Microencapsulated Isoniazid-Loaded Metal–Organic Frameworks for Pulmonary Administration of Antituberculosis Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. INH Encapsulation
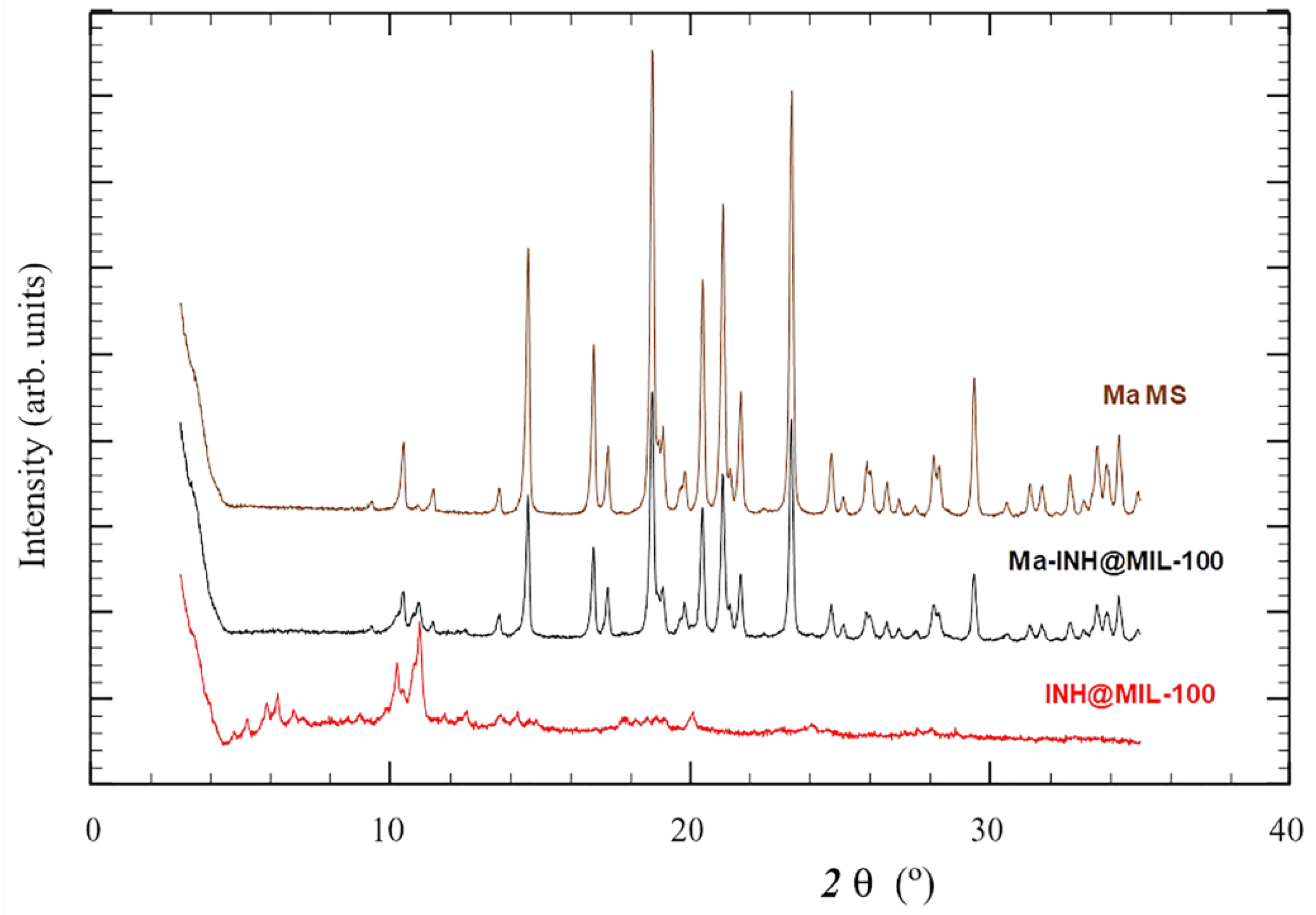
2.2. Preparation and Characterization of MS
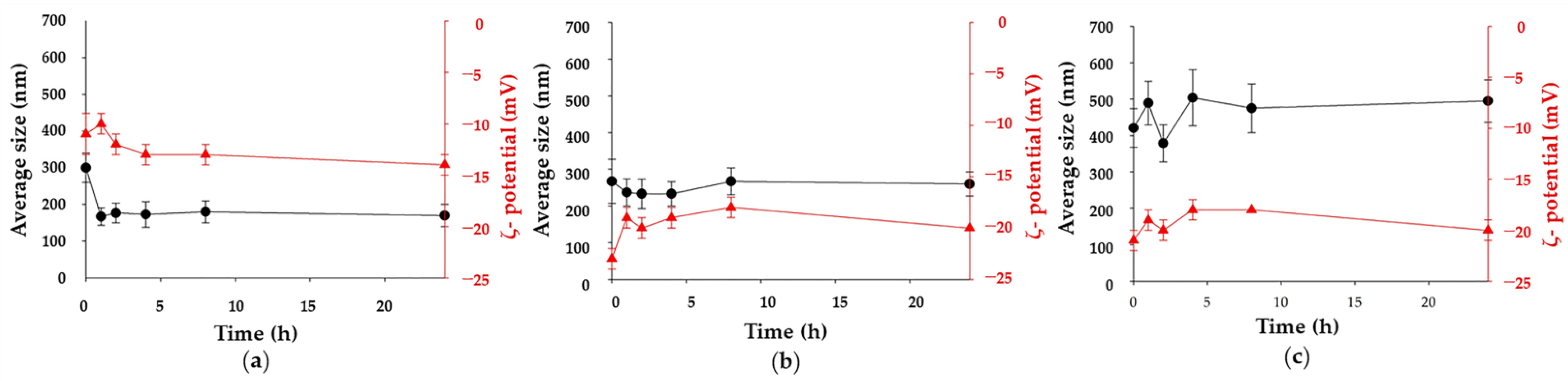
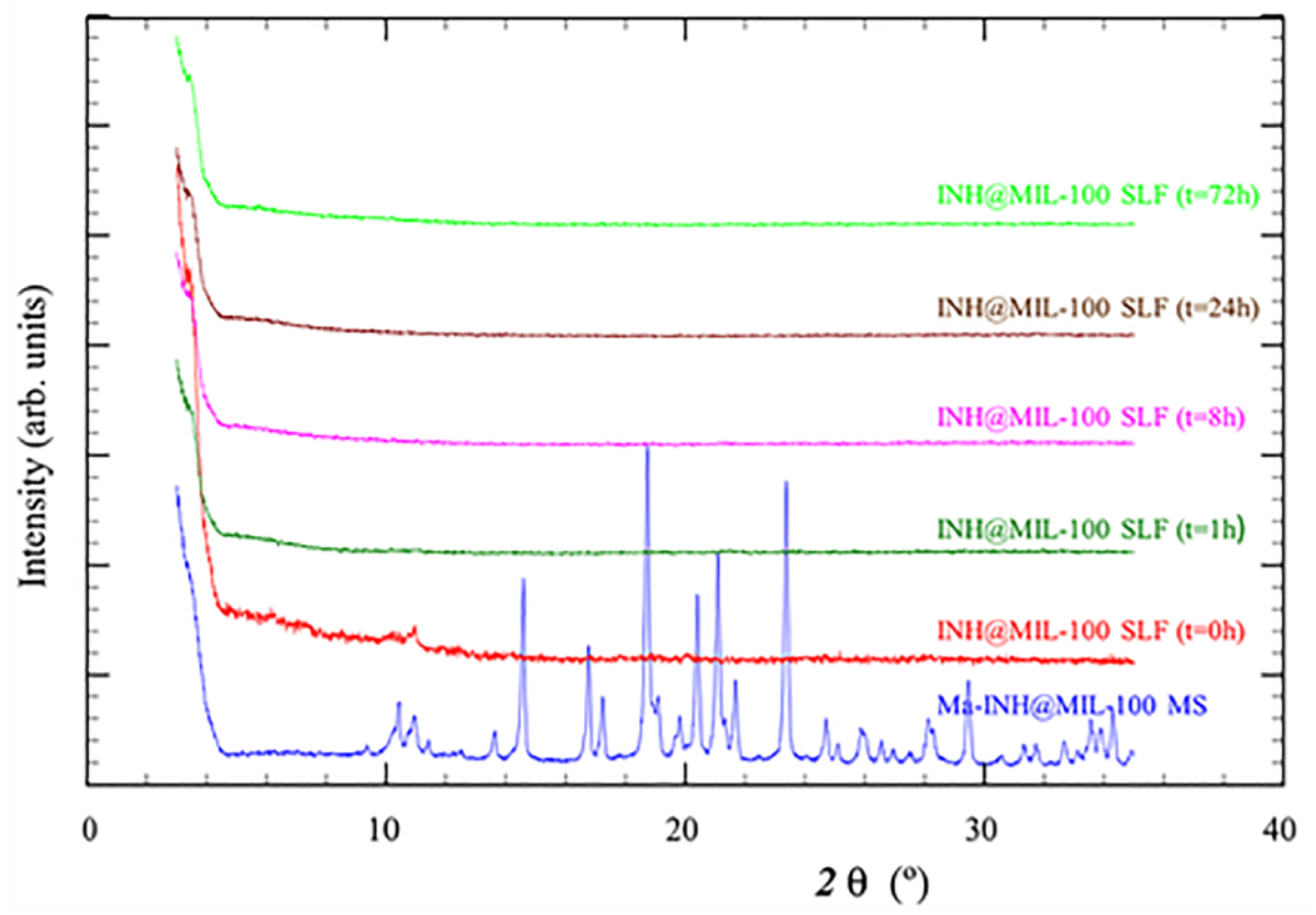
2.3. Ma-INH@MIL-100 MS: Colloidal and Chemical Stability, and INH Release
2.4. Characterization of Test Formulations for Cell Studies
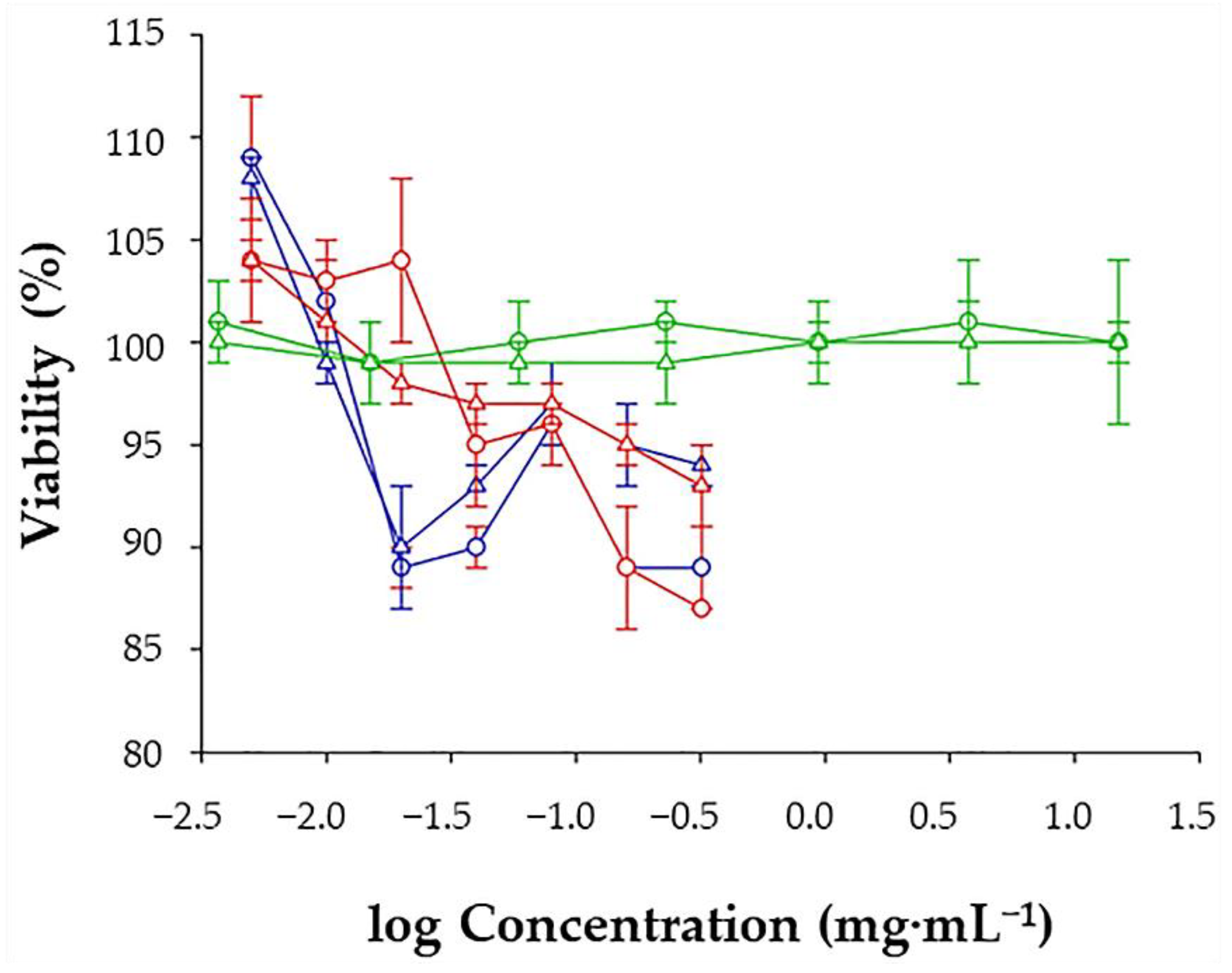
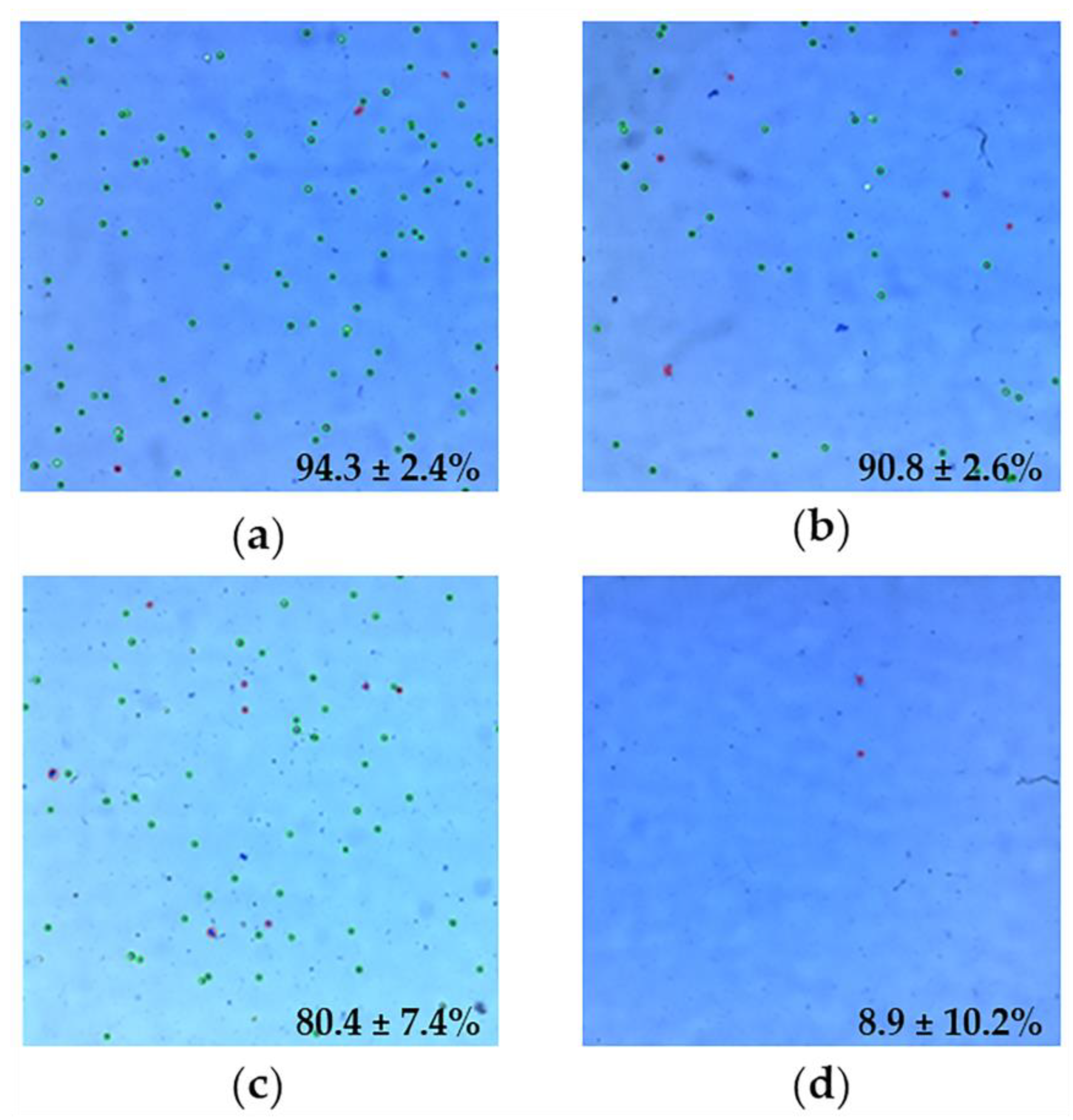
2.5. Cell Viability Studies
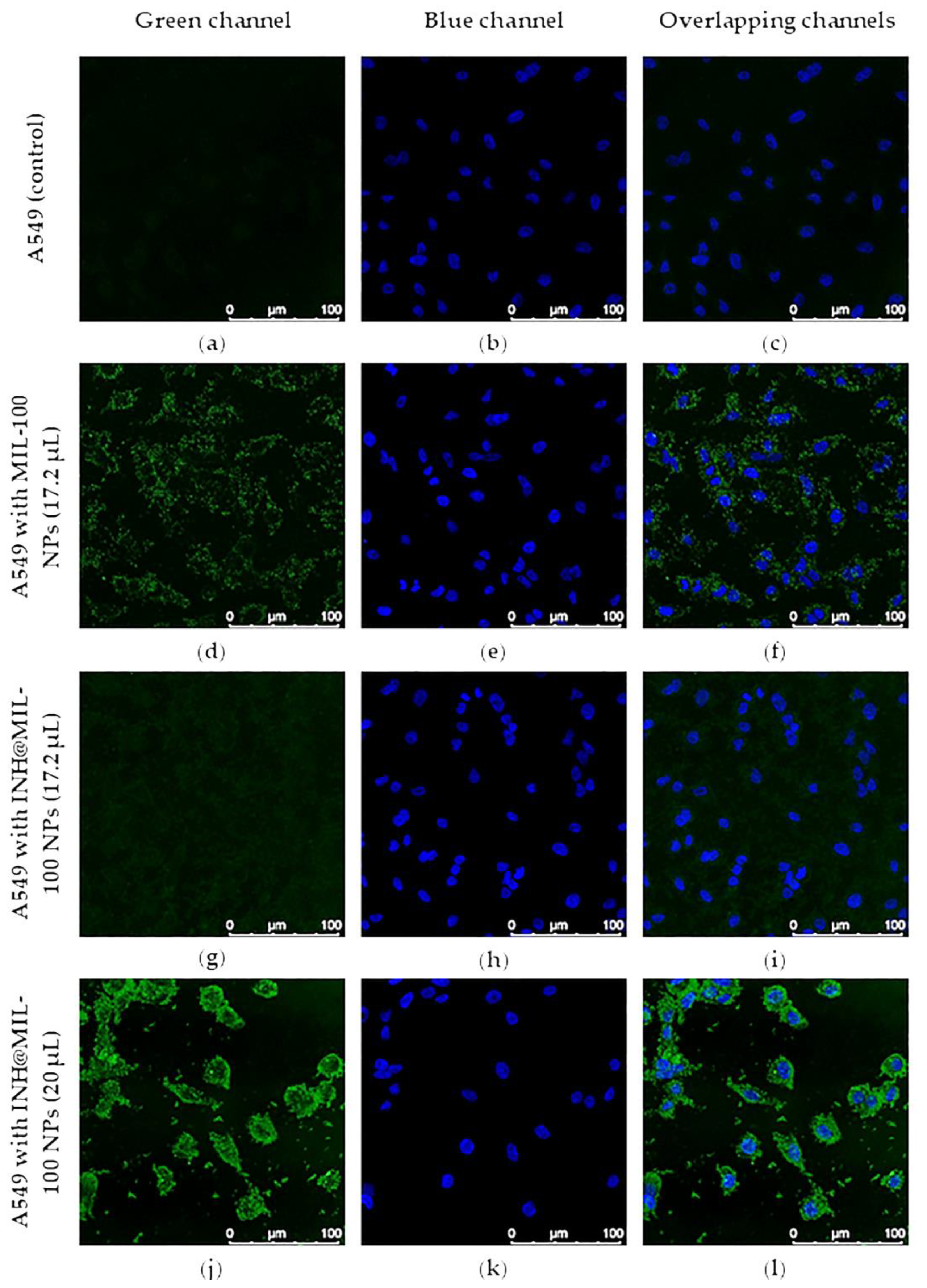
2.6. Intracellular Uptake and Distribution
2.7. Quantification of A549 Cells Internalized with NPs: Flow Cytometry
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MIL-100(Fe) and INH Encapsulation
3.3. Preparation of MS
3.4. Characterization of MS
3.5. Study of Composition and Structural Integrity of Ma-INH@MIL-100 MS
3.6. Ma-INH@MIL-100 MS: Colloidal and Chemical Stability, and INH Release
3.7. A549 Cell Line
3.8. Preparation and Characterization of Test Formulations for Cell Studies
3.9. Preparation of Test-Ma Solutions for the Viability Study
3.10. Cell Viability Studies
3.11. Intracellular Uptake and Distribution
3.12. Quantification of A549 Cells Internalized with NPs: Flow Cytometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Smulan, L.J.; Martinez, N.; Kiritsy, M.C.; Kativhu, C.; Cavallo, K.; Sassetti, C.M.; Singhal, A.; Remold, H.G.; Kornfeld, H. Sirtuin 3 downregulation in mycobacterium tuberculosis-infected macrophages reprograms mitochondrial metabolism and promotes cell death. mBio 2021, 12, e03140-20. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.C.D.Q.; Silva, D.R.; Dalcolmo, M.P. Tuberculosis: Where are we? J. Bras. Pneumol. 2018, 44, 82. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tuberculosis. Available online: https://www.who.int/health-topics/tuberculosis#tab=tab_1 (accessed on 4 September 2021).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 4 September 2021).
- Fogel, N. Tuberculosis: A disease without boundaries. Tuberculosis 2015, 95, 527–531. [Google Scholar] [CrossRef] [Green Version]
- Vishwa, B.; Moin, A.; Gowda, D.V.; Rizvi, S.M.D.; Hegazy, W.A.H.; Abu Lila, A.S.; Khafagy, E.-S.; Allam, A.N. Pulmonary targeting of inhalable moxifloxacin microspheres for effective management of tuberculosis. Pharmaceutics 2021, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for treatment of tuberculosis. Available online: https://www.who.int/tb/publications/2010/9789241547833/en/ (accessed on 4 September 2021).
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 4 September 2021).
- Lee, S.L.; Adams, W.P.; Li, B.V.; Conner, D.P.; Chowdhury, B.A.; Yu, L.X. In vitro considerations to support bioequivalence of locally acting drugs in dry powder inhalers for lung diseases. AAPS J. 2009, 11, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.; Rodrigues, M.; Domingues, R.; Torrado, E.; Reis, R.L.; Pedrosa, J.; Gomes, M.E. Exploring inhalable polymeric dry powders for anti-tuberculosis drug delivery. Mater. Sci. Eng. C 2018, 93, 1090–1103. [Google Scholar] [CrossRef]
- Grenha, A.; Carrión-Recio, D.; Teijeiro-Osorio, D.; Seijo, B.; Remuñán-López, C. Nano and micro-particulate carriers for pulmonary drug delivery. In Handbook of Particulate Drug Delivery, 1st ed.; Kumar, M.N.V., Ed.; American Scientific Publishers: Valencia, CA, USA, 2008; Volume 2, pp. 165–192. [Google Scholar]
- Brain, J.D. Inhalation, deposition, and fate of insulin and other therapeutic proteins. Diabetes Technol. Ther. 2007, 9, S4–S15. [Google Scholar] [CrossRef]
- Ogienko, A.; Bogdanova, E.; Trofimov, N.; Myz, S.; Kolesov, B.; Yunoshev, A.; Zubikov, N.; Manakov, A.; Boldyrev, V.; Boldyreva, E. Large porous particles for respiratory drug delivery. Glycine-based formulations. Eur. J. Pharm. Sci. 2017, 110, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Saxena, D.; Dwivedi, A.K.; Misra, A. Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm. Res. 2001, 18, 1405–1410. [Google Scholar] [CrossRef]
- D’Angelo, I.; Perfetto, B.; Costabile, G.; Ambrosini, V.; Caputo, P.; Miro, A.; Bianca, R.D.D.V.; Sorrentino, R.; Donnarumma, G.; Quaglia, F.; et al. Large porous particles for sustained release of a decoy oligonucelotide and poly(ethylenimine): Potential for combined therapy of chronic pseudomonas aeruginosa lung infections. Biomacromolecules 2016, 17, 1561–1571. [Google Scholar] [CrossRef]
- Muttil, P.; Kaur, J.; Kumar, K.; Yadav, A.; Sharma, R.; Misra, A. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci. 2007, 32, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Patel, L.; Dalwadi, S. Microparticles of rifampicin: Comparison of pulmonary route with oral route for drug uptake by alveolar macrophages, phagocytosis activity and toxicity study in albino rats. Drug Deliv. 2013, 21, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Kunda, N.K.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Polymeric nanoparticles for the delivery of miRNA to treat Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Pharm. Biopharm. 2019, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.S.; Rojas, S.; Salcedo-Abraira, P.; Simón-Yarza, T.; Remuñán-López, C.; Horcajada, P. Metal-organic framework microsphere formulation for pulmonary administration. ACS Appl. Mater. Interfaces 2020, 12, 25676–25682. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Paz, E.; Feijoo-Siota, L.; Gaspar, M.M.; Csaba, N.; Remuñán-López, C. Microencapsulated chitosan-based nanocapsules: A new platform for pulmonary gene delivery. Pharmaceutics 2021, 13, 1377. [Google Scholar] [CrossRef]
- Shah, K.; Chan, L.W.; Wong, T.W. Critical physicochemical and biological attributes of nanoemulsions for pulmonary delivery of rifampicin by nebulization technique in tuberculosis treatment. Drug Deliv. 2017, 24, 1631–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, S.; Feeney, O.; Meeusen, E.; Boyd, B.J.; McIntosh, M.P.; Pouton, C.W.; Whittaker, M.; Kaminskas, L.M. Local inflammation alters the lung disposition of a drug loaded pegylated liposome after pulmonary dosing to rats. J. Control. Release 2019, 307, 32–43. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.L.; Salgueiro, J.; Bernabeu, E.; Gonzalez, L.; Manca, M.L.; Amiano, N.; Valenti, D.; Manconi, M.; García, V.; et al. Pulmonary delivery of rifampicin-loaded soluplus micelles against Mycobacterium tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 53, 101170. [Google Scholar] [CrossRef]
- Baati, T.; Njim, L.; Neffati, F.; Kerkeni, A.; Bouttemi, M.; Gref, R.; Najjar, M.F.; Zakhama, A.; Couvreur, P.; Serre, C.; et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(III) metal-organic frameworks. Chem. Sci. 2013, 4, 1597–1607. [Google Scholar] [CrossRef]
- Nabi, B.; Rehman, S.; Aggarwal, S.; Baboota, S.; Ali, J. Nano-based anti-tubercular drug delivery: An emerging paradigm for improved therapeutic intervention. Drug Deliv. Transl. Res. 2020, 10, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- García-Márquez, A.; Demessence, A.; Platero-Prats, A.E.; Heurtaux, D.; Horcajada, P.; Serre, C.; Chang, J.-S.; Férey, G.; de la Peña-O’Shea, V.A.; Boissière, C.; et al. Green microwave synthesis of MIL-100(Al, Cr, Fe) nanoparticles for thin-film elaboration. Eur. J. Inorg. Chem. 2012, 100, 5165–5174. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Bosquillon, C.; Lombry, C.; Préat, V.; Vanbever, R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release 2001, 70, 329–339. [Google Scholar] [CrossRef]
- Weers, J.; Tarara, T. The PulmoSphere TM platform for pulmonary drug delivery. Ther. Deliv. 2014, 5, 277–295. [Google Scholar] [CrossRef]
- Gharse, S.; Fiegel, J. Large porous hollow particles: Lightweight champions of pulmonary drug delivery. Curr. Pharm. Des. 2016, 22, 2463–2469. [Google Scholar] [CrossRef] [Green Version]
- Bi, R.; Shao, W.; Wang, Q.; Zhang, N. Solid lipid nanoparticles as insulin inhalation carriers for enhanced pulmonary delivery. J. Biomed. Nanotechnol. 2009, 5, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadi, S.; Grenha, A.; Remuñán-López, C. Microspheres loaded with polysaccharide nanoparticles for pulmonary delivery: Preparation, structure and surface analysis. Carbohydr. Polym. 2011, 86, 25–34. [Google Scholar] [CrossRef]
- Das, S.; Tucker, I.; Stewart, P. Inhaled dry powder formulations for treating tuberculosis. Curr. Drug Deliv. 2015, 12, 26–39. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltonen, L.; Valo, H.; Kolakovic, R.; Laaksonen, T.; Hirvonen, J. Electrospraying, spray drying and related techniques for production and formulation of drug nanoparticles. Expert Opin. Drug Deliv. 2010, 7, 705–719. [Google Scholar] [CrossRef]
- Sham, J.O.-H.; Zhang, Y.; Finlay, W.H.; Roa, W.H.; Löbenberg, R. Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int. J. Pharm. 2004, 269, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Azarmi, S.; Tao, X.; Chen, H.; Wang, Z.; Finlay, W.H.; Löbenberg, R.; Roa, W.H. Formulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particles. Int. J. Pharm. 2006, 319, 155–161. [Google Scholar] [CrossRef]
- Guterres, S.S.; Beck, R.C.R.; Pohlmann, A.R. Spray-drying technique to prepare innovative nanoparticulated formulations for drug administration: A brief overview. Braz. J. Physics 2009, 39, 205–209. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Chow, A.H.; Tong, H.H.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Kim, R.Y.; Brown, A.C.; Donovan, C.; Vanka, K.S.; Mayall, J.R.; Liu, G.; Pillar, A.L.; Bernadette, J.-F.; Xenaki, D.; et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J. Pathol. 2020, 251, 49–62. [Google Scholar] [CrossRef]
- Allden, S.J.; Ogger, P.P.; Ghai, P.; McErlean, P.; Hewitt, R.; Toshner, R.; Walker, S.A.; Saunders, P.; Kingston, S.; Molyneaux, P.L.; et al. The transferrin receptor CD71 delineates functionally distinct airway macrophage subsets during idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 209–219. [Google Scholar] [CrossRef]
- Pietrangelo, A.; Gualdi, R.; Casalgrandi, G.; Montosi, G.; Ventura, E. Molecular and cellular aspects of iron-induced hepatic cirrhosis in rodents. J. Clin. Invest. 1995, 95, 1824–1831. [Google Scholar] [CrossRef]
- Tuderman, L.; Myllyla, R.; Kivirikko, K.I. Mechanism of the prolyl hydroxylase reaction 1. Role of co-substrates. JBIC Eur. J. Biochem. 1977, 80, 341–348. [Google Scholar] [CrossRef]
- Wyszogrodzka-Gaweł, G.; Dorożyński, P.; Giovagnoli, S.; Strzempek, W.; Pesta, E.; Węglarz, W.P.; Gil, B.; Menaszek, E.; Kulinowski, P. An inhalable theranostic system for local tuberculosis treatment containing an isoniazid loaded metal organic framework Fe-MIL-101-NH2-from raw MOF to drug delivery system. Pharmaceutics 2019, 11, 687. [Google Scholar] [CrossRef] [Green Version]
- Singco, B.; Liu, L.-H.; Chen, Y.-T.; Shih, Y.-H.; Huang, H.-Y.; Lin, C.-H. Approaches to drug delivery: Confinement of aspirin in MIL-100(Fe) and aspirin in the de novo synthesis of metal-organic frameworks. Microporous Mesoporous Mater. 2016, 223, 254–260. [Google Scholar] [CrossRef]
- Rojas, S.; Colinet, I.; Cunha, D.; Hidalgo, T.; Salles, F.; Serre, C.; Guillou, N.; Horcajada, P. Toward understanding drug incorporation and delivery from biocompatible metal-organic frameworks in view of cutaneous administration. ACS Omega 2018, 3, 2994–3003. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regi, M.; Sebban, M.; Taulelle, F.; Ferey, G. Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sailatha, E.; Seshadri, S.; Kumaresan, S. FTIR, FT Raman spectra and molecular structural confirmation of isoniazid. Indian J. Pure Appl. Phys. 2009, 47, 12–18. [Google Scholar]
- Leclerc, H.; Vimont, A.; Lavalley, J.-C.; Daturi, M.; Wiersum, A.D.; Llwellyn, P.L.; Horcajada, P.; Ferey, G.; Serre, C. Infrared study of the influence of reducible iron(iii) metal sites on the adsorption of CO, CO2, propane, propene and propyne in the mesoporous metal-organic framework MIL-100. Phys. Chem. Chem. Phys. 2011, 13, 11748–11756. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Serra, C.; Remuñán-López, C. Chitosan nanoparticle-loaded mannitol microspheres: Structure and surface characterization. Biomacromolecules 2007, 8, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.D.; Cavaco, J.S.; Guerreiro, F.; Lourenço, J.P.; da Costa, A.M.R.; Grenha, A. Inhalable antitubercular therapy mediated by locust bean gum microparticles. Molecules 2016, 21, 702. [Google Scholar] [CrossRef] [Green Version]
- Jensen, D.M.K.; Cun, D.; Maltesen, M.J.; Frokjaer, S.; Nielsen, H.M.; Foged, C. Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J. Control. Release 2010, 142, 138–145. [Google Scholar] [CrossRef]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar] [CrossRef]
- Maas, S.G.; Schaldach, G.; Littringer, E.M.; Mescher, A.; Griesser, U.J.; Braun, D.E.; Walzel, P.E.; Urbanetz, N.A. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Daviskas, E.; Anderson, S.D.; Eberl, S.; Young, I.H. Beneficial effect of inhaled mannitol and cough in asthmatics with mucociliary dysfunction. Respir. Med. 2010, 104, 1645–1653. [Google Scholar] [CrossRef] [Green Version]
- Elversson, J.; Millqvist-Fureby, A. In situ coating—An approach for particle modification and encapsulation of proteins during spray-drying. Int. J. Pharm. 2006, 323, 52–63. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Gaspar, M.M.; Eleutério, C.V.; Grenha, A.; Blanco, M.; Gonçalves, L.M.D.; Taboada, P.; Almeida, A.J.; Remuñán-López, C. Microencapsulated solid lipid nanoparticles as a hybrid platform for pulmonary antibiotic delivery. Mol. Pharm. 2017, 14, 2977–2990. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.M.; Gorman, E.M.; Munson, E.J.; Berkland, C. Pure insulin nanoparticle agglomerates for pulmonary delivery. Langmuir 2008, 24, 13614–13620. [Google Scholar] [CrossRef] [Green Version]
- Sinsuebpol, C.; Chatchawalsaisin, J.; Kulvanich, P. Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery. Drug Design Dev. Ther. 2013, 7, 861–873. [Google Scholar] [CrossRef] [Green Version]
- Al-Qadi, S.; Grenha, A.; Carrión-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release 2012, 157, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tewa-Tagne, P.; Briançon, S.; Fessi, H. Preparation of redispersible dry nanocapsules by means of spray-drying: Development and characterization. Eur. J. Pharm. Sci. 2007, 30, 124–135. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [Green Version]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remuñán-López, C.; Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef]
- Grall, R.; Hidalgo, T.; Delic, J.; Garcia-Marquez, A.; Chevillard, S.; Horcajada, P. In vitro biocompatibility of mesoporous metal (III; Fe, Al, Cr) trimesate MOF nanocarriers. J. Mater. Chem. B 2015, 3, 8279–8292. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Faria, V.; Gonçalves, L.M.D.; Taboada, P.; Remuñán-López, C.; Almeida, A.J. Rifabutin-loaded solid lipid nanoparticles for inhaled antitubercular therapy: Physicochemical and in vitro studies. Int. J. Pharm. 2016, 497, 199–209. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Model Lists of Essential Medicines. Available online: https://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 4 September 2021).
- U.S. Food & Drug Administration. Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 4 September 2021).
- Porru, D.; Parmigiani, A.; Tinelli, C.; Barletta, D.; Choussos, D.; di Franco, C.; Rovereto, B. Oral D-mannose in recurrent urinary tract infections in women: A pilot study. J. Clin. Urol. 2014, 7, 208–213. [Google Scholar] [CrossRef]
- Palazzo, F.; Giovagnoli, S.; Schoubben, A.; Blasi, P.; Rossi, C.; Ricci, M. Development of a spray-drying method for the formulation of respirable microparticles containing ofloxacin-palladium complex. Int. J. Pharm. 2013, 440, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pant, G.; Mitra, K.; Madan, J.; Chourasia, M.K.; Misra, A. Inhalable particles containing rapamycin for induction of autophagy in macrophages infected with Mycobacterium tuberculosis. Mol. Pharm. 2014, 11, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Bosquillon, C.; Préat, V.; Vanbever, R. Pulmonary delivery of growth hormone using dry powders and visualization of its local fate in rats. J. Control. Release 2004, 96, 233–244. [Google Scholar] [CrossRef]
- Rodrigues, S.; Cordeiro, C.; Seijo, B.; Remuñán-López, C.; Grenha, A. Hybrid nanosystems based on natural polymers as protein carriers for respiratory delivery: Stability and toxicological evaluation. Carbohydr. Polym. 2015, 123, 369–380. [Google Scholar] [CrossRef] [Green Version]


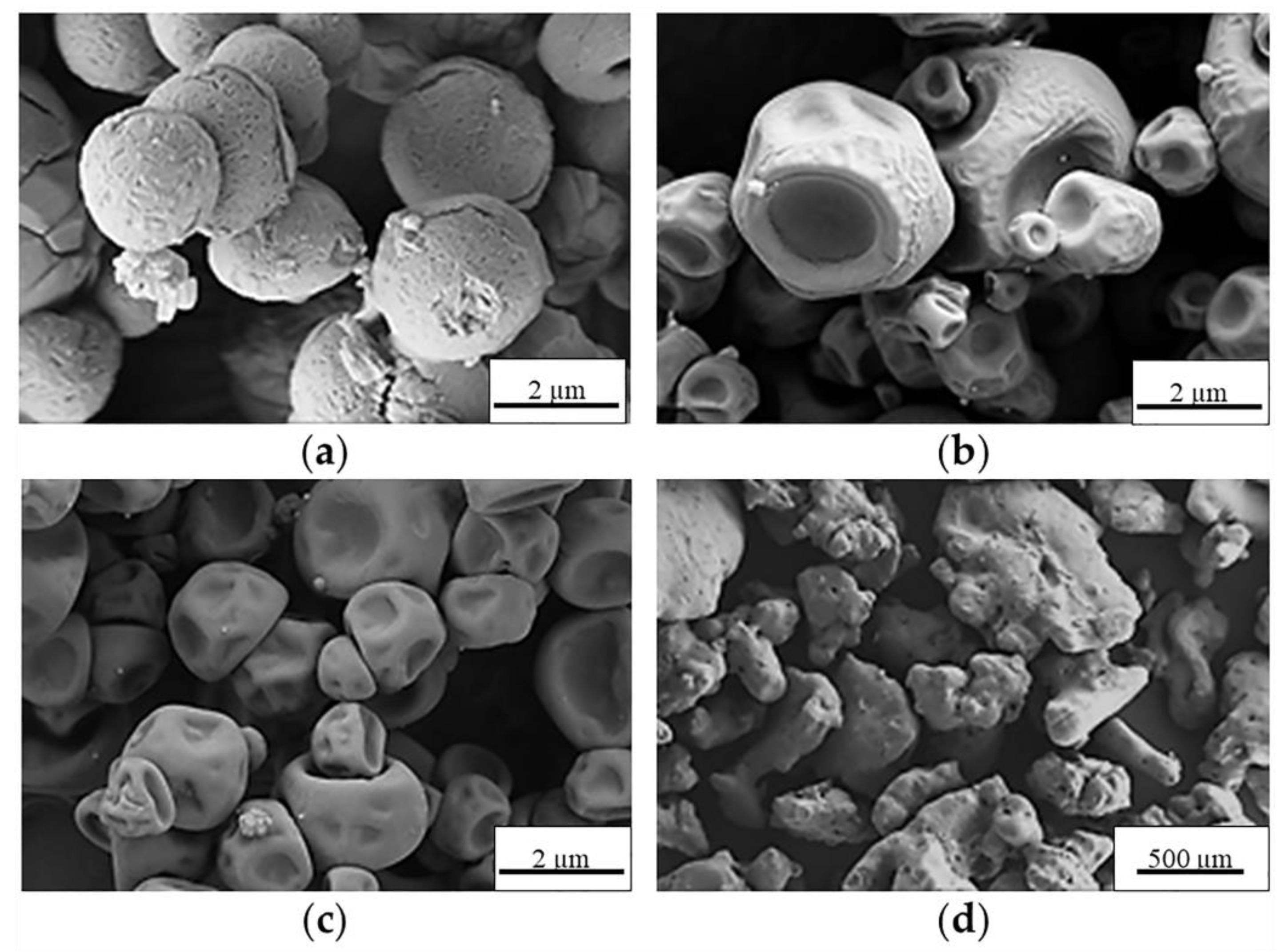
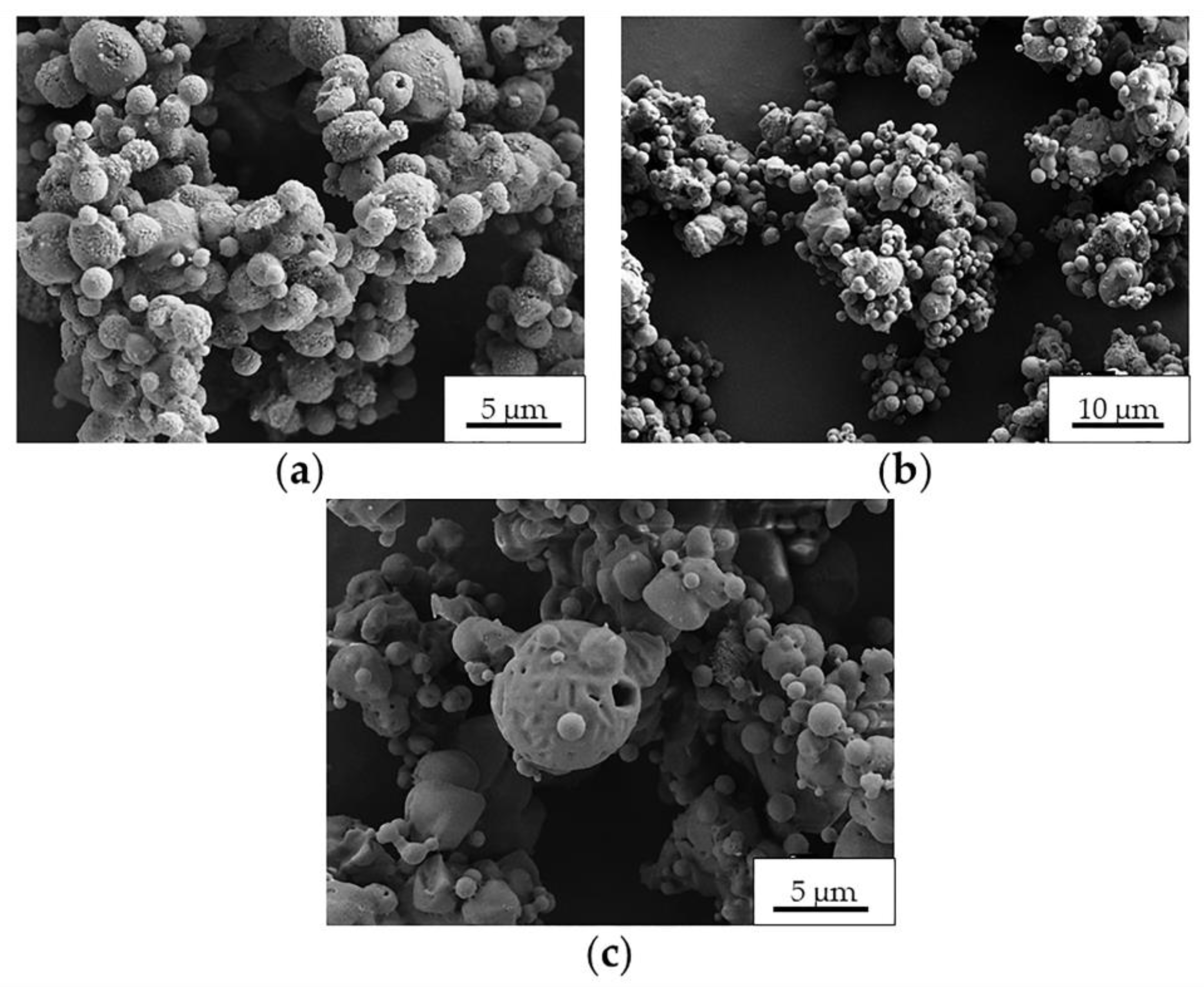
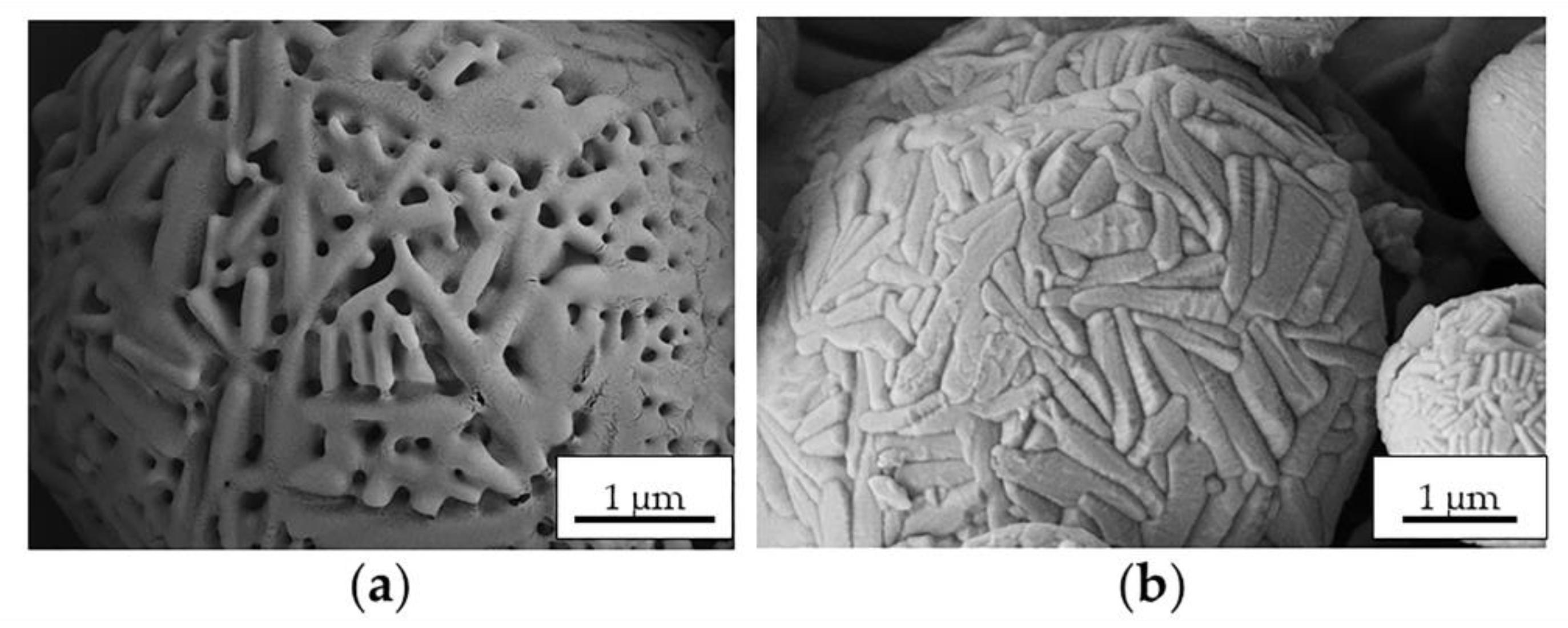
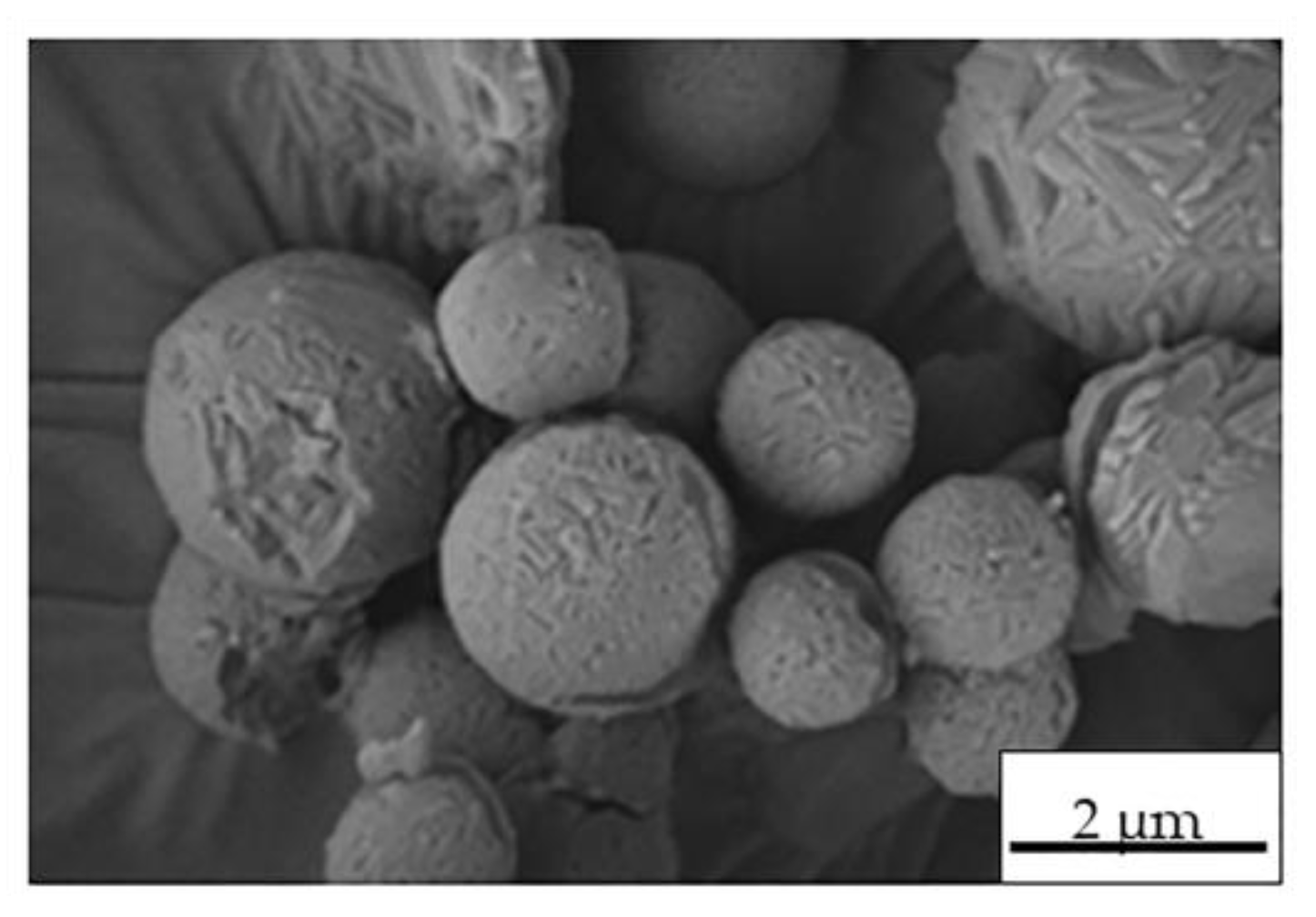




| Control MS | Process Yield (wt, %) | Morphology | Apparent Density (g·cm−3) |
|---|---|---|---|
| Ma MS | 58.5 ± 3.5 | Spherical | 0.43 ± 0.01 |
| α-CD MS | 74.0 ± 3.0 | Less spherical | 0.36 ± 0.01 |
| Dex MS | 10.5 ± 1.5 | Less spherical | - 1 |
| Tre MS | 27.0 ± 1.0 | Agglomerate | - 1 |
| Samples | Theoretical Values | Experimental Values | ||||||
|---|---|---|---|---|---|---|---|---|
| %C | %H | %N | %Fe | %C | %H | %N | %Fe | |
| Ma MS | 39.50 | 7.68 | - | - | 39.72 ± 0.24 | 7.50 ± 0.01 | - | - |
| Ma-MIL-100 MS | 38.89 | 7.08 | - | 2.50 | 39.79 ± 0.06 | 6.75 ± 0.07 | - | 2.68 ± 0.13 |
| Ma-INH@MIL-100 MS | 39.40 | 7.32 | 0.55 | 1.60 | 39.98 ± 0.09 | 7.24 ± 0.06 | 0.56 ± 0.07 | 1.25 ± 0.06 |
| Sample | Geometric Diameter (µm) | Real Density (g·cm−3) | Apparent Density (g·cm−3) | Aerodynamic Diameter (µm) |
|---|---|---|---|---|
| Ma MS | 2.3 ± 1.0 | 0.0666 ± 0.0001 | 0.43 ± 0.01 | 0.594 ± 0.010 |
| Ma-MIL-100 MS | 1.8 ± 0.7 | 0.0550 ± 0.0001 | 0.44 ± 0.02 | 0.422 ± 0.007 |
| Ma-INH@MIL-100 MS | 1.4 ± 0.4 | 0.0907 ± 0.0003 | 0.52 ± 0.01 | 0.422 ± 0.007 |
| NanoMOFs (mg·mL−1) | Ma (mg·mL−1) |
|---|---|
| 0.32 | 15.00 |
| 0.16 | 3.75 |
| 0.08 | 0.94 |
| 0.04 | 0.23 |
| 0.02 | 0.059 |
| 0.01 | 0.015 |
| 0.005 | 0.0037 |
| Sample | Volume of NP Dispersions (µL) | Number of Accumulations Per Plane |
|---|---|---|
| Without NPs | - | 2 |
| MIL-100 | 17.2 | 2 |
| INH@MIL-100 | 17.2 | 2 |
| INH@MIL-100 | 20.0 | 16 |
| INH@MIL-100 | 30.0 | 16 |
| INH@MIL-100 | 40.0 | 16 |
| INH@MIL-100 | 50.0 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Paz, C.; Fernández-Paz, E.; Salcedo-Abraira, P.; Rojas, S.; Barrios-Esteban, S.; Csaba, N.; Horcajada, P.; Remuñán-López, C. Microencapsulated Isoniazid-Loaded Metal–Organic Frameworks for Pulmonary Administration of Antituberculosis Drugs. Molecules 2021, 26, 6408. https://doi.org/10.3390/molecules26216408
Fernández-Paz C, Fernández-Paz E, Salcedo-Abraira P, Rojas S, Barrios-Esteban S, Csaba N, Horcajada P, Remuñán-López C. Microencapsulated Isoniazid-Loaded Metal–Organic Frameworks for Pulmonary Administration of Antituberculosis Drugs. Molecules. 2021; 26(21):6408. https://doi.org/10.3390/molecules26216408
Chicago/Turabian StyleFernández-Paz, Cristina, Estefanía Fernández-Paz, Pablo Salcedo-Abraira, Sara Rojas, Sheila Barrios-Esteban, Noemi Csaba, Patricia Horcajada, and Carmen Remuñán-López. 2021. "Microencapsulated Isoniazid-Loaded Metal–Organic Frameworks for Pulmonary Administration of Antituberculosis Drugs" Molecules 26, no. 21: 6408. https://doi.org/10.3390/molecules26216408
APA StyleFernández-Paz, C., Fernández-Paz, E., Salcedo-Abraira, P., Rojas, S., Barrios-Esteban, S., Csaba, N., Horcajada, P., & Remuñán-López, C. (2021). Microencapsulated Isoniazid-Loaded Metal–Organic Frameworks for Pulmonary Administration of Antituberculosis Drugs. Molecules, 26(21), 6408. https://doi.org/10.3390/molecules26216408






