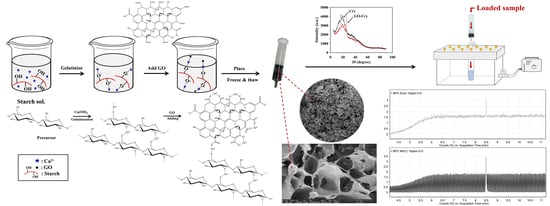
Greener Monolithic Solid Phase Extraction Biosorbent Based on Calcium Cross-Linked Starch Cryogel Composite Graphene Oxide Nanoparticles for Benzo(a)pyrene Analysis
Abstract
:1. Introduction
2. Results and Discussion
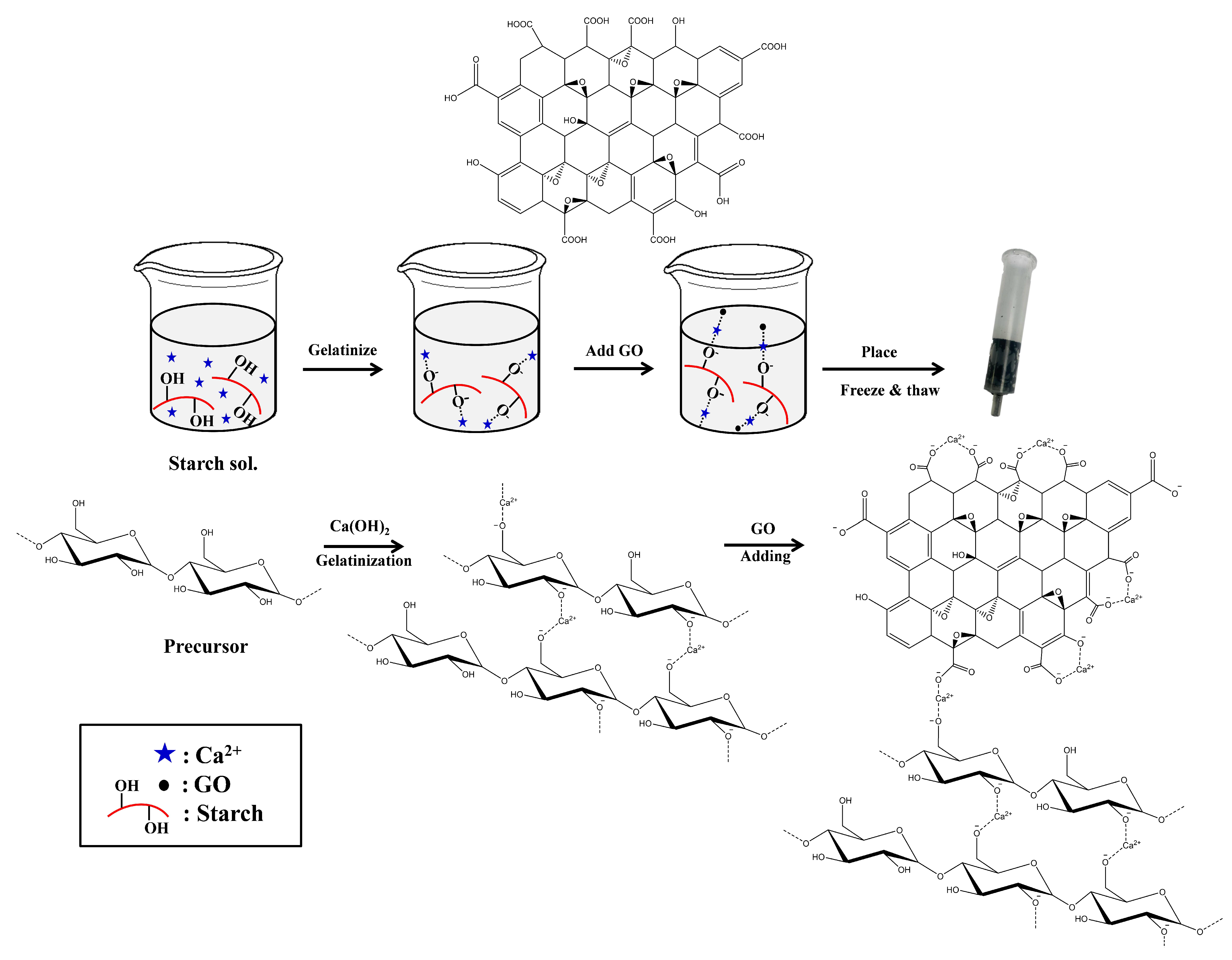
2.1. Preparation of GO-Cry
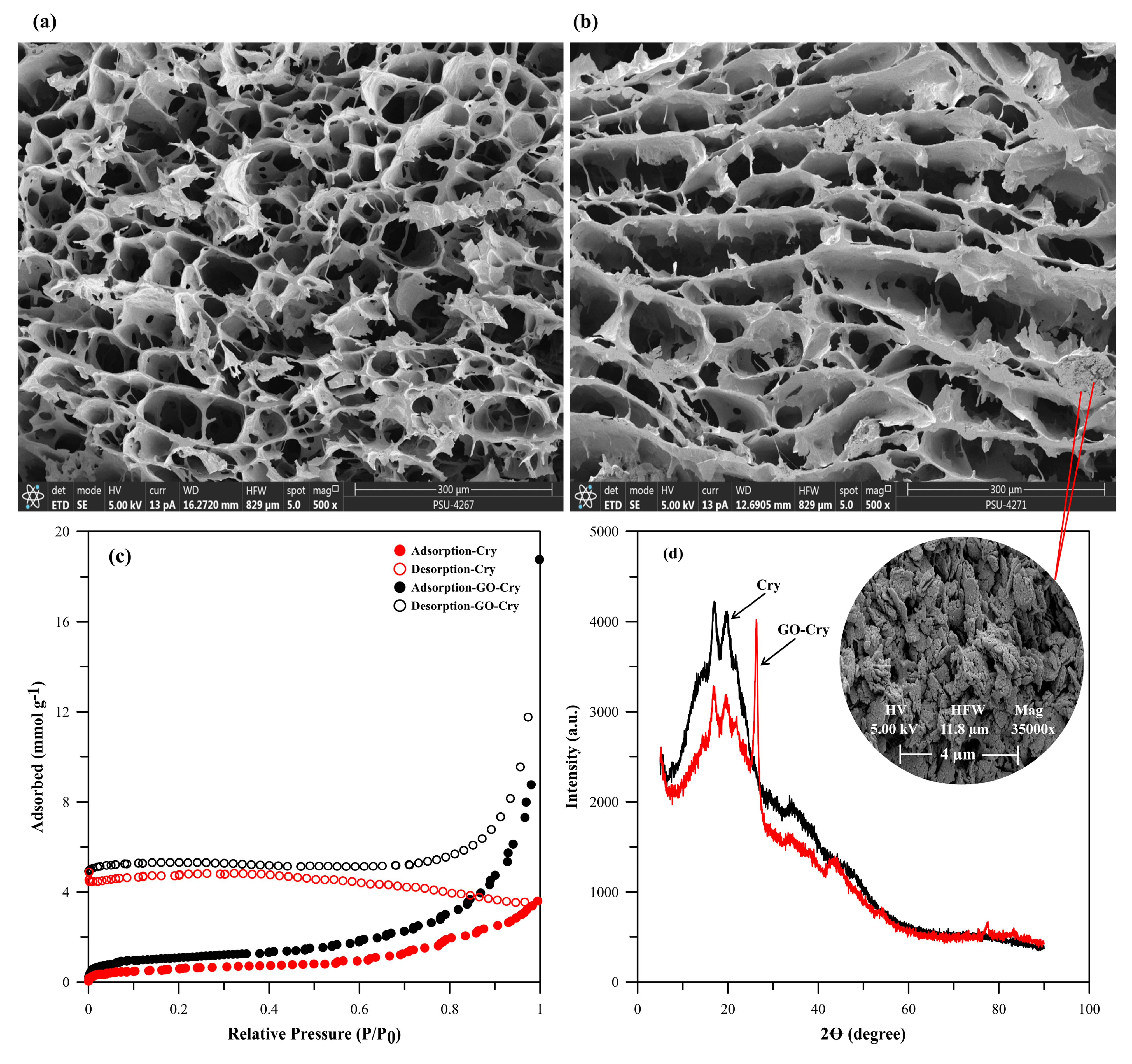
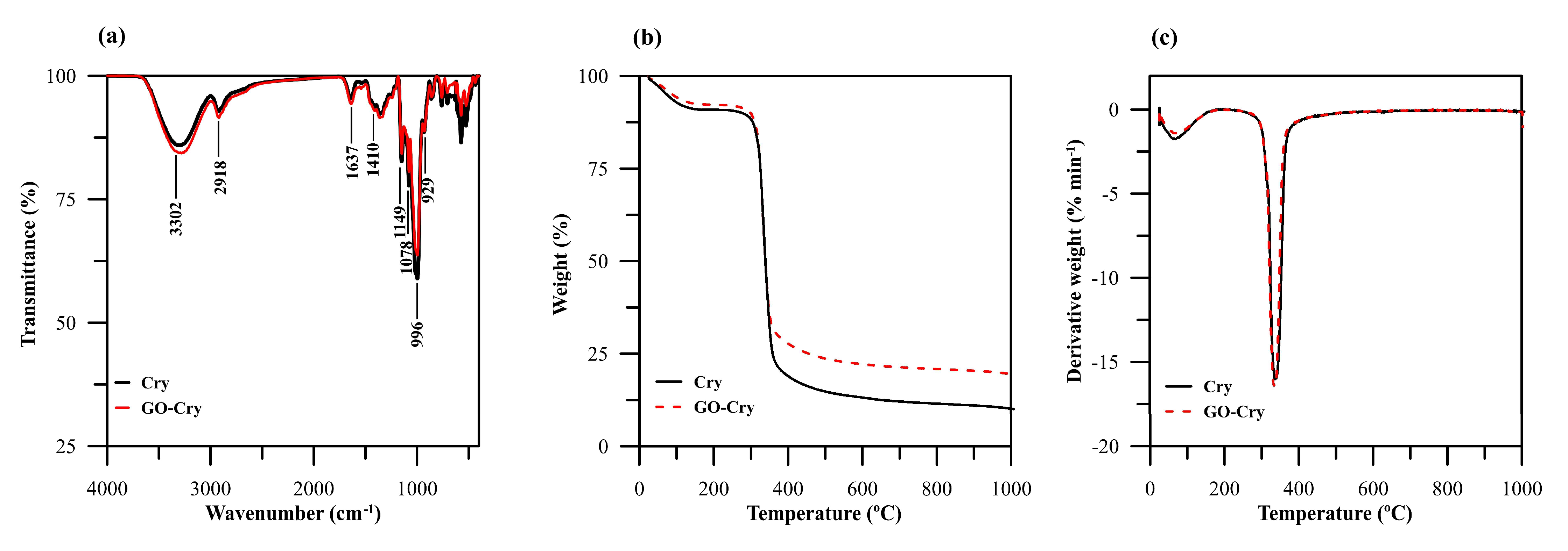
2.2. Characterization of GO-Cry
2.3. Optimization of SPE Conditions
2.4. Method Validation
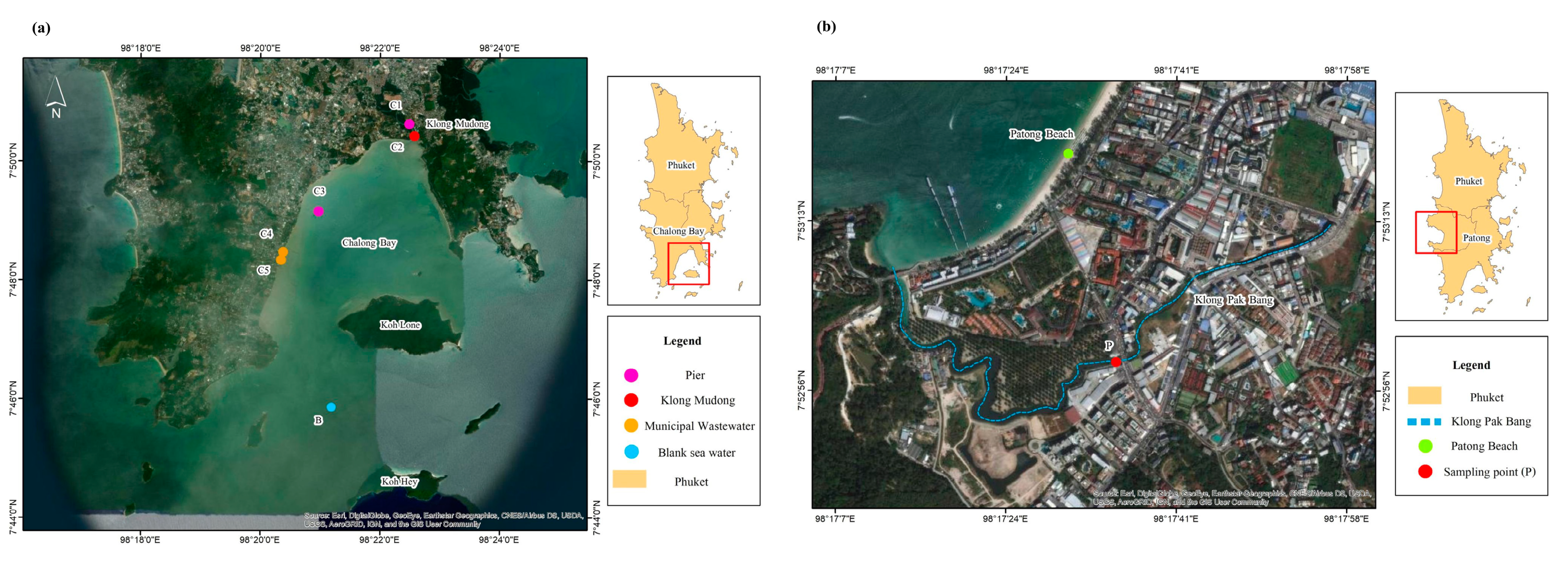
2.5. Analysis of Real Samples
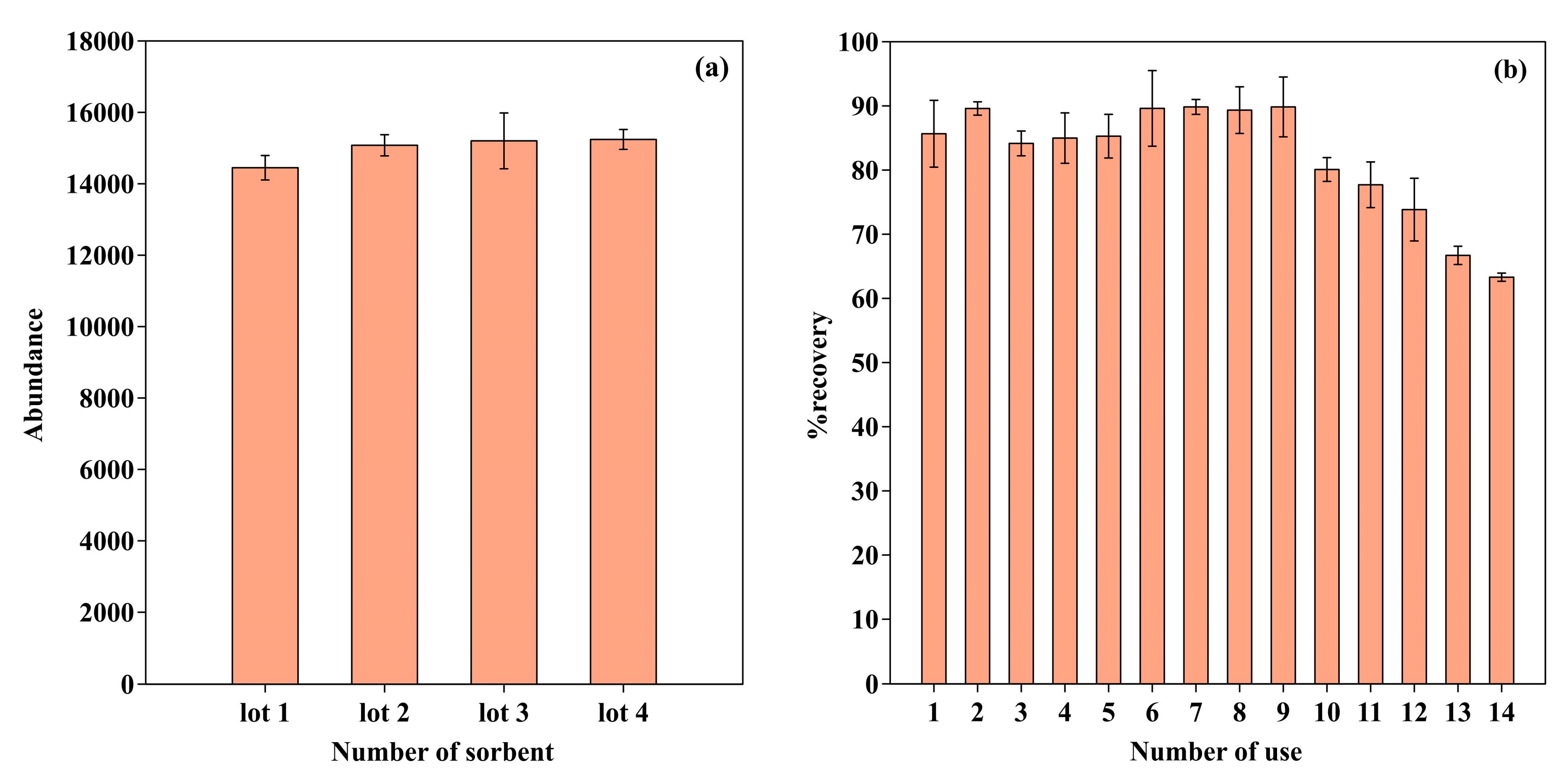
2.6. Reproducibility and Reusability
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO-Cry
3.3. Characterization of GO-Cry
3.4. Analysis of BaP using GC-MS
3.5. Solid Phase Extraction (SPE) for BaP Analysis
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Çorman, M.E.; Armutcu, C.; Uzun, L.; Denizli, A. Rapid, efficient and selective preconcentration of benzo[a]pyrene (BaP) by molecularly imprinted composite cartridge and HPLC. Mater. Sci. Eng. C 2017, 70, 41–53. [Google Scholar] [CrossRef]
- Hardonnière, K.; Saunier, E.; Lemarié, A.; Fernier, M.; Gallais, I.; Héliès-Toussaint, C.; Mograbi, B.; Antonio, S.; Bénit, P.; Rustin, P.; et al. The environmental carcinogen benzo[a]pyrene induces a Warburg-like metabolic reprogramming dependent on NHE1 and associated with cell survival. Sci. Rep. 2016, 6, 30776. [Google Scholar] [CrossRef]
- Purcaro, G.; Moret, S.; Conte, L.S. Rapid validated method for the analysis of benzo[a]pyrene in vegetable oils by using solid-phase microextraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1176, 231–235. [Google Scholar] [CrossRef]
- Cheng, X.; Forsythe, J.; Peterkin, E. Some factors affecting SPME analysis and PAHs in Philadelphia’s urban waterways. Water Res. 2013, 47, 2331–2340. [Google Scholar] [CrossRef]
- Ibrahim, W.A.W.; Nodeh, H.R.; Sanagi, M.M. Graphene-Based Materials as Solid Phase Extraction Sorbent for Trace Metal Ions, Organic Compounds, and Biological Sample Preparation. Crit. Rev. Anal. Chem. 2016, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zhang, X.; Mo, C.E.; Huang, Y.P.; Liu, Z.S. Preparation of graphene oxide incorporated monolithic chip based on deep eutectic solvents for solid phase extraction. Anal. Chim. Acta 2020, 1096, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Seidi, S.; Karimi, E.S.; Rouhollahi, A.; Baharfar, M.; Shanehsaz, M.; Tajik, M. Synthesis and characterization of polyamide-graphene oxide-polypyrrole electrospun nanofibers for spin-column micro solid phase extraction of parabens in milk samples. J. Chromatogr. A 2019, 1599, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rac, V.; Lević, S.; Balanč, B.; Graells, B.O.; Bijelić, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B 2019, 180, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; Daéid, N.N. Poly vinyl alcohol cryogel as a selective test kit for pre and post blast trinitrotoluene. Sens. Actuators B Chem. 2016, 222, 654–662. [Google Scholar] [CrossRef]
- Apopei Loghin, D.F.; Biliuta, G.; Coseri, S.; Dragan, E.S. Preparation and characterization of oxidized starch/poly(N,N-dimethylaminoethyl methacrylate) semi-IPN cryogels and in vitro controlled release evaluation of indomethacin. Int. J. Biol. Macromol. 2017, 96, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Apopei Loghin, D.F. Enhanced sorption of methylene blue from aqueous solutions by semi-IPN composite cryogels with anionically modified potato starch entrapped in PAAm matrix. Chem. Eng. J. 2013, 234, 211–222. [Google Scholar] [CrossRef]
- Seetapan, N.; Limparyoon, N.; Gamonpilas, C.; Methacanon, P.; Fuongfuchat, A. Effect of cryogenic freezing on textural properties and microstructure of rice flour/tapioca starch blend gel. J. Food Eng. 2015, 151, 51–59. [Google Scholar] [CrossRef]
- Charoenrein, S.; Preechathammawong, N. Effect of waxy rice flour and cassava starch on freeze–thaw stability of rice starch gels. Carbohydr. Polym. 2012, 90, 1032–1037. [Google Scholar] [CrossRef]
- Charles, A.L.; Cato, K.; Huang, T.C.; Chang, Y.H.; Ciou, J.Y.; Chang, J.S.; Lin, H.H. Functional properties of arrowroot starch in cassava and sweet potato composite starches. Food Hydrocoll. 2016, 53, 187–191. [Google Scholar] [CrossRef]
- Chanjarujit, W.; Hongsprabhas, P.; Chaiseri, S. Physicochemical properties and flavor retention ability of alkaline calcium hydroxide-mungbean starch films. Carbohydr. Polym. 2018, 198, 473–480. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Yu, J.; Copeland, L.; Wang, S. Phase transition and swelling behaviour of different starch granules over a wide range of water content. LWT-Food Sci. Technol. 2014, 59, 597–604. [Google Scholar] [CrossRef]
- Bertrand, R.; Holmes, W.; Orgeron, C.; Mclntyre, C.; Hernandez, R.; Revellame, E.D. Rapid Estimation of Parameters for Gelatinization of Waxy Corn Starch. Foods 2019, 8, 556. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.; Moo-Young, M. Medical Biothechnology and Healthcare. In Comprehensive Biotechnology, 2nd ed.; Cui, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Mattiasson, B.; Kumar, A.; Galeaev, I.Y. Macroporous Polymers: Production Properties and Biotechnological/Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Plieva, F.M.; Karlsson, M.; Aguilar, M.R.; Gomez, D.; Mikhalovsky, S.; Galaev, I.Y. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter 2005, 1, 303–309. [Google Scholar] [CrossRef]
- Okay, O. Polymeric Cryogels: Macroporous Gels with Remarkable Properties. In Basic Principles of Cryotropic Gelation, 1st ed.; Lozinsky, V.I., Ed.; Springer: New York, NY, USA, 2014; pp. 49–101. [Google Scholar]
- Zou, F.; Budtova, T. Tailoring the morphology and properties of starch aerogels and cryogels via starch source and process parameter. Carbohydr. Polym. 2021, 255, 117344. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.M.C.; Bueno, P.V.A.; Matsushita, A.F.Y.; Rubira, A.F.; Muniz, E.C.; Durães, L.; Murtinho, D.M.B.; Valente, A.J.M. Synthesis, characterization and sorption studies of aromatic compounds by hydrogels of chitosan blended with β-cyclodextrin-and PVA-functionalized pectin. RSC Adv. 2018, 8, 14609. [Google Scholar] [CrossRef] [Green Version]
- Tuncaboylu, D.C.; Abdurrahmanoglu, S.; Gazioglu, I. Rheological characterization of starch gels: A biomass based sorbent for removal of polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2019, 371, 406–414. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Jiang, G. Graphene and Graphene Oxide Sheets Supported on Silica as Versatile and High-Performance Adsorbents for Solid-Phase Extraction. Angew. Chem. Int. Ed. 2011, 50, 5913–5917. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Taweekarn, T.; Wongniramaikul, W.; Limsakul, W.; Sriprom, W.; Phawachalotorn, C.; Choodum, A. A novel colorimetric sensor based on modified mesoporous silica nanoparticles for rapid on-site detection of nitrite. Microchim. Acta 2020, 187, 643. [Google Scholar] [CrossRef]
- Lingling, Q.; Xu, T.; Zhaofeng, W.; Xinshan, P. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(propylene fumarate)/Polyethylene Glycol-Modified Graphene Oxide Nanocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef]
- Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene oxide/conducting polymer composite hydrogels. J. Mater. Chem. 2011, 21, 18653–18658. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Villegas, M.dlÁ.; Rincón-Londoño, N.; Real-López, A.D.; Rodríguez-García, M.E. The effect of Ca2+ ions on the pasting, morphological, structural, vibrational, and mechanical properties of corn starch–water system. J. Cereal Sci. 2018, 79, 174–182. [Google Scholar] [CrossRef]
- Pineda-Gómez, P.; Rosales-Rivera, A.; Rodríguez-García, M.E. Effect of the thermo-alkaline treatment over the thermal degradation of corn starch. Starch/Stärke 2012, 64, 776–785. [Google Scholar] [CrossRef]
- Boonkanon, C.; Phatthanawiwat, K.; Wongniramaikul, W.; Choodum, A. Curcumin nanoparticle doped starch thin film as a green colorimetric sensor for detection of boron. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117351. [Google Scholar] [CrossRef] [PubMed]
- Wongniramaikul, W.; Limsakul, W.; Choodum, A. A biodegradable colorimetric film for rapid low-cost field determination of formaldehyde contamination by digital image colorimetry. Food Chem. 2018, 249, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Chullasat, K.; Nurerk, P.; Kanatharana, P.; Kueseng, P.; Sukchuay, T.; Bunkoed, O. Hybrid monolith sorbent of polypyrrole-coated graphene oxide incorporated into a polyvinyl alcohol cryogel for extraction and enrichment of sulfonamides from water samples. Anal. Chim. Acta 2017, 961, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Taverniers, I.; Loose, M.D.; Bockstaele, E.V. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrA-Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- García-Falcón, M.S.; Pérez-Lamela, C.; Simal-Gándara, J. Strategies for the extraction of free and bound polycyclic aromatic hydrocarbons in run-off waters rich in organic matter. Anal. Chim. Acta 2004, 508, 177–183. [Google Scholar] [CrossRef]
- Filho, C.M.C.; Neto, M.N.L.; Teixeira, R.S.; Pais, A.A.C.C.; Valente, A.J.M. Development and optimization of an HPLC–DAD method for quantification of six petroleum hydrocarbon compounds in aqueous samples. J. Liq. Chromatogr. 2016, 39, 837–846. [Google Scholar] [CrossRef]
- Ledesma, E.; Rendueles, M.; Díaz, M. Spanish smoked meat products: Benzo(a)pyrene (BaP) contamination and moisture. J. Food Compos. Anal. 2015, 37, 87–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Lv, S.; Du, Z.; Liu, X. Simultaneous determination of 16 polycyclic aromatic hydrocarbons in reclaimed water using solid-phase extraction followed by ultra-performance convergence chromatography with photodiode array detection. J. Sep. Sci. 2016, 39, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Lin, J.M. Magnetic solid-phase extraction based on octadecyl functionalization of monodisperse magnetic ferrite microspheres for the determination of polycyclic aromatic hydrocarbons in aqueous samples coupled with gas chromatography-mass spectrometry. Talanta 2009, 77, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, Q.; Xia, J.; Xia, L.; Ding, M.; Tang, J. Graphene-based solid-phase extraction disk for fast separation and preconcentration of trace polycyclic aromatic hydrocarbons from environmental water samples. J. Sep. Sci. 2013, 36, 1834–1842. [Google Scholar] [CrossRef]
- Rawash, E.S.A.; Mohamed, G.; Souaya, E.; Khalil, L.H.; El-Chaghaby, G.A.; El-Gammai, M.H. Distribution and Health Hazards of Polycyclic Aromatic Hydrocarbons in Egyptian Milk and Dairy-Based Products. Beverages 2018, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Jayaramudu, T.; Ko, H.U.; Kim, H.C.; Kim, J.W.; Kim, J. Swelling Behavior of Polyacrylamide-Cellulose Nanocrystal Hydrogels: Swelling Kinetics, Temperature, and pH Effects. Materials 2019, 12, 2080. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Champ, S.; Huglin, M.B.; Lones, T.G.J. Rapid swelling and deswelling in cryogels of crosslinked poly(N-isopropylacrylamide-co-acrylic acid). Eur. Polym. J. 2004, 40, 467–476. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Cryogels-versatile tools in bioseparation. J. Chromatogr. A 2014, 1357, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Choodum, A.; Daéid, N.N. Development and validation of an analytical method for hydrocarbon residues using gas chromatography-mass spectrometry. Anal. Methods 2011, 3, 1136–1142. [Google Scholar] [CrossRef]
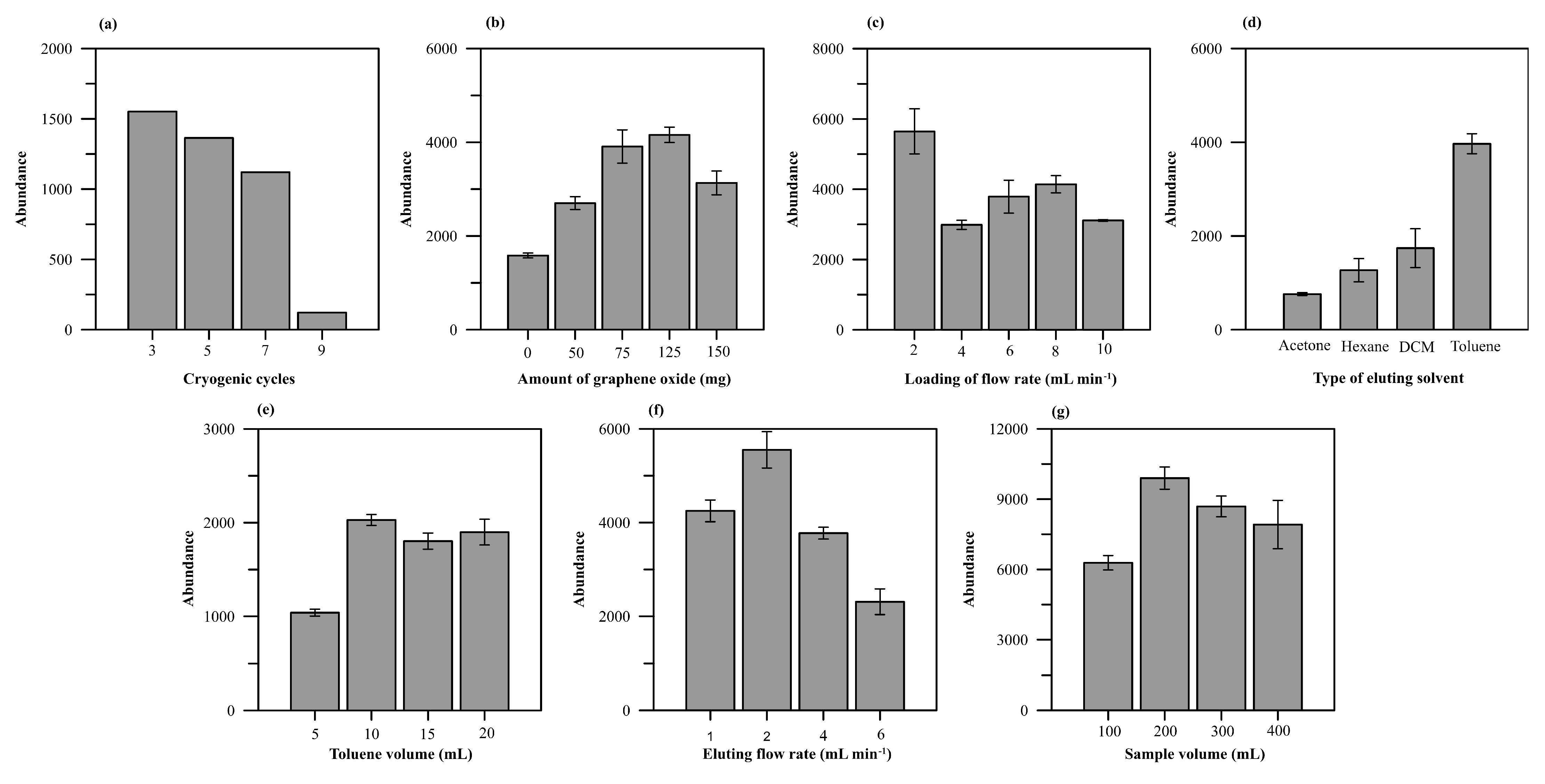

| Component | Study Value | Optimum Value |
|---|---|---|
| Rice flour (g) | 12.5 | 12.5 |
| Tapioca starch (g) | 0, 1.25, 2.50, 3.75, 5.00 | 3.75 |
| Arrowroot starch (g) | 0, 1.25, 2.50, 3.75, 5.00 | 0 |
| Limewater (mL) | 90, 100, 110, 120, 130, 150 | 130 |
| GO (mg) | 0, 50, 75, 125, 150 | 125 |
| Freeze–thaw cycle (cycle) | 3, 5, 7, 9 | 3 |
| Sample | December 2019 | October 2020 | ||
|---|---|---|---|---|
| Added (µg L−1) | Found (µg L−1) | %Recovery | Found (µg L−1) | |
| C1 | 0 50 100 | 10 ± 3 * 58 ± 2 106 ± 4 | 0 97 97 | ND ** |
| C2 | 0 50 100 | 7 ± 0 * 49 ± 3 108 ± 0 | 0 84 101 | ND ** |
| C3 | 0 50 100 | 36 ± 4 * 83 ± 3 138 ± 2 | 0 95 102 | 28 ± 1 * |
| C4 | 0 50 100 | ND ** 58 ± 1 95 ± 3 | 0 110 92 | ND ** |
| C5 | 0 50 100 | 5 ± 1 * 52 ± 3 89 ± 2 | 0 94 84 | ND ** |
| P | 0 50 100 | 5 ± 1 * 53 ± 3 105 ± 6 | 0 96 100 | ND ** |
| Method | Sample Preparation | Amount of Adsorbent | Sample Volume | LOD (µg L−1) | Reusability | Reference |
|---|---|---|---|---|---|---|
| GC/MS | Mega bond elut C18 SPE | 5 g | 4 mL | 0.05 | No | [43] |
| GC/MS | C18 Sep-Pak SPE | 500 mg | 100 mL | 4 | No | [44] |
| GC/MS | C18/Fe3O4/ µSPE | 50 mg | 20 mL | 5.6 | - | [45] |
| GC/MS | Graphene disk SPE | 200 mg | 1000 mL | 0.013 | 10 | [46] |
| GC/MS | GO-Cry SPE | 25 mg (GO) | 200 mL | 4.21 ± 0.06 | 10 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choodum, A.; Lamthornkit, N.; Boonkanon, C.; Taweekarn, T.; Phatthanawiwat, K.; Sriprom, W.; Limsakul, W.; Chuenchom, L.; Wongniramaikul, W. Greener Monolithic Solid Phase Extraction Biosorbent Based on Calcium Cross-Linked Starch Cryogel Composite Graphene Oxide Nanoparticles for Benzo(a)pyrene Analysis. Molecules 2021, 26, 6163. https://doi.org/10.3390/molecules26206163
Choodum A, Lamthornkit N, Boonkanon C, Taweekarn T, Phatthanawiwat K, Sriprom W, Limsakul W, Chuenchom L, Wongniramaikul W. Greener Monolithic Solid Phase Extraction Biosorbent Based on Calcium Cross-Linked Starch Cryogel Composite Graphene Oxide Nanoparticles for Benzo(a)pyrene Analysis. Molecules. 2021; 26(20):6163. https://doi.org/10.3390/molecules26206163
Chicago/Turabian StyleChoodum, Aree, Nareumon Lamthornkit, Chanita Boonkanon, Tarawee Taweekarn, Kharittha Phatthanawiwat, Wilasinee Sriprom, Wadcharawadee Limsakul, Laemthong Chuenchom, and Worawit Wongniramaikul. 2021. "Greener Monolithic Solid Phase Extraction Biosorbent Based on Calcium Cross-Linked Starch Cryogel Composite Graphene Oxide Nanoparticles for Benzo(a)pyrene Analysis" Molecules 26, no. 20: 6163. https://doi.org/10.3390/molecules26206163
APA StyleChoodum, A., Lamthornkit, N., Boonkanon, C., Taweekarn, T., Phatthanawiwat, K., Sriprom, W., Limsakul, W., Chuenchom, L., & Wongniramaikul, W. (2021). Greener Monolithic Solid Phase Extraction Biosorbent Based on Calcium Cross-Linked Starch Cryogel Composite Graphene Oxide Nanoparticles for Benzo(a)pyrene Analysis. Molecules, 26(20), 6163. https://doi.org/10.3390/molecules26206163