Properties of Ozone-Oxidized Tapioca Starch and Its Use in Coating of Fried Peanuts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics and Properties of Tapioca as Influenced by Ozone Oxidation under Different Conditions
2.1.1. Carbonyl (CBN) and Carboxyl (CBX) Contents
2.1.2. Amylose Content
2.1.3. Swelling Power (SP) and Solubility
2.1.4. Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
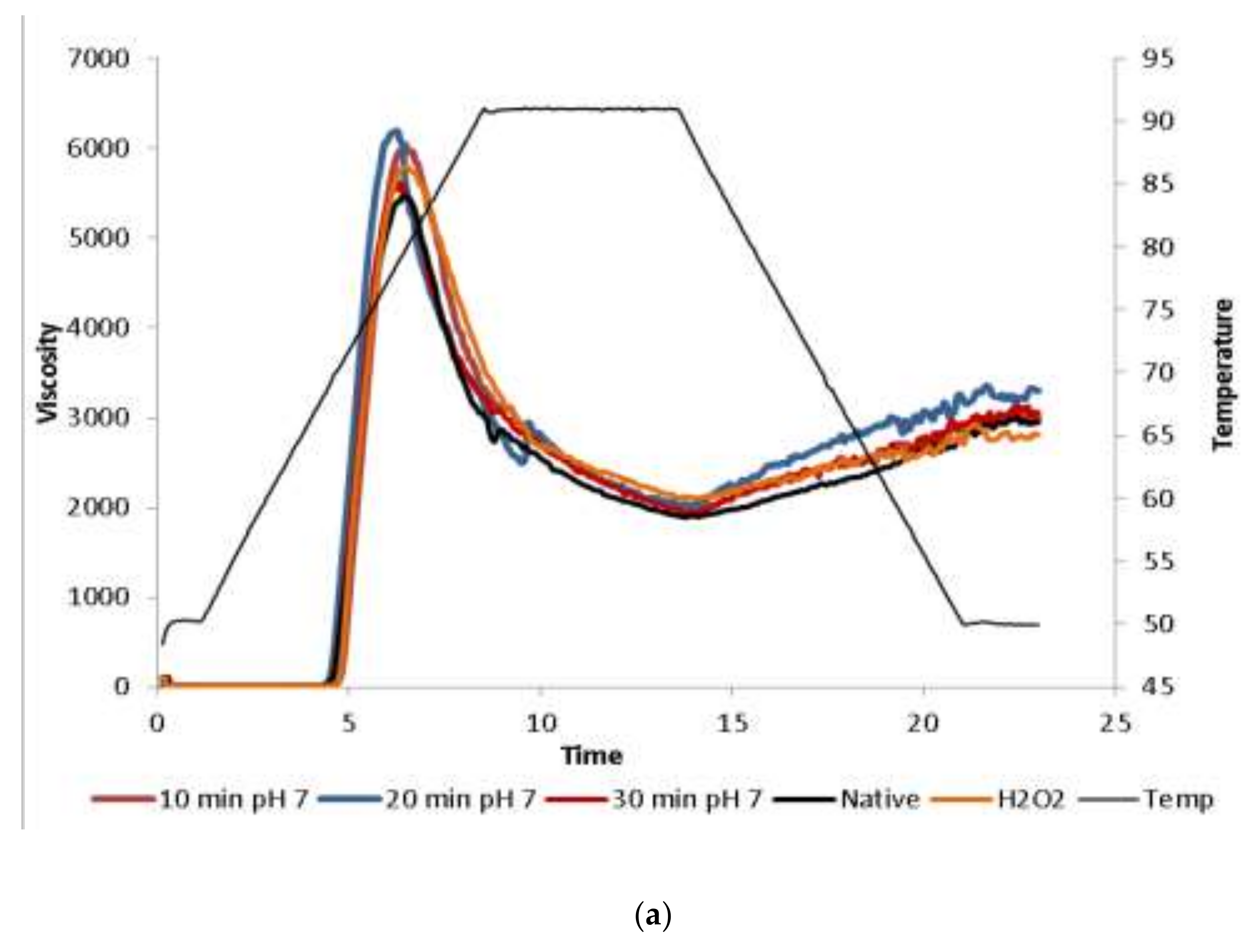
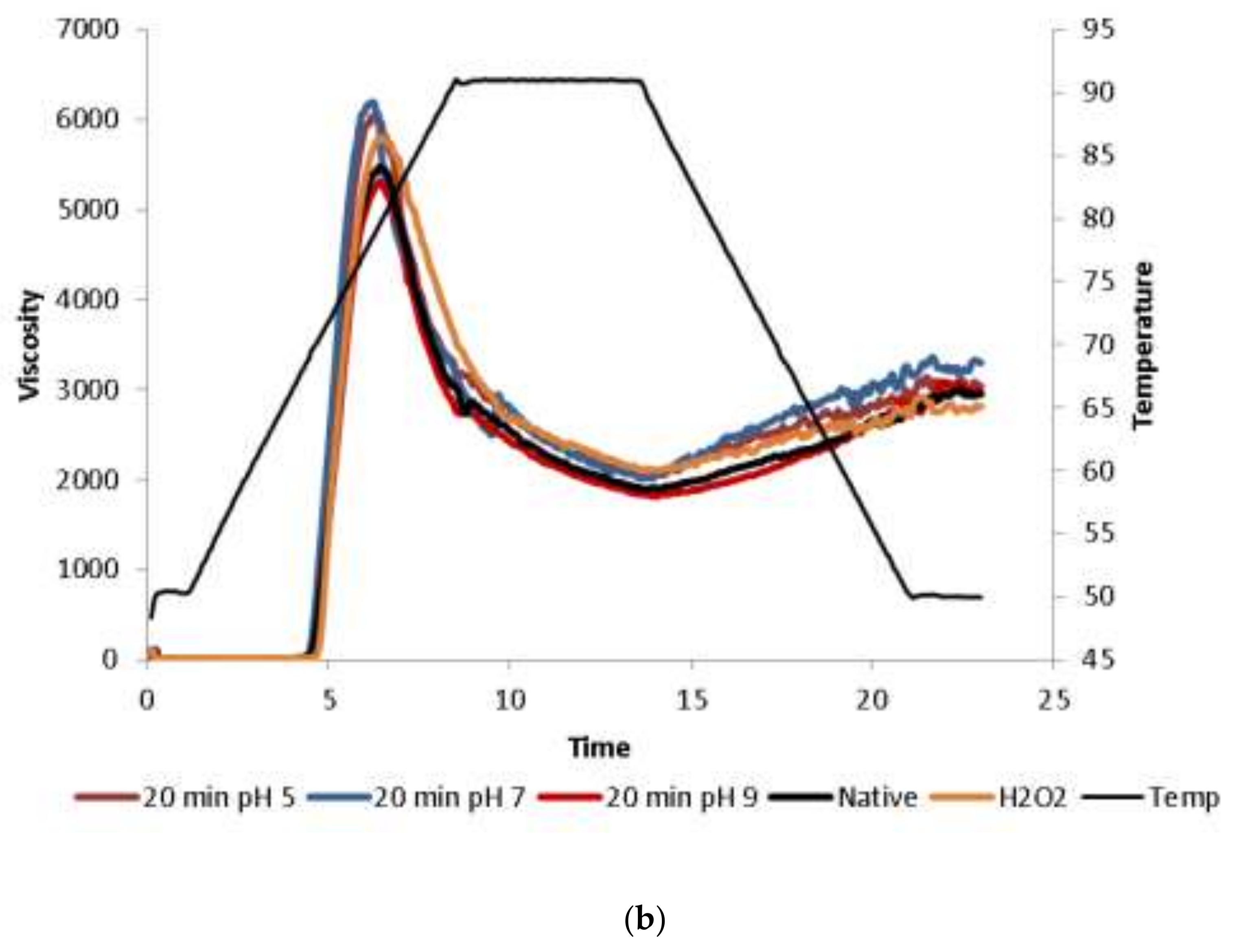
2.1.5. Pasting Properties
2.2. Characteristics and Properties of Fried Peanut Coated with Ozone-Oxidized Tapioca
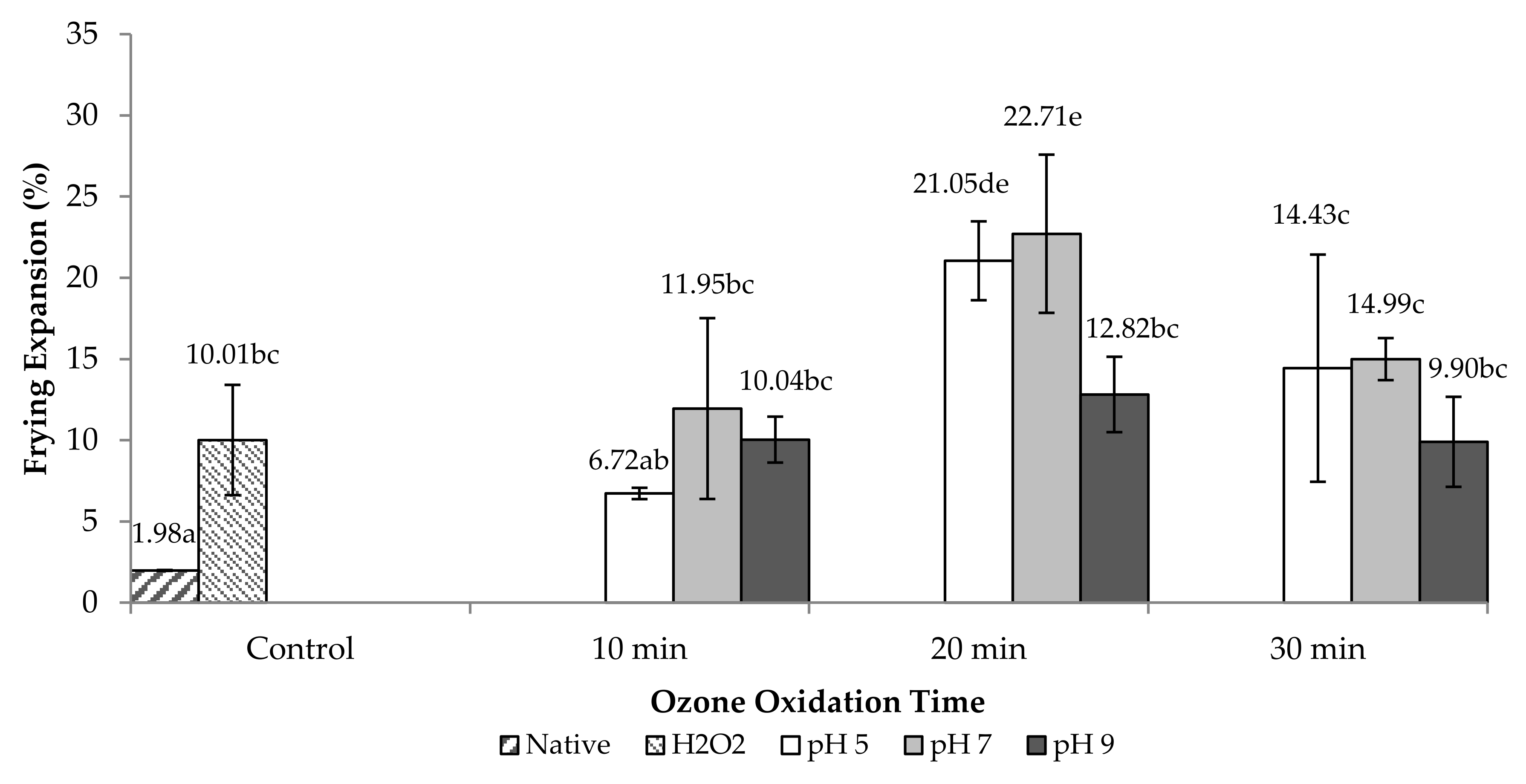
2.2.1. Frying Expansion (FE)
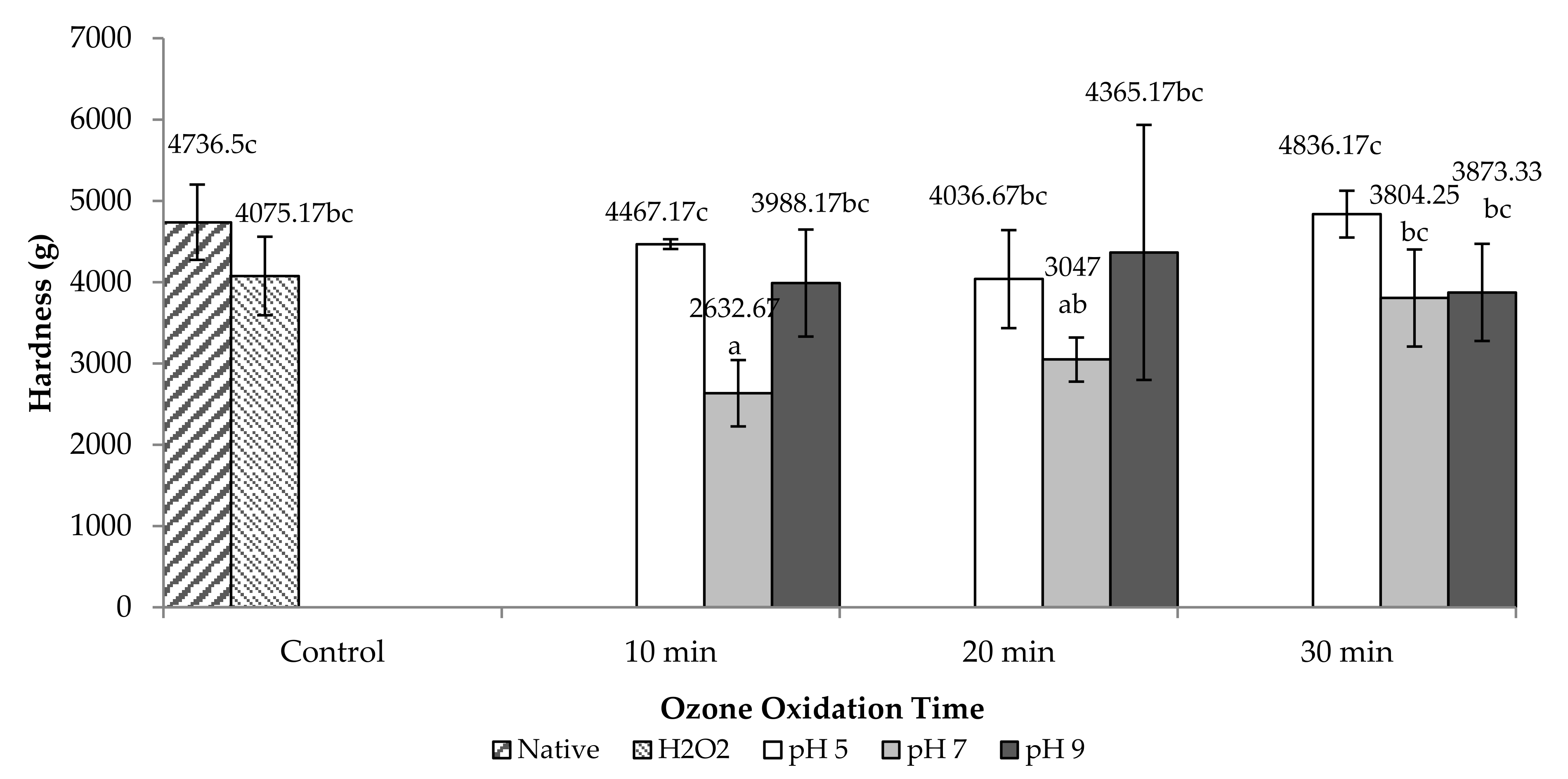
2.2.2. Hardness
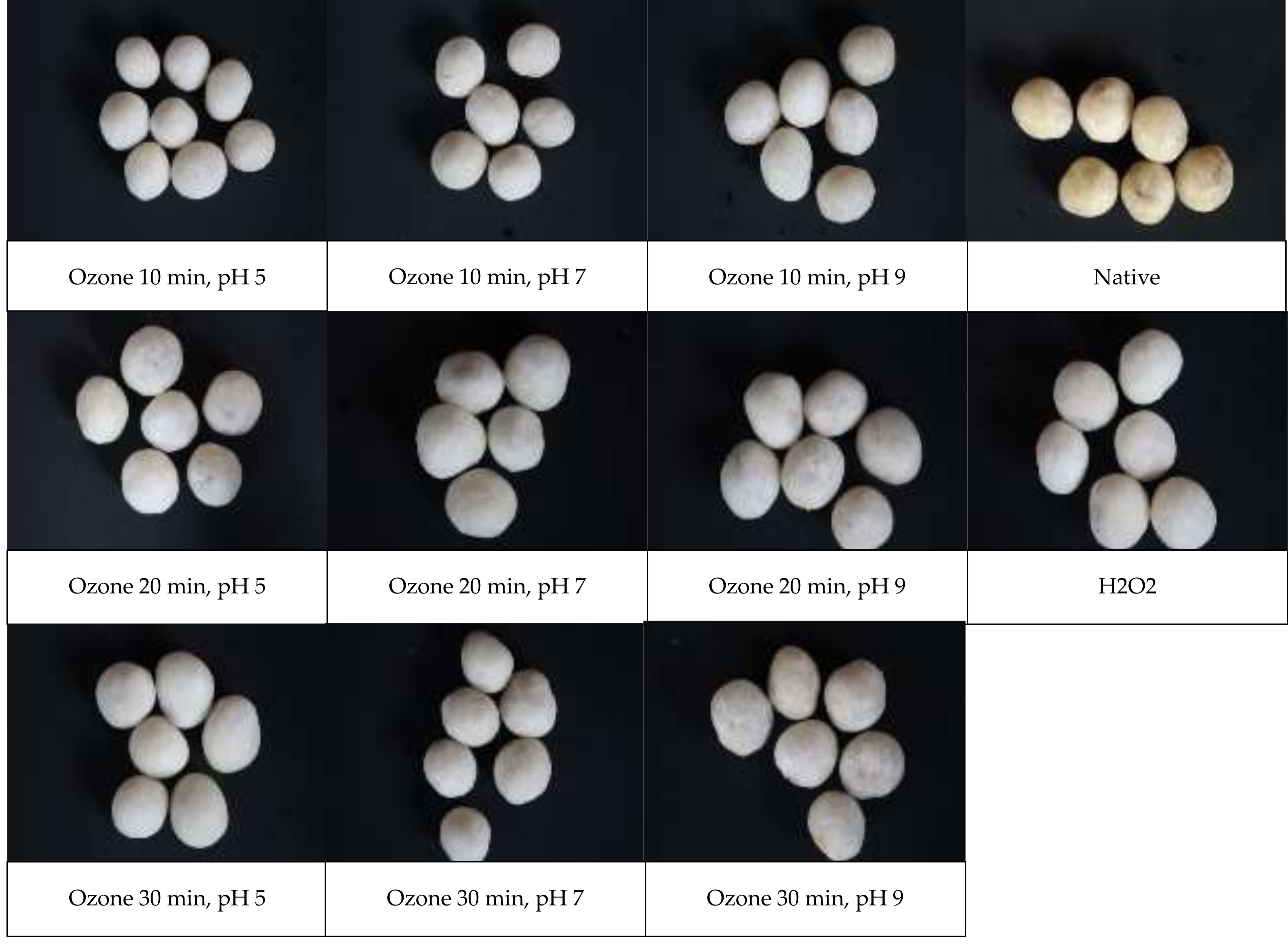
2.2.3. Microscopic Structure
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ozone-Oxidized Tapioca
3.2.1. Carbonyl (CBN) Content
3.2.2. Carboxyl (CBX) Content
3.2.3. Amylose Content
3.2.4. Swelling Power and Solubility
3.2.5. Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
3.2.6. Pasting Properties
3.3. Preparation of Fried Peanut Coated with Ozone-Oxidized Tapioca
3.3.1. Frying Expansion (FE)
3.3.2. Hardness
3.3.3. Microstructures
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Franco, C.M.L.; Ogawa, C.; Rabachini, T.; Rocha, T.D.S.; Cereda, M.P.; Jane, J.-I. Effect of lactic acid and UV irradiation on the cassava and corn starches. Braz. Arch. Biol. Technol. 2010, 53, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, A.C.; Mestres, C.; Lourdin, D.; Valle, G.D.; Colonna, P. Relationship between thermomechanical properties and baking expansion of sour cassava starch (Polvilho azedo). J. Sci. Food Agric. 2001, 81, 429–435. [Google Scholar] [CrossRef]
- Demiate, I.M.; Dupuy, N.; Huvenne, J.P.; Cereda, M.P.; Wosiacki, G. Relationship between baking behavior of modified cassava starches and starch chemical structure determined by FTIR spectroscopy. Carbohydr. Polym. 2000, 42, 149–158. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Starch-value addition by modification. Crit. Rev. Food Sci. Nutr. Polym. 2005, 45, 371–384. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr. Polym. 2003, 52, 207–217. [Google Scholar] [CrossRef]
- Chan, H.T.; Leh, C.P.; Bhat, R.; Senan, C.; Williams, P.A.; Karim, A.A. Molecular structure, rheological and thermal characteristics of ozone-oxidized starch. Food Chem. 2011, 126, 1019–1024. [Google Scholar] [CrossRef]
- Dias, A.R.G.; Zavareze, E.d.R.; Elias, M.C.; Helbig, E.; da Silva, D.O.; Ciacco, C.F. Pasting, expansion and textural properties of fermented cassava starch oxidised with sodium hypochlorite. Carbohydr. Polym. 2011, 84, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, H.P.S.; Manthey, F.A.; Simsek, S. Ozone gas affects physical and chemical properties of wheat (Triticum aestivum L.) starch. Carbohydr. Polym. 2012, 87, 1261–1268. [Google Scholar] [CrossRef]
- Oladebeye, A.O.; Oshodi, A.A.; Amoo, I.A.; Karim, A.A. Functional, thermal and molecular behaviours of ozone-oxidised cocoyam and yam starches. Food Chem. 2013, 141, 1416–1423. [Google Scholar] [CrossRef]
- Chan, H.T.; Bhat, R.; Karim, A.A. Physicochemical and functional properties of ozone-oxidized starch. J. Agric. Food Chem. 2009, 57, 5965–5970. [Google Scholar] [CrossRef]
- Klein, B.; Vanier, N.L.; Moomand, K.; Pinto, V.Z.; Colussi, R.; da Rosa Zavareze, E.; Dias, A.R.G. Ozone oxidation of cassava starch in aqueous solution at different pH. Food Chem. 2014, 155, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Rosal, R.; Perdigón-Melón, J.A.; Mezcua, M.; Agüera, A.; Hernando, M.D.; Letón, P.; Fernández-Alba, A.R.; García-Calvo, E. Ozone based technologies in water and wastewater treatment. In Handbook of Environmental Chemistry, Volume 5: Water Pollution; Barceló, D., Kostianoy, A.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 127–175. [Google Scholar]
- Kuakpetoon, D.; Wang, Y.-J. Characterization of different starches oxidized by hypochlorite. Starke 2001, 53, 211–218. [Google Scholar] [CrossRef]
- El-Sheikh, M.A.; Ramadan, M.A.; El-Shafie, A. Photo-oxidation of rice starch. Part I: Using hydrogen peroxide. Carbohydr. Polym. 2010, 80, 266–269. [Google Scholar] [CrossRef]
- Eriksson, M. Ozone Chemistry in Aqueous Solution. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 13 May 2005. [Google Scholar]
- Kuakpetoon, D.; Wang, Y.-J. Structural characteristics and physicochemical properties of oxidized corn starches varying in amylose content. Carbohydr. Res. 2006, 341, 1896–1915. [Google Scholar] [CrossRef]
- Dewi, A.M.P. Tapioca Oxidation with Hydrogen Peroxide and UV C Irradiation Catalyst, and the Application for Edible Film (Indonesian). Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2011. [Google Scholar]
- Lee, J.S.; Kumar, R.N.; Rozman, H.D.; Azemi, B.M.N. Pasting, Swelling, and Solubility Properties of UV initiated Starch-graft-Poly(AA). Food Chem. 2005, 91, 203–211. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Adeniyi Afolabi, T.; Lawal, O.S. Isolation, chemical modification and physicochemical characterisation of Bambarra groundnut (Voandzeia subterranean) starch and flour. Food Chem. 2002, 78, 305–311. [Google Scholar] [CrossRef]
- Uzomah, A.; Ibe, C. The functional properties, pasting and baking behaviour of chemically modified sour cassava starches. Afr. J. Food Sci. 2011, 5, 686–694. [Google Scholar]
- Lawal, O.S.; Adebowale, K.O.; Ogunsanwo, B.M.; Barba, L.L.; Ilo, N.S. Oxidized and acid thinned starch derivatives of hybrid maize: Functional characteristics, wide-angle X-ray diffractometry and thermal properties. Int. J. Biol. Macromol. 2005, 35, 71–79. [Google Scholar] [CrossRef]
- Farley, F.F.; Hixon, R.M. Oxidation of raw starch granules by electrolysis in alkaline sodium chloride solution. Ind. Eng. Chem. 1942, 34, 677–681. [Google Scholar] [CrossRef]
- Priadi, G. Effect of Oxidation of Shredded Acid Cassava with Hidrogen Peroxide and UV Catalyst in a Tumbler on the Baking Expansion (Indonesian). Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2013. [Google Scholar]
- Dana, D.; Saguy, I.S. Frying of nutritious foods: Obstacles and feasibility. Food Sci. Technol. Res. 2001, 7, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Nurul, H.; Boni, I.; Noryati, I. The effect of different ratios of Dory Fish to tapioca flour on the linear expansion, oil absorption, colour and hardness of fish crackers. Int. Food. Res. J. 2009, 16, 159–165. [Google Scholar]
- Ngadi, M.; Adedeji, A.A.; Kassama, L. Microstructural changes during frying of foods. In Advances in Deep-Frying of Foods; Sahin, S., Summu, S.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 169–200. [Google Scholar]
- Tsukakoshi, Y.; Naito, S.; Ishida, N. Fracture intermittency during a puncture test of cereal snacks and its relation to porous structure. Food Res. Int. 2008, 41, 909–917. [Google Scholar] [CrossRef]
- Satmalawati, M.M.E.M. Characterization of Oxidized Tapioca at Varying Dissolved Ozon and Starch Slurry Concentration (Indonesian). Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2011. [Google Scholar]
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized cassava starches. Carbohydr. Polym. 2010, 82, 446–453. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists (AOAC): Washington, DC, USA, 1984. [Google Scholar]
- Suprapti, M.L. Tapioca Flour: Making and its utilization (Indonesian); Kanisius: Yogyakarta, Indonesia, 2005. [Google Scholar]
- Nguyen, T.T.; Le, T.Q.; Songsermpong, S. Shrimp Cassava Cracker Puffed by Microwave Technique: Effect of Moisture and Oil Content on Some Physical Characteristics. Witthayasan Kasetsat 2013, 47, 434–446. [Google Scholar]
- Saeleaw, M.; Schleining, G. Effect of frying parameters on crispiness and sound emission of cassava crackers. J. Food Eng. 2011, 103, 229–236. [Google Scholar] [CrossRef]

| Oxidation Condition | Carbonyl (%) 1 | Carboxyl (%) 1 | Amylose (%) 1 | |
|---|---|---|---|---|
| Oxidation Time pH | ||||
| Native | 0.007 ± 0.011 a | 0.0000 ± 0.0015 a | 32.54 ± 3.96 a | |
| H2O2 | 0.270 ± 0.03 b | 0.0036 ± 0.0037 ab | 44.34 ± 1.29 d | |
| 10 min | pH 5 | 0.309 ± 0.03 bc | 0.0070 ± 0.0018 bc | 35.65 ± 0.97 ab |
| pH 7 | 0.353 ± 0.04 bc | 0.0088 ± 0.0030 c | 38.63 ± 0.70 bc | |
| pH 9 | 0.241 ± 0.03 b | 0.0063 ± 0.0001 bc | 37.54 ± 1.11 b | |
| 20 min | pH 5 | 0.353 ± 0.06 bc | 0.0061 ± 0.0026 bc | 37.92 ± 1.74 bc |
| pH 7 | 0.390 ± 0.10 c | 0.0103 ± 0.0021 c | 41.38 ± 1.43 cd | |
| pH 9 | 0.323 ± 0.02 bc | 0.0083 ± 0.0029 c | 37.80 ± 1.19 b | |
| 30 min | pH 5 | 0.314 ± 0.04 bc | 0.0071 ± 0.0015 bc | 39.07 ± 1.94 bc |
| pH 7 | 0.300 ± 0.09 bc | 0.0087 ± 0.0015 c | 38.41 ± 2.94 bc | |
| pH 9 | 0.269 ± 0.01 b | 0.0092 ± 0.0033 c | 38.11 ± 0.25 bc | |
| Oxidation Condition | Swelling Power (%) 1 | Solubility (%) 1 | WHC (%) 1 | OHC (%) 1 | |
|---|---|---|---|---|---|
| Oxidation Time pH | |||||
| Native | 13.27 ± 0.78 a | 0.18 ± 0.01 a | 0.76 ± 0.01 a | 1.20 ± 0.04 d | |
| H2O2 | 21.12 ±1.57 c | 0.34 ± 0.08 c | 0.97 ± 0.02 b | 0.76 ± 0.01 a | |
| 10 min | pH 5 | 16.54 ± 1.78 ab | 0.20 ± 0.02 a | 0.98 ± 0.03 b | 0.88 ± 0.03 bc |
| pH 7 | 17.10 ± 1.36 b | 0.25 ± 0.02 bc | 0.89 ± 0.08 b | 0.84 ± 0.05 b | |
| pH 9 | 15.70 ± 1.33 ab | 0.23 ± 0.06 ab | 0.91 ± 0.06 b | 0.87 ± 0.01 bc | |
| 20 min | pH 5 | 18.07 ± 3.25 bc | 0.23 ± 0.01 ab | 0.99 ± 0.05 b | 0.95 ± 0.02 c |
| pH 7 | 17.95 ± 1.22 bc | 0.27 ± 0.06 bc | 0.95 ± 0.03 b | 0.94 ± 0.05 c | |
| pH 9 | 16.54 ± 1.41 ab | 0.25 ± 0.08 bc | 0.92 ± 0.08 b | 0.91 ± 0.08 bc | |
| 30 min | pH 5 | 17.00 ± 1.32 b | 0.19 ± 0.04 a | 0.92 ± 0.10 b | 0.91 ± 0.06 bc |
| pH 7 | 17.86 ± 1.68 bc | 0.20 ± 0.01 a | 0.92 ± 0.03 b | 0.86 ± 0.02 bc | |
| pH 9 | 24.60 ± 2.73 d | 0.20 ± 0.02 a | 0.89 ± 0.04 b | 0.91 ± 0.03 bc | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranoto, Y.; Paramita, B.L.; Cahyanto, M.N.; Benjakul, S. Properties of Ozone-Oxidized Tapioca Starch and Its Use in Coating of Fried Peanuts. Molecules 2021, 26, 6281. https://doi.org/10.3390/molecules26206281
Pranoto Y, Paramita BL, Cahyanto MN, Benjakul S. Properties of Ozone-Oxidized Tapioca Starch and Its Use in Coating of Fried Peanuts. Molecules. 2021; 26(20):6281. https://doi.org/10.3390/molecules26206281
Chicago/Turabian StylePranoto, Yudi, Brigitta Laksmi Paramita, Muhammad Nur Cahyanto, and Soottawat Benjakul. 2021. "Properties of Ozone-Oxidized Tapioca Starch and Its Use in Coating of Fried Peanuts" Molecules 26, no. 20: 6281. https://doi.org/10.3390/molecules26206281
APA StylePranoto, Y., Paramita, B. L., Cahyanto, M. N., & Benjakul, S. (2021). Properties of Ozone-Oxidized Tapioca Starch and Its Use in Coating of Fried Peanuts. Molecules, 26(20), 6281. https://doi.org/10.3390/molecules26206281








