Assessment of the Activity of Decoquinate and Its Quinoline-O-Carbamate Derivatives against Toxoplasma gondii In Vitro and in Pregnant Mice Infected with T. gondii Oocysts
Abstract
1. Introduction
2. Results
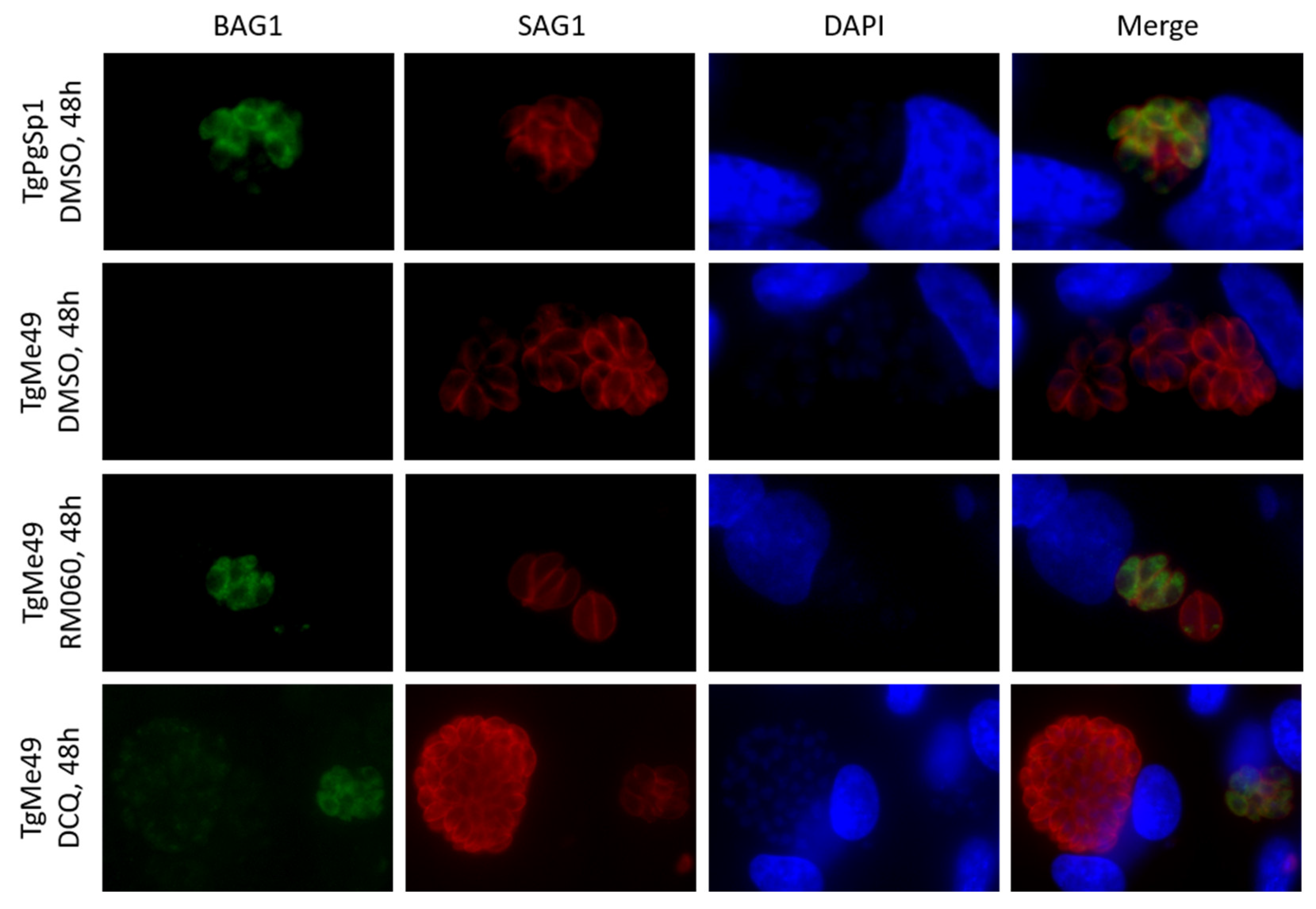
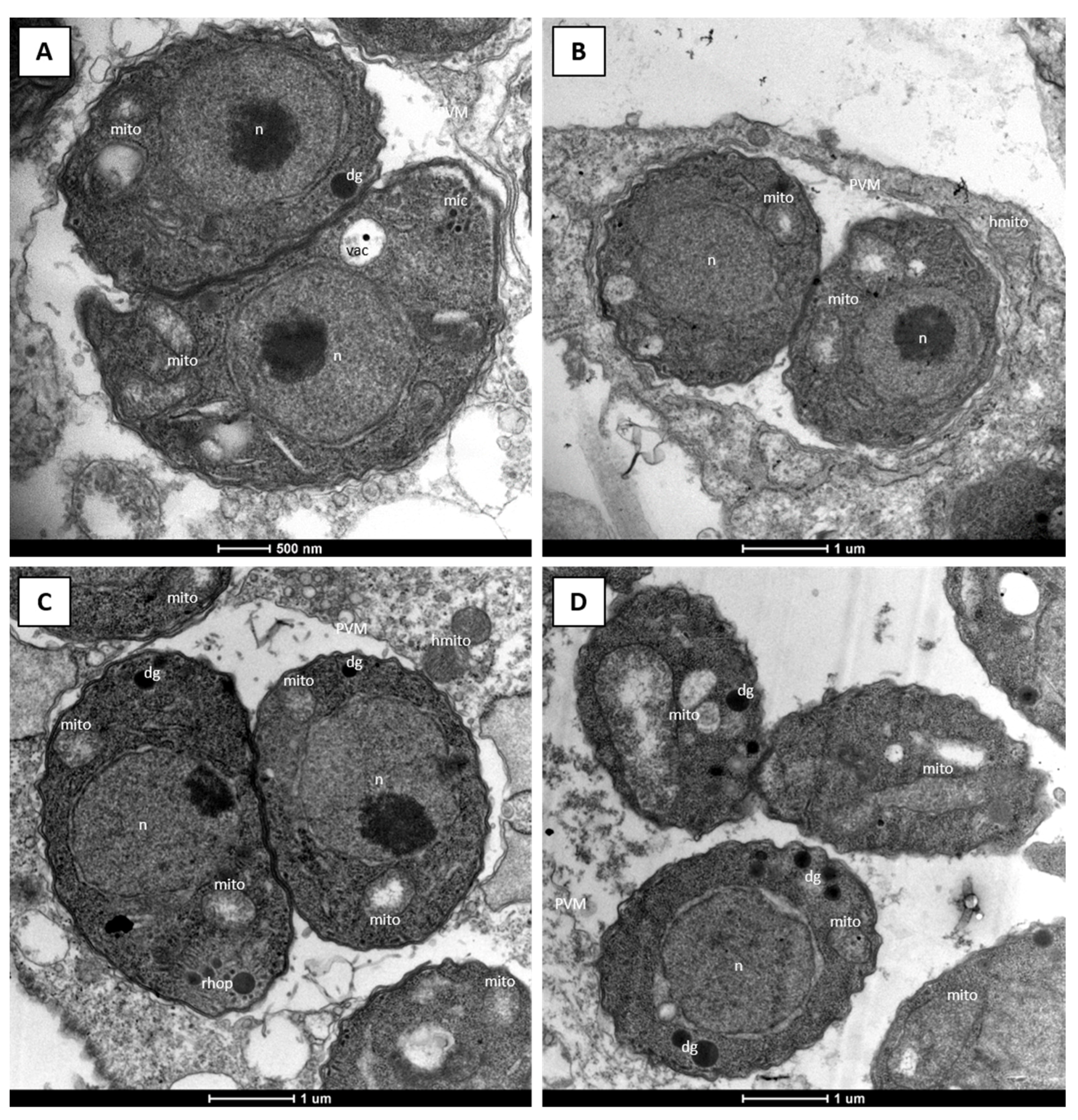
2.1. Efficacy of DCQ against T. gondii In Vitro and in CD1 Mice Infected with T. gondii Oocysts
2.2. Efficacies of the Quinoline-O-Carbamate Derivatives of DCQ In Vitro
2.3. Safety Assessment of DCQ and RMB060 during Pregnancy in BALB/c Mice
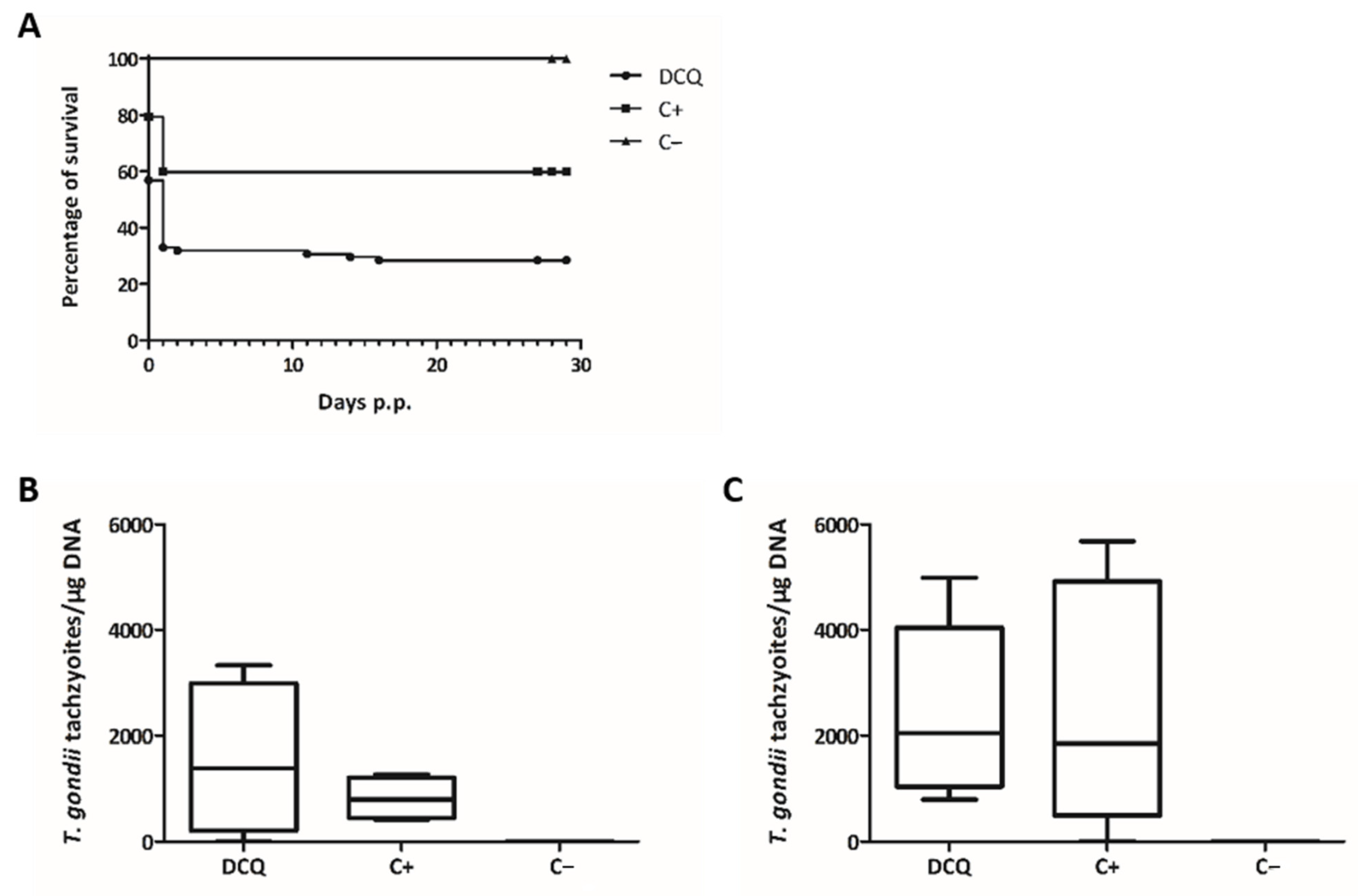
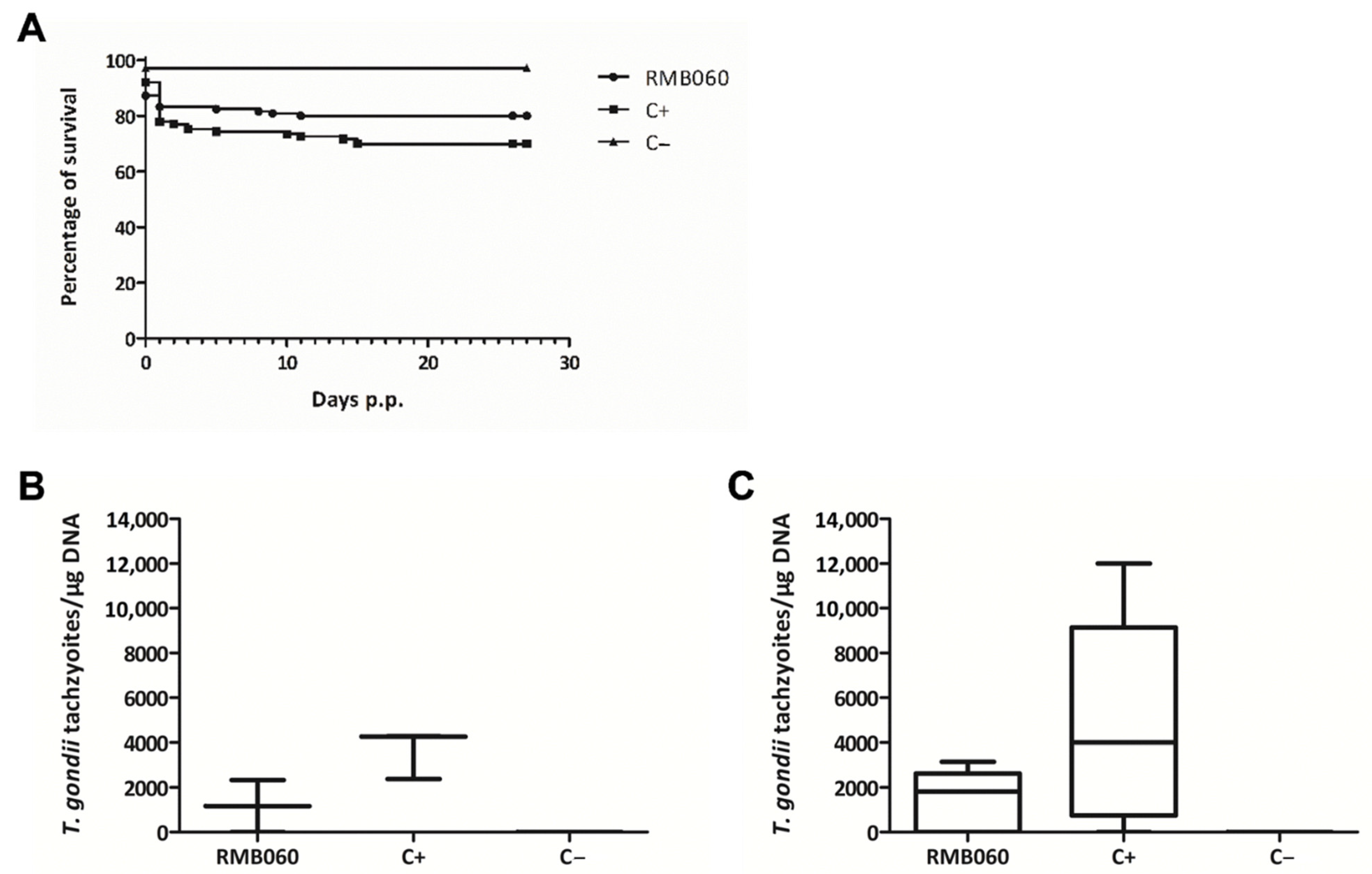
2.4. Efficacy of RMB060 in CD1 Mice Infected with T. gondii Oocysts
3. Discussion
4. Materials and Methods
4.1. Cell Culture Equipment and Media, Biochemicals, and Compounds
4.2. Host Cells and Parasites
4.3. Cytotoxicity, Anti-T. gondii Efficacy Assessments and Long-Term Drug Treatment In Vitro
4.4. Microscopy
4.5. Ethics Statement
4.6. Pregnancy Interference Test in BALB/c Mice
4.7. DCQ Efficacy Assessment in CD1 Mice Orally Infected with TgShSp1 Oocysts
4.8. RMB060 Efficacy Assessment in CD1 Mice Orally Infected with TgShSp1 Oocysts
4.9. Determination of Cerebral Parasite Load by Real-Time PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djurković-Djaković, O.; Dupouy-Camet, J.; Van der Giessen, J.; Dubey, J.P. Toxoplasmosis: Overview from a One Health Perspective. Food Waterborne Parasitol. 2019, 15, e00054. [Google Scholar] [CrossRef]
- Dubey, J.P. Outbreaks of Clinical Toxoplasmosis in Humans: Five Decades of Personal Experience, Perspectives and Lessons Learned. Parasit. Vectors 2021, 14, 263. [Google Scholar] [CrossRef]
- Melchor, S.J.; Ewald, S.E. Disease Tolerance in Toxoplasma Infection. Front. Cell. Infect. Microbiol. 2019, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Alday, P.H.; Doggett, J.S. Drugs in Development for Toxoplasmosis: Advances, Challenges, and Current Status. Drug Des. Devel. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef]
- Wei, H.X.; Wei, S.S.; Lindsay, D.S.; Peng, H.J. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma Gondii Medicines in Humans. PLoS ONE 2015, 10, e0138204. [Google Scholar] [CrossRef] [PubMed]
- Meneceur, P.; Bouldouyre, M.A.; Aubert, D.; Villena, I.; Menotti, J.; Sauvage, V.; Garin, J.F.; Derouin, F. In Vitro Susceptibility of Various Genotypic Strains of Toxoplasma Gondii to Pyrimethamine, Sulfadiazine, and Atovaquone. Antimicrob. Agents Chemother. 2008, 52, 1269–1277. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants: An Update. Vet. Clin. North. Am. Food Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug Repurposing and Human Parasitic Protozoan Diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.M.; Zach, S.J.; Wang, X.; Potluri, L.P.; Neville, A.J.; Vennerstrom, J.L.; Davis, P.H. Review of Experimental Compounds Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2016, 60, 7017–7034. [Google Scholar] [CrossRef] [PubMed]
- Secrieru, A.; Costa, I.C.C.; O’Neill, P.M.; Cristiano, M.L.S. Antimalarial Agents as Therapeutic Tools against Toxoplasmosis—A Short Bridge between Two Distant Illnesses. Molecules 2020, 25, 1574. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, R.; Vázquez, P.; Ferre, I.; Ortega-Mora, L.M. Treatment of Toxoplasmosis and Neosporosis in Farm Ruminants: State of Knowledge and Future Trends. Curr. Top. Med. Chem. 2018, 18, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Meléndez, A.; Rico-San Román, L.; Hemphill, A.; Balmer, V.; Ortega-Mora, L.M.; Álvarez-García, G. Repurposing of Commercially Available Anti-Coccidials Identifies Diclazuril and Decoquinate as Potential Therapeutic Candidates against Besnoitia Besnoiti Infection. Vet. Parasitol. 2018, 261, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and Efficacy of Deccox® (Decoquinate) for Chickens for Fattening. EFSA J. 2019, 17, e05541. [Google Scholar] [CrossRef] [PubMed]
- Quintero-de Leonardo, J.; Rosiles, R.; Bautista, J.; González-Monsón, N.; Sumano, H. Oral Pharmacokinetics and Milk Residues of Decoquinate in Milking Cows. J. Vet. Pharmacol. Ther. 2009, 32, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, A.P.; Pfefferkorn, E.R. Toxoplasma Gondii: Susceptibility and Development of Resistance to Anticoccidial Drugs In Vitro. Antimicrob. Agents Chemother. 1993, 37, 2358–2363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buxton, D.; Brebner, J.; Wright, S.; Maley, S.W.; Thomson, K.M.; Millard, K. Decoquinate and the Control of Experimental Ovine Toxoplasmosis. Vet. Rec. 1996, 138, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Pogany, S.; Tanol, M.; Baltezor, M.J. Decoquinate Prodrugs for Inhibiting or Treating a Parasitic Infection. International Patent Application No. PCT/US2012/042265, 2012. [Google Scholar]
- Beteck, R.M.; Seldon, R.; Coertzen, D.; van der Watt, M.E.; Reader, J.; Mackenzie, J.S.; Lamprecht, D.A.; Abraham, M.; Eribez, K.; Müller, J.; et al. Accessible and Distinct Decoquinate Derivatives Active against Mycobacterium Tuberculosis and Apicomplexan Parasites. Commun. Chem. 2018, 1, 62. [Google Scholar] [CrossRef]
- Andriole, V.T. The Quinolones: Past, Present, and Future. Clin. Infect. Dis. 2005, 41, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.M.; Flanigan, D.L.; Monastyrskyi, A.; LaCrue, A.N.; Sáenz, F.E.; Maignan, J.R.; Mutka, T.S.; White, K.L.; Shackleford, D.M.; Bathurst, I.; et al. Orally Bioavailable 6-Chloro-7-Methoxy-4(1H)-Quinolones Efficacious against Multiple Stages of Plasmodium. J. Med. Chem. 2014, 57, 8860–8879. [Google Scholar] [CrossRef] [PubMed]
- Miley, G.P.; Pou, S.; Winter, R.; Nilsen, A.; Li, Y.; Kelly, J.X.; Stickles, A.M.; Mather, M.W.; Forquer, I.P.; Pershing, A.M.; et al. ELQ-300 Prodrugs for Enhanced Delivery and Single-Dose Cure of Malaria. Antimicrob. Agents Chemother. 2015, 59, 5555–5560. [Google Scholar] [CrossRef]
- Ryley, J.F.; Peters, W. The Antimalarial Activity of Some Quinolone Esters. Ann. Trop. Med. Parasitol. 1970, 64, 209–222. [Google Scholar] [CrossRef]
- Stickles, A.M.; Smilkstein, M.J.; Morrisey, J.M.; Li, Y.; Forquer, I.P.; Kelly, J.X.; Pou, S.; Winter, R.W.; Nilsen, A.; Vaidya, A.B.; et al. Atovaquone and ELQ-300 Combination Therapy as a Novel Dual-Site Cytochrome Bc 1 Inhibition Strategy for Malaria. Antimicrob. Agents Chemother. 2016, 60, 4853–4859. [Google Scholar] [CrossRef] [PubMed]
- Beteck, R.M.; Smit, F.J.; Haynes, R.K.; N’Da, D.D. Recent Progress in the Development of Anti-Malarial Quinolones. Malar. J. 2014, 13, 339. [Google Scholar] [CrossRef]
- Doggett, J.S.; Schultz, T.; Miller, A.J.; Bruzual, I.; Pou, S.; Winter, R.; Dodean, R.; Zakharov, L.N.; Nilsen, A.; Riscoe, M.K.; et al. Orally Bioavailable Endochin-Like Quinolone Carbonate Ester Prodrug Reduces Toxoplasma Gondii Brain Cysts. Antimicrob. Agents Chemother. 2020, 64, e00535-20. [Google Scholar] [CrossRef]
- Doggett, J.S.; Nilsen, A.; Forquer, I.; Wegmann, K.W.; Jones-Brando, L.; Yolken, R.H.; Bordón, C.; Charman, S.A.; Katneni, K.; Schultz, T.; et al. Endochin-like Quinolones Are Highly Efficacious against Acute and Latent Experimental Toxoplasmosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15936–15941. [Google Scholar] [CrossRef]
- Martynowicz, J.; Doggett, J.S.; Sullivan, W., Jr. Efficacy of Guanabenz Combination Therapy against Chronic Toxoplasmosis across Multiple Mouse Strains. Antimicrob. Agents Chemother. 2020, 64, e00539-20. [Google Scholar] [CrossRef]
- Mitchell, P. Protonmotive Redox Mechanism of the Cytochrome b-c1 Complex in the Respiratory Chain: Protonmotive Ubiquinone Cycle. FEBS Lett. 1975, 56, 1–6. [Google Scholar] [CrossRef]
- Nam, T.; McNamara, C.W.; Bopp, S.; Dharia, N.V.; Meister, S.; Bonamy, G.M.C.; Plouffe, D.M.; Kato, N.; McCormack, S.; Bursulaya, B.; et al. A Chemical Genomic Analysis of Decoquinate, a Plasmodium Falciparum Cytochrome b Inhibitor. ACS Chem. Biol. 2011, 6, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, F.P.; Martin, C.; Buchholz, K.; Lafuente-Monasterio, M.J.; Rodrigues, T.; Sönnichsen, B.; Moreira, R.; Gamo, F.J.; Marti, M.; Mota, M.M.; et al. Drug Screen Targeted at Plasmodium Liver Stages Identifies a Potent Multistage Antimalarial Drug. J. Infect. Dis. 2012, 205, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Butler, J.M.; Blagburn, B.L. Efficacy of Decoquinate against Neospora Caninum Tachyzoites in Cell Cultures. Vet. Parasitol. 1997, 68, 35–40. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Nazir, M.M.; Maqbool, A.; Ellison, S.P.; Strobl, J.S. Efficacy of Decoquinate against Sarcocystis Neurona in Cell Cultures. Vet. Parasitol. 2013, 196, 21–23. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Toivio-Kinnucan, M.A.; Blagburn, B.L. Decoquinate Induces Tissue Cyst Formation by the RH Strain of Toxoplasma Gondii. Vet. Parasitol. 1998, 77, 75–81. [Google Scholar] [CrossRef]
- Li, Q.; Xie, L.; Caridha, D.; Zeng, Q.; Zhang, J.; Roncal, N.; Zhang, P.; Vuong, C.; Potter, B.; Sousa, J.; et al. Long-Term Prophylaxis and Pharmacokinetic Evaluation of Intramuscular Nano- and Microparticle Decoquinate in Mice Infected with P. Berghei Sporozoites. Malar. Res. Treat. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Woods, K.M.; Upton, S.J.; Blagburn, B.L. Activity of Decoquinate against Cryptosporidium Parvum in Cell Cultures and Neonatal Mice. Vet. Parasitol. 2000, 89, 307–311. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Reyes, S.; Zhang, J.; Zeng, Q.; Zhang, P.; Xie, L.; Lee, P.J.; Roncal, N.; Melendez, V.; et al. Nanoparticle Formulations of Decoquinate Increase Antimalarial Efficacy against Liver Stage Plasmodium Infections in Mice. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, M.M.H.J.; van Rooij, I.A.L.M.; Miller, R.K.; Zielhuis, G.A.; de Jong-van den Berg, L.T.W.; Roeleveld, N. Teratogenic Mechanisms of Medical Drugs. Hum. Reprod. Update 2010, 16, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Finnell, R.H. Teratology: General Considerations and Principles. J. Allergy Clin. Immunol. 1999, 103, S337–S342. [Google Scholar] [CrossRef]
- Pastor-Fernández, I.; Collantes-Fernández, E.; Jiménez-Pelayo, L.; Ortega-Mora, L.M.; Horcajo, P. Modeling the Ruminant Placenta-Pathogen Interactions in Apicomplexan Parasites: Current and Future Perspectives. Front. Vet. Sci. 2021, 7, 1234. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Manser, V.; Hemphill, A. In Vitro Treatment of Besnoitia Besnoiti with the Naphto-Quinone Buparvaquone Results in Marked Inhibition of Tachyzoite Proliferation, Mitochondrial Alterations and Rapid Adaptation of Tachyzoites to Increased Drug Concentrations. Parasitology 2019, 146, 112–120. [Google Scholar] [CrossRef]
- Müller, J.; Aguado, A.; Laleu, B.; Balmer, V.; Ritler, D.; Hemphill, A. In Vitro Screening of the Open Source Pathogen Box Identifies Novel Compounds with Profound Activities against Neospora Caninum. Int. J. Parasitol. 2017, 47, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Kropf, C.; Debache, K.; Rampa, C.; Barna, F.; Schorer, M.; Stephens, C.E.; Ismail, M.A.; Boykin, D.W.; Hemphill, A. The Adaptive Potential of a Survival Artist: Characterization of the in Vitro Interactions of Toxoplasma Gondii Tachyzoites with Di-Cationic Compounds in Human Fibroblast Cell Cultures. Parasitology 2012, 139, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Sharifiyazdi, H.; Namazi, F.; Oryan, A.; Shahriari, R.; Razavi, M. Point Mutations in the Theileria Annulata Cytochrome b Gene Is Associated with Buparvaquone Treatment Failure. Vet. Parasitol. 2012, 187, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, V.; Aubert, D.; Escotte-Binet, S.; Villena, I. The Role of ATP-Binding Cassette (ABC) Proteins in Protozoan Parasites. Mol. Biochem. Parasitol. 2009, 167, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Imhof, D.; Anghel, N.; Ritler, D.; Müller, J.; Boubaker, G.; Aguado-Martinez, A.; Ortega-Mora, L.M.; Ojo, K.K.; VanVoorhis, W.C.; et al. The Impact of BKI-1294 Therapy in Mice Infected With the Apicomplexan Parasite Neospora Caninum and Re-Infected During Pregnancy. Front. Vet. Sci. 2020, 7, 587570. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Müller, J.; Aguado-Martínez, A.; Rahman, M.; Balmer, V.; Manser, V.; Ortega-Mora, L.M.; Ojo, K.K.; Fan, E.; Maly, D.J.; et al. In Vitro and In Vivo Effects of the Bumped Kinase Inhibitor 1294 in the Related Cyst-Forming Apicomplexans Toxoplasma Gondii and Neospora Caninum. Antimicrob. Agents Chemother. 2015, 59, 6361–6374. [Google Scholar] [CrossRef] [PubMed]
- Anghel, N.; Imhof, D.; Winzer, P.; Balmer, V.; Ramseier, J.; Haenggeli, K.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Arnold, S.L.M.; et al. Endochin-like Quinolones (ELQs) and Bumped Kinase Inhibitors (BKIs): Synergistic and Additive Effects of Combined Treatments against Neospora Caninum Infection in Vitro and in Vivo. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, M.; Calero-Bernal, R.; Regidor-Cerrillo, J.; Vallejo, R.; Benavides, J.; Collantes-Fernández, E.; Ortega-Mora, L.M. Isolation, Genotyping, and Mouse Virulence Characterization of Toxoplasma Gondii From Free Ranging Iberian Pigs. Front. Vet. Sci. 2020, 7, 604782. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, R.; Ferre, I.; Regidor-Cerrillo, J.; Gutiérrez-Expósito, D.; Ferrer, L.M.; Arteche-Villasol, N.; Moreno-Gonzalo, J.; Müller, J.; Aguado-Martínez, A.; Pérez, V.; et al. Virulence in Mice of a Toxoplasma Gondii Type II Isolate Does Not Correlate With the Outcome of Experimental Infection in Pregnant Sheep. Front. Cell. Infect. Microbiol. 2019, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Imhof, D.; Anghel, N.; Winzer, P.; Balmer, V.; Ramseier, J.; Hänggeli, K.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Arnold, S.L.M.; et al. In Vitro Activity, Safety and in Vivo Efficacy of the Novel Bumped Kinase Inhibitor BKI-1748 in Non-Pregnant and Pregnant Mice Experimentally Infected with Neospora Caninum Tachyzoites and Toxoplasma Gondii Oocysts. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Anghel, N.; Imhof, D.; Balmer, V.; Ortega-Mora, L.M.; Ojo, K.K.; Van Voorhis, W.C.; Müller, J.; Hemphill, A. Neospora Caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Pathogens 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Pautas, C.; Ernault, P.; Foulet, F.; Cordonnier, C.; Bretagne, S. Real-Time PCR for Diagnosis and Follow-Up of Toxoplasma Reactivation after Allogeneic Stem Cell Transplantation Using Fluorescence Resonance Energy Transfer Hybridization Probes. J. Clin. Microbiol. 2000, 38, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Aguado-Martínez, A.; Ortega-Mora, L.M.; Moreno-Gonzalo, J.; Ferre, I.; Hulverson, M.A.; Choi, R.; McCloskey, M.C.; Barrett, L.K.; Maly, D.J.; et al. Development of a Murine Vertical Transmission Model for Toxoplasma Gondii Oocyst Infection and Studies on the Efficacy of Bumped Kinase Inhibitor (BKI)-1294 and the Naphthoquinone Buparvaquone against Congenital Toxoplasmosis. J. Antimicrob. Chemother. 2017, 72, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
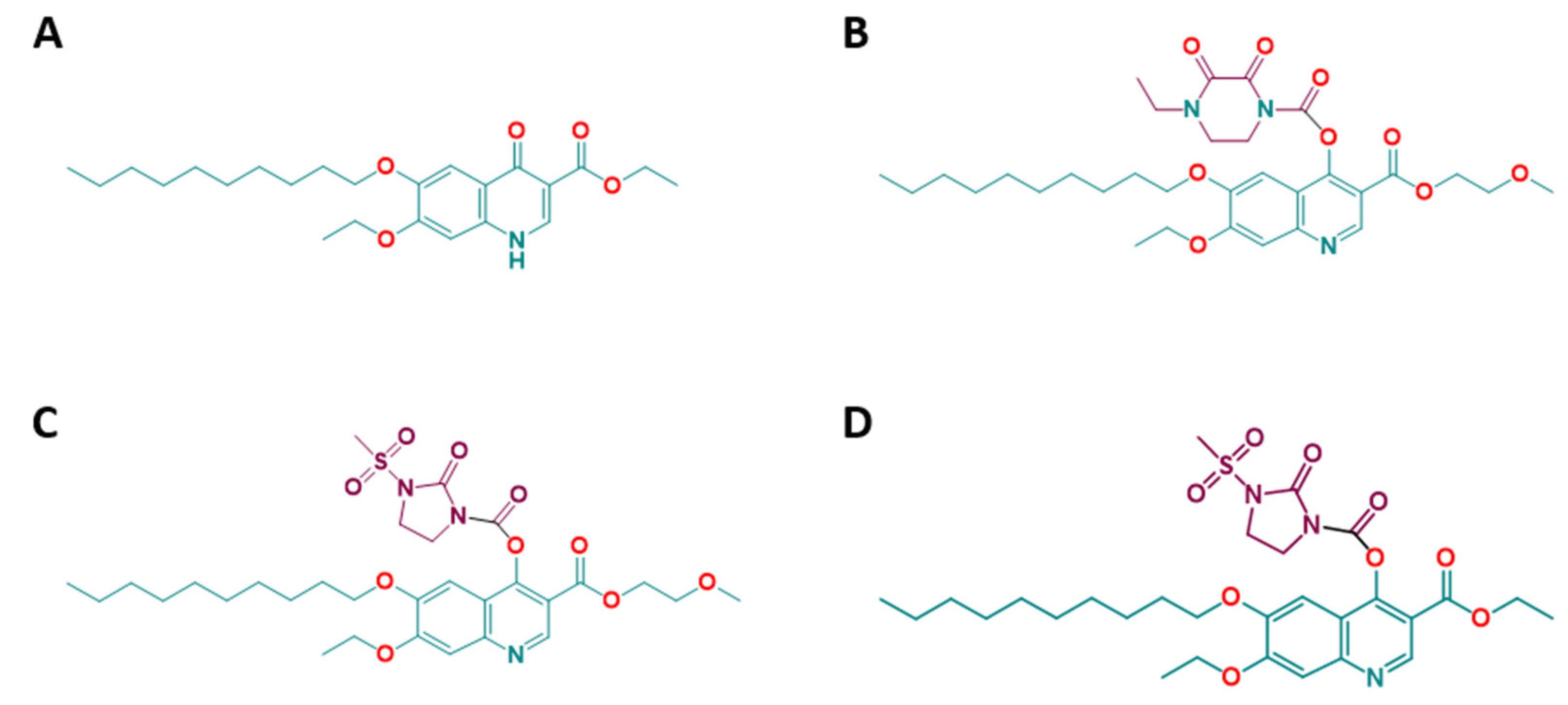

| Compounds | IC50 T. gondii (nM) [LS; LI] a Compound Added Prior to Infection | IC50 T. gondii (nM) [LS; LI] a Compound Added 3 h Post-infection | IC50 HFF (µM) | Selectivity Index |
|---|---|---|---|---|
| DCQ | 1.1 [3; 0.4] | 4.7 [10.1; 2.1] | >5 b | >4545 |
| RMB054 | 11.5 [16.6; 8] | 11.4 [15.5; 8.5] | >5 b | >435 |
| RMB055 | 199.6 [234.6; 169.8] | 226.7 [240.9; 213.4] | >10 b | >50 |
| RMB060 | 0.07 [0.14; 0.03] | 0.7 [1.22; 0.4] | >10 b | >142,857 |
| Treatment | Challenge | Seropositive for T. gondii | T. gondii Positive Non-Pregnant Mice | T. gondii Positive Dams | No. of Pups/No. of Dams | Neonatal Mortality | Postnatal Mortality | T. gondii Positive Pups |
|---|---|---|---|---|---|---|---|---|
| 10 mg/kg DCQ | TgShSp1 oocysts | 11/12 | 3/4 | 8/8 | 92/8 | 64/92 | 3/28 | 25/25 |
| Corn oil | TgShSp1 oocysts | 9/12 | 4/4 | 7/8 | 92/8 | 37/92 | 0/55 | 35/55 |
| Corn oil | PBS | 0/5 | 0/2 | 0/3 | 41/3 | 0/41 | 0/41 | 0/41 |
| Treatment | No. of Pregnant Mice/No. of Mice | No. of Non-Pregnant Mice/No. of Mice | Litter Size | Neonatal Mortality | Postnatal Mortality |
|---|---|---|---|---|---|
| 10 mg/kg DCQ | 2/6 | 4/6 | 7 | 0/7 | 0/7 |
| 10 mg/kg RMB060 | 4/6 | 2/6 | 27 | 3/27 | 0/24 |
| Corn oil | 4/6 | 2/6 | 19 | 1/19 | 0/18 |
| Treatment | Challenge | Seropositive for T. gondii | T. gondii Positive Non-Pregnant Mice | T. gondii Positive Dams | No. of Pups/No. of Dams | Neonatal Mortality | Postnatal Mortality | T. gondii Positive Pups |
|---|---|---|---|---|---|---|---|---|
| 10 mg/kg RMB060 | TgShSp1 oocysts | 8/12 | 1/2 | 7/10 | 125/10 | 21/125 | 4/104 | 62/100 |
| Corn oil | TgShSp1 oocysts | 10/11 | 3/3 | 8/9 | 113/9 | 26/113 | 8/87 | 70/79 |
| Corn oil | PBS | 0/5 | 0/3 | 0/2 | 35/2 | 1/35 | 0/34 | 0/34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramseier, J.; Imhof, D.; Anghel, N.; Hänggeli, K.; Beteck, R.M.; Balmer, V.; Ortega-Mora, L.-M.; Sanchez-Sanchez, R.; Ferre, I.; Haynes, R.K.; et al. Assessment of the Activity of Decoquinate and Its Quinoline-O-Carbamate Derivatives against Toxoplasma gondii In Vitro and in Pregnant Mice Infected with T. gondii Oocysts. Molecules 2021, 26, 6393. https://doi.org/10.3390/molecules26216393
Ramseier J, Imhof D, Anghel N, Hänggeli K, Beteck RM, Balmer V, Ortega-Mora L-M, Sanchez-Sanchez R, Ferre I, Haynes RK, et al. Assessment of the Activity of Decoquinate and Its Quinoline-O-Carbamate Derivatives against Toxoplasma gondii In Vitro and in Pregnant Mice Infected with T. gondii Oocysts. Molecules. 2021; 26(21):6393. https://doi.org/10.3390/molecules26216393
Chicago/Turabian StyleRamseier, Jessica, Dennis Imhof, Nicoleta Anghel, Kai Hänggeli, Richard M. Beteck, Vreni Balmer, Luis-Miguel Ortega-Mora, Roberto Sanchez-Sanchez, Ignacio Ferre, Richard K. Haynes, and et al. 2021. "Assessment of the Activity of Decoquinate and Its Quinoline-O-Carbamate Derivatives against Toxoplasma gondii In Vitro and in Pregnant Mice Infected with T. gondii Oocysts" Molecules 26, no. 21: 6393. https://doi.org/10.3390/molecules26216393
APA StyleRamseier, J., Imhof, D., Anghel, N., Hänggeli, K., Beteck, R. M., Balmer, V., Ortega-Mora, L.-M., Sanchez-Sanchez, R., Ferre, I., Haynes, R. K., & Hemphill, A. (2021). Assessment of the Activity of Decoquinate and Its Quinoline-O-Carbamate Derivatives against Toxoplasma gondii In Vitro and in Pregnant Mice Infected with T. gondii Oocysts. Molecules, 26(21), 6393. https://doi.org/10.3390/molecules26216393









