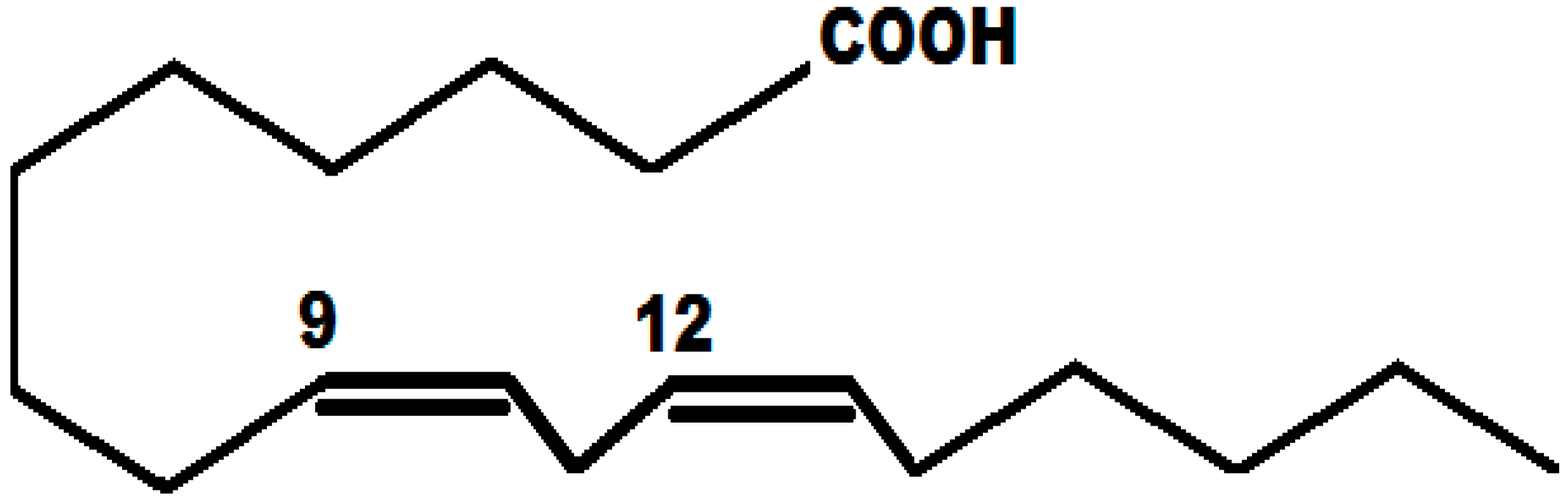
Experimental and Theoretical Studies of α-Linolenic Acid as Green Corrosion Inhibitor for Carbon Steel in 0.5 M Sulfuric Acid
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Testing Material
2.2. Testing Solution
2.3. Weight Loss Measurements
2.4. Electrochemical Techniques
2.4.1. Potentiodynamic Polarization Curves
2.4.2. Electrochemical Impedance Spectroscopy Studies
2.5. Computational Studies
3. Results and Discussion
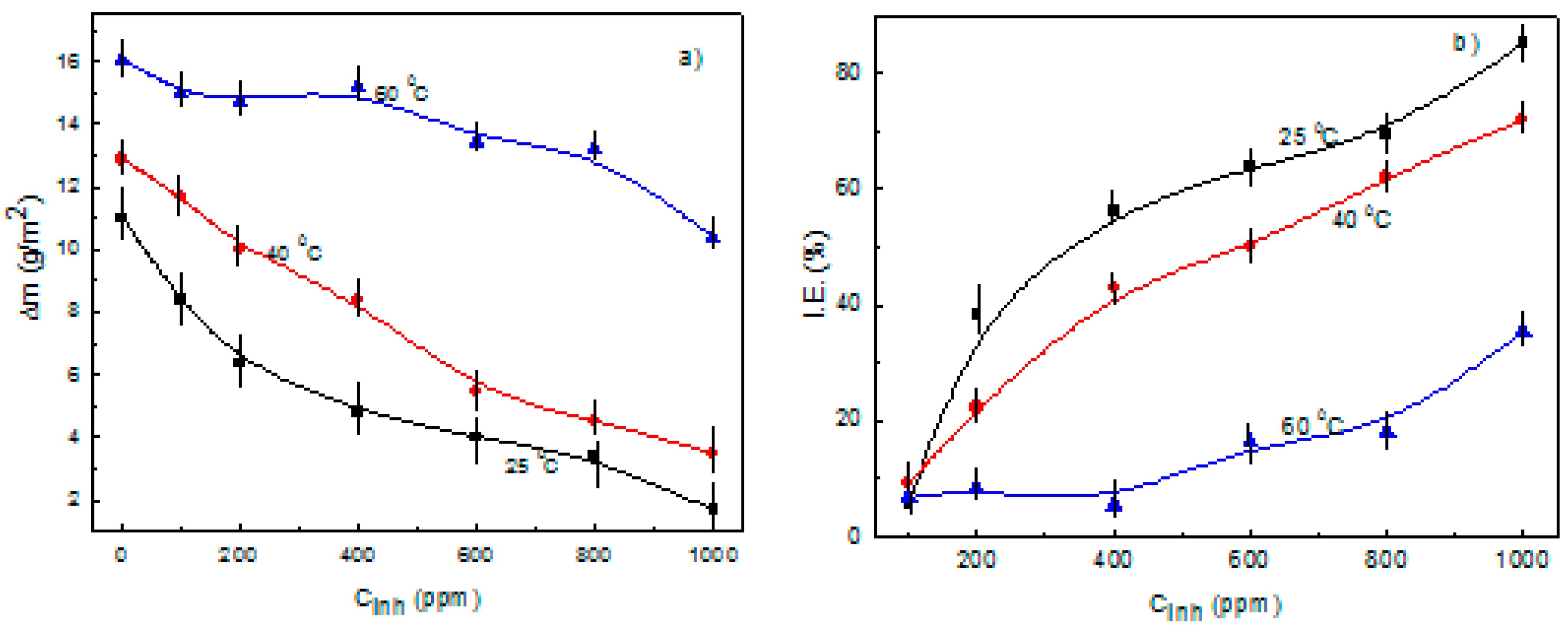
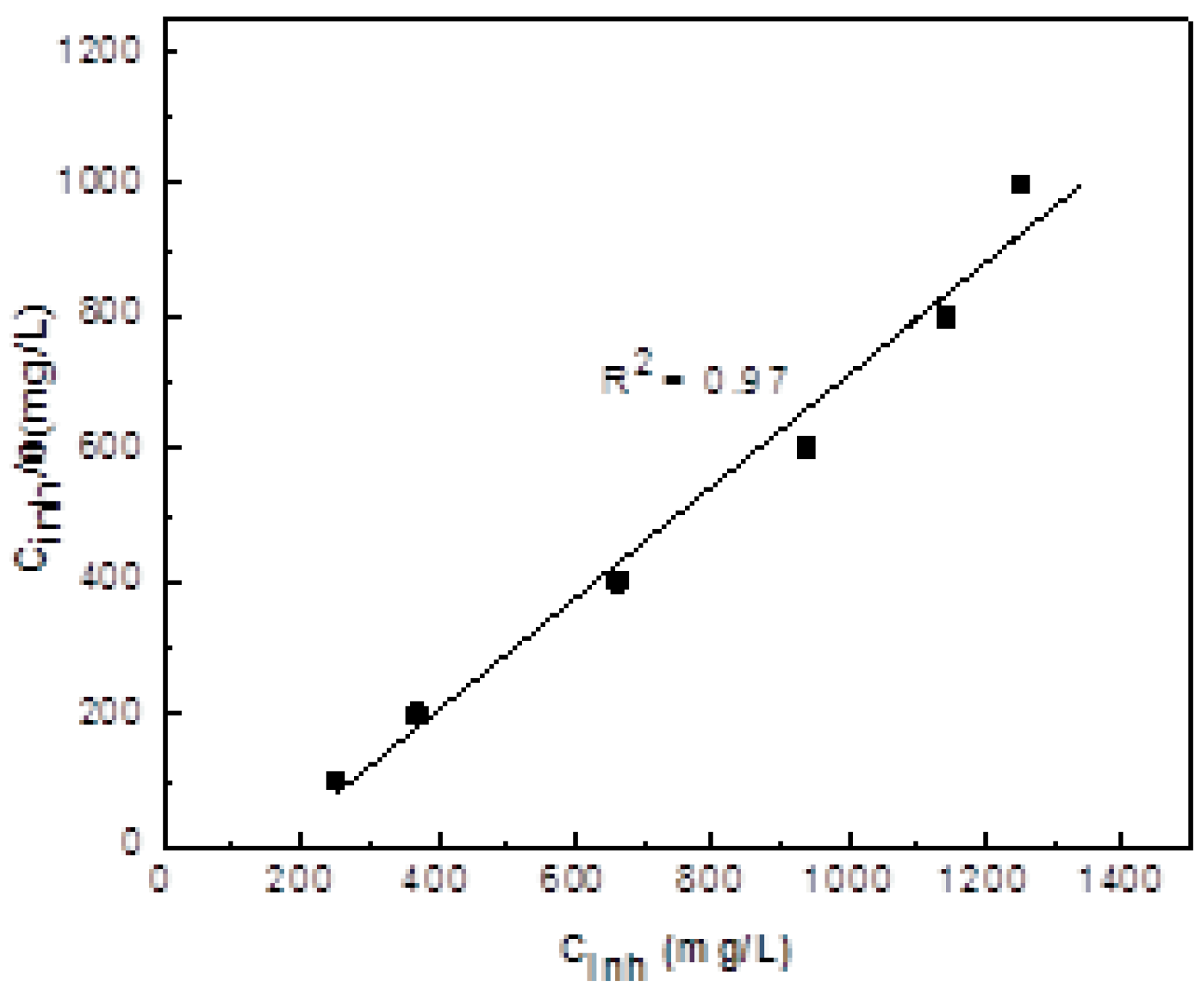
3.1. Weight Loss
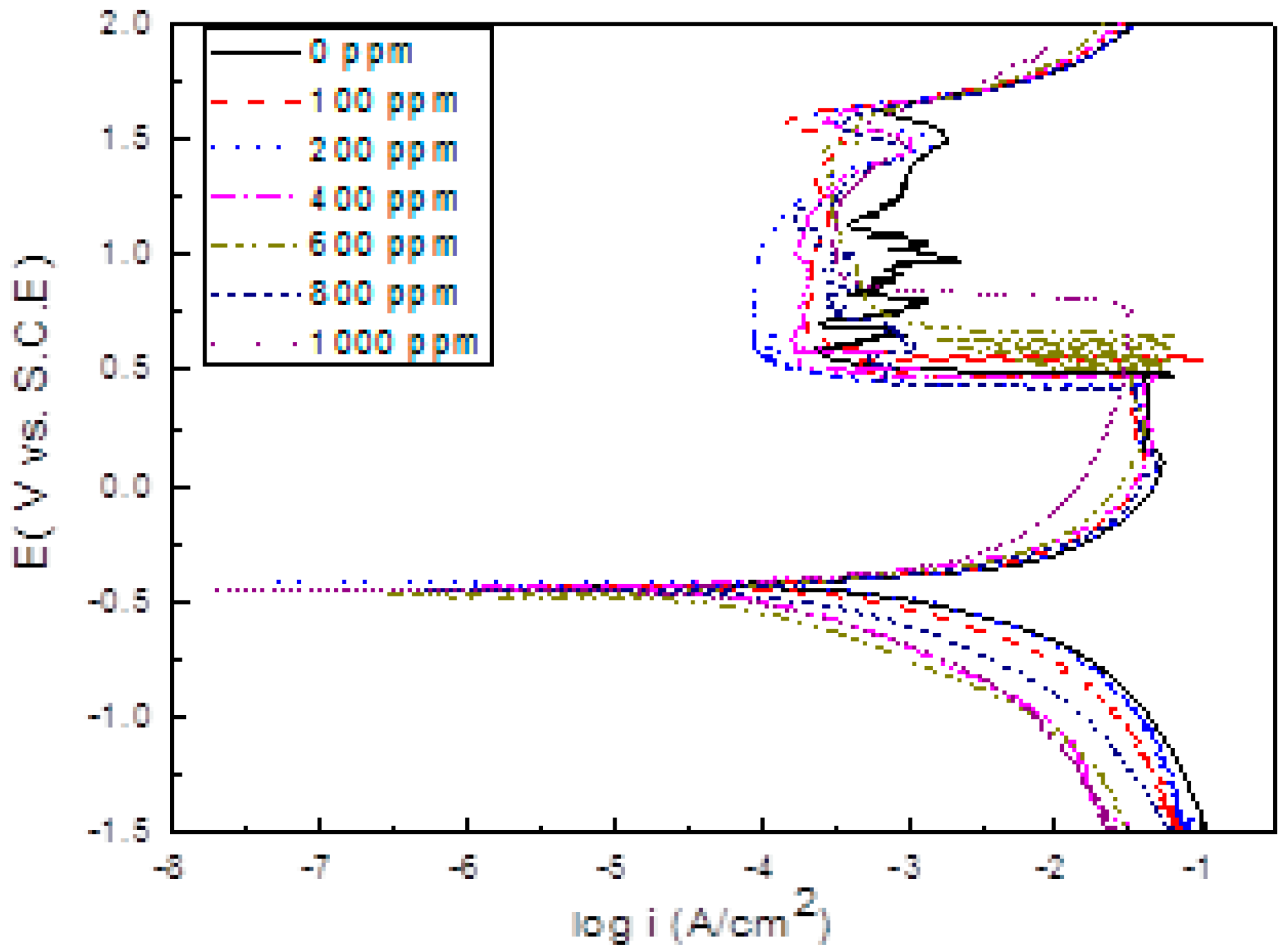
3.2. Polarization Curves
3.3. EIS Measurements
3.4. Theoretical Calculations
Neutral Molecule
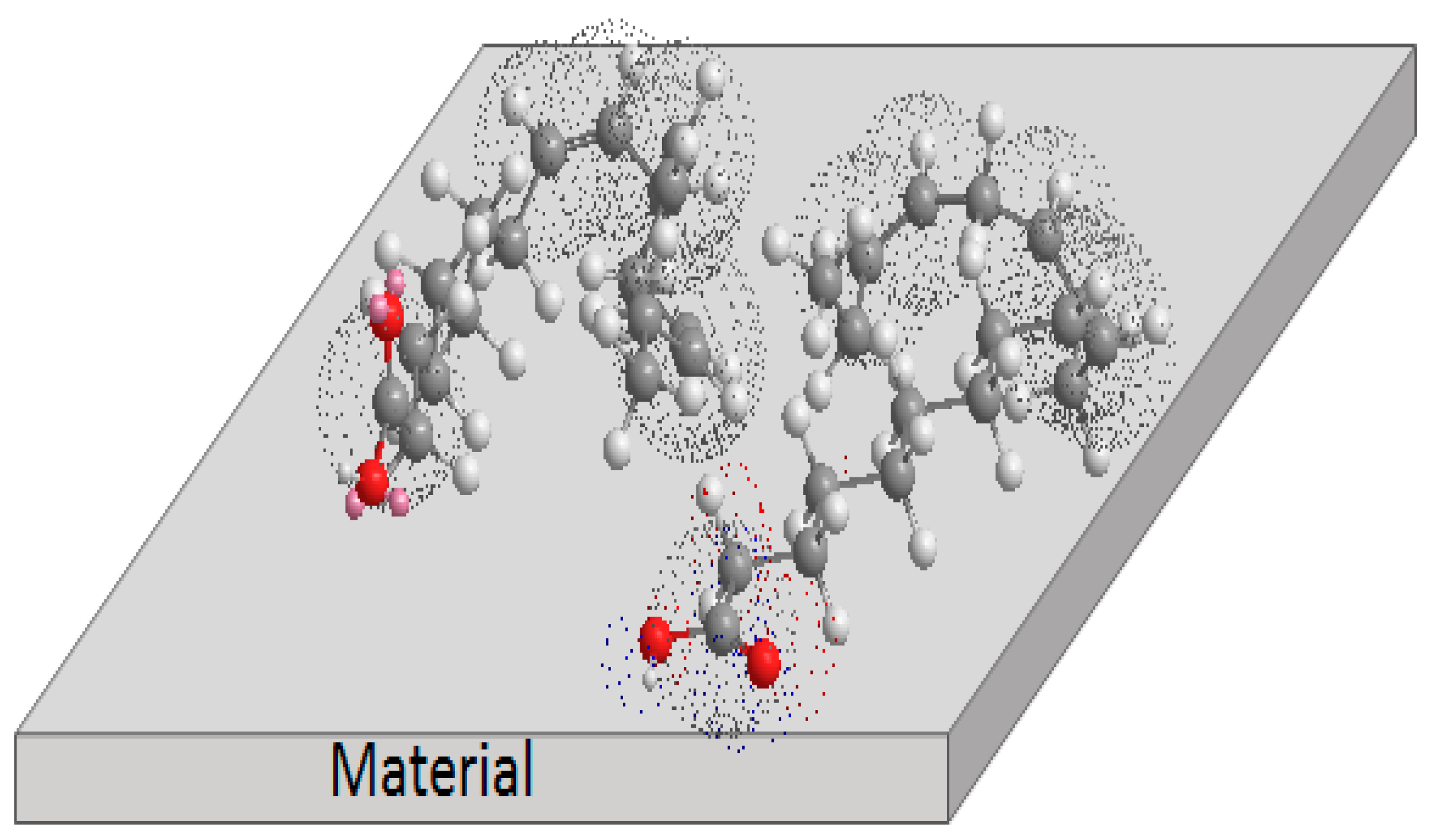
3.5. Corrosion Inhibition Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, L.G.; Wu, Y.; Wang, Y.M.; Jiang, X. Synergistic effect between cationic gemini surfactant and chloride ion for the corrosion inhibition of steel in sulphuric acid. Corros. Sci. 2008, 50, 576–582. [Google Scholar] [CrossRef]
- Li, Y.; Xin, Y.; Wang, X.; Li, S. Fixed Bed Reactor Pyrolysis of Rape Straw: Effect of Dilute Acid Pickling on the Production of Bio-oil and Enhancement of Sugars. Ind. Eng. Chem. Res. 2020, 59, 17564–17574. [Google Scholar] [CrossRef]
- El Kacimi, Y.; Touir, R.; Galai, M.; Alaoui, K.; Dkhireche, N.; Touhami, M.E. Relationship between silicon, phosphorus content and grain number in mild steels and its corrosion resistance in pickling hydrochloric acid. Int. J. Ind. Chem. 2020, 11, 111–122. [Google Scholar] [CrossRef]
- Prasad, A.R.; Kunyankandy, A.; Joseph, A. Economic Considerations. In Corrosion Inhibition in the Oil and Gas Industry; Saji, V.S., Umoren, S.A., Eds.; Wiley-VCH Verlag GmbH and Co.: Weinheim, Germany, 2020. [Google Scholar]
- Dohare, P.; Quraishi, M.A.; Verma, C.; Lgaz, H.; Salghi, R.; Ebenso, E.E. Ultrasound induced green synthesis of pyrazolo-pyridines as novel corrosion inhibitors useful for industrial pickling process: Experimental and theoretical approach. Results Phys. 2020, 13, 102344. [Google Scholar] [CrossRef]
- Shetty, P. Schiff bases: An overview of their corrosion inhibition activity in acid media against mild steel. Chem. Eng. Commun. 2020, 207, 985–1029. [Google Scholar] [CrossRef]
- Mohammed, N.J.; Othman, N.K.; Taib, M.F.M.; Samat, M.H.; Yahya, S. Experimental and Theoretical Studies on Extract of Date Palm Seed as a Green Anti-Corrosion Agent in Hydrochloric Acid Solution. Molecules 2021, 26, 3535. [Google Scholar] [CrossRef]
- Savita, N.C.; Qurashi, A.; Chauhan, D.S.; Quraishi, M.A. Frontiers and advances in green and sustainable inhibitors for corrosion applications: A critical review. J. Mol. Liq. 2020, 321, 114385. [Google Scholar]
- Varvara, S.; Caniglia, G.; Izquierdo, J.; Bostan, R.; Găină, L.; Bobis, O.; Souto, R.M. Multiscale electrochemical analysis of the corrosion control of bronze in simulated acid rain by horse-chestnut (Aesculus hippocastanum L.) extract as green inhibitor. Corros. Sci. 2020, 165, 108381. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Qiang, Y.; Xu, S.; Tan, B.; Chen, S. The synergistic corrosion inhibition study of different chain lengths ionic liquids as green inhibitors for X70 steel in acidic medium. Mater. Chem. Phys. 2018, 215, 229–241. [Google Scholar] [CrossRef]
- Luo, X.; Ci, C.; Li, J.; Lin, K.; Du, S.; Zhang, H.; Li, X.; Cheng, Y.F.; Zang, J.; Liu, Y. 4-aminoazobenzene modified natural glucomannan as a green eco-friendly inhibitor for the mild steel in 0.5 M HCl solution. Corros. Sci. 2019, 151, 132–142. [Google Scholar] [CrossRef]
- Aslam, R.; Huda, M.M.; Obot, I.B.; Alamri, A.H. Ionic liquids derived from α-amino acid ester salts as potent green corrosion inhibitors for mild steel in 1 M HCl. J. Mol. Liq. 2020, 318, 113982. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Sorour, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 2019, 150, 183–193. [Google Scholar] [CrossRef]
- Izadi, M.; Shahrabi, T.; Ramezanzadeh, B. Active corrosion protection performance of an epoxy coating applied on the mild steel modified with an eco-friendly sol-gel film impregnated with green corrosion inhibitor loaded nanocontainers. Appl. Surf. Sci. 2018, 440, 491–505. [Google Scholar] [CrossRef]
- Machado-Fernandes, C.; Alvarez, L.X.; Santos, N.E.; Maldonado-Barrios, A.C.; Ponzio, E.A. Green synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Mobin, M.; Basik, M.; Shoeb, M. A novel organic-inorganic hybrid complex based on Cissus quadrangularis plant extract and zirconium acetate as a green inhibitor for mild steel in 1 M HCl solution. Appl. Surf. Sci. 2019, 469, 387–403. [Google Scholar] [CrossRef]
- Sun, X.; Yu, L. Investigation of polyacrylamide containing capsaicin monomer as a novel corrosion inhibitor for mild steel in hydrochloric acid. Mater. Corros. 2018, 69, 1095–1103. [Google Scholar] [CrossRef]
- Zafari, S.; Sarabi, A.A.; Movassagh, B. A novel green corrosion inhibitor based on task-specific benzimidazolium ionic liquid for carbon steel in HCl. Corros. Eng. Sci. Technol. 2020, 55, 589–601. [Google Scholar] [CrossRef]
- Sanatgar, S.M.; Peyvandi, K. New edible additives as green inhibitors for preventing methane hydrate formation. J. Environ. Chem. Eng. 2019, 7, 103172. [Google Scholar] [CrossRef]
- Yang, H.M. Role of Organic and Eco-Friendly Inhibitors on the Corrosion Mitigation of Steel in Acidic Environments—A State-of-Art Review. Molecules 2021, 26, 3473. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- Guedes, L.A.L.; Bacca, K.G.; Lopes, N.F.; Da Costa, E.M. Tannin of Acacia mearnsii as green corrosion inhibitor for AA7075-T6 alluminum alloy in acidic medium. Mater. Corros. 2019, 70, 1288–1297. [Google Scholar] [CrossRef]
- Pérez-Miranda, S.; Zamudio-Rivera, L.S.; Cisneros-Dévora, R.; George-Téllez, R.; Fernández, F.J. Theoretical insight and experimental elucidation of desferrioxamine B from Bacillus sp. AS7 as a green corrosion inhibitor. Corros. Eng. Sci. Technol. 2020, 56, 93–101. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R.; Singh, G.; Kumar, A. Use of Sida cordifolia extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Environ. Chem. Eng. 2018, 6, 694–700. [Google Scholar] [CrossRef]
- Haris, N.I.N.; Sobri, S.; Yusof, Y.A.; Kassim, N. Oil palm empty fruit bunch extract and powder as an eco-friendly corrosion inhibitor for mild steel: A comparison study. Mater. Corros. 2019, 70, 2326–2333. [Google Scholar] [CrossRef]
- Anupama, K.K.; Ramya, K.; Shainy, K.M.; Joseph, A. Adsorption and electrochemical studies of Pimenta dioica leaf extracts as corrosion inhibitor for mild steel in hydrochloric acid. Mater. Chem. Phys. 2015, 167, 28–41. [Google Scholar] [CrossRef]
- Hussin, M.H.; Rahim, A.A.; Ibrahim, M.N.M.; Brosse, N. Improved corrosion inhibition of mild steel by chemically modified lignin polymers from Elaeis guineensis agricultural waste. Mater. Chem. Phys. 2015, 163, 201–212. [Google Scholar] [CrossRef]
- Zhang, B.; He, C.; Wang, C.; Sun, P.; Li, F.; Lin, Y. Synergistic corrosion inhibition of environment-friendly inhibitors on the corrosion of carbon steel in soft water. Corros. Sci. 2015, 94, 6–20. [Google Scholar] [CrossRef]
- Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R. Musa paradisica peel extract as green corrosion inhibitor for mild Steel in HCl solution. Corros. Sci. 2015, 90, 107–117. [Google Scholar] [CrossRef]
- Ngobiri, N.C.; Oguzie, E.E.; Li, Y.; Liu, L.; Oforka, N.C.; Akaranta, O. Eco-Friendly Corrosion Inhibition of Pipeline Steel Using Brassica oleracea. Int. J. Corros. 2015, 2015, 404139. [Google Scholar] [CrossRef] [Green Version]
- El Hamdani, N.; Fdil, R.; Tourabi, M.; Jama, C.; Bentiss, F. Alkaloids extract of Retama monosperma (L.) Boiss. seeds used as novel eco-friendly inhibitor for carbon steel corrosion in 1 M HCl solution: Electrochemical and surface studies. Appl. Surf. Sci. 2015, 357, 1294–1305. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P.; Poonkuzhali, K.; Palvannan, T. Corrosion inhibition of Eleusine aegyptiaca and Croton rottleri leaf extracts on cast iron surface in 1 M HCl medium. Appl. Surf. Sci. 2014, 314, 537–545. [Google Scholar] [CrossRef]
- Heuer, B.; Yaniv, Z.; Ravina, I. Effect of late salinization of chia (Salvia hispanica), stock (Matthiola tricuspidata) and evening primrose (Oenothera biennis) on their oil content and quality. Ind. Crops Prod. 2002, 15, 163–167. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gai, F. Fatty acid and nutritive quality of chia Salvia hispanica L. seeds and plant during growth. Anim. Feed Sci. Technol. 2009, 148, 267–275. [Google Scholar] [CrossRef]
- Chicco, A.G.; D’Alessandro, M.E.; Hein, G.J.; Oliva, E.M.; Lombardo, Y.B. Dietary chia seed (Salvia hispanica L.) rich in α-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br. J. Nutr. 2009, 101, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Ovando, A.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.). Food Sci. Technol. 2009, 42, 168–173. [Google Scholar]
- Hermoso-Diaz, I.A.; Velázquez-Gonzalez, M.A.; Lucio-Garcia, M.A.; Gonzalez-Rodriguez, J.G. A Study of Salvia hispanica as Green Corrosion Inhibitor for Carbon Steel in Sulfuric Acid. Chem. Sci. Rev. Lett. 2014, 3, 685–697. [Google Scholar]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, D.; Kumar, S.; Kumar, S.; Bahadur, I.; Ebenso, E.E. Electrochemical and Theoretical Studies on Amino Phosphonates as Efficient Corrosion Inhibitor for N80 Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2014, 9, 6580–6593. [Google Scholar]
- Obot, I.B.; Macdonalda, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Wazzan, N.A. DFT calculations of thiosemicarbazide, arylisothiocynates, and 1-aryl-2,5-dithiohydrazodicarbonamides as corrosion inhibitors of copper in an aqueous chloride solution. J. Ind. Eng. Chem. 2015, 26, 291–308. [Google Scholar] [CrossRef]
- Arslan, T.; Kandemirli, F.; Ebenso, E.E.; Love, I.; Alemu, H. Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci. 2009, 51, 35–47. [Google Scholar] [CrossRef]
- Gece, G. T The use of quantum chemical methods in corrosion inhibition studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Amovilli, C.; Barone, V.; Cammi, R.; Cancés, E.; Cossi, M.; Mennucci, B.; Pomelli, C.S.; Tomasi, J. Recent advances in the description of solvent effects with the polarizable continuum model. Adv. Quantum Chem. 1998, 32, 27–261. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Obot, I.B.; Gasem, Z.M. Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives. Corros. Sci. 2014, 83, 359–366. [Google Scholar] [CrossRef]
- Parr, R.G. Density functional theory of atoms and molecules. Horiz. Quantum Chem. 1980, 3, 5–15. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Maiti, B.; Sarkar, U. Philicity: A unified treatment of chemical reactivity and selectivity. J. Phys. Chem. 2003, 107, 4973–4975. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbe, A. Theoretical support for using the Delta f(r) descriptor. Chem. Phys. Lett. 2006, 425, 342–346. [Google Scholar] [CrossRef]
- Hsissou, R. Review on epoxy polymers and its composites as a potential anticorrosive coatings for carbon steel in 3.5% NaCl solution: Computational approaches. J. Mol. Liq. 2021, 336, 116307. [Google Scholar] [CrossRef]
- Verna, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Adsorption characteristics of Green 5-arylaminomethylene pyrimidine-2,4,6-triones on mild Steel Surface in acidic medium: Experimental and computational approach. Res. Phys. 2018, 8, 657–670. [Google Scholar]
- Free, M.L. Understanding the effect of surfactant aggregation on corrosion inhibition of mild steel in acid medium. Corros. Sci. 2002, 44, 2865–2870. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, S.; Li, W.; Hou, B. Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros. Sci. 2009, 51, 2588–2595. [Google Scholar] [CrossRef]
- Gusmano, G.; Labella, P.; Montesperelli, G.; Privitera, A.; Tassinari, S. Study of the Inhibition Mechanism of Imidazolines by Electrochemical Impedance Spectroscopy. Corrosion 2006, 62, 576–583. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, C.; Lu, M.; Chai, C.; Wu, Y. Evaluation of inhibition efficiency of an imidazoline derivative in CO2-containing aqueous solution. Mater. Chem. Phys. 2007, 105, 331–340. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.E.F. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid, Part I weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim. Acta 2007, 52, 3588–3600. [Google Scholar] [CrossRef]
- Bontiss, F.; Lagrence, M.; Traisnel, M. 2,5-Bis(n-Pyridyl)-1,3,4-Oxadiazoles as Corrosion Inhibitors for Mild Steel in Acidic Media. Corrosion 2000, 56, 733–742. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.G.; Ke, W. Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corros. Sci. 2005, 47, 2636–2658. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interf. Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Rahoma, A.A.B.; Mesmari, H. Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J. Mol. Struct. (Theochem) 2010, 969, 233–243. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.O.; Essien, K.E.; Obot, I.B.; Ebenso, E.E. Computational Simulation and Statistical Analysis on the Relationship between Corrosion Inhibition Efficiency and Molecular Structure of Some Phenanthroline Derivatives on Mild Steel Surface. Int. J. Electrochem. Sci. 2011, 6, 5649–5675. [Google Scholar]
- Obot, I.B.; Obi-Egbedi, N.O. Ginseng Root: A new Efficient and Effective Eco-Friendly Corrosion Inhibitor for Aluminium Alloy of type AA 1060 in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2009, 4, 1277–1288. [Google Scholar]
- Guo, L.; Safi, Z.S.; Kaya, S.; Shi, W.; Tüzün, B.; Altunay, N.; Kaya, C. Anticorrosive effects of some thiophene derivatives against the corrosion of iron: A computational study. Front. Chem. 2018, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Kadihum, A.; Mohamad, A.B.; How, C.K.; Junaedi, S. Inhibition of Mild Steel Corrosion in Sulfuric Acid Solution by New Schiff Base. Materials 2014, 7, 787–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]



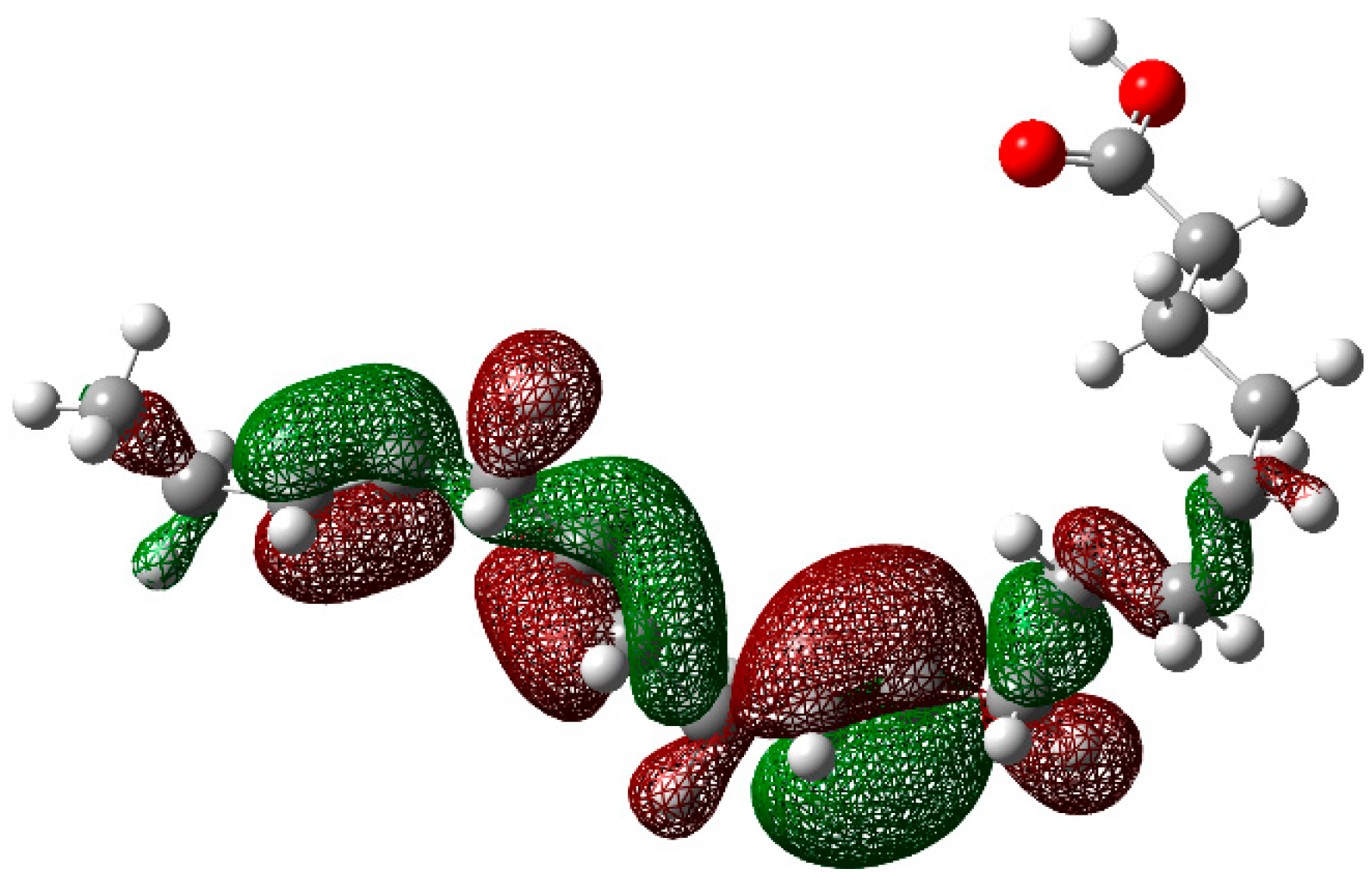
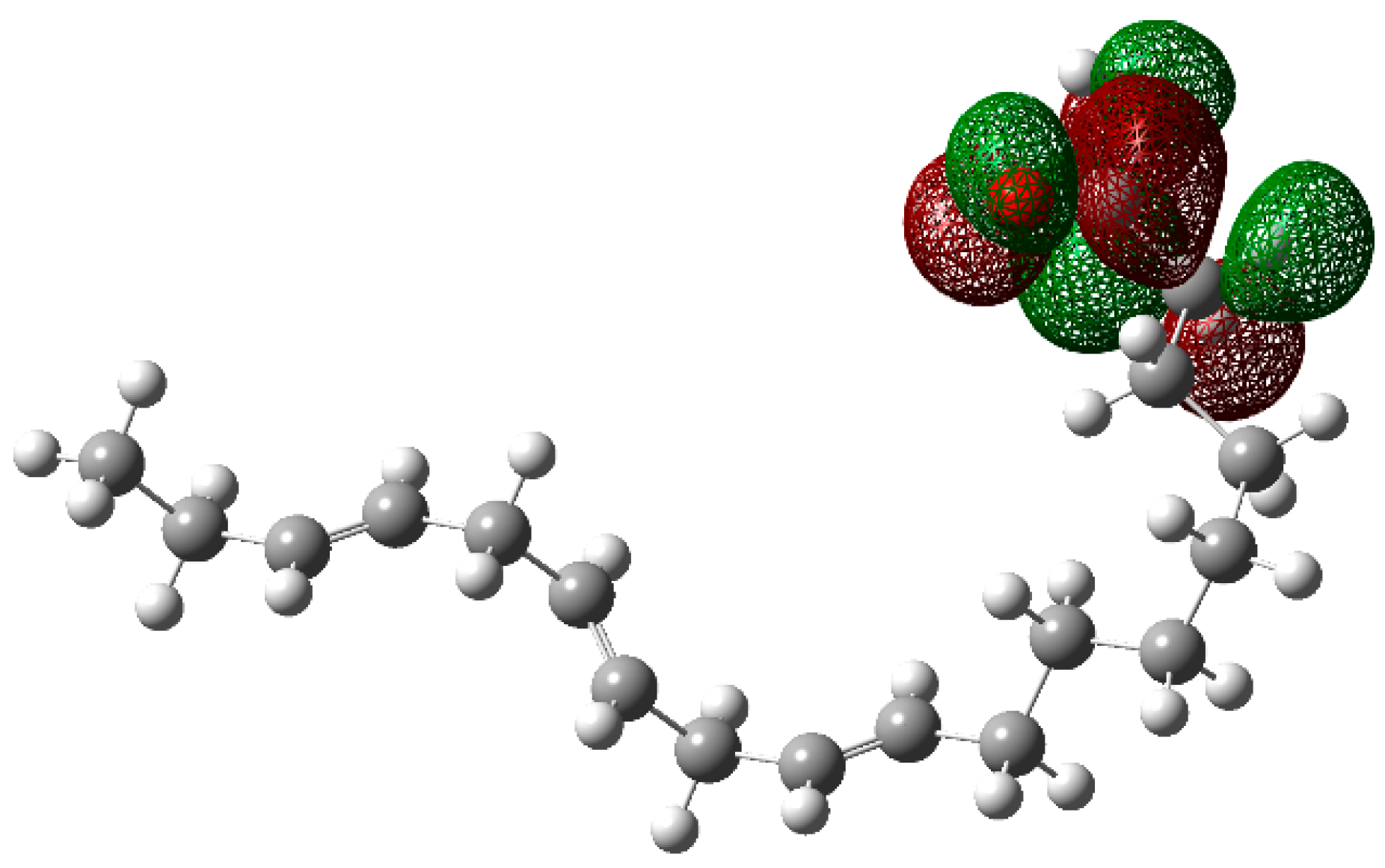

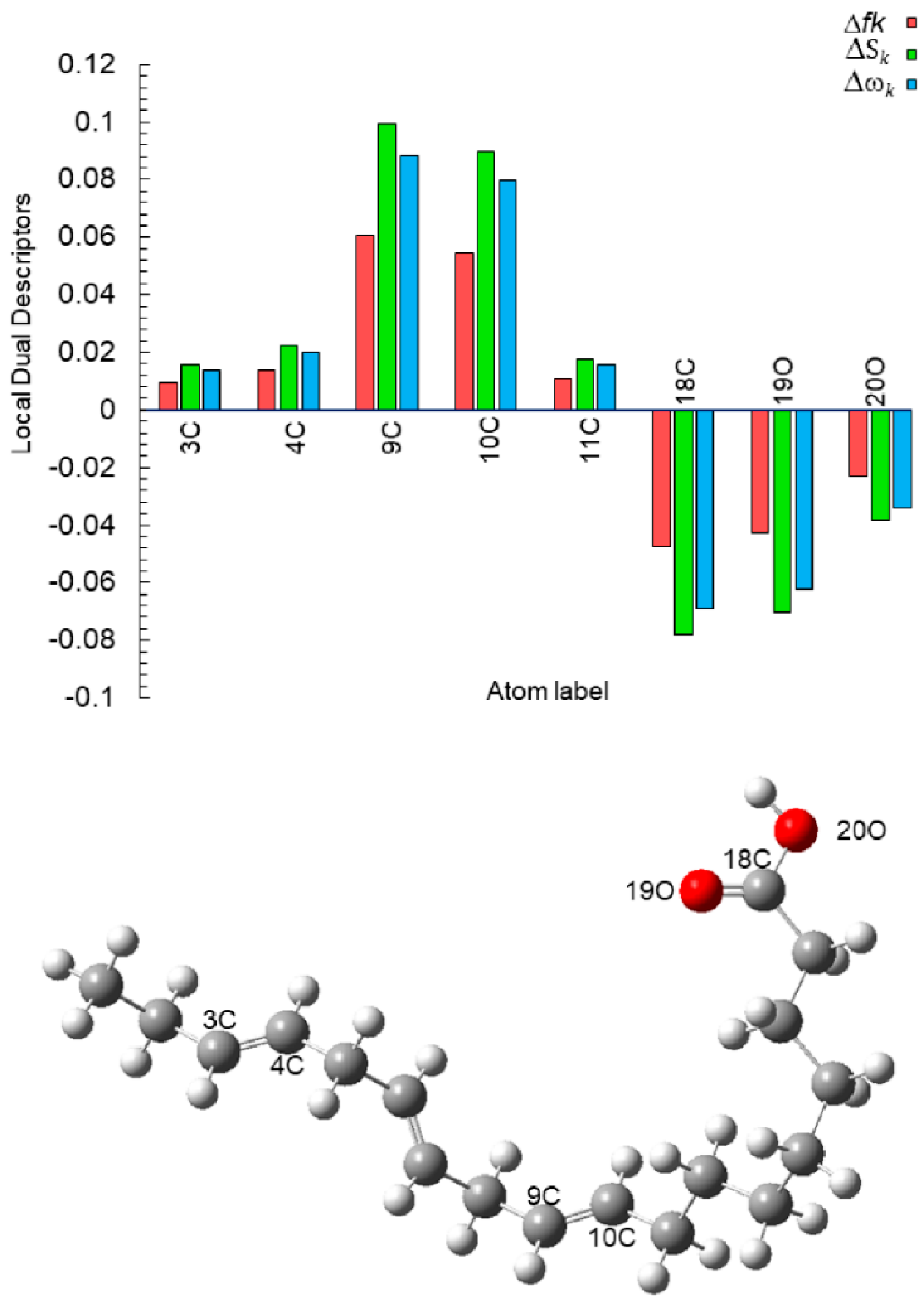

| Cinh (ppm) | Ea (kJ mol−1) |
|---|---|
| 0 | 33.41 |
| 100 | 60.18 |
| 200 | 63.42 |
| 400 | 83.47 |
| 600 | 83.64 |
| 800 | 93.66 |
| 1000 | 104.96 |
| Cinh (ppm) | Ecorr (mV) | Icorr (mA/cm2) | βa (mV/dec) | βc (mV/dec) | Ipas (mA/cm2) | Rp (Ohm cm2) | I.E. (%) |
|---|---|---|---|---|---|---|---|
| 0 | −428 | 5.49 | 42 | −88 | 0.30 | 18 | - |
| 100 | −451 | 4.89 | 38 | −140 | 0.29 | 75 | 24 |
| 200 | −423 | 1.58 | 45 | −160 | 0.08 | 106 | 75 |
| 400 | −439 | 0.09 | 40 | −165 | 0.16 | 540 | 96 |
| 600 | −473 | 0.05 | 50 | −190 | 0.25 | 594 | 97 |
| 800 | −454 | 0.15 | 60 | −270 | 0.16 | 184 | 96 |
| 1000 | −475 | 0.23 | 50 | −240 | 0.26 | 141 | 94 |
| Cinh (ppm) | Rs (Ohm cm2) | Rct (Ohm cm2) | CPEdl (µF cm2) | Rf (Ohm cm2) | CPEf (µF cm2) | I.E. (%) |
|---|---|---|---|---|---|---|
| 0 | 4 | 28 | 7.99 × 10−5 | - | - | - |
| 100 | 7 | 140 | 4.47 × 10−5 | 15 | 8.47 × 10−4 | 51 |
| 200 | 8 | 420 | 2.57 × 10−5 | 86 | 4.57 × 10−4 | 78 |
| 400 | 8 | 1130 | 1.13 × 10−5 | 110 | 1.13 × 10−4 | 97 |
| 600 | 10 | 1960 | 7.08 × 10−6 | 110 | 6.38 × 10−5 | 98 |
| 800 | 11 | 120 | 4.27 × 10−5 | 50 | 3.27 × 10−4 | 78 |
| 1000 | 11 | 108 | 3.31 × 10−5 | 20 | 6.31 × 10−4 | 72 |
| Chemical Properties | Values |
|---|---|
| EHOMO (Hartree) | −0.23646 |
| ELUMO (Hartree) | −0.00920 |
| EHOMO (eV) | −6.43440 |
| ELUMO (eV) | −0.25034 |
| GAP (eV) | 6.1841 |
| Electronegativity (eV) | 3.1 |
| Hardness (eV) | 3.29 |
| Ionization Potential (eV) | 6.39 |
| Dipole Moment | 1.9342 |
| Electrophilicity | 1.46 |
| Electron Affinity (eV) | −0.19 |
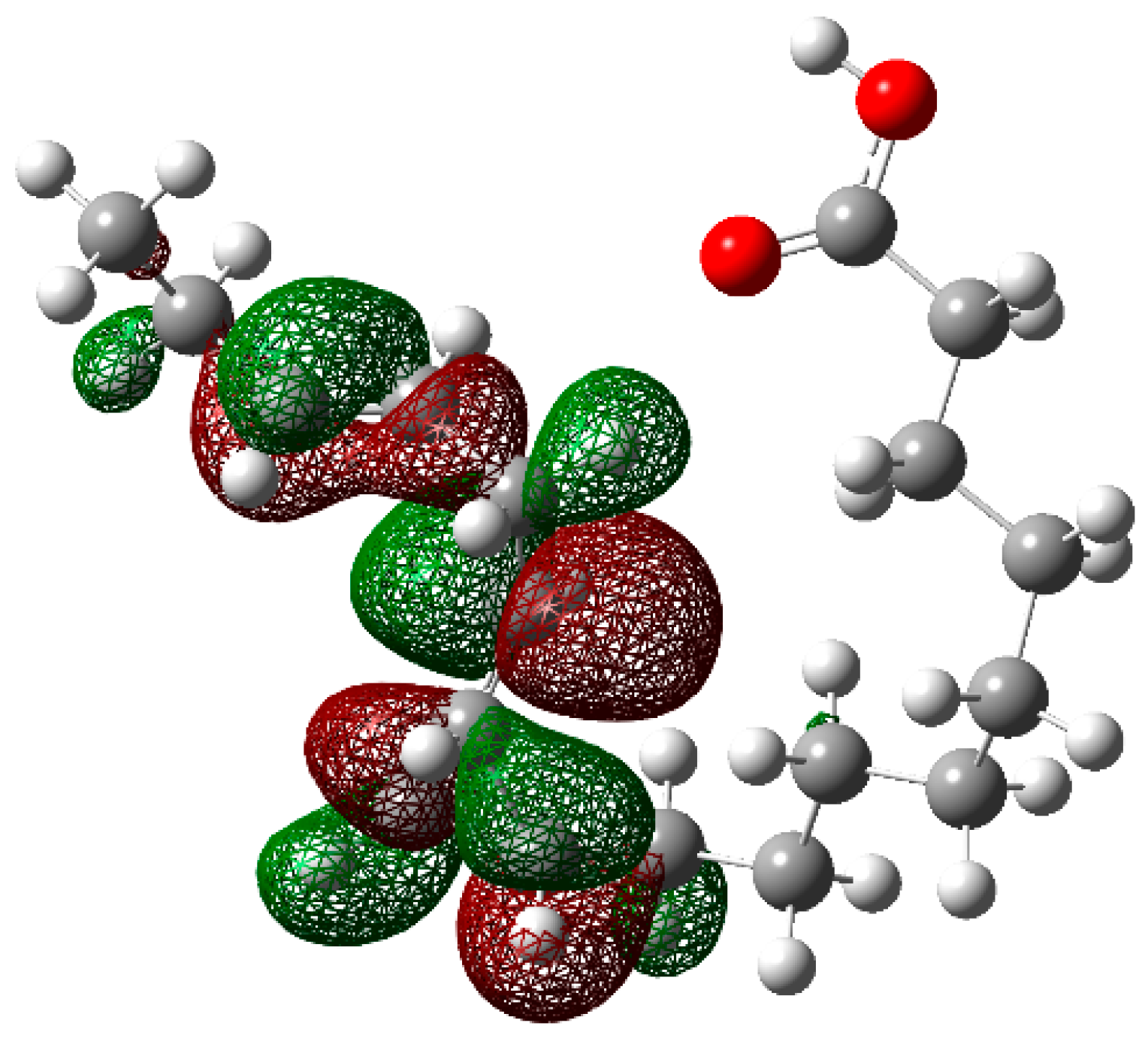
| Atom | fk+ | fk− | Δfk | Sk+ | Sk− | ΔSk | ωk+ | ωk− | Δωk |
|---|---|---|---|---|---|---|---|---|---|
| 3C | 0.0586 | 0.0680 | 0.0094 | 0.0964 | 0.1119 | 0.0155 | 0.0855 | 0.0993 | 0.0138 |
| 4C | 0.0475 | 0.0612 | 0.0137 | 0.0781 | 0.1006 | 0.0225 | 0.0693 | 0.0893 | 0.0199 |
| 7C | 0.0915 | 0.0592 | −0.0323 | 0.1505 | 0.0973 | −0.0532 | 0.1336 | 0.0864 | −0.0472 |
| 9C | 0.0477 | 0.1083 | 0.0605 | 0.0785 | 0.1781 | 0.0996 | 0.0697 | 0.1581 | 0.0884 |
| 10C | 0.0586 | 0.1133 | 0.0547 | 0.0964 | 0.1863 | 0.0899 | 0.0856 | 0.1653 | 0.0798 |
| 11C | 0.0103 | 0.0210 | 0.0107 | 0.0169 | 0.0345 | 0.0176 | 0.0150 | 0.0306 | 0.0156 |
| 18C | 0.0475 | 0.0002 | −0.0473 | 0.0782 | 0.0004 | −0.0778 | 0.0694 | 0.0003 | −0.0691 |
| 19O | 0.0428 | 0.0001 | −0.0426 | 0.0703 | 0.0002 | −0.0701 | 0.0624 | 0.0002 | −0.0623 |
| 20O | 0.0236 | 0.0004 | −0.0232 | 0.0389 | 0.0006 | −0.0382 | 0.0345 | 0.0006 | −0.0339 |
| Chemical Properties | Values |
|---|---|
| EHOMO (Hartree) | −0.26663 |
| ELUMO (Hartree) | −0.12105 |
| EHOMO (eV) | −7.25537 |
| ELUMO (eV) | −3.29393 |
| GAP (eV) | 3.961 |
| Electronegativity (eV) | 5.30 |
| Hardness (eV) | 1.95 |
| Ionization Potential (eV) | 7.26 |
| Dipole Moment | 9.1693 |
| Electrophilicity | 7.20 |
| Electron Affinity (eV) | 3.35 |
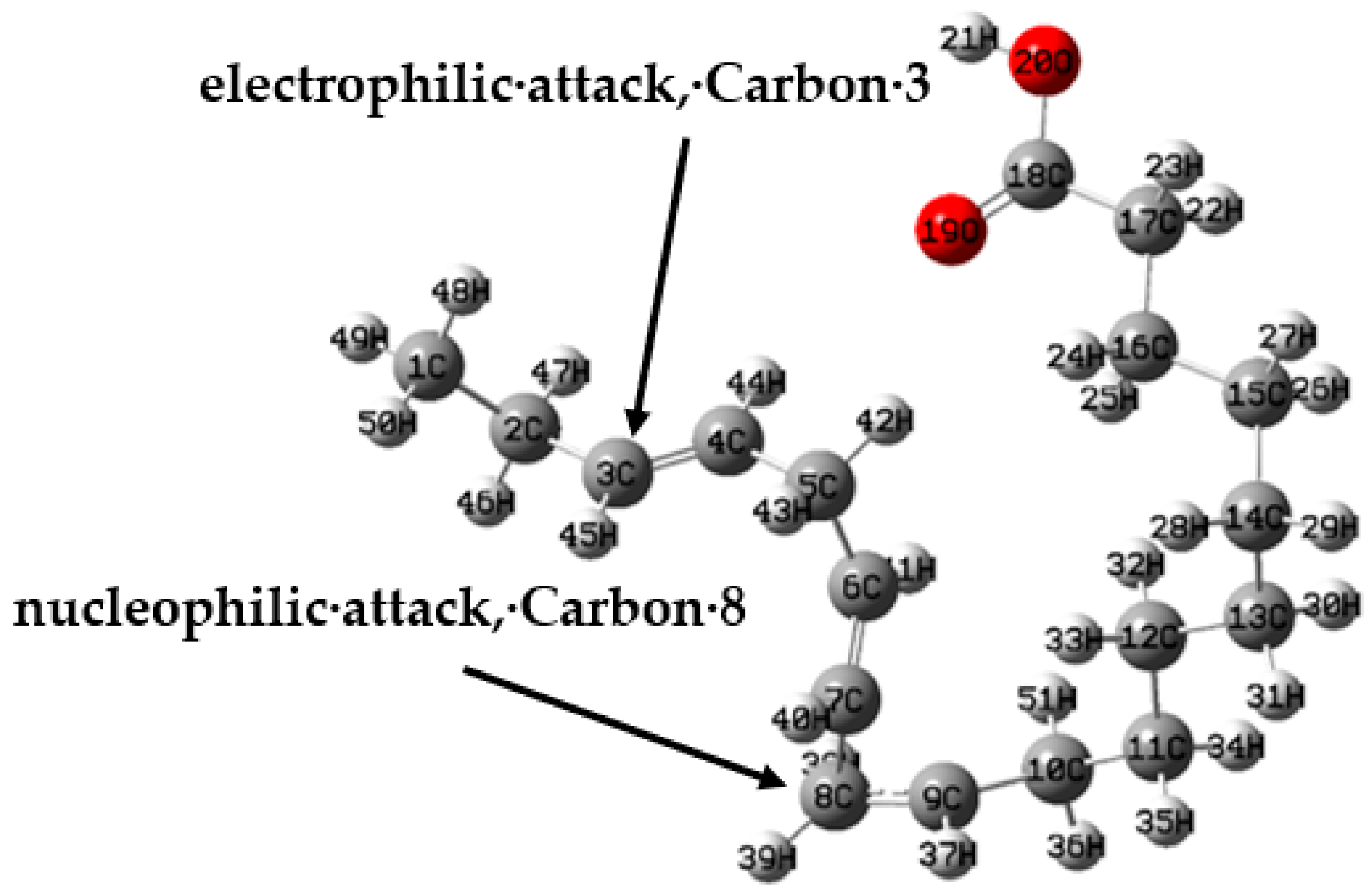
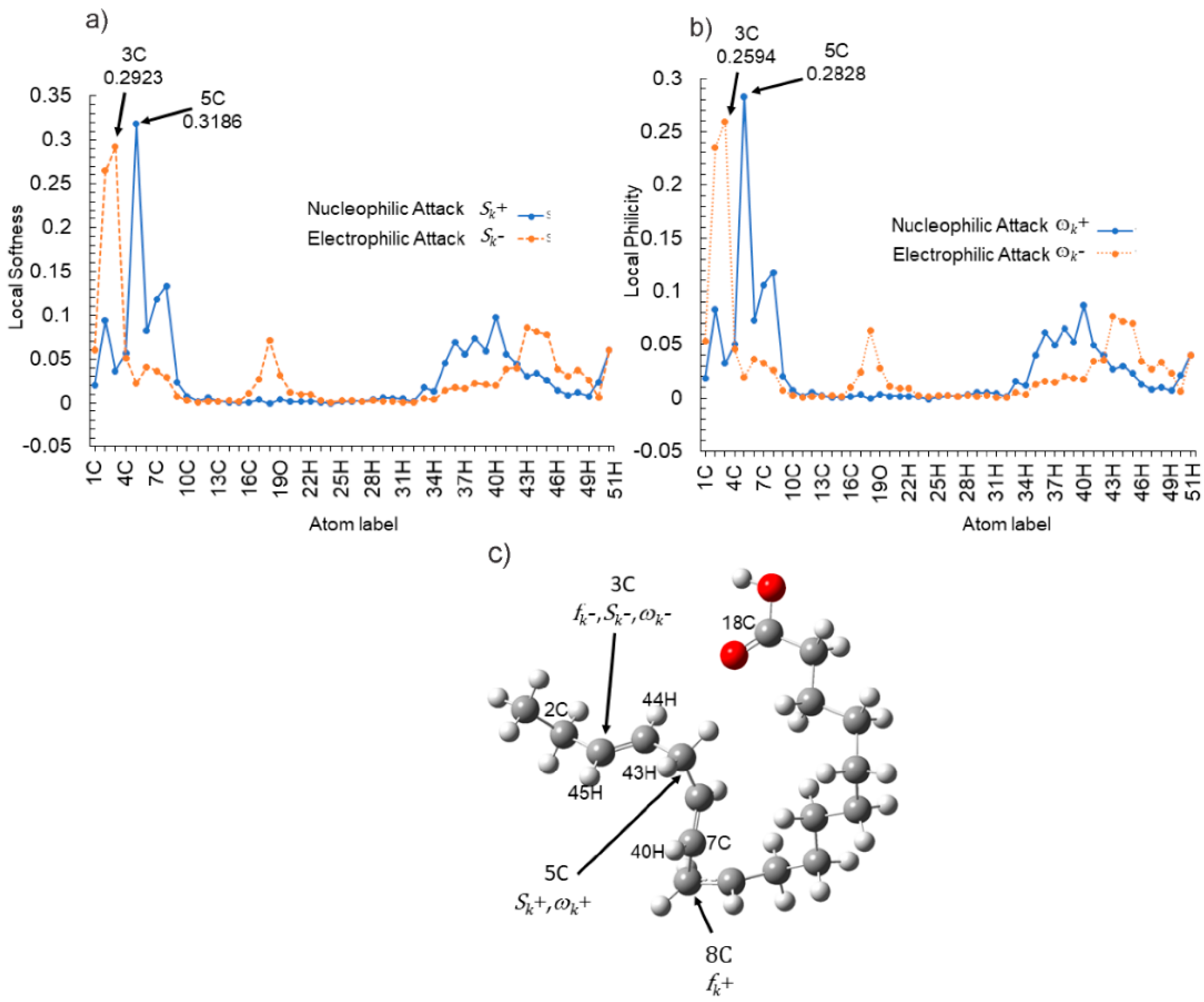
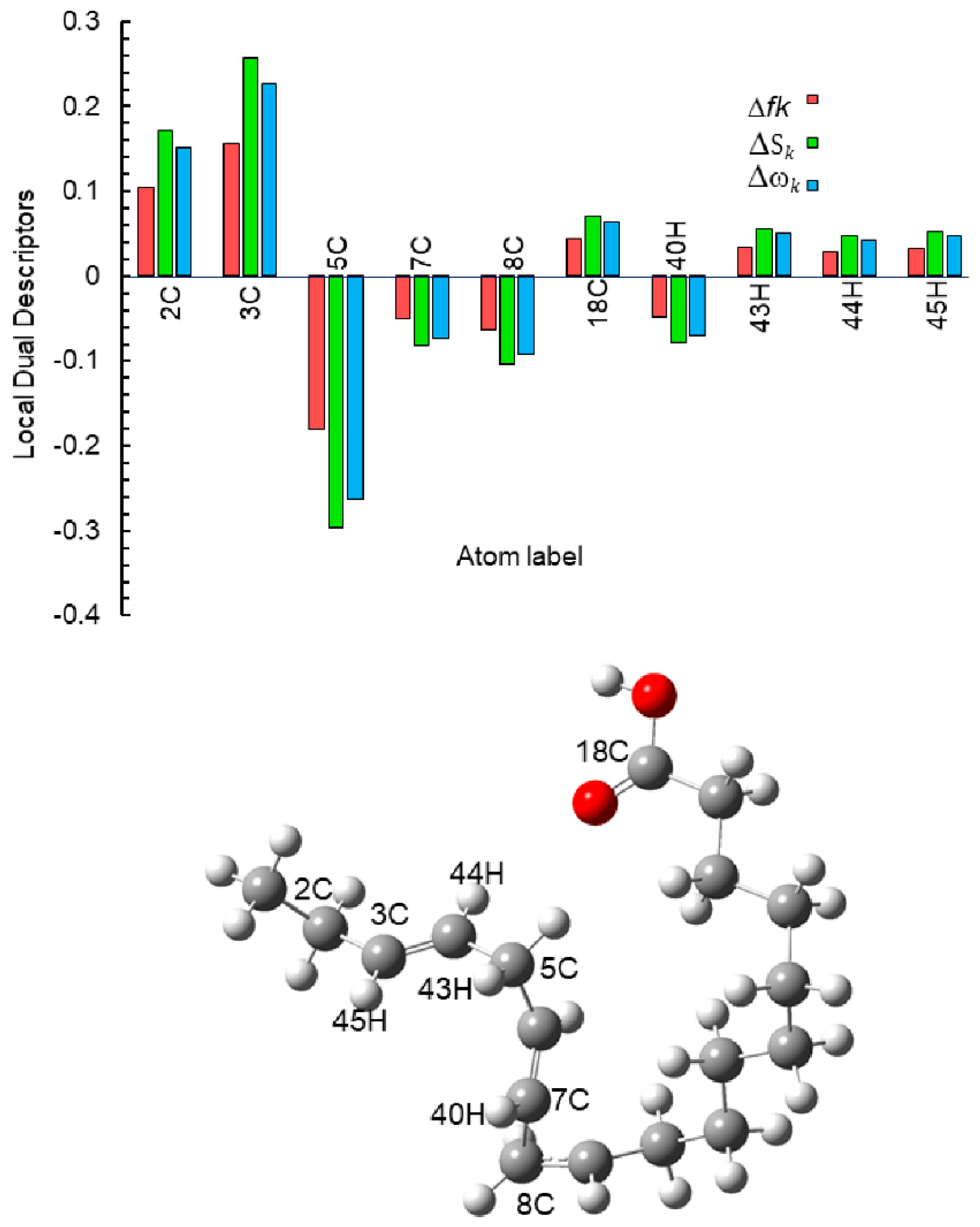
| Atom | fk+ | fk− | Δfk | Sk+ | Sk− | ΔSk | ωk+ | ωk− | Δωk |
|---|---|---|---|---|---|---|---|---|---|
| 2C | 0.0572 | 0.1610 | 0.1038 | 0.0940 | 0.2648 | 0.1708 | 0.0835 | 0.2350 | 0.1515 |
| 3C | 0.0220 | 0.1777 | 0.1557 | 0.0362 | 0.2923 | 0.2561 | 0.0321 | 0.2594 | 0.2273 |
| 5C | 0.1937 | 0.0133 | −0.1804 | 0.3187 | 0.0219 | −0.2968 | 0.2829 | 0.0195 | −0.2634 |
| 7C | 0.0720 | 0.0222 | −0.0498 | 0.1185 | 0.0366 | −0.0819 | 0.1052 | 0.0325 | −0.0727 |
| 8C | 0.0809 | 0.0175 | −0.0634 | 0.1331 | 0.0288 | −0.1043 | 0.1181 | 0.0256 | −0.0925 |
| 18C | −0.0004 | 0.0432 | 0.0436 | −0.0007 | 0.0710 | 0.0717 | −0.0006 | 0.0630 | 0.0636 |
| 40H | 0.0594 | 0.0121 | −0.0473 | 0.0977 | 0.0199 | −0.0779 | 0.0867 | 0.0176 | −0.0691 |
| 44H | 0.0206 | 0.0495 | 0.0290 | 0.0338 | 0.0815 | 0.0476 | 0.0300 | 0.0723 | 0.0423 |
| 45H | 0.0156 | 0.0477 | 0.0321 | 0.0257 | 0.0785 | 0.0528 | 0.0228 | 0.0697 | 0.0469 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermoso-Diaz, I.A.; Lopez-Cecenes, R.; Rios, J.P.F.-D.l.; Landeros-Martínez, L.L.; Sarmiento-Bustos, E.; Uruchurtu-Chavarin, J.; Gonzalez-Rodriguez, J.G. Experimental and Theoretical Studies of α-Linolenic Acid as Green Corrosion Inhibitor for Carbon Steel in 0.5 M Sulfuric Acid. Molecules 2021, 26, 6169. https://doi.org/10.3390/molecules26206169
Hermoso-Diaz IA, Lopez-Cecenes R, Rios JPF-Dl, Landeros-Martínez LL, Sarmiento-Bustos E, Uruchurtu-Chavarin J, Gonzalez-Rodriguez JG. Experimental and Theoretical Studies of α-Linolenic Acid as Green Corrosion Inhibitor for Carbon Steel in 0.5 M Sulfuric Acid. Molecules. 2021; 26(20):6169. https://doi.org/10.3390/molecules26206169
Chicago/Turabian StyleHermoso-Diaz, I.A., R. Lopez-Cecenes, J.P. Flores-De los Rios, L.L. Landeros-Martínez, E. Sarmiento-Bustos, J. Uruchurtu-Chavarin, and J.G. Gonzalez-Rodriguez. 2021. "Experimental and Theoretical Studies of α-Linolenic Acid as Green Corrosion Inhibitor for Carbon Steel in 0.5 M Sulfuric Acid" Molecules 26, no. 20: 6169. https://doi.org/10.3390/molecules26206169
APA StyleHermoso-Diaz, I. A., Lopez-Cecenes, R., Rios, J. P. F.-D. l., Landeros-Martínez, L. L., Sarmiento-Bustos, E., Uruchurtu-Chavarin, J., & Gonzalez-Rodriguez, J. G. (2021). Experimental and Theoretical Studies of α-Linolenic Acid as Green Corrosion Inhibitor for Carbon Steel in 0.5 M Sulfuric Acid. Molecules, 26(20), 6169. https://doi.org/10.3390/molecules26206169






