M.; Arai, T.; Taddei, P. Vibrational spectroscopic study of sulphated silk proteins. J. Mol. Struct. 2007, 834–836, 202–206. [Google Scholar] [CrossRef]
- Taddei, P.; Tsukada, M.; Freddi, G. Affinity of protein fibres towards sulfation. J. Raman Spectrosc. 2013, 44, 190–197. [Google Scholar] [CrossRef]
- Moore, W.H.; Krimm, S. Vibrational analysis of peptides, polypeptides, and proteins. I. Polyglycine I. Biopolymers 1976, 15, 439–2464. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Freddi, G.; Monti, P.; Bertoluzza, A.; Kasai, N. Structure and molecular conformation of tussah silk fibroin films—Effect of methanol. J. Polym. Sci. B 1995, 33, 1995–2001. [Google Scholar] [CrossRef]
- Pavoni, E.; Tozzi, S.; Tsukada, M.; Taddei, P. Structural study on methacrylamide-grafted tussah silk fibroin fibres. Int. J. Biol. Macromol. 2016, 88, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.; Arai, T.; Boschi, A.; Monti, P.; Tsukada, M.; Freddi, G. In vitro study of the proteolytic degradation of Antheraea pernyi silk fibroin. Biomacromolecules 2006, 6, 259–267. [Google Scholar] [CrossRef]
- Taddei, P.; Monti, P.; Freddi, G.; Arai, T.; Tsukada, M. IR study on the binding mode of metal cations to chemically modified Bombyx mori and tussah silk fibres. J. Mol. Struct. 2003, 651, 433–441. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nadiger, G.S. Crystallinity in silk fibers: Partial acid hydrolysis and related studies. J. Appl. Polym. Sci. 1980, 25, 921–932. [Google Scholar] [CrossRef]
- Freddi, G.; Monti, P.; Nagura, M.; Gotoh, Y.; Tsukada, M. Structure and molecular conformation of tussah silk fibroin films: Effect of heat treatment. J. Polym. Sci. B 1997, 35, 841–847. [Google Scholar] [CrossRef]
- Church, J.S.; Corino, G.L.; Woodhead, A.L. The analysis of merino wool cuticle and cortical cells by fourier transform raman spectroscopy. Biopolymers 1997, 42, 7–17. [Google Scholar] [CrossRef]
- Kuzuhara, A.; Hori, T. Reduction mechanism of tioglycolic acid on keratin fibres using microspectrophotometry and FT-raman spectroscopy. Polymer 2003, 44, 7963–7970. [Google Scholar] [CrossRef]
- Taddei, P.; Boga, C.; Micheletti, G.; Ballarin, B. Vibrational study on the interactions between yak keratin fibres and glyoxylic acid. J. Raman Spectrosc. 2015, 46, 100–108. [Google Scholar] [CrossRef]
- Frushour, B.G.; Koenig, J.L. Raman spectroscopic study of poly (β-benzyl-L-aspartate) and sequential polypeptides. Biopolymers 1975, 14, 2115–2135. [Google Scholar] [CrossRef]
- Kuzuhara, A. Analysis of structural changes in bleached keratin fibers (black and white human hair) using raman spectroscopy. Biopolymers 2006, 81, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, W.; Edwards, H.G.W.; Farwell, D.W.; Nutbrown, N. Fourier-transform raman spectroscopic study of human hair. Spectrochim. Acta Part A 1997, 53, 1021–1031. [Google Scholar] [CrossRef]
- Di Foggia, M.; Boga, C.; Micheletti, G.; Nocentini, B.; Taddei, P. Vibrational raman and IR data on brown hair subjected to bleaching. Data Brief 2021, 38, 107439. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared Characteristic Group Frequencies, 2nd ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1980. [Google Scholar]
- Pielesz, A.; Wlochowicz, A. Semiempirical infrared spectra simulations for some aromatic amines of interest for azo dye chemistry. Spectrochim. Acta Part A 2001, 57, 2637–2646. [Google Scholar] [CrossRef]
- Di Foggia, M.; Boga, C.; Micheletti, G.; Nocentini, B.; Taddei, P. Structural investigation on damaged hair keratin treated with α,β-unsaturated michael acceptors used as repairing agents. Int. J. Biol. Macromol. 2021, 167, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Parker, F.S. Applications of Infrared Spectroscopy in Biochemistry, Biology and Medicine; Plenum Press: New York, NY, USA, 1971; Volume 10. [Google Scholar]
- Signori, V.; Lewis, D.M. FTIR investigation of the damage produced on human hair by weathering and bleaching processes: Implementation of different sampling techniques and data processing. Int. J. Cosmet. Sci. 1997, 19, 1–13. [Google Scholar] [CrossRef]
- Carter, E.A.; Fredericks, P.M.; Church, J.S.; Denning, R.J. FT-raman spectroscopy of wool—I. Preliminary studies. Spectrochim. Acta Part A 1994, 50, 1927–1936. [Google Scholar] [CrossRef]
- Savige, W.E.; Eager, J.; MacLaren, J.A.; Roxburgh, M. The S-monoxides of cystine, cystamine and homocysteine. Tetrahedron Lett. 1964, 44, 3289–3293. [Google Scholar] [CrossRef]
- Furuzono, T.; Taguchi, T.; Kishida, A.; Akashi, M.; Tamada, Y. Preparation and characterisation of apatite deposited on silk fabrics using an alternate soaking process. J. Biomed. Mater Res. 2000, 50, 344–352. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.J.D.M.; Sone, E.D. In vitro models of collagen biomineralisation. J. Struct. Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Di Foggia, M.; Prati, C.; Gandolfi, M.G.; Taddei, P. An in vitro study on dentin demineralisation and remineralisation: Collagen rearrangements and influence on the enucleated phase. J. Inorg. Biochem. 2019, 193, 84–93. [Google Scholar] [CrossRef]




| Phosmer | Type of Fabric and % Weight Gain | Reaction Time (min) | Sample Acronym |
|---|---|---|---|
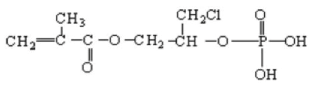 | B. mori silk fibroin, 8.4% | 40 | B m CL 8.4% |
| B. mori silk fibroin, 26.5% | 90 | B m CL 26.5% | |
| A. pernyi silk fibroin, 13.7% | 90 | T CL 13.7% | |
| Wool keratin, 15.8% | 40 | W CL 15.8% | |
| Wool keratin, 26.6% | 90 | W CL 26.6% | |
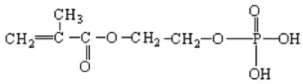 | B. mori silk fibroin, 7.8% | 40 | B m M 7.8% |
| B. mori silk fibroin, 22.8% | 90 | B m M 22.8% | |
| A. pernyi silk fibroin, 12.4% | 90 | T M 12.4% | |
| Wool keratin, 12.7% | 40 | W M 12.7% |
| Sample | Weight Gain % |
|---|---|
| B m control | 2.02 |
| B m CL 8.4% | 0.38 |
| B m CL 26.5% | −0.64 |
| B m M 7.8% | 1.28 |
| B m M 22.8% | 8.49 |
| T control | 0.85 |
| T CL 13.7% | 1.83 |
| T M 12.4% | 4.03 |
| W control | 3.53 |
| W CL 15.8% | 2.49 |
| W CL 26.6% | −0.39 |
| W M 12.7% | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Foggia, M.; Tsukada, M.; Taddei, P. Vibrational Study on Structure and Bioactivity of Protein Fibers Grafted with Phosphorylated Methacrylates. Molecules 2021, 26, 6487. https://doi.org/10.3390/molecules26216487
Di Foggia M, Tsukada M, Taddei P. Vibrational Study on Structure and Bioactivity of Protein Fibers Grafted with Phosphorylated Methacrylates. Molecules. 2021; 26(21):6487. https://doi.org/10.3390/molecules26216487
Chicago/Turabian StyleDi Foggia, Michele, Masuhiro Tsukada, and Paola Taddei. 2021. "Vibrational Study on Structure and Bioactivity of Protein Fibers Grafted with Phosphorylated Methacrylates" Molecules 26, no. 21: 6487. https://doi.org/10.3390/molecules26216487
APA StyleDi Foggia, M., Tsukada, M., & Taddei, P. (2021). Vibrational Study on Structure and Bioactivity of Protein Fibers Grafted with Phosphorylated Methacrylates. Molecules, 26(21), 6487. https://doi.org/10.3390/molecules26216487