2. Amide Receptors
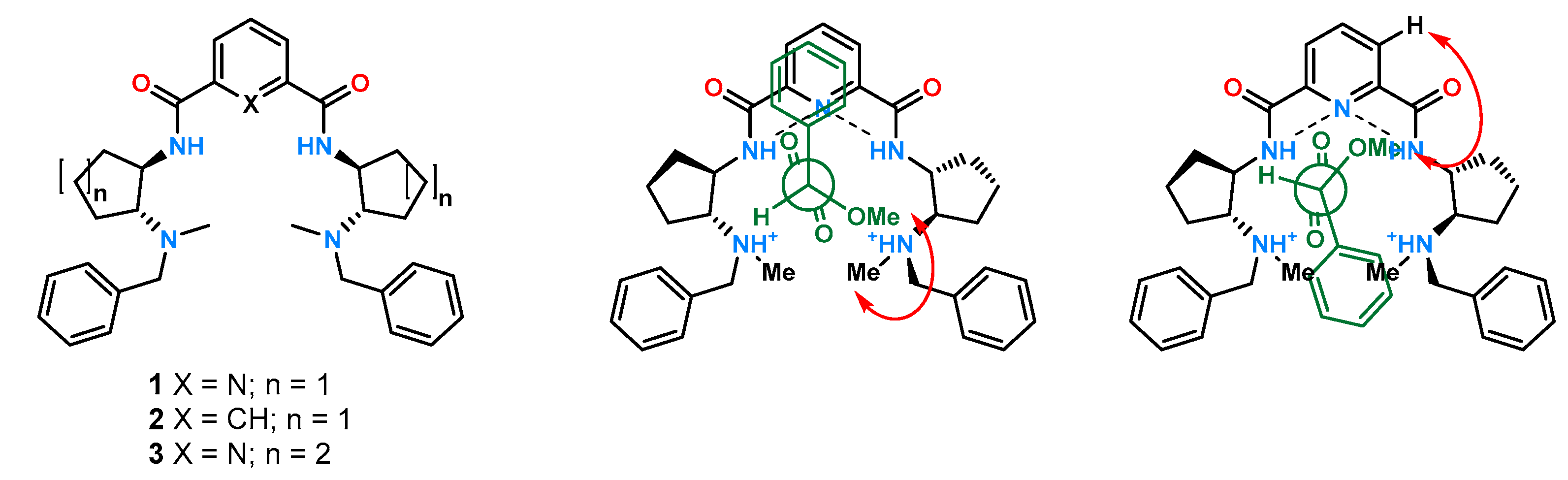
Diamide derivatives of simple aromatic compounds have found significant use as platforms in chiral anion recognition. Taking their advantages, a family of 1,2-disubstituted cyclic amine derivatives
1–
3 (
Figure 2) was obtained and investigated [
23,
24].
The ligands were designed to compare the enantiodiscrimination abilities of the chiral 1,3-diamide derivative of phthalic acid containing a five-membered cyclopentane ring 2 with the analogous 2,6-disubstituted pyridine derivative 1 and the effect of alkane ring size for the derivatives 1 and 3. The chiral recognition properties of receptors toward carboxylic acids were evaluated in CDCl3 using the 1H NMR technique by exploiting the ability of receptors to separate the reference protons in their racemic mixtures. Pyridine chiral solvating agents (CSAs) 1 and 3 resulted in a higher separation of the studied signals. Moreover, the ligand having a six-membered ring showed ΔΔδ ≥ 0.04 ppm for all tested guests. It was demonstrated that the tested receptors could be used as CSAs.
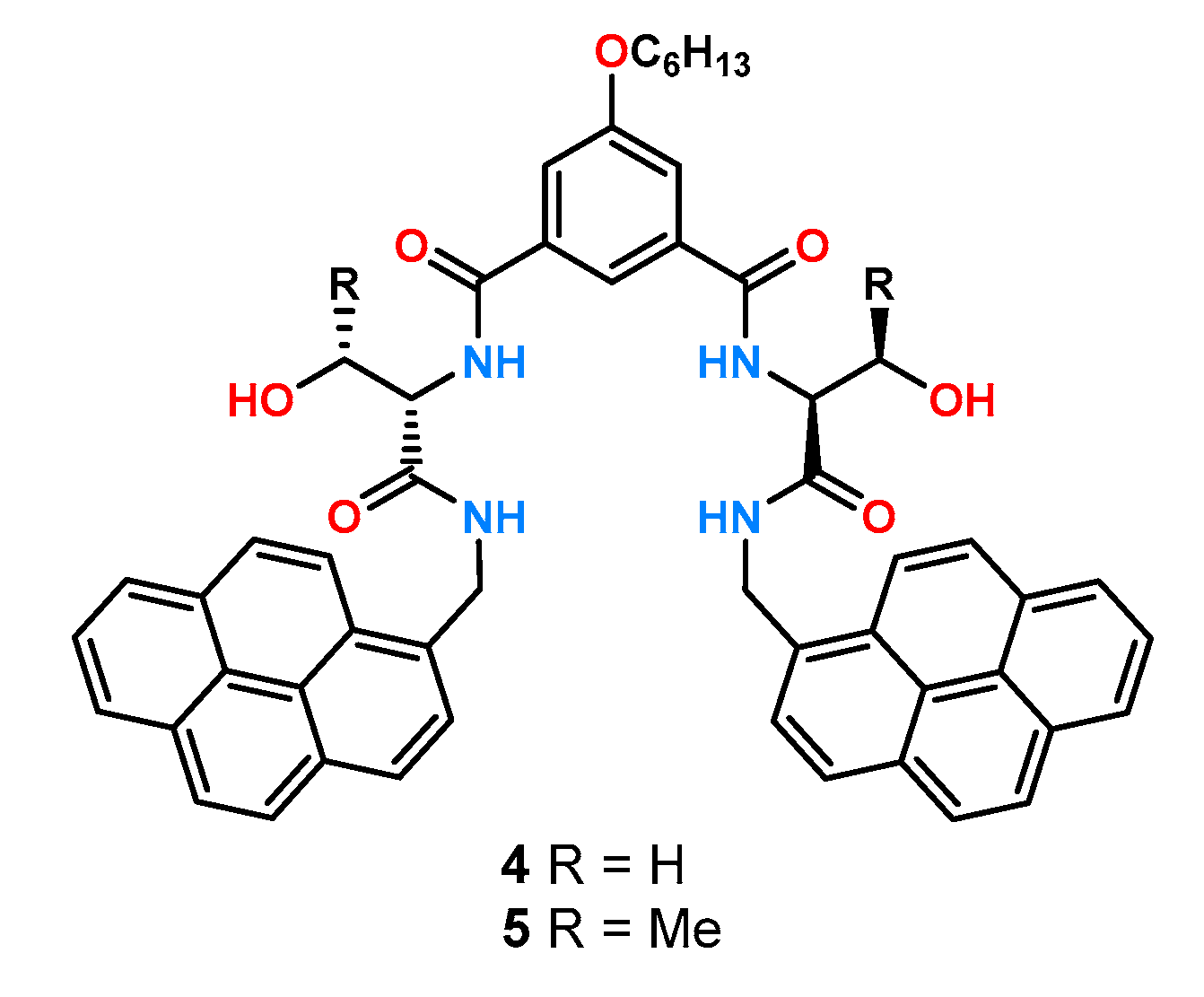
In 2018, a paper was published on treating the chiral recognition of tetraamide fluorescent receptors
4 and
5 using the geometry of a diamide derivative of isophthalic acid (
Figure 3) [
25].
Symmetrically attached pyrene units were responsible for the fluorescence response, while the blocks introducing chirality were serine or threonine derivatives. Comparison of the binding properties and ability to enantioselectively complex anions measured by fluorescence changes in acetonitrile showed that 5, having higher steric hindrance near the binding pocket, exhibited lower stability constants of the complexes formed (stability constants for the acetylated leucine derivative KD = and KL = and KD = and KL = , for receptors 4 and 5, respectively), while showing better chiral recognition (for leucine KD/KL = 3.9 and KD/KL = 4.6 for 4 and 5, respectively). The obtained enantioselectivity for the threonine host 5 was confirmed by titration under 1H NMR control in DMSO-d6, where the calculated ratio of stability constants was KD/KL = 2.10 for leucine. The results obtained indicate a significant effect of the solvent medium on chiral recognition.
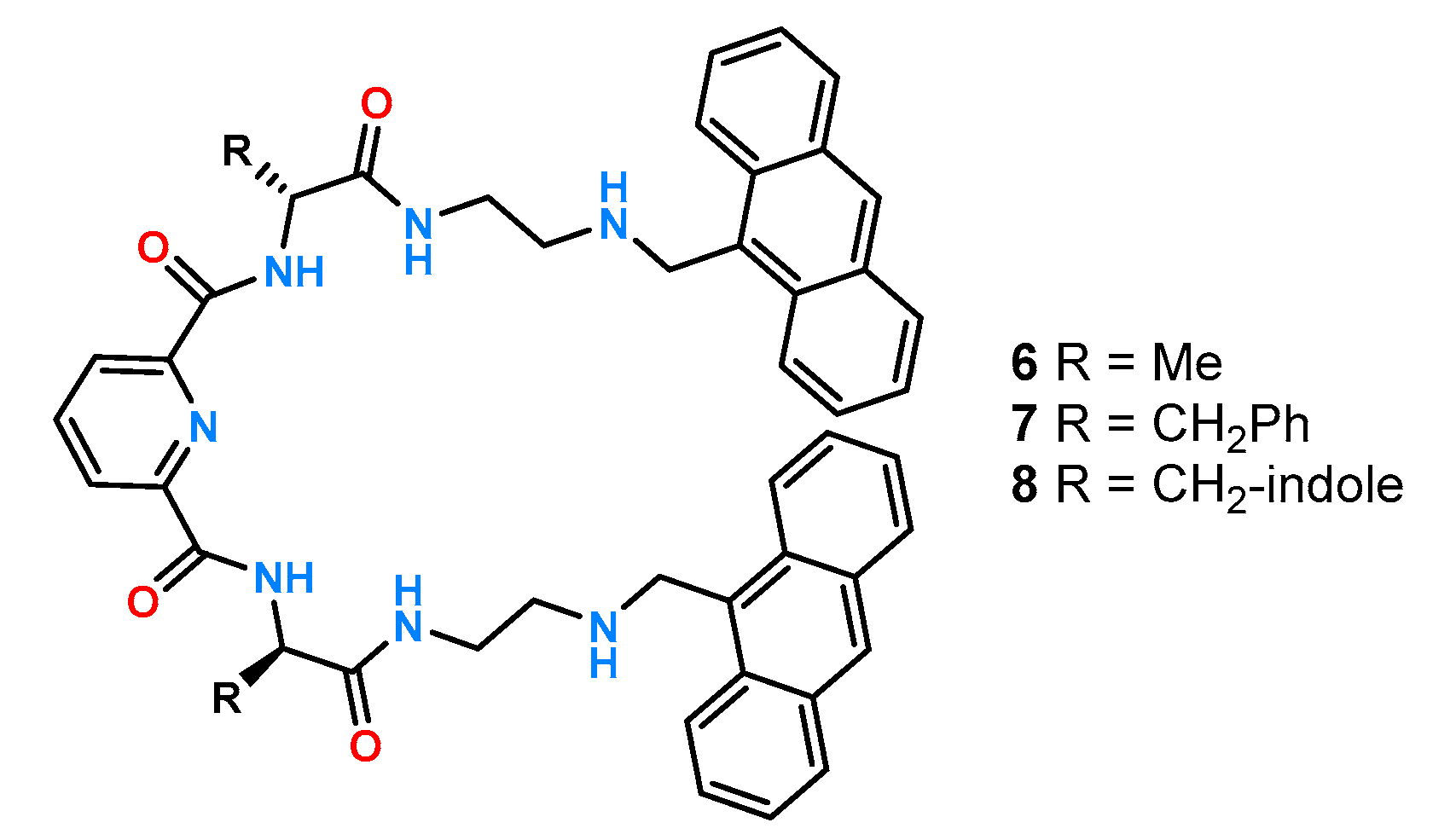
A similar approach was used to construct the group of receptors
6–
8 [
26], where a diamide derivative of pyridine acted as the binding pocket, while the chiral block was made up of alanine, phenylalanine, or tryptophan chiral linkers (
Figure 4).
Due to the attachment of anthracene units, it was possible to perform experiments by fluorescence titration in DMSO, while phenylalanine protected by a
tert-butoxycarbonyl group (Boc) was taken as the reference anion. The obtained results presented in
Table 1 show that the best enantioselective properties were exhibited by receptor
8 containing an indole derivative in its structure which allows the formation of additional hydrogen bonds.
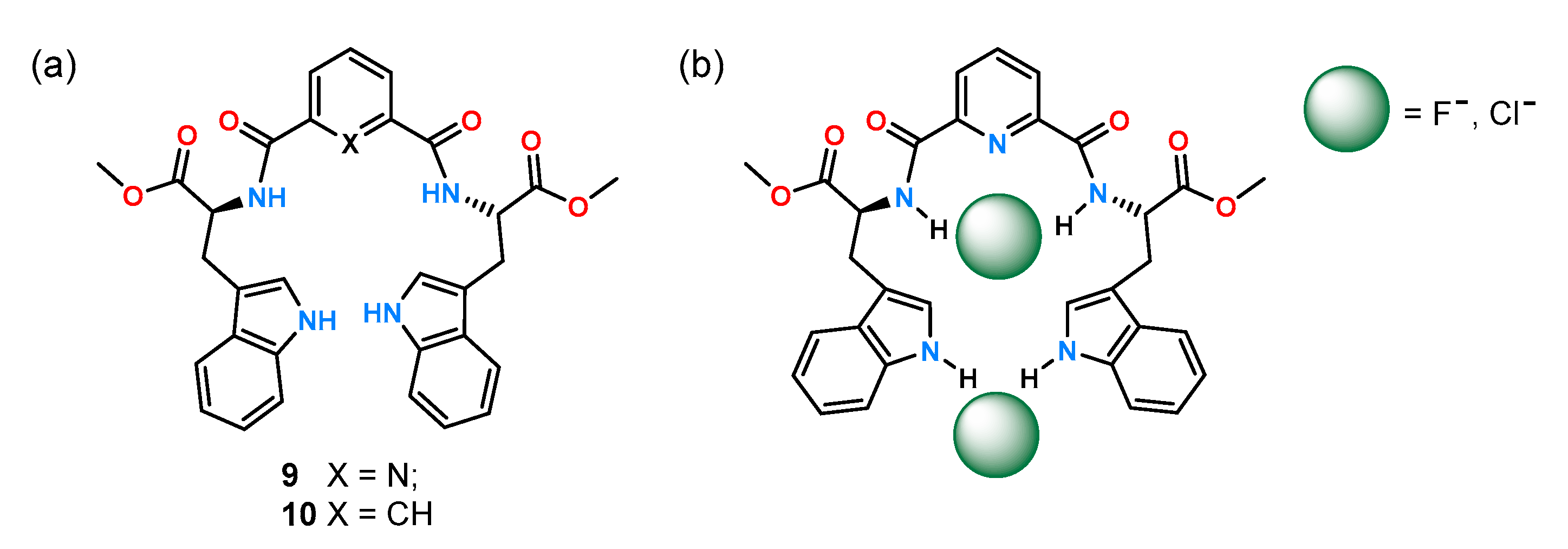
Previous work by the Gale group [
27] allowed preparation of the symmetric receptors
9 and
10 (
Figure 5) containing tryptophan moieties by the Caltagirone group [
28]. The anion binding properties of receptors to both achiral and chiral anions were evaluated by
1H NMR titration experiments in DMSO-
d6 with 0.5% H
2O.
While the isophthalic derivative 10 practically did not interact with the tested anions, receptor 9 formed complexes with stoichiometry 1:2 with chloride and fluoride anions and with an acetate complex with stoichiometry 1:1. Therefore, it was decided to continue the enantioselectivity studies only for receptor 9. As it turned out, this host showed greater affinity for the L enantiomer of the serine and alanine derivatives tested, and the chiral recognition for them was at the level of KL/KD = 1.40 and KL/KD = 1.65, respectively.
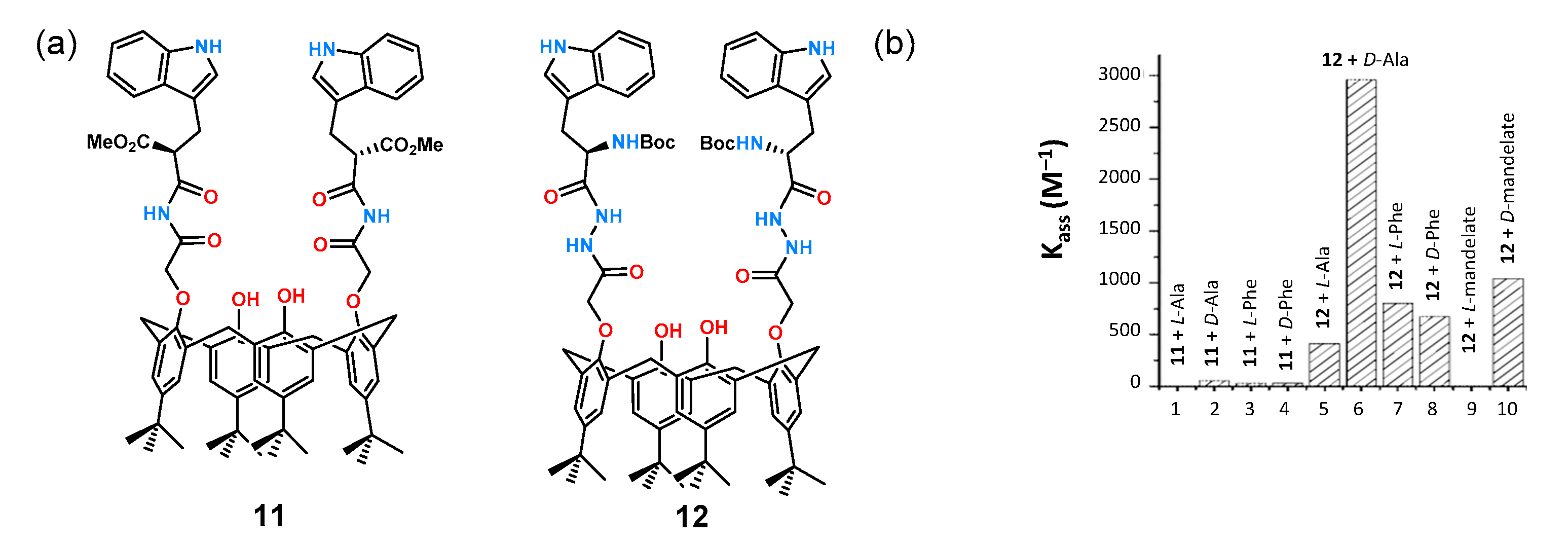
Tryptophan is commonly used as a chiral building block in the construction of receptors for anions because of its synthetic availability allowing straightforward preparation of a binding pocket around an inherently chiral molecule. A translation of this approach is shown in
Figure 6a for receptors
11 and
12 based on the calixarene scaffold [
29].
The receptors have two tryptophan molecules symmetrically attached via amide bonds to form an anion binding site. Affinity studies for a series of chiral carboxylate anions derived from mandelic acid, phenylglycine,
N-Boc-phenylglycine, and
N-Boc-phenylalanine were performed by fluorescence titration in DMSO. The titration results show the dependence of the stability constants (
Kass) of the formed complexes of receptors
11 and
12 with the aforementioned anions (
Figure 6b). As can be seen, receptor
11, having only two amide functions, practically did not interact with the tested anions, while receptor
12 showed very good chiral recognition toward a pair of enantiomers of alanine and mandelic acid anions.
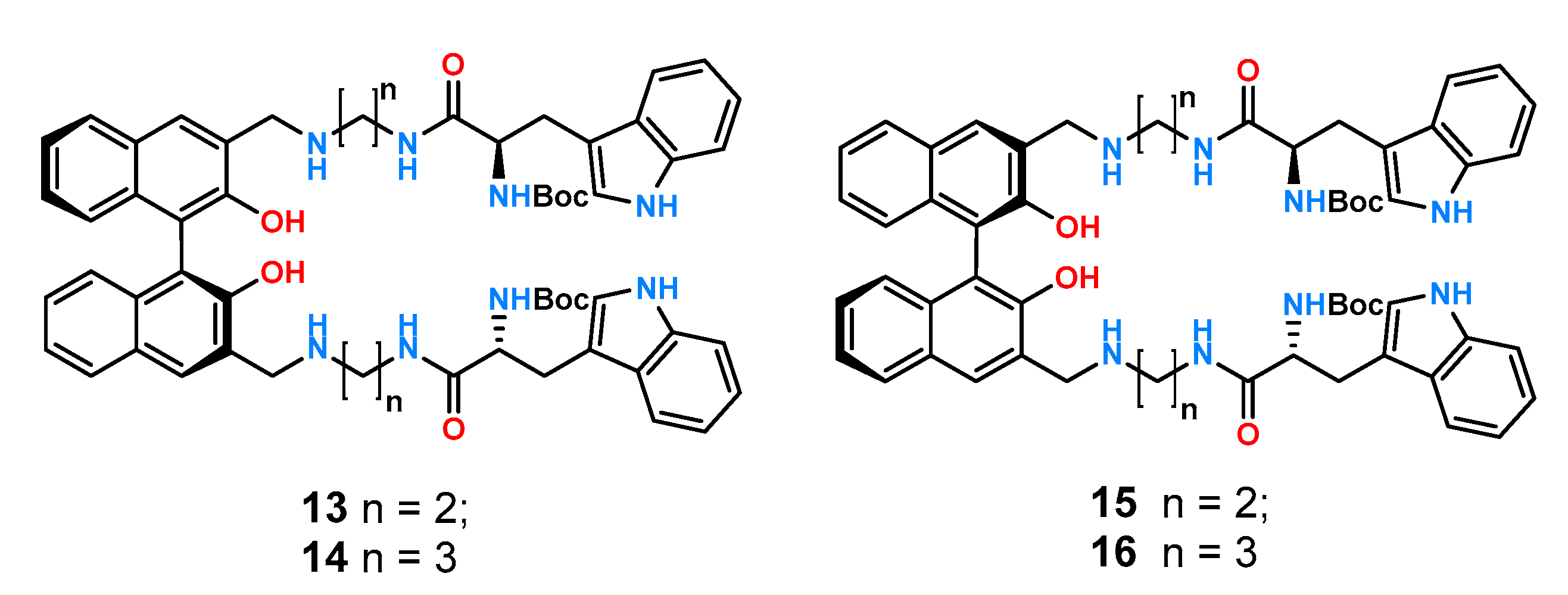
1,1′-Bi-2-naphthol (BINOL) is another structural motif important in the design of molecular receptors due to its inherent chirality, rigidity, and good synthetic availability. For these reasons, it was utilized in the construction of cation [
30,
31] and anion receptors [
32,
33] as well as in the preparation of chiral catalysts [
34,
35,
36]. Taking advantage of the rigid structure of BINOL, four new ligands
13–
16 (
Figure 7) were prepared and studied by fluorimetric titration experiments in chloroform [
37].
All receptors exhibited high association constants with the carboxylate anions tested which is understandable in such a non-competitive solvent. Furthermore, the highest chiral recognition was obtained for the pair of phenylalanine enantiomers protected by the Boc group KS/KR = 5.92 for 13 and KS/KR = 5.13 for 14, with enantioselectivity reversals of KS/KR = 1/5.18 and KS/KR = 1/4.49 for 15 and 16 for R-BINOL, respectively.
3. Urea and Thiourea Receptors
The urea and thiourea groups are important anion binding motifs in the construction of molecular receptors for chiral anions.
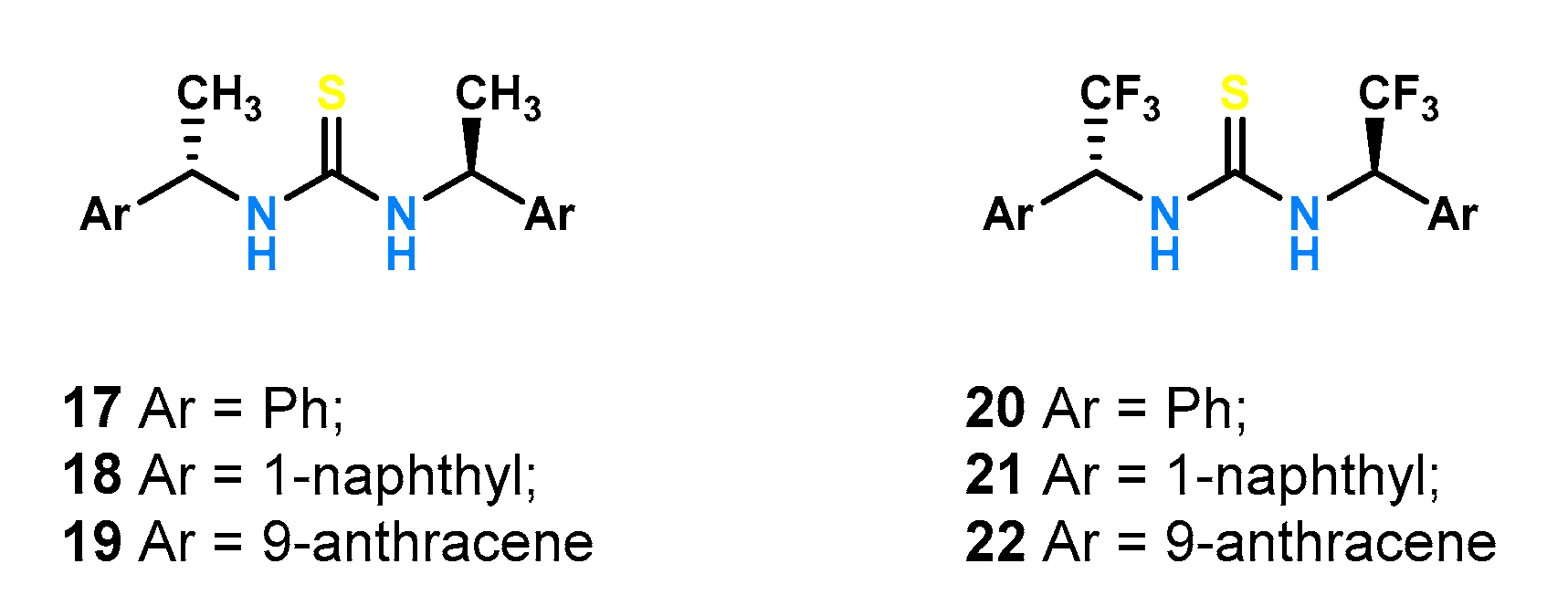
To investigate the effect of steric hindrance, receptors
17–
19 (
Figure 8) have been synthetized [
38]. The thiourea function was linked to a phenyl, 1-naphthyl, and 9-anthracene substituent, respectively, via a methine bridge. Additionally, for each receptor, its counterpart with a trifluoromethyl instead of a methyl group was obtained by synthesizing receptors
20–
22 (
Figure 8). Ligands dedicated to binding mandelates were evaluated using
1H NMR titrations in a competitive solvent such as DMSO-
d6.
Replacement of the methyl with a trifluoromethyl group (both of which create nearly identical steric hindrance) resulted in a tenfold increase in the stability constants of the resulting complexes with the highest KR = 447 M−1 and KS = 205 M−1 for the R- and S-enantiomers, respectively, and consequently the best chiral recognition (KS/KR = 2.18) recorded for the naphthyl substituted receptor 21 (compared to its counterpart with a methyl group 18: KR = 14 M−1 and KS = 18 M−1 and KS/KR = 1.29). Replacement of the naphthyl substituent by phenyl and anthracene resulted in the reduction of both binding affinity and enantioselectivity for mandelate, KS/KR = 1.64 and KS/KR = 1.38 for 20 and 22. These results highlight that even a small structural change in the receptor framework has a large effect on the chiral recognition properties.
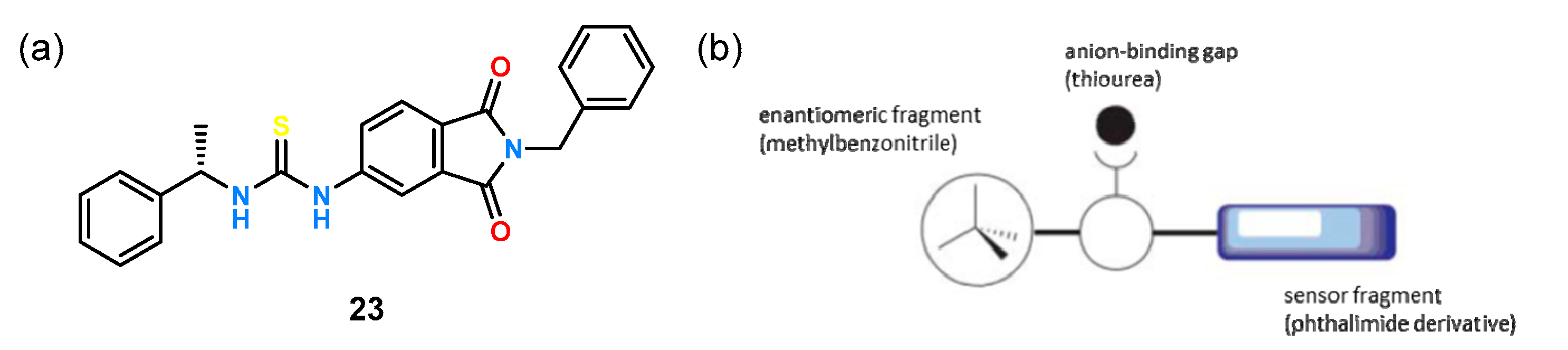
An example of an asymmetric thiourea receptor designed to act as a chiral chemosensor is ligand
23, shown in
Figure 9a [
39].
The thiourea group responsible for anion binding was combined on one side with a phthalimide derivative to serve as the sensing function and on the other side with a chiral benzyl derivative to serve as the enantioselective fragment (
Figure 9b). When evaluating the enantioselective properties of ligand
23 by UV-Vis titration experiments in acetonitrile, little change in absorption was observed when anions of the corresponding lactic acid enantiomers were added. Only the comparison of the change in absorption with the inverse of the guest concentration showed clear differences for the complexes formed and allowed the calculation of the corresponding stability constants for both guest enantiomers and the determination of K
D/K
L = 1.93.
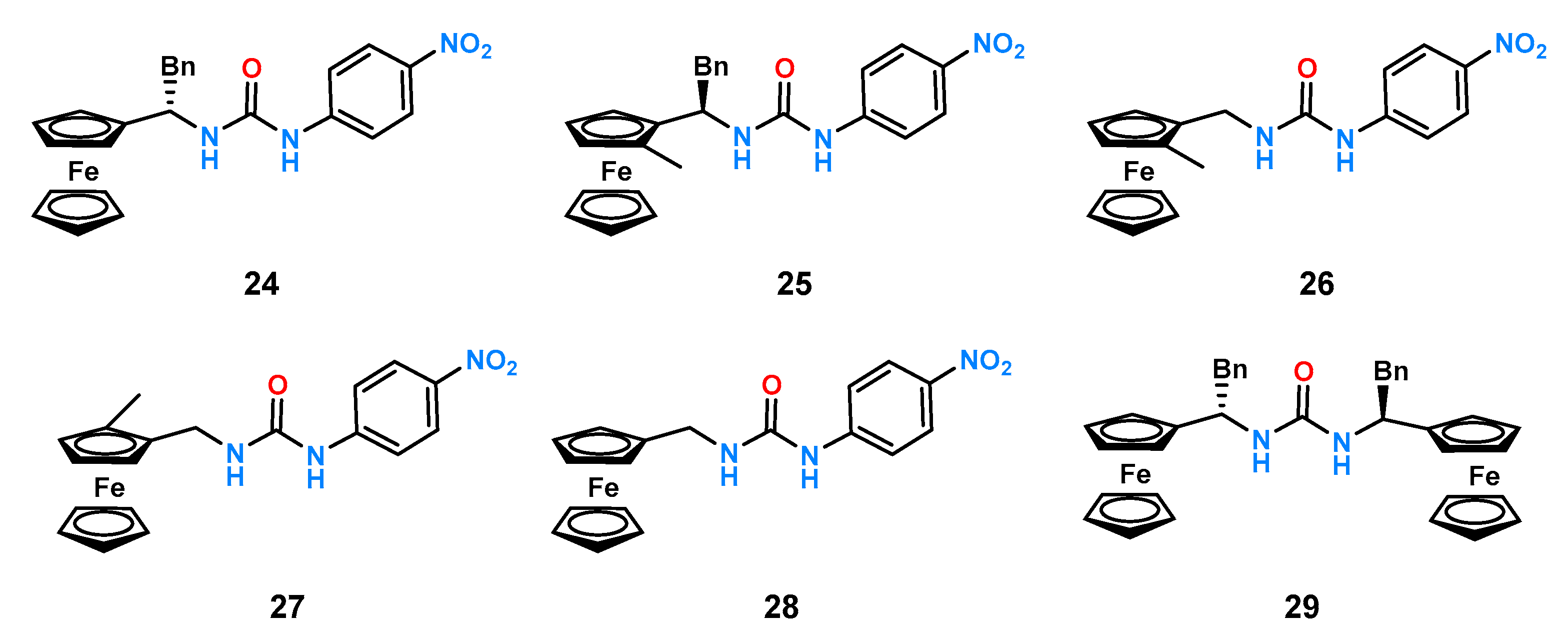
Another example of smartly designed ligands is the family of chiral, electrochemically functional receptors developed by Tucker’s group [
40,
41]. They investigated a series of ligands containing in their structure a (thio)urea unit as an anion binding center to which nitrobenzene was attached on one side (the presence of which made UV studies possible), while on the other, a ferrocene derivative was attached via a methine bridge as an electrochemically active unit. The effect of steric hindrance on chiral recognition was determined using UV-Vis titration experiments in acetonitrile. Evaluation of receptors
24–
29 with a set of selected chiral anions was performed (
Figure 10).
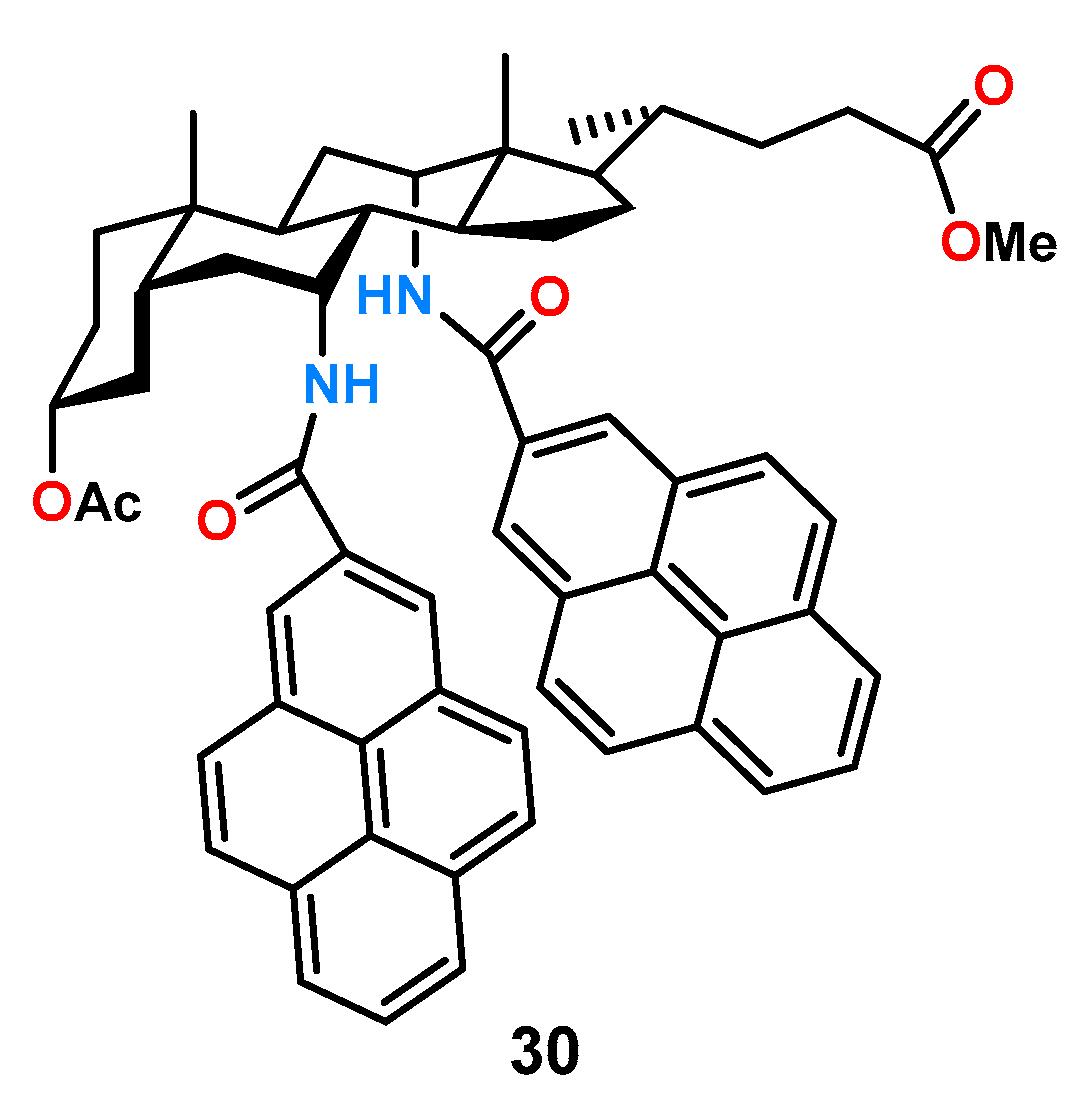
Cholic acid, due to its easy availability and inherent chirality, is attractive as a building block of chiral receptors for anion recognition. An example of such a ligand is the chiral receptor
30 (
Figure 11) which is also a fluorescent sensor due to the presence of two pyrene substituents [
21].
Studies on the potential enantioselectivity of ligand 30 were carried out by fluorescence titration experiments in acetonitrile with a pair of enantiomers of mandelic acid. The results show practically unnoticeable changes in fluorescence intensity for the R-enantiomer, while for the S-enantiomer, the change was significant and allowed the determination of the stability constant of the complex as KS = . Chiral recognition of mandelic acid anions was estimated as KS/KR = 5.0.
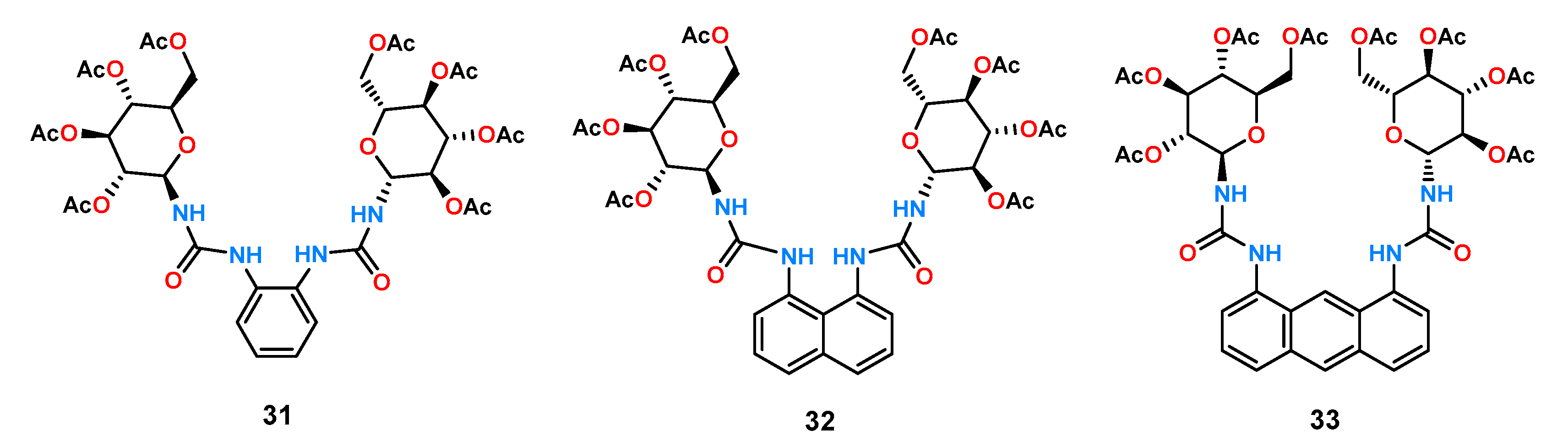
An example of a study of the effect of binding pocket size on anion association and chiral recognition by a family of three symmetrical urea-glucopyranose derivatives
31–
33 was published by the Jurczak group (
Figure 12) [
42].
The receptors tested differed in geometry and binding pocket size, with naphthalene receptor 32 having the smallest gap, benzene receptor 31 having a slightly larger, and anthracene receptor 33 having the largest one. Preliminary titration studies of the model carboxylates (acetate and benzoate), performed under 1H NMR control in DMSO-d6 containing 0.5% of water H2O, showed a stronger affinity for the smaller and more basic acetic anion for all three receptors. The stability constants of the complexes of both anions (KAcO/KBzO) compared with each other determine the dependence of the size of the binding pocket on the ratio of these constants. It turned out that, for receptor 33, this ratio was 10, while for the other two, it was 5 and 3 for 32 and 31, respectively. Then, using the same technique, the affinity of the obtained receptors for the anions of mandelic acid and tryptophan derivatives (N-Boc-Trp) was examined. The two receptors 31 and 32, which have smaller binding pockets, were characterized by low chiral anion recognitions (1.05 and 0.88 for 31 and 1.07 and 1.12 for 32 for mandelate and the protected amino acid, respectively). On the other hand, for receptor 33, relatively good results of KD/KL = 1.25 and 1.81 were obtained. Subsequently, analogous studies of this receptor with N-Boc-Phe and N-Boc-Val were carried out, for which satisfactory results of the ratio of constants of complexes KD/KL = 2.42 and 2.07 were obtained for valine and phenylalanine, respectively.
Analogous thiourea fluorescent chemosensors, using the anthracene platform, were published by Kim and coworkers [
43]. The receptors
34 and
35 (
Figure 13) were synthetized, and their chiral recognition for two sets of anions,
N-Boc and DBN-protected tetrabutylammonium salts of α-amino acid—alanine, valine, threonine, leucine, phenylglycine, and phenylalanine—was measured by the UV-Vis titration experiments in MeCN.
The addition of amino acid derivatives to both
34 and
35 receptor solutions resulted in a decrease in fluorescence intensity due to a chelation-enhanced quenching (CHEQ) reaction. For receptor
34, the CHEQ effect was explained by photoinduced charge transfer (PCT), while the CHEQ effect for receptor
35 was explained by photoinduced electron transfer (PET). Comparison of a series of results obtained for
N-blocked amino acids to anions having DNB as a protecting group showed that, in the second case, the effect of lowering the fluorescence intensity is even greater. This is explained by the presence of nitro groups which usually further decrease the fluorescence of both PET and PCT. The fluorescence lowering effect for receptor
34, with
d- and
l-
N-Boc-phenylglycine was 22.5% and 26.1%, respectively, while for
d- and
l-
N-DNB-phenylglycine, it was 66.6% and 63.9%. The obtained stability constants of the complexes were calculated using the out-dated Benesi–Hildebrandt approximation (vide infra). The best enantioselective binding result was obtained for
N-Boc-phenylglycine. Receptor
34 bound the
d- and
l-enantiomers with stability constants of 2160 and 11,800 M
−1, respectively, providing a ratio of K
D/K
L constants equal to 5.50. Different results were obtained for receptor
35, for which the stability constant for the formation of a complex with the
d-enantiomer was 2300 M
−1, while for the
l-enantiomer, it was 23,900 M
−1. The ratio of the K
L/K
D constants was 10.4. This unexpected relationship was explained by the formation of a CH-π interaction between the anthracene backbone and the methylene group in the receptor-
35–alanine complex. This type of interaction was impossible for
34 which lacks methylene groups in its structure, potentially responsible for the conformational lability of the entire system. However, a closer look at the Supporting Information indicates that the Korean authors misinterpreted the data. Jurczak and coworkers [
16,
44] questioned the results obtained by the Koreans by performing a competent
1H NMR titration of receptor
34 with
N-Boc-Ala and
N-Boc-Val. The determined chiral recognition in this case was 1.31 and 1.22 compared to the 5.50 and 4.30 presented in the paper for alanine and valine, respectively. The obtained ratios of stability constants were confirmed by fluorometric titration. The results obtained by Kim’s group are subject to a powerful error due to the use of the Benesi–Hildebrandt approximation because of the complicated fluorescence quenching mechanism.
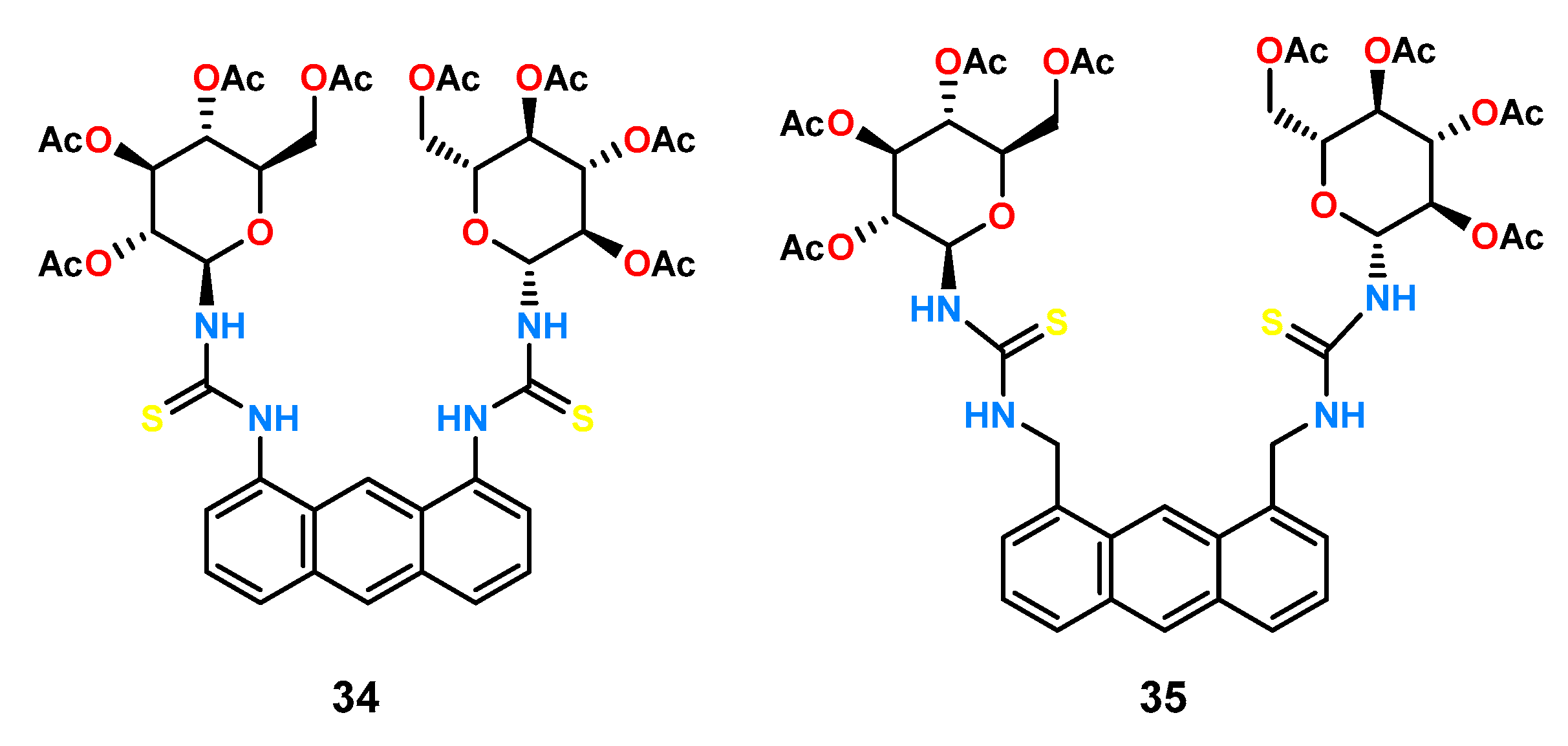
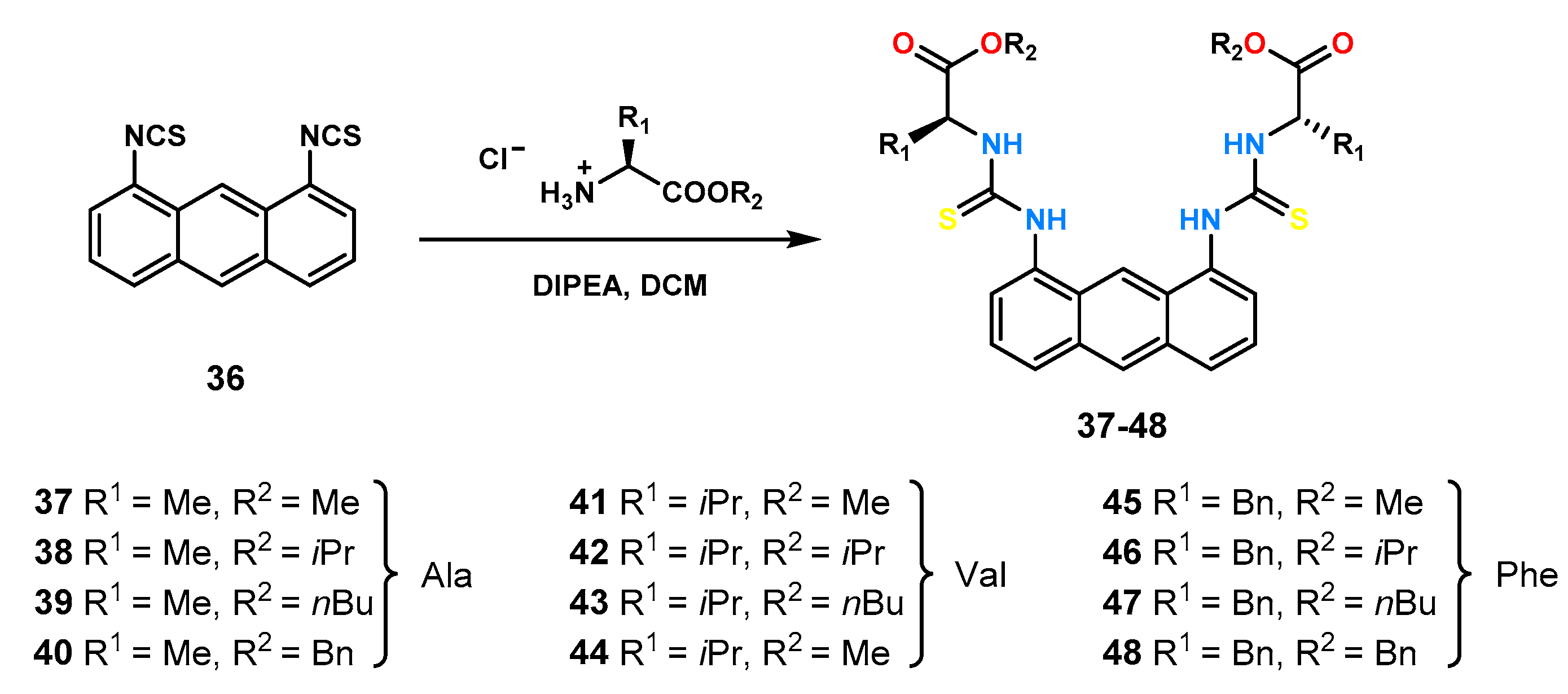
1,10-Dithiourea anthracene derivative was used to obtain a static library of thiourea receptors containing derivatives of different amino acids [
16]. Twelve new ligands (
Figure 14) were obtained by reacting dithioisocyanate
36 with ester derivatives of three amino acids (alanine, valine, and phenylalanine). Four esters of various sizes and shapes (methyl, isopropyl,
n-butyl, and benzyl) were used to investigate the effect of ester group size on the stability constant value and chiral recognition.
The ability to enantioselectively bind carboxylates was examined by competent titration under the control of
1H NMR in acetonitrile-
d3 with model pairs of enantiomers of mandelic acid and
N-Ac-Phe anions [
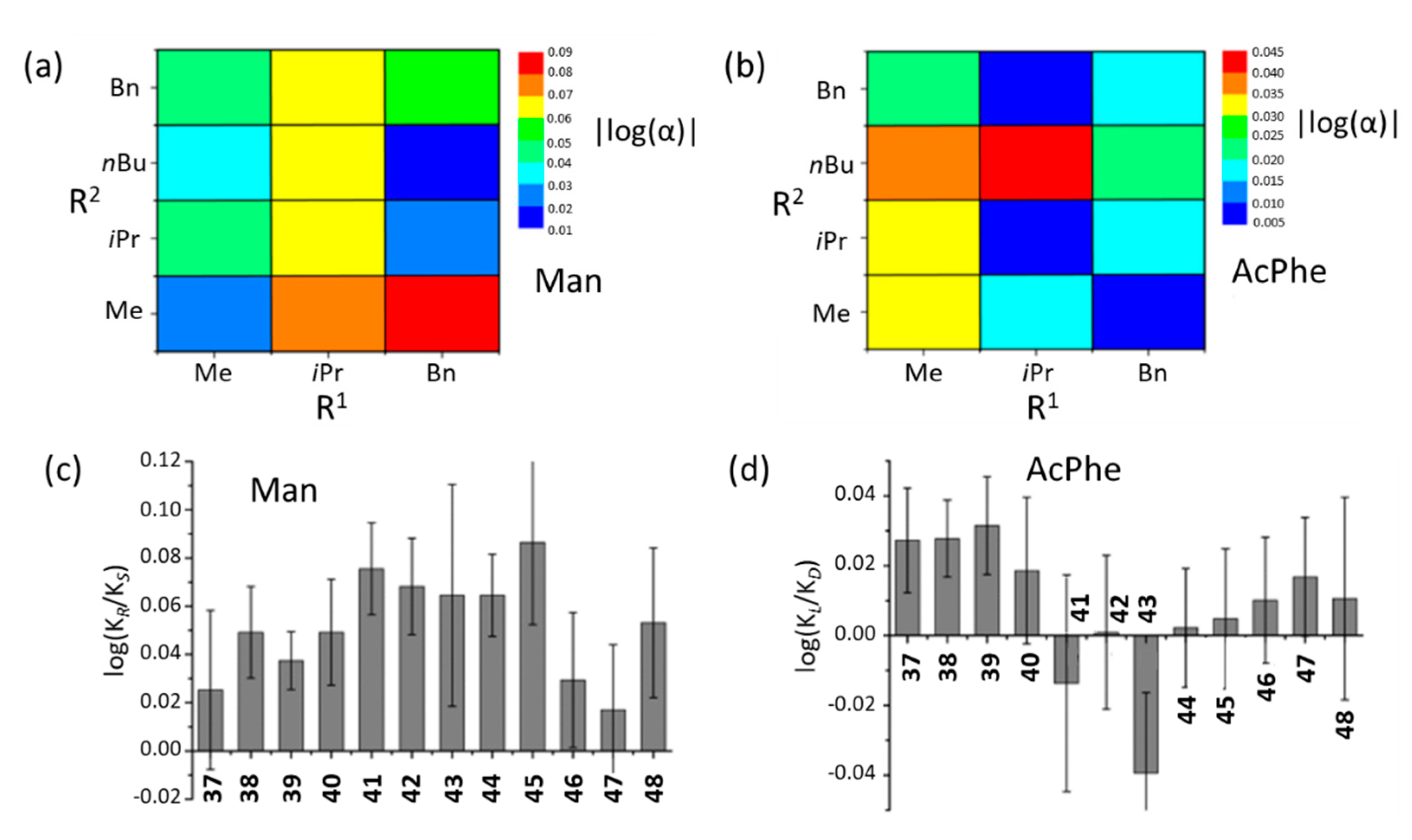
45]. A comparison was made by counting K′ = K/K (R
1 = Me) for the constant R
2 and the given anion. The highest constants were obtained for valine derivative receptors
41–
44 (K′ = 2.33–2.91), despite having the largest isopropyl substituent by volume (according to Taft steric parameters) [
46,
47], while phenylalanine derivatives showed medium affinity (K′ = 0.50–0.76) (
Figure 15a).
An analogous comparison was made for the substituent of the ester group assuming K′ = K/K (R
2 = Me). In this case, the substituent effect (
iPr <
nBu < Bn ≈ Me) was much smaller (
Figure 15b). All receptors tested showed a higher affinity for the
R-enantiomer of mandelate (
Figure 15c), whereas no such selectivity was present for the phenylalanine enantiomers (
Figure 15d). The obtained K
R/K
S ratios of the studied receptors were in the range of 1.0–1.22 which qualifies for quite low chiral recognition values. The described studies show a significant effect of steric substituents on the height of the persistence constant of the complexes.
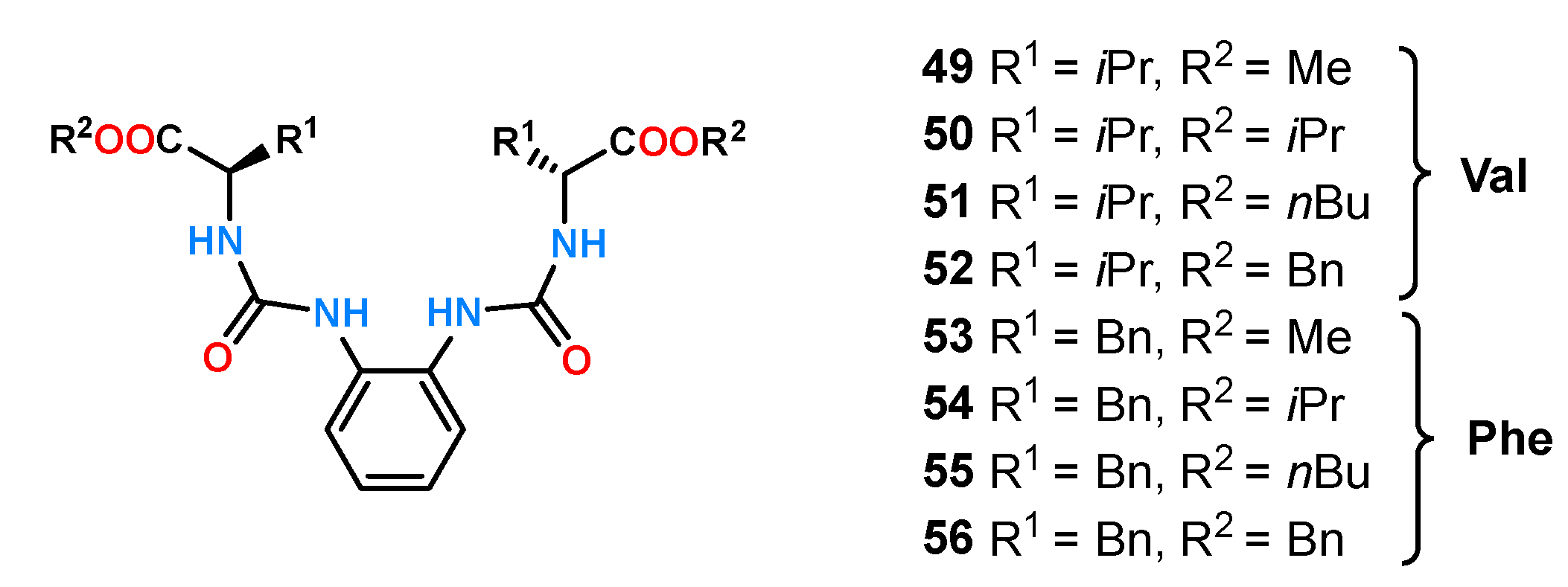
A similar analysis was performed for a family of 1,2-diurea benzene ligands [
15]. Using the UV-Vis titration experiments in MeCN, two groups of receptors derived from valine and phenylalanine with different substituents in the ester group (
49–
56,
Figure 16) were compared.
Very similar results were obtained as for the anthracene receptors described above. The enantioselectivity of the described receptors was low, and there was a strong dependence of the values of stability constants on the size of the substituents.
An example of a receptor with an intentionally introduced chromophore fragment that was designed for chiral recognition is compound
57 [
48]. In its structure, it contains thiourea groups responsible for carboxylate ion binding, glucopyranose fragments responsible for chirality, and an azophenol system as a chromophore (
Figure 17).
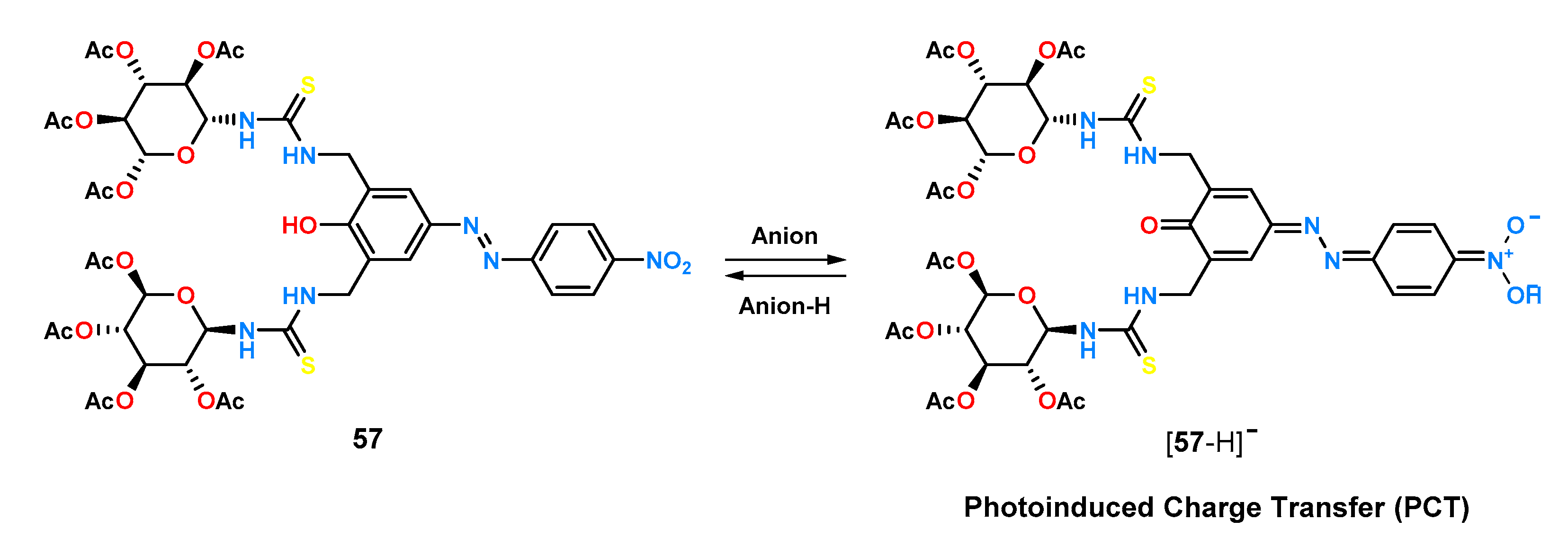
Receptor
57 exhibits pronounced colorimetric changes upon addition of anions which can be attributed to the anion-induced deprotonation of an azophenol moiety allowing the occurrence of a photoinduced charge transfer (PTC). This property was employed to study the chiral recognition properties of
57 using the UV-Vis titration technique in acetonitrile. Ligand
57 showed good enantioselectivity for anions derived from α-amino acids protected by a
tert-butoxycarbonyl (Boc) group, with a preference for
D-amino acids and a chiral recognition for alanine of 3.60 (
Table 2). For the anions of the same amino acids, protected by a dinitrobenzyl group (DNB), the enantioselectivity (K
D/K
L) was determined to be medium, ranking between 1.10 and 2.55. For sensor
57, tests were also carried out with the chiral salts of naproxen and 2-phenylpropionic acid, for which the ratio of the constants of the K
R/K
S complexes was 1.86 and 2.95, respectively.
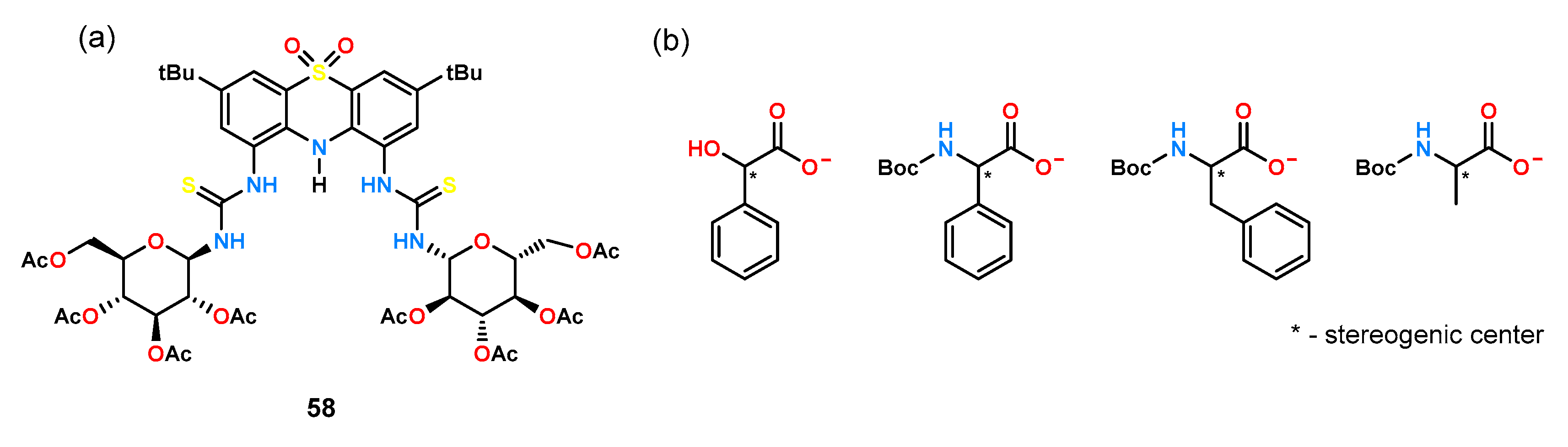
Huszthy’s group [
49], which has conducted extensive research on the fundamentals of chiral cation and anion recognition phenomena and their applications, has developed several diurea receptors with
C2 symmetry. Among others, they used a rigid platform of 5,5-dioxophenothiazine, whose diamine derivative was reacted with per-
O-acetylated glucosamine isocyanate to obtain receptor
58 (
Figure 18).
The ligand thus synthesized was tested for enantioselective anion recognition using UV-Vis titration experiments in MeCN, using TBA salts of acids with a stereogenic center on the α carbon atom (
Figure 18b,
Table 3). The studies showed that groups located at the stereogenic center have a strong effect on enantioselectivity. For anions having a phenyl group attached to the α carbon atom (mandelate, phenylglycine), chiral recognition was moderate. When the aromatic ring was offset from the stereogenic center by a methylene group (phenylalanine), negligible enantioselectivity was observed. In contrast, when the aromatic substituent was replaced with an aliphatic one (alanine), no enantiomeric differentiation by the receptor was observed at all. It was also observed that shifting the phenyl ring away from the carboxyl group by a methylene group reduces the interaction between the anion and the receptor. The effect of the anion protecting group on chiral recognition was also tested by measuring stability constants for amino acids protected by a formyl (Form), acetyl (Ac),
tert-butylxycarbonyl (Boc), and pivaloyl (Piv) group. Receptor
58 showed the best chiral recognition for amino acids protected with Boc and Piv; for amino acids protected with a formyl group, the effect on enantioselectivity was negligible.
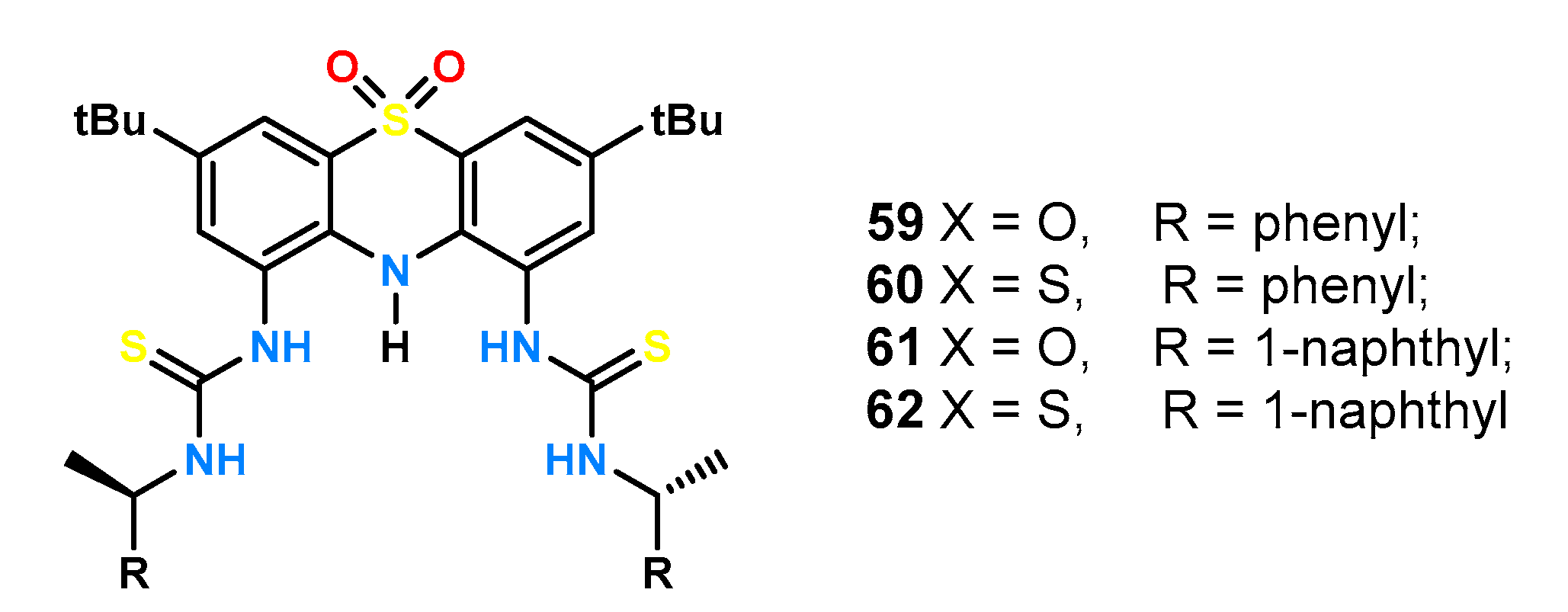
The same phenothiazine derivative was then used to obtain a series of ligands
59–
62 (
Figure 19) and to investigate their stereo-differentiating properties [
50]. The effects of the acidity of the hydrogen bond donors and the size of the aromatic substituent linked to the thio(urea) function via a methine bridge were examined.
An identical set of enantiomeric amino acid pairs was tested as for the glucopyrazone derivative (
Figure 18b). Urea derivatives
59 and
61 exhibited lower absorption and smaller fluorescence changes compared to thiourea ligands
60 and
62 making it impossible to determine reliable stability constants of the complexes by titrations under UV-Vis control in MeCN. Both, receptor
59, containing a benzyl unit in its structure, and receptor
61, being a naphthyl derivative, showed little enantioselectivity, with the receptor bound to the larger aromatic substituent, showing slightly higher differences, e.g., for
N-Boc-Phg ΔlogK = 0.24 compared to ΔlogK = 0.03 for
59 and
61, respectively. The results presented here suggest that chiral recognition depends on very subtle effects.
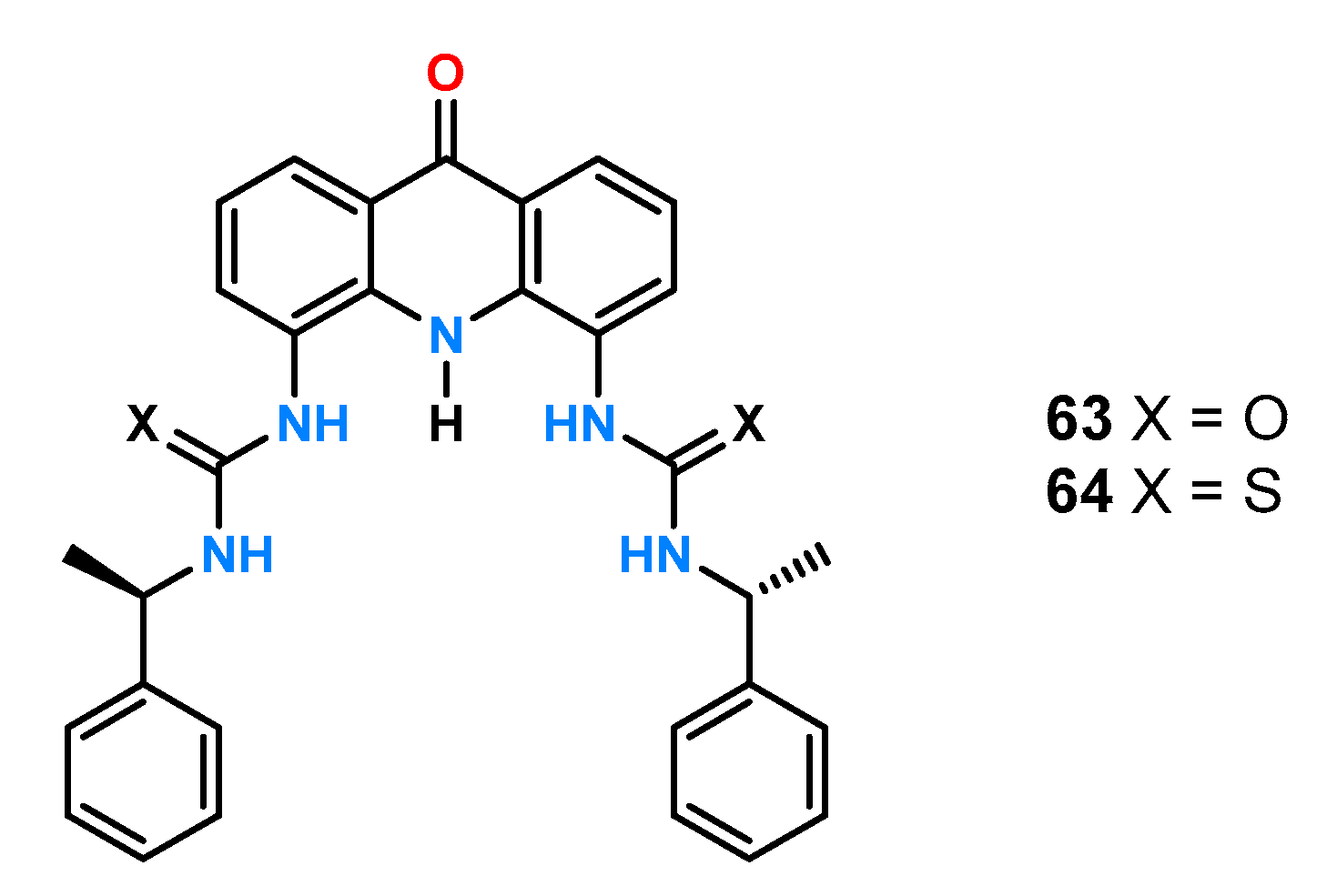
Huszthy and coworkers [
51] also obtained two receptors (urea
63 and thiourea
64,
Figure 20) based on the acridinium-9-one platform.
Results from the UV-Vis titration experiments in MeCN containing 1% DMSO using reference anions with a stereogenic center on the α-carbon atom (
Figure 18b) showed a particularly high degree of chiral recognition for
N-Boc-Ala by the urea receptor
63 (ΔlogK = −0.56; α = 3.6), compared to anions having an aromatic substituent (
Table 4). Furthermore, changing the urea group to thiourea had a significant effect on the chiral recognition of phenylglycine, with ΔlogK = 0.08; α = 1.2 for receptor
63 and ΔlogK = 0.26; α = 1.8 for ligand
64.
A few years ago, a study on the enantioselectivity of symmetric diurea receptors forming host:guest complexes with a stoichiometry of 1:2 was published [
52]. The authors obtained three receptors
65–
67 (
Figure 21), using a dibenzofuran derivative as the structural backbone of the receptors, and placed a source of chirality between the platform and hydrogen bonding donors. They decided to investigate their ability to enantioselectively bind carboxylate anions by titration experiments under
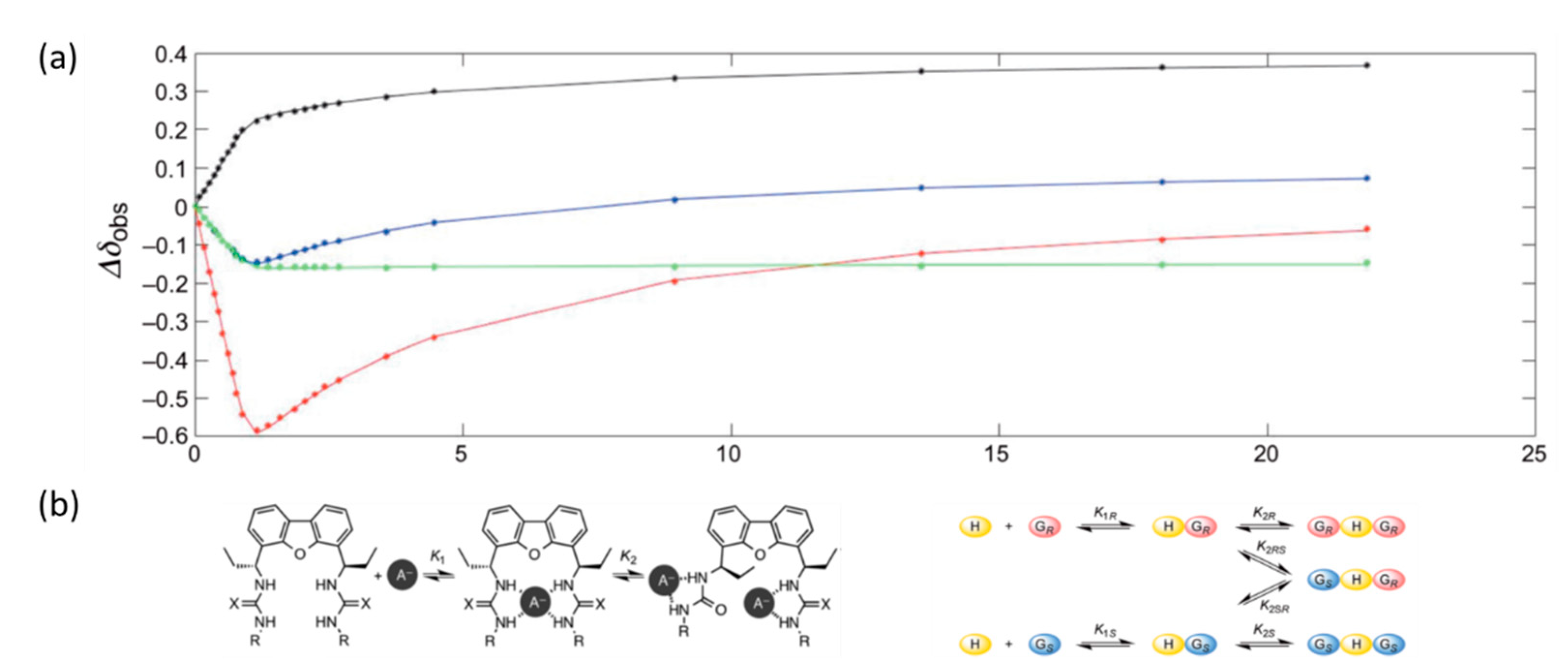
1H NMR control while planning to use them as chemical shift agents (CSAs).
The one from the hydrogen atom attached to the α carbon atom of the mandelic acid anion was chosen as the reference signal in this study. In preliminary studies, the effect of solvent on the potential for enantioselective recognition by receptor
65 was tested by conducting titrations of a racemic mixture of
DL-tetrabutylammonium mandelate (TBAM) in three solvents: CDCl
3, DMSO-
d6, and acetone-
d6. The largest separation of the enantiomeric signals after the addition of two equivalents of guest anions was obtained for chloroform ΔΔδ = 0.051 ppm, followed by acetone ΔΔδ = 0.042 ppm, and the smallest for DMSO ΔΔδ = 0.026ppm. Moreover, the best separation of the traced signal was achieved for 0.5 eq of the mixture in acetone (ΔΔδ = 0.063 ppm). The characteristic shifts of the signals in the
1H NMR spectrum (
Scheme 1a) during titration are indicative of conformational changes of the ligand and the formation of a complex with complex stoichiometry, in this case, mixed 1:1 and 1:2. The binding model proposed by the authors is shown in
Scheme 1b.
Receptors 66 and 67 also showed enantioselectivity toward the racemic mixture of DL-TBAM. The thiourea analog 66 behaved comparably to ligand 65, but a smaller chemical shift difference of ΔΔδ = 0.041 ppm was observed for the two equivalents. For the tosyl derivative, the value of ΔΔδ increased continuously with the addition of the receptor, reaching ΔΔδ = 0.53 ppm, suggesting the formation of a complex with a host:guest stoichiometry of 1:1. For the best receptor 65 in this combination, the authors tested its effect on a number of mixtures of racemic α-hydroxy and α-amino acid derivatives.
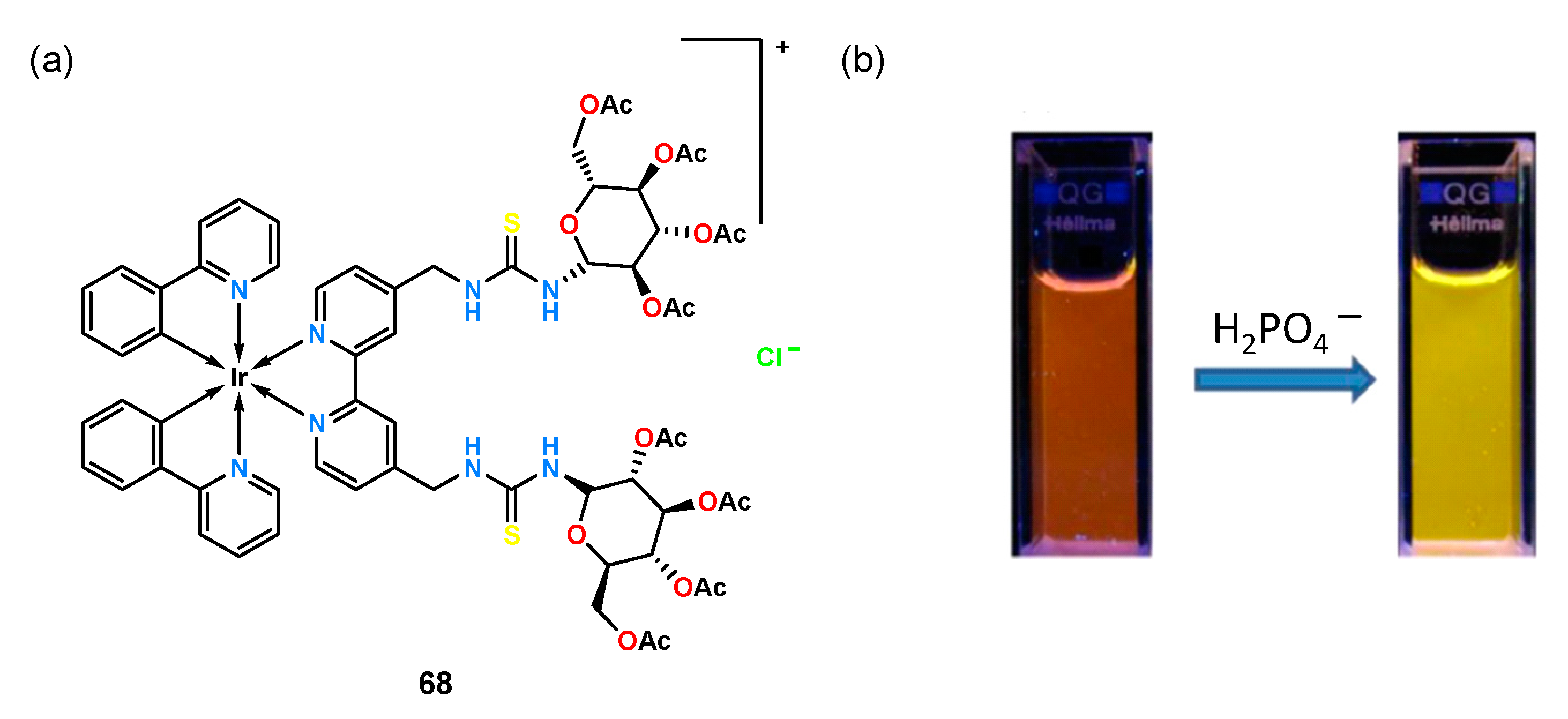
An approach combining the functions of a phosphorescent sensor with a preorganized carboxylate ion binding pocket and a chiral fragment was presented by Yoon and coworkers [
53]. They used an amine derivative of the iridium(III) complex as a rigid platform which they reacted with a thioisocyanate derivative of per-
O-acetylated glucosamine to obtain receptor
68 (
Figure 22).
Experiments for chiral recognition were performed by titration under UV-Vis and phosphorescence in acetonitrile with two sets of N-protected amino acids: alanine, valine, threonine, leucine, phenylglycine, and phenylalanine. Receptor 68 exhibited high association constants of the resulting complexes and had a higher affinity for the D-enantiomers when analyzing amino acids protected with a tert-butoxycarbonyl (Boc) group, with the best chiral recognition obtained for leucine (KD = , KL = ; KD/KL = 3.16). When ligand 68 was tested with a group of amino acids protected by a 3,5-dinitrobenzyl group, a greater phosphorescence quenching effect and a higher affinity for the L-isomers of the tested anions were observed compared to the previously tested set of amino acids. Receptor 68 showed the highest enantioselectivity for the threonine enantiomer pair equal to KL/KD = 3.04 (KD = , KL = ).
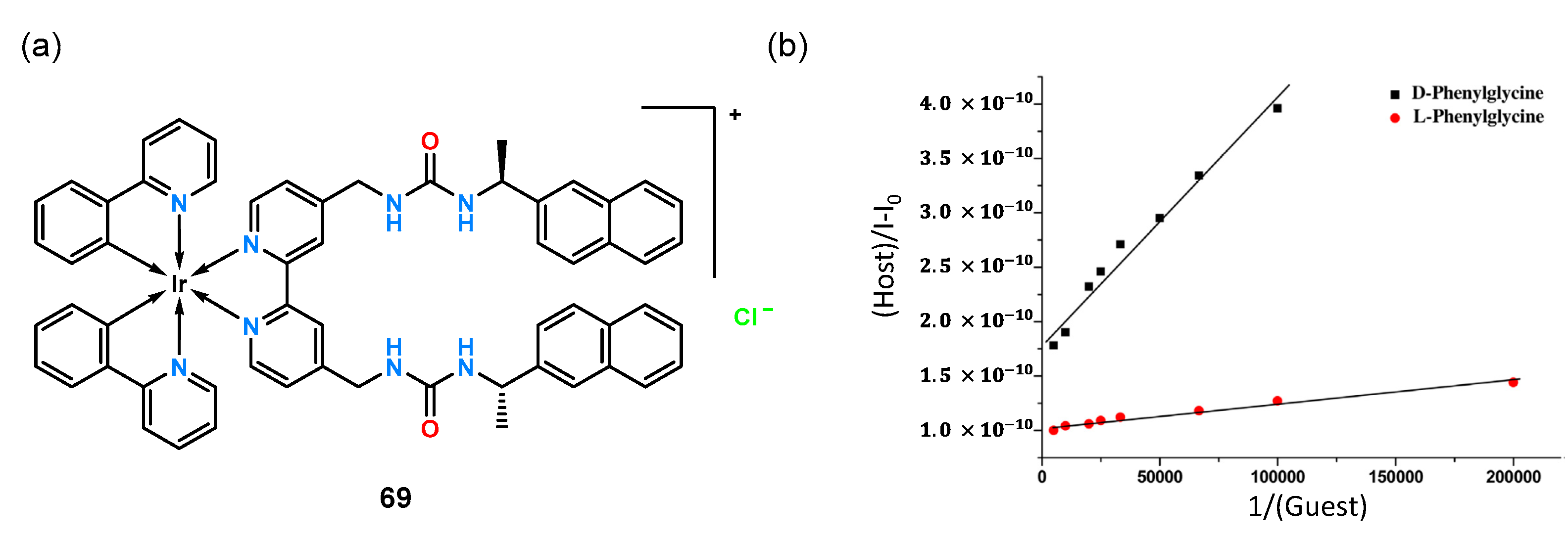
Using the same iridium platform, a diurea receptor
69 (
Figure 23a) was constructed with a chiral 1-naphthylethyl fragment attached [
54].
Analysis for chiral recognition was performed by UV-Vis titration experiments in MeCN, using, as before, the phorescence properties of the iridium(III) complex. The ligand tested exhibited extremely high
N-Boc-Phg enantioselectivity, binding the enantiomeric pair at a ratio of K
D/K
L = 5.0.
Figure 23b shows the linear relationship of the Benasi–Hildebrand equation of phosphorescence intensity to the inverse of the guest concentration which demonstrates the high value of chiral recognition for this anion.
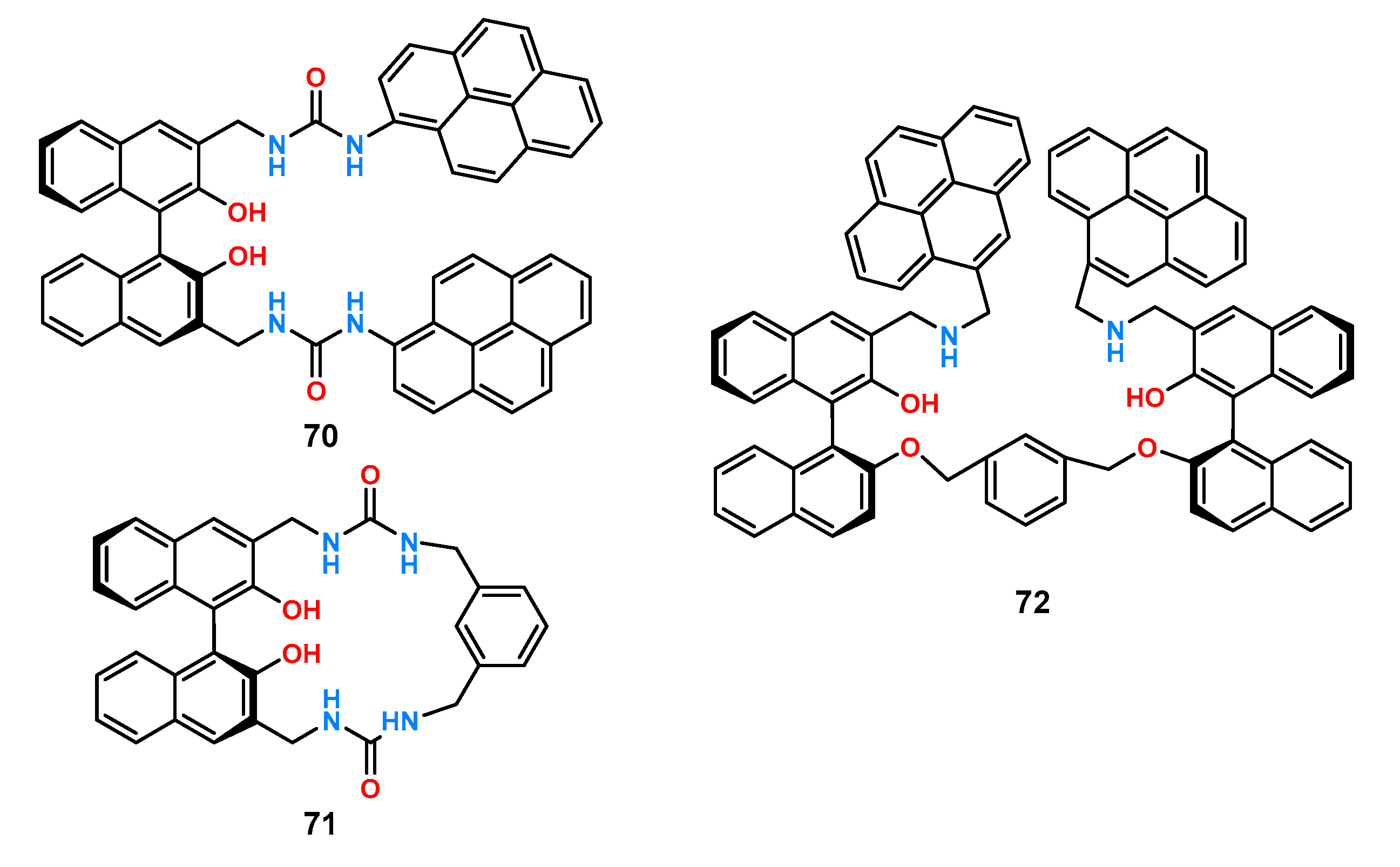
A representative group of chiral anion receptors having BINOL in their structure is ligands
70–
72 (
Figure 24) [
55]. Two urea groups were attached to the rigid platform to form a suitable pocket for binding carboxylate anions and, in addition, pyrene functions responsible for the fluorescence response (receptors
70 and
72).
Compounds 70–72 were screened for chiral recognition with a set of tetrabutylammonium salts of amino acids protected by a tert-butoxycarbonyl group (Phe, Leu, Ala, Ser) by fluorescence titration experiments in DMSO. All ligands exhibited higher affinity toward the d-enantiomers. Receptor 70 showed the highest chiral recognition for the alanine derivative with fluorescence quenching ΔID/ΔIL = 6.1 which was also confirmed by 1H NMR spectra by observing and comparing the N-H shifts of protons for both enantiomers (Δδ = 0.154 and Δδ = 0.139, for d- and l-alanine, respectively). Compared to the linear receptors 70 and 72, whose fluorescence intensity decreased in each case, the addition of an anion to the macrocyclic host 71 resulted in a different behavior, namely, a signal enhancement at 258 nm, a decrease at 342 nm, and an enhancement at 370 nm. The highest enantioselectivity was obtained for receptor 72 using the enantiomeric pair of alanine derivatives ΔID/ΔIL = 12.95.
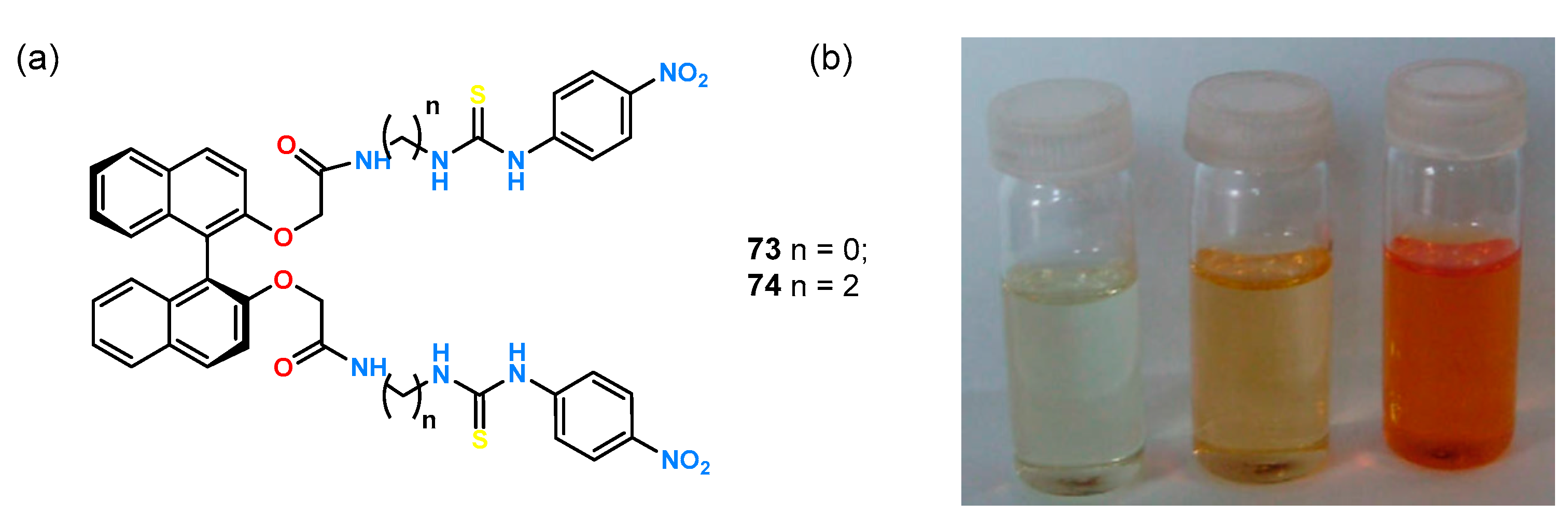
BINOL was also used to design colorimetric receptors
73 and
74 (
Figure 25) [
56]. Thiourea functions and
p-nitrophenyl groups were symmetrically attached to the chiral rigid platform. The receptors differed in the distance of the binding pocket from the chiral fragment by two methylene groups.
Preliminary enantioselectivity studies for both hosts were performed by fluorescence absorption experiments in DMSO for a pair of mandelates. While the addition of the enantiomeric guest to receptor 73 resulted in nearly identical fluorescence quenching for both isomers (from which it can be inferred that the ligand exhibits low enantioselectivity), the quenching efficiency for receptor 74 was equal to ΔID/ΔIL = 3.3. Experiments leading to the determination of stability constants and ΔID/ΔIL for anionic derivatives of malic acid and phenylglycine were also performed, but both 73 and 74 showed no chiral recognition toward the tested guests. To further evaluate receptor 74 association of a pair of enantiomeric mandelic acids, titrations were carried out under 1H NMR control in DMSO-d6 for the racemic mixture and the single isomers. Changes in the downfield shift of thiourea protons were observed, with maximum shifts of δ = 10.23 and δ = 9.20 ppm upon addition of d-enantiomer, and 10.97 and δ = 9.39 when l-enantiomer was added. The resulting enantioselectivity was explained by the presence of an additional π-π interaction of receptor 74 with d-mandelate, the presence of which was confirmed by molecular modeling.
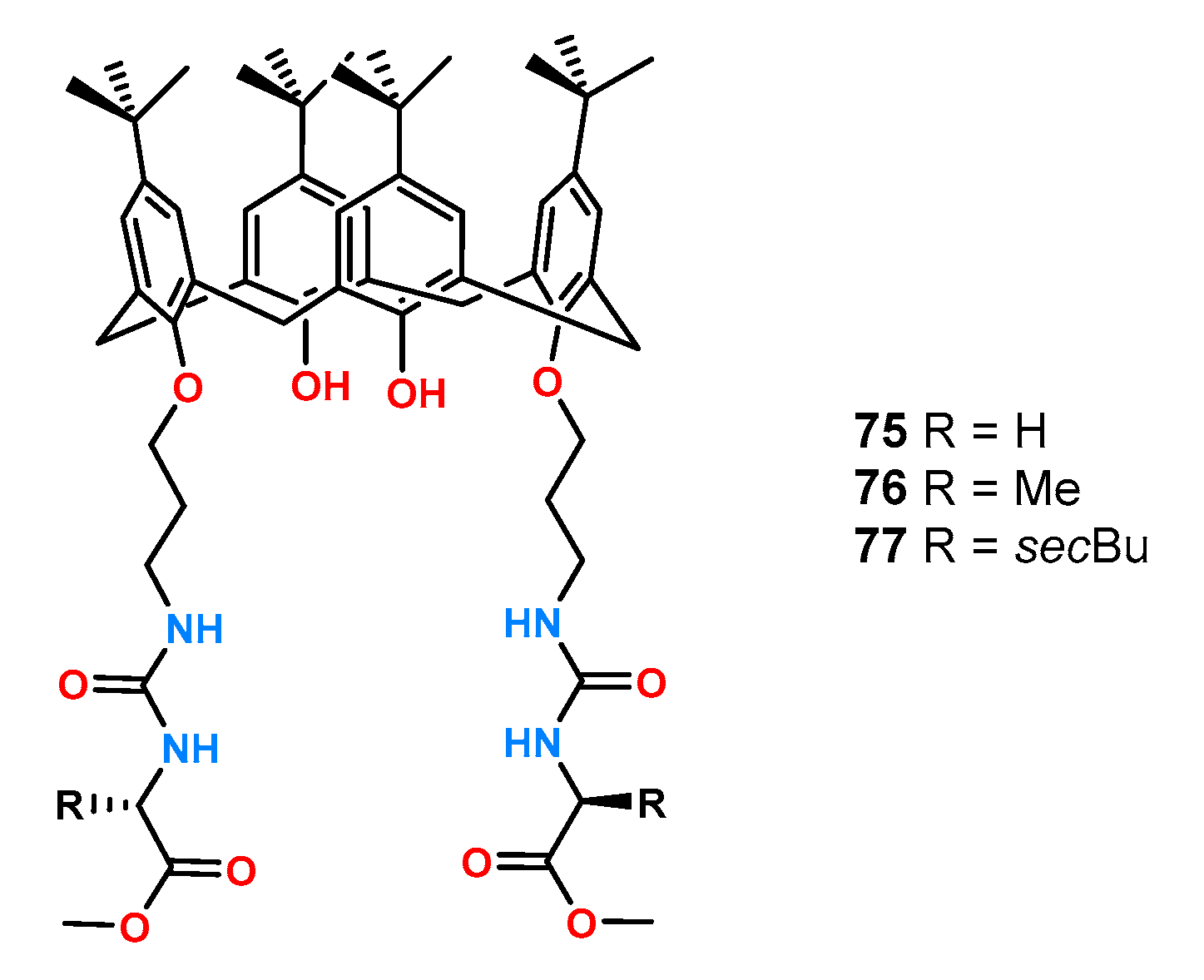
Receptors
75–
77 based on the calixarene (
Figure 26) were formed from the symmetric attachment of urea functions to the lower rim of calixarene and were examined by
1H NMR titration experiments in CDCl
3 and acetone-
d6 [
57].
Surprisingly, all ligands formed complexes with higher stability constants in acetone compared to chloroform, which is a less competent solvent medium. This is attributed to the strong intramolecular hydrogen bonds which were broken in a more polar solvent such as acetone, allowing the formation of complexes with the anion. The highest enantioselectivity toward the tested pair of phenylalanine enantiomers was characterized by receptor 77 (KD/KL = 4.14), compared to KD/KL = 3.55 and KD/KL = 1.96 for 76 and 75, respectively.
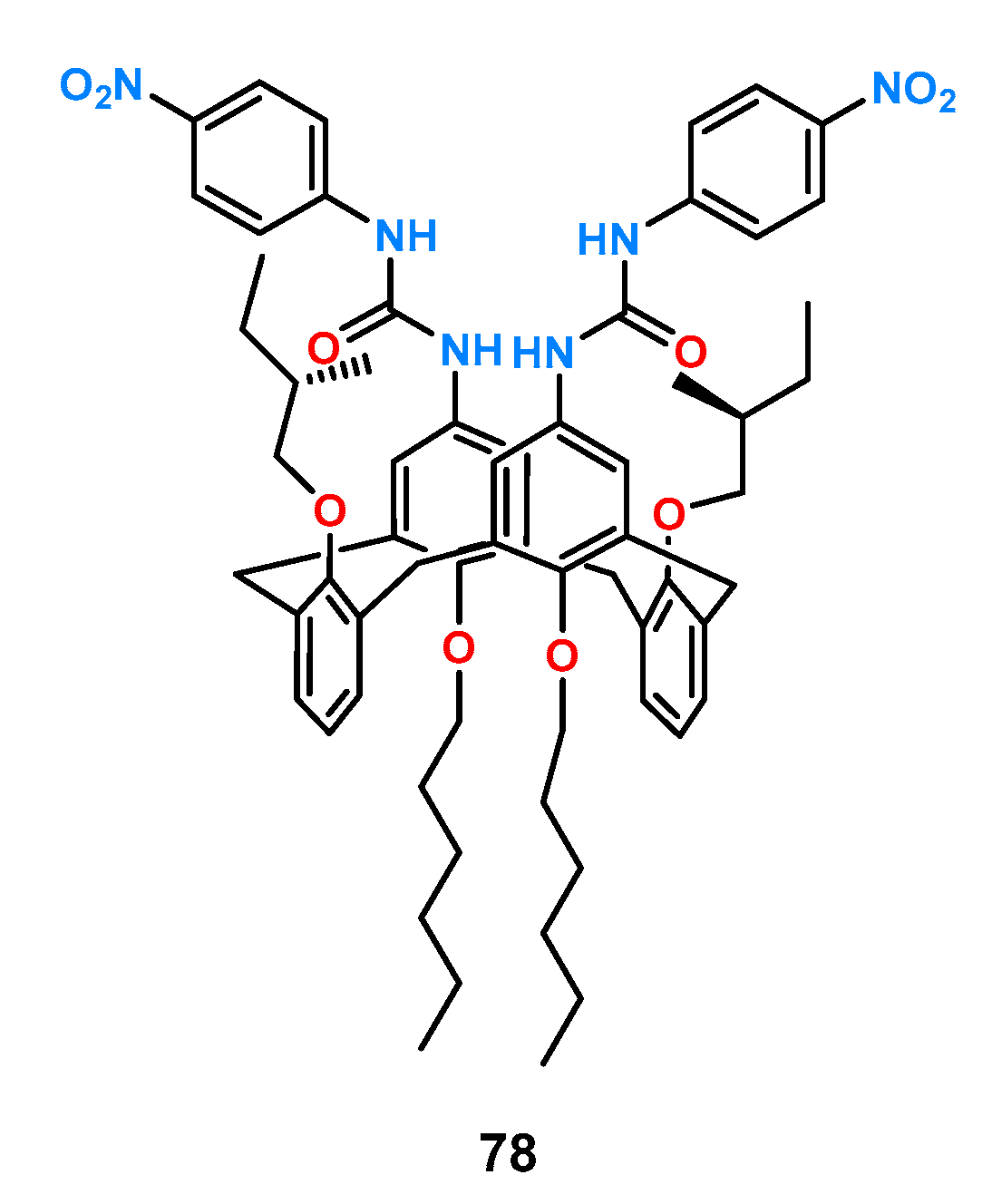
In the case of receptor
78, urea functions were attached to the core ring of calixarene (
Figure 27) [
58].
Its binding properties were examined by 1H NMR titration experiments in DMSO-d6. When this host was evaluated with a number of chiral carboxylates (Phe, N-Ac-Phe, N-Ac-Leu, N-Ac-Trp, and Man), it was shown that 78 did not show significant enantioselectivity toward the protected amino acids, whereas for free phenylalanine, the ratio of association constants for both stereoisomers was KD/KL = 2.86.
4. Hybrid Receptors
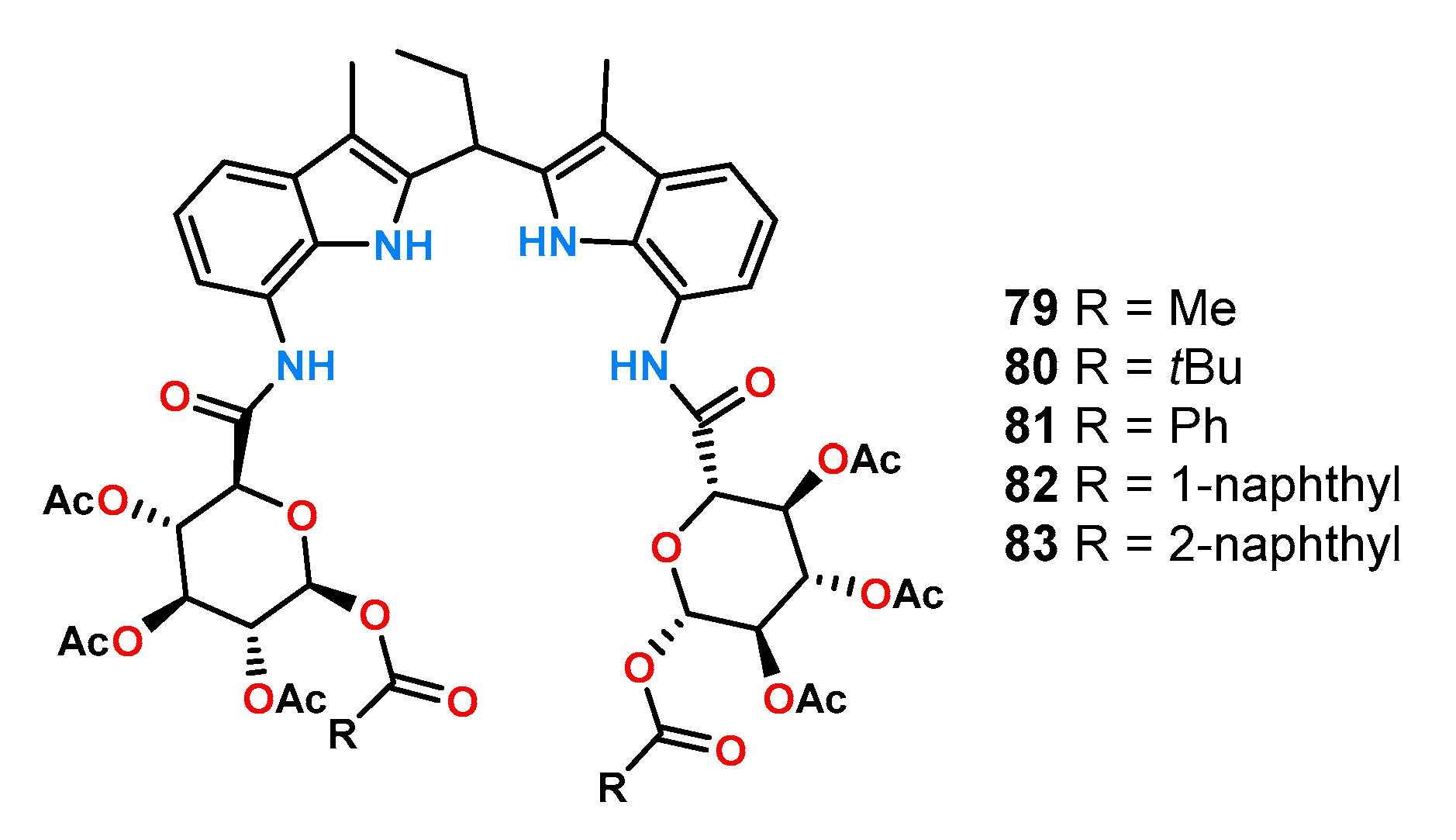
Among the effective receptors for chiral carboxylates, there are also ligands constructed with simultaneous insertion of hydrogen bonding donors of different nature into the receptor structure. A good representative example is the formation of a hybrid of two indole molecules via a methine bridge together with two amide functions which creates an anion binding pocket. Using this 7,7′-Diamino-2,2′-diindolylmethane – (DIM)-based hybrid concept, a series of chiral receptors
79–
83 was synthetized in the Jurczak group (
Figure 28) [
59].
These receptors were tested for their ability to recognize anions by the
1H NMR titration technique in a highly competitive solvent, such as DMSO-
d6 with 0.5% H
2O (
v/
v). The receptors differed in the protecting group of the hydroxyl group on the anomeric carbon atom. The results of chiral recognition of a range of anions using the receptors described above are given in
Table 5.
These results show very high ratios of association constants, which indicates an excellent matching of the bonding gap to the studied anions and appropriate localization of the chiral part. Moreover, a significant increase in chiral recognition was observed for receptors possessing an aromatic substituent which may indicate the presence of additional π-π interactions.
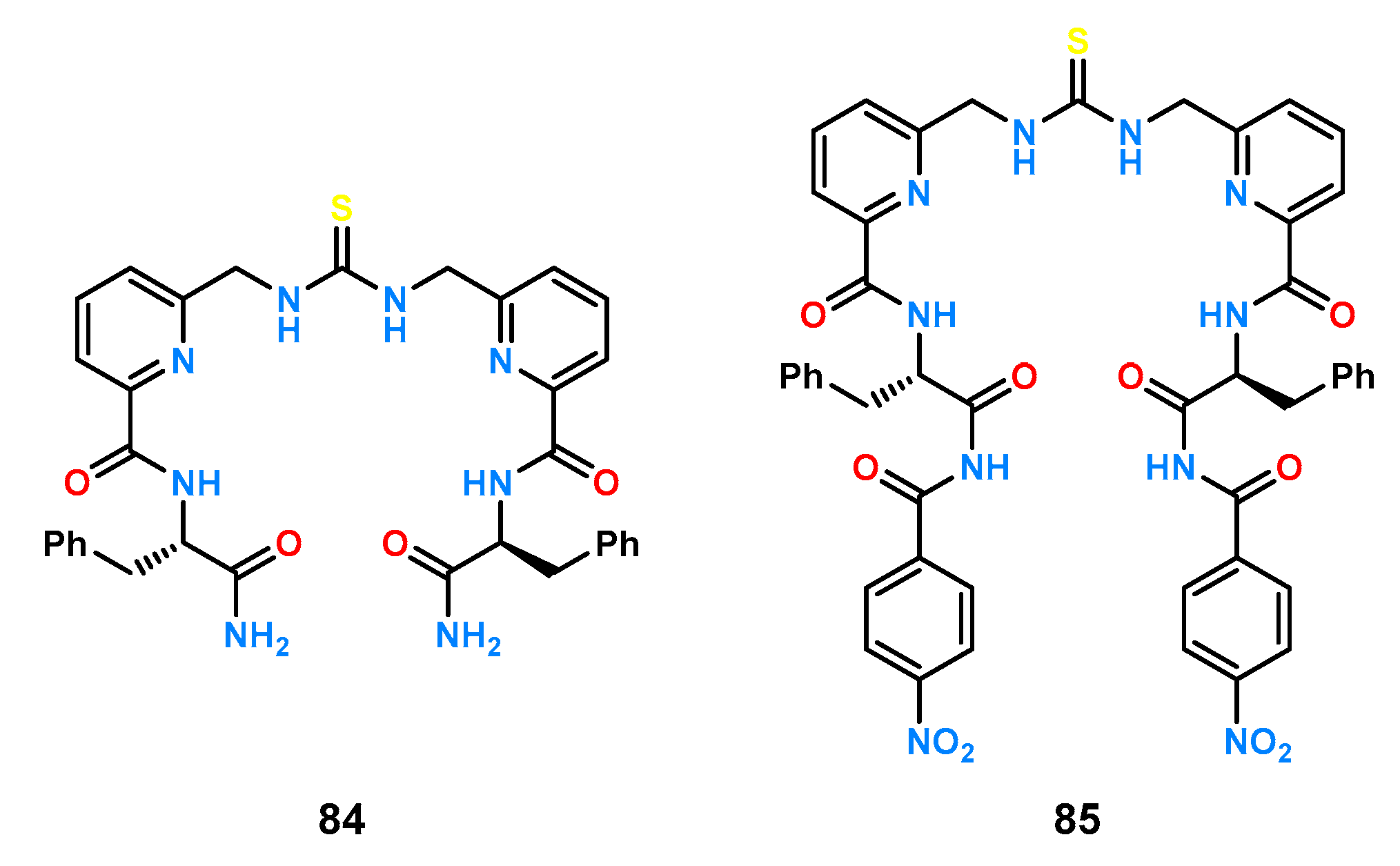
Another example of hybrid receptors is the acyclic ligands
84 and
85 with a central thiourea function (
Figure 29) [
60].
Titrations under 1H NMR control in a mixture of 10% DMSO-d6 in CDCl3 (v/v) of a series of protected amino acids (Ala, Phe, Asn, Gln, Trp, Ser) showed that receptor 84 exhibited little enantioselective amino acid binding properties. However, it exhibited high selectivity toward individual guest pairs; for example, the complex formed between 84 and N-Ac-Trp had 30 times higher stability than the 84 and N-Ac-Ser complex. In contrast, host 85 showed chiral recognition for the enantiomeric pair of N-acetylated tryptophan derivatives (KL = 1925, KD = 3785 M−1).
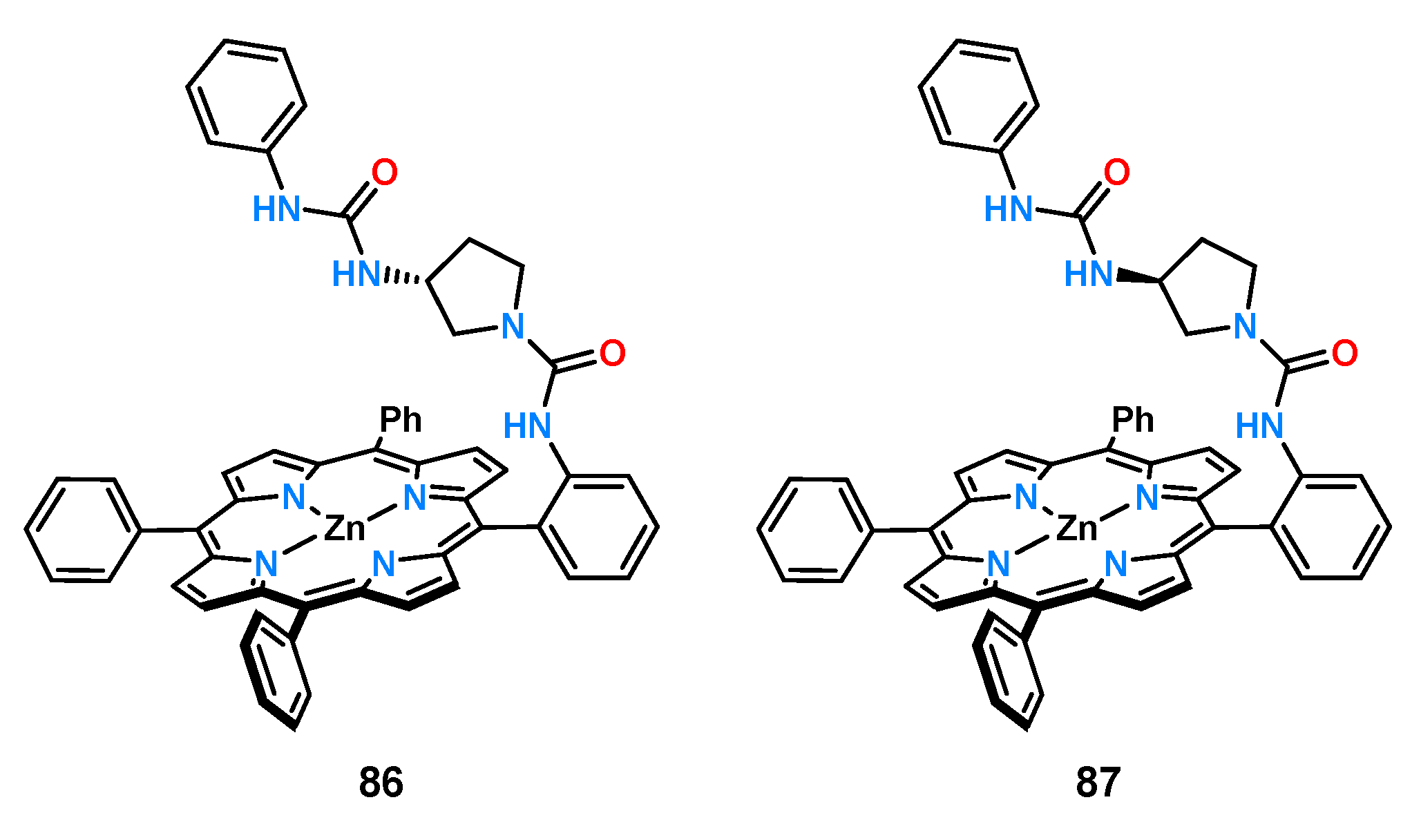
An interesting example demonstrating how small changes in receptor structure can affect chiral recognition are receptors
86 and
87 which differ in the absolute configuration of the carbon atom of the chiral fragment (
Figure 30) [
61].
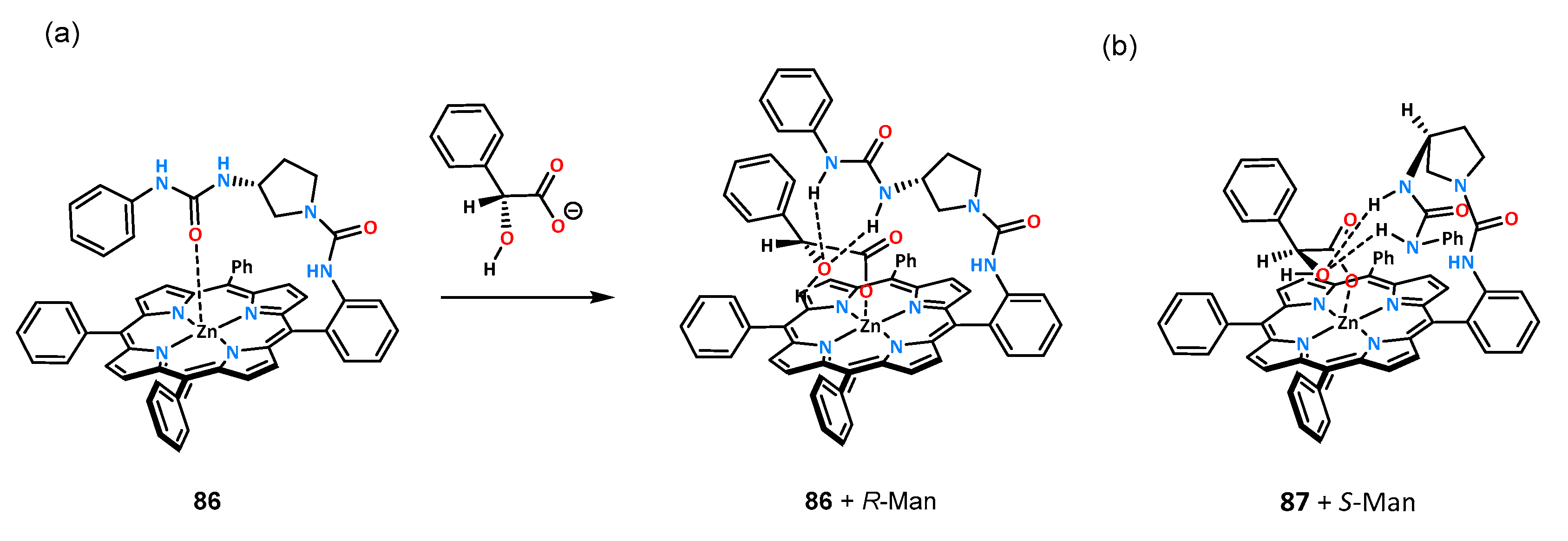
The binding properties of ligands constructed on a rigid porphyrin platform toward an enantiomeric pair of mandelic acid anions were investigated by titrations under UV-Vis and
1H NMR control in chloroform. The slight difference between the receptors resulted in a reversal of chiral recognition for mandelates. Receptor
86 showed a higher affinity for the
R-stereoisomer, with K
R/K
S = 3.0, while host
87 bound the
S-enantiomer with a higher association constant of K
R/K
S = 0.32. The authors also proposed a binding model for both receptors which is shown in
Scheme 2.
The oxygen atom of the urea carbonyl group for receptors in the free state (no guest addition) coordinates to the zinc atom located in the porphyrin system, and upon anion addition, the receptor begins to preorganize. The anion molecule interacts with the metal center on one side and with the urea function through hydrogen bonds on the other side. Ligand 86 may interact more favorably with R-mandelate due to its better fitting to the porphyrin site, and there is no steric interaction with the phenyl group; however, if the stereoisomer S binds in a similar manner, there will be an interaction of the phenyl substituent with the porphyrin surface.
The symmetrical molecular receptors
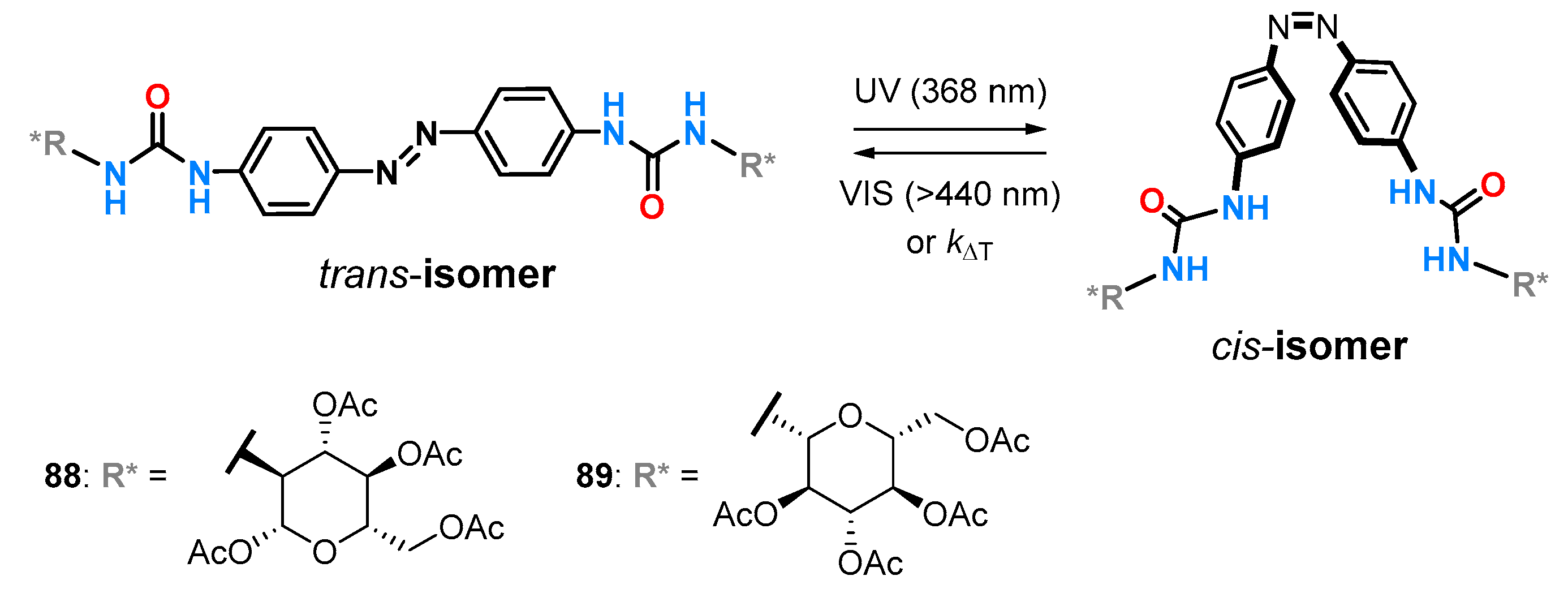
88 and
89 consisting of a photoswitchable azobenzene scaffold functionalized with urea hydrogen-bonding groups and
d-carbohydrates as chiral selectors were developed by Dąbrowa et al. to gain control over the chiral recognition of α-amino acid derived carboxylates (
Figure 31) [
18].
Upon irradiation with the UV light, the native and planar
trans-isomers of receptors
88 and
89 readily undergo
trans→
cis isomerization to the corresponding metastable and more compact
cis-isomers. The reverse
cis→
trans isomerization is triggered upon photoirradiation with visible light or upon heating. The
trans and
cis isomers were found to exhibit different affinities, selectivities, and binding modes toward tetrabutylammonium salts of amino acids protected by a
tert-butoxycarbonyl group (Boc-Phe and Boc-Trp) as revealed from UV-Vis and
1H NMR titration experiments in highly polar DMSO/H
2O (99.5/0.5
v/
v) solvent mixture (
Table 6).
Specifically, the binding affinity for the same enantiomerically pure guest was up to 3-fold higher for trans-isomers than for cis-isomers of receptors 88 and 89. Furthermore, both trans- and cis-isomers of 88 and 89 exhibited higher affinity to Boc-Trp over Boc-Phe, with a slight preference for the carboxylate guests derived from D-amino acids (except trans-88, which binds L-Boc-Phe more strongly than D-Boc-Phe). The preference for the Trp anion was attributed to a favorable intermolecular interaction between anion and receptor, in particular between indole NH proton and the carbonyl group of the sugar moieties. Moreover, the rate of thermal cis→trans isomerization was found to depend on the chiral binding ability properties of the cis-isomer; that is, a more strongly bound carboxylate enantiomer, as well as a higher enantiomer concentration, caused faster relaxation to the corresponding trans-isomer of receptors 88 and 89.