Embryonic Development of Avian Pineal Secretory Activity—A Lesson from the Goose Pineal Organs in Superfusion Culture
Abstract
:1. Introduction
2. Results
2.1. In Vitro Study
2.1.1. Experiment I
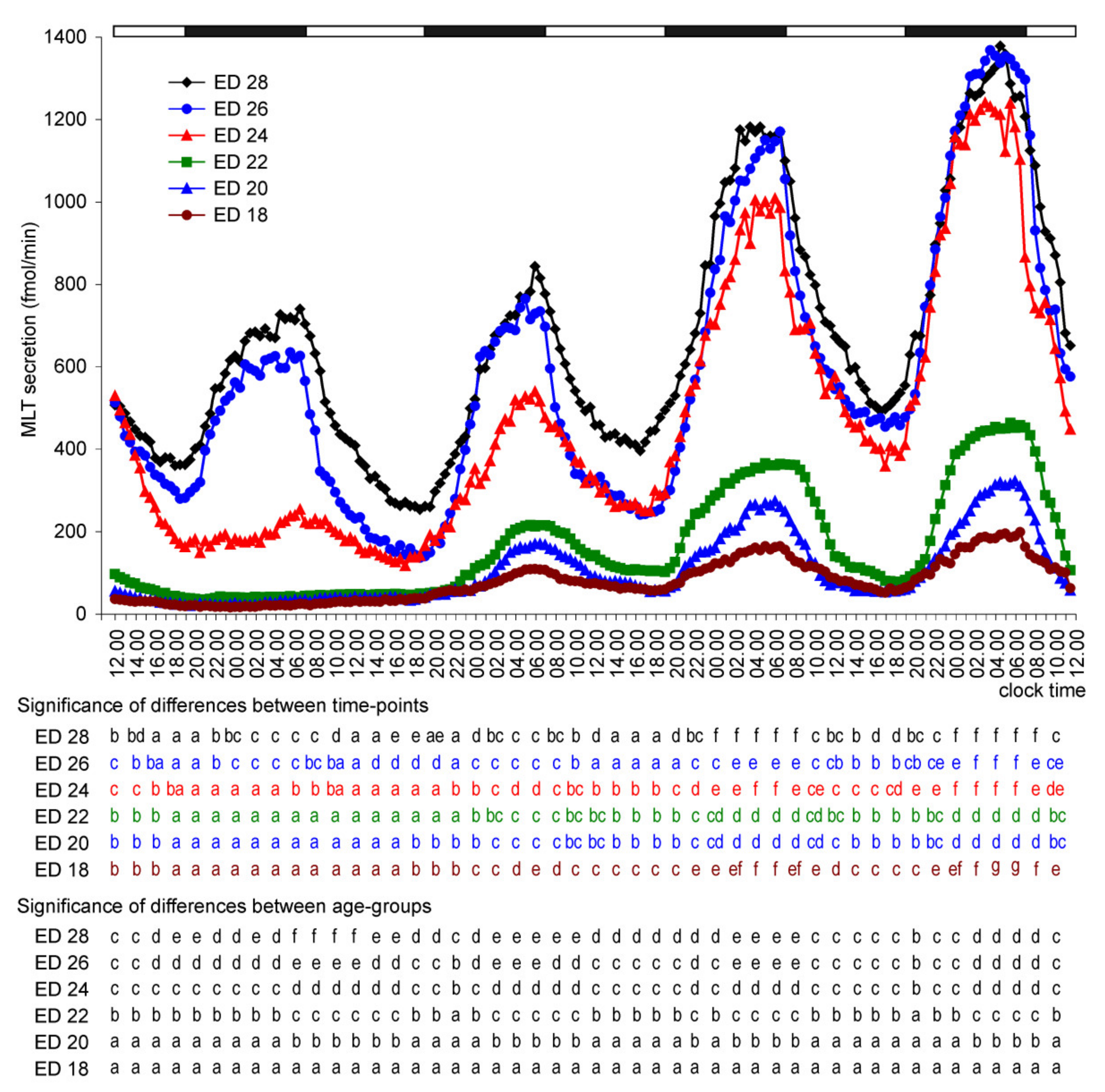
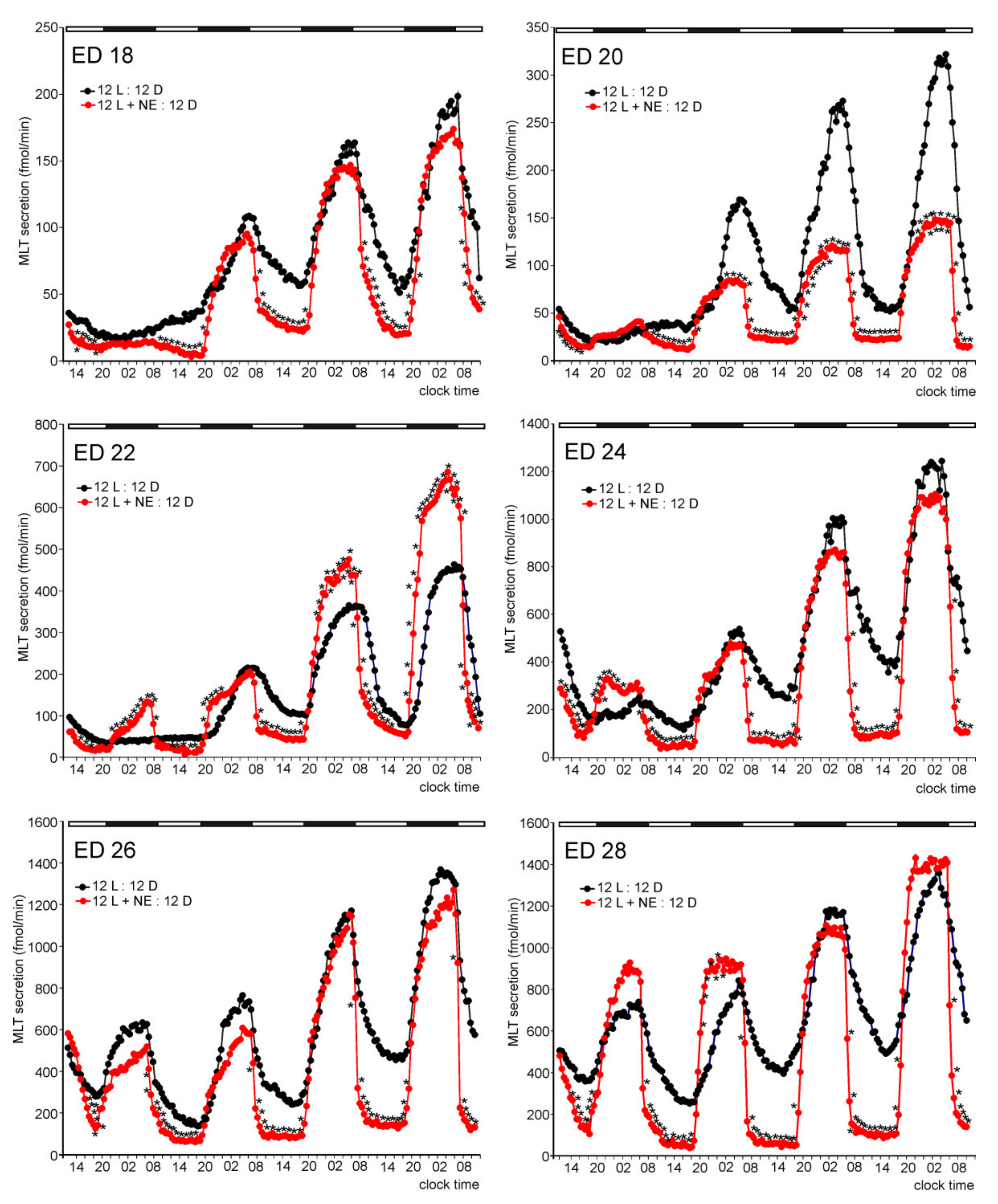
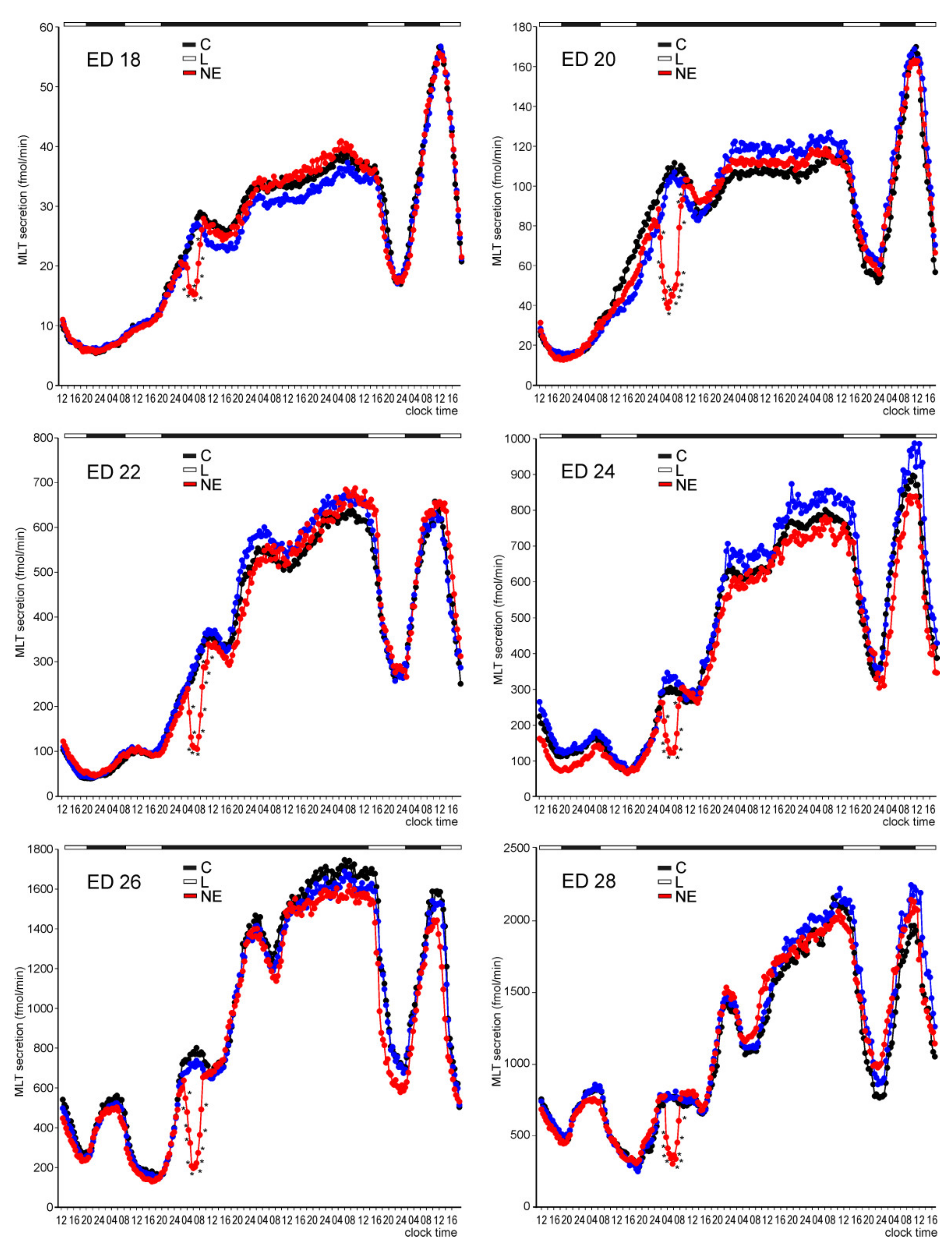
Melatonin Secretion from Embryonic Pineal Organs Incubated under Different Light Conditions and Treated with NE
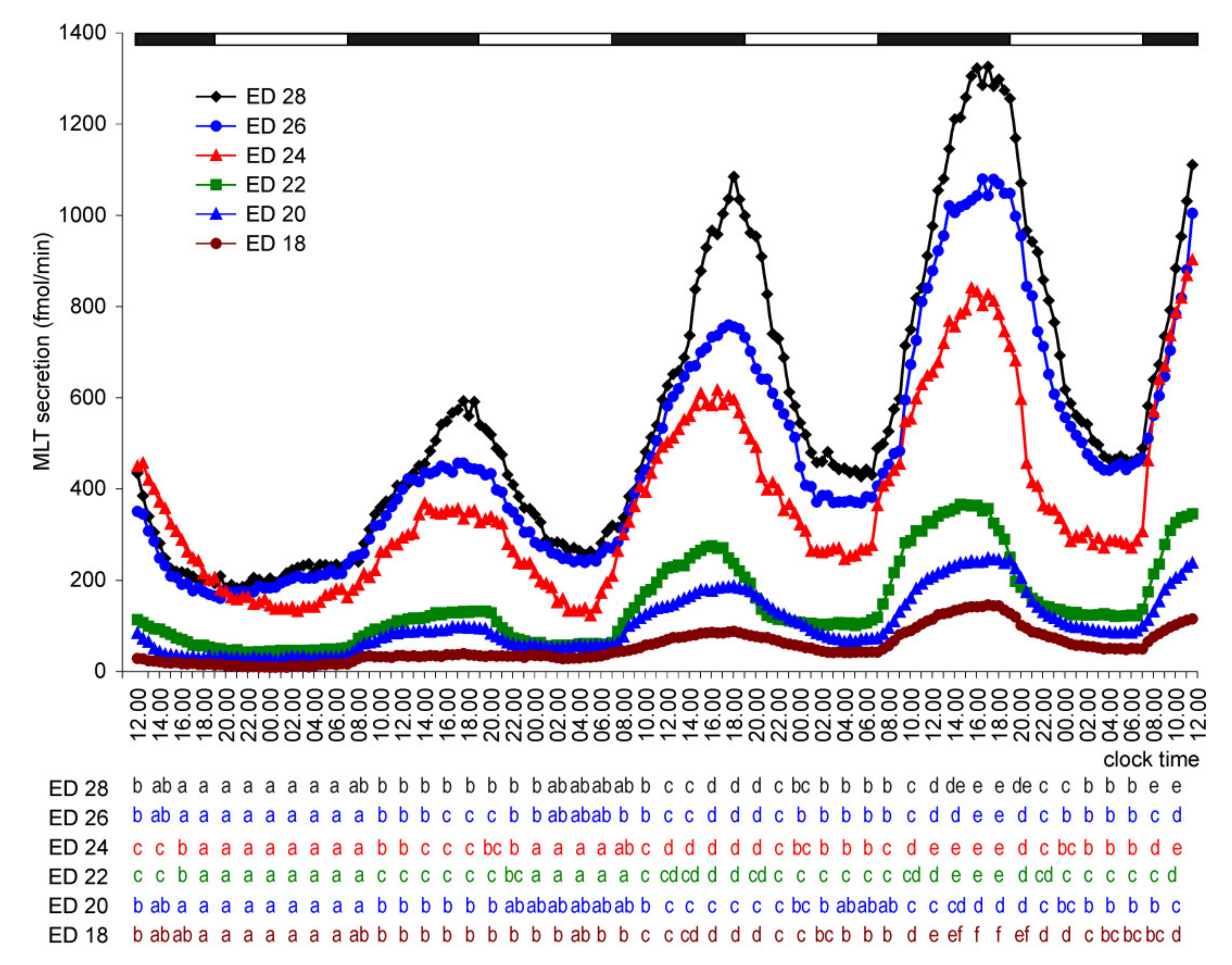
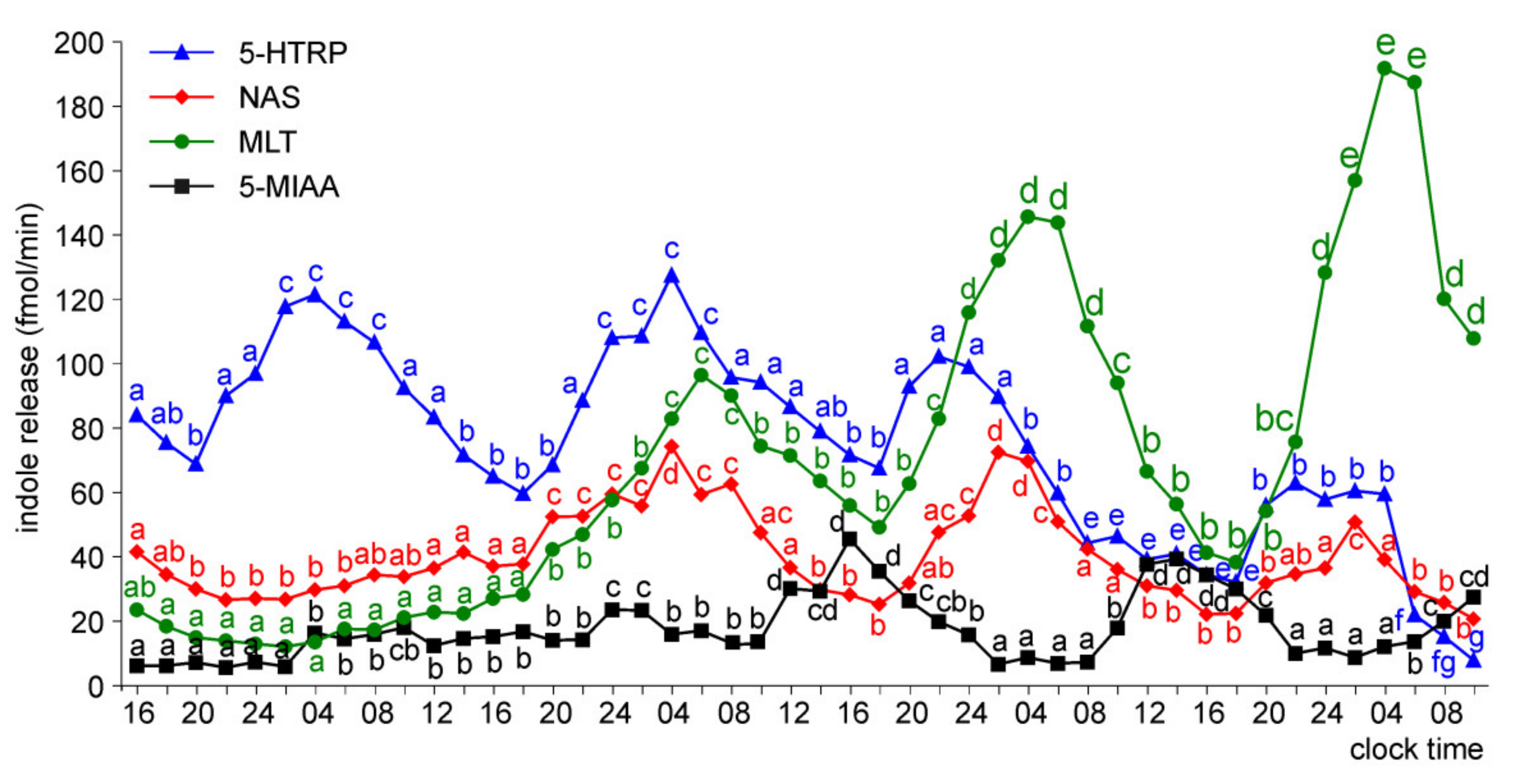
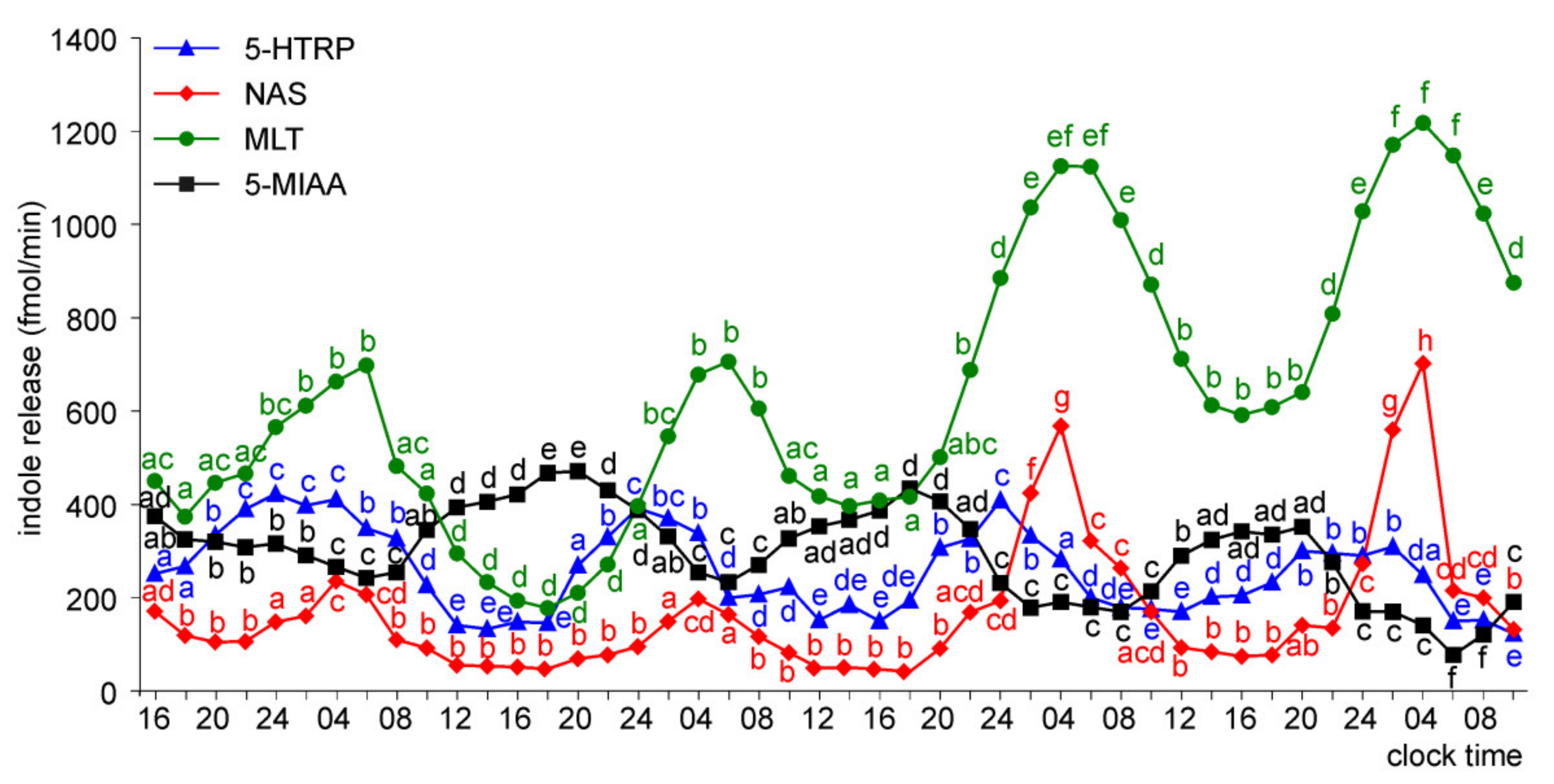
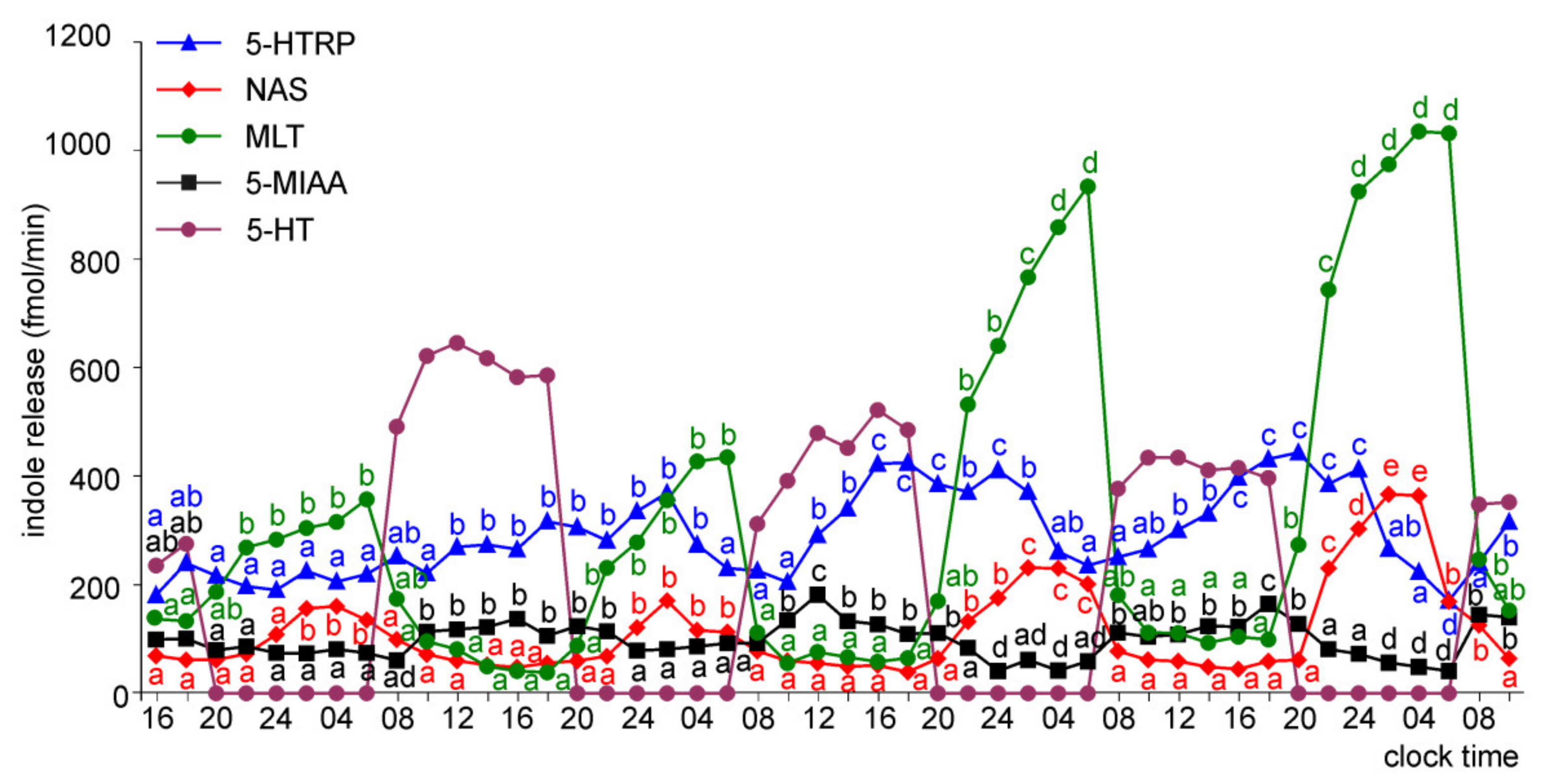
Release of 5-HTRP, 5-HT, NAS, MLT, and 5-MIAA from Embryonic Pineal Organs Incubated under 12L:12D Cycle
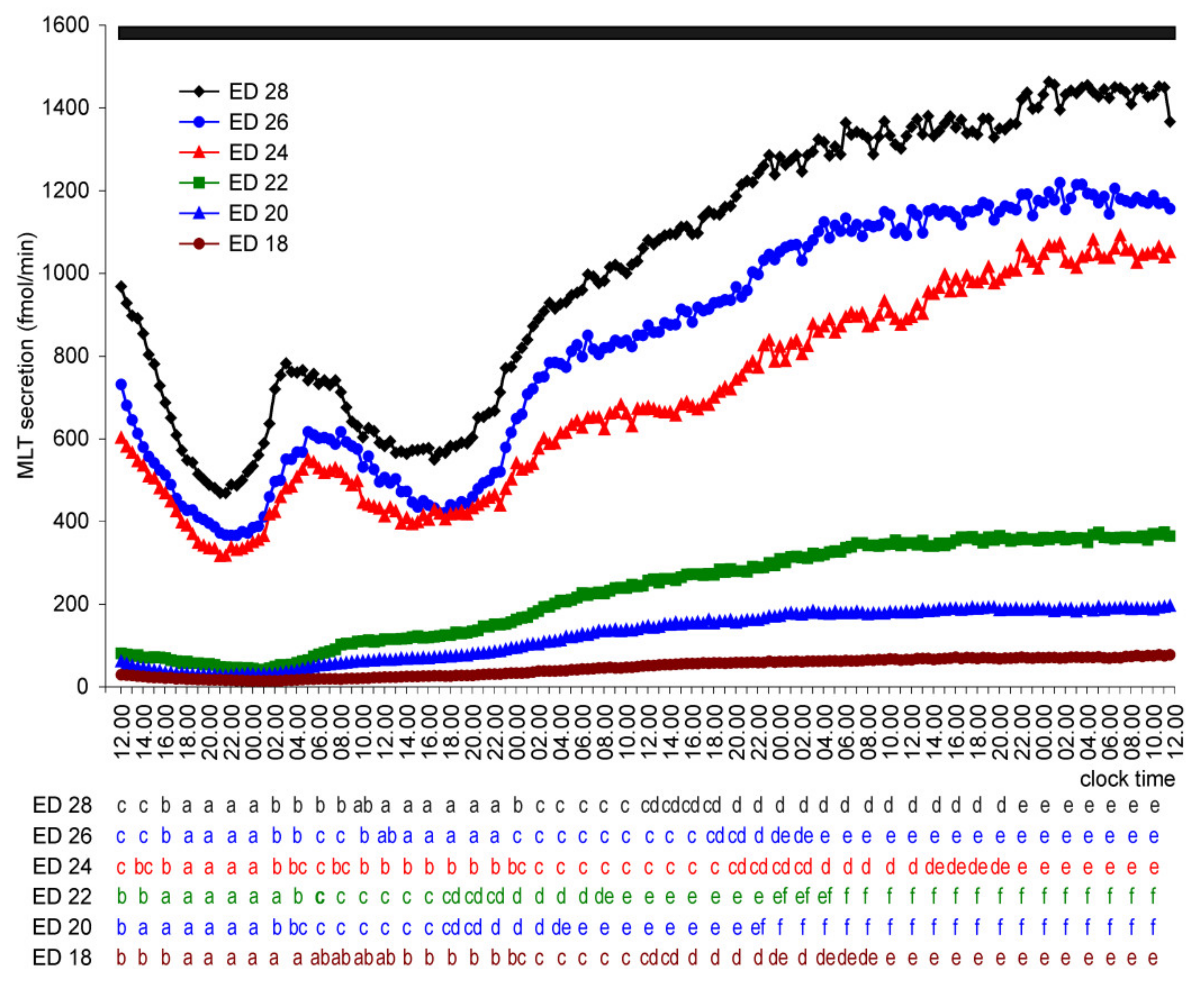
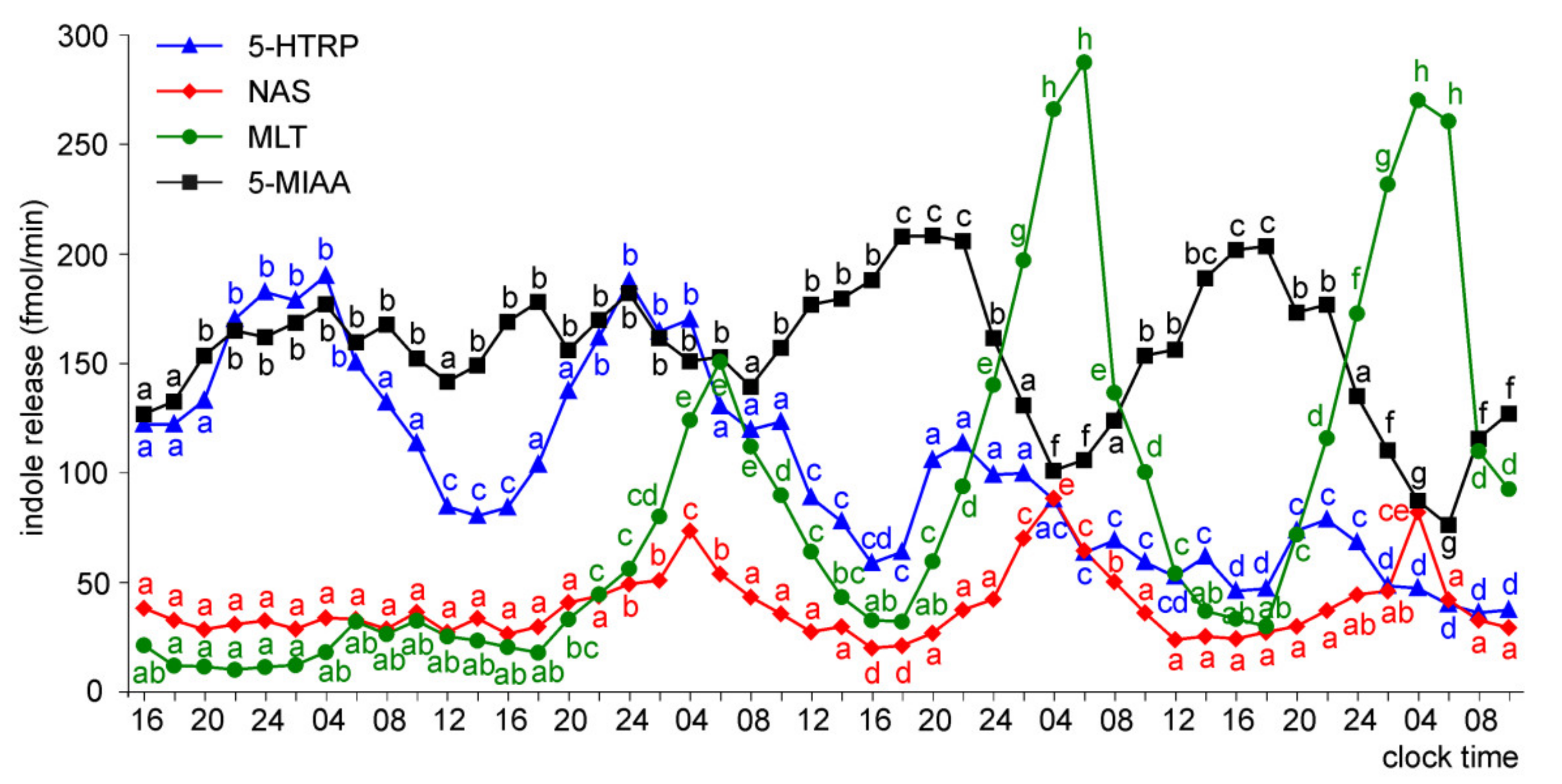
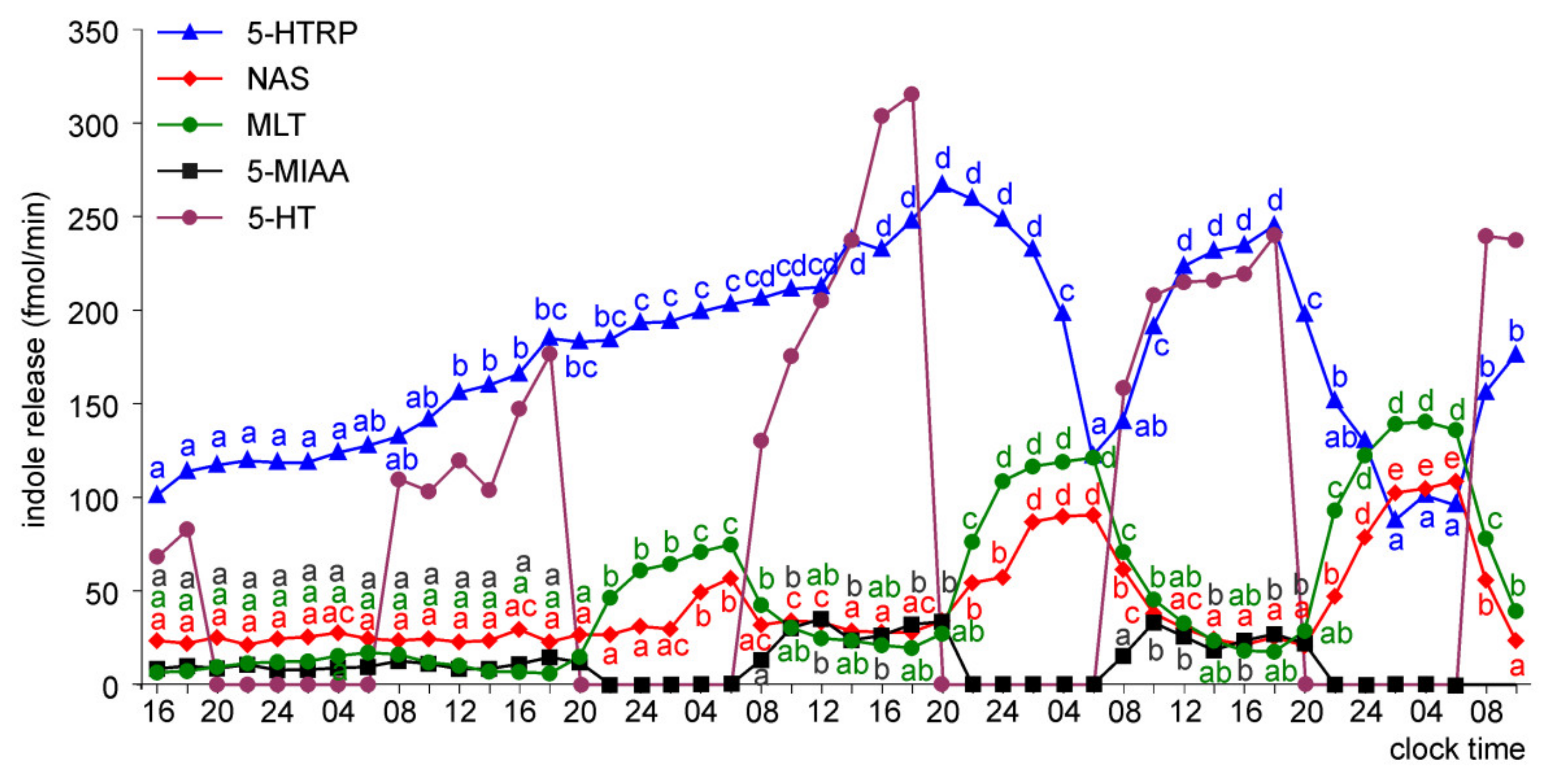
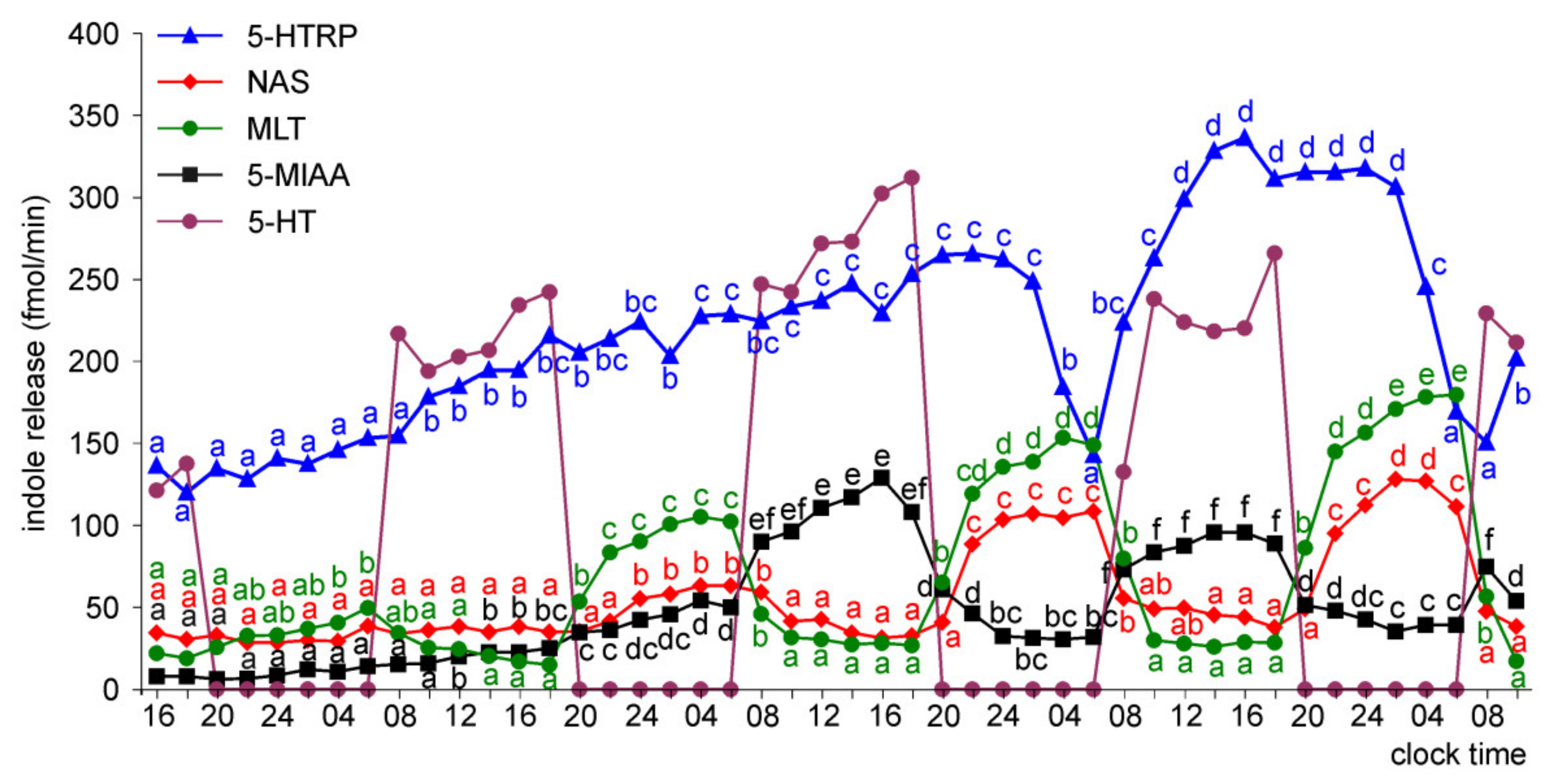
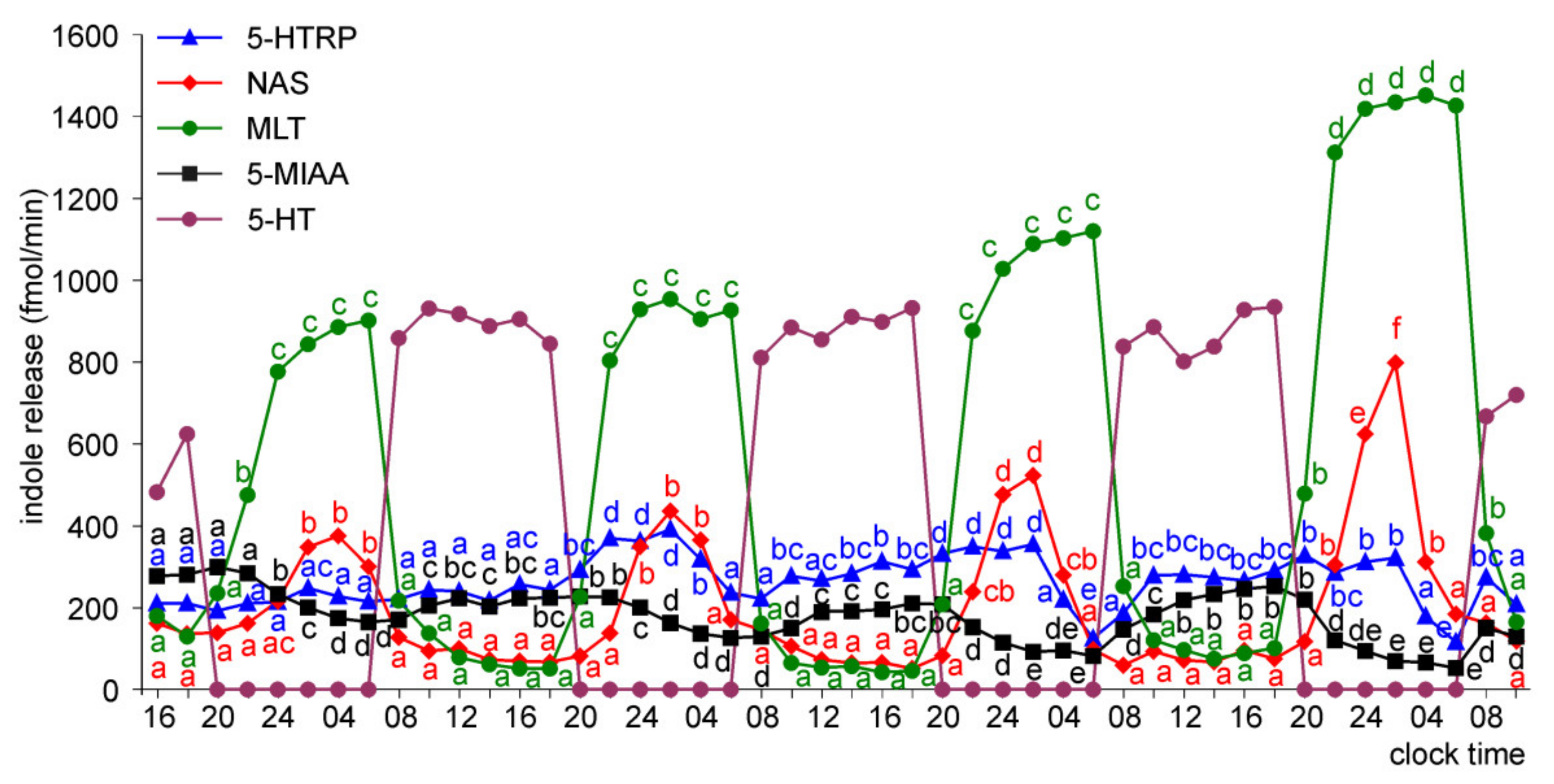
Release of 5-HTRP, 5-HT, NAS, MLT, and 5-MIAA from Embryonic Pineal Organs Incubated under 12 L:12D Cycle and Treated with NE during Photophases
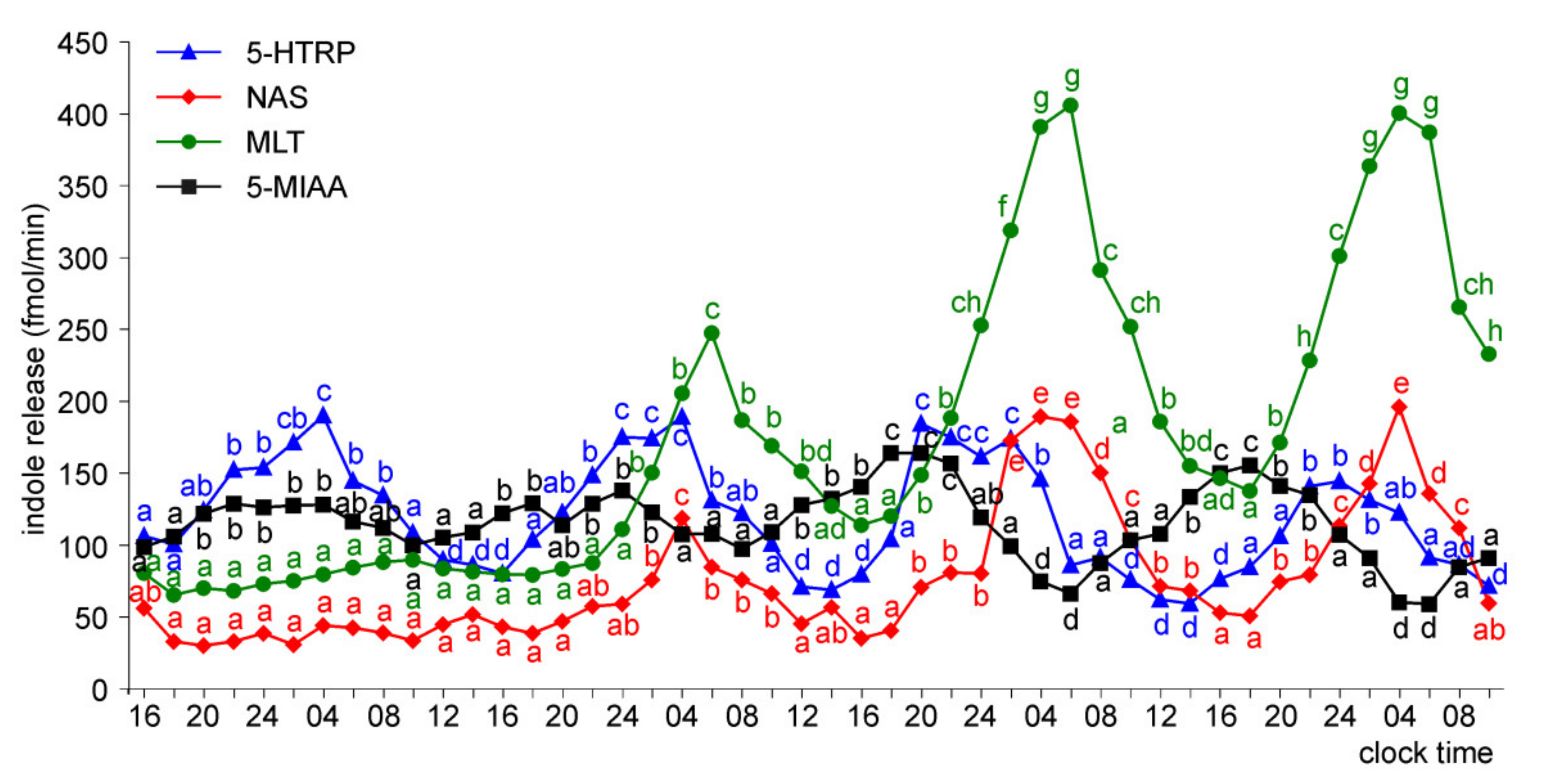
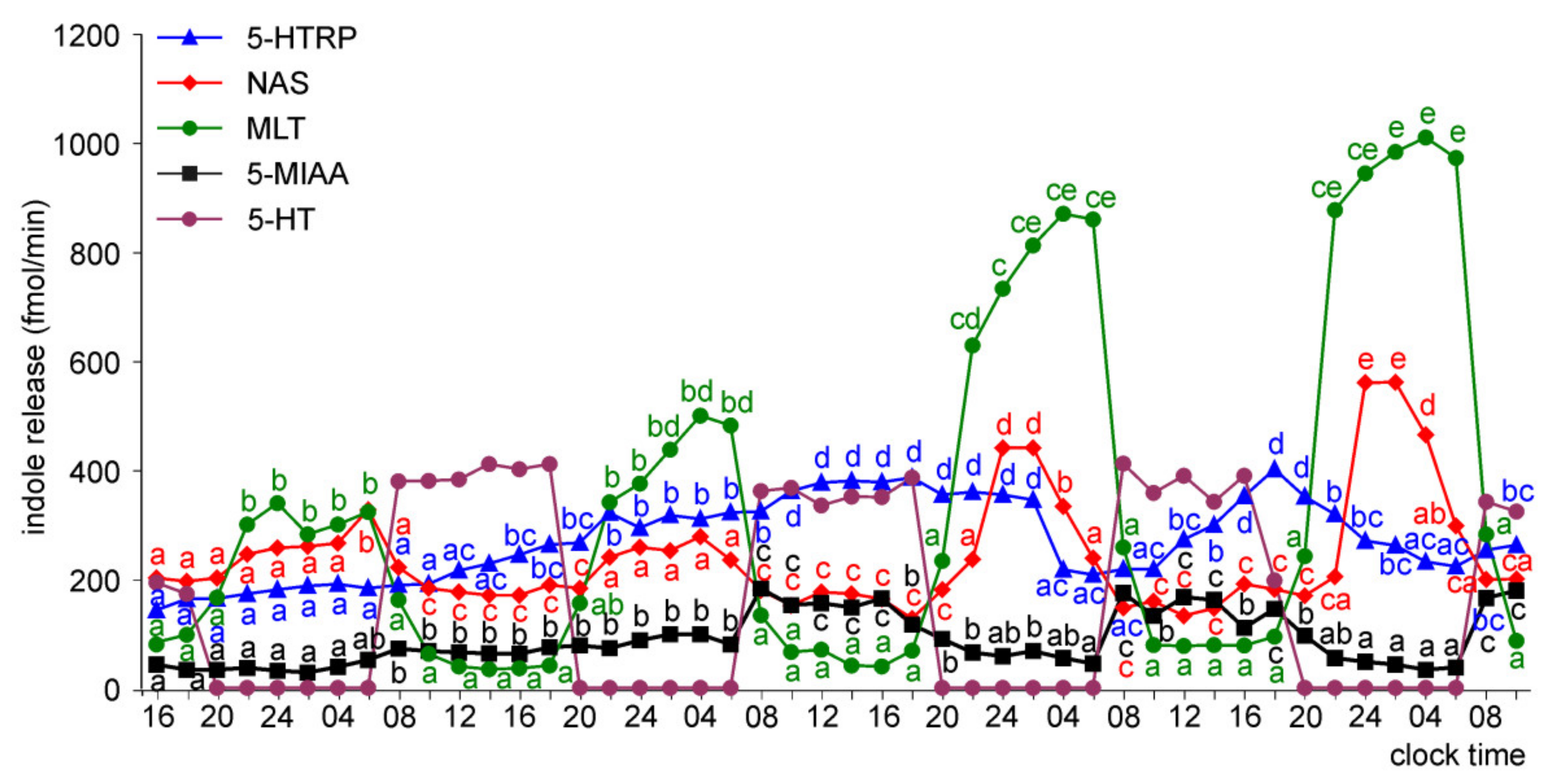
2.1.2. Experiment II
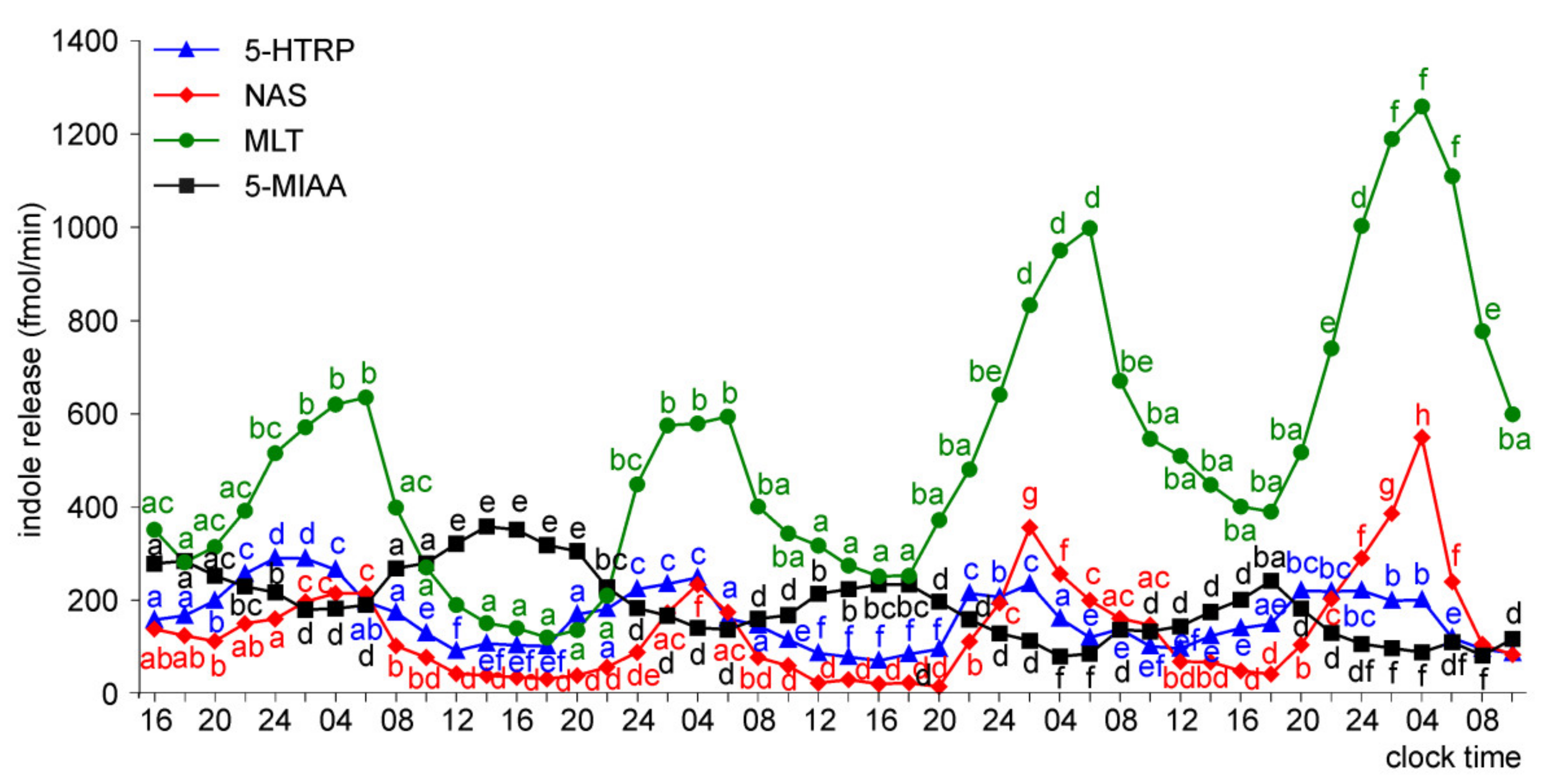
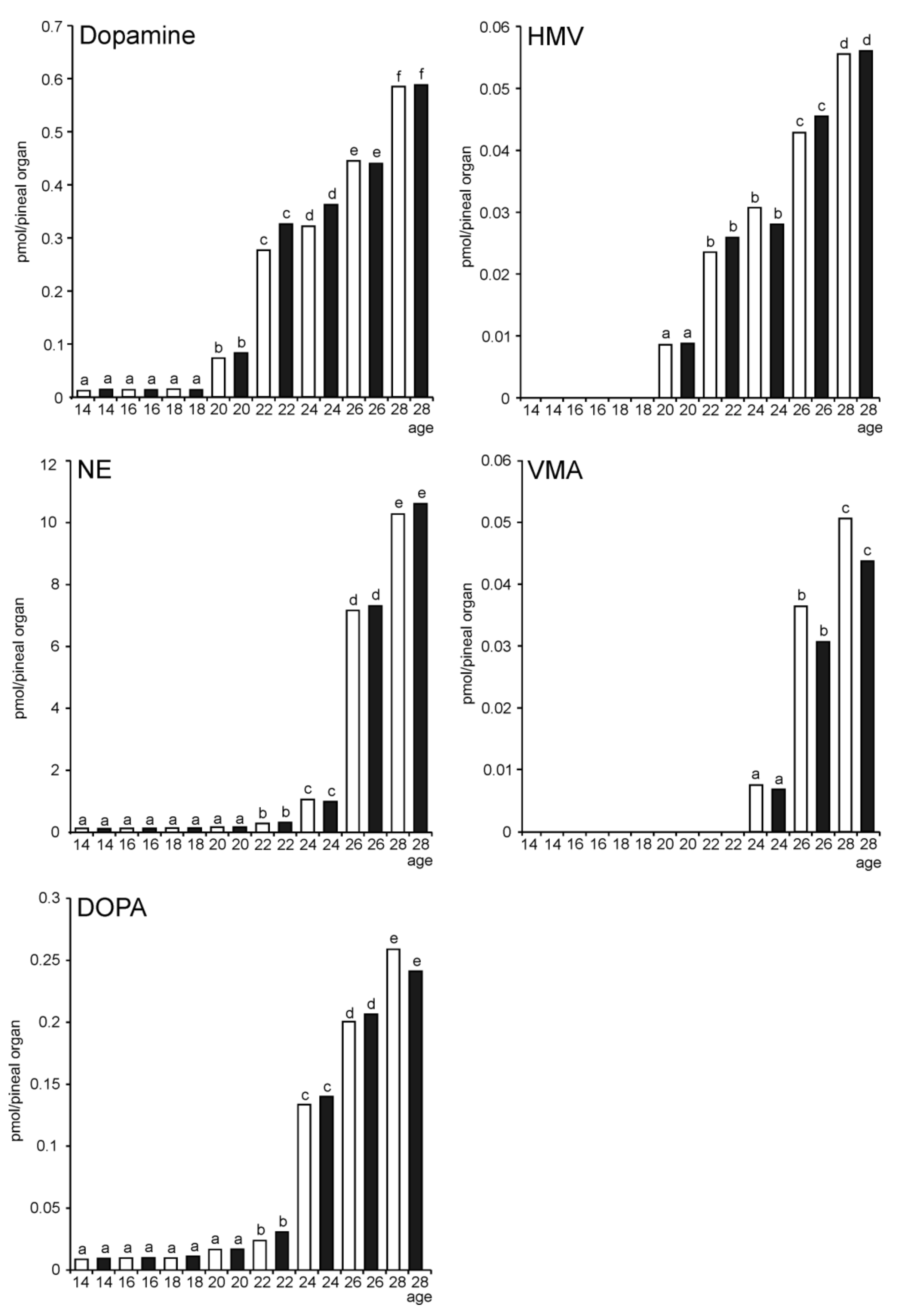
2.2. In Vivo Study
3. Discussion
4. Materials and Methods
4.1. Embryos
4.2. In Vitro Study
4.2.1. Culture Medium
4.2.2. Superfusion Culture Technique
4.2.3. Experiment I
4.2.4. Experiment II
4.3. In Vivo Study
4.4. Analytical Procedures
4.4.1. Chemicals
4.4.2. Melatonin Radioimmunoassay
4.4.3. Assay of 5-Hydroxyindoles and 5-Methoxyindoles in Culture Medium Samples
4.4.4. Assay of Catecholamines and Their Metabolites
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Drozdova, A.; Okuliarova, M.; Zeman, M. The effect of different wavelengths of light during incubation on the development of rhythmic pineal melatonin biosynthesis in chick embryos. Animal 2019, 13, 1635–1640. [Google Scholar] [CrossRef] [Green Version]
- Csernus, V.J.; Nagy, A.D.; Faluhelyi, N. Development of the rhythmic melatonin secretion in the embryonic chicken pineal gland. Gen. Comp. Endocrinol. 2007, 152, 148–153. [Google Scholar] [CrossRef]
- Bai, X.; Cao, J.; Dong, Y.; Wang, Z.; Chen, Y. Melatonin mediates monochromatic green light-induced satellite cell proliferation and muscle growth in chick embryo. PLoS ONE 2019, 14, e0216392. [Google Scholar] [CrossRef]
- Lamosová, D.; Zeman, M.; Macková, M.; Gwinner, E. Development of rhythmic melatonin synthesis in cultured pineal glands and pineal cells isolated from chick embryo. Experientia 1995, 51, 970–975. [Google Scholar] [CrossRef]
- Möller, W.; Möller, G. Structural and functional differentiation of the embryonic chick pineal organ in vivo and in vitro. A scanning electron-microscopic and radioimmunoassay study. Cell Tissue Res. 1990, 260, 337–348. [Google Scholar] [CrossRef]
- Zeman, M.; Gwinner, E.; Somogyiová, E. Development of melatonin rhythm in the pineal gland and eyes of chick embryo. Experientia 1992, 48, 765–768. [Google Scholar] [CrossRef]
- Zeman, M.; Gwinner, E.; Herichová, I.; Lamosová, D.; Kost’ál, L. Perinatal development of circadian melatonin production in domestic chicks. J. Pineal Res. 1999, 26, 28–34. [Google Scholar] [CrossRef]
- Herichová, I.; Zeman, M.; Macková, M.; Griac, P. Rhythms of the pineal N-acetyltransferase mRNA and melatonin concentrations during embryonic and post-embryonic development in chicken. Neurosci. Lett. 2001, 298, 123–126. [Google Scholar] [CrossRef]
- Zeman, M.; Pavlik, P.; Lamosová, D.; Herichová, I.; Gwinner, E. Entrainment of rhythmic melatonin production by light and temperature in the chick embryo. Avian Poult. Biol. Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Herichová, I.; Monosíková, J.; Zeman, M. Ontogeny of melatonin, Per2 and E4bp4 light responsiveness in the chicken embryonic pineal gland. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 44–50. [Google Scholar] [CrossRef]
- Hanuszewska, M.; Prusik, M.; Lewczuk, B. Embryonic Ontogeny of 5-Hydroxyindoles and 5-Methoxyindoles Synthesis Pathways in the Goose Pineal Organ. Int. J. Mol. Sci. 2019, 20, 3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziółkowska, N.; Lewczuk, B.; Prusik, M. Diurnal and circadian variations in indole contents in the goose pineal gland. Chronobiol. Int. 2018, 35, 1560–1575. [Google Scholar] [CrossRef] [PubMed]
- Adamska, I.; Lewczuk, B.; Markowska, M.; Majewski, P.M. Daily profiles of melatonin synthesis-related indoles in the pineal glands of young chickens (Gallus gallus domesticus L.). J. Photochem. Photobiol. B Biol. 2016, 164, 335–343. [Google Scholar] [CrossRef]
- Lewczuk, B.; Ziółkowska, N.; Prusik, M.; Przybylska-Gornowicz, B. Diurnal profiles of melatonin synthesis-related indoles, catecholamines and their metabolites in the duck pineal organ. Int. J. Mol. Sci. 2014, 15, 12604–12630. [Google Scholar] [CrossRef] [Green Version]
- Grady, R.K., Jr.; Caliguri, A.; Mefford, I.N. Day/night differences in pineal indoles in the adult pigeon (Columba livia). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1984, 78, 141–143. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B. Norepinephrine Is a Major Regulator of Pineal Gland Secretory Activity in the Domestic Goose (Anser anser). Front. Physiol. 2021, 12, 664117. [Google Scholar] [CrossRef]
- Takahashi, J.S.; Hamm, H.; Menaker, M. Circadian rhythms of melatonin release from individual superfused chicken pineal glands in vitro. Proc. Natl. Acad. Sci. USA 1980, 77, 2319–2322. [Google Scholar] [CrossRef] [Green Version]
- Robertson, L.M.; Takahashi, J.S. Circadian clock in cell culture: II. In vitro photic entrainment of melatonin oscillation from dissociated chick pineal cells. J. Neurosci. 1988, 8, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Csernus, V.; Faluhelyi, N.; Nagy, A.D. Features of the circadian clock in the avian pineal gland. Ann. N. Y. Acad. Sci. 2005, 1040, 281–287. [Google Scholar] [CrossRef]
- Csernus, V.; Becher, P.; Mess, B. Wavelength dependency of light-induced changes in rhythmic melatonin secretion from chicken pineal gland in vitro. Neuro. Endocrinol. Lett. 1999, 20, 299–304. [Google Scholar]
- Prusik, M.; Lewczuk, B. Roles of Direct Photoreception and the Internal Circadian Oscillator in the Regulation of Melatonin Secretion in the Pineal Organ of the Domestic Turkey: A Novel In Vitro Clock and Calendar Model. Int. J. Mol. Sci. 2019, 20, 4022. [Google Scholar] [CrossRef] [Green Version]
- Martyniuk, K.; Hanuszewska, M.; Lewczuk, B. Metabolism of Melatonin Synthesis-Related Indoles in the Turkey Pineal Organ and Its Modification by Monochromatic Light. Int. J. Mol. Sci. 2020, 21, 9750. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Nowicki, M. Post-hatching development of the turkey pineal organ: Histological and immunohistochemical studies. Neuro. Endocrinol. Lett. 2005, 26, 383–392. [Google Scholar] [PubMed]
- Petrusewicz-Kosińska, M.; Przybylska-Gornowicz, B.; Ziółkowska, N.; Martyniuk, K.; Lewczuk, B. Developmental morphology of the turkey pineal organ. Immunocytochemical and ultrastructural studies. Micron. 2019, 122, 8–20. [Google Scholar] [CrossRef]
- Prusik, M.; Lewczuk, B.; Nowicki, M.; Przybylska-Gornowicz, B. Histology and ultrastructure of the pineal gland of the domestic goose. Histol. Histopathol. 2006, 21, 1075–1090. [Google Scholar]
- Prusik, M.; Lewczuk, B. Structure of the avian pineal gland. Med. Weter. 2008, 64, 734–848. (In Polish) [Google Scholar]
- Fejér, Z.; Röhlich, P.; Szél, A.; Dávid, C.; Zádori, A.; Manzano, M.J.; Vígh, B. Comparative ultrastructure and cytochemistry of the avian pineal organ. Microsc. Res. Tech. 2001, 53, 12–24. [Google Scholar] [CrossRef]
- Boya, J.; Calvo, J. Ultrastructural study of the post-hatching evolution of the pineal gland of the chicken (Gallus gallus). Cells Tissues Organs 1980, 107, 143–168. [Google Scholar] [CrossRef]
- Boya, J.; Calvo, J. Evolution of the pineal gland in the adult chicken. Cells Tissues Organs 1979, 104, 104–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akasaka, K.; Nasu, T.; Katayama, T.; Murakami, N. Development of regulation of melatonin release in pineal cells in chick embryo. Brain Res. 1995, 692, 283–286. [Google Scholar] [CrossRef]
- Macková, M.; Lamosová, D.; Zeman, M. Regulation of rhythmic melatonin production in pineal cells of chick embryo by cyclic AMP. Cell. Mol. Life Sci. 1998, 54, 461–466. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Csernus, V. The effects of environmental illumination on the in vitro melatonin secretion from the embryonic and adult chicken pineal gland. Gen. Comp. Endocrinol. 2007, 152, 154–158. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Reglodi, D.; Csernus, V. Development of the circadian melatonin rhythm and its responsiveness to PACAP in the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2005, 1040, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bai, S.; Qin, X.; Zhang, J.; Irwin, D.M.; Zhang, S.; Wang, Z. Comparison of whole embryonic development in the duck (Anas platyrhynchos) and goose (Anser cygnoides) with the chicken (Gallus gallus). Poult. Sci. 2019, 98, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Kommedal, S.; Csernus, V.; Nagy, A.D. The embryonic pineal gland of the chicken as a model for experimental jet lag. Gen. Comp. Endocrinol. 2013, 188, 226–231. [Google Scholar] [CrossRef]
- Binkley, S.; Geller, E.B. Pineal Enzymes in Chickens: Development of Daily Rhythmicity. Gen. Comp. Endocrinol. 1975, 27, 424–429. [Google Scholar] [CrossRef]
- Zeman, M.; Illnerová, H. Ontogeny of N-acetyltransferase activity rhythm in pineal gland of chick embryo. Comp. Biochem. Physiol. A Comp. Physiol. 1990, 97, 175–178. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Monochromatic light affects the development of chick embryo liver via an anti-oxidation pathway involving melatonin and the melatonin receptor Mel1c. Can. J. Anim. Sci. 2014, 94, 391–400. [Google Scholar] [CrossRef]
- Archer, G.S.; Mench, J.A. The effects of the duration and onset of light stimulation during incubation on the behavior, plasma melatonin levels, and productivity of broiler chickens. J. Anim. Sci. 2014, 92, 1753–1758. [Google Scholar] [CrossRef]
- Prusik, M.; Lewczuk, B.; Ziółkowska, N.; Przybylska-Gornowicz, B. Regulation of melatonin secretion in the pineal organ of the domestic duck-an in vitro study. Pol. J. Vet. Sci. 2015, 18, 635–644. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Matkovits, A.; Párniczky, A.; Csernus, V. The in vitro and in ovo effects of environmental illumination and temperature on the melatonin secretion from the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2009, 1163, 383–385. [Google Scholar] [CrossRef]
- Voisin, P.; Van Camp, G.; Collin, J.P. N-acetylation of serotonin is correlated with alpha 2- but not with beta-adrenergic regulation of cyclic AMP levels in cultured chick pineal cells. J. Neurochem. 1990, 54, 1953–1960. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Berezińska, M.; Stasikowska, O.; Lorenc, A.; Skene, D.J.; Nowak, J.Z. Posthatching developmental changes in noradrenaline content in the chicken pineal gland. J. Pineal Res. 2005, 38, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Faluhelyi, N.; Reglodi, D.; Lengvári, I.; Csernus, V. Development of the circadian melatonin rhythm and the effect of PACAP on melatonin release in the embryonic chicken pineal gland. An in vitro study. Regul. Pept. 2004, 123, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Faluhelyi, N.; Reglodi, D.; Csernus, V. The effects of PACAP and VIP on the in vitro melatonin secretion from the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2006, 1070, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Sato, T. Sensory and endocrine characteristics of the avian pineal organ. Microsc. Res. Tech. 2001, 53, 2–11. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanuszewska-Dominiak, M.; Martyniuk, K.; Lewczuk, B. Embryonic Development of Avian Pineal Secretory Activity—A Lesson from the Goose Pineal Organs in Superfusion Culture. Molecules 2021, 26, 6329. https://doi.org/10.3390/molecules26216329
Hanuszewska-Dominiak M, Martyniuk K, Lewczuk B. Embryonic Development of Avian Pineal Secretory Activity—A Lesson from the Goose Pineal Organs in Superfusion Culture. Molecules. 2021; 26(21):6329. https://doi.org/10.3390/molecules26216329
Chicago/Turabian StyleHanuszewska-Dominiak, Maria, Kamila Martyniuk, and Bogdan Lewczuk. 2021. "Embryonic Development of Avian Pineal Secretory Activity—A Lesson from the Goose Pineal Organs in Superfusion Culture" Molecules 26, no. 21: 6329. https://doi.org/10.3390/molecules26216329
APA StyleHanuszewska-Dominiak, M., Martyniuk, K., & Lewczuk, B. (2021). Embryonic Development of Avian Pineal Secretory Activity—A Lesson from the Goose Pineal Organs in Superfusion Culture. Molecules, 26(21), 6329. https://doi.org/10.3390/molecules26216329






