Evaluation of the Relationship among Biogenic Amines, Nitrite and Microbial Diversity in Fermented Mustard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Media
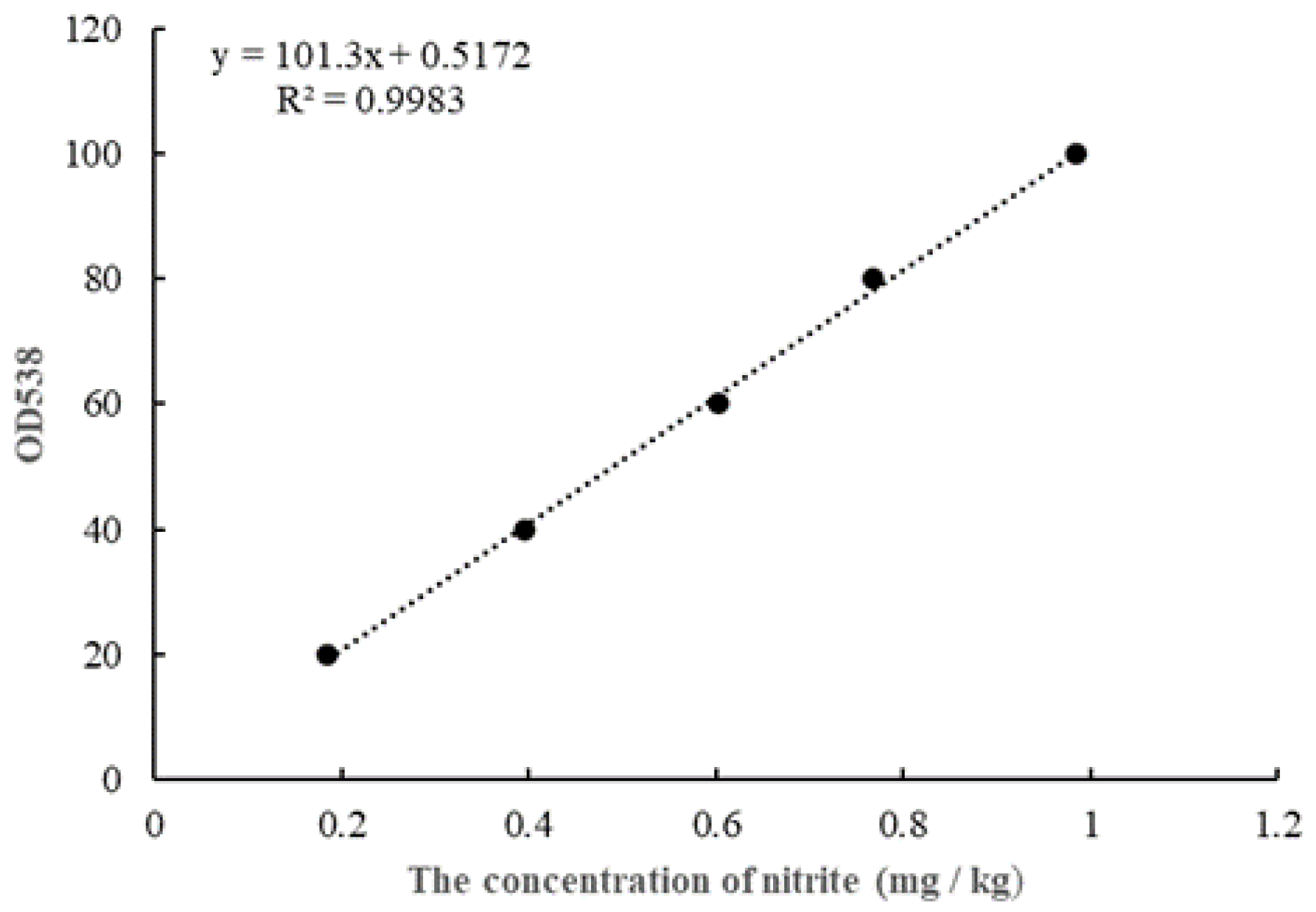
2.2. Determination of Nitrite Concentration
2.3. Determination of BAs Concentrations
2.4. Microbial Community Analysis
2.5. Isolation and Purified of Lactic Acid Bacteria (LAB) from FM Samples
2.6. Strains Identification
2.7. Evaluation of BAs and Nitrite Production Ability
2.8. Evaluation of BAs and Nitrite Degradation Ability
2.9. Fermented Mustard Product Model Analysis
3. Results and Discussion
3.1. BAs Contents in FM Samples
3.2. Nitrite Contents in FM Samples
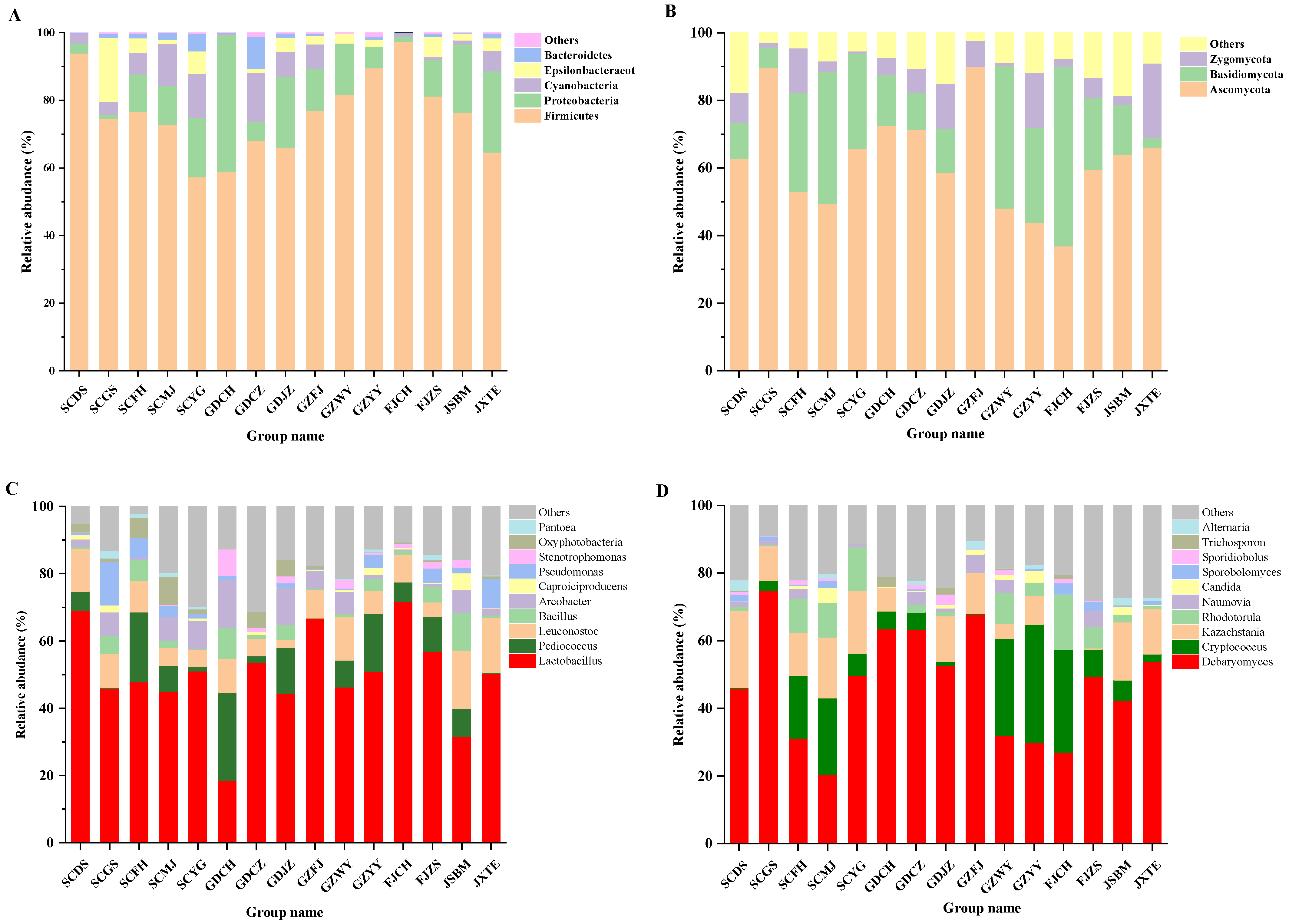
3.3. Microbial Communities in FM Samples
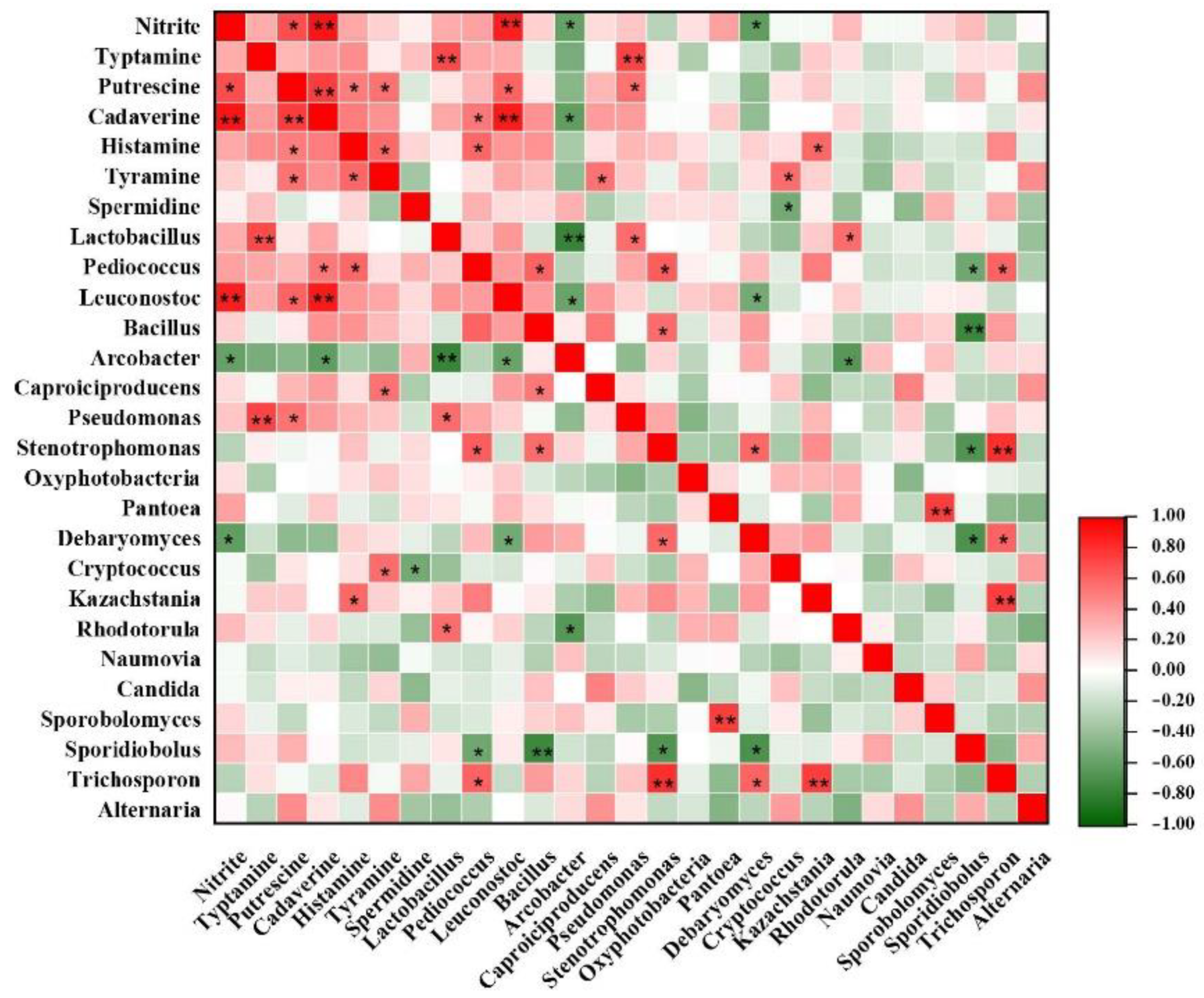
3.4. Correlations among Microbial Communities, Six Main BAs and Nitrite
3.5. Microbial Contribution to BAs and Nitrite Contents in FM
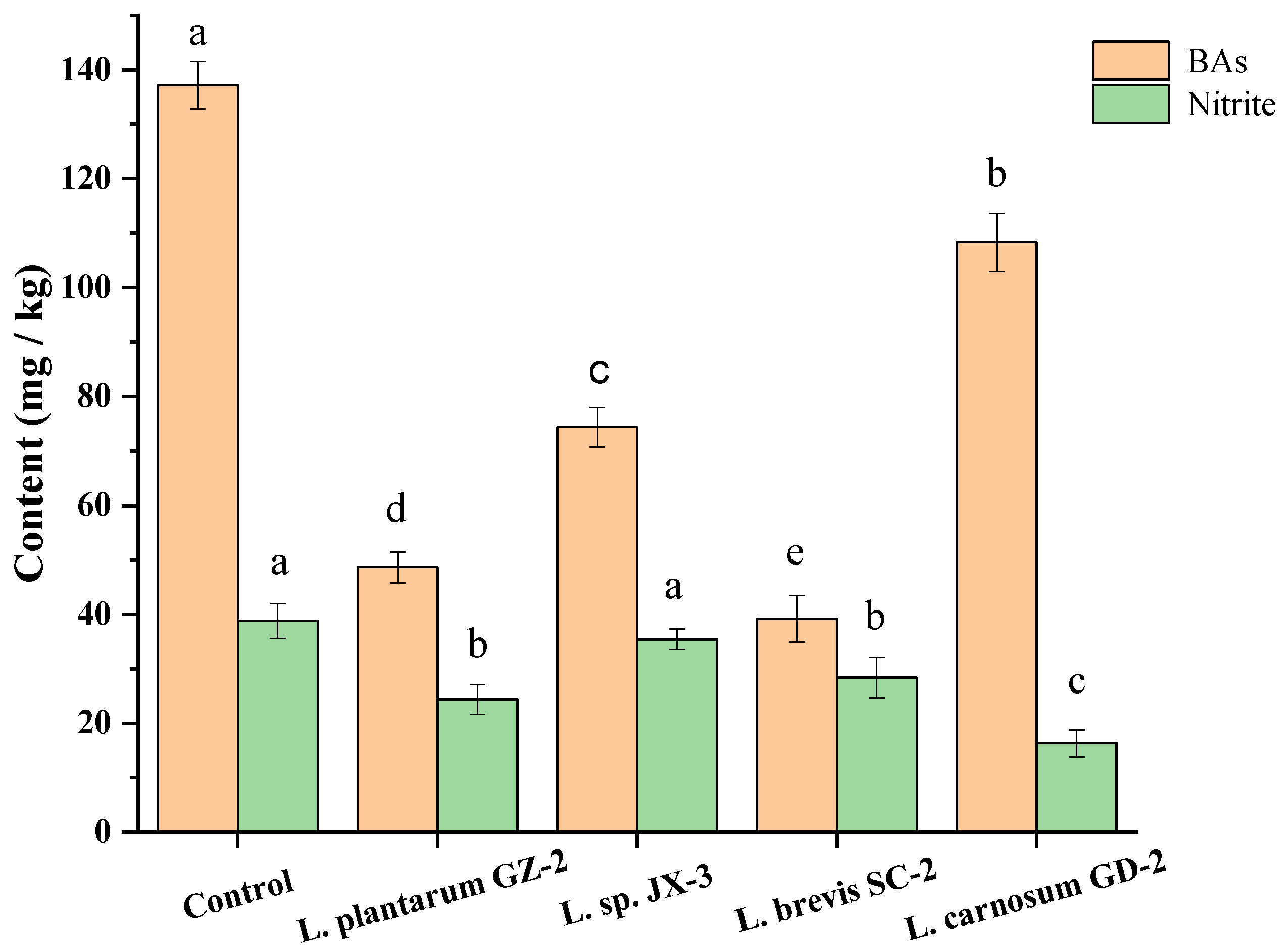
3.6. The BAs and Nitrite Controlling Capacity of Selected Strains in FM Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, A.-J.; Luo, W.; Peng, Y.-T.; Niu, K.-L.; Liu, X.-Y.; Shen, G.-H.; Zhang, Z.-Q.; Wan, H.; Luo, Q.-Y.; Li, S.-S. Quality and microbial flora changes of radish paocai during multiple fermentation rounds. Food Control 2019, 106, 106733. [Google Scholar] [CrossRef]
- Liu, A.; Li, X.; Pu, B.; Ao, X.; Zhou, K.; He, L.; Chen, S.; Liu, S. Use of psychrotolerant lactic acid bacteria (Lactobacillus spp. and Leuconostoc spp.) Isolated from Chinese Traditional Paocai for the Quality Improvement of Paocai Products. J. Agric. Food Chem. 2017, 65, 2580–2587. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.; Lee, J.H.; Mah, J.-H. Occurrence and reduction of biogenic amines in Kimchi and Korean fermented seafood products. Foods 2019, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Yoo, M.; Shin, D. The identification and quantification of biogenic amines in Korean turbid rice wine, Makgeolli by HPLC with mass spectrometry detection. LWT-Food Sci. Technol. 2015, 62, 350–356. [Google Scholar] [CrossRef]
- Świder, O.; Roszko, M.Ł.; Wójcicki, M.; Szymczyk, K. Biogenic Amines and Free Amino Acids in Traditional Fermented Vegetables—Dietary Risk Evaluation. J. Agric. Food Chem. 2020, 68, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Feddern, V.; Mazzuco, H.; Fonseca, F.N.; de Lima, G.J.M.M. A review on biogenic amines in food and feed: Toxicological aspects, impact on health and control measures. Anim. Prod. Sci. 2019, 59, 608–618. [Google Scholar] [CrossRef]
- Brink, B.T.; Damink, C.T.; Joosten, H.; Veld, J.H.H.I.T. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jin, Y.H.; Park, Y.K.; Yun, S.J.; Mah, J.-H. Formation of Biogenic Amines in Pa (Green Onion) Kimchi and Gat (Mustard Leaf) Kimchi. Foods 2019, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zou, H.; Qu, C.; Zhang, L.; Liu, T.; Wu, H.; Li, Y. Dominant Microorganisms during the Spontaneous Fermentation of Suan Cai, a Chinese Fermented Vegetable. Food Sci. Technol. Res. 2014, 20, 915–926. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Johanningsmeier, S.D.; Price, R.; Reynolds, R.; Truong, V.-D.; Payton, S.C.; Breidt, F. Evaluation of nitrate and nitrite contents in pickled fruit and vegetable products. Food Control 2018, 90, 304–311. [Google Scholar] [CrossRef]
- Huang, T.-T.; Wu, Z.-Y.; Zhang, W.-X. Effects of garlic addition on bacterial communities and the conversions of nitrate and nitrite in a simulated pickle fermentation system. Food Control 2020, 113, 107215. [Google Scholar] [CrossRef]
- Bollenbach, A.; Hanff, E.; Tsikas, D. Investigation of NG-hydroxy-l-arginine interference in the quantitative determination of nitrite and nitrate in human plasma and urine by GC-NICI-MS. J. Chromatogr. B 2018, 1100–1101, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Hu, Z.; Yang, X.; Gao, Y.; Ma, C. Nitrite-induced acute kidney injury with secondary hyperparathyroidism: Case report and literature review. Medicine 2018, 97, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, P.; Zhang, G.; Mao, X.; Zhao, Y.; Wang, J.; Duan, C.; Li, C.; Li, X. Residual nitrite and biogenic amines of traditional northeast sauerkraut in China. Int. J. Food Prop. 2017, 20, 2448–2455. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ma, Y.; Chen, M.; Wang, Y.; Lei, S.; Li, F.; Liu, D. Effect of pH on Nitrite Reduction of Pickled Chinese Cabbage. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Li, L.; Zou, D.; Ruan, L.; Wen, Z.; Chen, S.; Xu, L.; Wei, X. Evaluation of the Biogenic Amines and Microbial Contribution in Traditional Chinese Sausages. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ruan, L.; Ji, A.; Wen, Z.; Chen, S.; Wang, L.; Wei, X. Biogenic amines analysis and microbial contribution in traditional fermented food of Douchi. Sci. Rep. 2018, 8, 12567–12573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, C.M.; Schieberle, P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agric. Food Chem. 2012, 60, 3026–3032. [Google Scholar] [CrossRef]
- Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guidance; Food and Drug Administration: Washington, DC, USA, 2011.
- Kim, B.; Byun, B.Y.; Mah, J.-H. Biogenic amine formation and bacterial contribution in Natto products. Food Chem. 2012, 135, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- Peñas, E.; Frias, J.; Sidro, B.; Vidal-Valverde, C. Impact of fermentation conditions and refrigerated storage on microbial quality and biogenic amine content of sauerkraut. Food Chem. 2010, 123, 143–150. [Google Scholar] [CrossRef]
- Yan, P.; Chai, Z.; Chang, X.; Zhao, W.; Yue, H.; Zhang, T. Screening and identification of microorganism degrading nitrite in Chinese sauerkraut. Agro Food Ind. Hi-tech 2015, 26, 20–23. [Google Scholar]
- Yu, S.M.; Zhang, Y. Effects of Lactic Acid Bacteria on Nitrite Degradation during Pickle Fermentation. Adv. Mater. Res. 2013, 781–784, 1656–1660. [Google Scholar] [CrossRef]
- Guarcello, R.; De Angelis, M.; Settanni, L.; Formiglio, S.; Gaglio, R.; Minervini, F.; Moschetti, G.; Gobbetti, M. Selection of Amine-Oxidizing Dairy Lactic Acid Bacteria and Identification of the Enzyme and Gene Involved in the Decrease of Biogenic Amines. Appl. Environ. Microbiol. 2016, 82, 6870–6880. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Liu, T.; Hou, J.; Pan, L.; Sadiq, F.A.; Yuan, L.; Yang, H.; He, G. Analysis of bacterial diversity and biogenic amines content during the fermentation processing of stinky tofu. Food Res. Int. 2018, 111, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yu, M.; Liu, X.; Meng, L.; Wang, Q.; Xue, Y.; Wu, J.; Yue, X. Changes in flavour and microbial diversity during natural fermentation of suan-cai, a traditional food made in Northeast China. Int. J. Food Microbiol. 2015, 211, 23–31. [Google Scholar] [CrossRef]
- Xiong, T.; Li, X.; Guan, Q.; Peng, F.; Xie, M. Starter culture fermentation of Chinese sauerkraut: Growth, acidification and metabolic analyses. Food Control 2014, 41, 122–127. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Liu, X.-W.; Huang, J.-L.; Baloch, S.; Xu, X.; Pei, X.-F. Microbial diversity and chemical analysis of Shuidouchi, traditional Chinese fermented soybean. Food Res. Int. 2019, 116, 1289–1297. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.; Hou, Q.; Xu, H.; Zheng, Y.; Zhang, H.; Zhang, L. Assessment of bacterial profiles in aged, home-made Sichuan paocai brine with varying titratable acidity by PacBio SMRT sequencing technology. Food Control 2017, 78, 14–23. [Google Scholar] [CrossRef]
- Liang, H.; Chen, H.; Zhang, W.; Yu, C.; Ji, C.; Lin, X. Investigation on microbial diversity of industrial Zhacai paocai during fermentation using high-throughput sequencing and their functional characterization. LWT-Food Sci. Technol. 2018, 91, 460–466. [Google Scholar] [CrossRef]
- Park, E.-J.; Chun, J.; Cha, C.-J.; Park, W.-S.; Jeon, C.O.; Bae, J.-W. Bacterial community analysis during fermentation of ten representative kinds of kimchi with barcoded pyrosequencing. Food Microbiol. 2012, 30, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiong, T.; Peng, Z.; Liu, C.; Huang, T.; Yu, H.; Xie, M. Correlation between microbiota and flavours in fermentation of Chinese Sichuan Paocai. Food Res. Int. 2018, 114, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Wei, B.; Huang, T.; Xiao, Y.; Peng, Z.; Xie, M.; Xiong, T. Bacterial community and composition in Jiang-shui and Suan-cai revealed by high-throughput sequencing of 16S rRNA. Int. J. Food Microbiol. 2019, 306, 108271–108282. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.R.; Srinivasan, S.; Ravi, A.V. Inhibition of quorum sensing-mediated virulence in Serratia marcescens by Bacillus subtilis R-18. Microb. Pathog. 2018, 120, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yang, H.S.; Li, J.; Han, S.K.; Chang, H.C.; Kim, H.Y. Identification of lactic acid bacteria in salted Chinese cabbage by SDS-PAGE and PCR-DGGE. J. Sci. Food Agric. 2014, 94, 296–300. [Google Scholar] [CrossRef]
- Kirschbaum, J.; Rebscher, K.; Brückner, H. Liquid chromatographic determination of biogenic amines in fermented foods after derivatization with 3, 5-dinitrobenzoyl chloride. J. Chromatogr. A 2000, 881, 517–530. [Google Scholar] [CrossRef]
- Aflaki, F.; Ghoulipour, V.; Saemian, N.; Salahinejad, M. A simple method for benzoyl chloride derivatization of biogenic amines for high performance liquid chromatography. Anal. Methods 2014, 6, 1482–1487. [Google Scholar] [CrossRef]
- Jin, Y.H.; Lee, J.H.; Park, Y.K.; Lee, J.-H.; Mah, J.-H. The occurrence of biogenic amines and determination of biogenic amine-producing lactic acid bacteria in Kkakdugi and Chonggak kimchi. Foods 2019, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Liu, X.; Wang, G.; Zhang, H.; Xiong, Z.; Sun, Y.; Ai, L. Characterization and selection of Lactobacillus brevis starter for nitrite degradation of Chinese pickle. Food Control 2017, 78, 126–131. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A Holistic Review on Euro-Asian Lactic Acid Bacteria Fermented Cereals and Vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Kang, K.H.; Lee, S.; Kim, S.J.; Kim, J.G.; Chung, M.J. Kimchi probiotic bacteria contribute to reduced amounts of N-nitrosodimethylamine in lactic acid bacteria-fortified kimchi. LWT-Food Sci. Technol. 2017, 84, 196–203. [Google Scholar] [CrossRef]
- Rabie, M.A.; Siliha, H.; Saidy, S.; Badawy, A.A.; Malcata, F.X. Reduced biogenic amine contents in sauerkraut via addition of selected lactic acid bacteria. Food Chem. 2011, 129, 1778–1782. [Google Scholar] [CrossRef]

| Number | Region | The Abbreviation of Brand Name | Label |
|---|---|---|---|
| 1 | Sichuan (SC) | DS | SCDS |
| 2 | Sichuan (SC) | GS | SCGS |
| 3 | Sichuan (SC) | FH | SCFH |
| 4 | Sichuan (SC) | MJ | SCMJ |
| 5 | Sichuan (SC) | YG | SCYG |
| 6 | Guangdong (GD) | CH | GDCH |
| 7 | Guangdong (GD) | CZ | GDCZ |
| 8 | Guangdong (GD) | JZ | GDJZ |
| 9 | Guizhou (GZ) | FJ | GZFJ |
| 10 | Guizhou (GZ) | WY | GZWY |
| 11 | Guizhou (GZ) | YY | GZYY |
| 12 | Fujian (FJ) | CH | FJCH |
| 13 | Fujian (FJ) | ZS | FJZS |
| 14 | Jiangsu (JS) | BM | JSBM |
| 15 | Jiangxi (JX) | TE | JXTE |
| Parameters | Putrescine | Cadaverine | Tyramine | Histamine | Tryptamine | Spermidine |
|---|---|---|---|---|---|---|
| LOD (mg/kg) | 0.25 | 0.24 | 0.31 | 0.42 | 0.39 | 0.19 |
| LOQ (mg/kg) | 0.86 | 0.76 | 0.93 | 1.27 | 1.11 | 0.53 |
| Group Sample. | Total BAs (mg/kg) | Tryptamine (mg/kg) | Putrescine (mg/kg) | Cadaverine (mg/kg) | Histamine (mg/kg) | Tyramine (mg/kg) | Spermidine (mg/kg) |
|---|---|---|---|---|---|---|---|
| GZYY | 259.86 ± 6.23 a | 35.74 ± 1.34 a | 39.71 ± 3.95 b | 97.92 ± 2.33 a | 37.85 ± 1.66 b | 45.25 ± 0.46 a | 3.39 ± 0.19 a |
| SCDS | 199.45 ± 2.43 b | 13.75 ± 1.49 g,h | 61.75 ± 1.35 a | 62.80 ± 0.46 d | 31.35 ± 0.31 c | 29.79 ± 1.42 e | ND |
| SCFH | 195.92 ± 3.5 b,c | 14.60 ± 1.22 f,g,h | 24.72 ± 1.27 d | 81.89 ± 0.18 b | 24.55 ± 1.1 f | 47.64 ± 1.21 a | 2.52 ± 0.06 a,b |
| FJZS | 189.50 ± 0.74 c,b | 17.59 ± 0.04 e | 31.60 ± 0.12 c | 74.68 ± 1.53 c | 27.22 ± 0.82 d,e | 36.11 ± 1.26 c | 2.30 ± 0.02 a,b |
| JSBM | 184.90 ± 3.47 c,d | 11.76 ± 0.08 i,j | 27.50 ± 0.52 d | 75.16 ± 0.06 c | 29.61 ± 1.77 c,d | 40.88 ± 1.32 b | ND |
| FJCH | 157.17 ± 4.68 e | 26.76 ± 0.61 b | 26.14 ± 1.76 d | 49.11 ± 1.7 e | 25.09 ± 1.47 f | 37.03 ± 1.13 e,f | 2.54 ± 0.13 a,b |
| GDJZ | 137.70 ± 1.13 f | 20.45 ± 0.89 d | 18.02 ± 0.93 e,f | 10.57 ± 0.34 h | 62.05 ± 0.88a | 23.35 ± 0.15 g | 3.27 ± 0.02 a |
| SCMJ | 125.88 ± 3.58 g | 14.28 ± 0.26 f,g,h | 15.74 ± 0.51 f,g | 41.2 ± 1.17 f | 19.93 ± 0.62 g | 32.30 ± 0.93d | ND |
| GDCH | 102.84 ± 4.84 h | 15.73 ± 0.72 f | 14.72 ± 1.2 f,g | 26.5 ± 0.68 g | 25.29 ± 0.67 e,f | 17.47 ± 1.43 i | 3.13 ± 0.15 a |
| GZWY | 83.22 ± 3.85 i | 24.80 ± 0.29 c | 13.65 ± 1.64fg | 11.97 ± 0.60 h | 12.61 ± 0.9 h | 20.18 ± 0.41 h | ND |
| SCGS | 72.83 ± 1.72 j | 13.89 ± 0.64 g,h | 8.21 ± 1.3 h | 9.68 ± 0.4 h | 11.62 ± 1.08 h | 26.4 ± 0.78 f | 3.03 ± 0.13 a |
| GDCZ | 69.45 ± 4.04 j | 10.34 ± 0.89 j | 19.32 ± 1.17 e | 6.54 ± 0.24 i | ND | 29.87 ± 1.93 e | 3.38 ± 1.59 a |
| JXTE | 38.91 ± 2.15 k | 14.89 ± 0.21 f,g | 4.58 ± 1.34 i | ND | 5.18 ± 0.14 i | 12.88 ± 2.07 j | 2.39 ± 0.22 a,b |
| GZFJ | 35.40 ± 3.23 k | 11.02 ± 0.22 i,j | 4.99 ± 0.76 i | ND | ND | 16.68 ± 1.42i | 1.96 ± 0.83 b |
| SCYG | 34.57 ± 4.50 k | 12.85 ± 0.14 h,i | 3.79 ± 0.98 i | ND | 4.90 ± 1.96 i | 13.03 ± 1.7 i | ND |
| Samples | Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|
| Observed OUTs | Chao1 | Shannon | Goods Coverage (%) | Observed OUTs | Chao1 | Shannon | Goods Coverage (%) | |
| GZYY | 282 ± 12 | 283 ± 19 | 5.43 ± 0.34 | 99 | 22 ± 2 | 0.04 ± 0 | 23 ± 3 | 99 |
| SCDS | 258 ± 14 | 261 ± 35 | 5.77 ± 0.14 | 99 | 15 ± 1 | 0.03 ± 0 | 18 ± 2 | 99 |
| SCFH | 349 ± 36 | 348 ± 44 | 5.03 ± 0.23 | 99 | 27 ± 3 | 0.27 ± 0.01 | 25 ± 4 | 99 |
| FJZS | 337 ± 10 | 342 ± 28 | 4.78 ± 0.16 | 99 | 32 ± 3 | 0.3 ± 0.02 | 32 ± 5 | 99 |
| JSBM | 302 ± 16 | 303 ± 26 | 5.67 ± 0.28 | 99 | 14 ± 1 | 0.02 ± 0 | 14 ± 3 | 99 |
| FJCH | 170 ± 8 | 170 ± 13 | 4.51 ± 0.24 | 99 | 13 ± 4 | 0.04 ± 0 | 15 ± 4 | 99 |
| GDJZ | 312 ± 23 | 314 ± 19 | 4.94 ± 0.26 | 99 | 60 ± 6 | 0.57 ± 0.04 | 64 ± 7 | 99 |
| SCMJ | 299 ± 14 | 307 ± 27 | 5.63 ± 0.46 | 99 | 19 ± 2 | 0.04 ± 0 | 19 ± 4 | 99 |
| GDCH | 292 ± 8 | 298 ± 40 | 5.74 ± 0.2 | 99 | 29 ± 3 | 0.44 ± 0.13 | 29 ± 5 | 99 |
| GZWY | 186 ± 6 | 188 ± 16 | 4.55 ± 0.16 | 99 | 17 ± 6 | 0.04 ± 0 | 19 ± 4 | 99 |
| SCGS | 421 ± 15 | 436 ± 37 | 5.46 ± 0.26 | 99 | 26 ± 3 | 0.29 ± 0.01 | 25 ± 3 | 99 |
| GDCZ | 404 ± 31 | 408 ± 35 | 5.33 ± 0.18 | 99 | 30 ± 2 | 0.22 ± 0.03 | 32 ± 5 | 99 |
| JXTE | 390 ± 22 | 394 ± 46 | 5.15 ± 0.45 | 99 | 37 ± 4 | 0.34 ± 0.04 | 40 ± 6 | 99 |
| GZFJ | 187 ± 22 | 187 ±1 8 | 5.40 ± 0.23 | 99 | 22 ± 5 | 0.04 ± 0 | 21 ± 3 | 99 |
| SCYG | 436 ± 28 | 408 ± 51 | 5.49 ± 0.35 | 99 | 25 ± 3 | 0.29 ± 0.02 | 25 ± 2 | 99 |
| Strain | Tryptamine (Tryptophan) (mg/kg) | Putrescine (Agmatine Sulfate Salt) (mg/kg) | Cadaverine (Lysine) (mg/kg) | Histamine (Histidin) (mg/kg) | Tyramine (Tyrosine) (mg/kg) | Total BAs (mg/kg) | Nitrite (mg/kg) |
|---|---|---|---|---|---|---|---|
| SC-1 | 7.54 ± 0.93 c | 13.45 ± 1.14 c | ND | ND | 26.31 ± 1.05 b | 47.3 ± 1.67 c | ND |
| SC-2 | ND | ND | ND | 8.53 ± 0.76 b | 5.42 ± 0.68 g | 13.95 ± 0.96 h | 39.52 ± 1.87 c |
| SC-3 | ND | 5.34 ± 0.93 e | 10.65 ± 0.57 b | ND | 20.31 ± 1.34 c | 36.30 ± 1.08 d | 53.31 ± 3.34 a |
| SC-4 | 18.24 ± 1.07 a | ND | 5.42 ± 0.37 c | 23.51 ± 1.24 a | 30.54 ± 1.73 a | 77.71 ± 1.73 a | 8.62 ± 1.26 f |
| SC-5 | 12.43 ± 1.31 b | 10.24 ± 1.05 d | 17.61 ± 1.64 a | ND | 6.31 ± 0.61 g | 56.59 ± 1.15 b | 36.42 ± 1.71 c |
| JX-1 | 10.34 ± 0.92 bc | 4.65 ± 0.61 e | ND | ND | 14.65 ± 0.91 d | 29.64 ± 0.83 e | 16.34 ± 1.93 e |
| JX-2 | ND | 17.15 ± 0.98 b | ND | 10.32 ± 0.37 b | ND | 27.47 ± 0.79 f | 25.86 ± 2.76 d |
| JX-3 | 5.85 ± 0.43 d | ND | ND | ND | 7.12 ± 0.61 f,g | 12.97 ± 0.92 h | ND |
| GD-1 | 4.50 ± 0.82 d,e | ND | ND | ND | 8.62 ± 0.34 e | 13.12 ± 0.71 h | 37.65 ± 2.08 c |
| GD-2 | 7.45 ± 0.34 c | 23.65 ± 1.62 a | 9.61 ± 0.83 b | ND | 15.43 ± 0.57 d | 56.14 ± 1.01 b | 4.86 ± 1.17 g |
| GZ-1 | 3.43 ± 0.72 e | 9.15 ± 0.93 d | ND | ND | 7.61 ± 0.81 f | 20.19 ± 0.54 g | 46.17 ± 2.43 b |
| GZ-2 | ND | 4.65 ± 0.76 e | ND | ND | ND | 4.65 ± 0.49 i | NG |
| GZ-3 | 8.64 ± 0.87 c | 16.54 ± 1.21 b | ND | ND | 9.31 ± 0.69 e | 34.49 ± 1.73d | 16.23 ± 1.72 e |
| Strain | Tryptamine (%) | Putrescine (%) | Cadaverine (%) | Histamine (%) | Tyramine (%) | Nitrite (%) |
|---|---|---|---|---|---|---|
| SC-1 | 0 | 8.32 ± 1.14 c | 0 | 0 | 17.32 ± 2.16 a | 44.26 ± 2.34 a |
| SC-2 | 0 | 0 | 0 | 0 | 0 | 13.46 ± 3.46 d |
| SC-3 | 0 | 0 | 0 | 0 | 0 | 0 |
| SC-4 | 10.32 ± 1.24 c | 0 | 17.32 ± 1.68 a | 23.65 a | 0 | 14.62 ± 2.17 d |
| SC-5 | 0 | 0 | 0 | 0 | 0 | 0 |
| JX-1 | 15.36 ± 0.94 a | 0 | 0 | 0 | 0 | 6.32 ± 1.92 e |
| JX-2 | 0 | 21.35 ± 2.16 a | 0 | 0 | 0 | 0 |
| JX-3 | 0 | 0 | 0 | 0 | 0 | 3.26 ± 1.14 f |
| GD-1 | 0 | 0 | 0 | 0 | 0 | 0 |
| GD-2 | 0 | 0 | 10.64 ± 0.92 b | 0 | 9.56 ± 1.16 b | 28.32 ± 2.09 b |
| GZ-1 | 0 | 0 | 0 | 0 | 0 | 4.56 ± 1.21 e,f |
| GZ-2 | 18.62 ± 1.86 b | 16.35 ± 1.34 b | 0 | 0 | 0 | 21.62 ± 3.42 c |
| GZ-3 | 0 | 0 | 0 | 0 | 0 | 0 |
| SC-1 | 0 | 0 | 0 | 0 | 0 | 38.41 ± 3.91 a |
| SC-2 | 0 | 0 | 0 | 0 | 0 | 7.32 ± 1.73 e |
| Isolates | Closest Strains | Identities (%) | Accession No. |
|---|---|---|---|
| GZ-2 | Lactobacillus plantarum MLG5-1 | 99 | EU600906.1 |
| JX-3 | Lactobacillus plantarum. KLDS 1.0702 | 99 | MT473388.1 |
| SC-2 | Lactobacillus brevis ATCC 14869 | 99 | NR044704.2 |
| GD-2 | Leuconostoc carnosum JB16 | 99 | HV538100.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Li, L.; Xu, Y.; An, K.; Shi, Q.; Yu, Y.; Xu, Z. Evaluation of the Relationship among Biogenic Amines, Nitrite and Microbial Diversity in Fermented Mustard. Molecules 2021, 26, 6173. https://doi.org/10.3390/molecules26206173
Yu Y, Li L, Xu Y, An K, Shi Q, Yu Y, Xu Z. Evaluation of the Relationship among Biogenic Amines, Nitrite and Microbial Diversity in Fermented Mustard. Molecules. 2021; 26(20):6173. https://doi.org/10.3390/molecules26206173
Chicago/Turabian StyleYu, Yangyang, Lu Li, Yujuan Xu, Kejing An, Qiao Shi, Yuanshan Yu, and Zhenlin Xu. 2021. "Evaluation of the Relationship among Biogenic Amines, Nitrite and Microbial Diversity in Fermented Mustard" Molecules 26, no. 20: 6173. https://doi.org/10.3390/molecules26206173
APA StyleYu, Y., Li, L., Xu, Y., An, K., Shi, Q., Yu, Y., & Xu, Z. (2021). Evaluation of the Relationship among Biogenic Amines, Nitrite and Microbial Diversity in Fermented Mustard. Molecules, 26(20), 6173. https://doi.org/10.3390/molecules26206173






