DCMC as a Promising Alternative to Bentonite in White Wine Stabilization. Impact on Protein Stability and Wine Aromatic Fraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Wine Characterization
2.2. Effects of Fining Treatments on Wine Stability and Protein Content
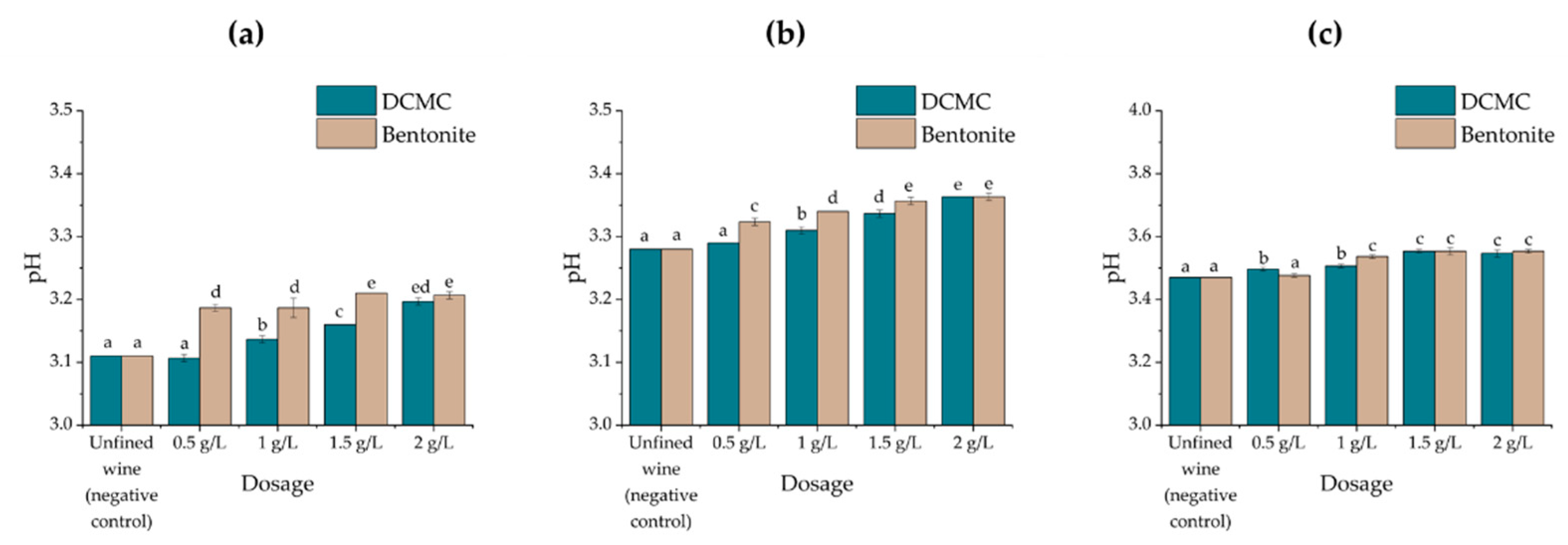
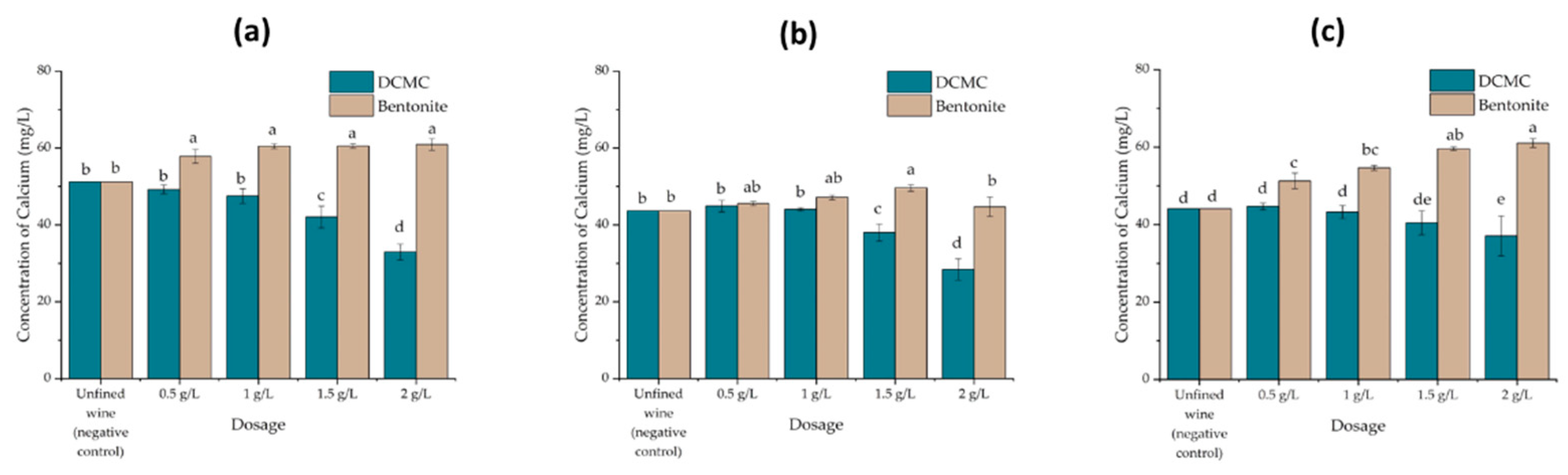
2.3. Effect of Wine Fining Treatments on Wine Chemical Composition
2.4. Effect of Wine Fining Treatments in the Profile of Volatile Organic Compounds
3. Materials and Methods
3.1. Reagents and Materials
3.2. Wines
3.3. Heat Stability Test (HST)
3.4. Analytical Enological Parameters
3.5. Protein Quantification
3.6. Fining Experiments
3.7. Determination of Wine Volatile Organic Compounds
3.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Monteiro, S.; Picarra-Pereira, M.A.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. The diversity of pathogenesis-related proteins decreases during grape maturation. Phytochemistry 2007, 68, 416–425. [Google Scholar] [CrossRef]
- Monteiro, S.; Picarra-Pereira, M.A.; Teixeira, A.R.; Loureiro, V.B.; Ferreira, R.B. Environmental conditions during vegetative growth determine the major proteins that accumulate in mature grapes. J. Agric. Food Chem. 2003, 51, 4046–4053. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Picarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Blade, W.H.; Boulton, R. Adsorption of Protein by Bentonite in A Model Wine Solution. Am. J. Enol. Vitic. 1988, 39, 193–199. [Google Scholar]
- Sommer, S.; Tondini, F. Sustainable Replacement Strategies for Bentonite in Wine Using Alternative Protein Fining Agents. Sustainability 2021, 13, 1860. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Qi, G.; Smith, P.; Bindon, K.; Vasilev, K. Regeneration of Magnetic Nanoparticles Used in the Removal of Pathogenesis-Related Proteins from White Wines. Foods 2020, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; McRae, J.M.; Wollan, D.; Muhlack, R.A.; Godden, P.; Wilkinson, K.L. Use of ultrafiltration and proteolytic enzymes as alternative approaches for protein stabilisation of white wine. Aust. J. Grape Wine Res. 2021, 27, 234–245. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Esti, M. Papain Covalently Immobilized on Chitosan-Clay Nanocomposite Films: Application in Synthetic and Real White Wine. Nanomaterials 2020, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Lucchetta, M.; Waters, E.J. Protein stabilisation of white wines using zirconium dioxide enclosed in a metallic cage. Aust. J. Grape Wine Res. 2011, 17, 28–35. [Google Scholar] [CrossRef]
- Sarmento, M.R.; Oliveira, J.C.; Boulton, R.B. Selection of low swelling materials for protein adsorption from white wines. Int. J. Food Sci. Technol. 2000, 35, 41–47. [Google Scholar] [CrossRef]
- de Bruijn, J.; Martinez-Oyanedel, J.; Loyola, C.; Seiter, J.; Lobos, F.; Perez-Arias, R. Fractionation of Sauvignon wine macromolecules by ultrafiltration and diafiltration: Impact of protein composition on white wine haze stability. Int. J. Food Sci. Technol. 2011, 46, 1691–1698. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Robinson, E.M.C.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Godden, P.W.; Smith, P.A.; Waters, E.J. Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine-Stabilization and Treatments, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006; Volume 2. [Google Scholar]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of Combined Effect of Proteins and Bentonite Fining on the Wine Aroma Loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef]
- Santos, C.V.A.; da Silva, M.G.; Cabrita, M.J. Impact of SO2 and bentonite addition during fermentation on volatile profile of two varietal white wines. LWT 2020, 133, 109893. [Google Scholar] [CrossRef]
- Chagas, R.; Gericke, M.; Ferreira, R.B.; Heinze, T.; Ferreira, L.M. Synthesis and characterization of dicarboxymethyl cellulose. Cellulose 2020, 27, 1965–1974. [Google Scholar] [CrossRef] [Green Version]
- Gago, D.; Chagas, R.; Ferreira, L.M.; Velizarov, S.; Coelhoso, I. A Novel Cellulose-Based Polymer for Efficient Removal of Methylene Blue. Membranes 2020, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gago, D.; Chagas, R.; Ferreira, L.M. The Effect of Dicarboxymethyl Cellulose on the Prevention of Protein Haze Formation on White Wine. Beverages 2021, 7, 57. [Google Scholar] [CrossRef]
- Chagas, R.; Laia, C.A.T.; Ferreira, R.B.; Ferreira, L.M. Sulfur dioxide induced aggregation of wine thaumatin-like proteins: Role of disulfide bonds. Food Chem. 2018, 259, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocock, K.F.; Waters, E.J. Protein haze in bottled white wines: How well do stability tests and bentonite fining trials predict haze formation during storage and transport? Aust. J. Grape Wine Res. 2006, 12, 212–220. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chagas, R.; Ferreira, L.M.; Laia, C.A.T.; Monteiro, S.; Ferreira, R.B. The challenging SO2-mediated chemical build-up of protein aggregates in wines. Food Chem. 2016, 192, 460–469. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.; Schulkin, A.; Dambergs, R. Effect of white wine composition on protein haze potential. Aust. J. Grape Wine Res. 2018, 24, 498–503. [Google Scholar] [CrossRef]
- Vernhet, A.; Meistermann, E.; Cottereau, P.; Charrier, F.; Chemardin, P.; Poncet-Legrand, C. Wine Thermosensitive Proteins Adsorb First and Better on Bentonite during Fining: Practical Implications and Proposition of Alternative Heat Tests. J. Agric. Food Chem. 2020, 68, 13450–13458. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hider, R.; Zhao, J.; Tian, B. Effect of bentonite fining on proteins and phenolic composition of Chardonnay and Sauvignon Blanc wines. S. Afr. J. Enol. Vitic. 2020, 41, 113–120. [Google Scholar] [CrossRef]
- Dordoni, R.; Colangelo, D.; Giribaldi, M.; Giuffrida, M.G.; De Faveri, D.M.; Lambri, M. Effect of bentonite characteristics on wine proteins, polyphenols, and metals under conditions of different pH. Am. J. Enol. Vitic. 2015, 66, 518–530. [Google Scholar] [CrossRef]
- Mateus, E.; Barata, R.C.; Zrostlikova, J.; da Silva, M.; Paiva, M.R. Characterization of the volatile fraction emitted by Pinus spp. by one- and two-dimensional chromatographic techniques with mass spectrometric detection. J. Chromatogr. A 2010, 1217, 1845–1855. [Google Scholar] [CrossRef]
- Martins, N.; Garcia, R.; Mendes, D.; Freitas, A.M.C.; da Silva, M.G.; Cabrita, M.J. An ancient winemaking technology: Exploring the volatile composition of amphora wines. LWT 2018, 96, 288–295. [Google Scholar] [CrossRef]
- Pereira, C.; Mendes, D.; Dias, T.; Garcia, R.; da Silva, M.G.; Cabrita, M.J. Revealing the yeast modulation potential on amino acid composition and volatile profile of Arinto white wines by a combined chromatographic-based approach. J. Chromatogr. A 2021, 1641, 461991. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R.; Kamalinejad, M.; Nasiri, A. Composition of the essential oil of Salvia mirzayanii Rech. f. & Esfand from Iran. Flavour Frag. J. 2002, 17, 465–467. [Google Scholar] [CrossRef]
- Demyttenaere, J.C.R.; Dagher, C.; Sandra, P.; Kallithraka, S.; Verhe, R.; De Kimpe, N. Flavour analysis of Greek white wine by solid-phase microextraction-capillary gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 985, 233–246. [Google Scholar] [CrossRef]
- Shellie, R.A.; Marriott, P.J. Comprehensive two-dimensional gas chromatography-mass spectrometry analysis of Pelargonium graveolens essential oil using rapid scanning quadrupole mass spectrometry. Analyst 2003, 128, 879–883. [Google Scholar] [CrossRef]
- Palmeira, S.F.; Moura, F.D.; Alves, V.D.; de Oliveira, F.M.; Bento, E.S.; Conserva, L.M.; Andrade, E.H.D. Neutral components from hexane extracts of Croton sellowii. Flavour Frag. J. 2004, 19, 69–71. [Google Scholar] [CrossRef]
- Adams, R.P.; Morris, J.A.; Pandey, R.N.; Schwarzbach, A.E. Cryptic speciation between Juniperus deltoides and Juniperus oxycedrus (Cupressaceae) in the Mediterranean. Biochem. Syst. Ecol. 2005, 33, 771–787. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Kibaris, A.C.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Flavour compounds of Greek cotton honey. J. Sci. Food Agric. 2005, 85, 1444–1452. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M. Aroma compounds in Oregon Pinot Noir wine determined by aroma extract dilution analysis (AEDA). Flavour Frag. J. 2005, 20, 22–29. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Munoz, Y.; Marti, M.P.; Marbot, R. Volatile components from Mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Shatar, S. Essential oil of Ferula ferulaoides from Western Mongolia. Chem. Nat. Compd. 2005, 41, 607–608. [Google Scholar] [CrossRef]
- Wu, S.M.; Zorn, H.; Krings, U.; Berger, R.G. Characteristic volatiles from young and aged fruiting of wild Polyporus sulfureus (Bull.: Fr.) fr. J. Agric. Food Chem. 2005, 53, 4524–4528. [Google Scholar] [CrossRef]
- Adams, R.P.; Elizondo, M.S.G.; Elizondo, M.G.; Slinkman, E. DNA fingerprinting and terpenoid analysis of Juniperus blancoi var. huehuentensis (Cupressaceae), a new subalpine variety from Durango, Mexico. Biochem. Syst. Ecol. 2006, 34, 205–211. [Google Scholar] [CrossRef]
- Bylaite, E.; Meyer, A.S. Characterisation of volatile aroma compounds of orange juices by three dynamic and static headspace gas chromatography techniques. Eur. Food Res. Technol. 2006, 222, 176–184. [Google Scholar] [CrossRef]
- Fan, W.L.; Qian, M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Mevy, J.P.; Bessiere, J.M.; Greff, S.; Zombre, G.; Viano, J. Composition of the volatile oil from the leaves of Ximenia americana L. Biochem. Syst. Ecol. 2006, 34, 549–553. [Google Scholar] [CrossRef]
- Xian, Q.M.; Chen, H.D.; Zou, H.X.; Yin, D.Q. Chemical composition of essential oils of two submerged macrophytes, Ceratophyllum demersum L. and Vallisneria spiralis L. Flavour Frag. J. 2006, 21, 524–526. [Google Scholar] [CrossRef]
- Zhao, C.X.; Li, X.N.; Liang, Y.Z.; Fang, H.Z.; Huang, L.F.; Guo, F.Q. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemom. Intell. Lab. Syst. 2006, 82, 218–228. [Google Scholar] [CrossRef]
- Baccouri, B.; Ben Temime, S.; Campeol, E.; Cioni, P.L.; Daoud, D.; Zarrouk, M. Application of solid-phase microextraction to the analysis of volatile compounds in virgin olive oils from five new cultivars. Food Chem. 2007, 102, 850–856. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Perez-Cacho, P.R.; Davenport, T.; Rouseff, R. Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. J. Agric. Food Chem. 2007, 55, 1939–1944. [Google Scholar] [CrossRef]
- Methven, L.; Tsoukka, M.; Oruna-Concha, M.J.; Parker, J.K.; Mottram, D.S. Influence of sulfur amino acids on the volatile and nonvolatile components of cooked salmon (Salmo salar). J. Agric. Food Chem. 2007, 55, 1427–1436. [Google Scholar] [CrossRef]
- Steinhaus, P.; Schieberle, P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007, 55, 6262–6269. [Google Scholar] [CrossRef]
- Cho, I.H.; Namgung, H.J.; Choi, H.K.; Kim, Y.S. Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing.). Food Chem. 2008, 106, 71–76. [Google Scholar] [CrossRef]
- Cabrita, M.J.; Freitas, A.M.C.; Laureano, O.; Borsa, D.; Di Stefano, R. Aroma compounds in varietal wines from Alentejo, Portugal. J. Food Compos. Anal. 2007, 20, 375–390. [Google Scholar] [CrossRef]
- Rocha, S.M.; Coutinho, P.; Barros, A.; Delgadillo, I.; Coimbra, M.A. Rapid tool for distinction of wines based on the global volatile signature. J. Chromatogr. A 2006, 1114, 188–197. [Google Scholar] [CrossRef] [PubMed]
- ben Hammouda, I.; Freitas, F.; Ammar, S.; Da Silva, M.; Bouaziz, M. Comparison and characterization of volatile compounds as markers of oils stability during frying by HS-SPME-GC/MS and Chemometric analysis. J. Chromatogr. B 2017, 1068, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Kramling, T.E.; Singleton, V.L. An Estimate of Nonflavonoid Phenols in Wines. Am. J. Enol. Vitic. 1969, 20, 86–92. [Google Scholar]
- Somers, T.C.; Evans, M.E. Spectral Evaluation of Young Red Wines—Anthocyanin Equilibria, Total Phenolics, Free and Molecular SO2, Chemical Age. J. Sci. Food Agric. 1977, 28, 279–287. [Google Scholar] [CrossRef]
- Gazzola, D.; Vincenzi, S.; Pasini, G.; Lomolino, G.; Curioni, A. Advantages of the KDS/BCA Assay over the Bradford Assay for Protein Quantification in White Wine and Grape Juice. Am. J. Enol. Vitic. 2015, 66, 227–233. [Google Scholar] [CrossRef]
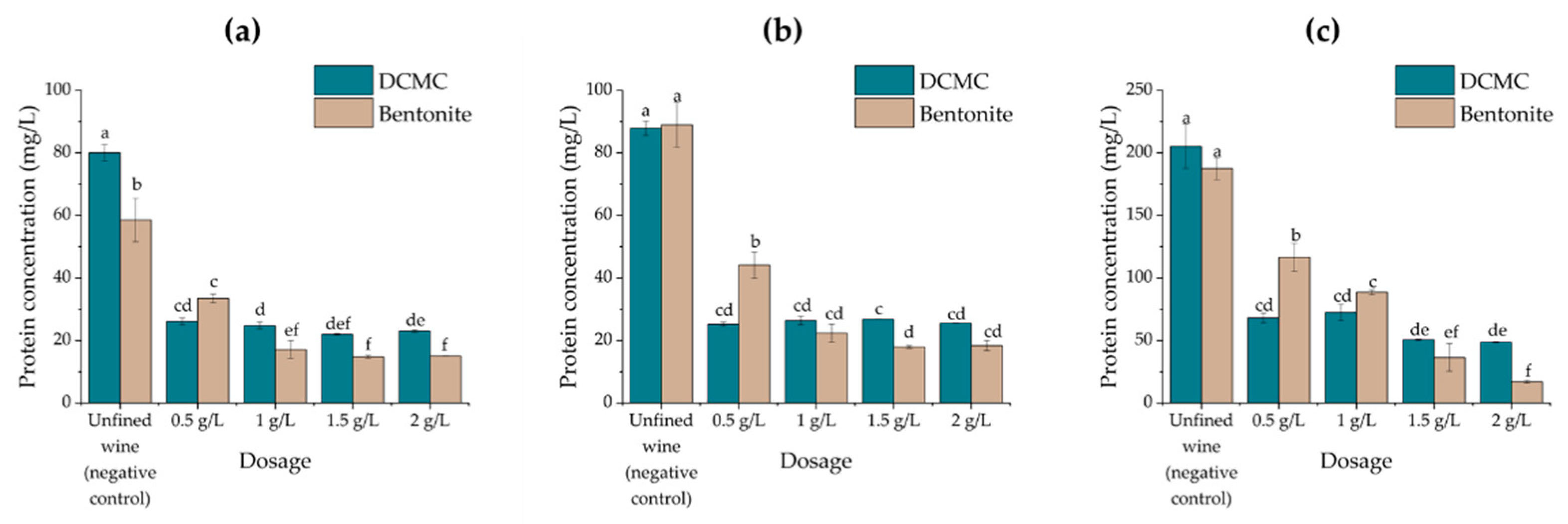
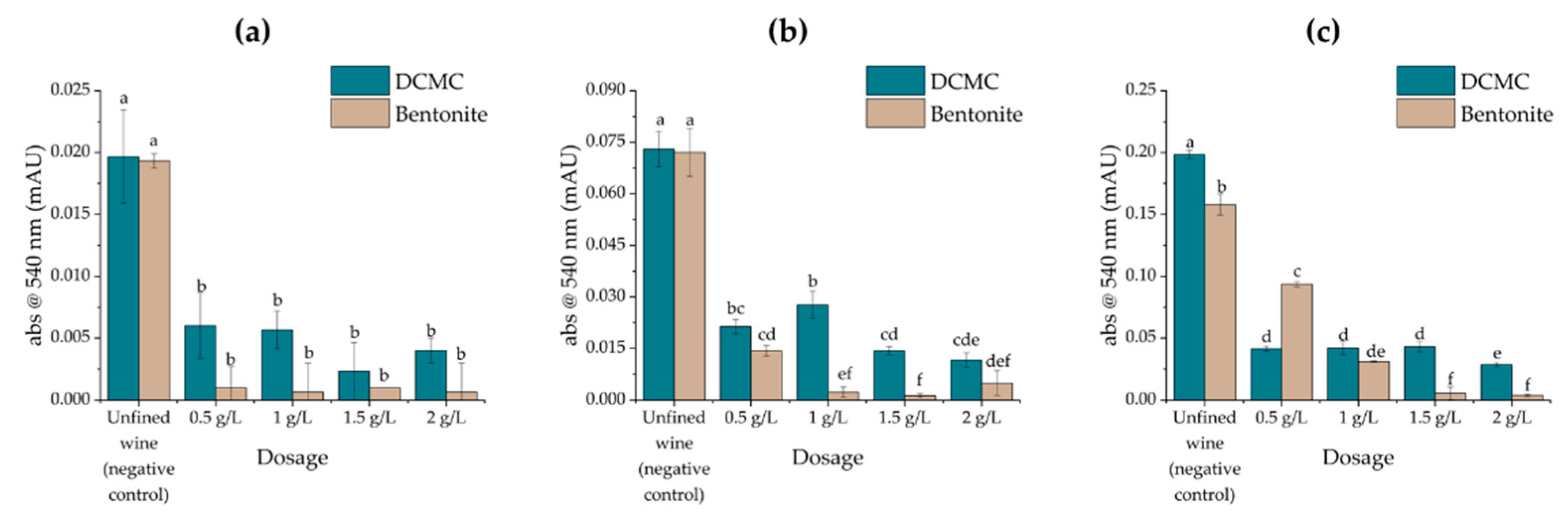


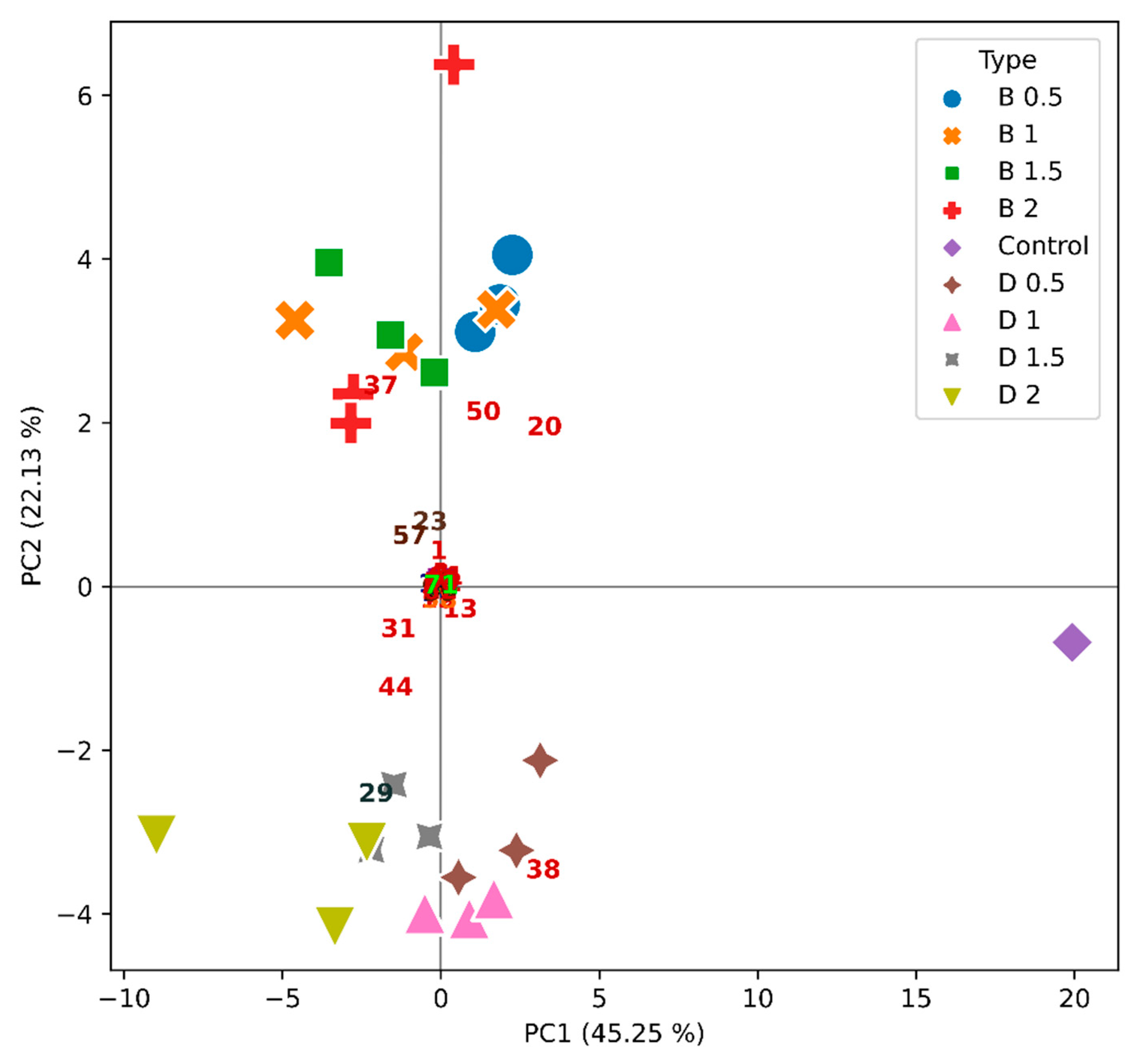
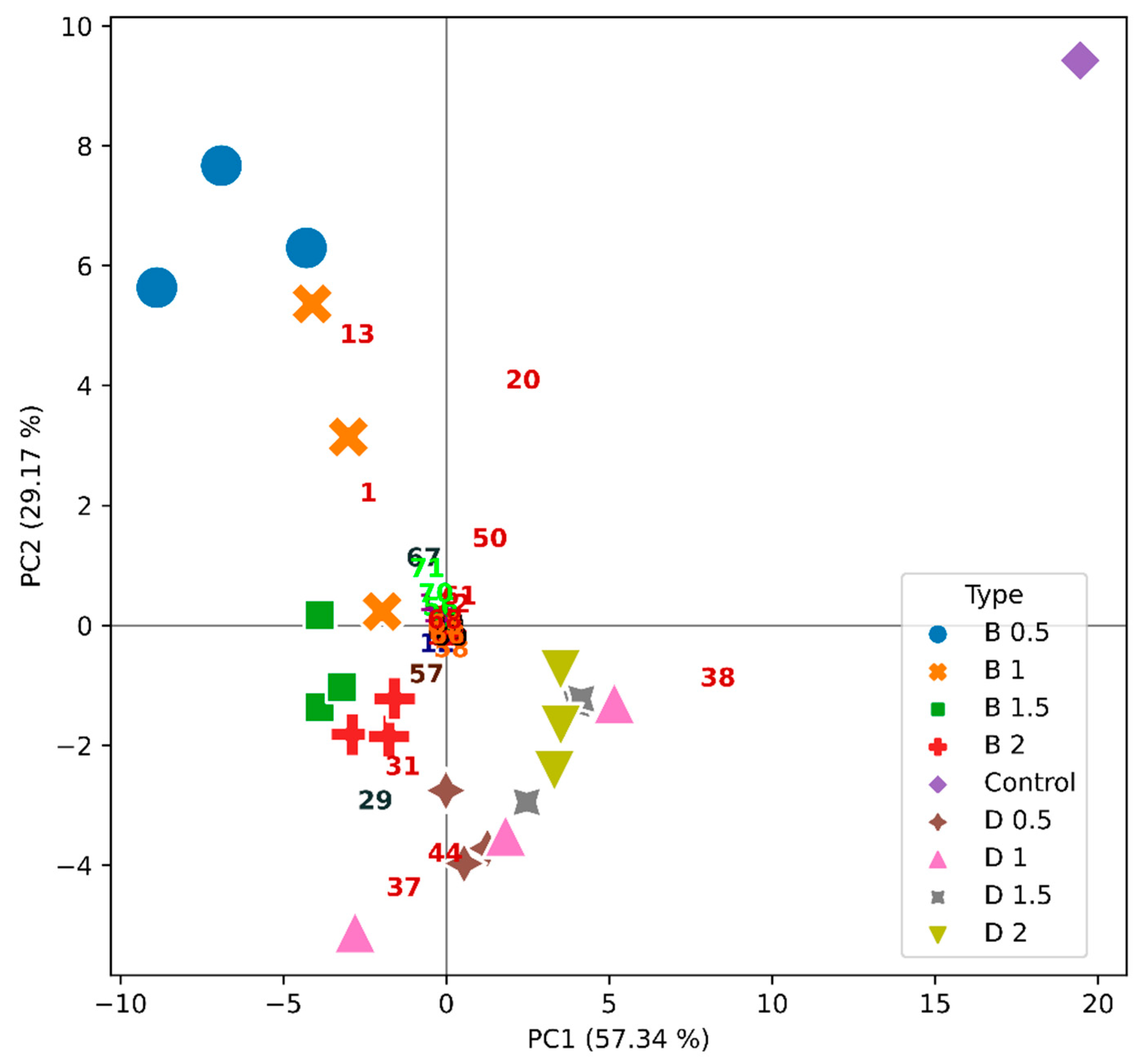

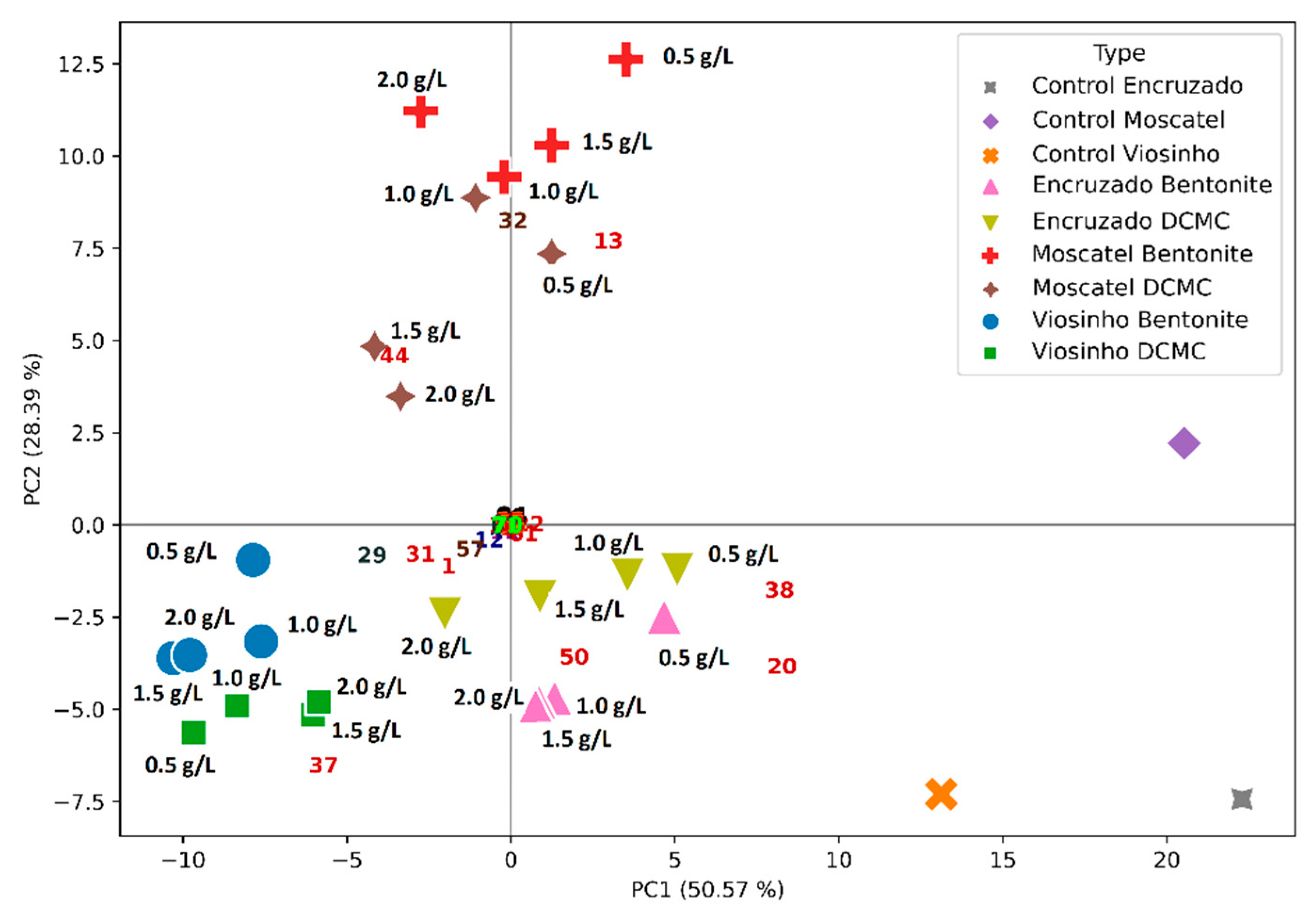
| Wine | Protein Concentration (mg/L) | HST | Stability 1 |
|---|---|---|---|
| Encruzado | 80.1 ± 8 | 0.019 ± 0.002 | stable |
| Viosinho | 87.9 ± 6 | 0.073 ± 0.008 | unstable |
| Moscatel de Setúbal | 218.7 ± 10 | 0.190 ± 0.01 | unstable |
| Peak # | Chemical Family | Compound Name | LRI Calc a | LRI Lit a* | Δ (LRI Calc-LRI Lit) |
|---|---|---|---|---|---|
| 6 | Alcohol | 3-Methyl-1-pentan-1-ol | 851 | 843 | 8 |
| 9 | Alcohol | 3-Hexen-1-ol (isomer) | 860 | 855 | 5 |
| 12 | Alcohol | Hexan-1-ol | 872 | 870 | 2 |
| 24 | Alcohol | 2-Ethylhexanol | 1030 | 1029 | 1 |
| 25 | Alcohol | Benzyl alcohol | 1033 | 1033 | 0 |
| 28 | Alcohol | Octan-1-ol | 1071 | 1078 | −7 |
| 29 | Aldehyde | 4-Methyl benzaldehyde | 1078 | 1079 | −1 |
| 39 | Aldehyde | Decanal | 1203 | 1205 | −2 |
| 5 | Alkanes | 2,4-Dimethyl-hept-1-ene | 845 | 842 | 3 |
| 33 | Alkanes | Cosmene | 1129 | 1134 | −5 |
| 48 | Alkanes | Tridecane | 1296 | 1300 | −4 |
| 56 | Alkanes | Pentadecane | 1498 | 1500 | −2 |
| 67 | Alkanes | Heptadecane | 1693 | 1700 | −7 |
| 70 | Alkanes | Nonadecane | 1895 | 1900 | −5 |
| 71 | Alkanes | Eicosane | 1995 | 2000 | −5 |
| 10 | Aromatic | Ethylbenzene | 862 | 864 | −2 |
| 11 | Aromatic | Xylene | 869 | 866 | 3 |
| 19 | Aromatic | 1,3,5-Trimethyl-benzene | 990 | 995 | −5 |
| 55 | Aromatic | 3-Ethyl-3-phenyl-pent-1-ene | 1477 | NC | |
| 1 | Ester | Ethyl acetate | NC | 610 | NC |
| 2 | Ester | Ethyl isobutyrate | NC | 756 | NC |
| 3 | Ester | Ethyl butanoate | 814 | 806 | 8 |
| 4 | Ester | Ethyl lactate | 824 | 815 | 9 |
| 7 | Ester | Ethyl 2-methylbutanoate | 856 | 856 | 0 |
| 8 | Ester | Ethyl 3-methylbutanoate | 859 | 867 | −8 |
| 13 | Ester | Isoamyl acetate | 880 | 878 | 2 |
| 15 | Ester | 1-Ethoxypropan-2-yl acetate | 937 | 965 | −28 |
| 20 | Ester | Ethyl hexanoate | 1000 | 1001 | −1 |
| 22 | Ester | Hexyl acetate | 1014 | 1015 | −1 |
| 31 | Ester | 2-Butoxyethyl acetate | 1093 | 1090 | 3 |
| 37 | Ester | Diethyl succinate | 1181 | 1182 | −1 |
| 38 | Ester | Ethyl octanoate | 1196 | 1199 | −3 |
| 43 | Ester | Ethyl phenylacetate | 1242 | 1244 | −2 |
| 44 | Ester | 2-Phenethyl acetate | 1253 | 1257 | −4 |
| 47 | Ester | Ethyl nonanoate | 1293 | 1294 | −1 |
| 50 | Ester | Ethyl decanoate | 1394 | 1397 | −3 |
| 52 | Ester | Ethyl-3-methylbutyl butanedioate | 1428 | 1433 | −5 |
| 61 | Ester | Ethyl dodecanoate | 1589 | 1597 | −8 |
| 62 | Ester | 1-O-(2-Methylpropyl) 4-O-propan-2-yl-2,2-dimethyl-3-propan-2-yl-butanedioate | 1592 | 1581 | 11 |
| 68 | Ester | Ethyl tetradecanoate | 1789 | 1793 | −4 |
| 14 | Ketone | 6-Methyl-2-heptanone | 928 | 932 | −4 |
| 27 | Ketone | 2-Nonanone | 1055 | 1091 | −36 |
| 54 | Ketone | Geranyl acetone | 1451 | 1452 | −1 |
| 17 | Miscellaneous | 3-(Methylthio)-1-propanol (methionol) | 977 | 981 | −4 |
| 18 | Miscellaneous | 3(2H)-Thiophenone, dihydro-2-methyl- | 984 | 996 | −12 |
| 32 | Miscellaneous | ethyl 3-(methylthio)-propanoate | 1100 | 1098 | 2 |
| 45 | Miscellaneous | Vitispirane | 1275 | 1281 | −6 |
| 49 | Miscellaneous | Isobutyl-2,2,4-trimethyl-3-hydroxy-pentanoate | 1348 | NC | |
| 57 | Miscellaneous | 2,4-Di-tert-butylphenol | 1511 | 1513 | −2 |
| 59 | Miscellaneous | Dehydro-ar-himachalene | 1537 | 1514 | 23 |
| 23 | Terpenoid | Limonene | 1026 | 1028 | −2 |
| 26 | Terpenoid | β-Ocimene | 1038 | 1049 | −11 |
| 30 | Terpenoid | Terpinolene | 1086 | 1088 | −2 |
| 34 | Terpenoid | Nerol oxide | 1153 | 1153 | 0 |
| 36 | Terpenoid | Linalool | 1171 | 1099 | 72 |
| 40 | Terpenoid | Nerol | 1226 | 1228 | −2 |
| 46 | Terpenoid | Geraniol | 1285 | 1267 | 18 |
| 60 | Terpenoid | α-Calacorene | 1540 | 1542 | −2 |
| 63 | Terpenoid | α-Corocalene | 1617 | 1623 | −6 |
| 16 | Unknown | Unknown | 971 | ||
| 35 | Unknown | Unknown | 1167 | ||
| 41 | Unknown | Unknown | 1231 | ||
| 42 | Unknown | Unknown | 1236 | ||
| 51 | Unknown | Unknown | 1415 | ||
| 58 | Unknown | Unknown | 1519 | ||
| 64 | Unknown | Unknown | 1639 | ||
| 65 | Unknown | Unknown | 1658 | ||
| 66 | Unknown | Unknown | 1663 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saracino, F.; Brinco, J.; Gago, D.; Gomes da Silva, M.; Boavida Ferreira, R.; Ricardo-da-Silva, J.; Chagas, R.; Ferreira, L.M. DCMC as a Promising Alternative to Bentonite in White Wine Stabilization. Impact on Protein Stability and Wine Aromatic Fraction. Molecules 2021, 26, 6188. https://doi.org/10.3390/molecules26206188
Saracino F, Brinco J, Gago D, Gomes da Silva M, Boavida Ferreira R, Ricardo-da-Silva J, Chagas R, Ferreira LM. DCMC as a Promising Alternative to Bentonite in White Wine Stabilization. Impact on Protein Stability and Wine Aromatic Fraction. Molecules. 2021; 26(20):6188. https://doi.org/10.3390/molecules26206188
Chicago/Turabian StyleSaracino, Francesco, João Brinco, Diana Gago, Marco Gomes da Silva, Ricardo Boavida Ferreira, Jorge Ricardo-da-Silva, Ricardo Chagas, and Luísa Maria Ferreira. 2021. "DCMC as a Promising Alternative to Bentonite in White Wine Stabilization. Impact on Protein Stability and Wine Aromatic Fraction" Molecules 26, no. 20: 6188. https://doi.org/10.3390/molecules26206188
APA StyleSaracino, F., Brinco, J., Gago, D., Gomes da Silva, M., Boavida Ferreira, R., Ricardo-da-Silva, J., Chagas, R., & Ferreira, L. M. (2021). DCMC as a Promising Alternative to Bentonite in White Wine Stabilization. Impact on Protein Stability and Wine Aromatic Fraction. Molecules, 26(20), 6188. https://doi.org/10.3390/molecules26206188








