Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates
Abstract
1. Introduction
2. Antiviral Activity from Plant Extracts and Secondary Metabolites
2.1. Influenza Virus
2.2. Human Immunodeficiency Virus (HIV)
2.3. Arthropod-Borne Flaviviruses
2.4. Herpes Simplex Virus
2.5. Hepatitis Virus
3. Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 229E | Human coronavirus |
| AV | Adenovirus |
| B/Lee/40 | Influenza B/Lee/40 |
| CHIKV | Chikungunya virus |
| CV-B | Coxsackievirus B |
| CV | Coxsackieviruses |
| CyHV-3 | Cyprinid herpesvirus 3 |
| DENV | Dengue virus |
| EBV | Epstein–Barr virus |
| EHV-1 | Equid herpesvirus 1 |
| EV | Ebola virus |
| FV | Flavivirus |
| H1N1 | Influenza virus |
| H3N2 | Influenza A/Victoria virus |
| H5N1 | Avian influenza virus |
| H9N2 | Novel Reassortant avian influenza A virus |
| HAV | Hepatitis A Virus |
| HBV | Hepatitis B Virus |
| HCoV 229E | Human coronavirus |
| HCV | Hepatitis C virus |
| HIV-1 | Human immunodeficiency virus 1 |
| HIV-2 | Human immunodeficiency virus 2 |
| HIV | Human immunodeficiency virus |
| HR3V | Human rhinovirus 3 virus |
| HRoV | Human rotavirus |
| HSV | Herpes simplex viruses |
| HRV | Human rhino virus |
| HSV-1 | Herpes simplex viruses 1 |
| HSV-2 | Herpes simplex viruses 2 |
| HuNoVs | Human noroviruses |
| MV | Measles virus |
| MNV-1 | Murine Norovirus-1 |
| NDV | Newcastle disease virus |
| POV | Poliovirus |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PRV | Pseudorabies Virus |
| PV | Pestivirus |
| PV1 | Picornavirus |
| RSV | Respiratory syncytial virus |
| SARS-CoV-2 | COVID19 |
| SFV | Semliki forest virus |
| SINV | Sindbis virus |
| SuHV-1 | Suid herpesvirus 1 |
| TMV | Tobacco mosaic virus |
| VSV | Vesicular stomatitis virus |
| VV | Vaccinia virus |
| WNV | West Nile virus |
| WSSV | White spot syndrome virus |
| YFV | Yellow fever virus |
| ZIKV | Zika virus |
References
- Lorizate, M.; Krausslich, H.-G. Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 2011, 3, a004820. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef]
- Aleksandr, I.; Eva, Z.; Suvi, K.; Marten, S.; Hilde, L.; Mona, T.; Laura, K.; Henrik, P.; Mira, L.; Hannimari, K.K.; et al. Novel ac-tivities of safe-in-human broad-spectrum antiviral agents. Antivir. Res. 2018, 154, 174–182. [Google Scholar]
- WHO. WHO Publishes List of Top Emerging Diseases Likely to Cause Major Epidemics. Available online: https://www.who.int/medicines/ebola-treatment/WHO-list-oftop-emerging-diseases/en/ (accessed on 15 December 2020).
- Guangxiang, L.; Shou-Jiang, G. Global health concerns by emerging viral infections. J Med. Virol. 2020, 92, 399–400. [Google Scholar]
- Li, M.; Lei, Y. Antiviral Effects of Plant-Derived Essential Oils and Their Compounds: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar]
- Divyabharathi, M.; Ashish, W.; Praveen, T.K. Drug Repurposing in Antiviral Research: A Current Scenario. J. Young Pharm. 2019, 11, 117–121. [Google Scholar]
- Daisuke, A.; Hideki, N. Pathogenic Viruses Commonly Present in the Oral Cavity and Relevant Antiviral Compounds De-rived from Natural Products. Medicines 2018, 5, 120. [Google Scholar]
- Clark, A.M. Natural Products as a Resource for New Drugs. Pharm. Res. 1996, 13, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef]
- El Sayed, K.A. Natural Products as Antiviral Agents. Stereoselective Synth. (Part K) 2000, 24, 473–572. [Google Scholar] [CrossRef]
- Goh, V.S.L.; Mok, C.-K.; Chu, J.J.H. Antiviral Natural Products for Arbovirus Infections. Molecules 2020, 25, 2796. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P. Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef]
- Riccio, G.; Ruocco, N.; Mutalipassi, M.; Constantini, M.; Zupo, V.; Coppola, D.; de Pascale, D.; Lauritano, C. Ten-Year Re-search Update Review: Antiviral Activities from Marine Organisms. Biomolecules 2020, 10, 2627. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Pereira, W.L.; da Silveira Oliveira, A.F.C.; da Silva, A.M.; de Oliveira, A.S.; da Silva, M.L.; da Silva, C.C.; de Paula, S.O. Natural products as source of potential dengue antivirals. Molecules 2014, 19, 8151–8176. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2014. J. Nat. Prod. 2014, 79, 629–661. [Google Scholar] [CrossRef]
- Mohan, S.; Taha, M.M.E.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Tripathi, G. Phytochemicals as Potential Curative Agents against Viral Infection: A Review. Curr. Org. Chem. 2020, 24, 2356–2366. [Google Scholar] [CrossRef]
- Remali, J.; Aizat, W.M. A Review on Plant Bioactive Compounds and Their Modes of Action against Coronavirus Infection. Front. Pharmacol. 2021, 11, 589044. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Attah, A.F.; Fagbemi, A.A.; Olubiyi, O.; Dada-Adegbola, H.; Oluwadotun, A.; Elujoba, A.; Babalola, C.P. Therapeutic Potentials of Antiviral Plants Used in Traditional African Medicine With COVID-19 in Focus: A Nigerian Perspective. Front. Pharmacol. 2021, 11, 589044. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. AJTCAM 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Paine, M.F.; McCune, J.S.; Oberlies, N.H.; Cech, N.B. Selection and characterization of botanical natural products for research studies: A NaPDI center recommended approach. Nat. Prod. Rep. 2019, 36, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- CDC. Key Facts about Influenza (Flu). Available online: https://www.cdc.gov/flu/about/keyfacts.htm (accessed on 15 December 2020).
- CDC. Influenza (Flu) Types of Iinfluenza Viruses, Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD). Available online: https://www.cdc.gov/flu/about/viruses/types.htm (accessed on 20 September 2020).
- CDC. Influenza (Flu) 2009 H1N1 Pandemic Timeline. Available online: https://www.cdc.gov/flu/pandemic-resources/2009-pandemic-timeline.html (accessed on 15 December 2020).
- Nabel, G.J.; Fauci, A.S. Induction of unnatural immunity: Prospects for a broadly protective universal influenza vaccine. Nat. Med. 2010, 16, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Gondim, A.C.S.; da Silva, S.R.; Mathys, L.; Noppen, S.; Liekens, S.; Sampaio, A.H.; Nagano, C.S.; Rocha, C.R.C.; Nascimento, K.S.; Cavada, B.S.; et al. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. MedChemComm 2019, 10, 390–398. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhou, B.; Lu, J.; Chen, Q.L.; Ti, H.; Huang, W.Y.; Li, J.; Yang, Z.F.; Jiang, Z.; Wang, X.H. Inhibition of influenza virus via a sesquiterpene fraction isolated from Laggera pterodonta by targeting the NF-κB and p38 pathways. BMC Complement. Altern. Med. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Yu, W.-Y.; Fang, J.; Zhang, H.-H.; Ma, Y.; Yu, B.; Wu, F.; Wu, X.-N. Mosla scabra flavonoids ameliorate the influenza A virus-induced lung injury and water transport abnormality via the inhibition of PRR and AQP signaling pathways in mice. J. Ethnopharmacol. 2016, 179, 146–155. [Google Scholar] [CrossRef]
- Chavan, R.D.; Shinde, P.; Girkar, K.; Madage, R.; Chowdhary, A. Assessment of Anti-Influenza Activity and Hemagglutina-tion Inhibition of Plumbago indica and Allium sativum Extracts. Pharmacogn. Res. 2016, 8, 105–111. [Google Scholar] [CrossRef]
- Makau, J.N.; Watanabe, K.; Mohammed, M.M.; Nishida, N. Antiviral Activity of Peanut (Arachis hypogaea L.) Skin Extract against Human Influenza Viruses. J. Med. Food 2018, 21, 777–784. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Ke, C.; Zhang, H.; Zhang, S.; Tang, W.; Liu, C.; Liu, G.; Chen, S.; Hu, A.; et al. Ethanol extract of Caesal-pinia decapetala inhibits influenza virus infection in vitro and in vivo. Viruses 2020, 12, 557. [Google Scholar] [CrossRef]
- Wang, J.-F.; He, W.-J.; Zhang, X.-X.; Zhao, B.-Q.; Liu, Y.-H.; Zhou, X.-J. Dicarabrol, a new dimeric sesquiterpene from Carpesium abrotanoides L. Bioorg. Med. Chem. Lett. 2015, 25, 4082–4084. [Google Scholar] [CrossRef] [PubMed]
- Enkhtaivan, G.; John, K.M.; Ayyanar, M.; Sekar, T.; Jin, K.-J.; Kim, D.H. Anti-influenza (H1N1) potential of leaf and stem bark extracts of selected medicinal plants of South India. Saudi J. Biol. Sci. 2015, 22, 532–538. [Google Scholar] [CrossRef]
- Hossan, S.; Fatima, A.; Rahmatullah, M.; Khoo, T.J.; Nissapatorn, V.; Galochkina, A.V.; Slita, A.V.; Shtro, A.A.; Nikolaeva, Y.; Zarubaev, V.V.; et al. Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch. Virol. 2018, 163, 2121–2131. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; John, K.M.; Pandurangan, M.; Hur, J.H.; Leutou, A.S.; Kim, D.H. Extreme effects of Seabuckthorn extracts on influenza viruses and human cancer cells and correlation between flavonol glycosides and biological activities of extracts. Saudi J. Biol. Sci. 2017, 24, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.H.A.; Ibrahim, E.A.; Kandeil, A.; El Baz, F.K. Sterols Bioactivity of Ruta graveolens L. and Murraya paniculata L. Int. J. Pharm. Pharm. Sci. 2017, 9, 103–108. [Google Scholar] [CrossRef]
- Priya, N.C.; Kumari, P.S. Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines. Int. J. Pharm. Sci. Rev. Res. 2017, 44, 197–202. [Google Scholar]
- Tran, T.T.; Kim, M.; Jang, Y.; Lee, H.W.; Nguyen, H.T.; Nguyen, T.N.; Park, H.W.; Le Dang, Q.; Kim, J.-C. Characterization and mechanisms of anti-influenza virus metabolites isolated from the Vietnamese medicinal plant Polygonum chinense. BMC Complement. Altern. Med. 2017, 17, 162. [Google Scholar] [CrossRef]
- Heo, Y.; Cho, Y.; Ju, K.S.; Cho, H.; Park, K.H.; Choi, H.; Yoon, J.K.; Moon, C.; Kim, Y.B. Antiviral activity of Poncirus trifo-liata seed extract against oseltamivir-resistant influenza virus. J. Microbiol. 2018, 56, 586–592. [Google Scholar] [CrossRef]
- Choi, J.-G.; Jin, Y.-H.; Kim, J.-H.; Oh, T.W.; Yim, N.-H.; Cho, W.-K.; Ma, J.Y. In vitro Anti-viral Activity of Psoraleae Semen Water Extract against Influenza A Viruses. Front. Pharmacol. 2016, 7, 460. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Hu, P.; Zhong, S.; Chen, Y.; Li, L.; Li, X.-Z.; Li, C.; Li, J.; Wang, Y.-T. Extraction and Separation of Polysaccharides from Radix isatidis and their Effects towards Hemagglutinin Protein of Influenza Virus. Asian J. Chem. 2015, 27, 1615–1620. [Google Scholar] [CrossRef]
- Romero-Perez, G.; Egashira, M.; Harada, Y.; Tsuruta, T.; Oda, Y.; Ueda, F.; Tsukahara, T.; Tsukamota, Y.; Inoue, R. Orally ad-ministered Salacia reticulata extract reduces h1n1 influenza clinical symptoms in Murine lung Tissues Putatively Due to enhanced natural Killer cell activity. Front. Immunol. 2016, 7, 115–125. [Google Scholar] [CrossRef]
- Nie, L.-X.; Wu, Y.-L.; Dai, Z.; Ma, S.-C. Antiviral activity of Isatidis Radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action. J. Ethnopharmacol. 2020, 251, 112550. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, B.; Lu, Y.; Qian, C.; Feng, Y.; Fang, L.; Ding, Z.; Cheng, D. Antiviral activity of phenanthrenes from the me-dicinal plant bletilla striata against influenza a virus. BMC Complement. Altern. Med. 2017, 17, 273. [Google Scholar] [CrossRef]
- Law, A.H.-Y.; Yang, C.L.-H.; Lau, A.S.-Y.; Chan, G.C.-F. Antiviral effect of forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit against influenza A virus through reduction of viral M1 protein. J. Ethnopharmacol. 2017, 209, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Fanhchaksai, K.; Kodchakorn, K.; Pothacharoen, P.; Kongtawalert, P. Effect of sesamin against cytokine production from influenza typeA H1N1-induced peripheral blood mononuclear cells:computational and experimental studies. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 107–119. [Google Scholar] [CrossRef]
- Zhao, L.; Xiang, K.-L.; Liu, R.-X.; Xie, Z.-P.; Zhang, S.-M.; Dai, S.-J. Anti-inflammatory and anti-viral labdane diterpenoids from the fruits of Forsythia suspensa. Bioorg. Chem. 2020, 96, 103651. [Google Scholar] [CrossRef]
- Tan, Y.P.; Xue, Y.; Savchenko, A.I.; Houston, S.D.; Modhiran, N.; McMillan, C.L.D.; Boyle, G.M.; Bernhardt, P.V.; Young, P.R.; Watterson, D.; et al. Basimarols A, B, and C, Highly Oxygenated Pimarane Diterpenoids from Basilicum polystachyon. J. Nat. Prod. 2019, 82, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Azman, N.S.N.; Hossan, S.; Nissapatorn, V.; Uthaipibull, C.; Prommana, P.; Jin, K.T.; Rahmatullah, M.; Mahboob, T.; Raju, C.S.; Jindal, H.M.; et al. Anti-infective activities of 11 plants species used in traditional medicine in Malaysia. Exp. Parasitol. 2018, 194, 67–78. [Google Scholar] [CrossRef]
- Gong, K.-K.; Li, P.-L.; Qiao, D.; Zhang, X.-W.; Chu, M.-J.; Qin, G.-F.; Tang, X.-L.; Li, G.-Q. Cytotoxic and antiviral triterpenoids from the mangrove plant Sonneratia paracaseolaris. Molecules 2017, 22, 1319. [Google Scholar] [CrossRef]
- Bang, S.; Ha, T.K.Q.; Lee, C.; Li, W.; Oh, W.-K.; Shim, S.H. Antiviral activities of compounds from aerial parts of Salvia plebeia R. Br. J. Ethnopharmacol. 2016, 192, 398–405. [Google Scholar] [CrossRef]
- Peng, M.-H.; Dai, W.-P.; Liu, S.-J.; Yu, L.-W.; Wu, Y.-N.; Liu, R.; Chen, X.-L.; Lai, X.-P.; Li, X.; Zhao, Z.-X.; et al. Bioactive glycosides from the roots ofIlex asprella. Pharm. Biol. 2016, 54, 2127–2134. [Google Scholar] [CrossRef]
- Hu, C.-L.; Xiong, J.; Li, J.-Y.; Gao, L.-X.; Wang, W.-X.; Cheng, K.-J.; Yang, G.-X.; Li, J.; Hu, J.-F. ChemInform Abstract: Rare Sesquiterpenoids from the Shed Trunk Barks of the Critically Endangered Plant Abies beshanzuensis and Their Bioactivities. Eur. J. Org. Chem. 2016, 47, 1832–1835. [Google Scholar] [CrossRef]
- Ha, T.K.Q.; Dao, T.T.; Nguyen, N.H.; Kim, J.; Kim, E.; Cho, T.O.; Oh, W.K. Antiviral phenolics from the leaves of Cleistocalyx operculatus. Fitoterapia 2016, 110, 135–141. [Google Scholar] [CrossRef]
- Dao, N.T.; Jang, Y.; Kim, M.; Nguyen, H.H.; Pham, D.Q.; Le Dang, Q.; Van Nguyen, M.; Yun, B.-S.; Pham, Q.M.; Kim, J.-C.; et al. Chemical Constituents and Anti-influenza Viral Activity of the Leaves of Vietnamese Plant Elaeocarpus tonkinensis. Rec. Nat. Prod. 2018, 13, 71–80. [Google Scholar] [CrossRef]
- UNAIDS. UNAIDS Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 15 December 2020).
- Arts, E.J.; Hazuda, D.J. HIV-1 Antiretroviral Drug Therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed]
- Cary, D.C.; Peterlin, B.M. Natural Products and HIV/AIDS. AIDS Res. Hum. Retrovir. 2018, 34, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ticona, L.A.; Bermejo, P.; Guerra, J.A.; Abad, M.J.; Beltran, M.; Lazaro, R.M.; Alcami, J.; Bedoya, L.M. Ethanolic extract of Artemisia campestris subsp. glutinosa (Besser) Batt. inhibits HIV-1 replication in vitro through the activity of terpenes and flavonoids on viral entry and NF-κB pathway. J. Ethnopharmacol. 2020, 263, 113163. [Google Scholar]
- Tietjen, I.; Gatonye, T.; Ngwenya, B.N.; Namushe, A.; Simonambanga, S.; Muzila, M.; Mwimanzi, P.; Xiao, J.; Fedida, D.; Brumme, Z.; et al. Croton megalobotrys Müll Arg. and Vitex doniana (Sweet): Traditional medicinal plants in a three-step treatment regimen that inhibit in vitro replication of HIV-1. J. Ethnopharmacol. 2016, 191, 331–340. [Google Scholar] [CrossRef]
- Sanna, G.; Farci, P.; Busonera, B.; Murgia, G.; La Colla, P.; Giliberti, G. Antiviral properties from plants of the Mediterranean flora. Nat. Prod. Res. 2015, 29, 2065–2070. [Google Scholar] [CrossRef]
- Gurrapu, S.; Mamidala, E. In vitro HIV-1 reverse transcriptase inhibition by alkaloids isolated from leaves of eclipta alba. Int. Res. J. Pharm. 2018, 9, 66–70. [Google Scholar] [CrossRef]
- Liu, O.; Li, W.; Huang, L.; Asada, Y.; Morris-Natschke, S.L.; Chen, C.; Lee, K.; Koike, K. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenane esters from Euphorbia kansui. Eur. J. Med. Chem. 2018, 156, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Paul, K.S. Phytochemistry and medicinal value of Harad (Terminalia chebula Retz.) the ‘king of medicinal plants. Pharma Chem. 2018, 10, 186–195. [Google Scholar]
- Chen, H.; Zhang, R.; Luo, R.-H.; Yang, L.-M.; Wang, R.-R.; Hao, X.-J.; Zheng, Y.-T. Anti-HIV Activities and Mechanism of 12-O-Tricosanoylphorbol-20-acetate, a Novel Phorbol Ester from Ostodes katharinae. Molecules 2017, 22, 1498. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Wang, X.-Y.; Zhou, Y.-P.; Lu, R.; Chen, C.-H.; Zhang, M.-H.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Lee, K.-H.; Wang, Y.-S. Carbazole Alkaloids from Clausena anisum-olens: Isolation, Characterization, and Anti-HIV Evaluation. Molecules 2020, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Yan, G.; Guo, J.-M.; Liu, Y.-Y.; Li, Y.-J.; Zhao, Y.-Y.; Qiang, L.; Fu, Y.-H. Prenylated Coumarins from the Fruits of Manilkara zapota with Potential Anti-inflammatory Effects and Anti-HIV Activities. J. Agric. Food Chem. 2019, 67, 11942–11947. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Boutet-Mercey, S.; Cachet, X.; De La Torre, E.; Laboureur, L.; Gallard, J.-F.; Retailleau, P.; Brunelle, A.; Dorrestein, P.C.; Costa, J.; et al. Environmentally Friendly Procedure Based on Supercritical Fluid Chromatography and Tandem Mass Spectrometry Molecular Networking for the Discovery of Potent Antiviral Compounds from Euphorbia semiperfoliata. J. Nat. Prod. 2017, 80, 2620–2629. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, J.; Zhang, M.; Liu, X.; Ba, M.; Tao, X.; Yu, L.; Guo, Y.; Dai, J. Oxazole-containing diterpenoids from cell cultures of Salvia miltiorrhiza and their anti-HIV-1 activities. J. Nat. Prod. 2017, 80, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.T.; Serrano, M.L.; Suárez, A.I.; Baptista, J.; Pujol, F.H.; Rangel, H.R. Methoxyflavones from Marcetia taxifolia as HIV-1 Reverse Transcriptase Inhibitors. Nat. Prod. Commun. 2017, 12, 1677–1680. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Liu, K.-L.; Wang, D.-Y.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S.; et al. Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochemistry 2017, 136, 94–100. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Wang, D.-Y.; Liu, K.-L.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S.; et al. Potent Inhibitor of Drug-Resistant HIV-1 Strains Identified from the Medicinal Plant Justicia gendarussa. J. Nat. Prod. 2017, 80, 1798–1807. [Google Scholar] [CrossRef]
- Esposito, F.; Carli, I.; DEL Vecchio, C.; Xu, L.; Corona, A.; Grandi, N.; Piano, D.; Maccioni, E.; Distinto, S.; Parolin, C.; et al. Sennoside A, derived from the traditional chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine 2016, 23, 1383–1391. [Google Scholar] [CrossRef]
- Chao, C.-H.; Cheng, J.-C.; Shen, D.-Y.; Huang, H.-C.; Wu, Y.-C.; Wu, T.-S. 13-Methyl-3,4-seco-ent-podocarpanes, rare C18-diterpenoids from the roots of Flueggea virosa. RSC Adv. 2016, 6, 34708–34714. [Google Scholar] [CrossRef]
- Yan, H.; Ba, M.-Y.; Li, X.-H.; Guo, J.-M.; Qin, X.-J.; He, L.; Zhang, Z.-Q.; Guo, Y.; Liu, H.-Y. Lindenane sesquiterpenoid dimers from Chloranthus japonicus inhibit HIV-1 and HCV replication. Fitoterapia 2016, 115, 64–68. [Google Scholar] [CrossRef]
- Win, N.N.; Ito, T.; Matsui, T.; Aimaiti, S.; Kodama, T.; Ngwe, H.; Okamota, Y.; Tanaka, M.; Asakawa, Y.; Abe, I.; et al. Isopi-marane diterpenoids from Kaempferia pulchrarhizomes collected in Myanmar and their Vpr inhibitory activity. Bioorg. Med. Chem. Lett. 2016, 26, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Olivon, F.; Palenzuela, H.; Girard-Valenciennes, E.; Neyts, J.; Pannecouque, C.; Roussi, F.; Grondin, I.; Leyssen, P.; Litaudon, M. Antiviral Activity of Flexibilane and Tigliane Diterpenoids from Stillingia lineata. J. Nat. Prod. 2015, 78, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Tamayose, C.I.; Torres, P.B.; Roque, N.; Ferreira, M.J.P. HIV-1 reverse transcriptase inhibitory activity of flavones and chlorogenic acid derivatives from Moquiniastrum floribundum (Asteraceae). S. Afr. J. Bot. 2019, 123, 142–146. [Google Scholar] [CrossRef]
- CDC. About Dengue: What You Need to Know. Available online: https://www.cdc.gov/dengue/about/index.html (accessed on 15 December 2020).
- CDC. Dengue Vaccine. Available online: https://www.cdc.gov/dengue/prevention/dengue-vaccine.html (accessed on 15 December 2020).
- Dighe, S.N.; Ekwudu, O.; Dua, K.; Chellappan, D.K.; Katavic, P.L.; Collet, T.A. Recent update on anti-dengue drug discovery. Eur. J. Med. Chem. 2019, 176, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Angelina, M.; Hanafi, M.; Suyatna, F.D.; Dewi, B.E. Drug of Action Cassia Alata Leaves Extract as Antiviral to Dengue Virus Serotype-2 In vitro. Pharmacogn. J. 2020, 12, 864. [Google Scholar] [CrossRef]
- CDC. West Nile Virus. Available online: https://www.cdc.gov/westnile/index.html (accessed on 15 December 2020).
- Acharya, D.; Bai, F. An Overview of Current Approaches toward the Treatment and Prevention of West Nile Virus Infection. Methods Mol. Biol. 2016, 1435, 249–291. [Google Scholar]
- Abubakr, M.; Mandal, S.C.; Banerjee, S. Natural Compounds against Flaviviral Infections. Nat. Prod. Commun. 2013, 8, 1487–1492. [Google Scholar] [CrossRef]
- CDC. Chikungunya Virus. Available online: https://www.cdc.gov/chikungunya/index.html (accessed on 15 December 2020).
- Pérez-Pérez, M.-J.; Delang, L.; Ng, L.F.P.; Priego, E.-M. Chikungunya virus drug discovery: Still a long way to go? Expert Opin. Drug Discov. 2019, 14, 855–866. [Google Scholar] [CrossRef]
- Panya, A.; Yongpitakwattana, P.; Budchart, P.; Sawasdee, N.; Krobthong, S.; Paemanee, A.; Roytrakul, S.; Rattanabunyong, S.; Choowongkomon, K.; Yenchitsomanus, P.-T. Novel bioactive peptides demonstrating anti-dengue virus activity isolated from the Asian medicinal plant Acacia Catechu. Chem. Biol. Drug Des. 2019, 93, 100–109. [Google Scholar] [CrossRef]
- Leite, F.C.; Mello, C.S.; Fialho, L.G.; Marinho, C.F.; Lima, A.L.A.; Filho, J.M.B.; Kubelka, C.F.; Piuvezam, M.R. Cissampelos sympodialish as anti-viral effect inhibiting denguenon-structural viral protein-1 and pro-inflammatory mediators. Rev. Bras. Farmacogn. 2016, 26, 502–506. [Google Scholar] [CrossRef][Green Version]
- Panraksa, P.; Ramphan, S.; Khongwichit, S.; Smith, D.R. Activity of andrographolide against dengue virus. Antivir. Res. 2017, 139, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Maryam, M.; Te, K.K.; Wong, F.C.; Chai, T.T.; Low, G.K.K.; Gan, S.C.; Chee, H.Y. Antiviral activity of traditional Chinese medicinal plants Dryopteris crassirhizoma and Morus alba against dengue virus. J. Integr. Agric. 2020, 19, 1085–1096. [Google Scholar] [CrossRef]
- Nothias-Scaglia, L.F.; Dumontet, V.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. LC-MS2-Based derep-lication of Euphorbia extracts with anti-Chikungunya virus activity. Fitoterapia 2015, 105, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.K.; Shyamala, G.; Paulpandi, M.; Narayanasamy, A.; Siram, K.; Karuppaiah, A.; Sankar, V. Development and Characterization of Phytoniosome Nano Vesicle Loaded with Aqueous Leaf Extracts of Justicia adhatoda and Psidium guajoava Against Dengue Virus (DEN-2). J. Clust. Sci. 2021, 32, 297–304. [Google Scholar] [CrossRef]
- Zakaria, I.I.; Salin, N.H.; Amanah, A.; Othman, S.; Khairuddin, F.; Khawory, M.H.; Wahab, R.A.; Rahaman, M.R.A.; Chern, P.P.; Johari, N.A.; et al. Potential anti-viral compounds from Malaysian Plant Natural Product Repository and Data-base (MyNature50000) for DENV2. Biotechnol. Biotechnol. Equip. 2019, 33, 379–389. [Google Scholar] [CrossRef]
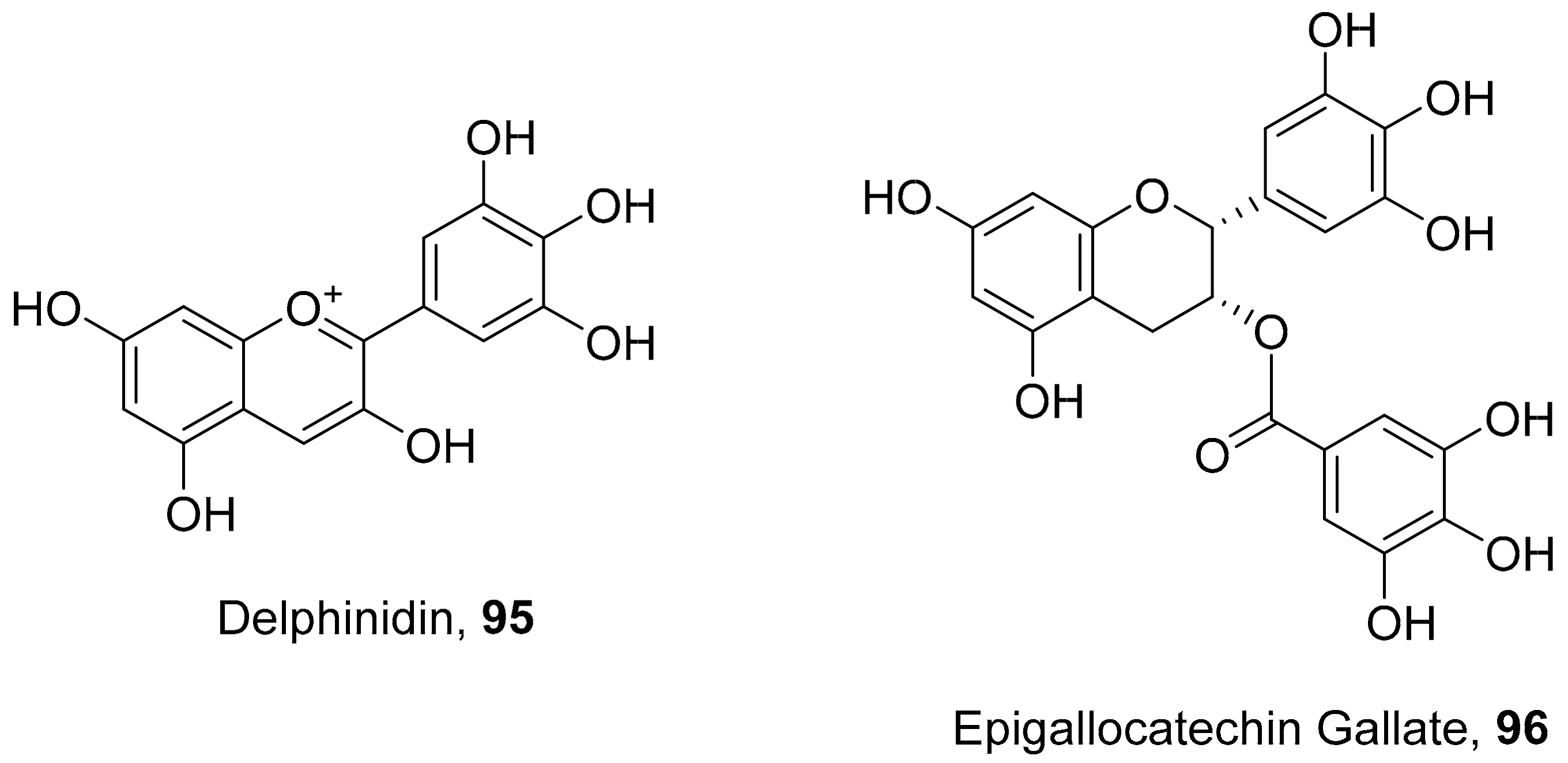
- Vázquez-Calvo, Á.; de Oya, N.J.; Martin-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Yuen, P.T.; Sevan, D.H.; Naphak, M.; Andrei, I.S.; Glen, M.B.; Paul, R.Y.; Daniel, W.; Craig, M.W. Stachyonic Acid: A Dengue Virus Inhibitor from Basilicum polystachyon. Chem. Eur. J. 2019, 25, 5664–5667. [Google Scholar]
- Gómez-Calderónet, C.; Mesa-Castro, C.; Robledo, S.; Gómez, S.; Bolivar-Avila, S.; Diaz-Caastillo, F.; Martinez-Gutierrez, M. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymose on Dengue and Chickugunya virus infections. BMC Complement. Altern. Med. 2017, 17, 57. [Google Scholar]
- Qian, X.-J.; Jin, Y.-S.; Chen, H.-S.; Xu, Q.-Q.; Ren, H.; Zhu, S.-Y.; Tang, H.-L.; Wang, Y.; Zhao, P.; Qi, Z.-T.; et al. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J. Gen. Virol. 2016, 97, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Sanna, G.; Madeddu, S.; Giliberti, G.; Nikoletta, G.N.; Cottiglia, F.; Caboni, P.; De Logu, A.; Agus, E. Limonoids from Melia azedarach Fruits as Inhibitors of Flaviviruses and Mycobacterium tuberculosis. PLoS ONE 2015, 10, e0141272. [Google Scholar] [CrossRef] [PubMed]
- Techer, S.; Girard-Valenciennes, E.; Retailleau, P.; Neyts, J.; Gueritte, F.; Leyssen, P.; Litaudon, M.; Smadja, J.; Grondin, I. Tonantzitlolones from Stillingia lineata ssp. lineata as potential inhibitors of chikungunya virus. Phytochem. Lett. 2015, 12, 313–319. [Google Scholar] [CrossRef]
- Arduino, P.G.; Porter, S.R. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features. J. Oral Pathol. Med. 2007, 37, 107–121. [Google Scholar] [CrossRef]
- WHO. Herpes Simplex Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 15 December 2020).
- CDC. Genital herpes-CDC Fact Sheet (Detailed). Available online: https://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm (accessed on 6 October 2021).
- CDC Genital Herpes Treatment and Care. Available online: https://www.cdc.gov/std/herpes/treatment.htm (accessed on 6 October 2021).
- Itzhaki, R.F.; Wozniak, M.A. Herpes simplex virus type 1, apolipoprotein E, and cholesterol: A dangerous liaison in Alzheimer’s disease and other disorders. Prog. Lipid Res. 2006, 45, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmova-Ilieva, N.; Jacquet, R.; Deffieux, D.; Pouységu, L.; Sylla, T.; Chassaing, S.; Nikolova, I.; Quideau, S.; Galabov, A.S. Anti-Herpes Simplex Virus Type 1 Activity of Specially Selected Groups of Tannins. Drug Res. 2019, 69, 374-373. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Jacquet, R.; Quideau, S.; Galabov, A.S. Ellagitannins as synergists of ACV on the replication of ACV-resistant strains of HSV 1 and 2. Antivir. Res. 2014, 110, 104–114. [Google Scholar] [CrossRef]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef]
- Dias, M.M.; Zuza, O.; Riani, L.R.; Faria-Pinto, P.; Pinto, P.L.S.; Silva, M.P.; de Moraes, J.; Ataíde, A.C.Z.; Silva, F.D.O.; Cecílio, A.B.; et al. In vitro schistosomicidal and antiviral activities of Arctium lappa L. (Asteraceae) against Schistosoma mansoni and Herpes simplex virus-1. Biomed. Pharmacother. 2017, 94, 489–498. [Google Scholar] [CrossRef]
- Cho, W.-K.; Weeratunga, P.; Lee, B.-H.; Park, J.-S.; Kim, C.-J.; Ma, J.Y.; Lee, J.-S. Epimedium koreanum Nakai Displays Broad Spectrum of Antiviral Activity in Vitro and in Vivo by Inducing Cellular Antiviral State. Viruses 2015, 7, 352–377. [Google Scholar] [CrossRef]
- Okba, M.M.; Gedaily, R.A.E.; Ashour, R.M. UPLC-PDA-ESI-qTOF-MS profiling and potent anti-HSV-II activity of Eucalyptus sideroxylon leaves. J. Chromatogr. B 2017, 1068–1069, 335–342. [Google Scholar] [CrossRef]
- Bús, C.; Kúsz, N.; Jakab, G.; Tahaei, S.A.S.; Zupkó, I.; Endrész, V.; Bogdanov, A.; Burián, K.; Csupor-Löffler, B.; Hohmann, J.; et al. Phenanthrenes from Juncus Compressus Jacq. with Promising Antiproliferative and Anti-HSV-2 Activities. Molecules 2018, 23, 2085. [Google Scholar] [CrossRef]
- Houston, D.M.J.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE 2017, 12, e0179291. [Google Scholar]
- Duman, R.; Hasan, H.; Dogan, H.H.; Dinc, M.; Tuncer, P. Cytotoxic and antiviral activity of Ribes Uva Crispa Linn. and Ribes Multflorum kit. Ex romer and schultes extracts. Int. J. Pharm. Sci. Res. 2018, 9, 1779–1787. [Google Scholar]
- Al-Megrin, W.A.; Alsadhan, N.A.; Metwally, D.M.; Al-Talhi, R.A.; El-Khadragy, M.F.; Abdel-Hafez, L.J.M. Potential antiviral agents of Rosmarinus officinalis extract against herpes viruses 1 and 2. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Twilley, D.; Langhansová, L.; Palaniswamy, D.; Lall, N. Evaluation of traditionally used medicinal plants for anticancer, antioxidant, anti-inflammatory and anti-viral (HPV-1) activity. S. Afr. J. Bot. 2017, 112, 494–500. [Google Scholar] [CrossRef]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement. Altern. Med. 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Boff, L.; Silva, I.T.; Argenta, D.F.; Farias, L.M.; Alvarenga, L.F.; Pádua, R.M.; Braga, F.C.; Leite, J.P.V.; Kratz, J.M.; Simões, C.M.O. Strychnos pseudoquina A. St. Hil.: A Brazilian medicinal plant with promising in vitro antiherpes activity. J. Appl. Microbiol. 2016, 121, 1519–1529. [Google Scholar] [CrossRef]
- Kerl, T.; Berger, F.; Schmalz, H.-G. Total Synthesis of the Antiviral Natural Product Houttuynoid B. Chem. Eur. J. 2016, 22, 2935–2938. [Google Scholar] [CrossRef]
- Goswami, D.; Mahapatra, A.D.; Banerjee, S.; Kar, A.; Ojha, D.; Mukherjee, P.K.; Chattopadhyay, D. Boswellia serrata oleo-gun-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-κB and p38 MAP kinase signaling. Phytomedicine 2018, 51, 94–103. [Google Scholar] [CrossRef]
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem. Toxicol. 2017, 109, 1026–1031. [Google Scholar] [CrossRef]
- Liao, H.-B.; Huang, G.-H.; Yu, M.-H.; Lei, C.; Hou, A.-J. Five Pairs of Meroterpenoid Enantiomers from Rhododendron capi-tatum. J. Org. Chem. 2017, 82, 1632–1637. [Google Scholar] [CrossRef]
- Su, F.; Zhao, Z.; Ma, S.; Wang, R.; Li, Y.; Liu, Y.; Li, Y.; Li, L.; Qu, J.; Yu, S. Cnidimonins A–C, Three Types of Hybrid Dimer from Cnidium monnieri: Structural Elucidation and Semisynthesis. Org. Lett. 2017, 19, 4920–4923. [Google Scholar] [CrossRef]
- Ürményi, F.G.G.; Saraiva, G.D.N.; Casanova, L.M.; Matos, A.D.S.; Camargo, L.M.D.M.; Romanos, M.T.V.; Costa, S.S. Anti-HSV-1 and HSV-2 Flavonoids and a New Kaempferol Triglycoside from the Medicinal Plant Kalanchoe daigremontiana. Chem. Biodivers. 2016, 13, 1707–1714. [Google Scholar] [CrossRef]
- Ma, F.; Shen, W.; Zhang, X.; Li, M.; Wang, Y.; Zou, Y.; Li, Y.; Wang, H. Anti-HSV Activity of Kuwanon X from Mulberry Leaves with Genes Expression Inhibitory and HSV-1 Induced NF-κB Deactivated Properties. Biol. Pharm. Bull. 2016, 39, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.; Prince, D.; Lo, C.Y.; Lee, L.H.; Chu, T.C. Antiviral activity of theaflavin digallate against herpes simplex virus type 1. Antivir. Res. 2015, 118, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Cryer, M.; Lane, K.; Greer, M.; Cates, R.; Burt, S.; Andrus, M.; Zou, J.; Rogers, P.; Hansen, M.D.H.; Burgado, J.; et al. Isolation and identification of compounds from Kalanchoe pinnata having human alphaherpesvirus and vaccinia virus antiviral activity. Pharm. Biol. 2017, 55, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- CDC. Global Viral Hapatitus: Millions of People are Affected. Available online: https://www.cdc.gov/hepatitis/global/index.htm (accessed on 15 December 2020).
- WHO. WHO Guidelines on Hepatitis B and C Testing; World Health Organization: Geneva, Switzerland, 2017; pp. 1–32. [Google Scholar]
- CDC. Viral Hepatitis. Available online: https://www.cdc.gov/hepatitis/abc/index.htm (accessed on 15 December 2020).
- Arbab, A.H.; Parvez, M.K.; Al-Dosari, M.S.; Al-Rehaily, A.J. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp. Ther. Med. 2017, 14, 626–634. [Google Scholar] [CrossRef]
- Tumewu, L.; Apryani, E.; Santi, M.R.; Wahyuni, T.S.; Permanasari, A.A.; Adianti, M.; Aoki, C.; Widyawaruyanti, A.; Hafid, A.F.; Lusida, M.I.; et al. AntiHepatitis C Virus Activity of Alectryon serratus Leaves Extract. Procedia Chem. 2016, 18, 169–173. [Google Scholar] [CrossRef][Green Version]
- Bose, M.; Kamra, M.; Mullick, R.; Bhattacharya, S.; Das, S.; Karande, A.A. A plant-derived dehydrorotenoid: A new inhibitor of hepatitis C virus entry. FEBS Lett. 2017, 591, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, T.S.; Permatasari, A.A.; Widiandani, T.; Fuad, A.; Widyawaruyanti, A.; Aoki-Utsubo, C.; Hotta, H. Antiviral Activities of Curcuma Genus against Hepatitis C Virus. Nat. Prod. Commun. 2018, 13, 1579. [Google Scholar] [CrossRef]
- Ghanem, K.Z.; Mahran, M.Z.; Ramadan, M.M.; Ghanem, H.Z.; Fadel, M.; Mahmoud, M.H. A comparative study on flavour components and therapeutic properties of unfermented and fermented defatted soybean meal extract. Sci. Rep. 2020, 10, 5998. [Google Scholar] [CrossRef]
- Sahli, R.; Rivière, C.; Neut, C.; Bero, J.; Sahuc, M.-E.; Smaoui, A.; Beaufay, C.; Roumy, V.; Hennebelle, T.; Rouillé, Y.; et al. An ecological approach to discover new bioactive extracts and products: The case of extremophile plants. J. Pharm. Pharmacol. 2017, 69, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Matsuhisa, K.; Yamane, S.; Okamoto, T.; Watari, A.; Kondoh, M.; Matsuura, Y.; Yagi, K. Anti-HCV effect of Lentinula edodes mycelia solid culture extracts and low-molecular-weight lignin. Biochem. Biophys. Res. Commun. 2015, 462, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-C.; Chang, S.-P.; Lin, L.-C.; Li, C.-L.; Richardson, C.D.; Lin, C.-C.; Lin, L.-T. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antivir. Res. 2015, 118, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Aarthi, C.; Babu, P.B.R. Studies On in vitro Culture and Secondary Metabolite Production of Phyllanthus reticulates Poir. J. Pharm. Sci. Res. 2017, 9, 307–313. [Google Scholar]
- Ezzikouri, S.; Nishimura, T.; Kohara, M.; Benjelloun, S.; Kino, Y.; Inoue, K.; Matsumori, A.; Tsukiyama-Kohara, K. Inhibitory effects of Pycnogenol® on hepatitis C virus replication. Antivir. Res. 2015, 113, 93–102. [Google Scholar] [CrossRef]
- Rehman, S.; Ijaz, B.; Fatima, N.; Muhammad, S.A.; Riazuddin, S. Therapeutic potential of Taraxacum officinale against HCVNS5B polymerase: In-vitro and In silico study. Biomed. Pharmacother. 2016, 83, 881–891. [Google Scholar] [CrossRef]
- Ganta, K.K.; Mandal, A.; Debnath, S.; Hazra, B.; Chaubey, B. Anti-HCV Activity from Semi-purified Methanolic Root Extracts ofValeriana wallichii. Phytother. Res. 2017, 31, 433–440. [Google Scholar] [CrossRef]
- Gu, C.; Yin, A.P.; Yuan, H.Y.; Yang, K.; Luo, J.; Zhan, Y.J.; Yang, C.R.; Zuo, D.M.; Li, H.Z.; Xu, M. New anti-HBV nor-bisabolane sesquiterpenes from Phyllantus acidus. Fitoterapia 2019, 137, 104151. [Google Scholar] [CrossRef]
- Lee, S.; Mailar, K.; Kim, M.I.; Park, M.; Kim, J.; Min, D.-H.; Heo, T.-H.; Bae, S.K.; Choi, W.; Lee, C. Plant-Derived Purification, Chemical Synthesis, and In Vitro/In Vivo Evaluation of a Resveratrol Dimer, Viniferin, as an HCV Replication Inhibitor. Viruses 2019, 11, 890. [Google Scholar] [CrossRef]
- Li, S.-F.; Jiao, Y.-Y.; Zhang, Z.-Q.; Chao, J.-B.; Jia, J.; Shi, X.-L.; Zhang, L.-W. Diterpenes from buds of Wikstroemia chamaedaphne showing anti-hepatitis B virus activities. Phytochemistry 2018, 151, 17–25. [Google Scholar] [CrossRef]
- Subaiea, G.M.; Aljofan, M.; Devadasu, V.R.; Alshammari, T.M. Acute toxicity testing of newly discovered potential antihep-atitis B virus agents of plant origin. Asian J. Pharm. Clin. Res. 2017, 10, 210–213. [Google Scholar]
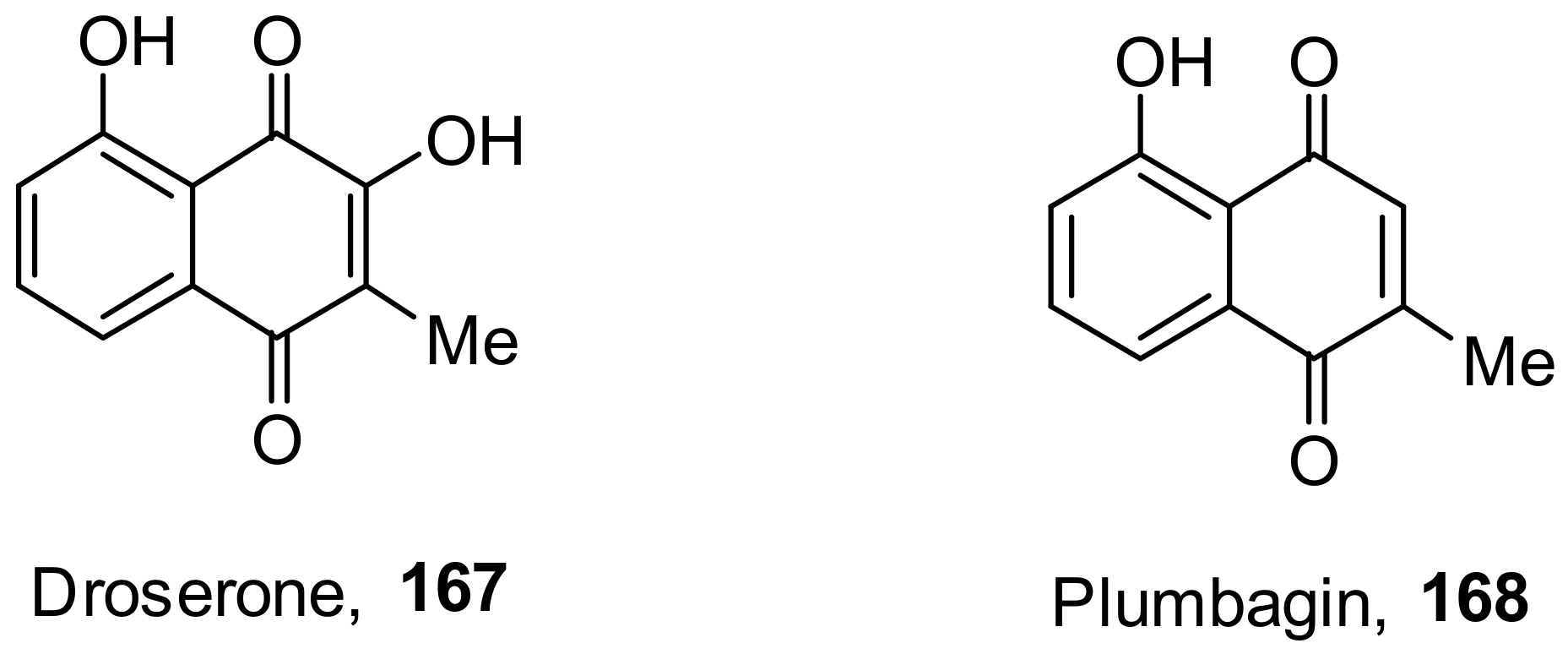
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J. Plumbagin, a Plant-Derived Compound, Exhibits Antifungal Combinatory Effect with Amphotericin B against Candida albicans Clinical Isolates and Anti-hepatitis C Virus Activity. Phytother. Res. 2016, 30, 1487–1492. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Koutsoudakis, G.; Saludes, V.; Pérez-Vilaró, G.; Turpeinen, A.; Matilla, S.; Pirttilä, A.M.; Fontaine-Vive, F.; Me-hiri, M.; Meyerhans, A.; et al. Pan-genotypic Hepatitis C Virus Inhibition by Natural Products Derived from the Wild Egyptian Artichoke. J. Virol. 2016, 90, 1918–1930. [Google Scholar] [CrossRef]
- Xu, J.; Gu, W.; Li, C.; Li, X.; Xing, G.; Li, Y.; Song, Y.; Zheng, W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 2016, 70, 584–591. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.B.; Hou, L.J.; Lv, H.X.; Liu, A.M.; Zeng, P.; Li, A.H. A New Selaginellin from Selaginella moellendorffii In-hibits Hepatitis B Virus Gene Expression and Replication. Chem. Nat. Compd. 2016, 52, 624–629. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Liu, C.-H.; Burnouf, T.; Wang, G.-H.; Chang, S.-P.; Jassey, A.; Tai, C.-J.; Tai, C.-J.; Huang, C.-J.; Richardson, C.D.; et al. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antivir. Res. 2016, 130, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-J.; Tao, H.-M.; Min, Q.-X.; Zhu, Q.-H. Anti-hepatitis B virus activities of friedelolactones from Viola diffusa Ging. Phytomedicine 2015, 22, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Y.; Yu, Y.-J.; Huang, C.-G.; Huang, X.-Z.; Hu, Q.-F.; Yang, G.-Y.; Wang, H.-B.; Zhang, X.-Y.; Li, G.-P. Icetexane Diterpenoids from Perovskia atriplicifolia. Planta Med. 2015, 81, 241–246. [Google Scholar] [CrossRef]
- Jardim, A.C.G.; Igloi, Z.; Shimizu, J.F.; Santos, V.A.F.F.M.; Felippe, L.G.; Mazzeu, B.F.; Amako, Y.; Furlan, M.; Harris, M.; Rahal, P. Natural compounds isolated from Brazilian plants are potent inhibitors of hepatitis C virus replication in vitro. Antivir. Res. 2014, 115, 39–47. [Google Scholar] [CrossRef]
- Liu, J.-F.; Wang, L.; Wang, Y.-F.; Song, X.; Yang, L.-J.; Zhang, Y.-B. Sesquiterpenes from the fruits of Illicium jiadifengpi and their anti-hepatitis B virus activities. Fitoterapia 2015, 104, 41–44. [Google Scholar] [CrossRef]
- Parvez, M.K.; Al-Dosari, M.S.; Alam, P.; Rehman, M.; Alajmi, M.F.; Alqahtani, A.S. The anti-hepatitis B virus therapeutic potential of anthraquinones derived from Aloe vera. Phytother. Res. 2019, 33, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Sun, L.; Zou, S.; Chen, J.; Mao, H.; Zhang, Y.; Liao, N.; Zhang, R. Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate. Molecules 2019, 24, 1835. [Google Scholar] [CrossRef]
- Winer, H.; Ozer, J.; Shemer, Y.; Reichenstein, I.; Eilam-Frenkel, B.; Benharroch, D.; Golan-Goldhirsh, A.; Gopas, J. Nuphar lu-tea extracts exhibit anti-viral activity against the measles virus. Molecules 2020, 25, 1657. [Google Scholar] [CrossRef] [PubMed]
- Lieberherr, C.; Zhang, G.; Grafen, A.; Singethan, K.; Kendl, S.; Vogt, V.; Maier, J.; Bringmann, G.; Schneider-Schaulies, J. The Plant-Derived Naphthoquinone Droserone Inhibits In Vitro Measles Virus Infection. Planta Med. 2016, 83, 232–238. [Google Scholar] [CrossRef]
- Arjin, C.; Pringproa, K.; Hongsibsong, S.; Ruksiriwanich, W.; Seel-Audom, M.; Mekchay, S.; Sringarm, K. In vitro screening antiviral activity of Thai medicinal plants against porcine reproductive and respiratory syndrome virus. BMC Vet. Res. 2020, 16, 102. [Google Scholar] [CrossRef]
- Thabti, I.; Albert, Q.; Philippot, S.; Dupire, F.; Westerhuis, B.; Fontanay, S.; Risler, A.; Kassab, T.; Elfalleh, W.; Aferchichi, A.; et al. Advances on Antiviral Activity of Morus spp. Plant Extracts: Human Coronavirus and Virus-Related Respiratory Tract Infections in the Spotlight. Molecules 2020, 25, 1876. [Google Scholar] [CrossRef] [PubMed]
- Civra, A.; Francese, R.; Sinato, D.; Donalisio, M.; Cagno, V.; Rubiolo, P.; Ceylan, R.; Uysal, A.; Zengin, G.; Lembo, D. In vitro screening for antiviral activity of Turkish plants revealing methanolic extract of Rindera lanata var. lanata active against human rotavirus. BMC Complement. Altern. Med. 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Snene, A.; El Mokni, R.; Jmii, H.; Jlassi, I.; Jaïdane, H.; Falconieri, D.; Piras, A.; Dhaouadi, H.; Porcedda, S.; Hammami, S. In vitro antimicrobial, antioxidant and antiviral activities of the essential oil and various extracts of wild (Daucus virgatus (Poir.) Maire) from Tunisia. Ind. Crop. Prod. 2017, 109, 109–115. [Google Scholar] [CrossRef]
- Fu, Q.; Gao, L.; Fu, X.; Meng, Q.; Lu, Z. Scutellaria baicalensis Inhibits Coxsackievirus B3-Induced Myocarditis Via AKT and p38 Pathways. J. Microbiol. Biotechnol. 2019, 29, 1230–1239. [Google Scholar] [CrossRef]
- Kim, J.W.; Ha, T.-K.-Q.; Cho, H.M.; Kim, E.; Shim, S.H.; Yang, J.-L.; Oh, W.K. Antiviral escin derivatives from the seeds of Aesculus turbinata Blume (Japanese horse chestnut). Bioorg. Med. Chem. Lett. 2017, 27, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gong, X.; Tan, J.Y.; Kang, L.; Li, D.; Vikash; Yang, J.; Du, G. In vitro Antiviral Activity of Rubia cordifolia Aerial Part Extract against Rotavirus. Front. Pharmacol. 2016, 7, 308–318. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Y.; Zhao, X.; Tian, X.; Ning, J.; Zhang, B.; Deng, S.; Li, D.; Ma, X.; Wang, C. Unusual ent-atisane type diterpenoids with 2-oxopropyl skeleton from the roots of Euphorbia ebracteolate and their antiviral activity against human rhinovirus 3 and enterovirus 71. Bioorg. Med. 2018, 81, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.; Wang, J.; Hu, X. A New Triterpenoid from the Bulbs of EW Lilium speciosum var. gloriosoides. Chem. Nat. Compd. 2019, 55, 289–291. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Wang, J.; Hu, X. Flavonoid Glycosides from Bulbs of Lilium speciosum var. gloriosoides and their Po-tential Antiviral Activity against RSV. Chem. Nat. Compd. 2019, 55, 461–464. [Google Scholar] [CrossRef]
- Tang, W.; Li, M.; Liu, Y.; Liang, N.; Yang, Z.; Zhao, Y.; Wu, S.; Lu, S.; Li, Y.; Liu, F. Small molecule inhibits respiratory syncytial virus entry and infection by blocking the interaction of the viral fusion protein with the cell membrane. FASEB J. 2019, 33, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; Fais, A.; Pintus, F.; Santos-Buelga, C.; González-Paramás, A.M.; Piras, V.; Orrù, G.; Mameli, A.; Tramontano, E.; Frau, A. Broad-range potential of Asphodelus microcarpus leaves extract for drug development. BMC Microbiol. 2017, 17, 159. [Google Scholar] [CrossRef]
- Biedenkopf, N.; Lange-Gruenweller, K.; Schulte, F.W.; Weisser, A.; Mueller, C.; Becker, D.; Becker, S.; Hartmann, R.K.; Gruenweller, A. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antivir. Res. 2017, 137, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Kumar, S.; Misra, U.K.; Kalita, J.; Khanna, V.K.; Khan, M.Y. Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem. Int. 2009, 55, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.; Khsirsagar, M.; Kamble, S.; Bandgar, B.; Dhole, N.; Shisode, K.; Chaudhari, A. Antioxidant and Anti-inflammatory Related Activities of Selected Synthetic Chalcones: Structure-Activity Relationship Studies Using Computational Tools. Chem. Pharm. Bull. 2008, 56, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, R.C.; Steel, J.J.; Moon, S.L.; Soltani, E.; Geiss, B.J. Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology 2015, 475, 219–229. [Google Scholar] [CrossRef] [PubMed]
- StarDrop. A Complete Platform for Small Molecule Design, Optimisation and Data Analysis. Available online: https://www.optibrium.com/stardrop/ (accessed on 6 October 2021).
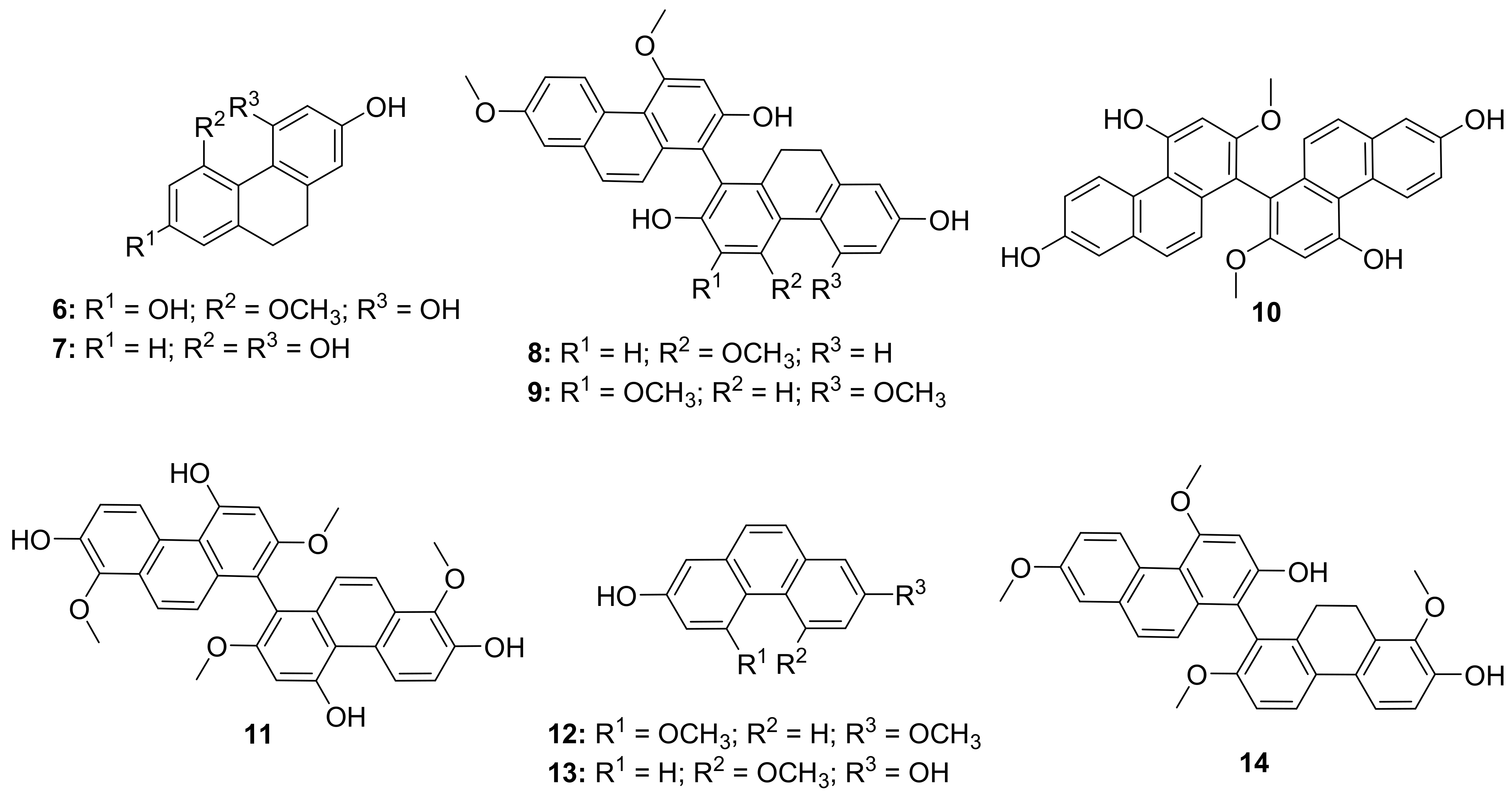
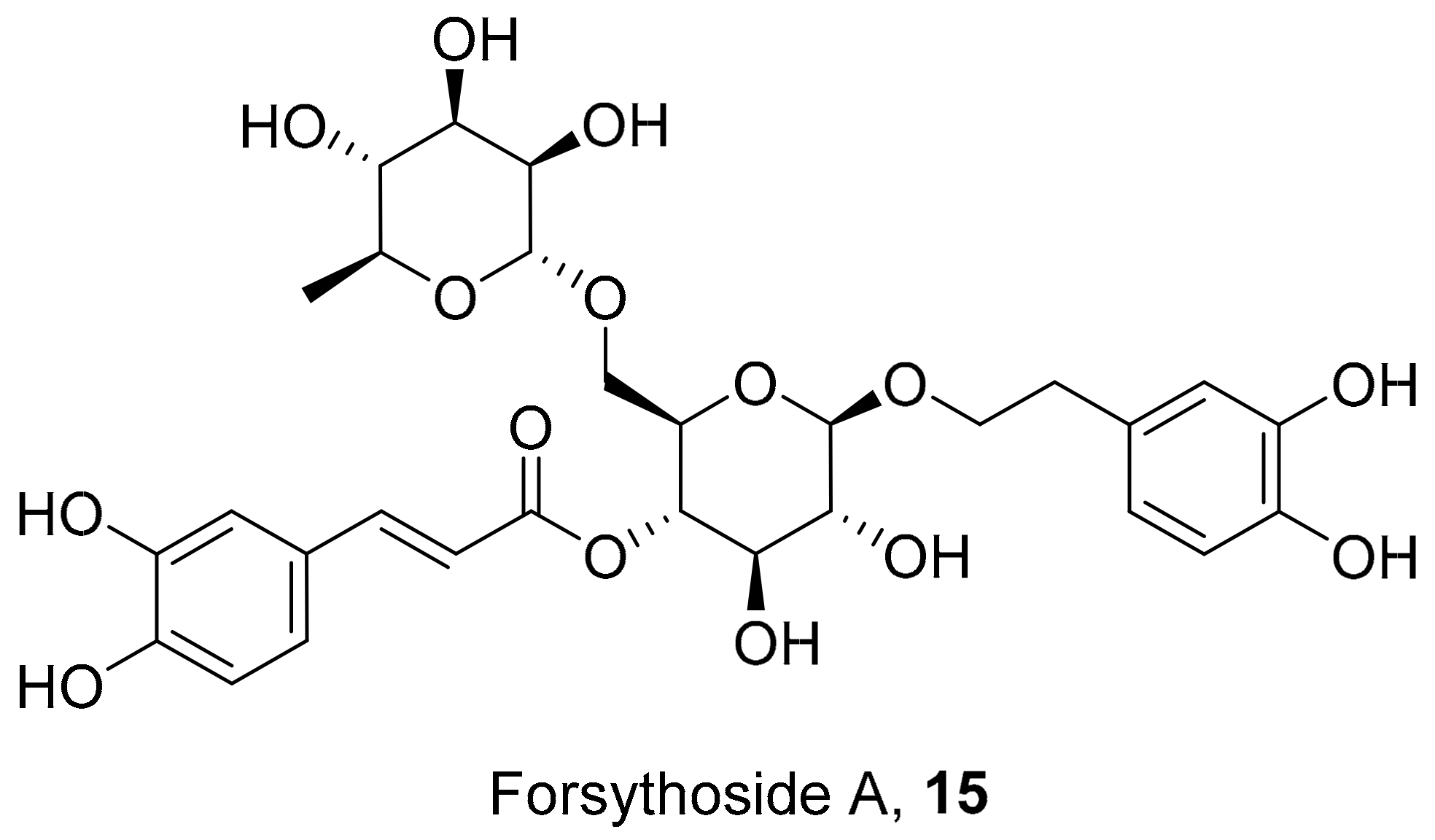
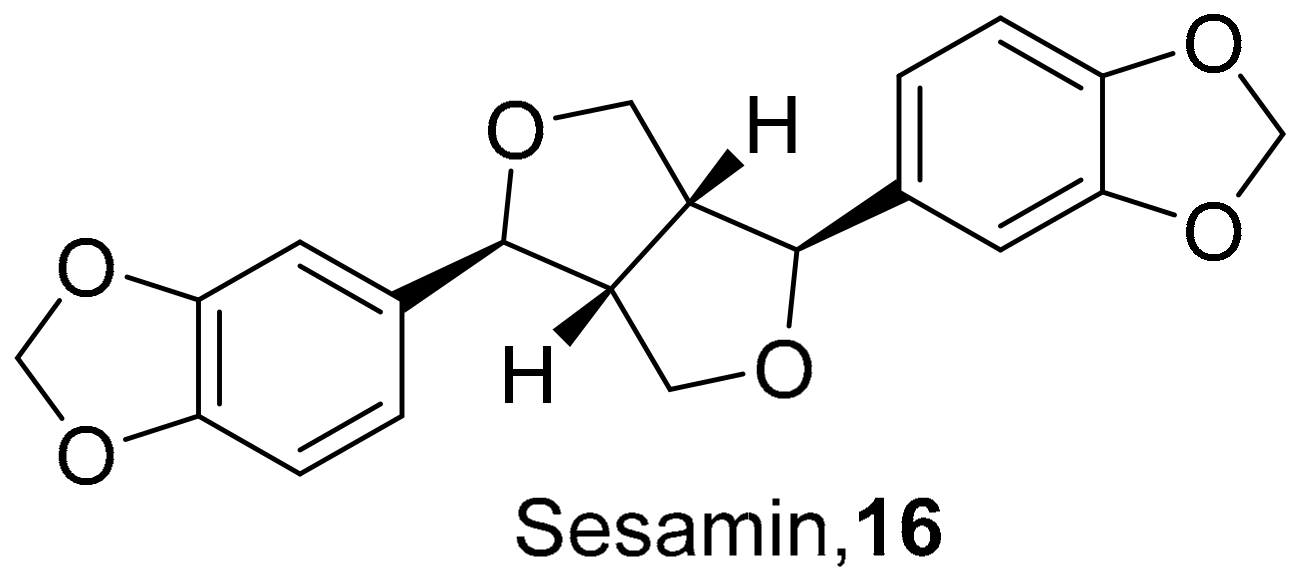
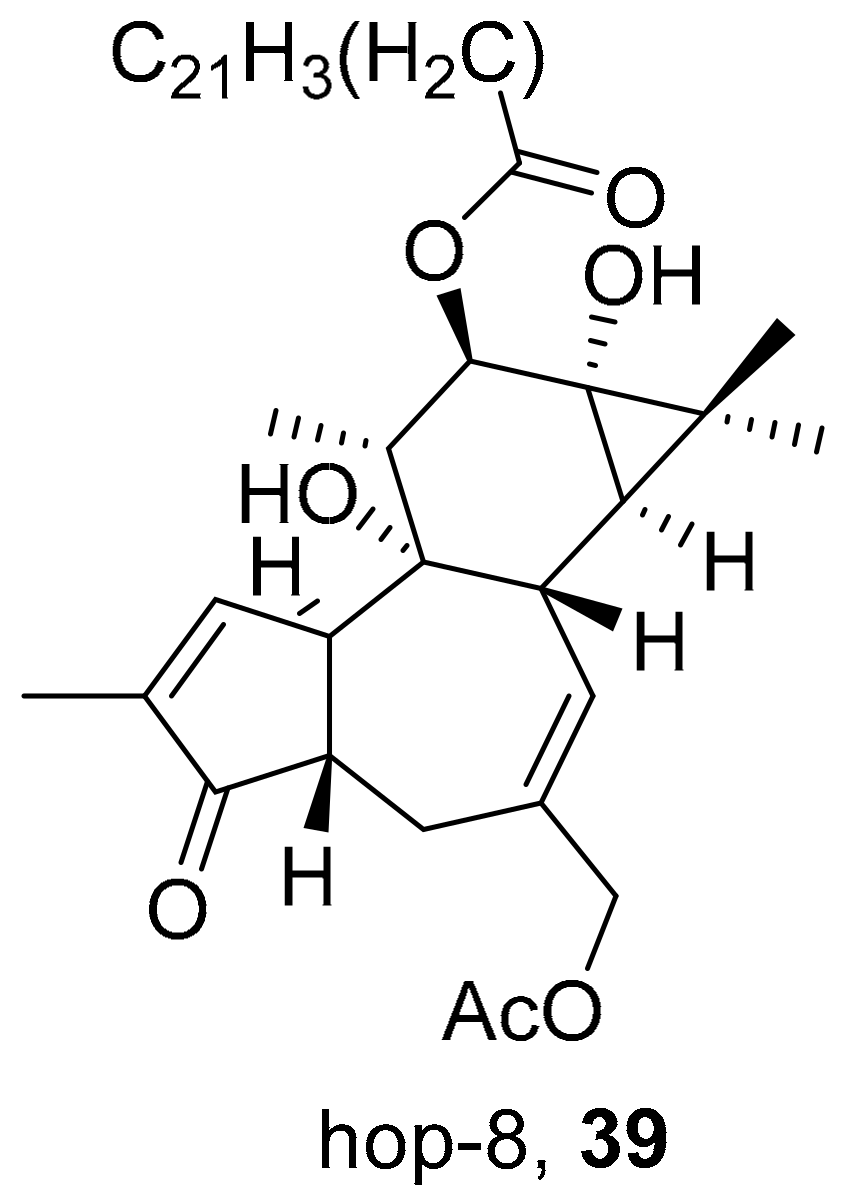
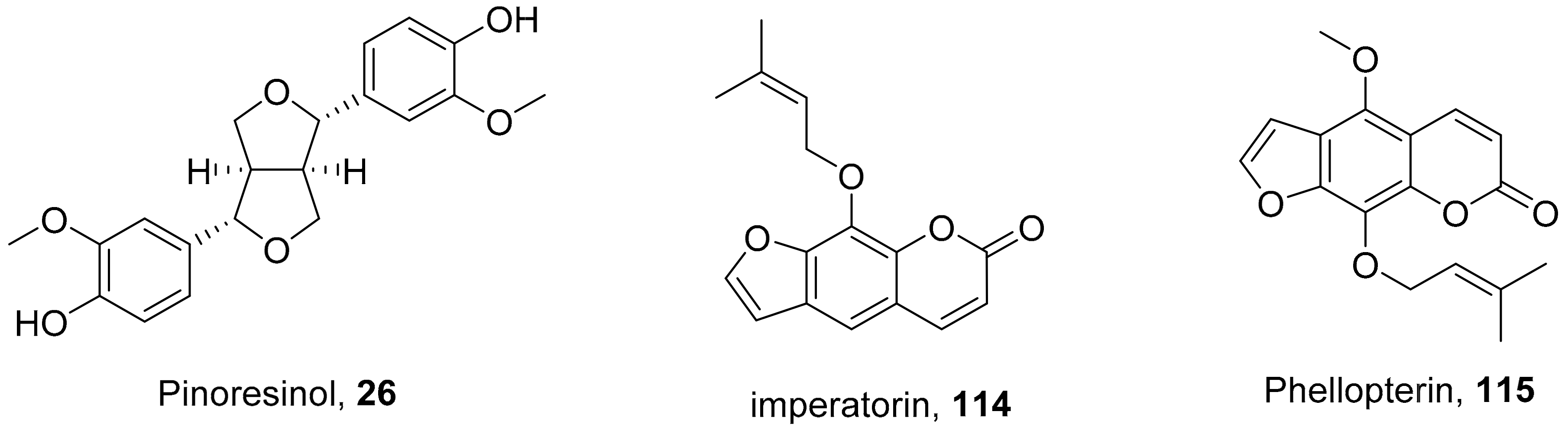
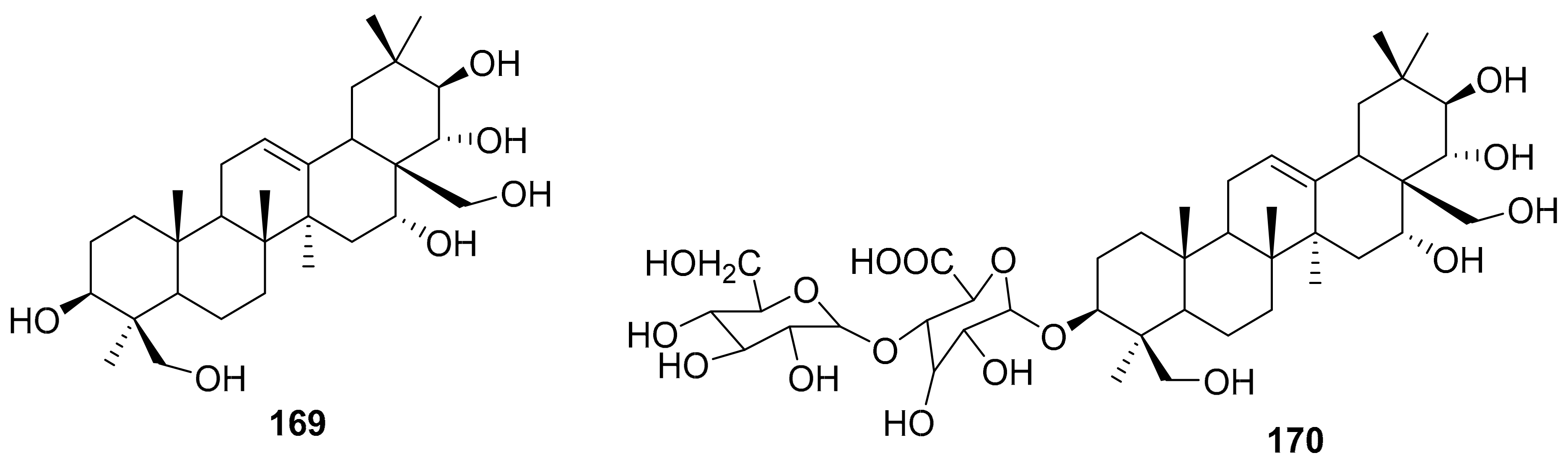
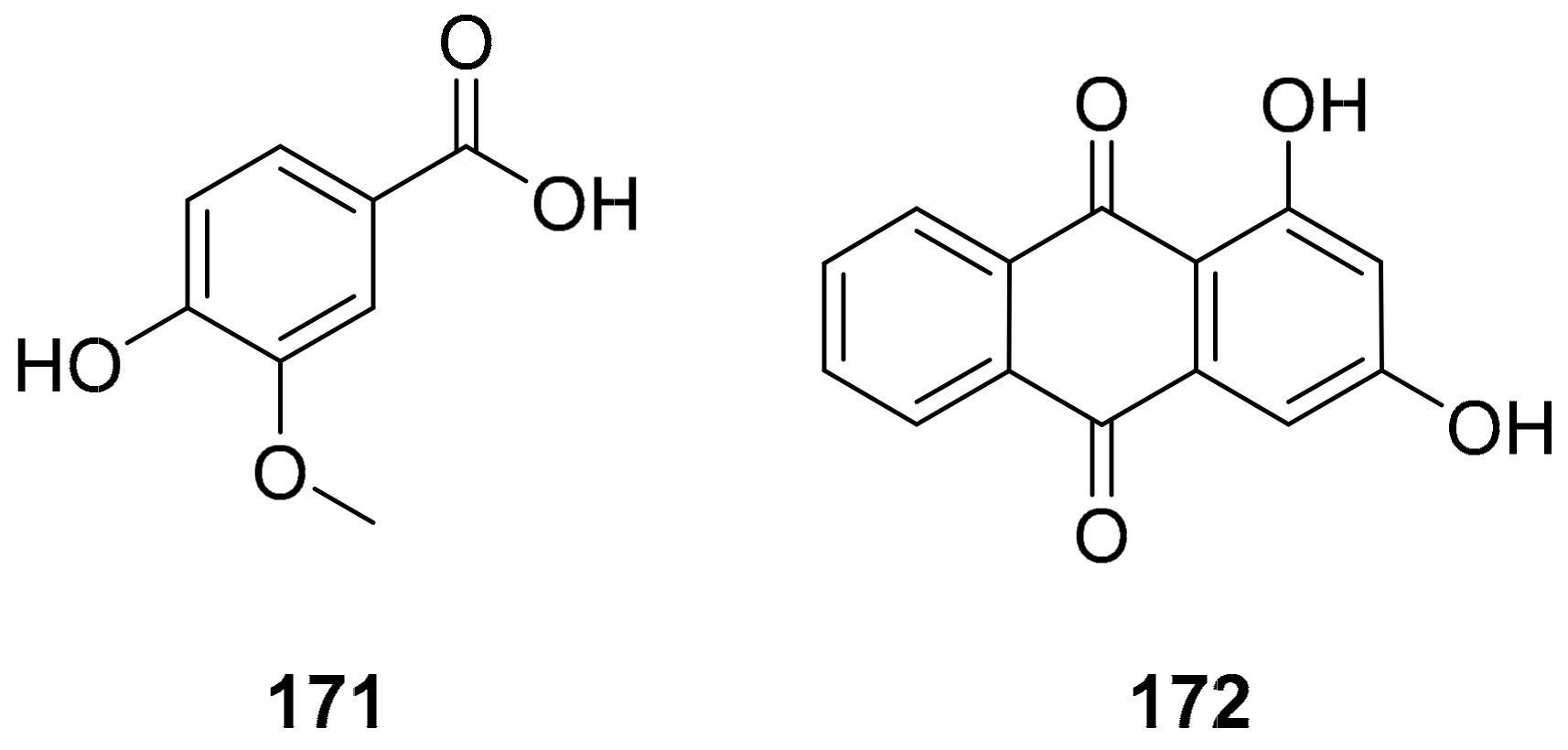

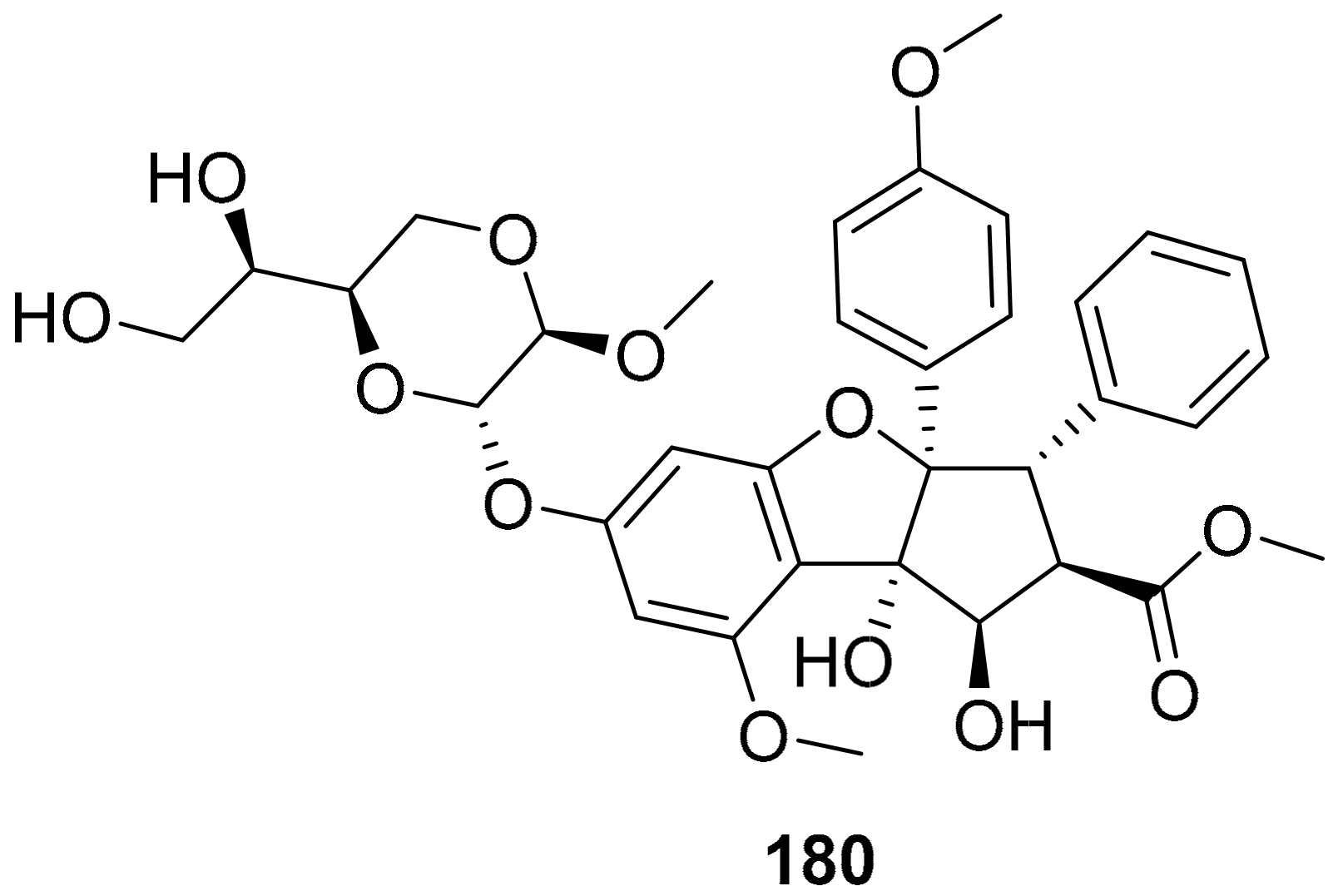
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Allium sativum | Methanolic extract of roots | H1N1 | EC50: 5 mg/mL | [32] |
| 2 | Plumbago indica | Ethanolic extract of roots | H1N1 | EC50: 1 mg/mL | [32] |
| 3 | Arachis hypogaea L. | Peanut skin extracted with hexane | H1N1 | IC50: 1.0–1.5 µg/mL | [33] |
| 4 | Caesalpinia decapetala | Aqueous ethanolic extract of leaves | H1N1 | EC50: 5.7 µg/mL | [34] |
| 5 | Carpesium abrotanoides L. | Dried herbal ethanolic extraction | H1N1 | IC50: 15.9 µM | [35] |
| H3N2 | IC50: 11.6 µM | ||||
| 6 | Cayratia pedata | DMSO extract of leaves | H1N1 | IC50: 65.99 µg/mL | [36] |
| 7 | Cayratia pedata | DMSO extract of stem bark | H1N1 | IC50: 20.50 µg/mL | [36] |
| 8 | Diotacanthus albiflorus | DMSO extract of leaves | H1N1 | IC50: 60.09 µg/mL | [36] |
| 9 | Diotacanthus albiflorus | DMSO extract of stem bark | H1N1 | IC50: 33.98 µg/mL | [36] |
| 10 | Embelia Ribes | Fruits extracted with ethyl acetate | H1N1 | IC50: 0.2 µM | [37] |
| 11 | Hippophae rhamnoides L. | Methanolic extracts of leaves | H1N1 | IC50: 7.2l µg/mL | [38] |
| 12 | Hippophae rhamnoides L. | Ethyl acetate extracts of leaves | H1N1 | IC50: 10.3l µg/mL | [38] |
| 13 | Murraya paniculata L. | Petroleum ether extraction of plant leaves | H5N1 | IC50: 0.15 µg/mL | [39] |
| 14 | Piper longum | Methanolic and chloroform extract from seeds | H1N1 | IC50: 33.43–46.24 µg/mL | [40] |
| 15 | Piper nigrum | Methanolic and chloroform extract from seeds | H1N1 | IC50: 17.47 µg/mL | [40] |
| 16 | Polygonum chinense Linn | Methanolic extract of dried and ground whole plant | H1N1 | EC50: 38.4–55.5 µg/mL | [41] |
| 17 | Poncirus trifoliata | Seeds extracted with ethanol | H1N1 | EC50: 2.51 µg/mL | [42] |
| 18 | Psoralae Semen | Aqueous extract of unknown part | H1N1 | Inhibitory (%): 30 | [43] |
| 19 | Radix isatidis | Hot methanol and ethanol extraction | H1N1 | IC50: 3.34 mg/mL | [44] |
| 20 | Ruta graveolens L. | Petroleum ether extraction of plant leaves | H5N1 | IC50: 7.8 µg/mL | [39] |
| 21 | Strychnos minor | DMSO extract of leaves | H1N1 | IC50: 46.69 µg/mL | [36] |
| 22 | Strychnos minor | DMSO extract of stem bark | H1N1 | IC50: 22.43 µg/mL | [36] |
| 23 | Strychnos nux-vomica | DMSO extract of leaves | H1N1 | IC50: 33.36 µg/mL | [36] |
| 24 | Strychnos nux-vomica | DMSO extract of stem bark | H1N1 | IC50: 23.60 µg/mL | [36] |
| S. No. | Plant Name (Part) | Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Forsythia suspensa (Fruits) | 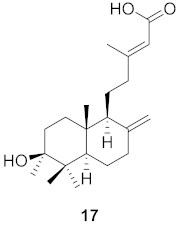 | H1N1 | IC50: 19.9 µM | [50] |
| 2 | Forsythia suspensa (Fruits) |  | H1N1 | IC50: 18.4 µM | [50] |
| 3 | Forsythia suspensa (Fruits) |  | H1N1 | IC50: 26.2 µM | [50] |
| 4 | Forsythia suspensa (Fruits) | 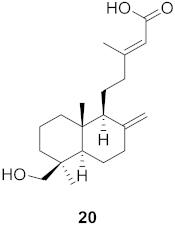 | H1N1 | IC50: 25.7 µM | [50] |
| 5 | Forsythia suspensa (Fruits) |  | H1N1 | IC50: 24.1 µM | [50] |
| 6 | Forsythia suspensa (Fruits) | 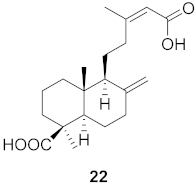 | H1N1 | IC50: 24.9 µM | [50] |
| 7 | Forsythia suspensa (Fruits) | 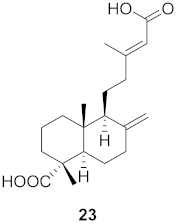 | H1N1 | IC50: 23.5 µM | [50] |
| 8 | Forsythia suspensa (Fruits) | 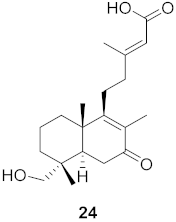 | H1N1 | IC50: 18.6 µM | [50] |
| 9 | Basilicum polystachyon (Whole plant) |  | H1N1 | IC50: 4.1 μM | [51] |
| H3N3 | IC50: 18 μM | ||||
| 10 | Curcuma aeruginosa (Rhizomes) | 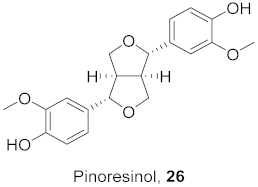 | H1N1 | IC50: 30.4 μg/mL | [52] |
| 11 | Sonneratia paracaseolaris |  | H1N1 | IC50: 28.4 µg/mL | [53] |
| 12 | Salvia plebeian R. Br (Roots) |  | H1N1 | IC50: 16.65–19.83 µM | [54] |
| 13 | Ilex asprella (Roots) | 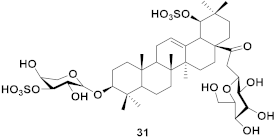 | H1N1 | EC50: 4.1 µM | [55] |
| 14 | Ilex asprella(Roots) |  | H1N1 | EC50: 1.7 µM | [55] |
| 15 | Abies beshanzuensis (Bark) | 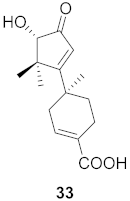 | H3N2 | IC50: 30.8 µg/mL | [56] |
| 16 | Abies beshanzuensis (Bark) |  | H3N2 | IC50: 30.9 µg/mL | [56] |
| 17 | Cleistocalyx operculatus (Leaves) | 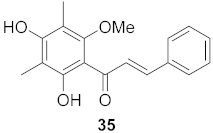 | H1N1 | IC50: 5.07 µM | [57] |
| H9N2 | IC50: 9.34 µM | ||||
| 18 | Cleistocalyx operculatus (Leaves) | 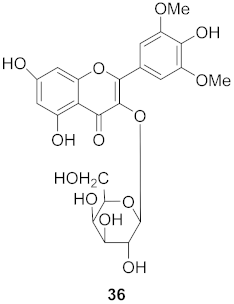 | H1N1 | IC50: 5.07 µM | [57] |
| H9N2 | IC50: 9.34 µM | ||||
| 19 | Elaeocarpus tonkinesis (Leaves and Twigs) | 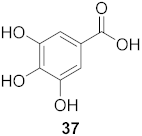 | H1N1 | EC50: 8.1 µg/mL | [58] |
| 20 | Elaeocarpus tonkinesis (Leaves and Twigs) | 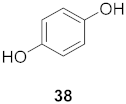 | H1N1 | EC50: 19.7 µg/mL | [58] |
| S. No. | Plant name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Artemisia campestris | Aqueous ethanolic extract of the whole plant | HIV-1 | IC50: 14.62 μg/mL | [62] |
| 2 | Cassia Siberiana | Chloroform methanolic extract of roots | HIV-1 | IC50: 84.8 μg/mL | [63] |
| 3 | Croton megalobotrys | Chloroform methanolic extract of bark | HIV-1 | IC50: 0.05 μg/mL | [63] |
| 4 | Daphne gnidium L. | Ethyl acetate extraction of branches | HIV-1 | EC50: 0.08 μg/mL | [64] |
| 5 | Eclipta alba | Leaves extracted with chloroform | HIV-1 | IC50: 250 μg/mL | [65] |
| 6 | Euphorbia kansui | Methanolic extract of roots | HIV-1 | EC50: 110 ng/mL | [66] |
| 7 | Terminalia chebula | Methanolic/aqueous extract of fruit | HIV-1 | IC50: ≤5 μg/mL | [67] |
| 8 | Vitex doniana | Chloroform methanolic extract of roots | HIV-1 | IC50: 25 μg/mL | [63] |
| S. No. | Plant Name (Part) | Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Clausena anisum-olens (Leaves and twigs) | 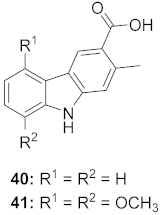 | HIV | 40: EC50: 2.4 μg/mL 41: EC50: 3.7 μg/mL | [69] |
| 2 | Manilkara zapota (Fruit) | 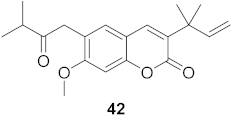 | HIV | EC50: 8.69 μM | [70] |
| 3 | Manilkara zapota (Fruit) | 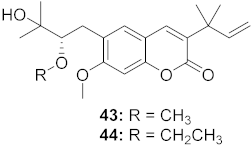 | HIV | 43: EC50: 0.33 μM 44: EC50: 0.42 μM | [70] |
| 4 | Manilkara zapota (Fruit) | 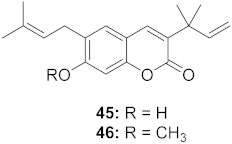 | HIV | 45: EC50: 2.28 μM 46: EC50: 3.49 μM | [70] |
| 5 | Manilkara zapota (Fruit) | 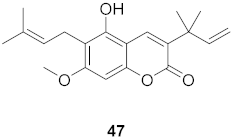 | HIV | EC50: 4.26 μM | [70] |
| 6 | Manilkara zapota (Fruit) | 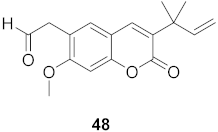 | HIV | EC50: 0.97 μM | [70] |
| 7 | Manilkara zapota (Fruit) | 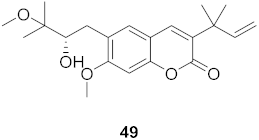 | HIV | EC50: 5.26 μM | [70] |
| 8 | Manilkara zapota (Fruit) | 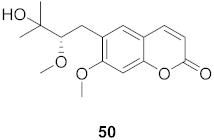 | HIV | EC50: 6.73 μM | [70] |
| 9 | Manilkara zapota (Fruit) |  | HIV | EC50: 0.12 μM | [70] |
| 10 | Euphorbia semiperfoliata (Whole plant) | 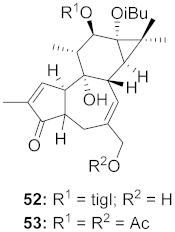 | HIV-1 | 52: EC50: 0.013 μM 53: EC50: 0.054 μM | [71] |
| 11 | Salvia miltiorrhiza Bunge |  | HIV-1 | 54: IC50: 0.03 μM 55: IC50: 1.2 μM | [72] |
| 12 | Marcetia taxifolia (Aerial parts) | 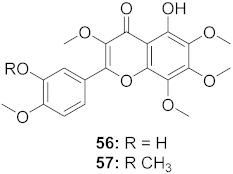 | HIV-1 | 56: IC50: 4.1 μM 57: IC50: 0.4 μM | [73] |
| 13 | Justicia gendarussa (Stem and bark) | 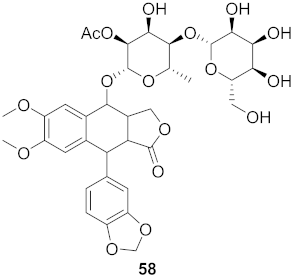 | HIV-1 | IC50: 15–21 nM | [74] |
| 14 | Justicia gendarussa (Root and stem) | 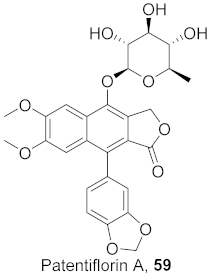 | HIV- 1 | IC50: 26.9 nM | [75] |
| 15 | Rheum palmatum L. and Rheum officinale Baill (Roots) |  | HIV | IC50: 1.9 μM | [76] |
| 16 | Rheum palmatum L. and Rheum officinale Baill (Roots) |  | HIV | IC50: 2.1 μM | [76] |
| 17 | Flueggea virosa (Roots) |  | HIV | 62: EC50: 19.2 μM 63: EC50: 20.5 μM 64: EC50: 40.1 μM | [77] |
| 18 | Flueggea virosa (Roots) | 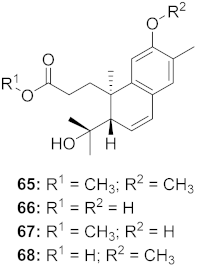 | HIV | 65: EC50: 51.8 μM 66: EC50: >100 μM 67: EC50: 87.8 μM 68: EC50: 7.1 μM | [77] |
| 19 | Flueggea virosa (Roots) | 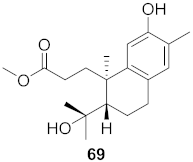 | HIV | EC50: 58.0 μM | [77] |
| 20 | Flueggea virosa (Roots) | 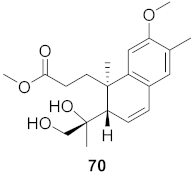 | HIV | EC50: >100 μM | [77] |
| 21 | Flueggea virosa (Roots) | 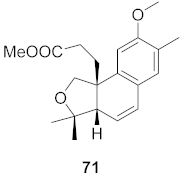 | HIV | EC50: 53.9 μM | [77] |
| 22 | Flueggea virosa (Roots) | 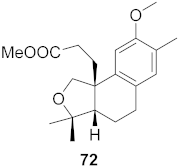 | HIV | EC50: 48.6 μM | [77] |
| 23 | Flueggea virosa (Roots) |  | HIV | 73: EC50: 40.6 μM 74: EC50: >100 μM | [77] |
| 24 | Flueggea virosa (Roots) | 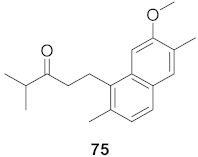 | HIV | EC50: 69.4 μM | [77] |
| 25 | Chloranthus japonicus (Roots) | 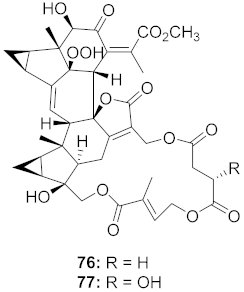 | HIV-1 | 76: EC50: 3.08 μM 77: EC50: 3.29 μM | [78] |
| 26 | Chloranthus japonicus (Roots) | 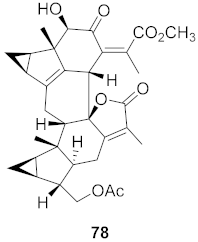 | HIV-1 | EC50: 5.41 μM | [78] |
| 27 | Kaempferia pulchra (Rhizomes) | 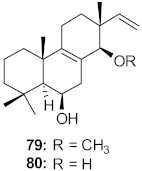 | HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 28 | Kaempferia pulchra (Rhizomes) | 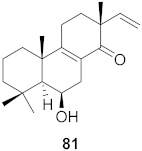 | HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 29 | Kaempferia pulchra (Rhizomes) | 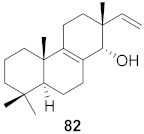 | HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 30 | Kaempferia pulchra (Rhizomes) |  | HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 31 | Kaempferia pulchra (Rhizomes) | 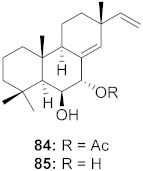 | HIV-1 | IC50: 1.56–6.25 μM | [79] |
| 32 | Stillingia lineata (Bark) | 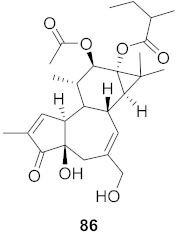 | HIV-1 | EC50: 0.271 μM | [80] |
| HIV-2 | EC50: 0.107 μM | ||||
| 33 | Stillingia lineata (Bark) | 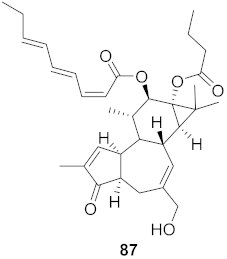 | HIV-1 | EC50: 0.233 μM | [80] |
| HIV-2 | EC50: 0.174 μM | ||||
| 34 | St35illingia lineata (Bark) | 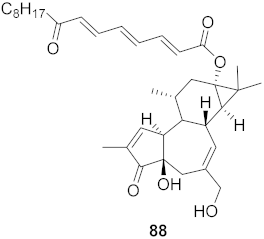 | HIV-1 | EC50: 0.043 μM | [80] |
| HIV-2 | EC50: 0.018 μM | ||||
| 35 | Moquiniastrm floribundum (Leaves) | 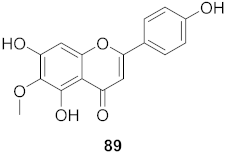 | HIV-1 | IC50: 0.345 mM | [81] |
| 36 | Moquiniastrm floribundum (Leaves) | 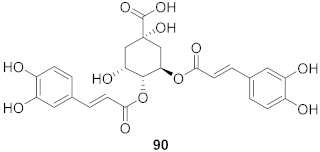 | HIV-1 | IC50: 0.240 mM | [81] |
| 37 | Moquiniastrm floribundum (Leaves) | 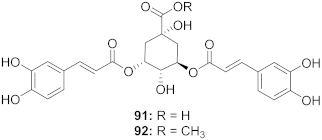 | HIV-1 | 91: IC50: 0.315 mM 92: IC50: 0.250 mM | [81] |
| 38 | Moquiniastrm floribundum (Leaves) | 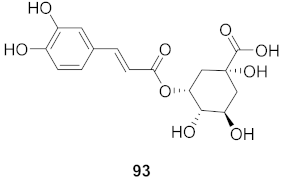 | HIV-1 | IC50: 0.374 mM | [81] |
| 39 | Moquiniastrm floribundum (Leaves) | 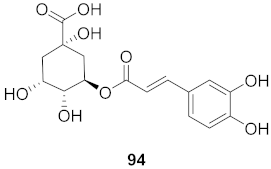 | HIV-1 | IC50: 0.489 mM | [81] |
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Andrographis paniculata | Pure andrographolide in DMSO | DENV2 | EC50: 21.304 µM | [93] |
| 2 | Dryopteris crassirhizoma | Aqueous extract of whole plant | DENV | IC50: 130 µg/mL | [94] |
| 3 | Euphorbia amygdaloides semiperfoliata | Ethyl acetate extract of whole plant | CHIKV | EC50: <0.8 µg/mL | [95] |
| 4 | Euphorbia characias | Ethyl acetate extract of stems | CHIKV | EC50: 2.9 µg/mL | [95] |
| 5 | Euphorbia hyberna insularis | Ethyl acetate extract of aerial parts | CHIKV | EC50: 1.0 µg/mL | [95] |
| 6 | Euphorbia pithyusa | Ethyl acetate extract of leaves | CHIKV | EC50: <0.8 µg/mL | [95] |
| 7 | Euphorbia pithyusa | Ethyl acetate extract of stems | CHIKV | EC50: <0.8 µg/mL | [95] |
| 8 | Euphorbia pithyusa | Methanolic and ethyl acetate extract of roots. | CHIKV | EC50: <0.8 µg/mL | [95] |
| 9 | Euphorbia segetalis pinea | Ethyl acetate extract of roots | CHIKV | EC50: 1.8 µg/mL | [95] |
| 10 | Euphorbia segetalis pinea | Ethyl acetate extract of arial parts | CHIKV | EC50: 3.7 µg/mL | [95] |
| 11 | Euphorbia segetalis pinea | Ethyl acetate extract of stems | CHIKV | EC50: 3.5 µg/mL | [95] |
| 12 | Euphorbia spinosa | Ethyl acetate extract of roots | CHIKV | EC50: <0.8 µg/mL | [95] |
| 13 | Euphorbia spinosa | Methanolic extract of roots | CHIKV | EC50: 2.3 µg/mL | [95] |
| 14 | Euphorbia spinosa | Ethyl acetate extract of stems | CHIKV | EC50: 3.4 µg/mL | [95] |
| 15 | Justicia adhatoda | Aqueous extract of leaf | DENV | IC50: 60 µg/mL | [96] |
| 16 | Morus alba | Aqueous extract of the whole plant | DENV | IC50: 221 µg/mL | [94] |
| 17 | Psidium guajoava | Aqueous extract of leaf | DENV | IC50: 60 µg/mL | [96] |
| 18 | Syzygium campanulatum | Ethyl acetate extract of leaves | DENV2 | Inhibitory (%): 64.77 | [97] |
| 19 | Syzygium grande | Ethyl acetate extract of leaves | DENV2 | Inhibition (%): 61.46 | [97] |
| S. No. | Plant Name (Part) | Compound | Virus | Activity | Ref |
|---|---|---|---|---|---|
| 1 | Basilicum poly-stachyon | 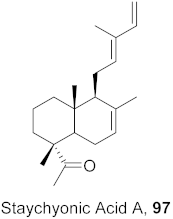 | DENV | IC50: 1.4 µM | [99] |
| 2 | Basilicum polystachyon (Whole plant) | 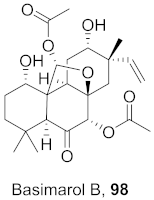 | WNV | IC50: 100 μM | [51] |
| 3 | Mammea americana (Seeds) |  | DENV2 | EC50: 9.6 µg/mL | [100] |
| CHIKV | EC50: 10.7 µg/mL | ||||
| 4 | Mammea americana (Seeds) |  | DENV2 | EC50: 2.6 µg/mL | [100] |
| CHIKV | EC50: 0.5 µg/mL | ||||
| 5 | Diospyros Ebenaceae (Bark) | 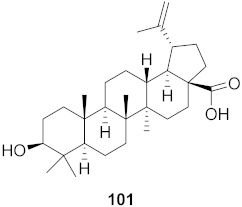 | DENV | IC50: 6.6 µM | [101] |
| 6 | Diospyros Ebenaceae (Bark) | 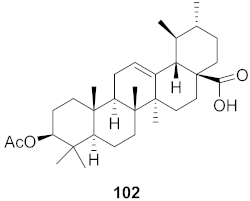 | DENV | IC50: 7.0 µM | [101] |
| 7 | Diospyros Ebenaceae (Bark) |  | DENV | IC50: 6.1 µM | [101] |
| 8 | Diospyros Ebenaceae (Bark) | 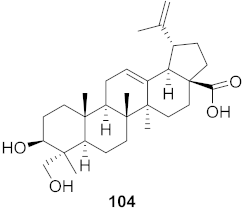 | DENV | IC50: 5.3 µM | [101] |
| 9 | Melia azedarach (Fruits) |  | DENV2 | EC50: 3.0 µM | [102] |
| 10 | Melia azedarach (Fruits) |  | DENV2 | EC50: 12 µM | [102] |
| 11 | Stillingia lineata (Bark) | 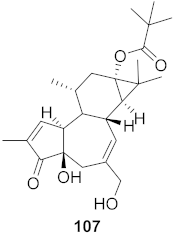 | CHIKV | EC50: 1.2 μM | [80] |
| 12 | Stillingia lineata (Stem bark) | 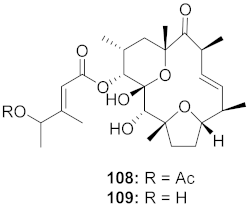 | CHIKV | 108: EC50: 7 μM 109: EC50: 34 μM | [103] |
| 13 | Stillingia lineata (Bark) |  | CHIKV | EC50: 3.3 μM | [80] |
| 14 | Stillingia lineata (Bark) | 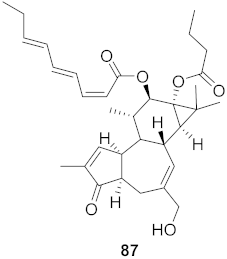 | CHIKV | EC50: 1.4 μM | [80] |
| SINV | EC50: 5.0 μM | ||||
| 15 | Stillingia lineata (Bark) | 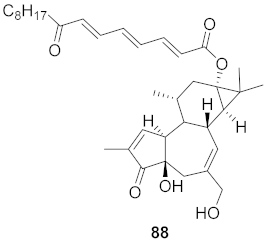 | CHIKV | EC50: 2.2 μM | [80] |
| SINV | EC50: 11 μM | ||||
| 16 | Euphorbia semiperfoliata (Whole plant) | 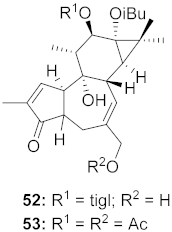 | CHIKV | 52: EC50: 1.0 μM 53: EC50: 0.44 μM | [71] |
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Arctium lappa L. | Ethanolic extract of fruits | HSV-1 | IC50: 400 μg/mL | [112] |
| 2 | Epimedium koreanum | Herbal aqueous extraction | HSV | EC50: 0.62 μg/mL | [113] |
| 3 | Eucalyptus sideroxylon Cunn. ex Woolls | Methanolic extract of leaves | HSV-2 | IC50: 199.34 μg/mL | [114] |
| 4 | Juncus Compressus | Whole plant extracted with methanol | HSV-2 | IC50: 12.4 μM | [115] |
| 5 | Punica granatum L. | Aqueous extract of pomegranate rind | HSV | EC50: 0.02 μg/mL | [116] |
| 6 | Ribes multiflorum | Methanol and aqueous extraction of leaves and fruit | HSV-1 | EC50: 9710 μg/mL | [117] |
| 7 | Ribes uva-crispa | Methanol and aqueous extraction of leaves and fruit | HSV-1 | EC50: 9710 μg/mL | [117] |
| 8 | Rosmarinus officinalis | Aqueous extract of the whole plant | HSV-1 | EC50: 67.34 μg/mL | [118] |
| 9 | Syzygium jambos | Ethanolic extract of leaves | HSV-1 | IC50: 50.00 μg/mL | [119] |
| 10 | Terminalia chebula Retz | Ethanolic extract of fruit | HSV-2 | IC50: 0.01 μg/mL | [120] |
| S. No. | Plant Name (Part) | Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Houttuynia cordata | 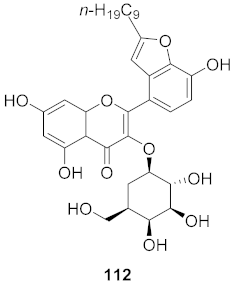 | HSV | IC50: 23.5 μM | [122] |
| 2 | Boswellia serrata (Oleo-gum-resin) | 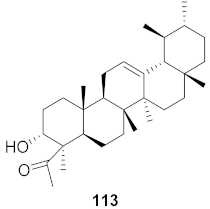 | HSV-1 | EC50: 5.2 μg/mL | [123] |
| 3 | Angelica archangelica L. (Fruits) | 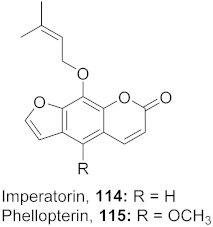 | HSV-1 | 114: IC50: 15.62 μg/mL 115: IC50: 3.90 μg/mL | [124] |
| 4 | Rhododendron capitatum (Aerial parts) | 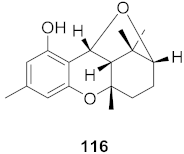 | HSV-1 | IC50: 4.2 μM | [125] |
| 5 | Cnidium monnieri (Fruit) |  | HSV-1 | IC50: 1.23 μM | [126] |
| 6 | Kalanchoe daigremontiana (Leaves) | 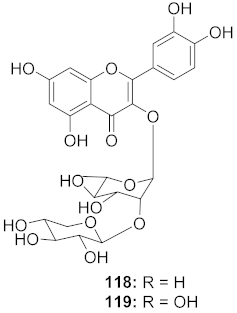 | HSV-1 | EC50: 0.97 μg/mL | [127] |
| HSV-2 | EC50: 0.72 μg/mL | ||||
| 7 | Morus alba L. | 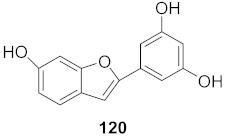 | HSV-1 | IC50: 2.2 ± 0.1 μg/mL | [128] |
| HSV-2 | IC50: 2.5 ± 0.3 μg/mL | ||||
| 8 | Morus alba L. | 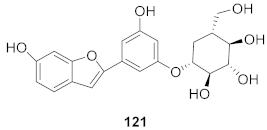 | HSV-1 | IC50: 5.0 μg/mL | [128] |
| HSV-2 | IC50: 3.2 μg/mL | ||||
| 9 | Morus alba L. |  | HSV-1 | IC50: 8.4 μg/mL | [128] |
| HSV-2 | IC50: 8.2 ± 0.4 μg/mL | ||||
| 10 | Morus alba L. | 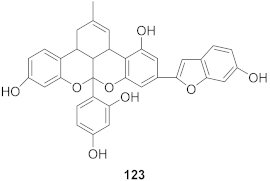 | HSV-1 | IC50: 5.2 μg/mL | [128] |
| HSV-2 | IC50: 3.7 μg/mL | ||||
| 11 | Morus alba L. | 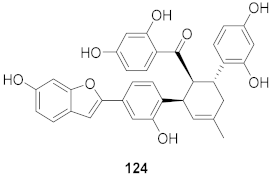 | HSV-1 | IC50: 12.5 μg/mL | [128] |
| HSV-2 | IC50: 12.5 μg/mL | ||||
| 12 | Morus alba L. | 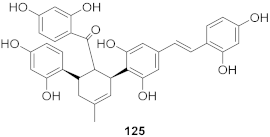 | HSV-1 | IC50: 6.3 μg/mL | [128] |
| HSV-2 | IC50: 25.0 μg/mL | ||||
| 13 | Camellia sinensis (Leaves) | 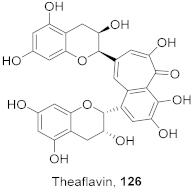 | HSV-1 | EC50: 50 μM | [129] |
| 14 | Camellia sinensis (Leaves) |  | HSV-1 | EC50: 25 μM | [129] |
| 15 | Camellia sinensis (Leaves) | 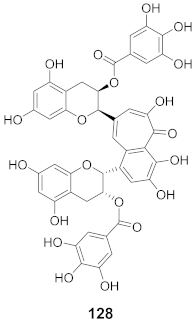 | HSV-1 | EC50: 20 μM | [129] |
| 16 | Kalanchoe pinnata (Root) | 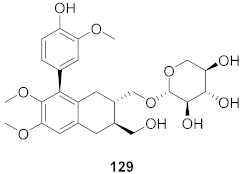 | HSV-1 | EC50: 0.97 μg/mL | [130] |
| HSV-2 | EC50: 0.72 μg/mL | ||||
| 17 | Kalanchoe pinnata (Root) | 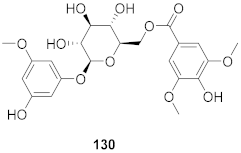 | HSV-1 | EC50: 0.97 μg/mL | [130] |
| HSV-2 | EC50: 0.72 μg/mL |
| S. No. | Plant Name | Plant Extract | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Abutilon figarianum | Ethanolic then dichloromethane extract of whole plant | HBV | IC50: 99.76 μg/mL | [134] |
| 2 | Acacia oerfota | Total ethanolic extract of whole plant | HBV | IC50: 101.46 μg/mL | [134] |
| 3 | Alectryon serratus | Ethanolic extract of leaves | HCV | IC50: 9.8 μg/mL | [135] |
| 4 | Alectryon serratus | Chloroform methanolic extract of leaves | HCV | IC50: 1.2 μg/mL | [135] |
| 5 | Alectryon serratus | Chloroform methanolic and water extract of leaves | HCV | IC50: 0.43 μg/mL | [135] |
| 6 | Boerhavia diffusa | Methanolic extract of whole plant | HCV | IC50: 12.5–25 μM | [136] |
| 7 | Capparis decidua | Ethanolic then aqueous extract of the whole plant | HBV | IC50: 66.82 μg/mL | [134] |
| 8 | Coccinea grandis | Total ethanolic extract of whole plant | HBV | IC50: 31.57 μg/mL | [134] |
| 9 | Corallocarpus epigeus | Total ethanolic extract of whole plant | HBV | IC50: 71.9 μg/mL | [134] |
| 10 | Curcuma domestica | Dried powder of rhizomes was extracted with ethanol | HCV | IC50:1.68 μg/mL | [137] |
| 11 | Curcuma heyneana | Dried powder of rhizomes was extracted with ethanol | HCV | IC50: 5.49 μg/mL | [137] |
| 12 | Curcuma xanthorrhiza | Dried powder of rhizomes was extracted with ethanol | HCV | IC50:4.93 μg/mL | [137] |
| 13 | Fumaria parviflora | Ethanolic then hexane extract of the whole plant | HBV | IC50: 35.44 μg/mL | [134] |
| 14 | Glycine max | A fermented extract of defatted soybean meal | HAV | IC50: 27 μg/mL | [138] |
| 15 | Glycine max | A fermented extract of defatted soybean meal with Aspergillus fumigatus F-993 | HAV | IC50: 8.60 μg/mL | [138] |
| 16 | Glycine max | A fermented extract of defatted soybean meal with A. awamori FB-113 | HAV | IC50: 16.88 μg/mL | [138] |
| 17 | Guiera senegalensis | Ethanolic then dichloromethane extract of the whole plant | HBV | IC50: 10.65 μg/mL | [134] |
| 18 | Indigofera caerulea | Methanolic extract of whole plant | HBV | IC50: 73.21 μg/mL | [134] |
| 19 | Juncus maritimus Lam. | Methanolic extract of rhizomes | HCV | Inhibition (%): >50 | [139] |
| 20 | Lentinula edodes | Hot water extraction of mycelia | HCV | IC50: 5 μg/mL | [140] |
| 21 | Limonium sinense | Aqueous extract from underground part of plant | HCV | EC50: 9.71 μg/mL CC50: 343.47 μg/mL | [141] |
| 22 | Phyllanthus reticulates Poir. | Aqueous and ethanol extracts | HBV | EC50: 0.56μg/mL | [142] |
| 23 | Pinus pinaster | Pine extract from bark | HCV | IC50: 5.78 μg/mL EC50: 4.33 μg/mL | [143] |
| 24 | Pulicaria crispa | Ethyl acetate extract of whole plant | HBV | IC50: 14.45 μg/mL | [134] |
| 25 | Taraxacum officinale | Methanolic extract of leaves | HCV | Inhibition (%): >65% | [144] |
| 26 | Valeriana wallichii | Methanolic extract of roots | HCV | CC50: 252.2 μg/mL | [145] |
| S. No. | Plant Name (Part) | Compound | Virus | Activity | Ref. |
|---|---|---|---|---|---|
| 1 | Phyllantus acidus (Stem) | 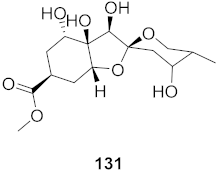 | HBV | IC50: 11.2 μM | [146] |
| 2 | Phyllantus acidus (Stem) | 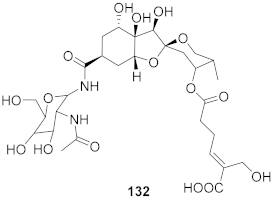 | HBV | IC50: 57.1 μM | [146] |
| 3 | Vitis vinifera (Root) | 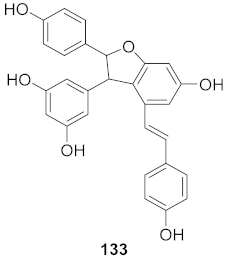 | HCV | EC50: 0.006 μM | [147] |
| 4 | Vitis vinifera (Root) | 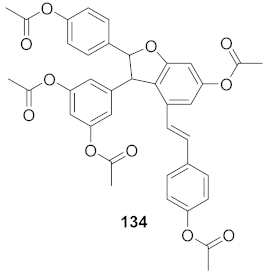 | HCV | EC50: 2.37 μM | [147] |
| 5 | Wikstroemia chamaedaphne (Buds) |  | HBV | IC50: 46.5 μg/mL | [148] |
| 6 | Wikstroemia chamaedaphne (Buds) | 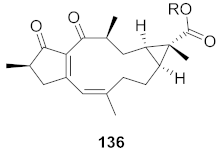 | HBV | IC50: 88.3 μg/mL | [148] |
| 7 | Multiple Fumaria and Corydalis species from Turkey | 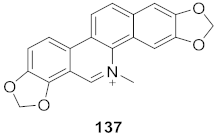 | HBV | IC50: 15 mg | [149] |
| 8 | Multiple Fumaria and Corydalis species from Turkey |  | HBV | IC50: 23 mg | [149] |
| 9 | Candida albicans (Root) | 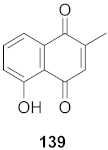 | HCV | IC50: 0.57 μM/L | [150] |
| 10 | Cyanara Cardunculus L.var. sylvestris (Lam.) Fiori (Leaves) | 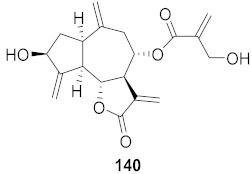 | HCV | EC50: 0.4–1.4 μM | [151] |
| 11 | Cyanara Cardunculus L.var. sylvestris (Lam.) Fiori (Leaves) | 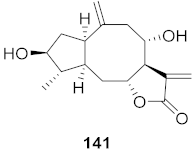 | HCV | EC50: 2.7–14.0 μM | [151] |
| 12 | Green Tea | 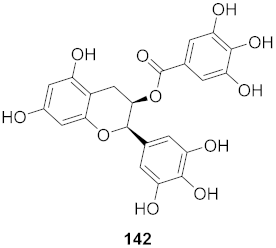 | HBV | CC50: 247.28 μM | [152] |
| 13 | Caulis trachelospermi |  | HCV | IC50: 0.325 μg/mL | [101] |
| 14 | Selaginella moellendorffii | 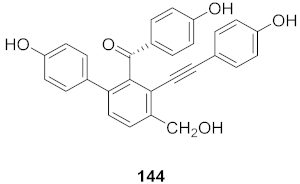 | HBV | IC50: 0.026 μg/mL | [153] |
| 15 | Phyllanthus urinaria | 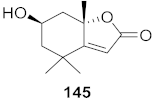 | HCV | EC50: 2.48 μM | [154] |
| 16 | Viola diffusa Ging (Whole plant) | 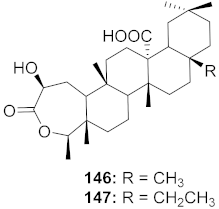 | HBV | 146: IC50: 26.2 μM 147: IC50: 33.7 μM | [155] |
| 17 | Viola diffusa Ging (Whole plant) | 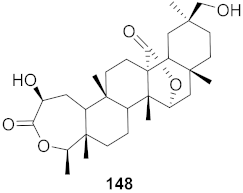 | HBV | IC50: 104.0 μM | [155] |
| 18 | Viola diffusa Ging (Whole plant) | 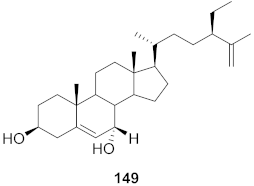 | HBV | IC50: 62.0 μM | [155] |
| 19 | Viola diffusa Ging (Whole plant) |  | HBV | IC50: 32.7 μM | [155] |
| 20 | Viola diffusa Ging (Whole plant) | 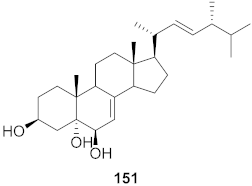 | HBV | IC50: 112.8 μM | [155] |
| 21 | Perovskia atriplicifolia (Whole plant) |  | HBV | IC50: 1.03 μM | [156] |
| 22 | Perovskia atriplicifolia (Whole plant) | 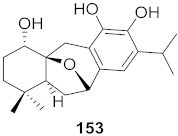 | HBV | IC50: 0.59 μM | [156] |
| 23 | Maytrenus ilicifolia (Root bark) | 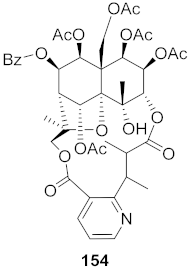 | HCV | EC50: 2.3 μM | [157] |
| 24 | Peperomia blanda (Aerial parts) | 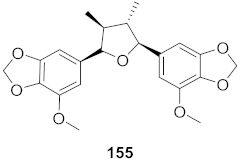 | HCV | EC50: 4.0 μM | [157] |
| 25 | Peperomia blanda (Aerial parts) |  | HCV | EC50: 8.2 μM | [157] |
| 26 | Peperomia blanda (Aerial parts) | 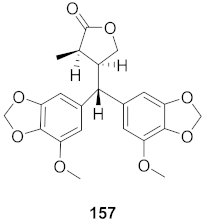 | HCV | EC50: 38.9 μM | [157] |
| 27 | Illicium jiadifengpi (Fruits) | 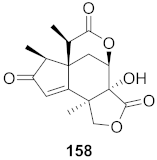 | HBV | Inhibitory (%): 28.85 | [158] |
| 28 | Illicium jiadifengpi (Fruits) | 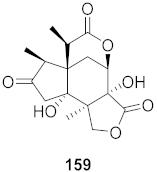 | HBV | Inhibitory (%): 37.93 | [158] |
| 29 | Chloranthus japonicus (Roots) | 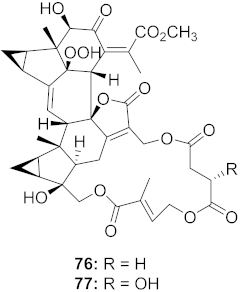 | HCV | 76: EC50: 3.07 μM 77: EC50: 9.34 μM | [78] |
| 30 | Chloranthus japonicus (Roots) | 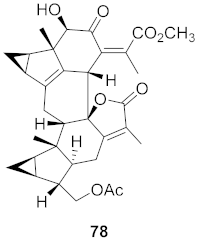 | HCV | EC50: 1.62 μM | [78] |
| 31 | Aloe vera (Leaves) | 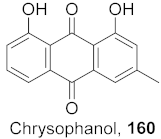 | HBV | Inhibitory (%): 62 | [159] |
| 32 | Aloe vera (Leaves) | 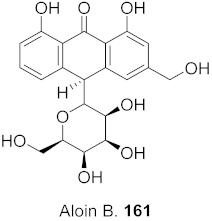 | HBV | Inhibitory (%): 61 | [159] |
| 33 | Aloe vera (Leaves) | 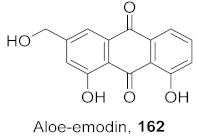 | HBV | Inhibitory (%): 83 | [159] |
| S. No. | Plant Name (Part) | Compound | Virus | IC50/EC50 | Ref. |
|---|---|---|---|---|---|
| 1 | Lilium speciosum var gloriosoides Barker (Bulbs) | 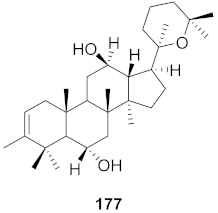 | RSV | IC50: 2.9 μg/mL | [171] |
| 2 | Lilium speciosum var gloriosoides Barker (Bulbs) | 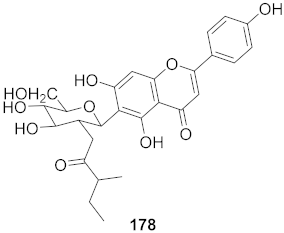 | RSV | IC50: 2.1 μg/mL | [172] |
| 3 | Erycibe obtusifolia | 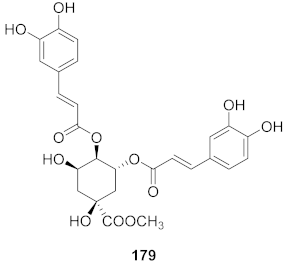 | RSV A2 | EC50: 0.52 μg/mL | [173] |
| RSV Long | EC50: 0.59 μg/mL |
| Entry | Compound | logP | HIA | BBB | hERG pIC50 | Rotatable Bonds | HBD | HBA | MW |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 1.991 | + | - | 3.836 | 8 | 2 | 9 | 494.6 |
| 2 | 31 | 1.129 | - | - | 4.580 | 10 | 8 | 18 | 925.1 |
| 3 | 32 | −0.989 | - | - | 4.561 | 13 | 7 | 15 | 626.6 |
| 4 | 43 | 3.258 | + | - | 5.332 | 7 | 1 | 5 | 360.4 |
| 5 | 44 | 3.635 | + | - | 5.456 | 8 | 1 | 5 | 374.5 |
| 6 | 48 | 2.752 | + | + | 4.855 | 5 | 0 | 4 | 286.3 |
| 7 | 51 | 2.307 | + | + | 4.953 | 4 | 0 | 4 | 260.3 |
| 8 | 54 | 5.480 | + | + | 6.054 | 0 | 0 | 3 | 289.3 |
| 9 | 55 | 7.230 | + | + | 6.668 | 2 | 0 | 3 | 379.5 |
| 10 | 56 | 2.076 | - | - | 4.810 | 6 | 2 | 9 | 404.4 |
| 11 | 57 | 2.270 | - | - | 4.872 | 7 | 1 | 9 | 418.4 |
| 12 | 86 | 3.411 | + | - | 4.463 | 7 | 2 | 7 | 474.6 |
| 13 | 88 | 4.945 | + | - | 5.576 | 14 | 2 | 6 | 578.8 |
| 14 | 97 | 5.158 | + | + | 5.625 | 4 | 0 | 1 | 300.5 |
| 15 | 100 | 4.480 | + | - | 5.680 | 7 | 2 | 5 | 372.5 |
| 16 | 105 | 5.770 | + | - | 5.498 | 5 | 1 | 5 | 554.8 |
| 17 | 107 | 3.564 | + | - | 4.708 | 4 | 2 | 5 | 416.6 |
| 18 | 117 | 4.049 | + | - | 5.981 | 5 | 4 | 9 | 528.5 |
| 19 | 118 | −0.320 | - | - | 4.146 | 5 | 8 | 14 | 564.5 |
| 20 | 119 | −0.504 | - | - | 3.960 | 5 | 9 | 15 | 580.5 |
| 21 | 129 | 0.348 | - | - | 4.587 | 8 | 5 | 10 | 506.5 |
| 22 | 130 | −0.473 | - | - | 4.216 | 9 | 5 | 12 | 482.4 |
| 23 | 133 | 3.561 | + | - | 5.617 | 4 | 5 | 6 | 454.5 |
| 24 | 139 | 2.345 | + | + | 3.865 | 0 | 1 | 3 | 188.2 |
| 25 | 140 | 1.284 | + | - | 3.963 | 4 | 2 | 6 | 346.4 |
| 26 | 143 | 2.531 | + | - | 4.917 | 7 | 2 | 7 | 388.4 |
| 27 | 144 | 3.605 | + | - | 5.396 | 6 | 4 | 5 | 436.5 |
| 28 | 153 | 3.472 | + | - | 5.208 | 1 | 3 | 4 | 332.4 |
| 29 | 179 | 1.414 | - | - | 4.310 | 10 | 6 | 12 | 530.5 |
| 30 | 180 | 0.821 | + | - | 4.432 | 11 | 4 | 13 | 654.7 |
| 31 | Ribavirin | −1.85 | 3.325 | 3 | 4 | 9 | 244.2 | ||
| 32 | Oseltamivir | 1.767 | + | - | 3.737 | 9 | 2 | 6 | 312.4 |
| 33 | Efavirenz | 4.013 | + | + | 4.992 | 3 | 1 | 3 | 315.7 |
| 34 | Zidovudine | −0.018 | + | - | 4.089 | 3 | 2 | 9 | 267.2 |
| 35 | Prostratin | 1.971 | + | - | 4.365 | 3 | 3 | 6 | 390.5 |
| 36 | Nevaripine | 1.828 | + | - | 4.890 | 1 | 0 | 5 | 300.7 |
| 37 | Honokiol | 4.362 | + | + | 5.358 | 5 | 2 | 2 | 266.3 |
| 38 | Myricetin | 1.303 | - | - | 4.274 | 1 | 6 | 8 | 318.2 |
| 39 | Foscarnet | −1.535 | + | - | 2.667 | 1 | 3 | 5 | 126.0 |
| 40 | Acyclovir | −1.649 | - | - | 4.302 | 4 | 3 | 8 | 225.2 |
| 41 | Lamivudine | −1.036 | + | - | 3.955 | 2 | 2 | 6 | 229.3 |
| 42 | Plumbagin | 2.345 | + | + | 3.865 | 0 | 1 | 3 | 188.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, E.; Stewart, L.E.; Darley, B.A.; Pham, A.M.; Esteban, I.; Panda, S.S. Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules 2021, 26, 6197. https://doi.org/10.3390/molecules26206197
Thomas E, Stewart LE, Darley BA, Pham AM, Esteban I, Panda SS. Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules. 2021; 26(20):6197. https://doi.org/10.3390/molecules26206197
Chicago/Turabian StyleThomas, Eyana, Laura E. Stewart, Brien A. Darley, Ashley M. Pham, Isabella Esteban, and Siva S. Panda. 2021. "Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates" Molecules 26, no. 20: 6197. https://doi.org/10.3390/molecules26206197
APA StyleThomas, E., Stewart, L. E., Darley, B. A., Pham, A. M., Esteban, I., & Panda, S. S. (2021). Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules, 26(20), 6197. https://doi.org/10.3390/molecules26206197