Voltammetric Determination of Isopropylmethylphenols in Herbal Spices
Abstract
:1. Introduction
2. Results and Discussion
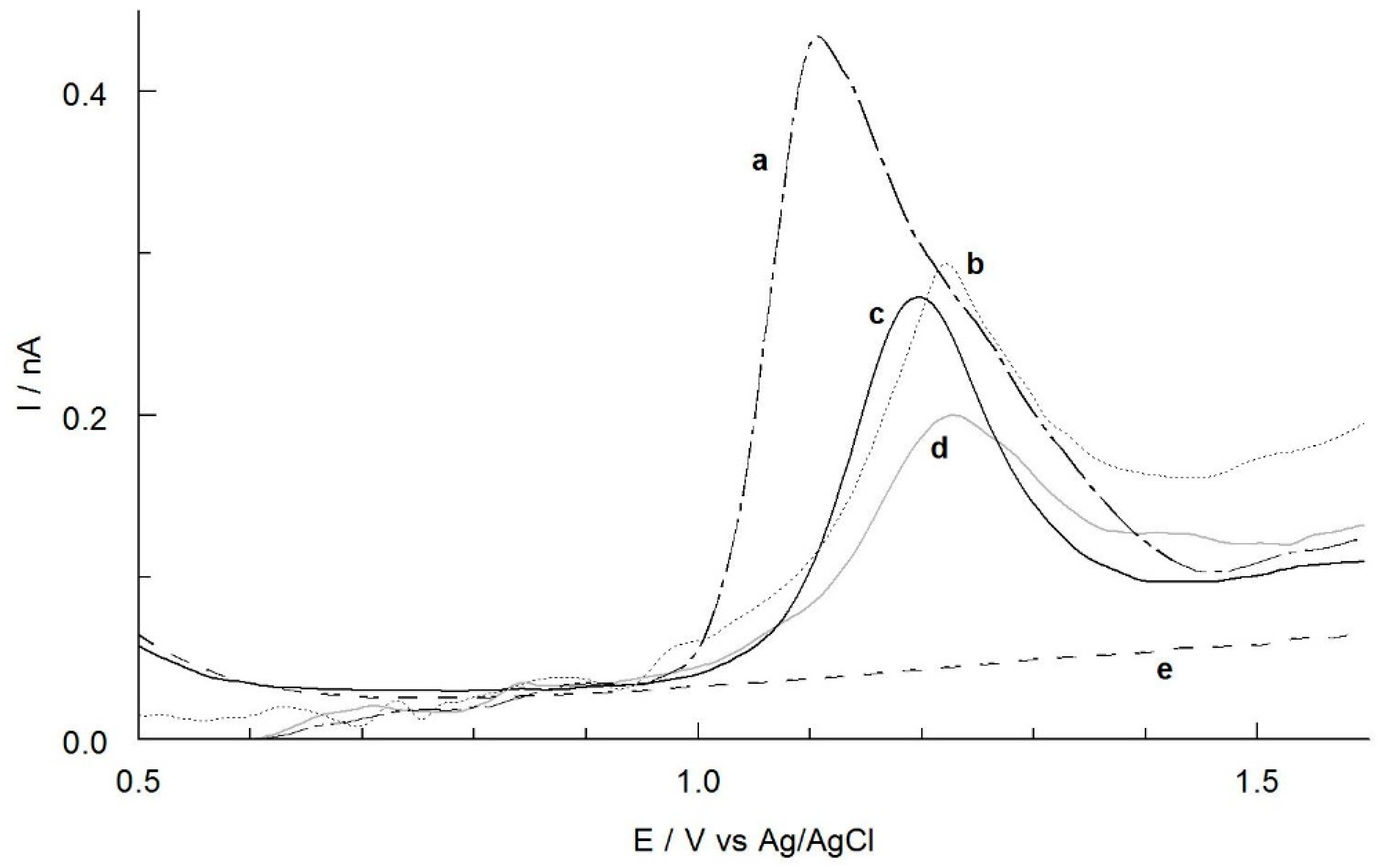
2.1. Influence of the Electrode Material and Composition of Solution on THY and CAR Oxidation Curves
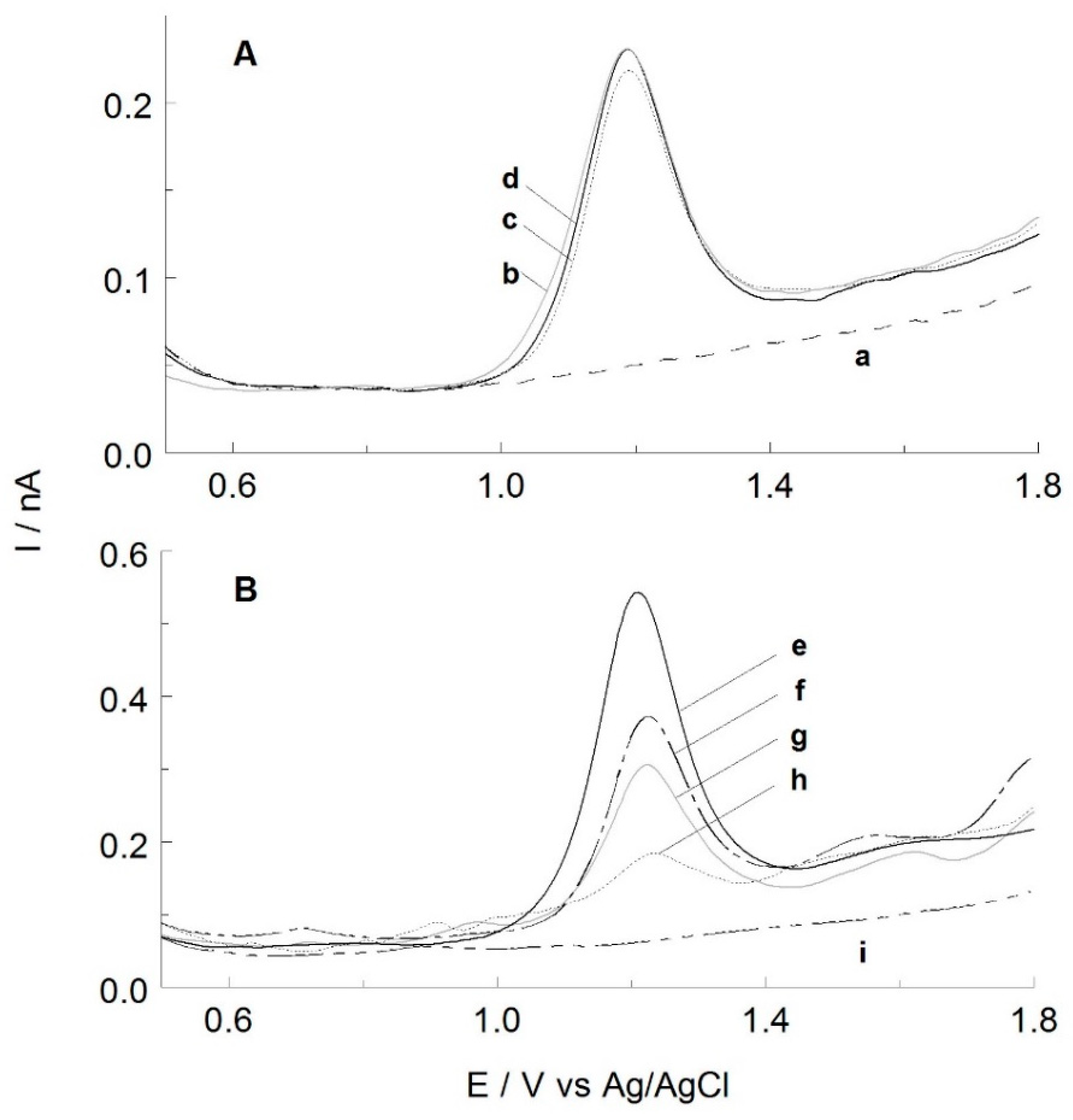
2.2. Identification of THY and CAR
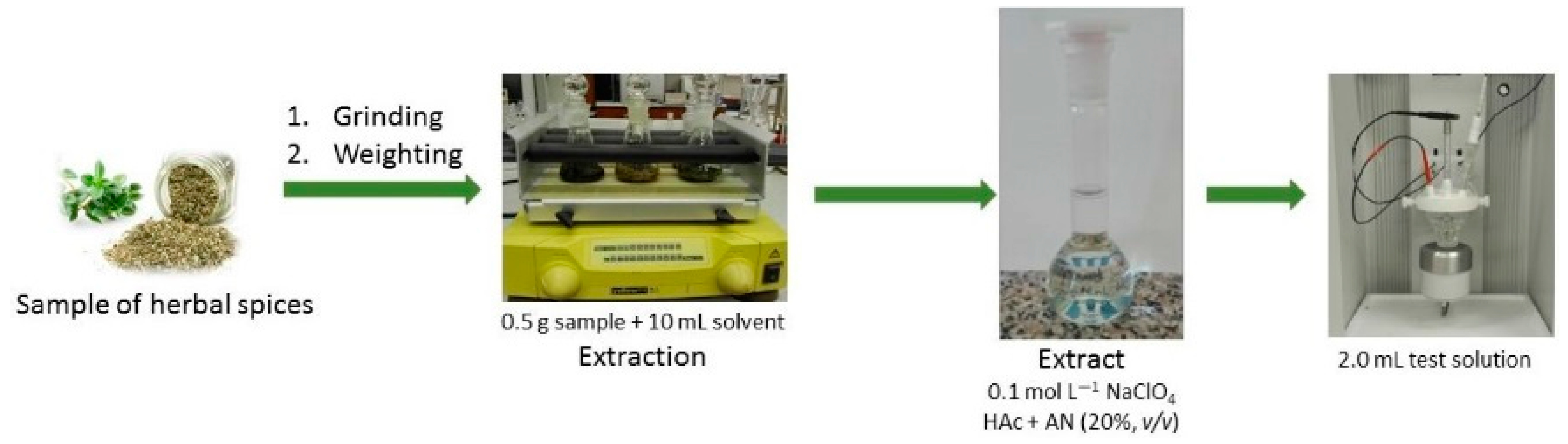
2.3. Optimization of Extraction Condition
2.4. Identification of THY and CAR in Real Samples
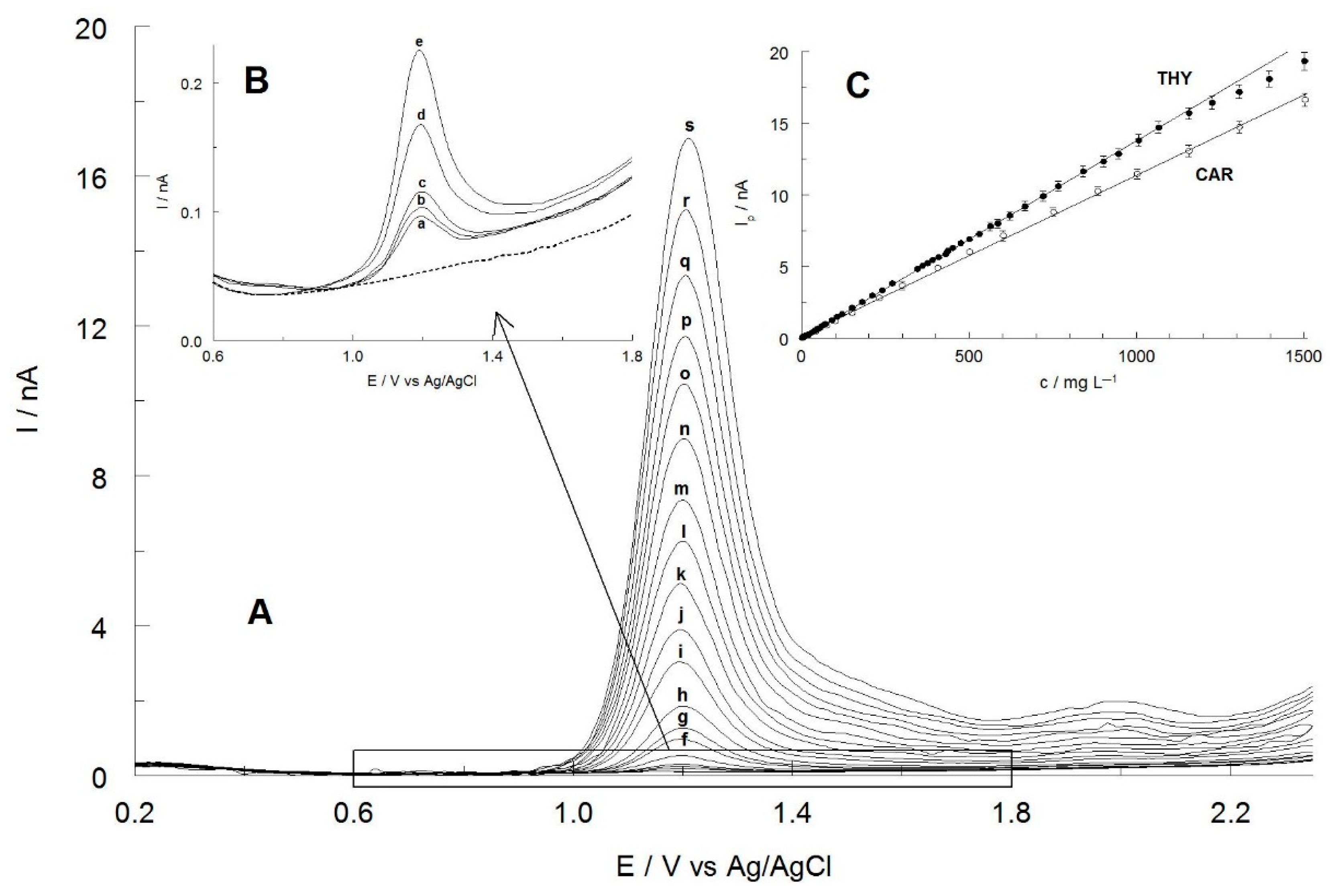
2.5. Calibration Curves, Repeatability and Reproducibility
2.6. Interferences
2.7. Recovery Studies
2.8. Real Sample Analysis
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Materials
3.3. Electrochemical Measurements
3.4. GC Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Silva, L.P.; Ferreira, O.; Schröder, B.; Coutinho, J.A.P.; Pinho, S.P. Terpenes solubility in water and their environmental distribution. J. Mol. Liq. 2017, 241, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Breitmaier, E. Terpenes—Flavors, Fragrances, Pharmaca, Pheromones; WILEY-VCH: Weinheim, Germany, 2006; ISBN 9783527317868. [Google Scholar]
- Cattelan, M.G.; de Nishiyama, Y.P.O.; Gonçalves, T.M.V.; Coelho, A.R. Combined effects of oregano essential oil and salt on the growth of Escherichia coli in salad dressing. Food Microbiol. 2018, 73, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fadil, M.; Fikri-Benbrahim, K.; Rachiq, S.; Ihssane, B.; Lebrazi, S.; Chraibi, M.; Haloui, T.; Farah, A. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur. J. Pharm. Biopharm. 2018, 126, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Starliper, C.E.; Ketola, H.G.; Noyes, A.D.; Schill, W.B.; Henson, F.G.; Chalupnicki, M.A.; Dittman, D.E. An investigation of the bactericidal activity of selected essential oils to Aeromonas spp. J. Adv. Res. 2015, 6, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Bertuola, M.; Fagali, N.; Fernández Lorenzo de Mele, M. Detection of carvacrol in essential oils by electrochemical polymerization. Heliyon 2020, 6, e03714. [Google Scholar] [CrossRef]
- Zarrini, G.; Delgosha, Z.B.; Moghaddam, K.M.; Shahverdi, A.R. Post-antibacterial effect of thymol. Pharm. Biol. 2010, 48, 633–636. [Google Scholar] [CrossRef]
- Ribeiro, A.R.S.; Diniz, P.B.F.; Pinheiro, M.S.; Albuquerque-Júnior, R.L.C.; Thomazzi, S.M. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, ATP-sensitive K+ channels, and gastric mucus secretion. Chem. Biol. Interact. 2016, 244, 121–128. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety. A Guide for Health Care Professionals, 2nd ed.; Elsevier: Edinburgh, Australia, 2014; ISBN 9780443062414. [Google Scholar]
- Fiori, G.M.L.; Bonato, P.S.; Pereira, M.P.M.; Contini, S.H.T.; Pereira, A.M.S. Determination of thymol and carvacrol in plasma and milk of dairy cows using solid-phase microextraction. J. Braz. Chem. Soc. 2013, 24, 837–846. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Evaluation of three headspace sorptive extraction coatings for the determination of volatile terpenes in honey using gas chromatography-mass spectrometry. J. Chromatogr. A 2015, 1399, 18–24. [Google Scholar] [CrossRef]
- Nozal, M.; Bernal, J.; Jiménez, J.; González, M.; Higes, M. Extraction of thymol, eucalyptol, menthol, and camphor residues from honey and beeswax. J. Chromatogr. A 2002, 954, 207–215. [Google Scholar] [CrossRef]
- Kohlert, C.; Abel, G.; Schmid, E.; Veit, M. Determination of thymol in human plasma by automated headspace solid-phase microextraction-gas chromatographic analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 767, 11–18. [Google Scholar] [CrossRef]
- Viñas, P.; Soler-Romera, M.J.; Hernández-Córdoba, M. Liquid chromatographic determination of phenol, thymol and carvacrol in honey using fluorimetric detection. Talanta 2006, 69, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.; Dowlatshah, S.; Nouraei, N.; Heidari, N.; Yazdankhah, F. A solid-phase microextraction platinized stainless steel fiber coated with a multiwalled carbon nanotube-polyaniline nanocomposite film for the extraction of thymol and carvacrol in medicinal plants and honey. J. Chromatogr. A 2015, 1406, 87–93. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacogn. Mag. 2010, 6, 154–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedić, M.; Bečić, E.; Imamović, B.; Žiga, N.; Medanhodžić-Vuk, S.; Šober, M. HPLC method for determination the content of thymol and carvacrol in Thyme tincture. Bull. Chem. Technol. Bosnia Herzegovina 2018, 50, 1–6. [Google Scholar]
- Louchard, B.O.; Costa, L.C.; Silva, A.R.; Leal, L.K.A. Validation of A High Performance Liquid Chromatography Method to Quantify Thymol in Nanocapsules of Bioactive Essential Oil from Lippia Sidoides. Int. J. Complement. Altern. Med. 2017, 10, 00330. [Google Scholar] [CrossRef]
- Zima, J.; Cienciala, M.; Barek, J.; Moreira, J.C. Determination of thymol using HPLC-ED with glassy carbon paste electrode. Chem. Anal. 2007, 52, 1049–1057. [Google Scholar]
- Angelo, T.; Pires, F.Q.; Gelfuso, G.M.; da Silva, J.K.R.; Gratieri, T.; Cunha-Filho, M.S.S. Development and validation of a selective HPLC-UV method for thymol determination in skin permeation experiments. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1022, 81–86. [Google Scholar] [CrossRef]
- Martel, A.C.; Zeggane, S. Determination of acaricides in honey by high-performance liquid chromatography with photodiode array detection. J. Chromatogr. A 2002, 954, 173–180. [Google Scholar] [CrossRef]
- Tonello, N.; D’Eramo, F.; Marioli, J.M.; Crevillen, A.G.; Escarpa, A. Extraction-free colorimetric determination of thymol and carvacrol isomers in essential oils by pH-dependent formation of gold nanoparticles. Microchim. Acta 2018, 185, 352. [Google Scholar] [CrossRef]
- Fadhil, G. Spectrophotometric Determination of Thymol in Pharmaceutical Preparations Via Oxidative Coupling Reaction with 2,4-dinitrophenylhydrazine in the Presence of Potassium Periodate. Iraqi J. Sci. 2014, 55, 27–34. [Google Scholar]
- Blanc, R.; González-Casado, A.; Navalón, A.; Vílchez, J.L. On the estimate of blanks in differential pulse voltammetric techniques: Application to detection limits evaluation as recommended by IUPAC. Anal. Chim. Acta 2000, 403, 117–123. [Google Scholar] [CrossRef]
- Stanković, D.M. Sensitive voltammetric determination of thymol in essential oil of Carum copticum seeds using boron-doped diamond electrode. Anal. Biochem. 2015, 486, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kowalcze, M.; Jakubowska, M. Voltammetric determination of carvacrol on Boron Doped Diamond Electrode. Anal. Chim. Acta 2019, 1045, 98–107. [Google Scholar] [CrossRef]
- Kowalcze, M.; Jakubowska, M. Multivariate approach in voltammetric identification and simultaneous determination of eugenol, carvacrol, and thymol on boron-doped diamond electrode. Mon. Chem. 2019, 150, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Tonello, N.; Moressi, M.B.; Robledo, S.N.; D’Eramo, F.; Marioli, J.M. Square wave voltammetry with multivariate calibration tools for determination of eugenol, carvacrol and thymol in honey. Talanta 2016, 158, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.K.; Budnikov, H.C. Evaluation of the antioxidant properties of spices by cyclic voltammetry. J. Anal. Chem. 2014, 69, 990–997. [Google Scholar] [CrossRef]
- Robledo, S.N.; Pierini, G.D.; Nieto, C.H.D.; Fernández, H.; Zon, M.A. Development of an electrochemical method to determine phenolic monoterpenes in essential oils. Talanta 2019, 196, 362–369. [Google Scholar] [CrossRef]
- Zhao, X.; Du, Y.; Ye, W.; Lu, D.; Xia, X.; Wang, C. Sensitive determination of thymol based on CeO2 nanoparticle–decorated graphene hybrid film. New J. Chem. 2013, 37, 4045. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.N.; Budnikov, H. Voltammetric Determination of Thymol in Oregano Using CeO2-Modified Electrode in Brij® 35 Micellar Medium. Food Anal. Methods 2017, 10, 129–136. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Romashkina, S.A.; Ziganshina, E.R.; Budnikov, H.C. Voltammetric Determinations of Thymol on an Electrode Modified by Coimmobilized Carboxylated Multiwalled Carbon Nanotubes and Surfactants. J. Anal. Chem. 2018, 73, 63–70. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. MWNT-Based Electrode for the Voltammetric Quantification of Carvacrol. Food Anal. Methods 2021, 14, 401–410. [Google Scholar] [CrossRef]
- Piech, R.; Paczosa-Bator, B. Application of glassy carbon electrode modified with Nafion/MWCNTs for sensitive voltammetric determination of thymol. Acta Pol. Pharm. Drug Res. 2015, 72, 1081–1088. [Google Scholar]
- Gan, T.; Lv, Z.; Deng, Y.; Sun, J.; Shi, Z.; Liu, Y. Facile synthesis of monodisperse Ag@C@Ag core–double shell spheres for application in the sensing of thymol and phenol simultaneous. New J. Chem. 2015, 39, 6244–6252. [Google Scholar] [CrossRef]
- Fuentes, F.G.; Gil, M.Á.L.; Mendoza, S.; Escarpa, A. Electrochemical screening of biomarkers in chemotype Mexican oregano oils on single-walled carbon nanotubes screen-printed electrodes. Electroanalysis 2011, 23, 2212–2216. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Beitollahi, H.; Rohani, T.; Allahabadi, H. Carvacrol electrochemical reaction characteristics on screen printed electrode modified with La2O3/Co3O4 nanocomposite. J. Electrochem. Sci. Eng. 2019, 9, 113–123. [Google Scholar] [CrossRef]
- Aghamohseni, B.; Hassaninejad-Darzi, S.K.; Asadollahi-Baboli, M. A new sensitive voltammetric determination of thymol based on MnY nanozeolite modified carbon paste electrode using response surface methodology. Microchem. J. 2019, 145, 819–832. [Google Scholar] [CrossRef]
- Naskar, H.; Biswas, S.; Tudu, B.; Bandyopadhyay, R.; Pramanik, P. Voltammetric Detection of Thymol (THY) Using Polyacrylamide Embedded Graphite Molecular Imprinted Polymer (PAM@G-MIP) Electrode. IEEE Sens. J. 2019, 19, 8583–8589. [Google Scholar] [CrossRef]
- Izutsu, K. Electrochemistry in Nonaqueous Solutions; WILEY-VCH: Weinheim, Germany, 2009; ISBN 9783527315659. [Google Scholar]
- Bond, A.M. Past, present and future contributions of microelectrodes to analytical studies employing voltammetric detection. A review. Analyst 1994, 119, R1–R21. [Google Scholar] [CrossRef]
- Stojek, Z. New possibilities in analytical chemistry connected with voltammetric applications of microelectrodes. Mikrochim. Acta 1991, 104, 353–361. [Google Scholar] [CrossRef]
- Michalkiewicz, S.; Tutaj, M.; Kaczor, M.; Malyszko, J. Electrochemical behavior of tocopherols on microelectrodes in acetic acid medium. Electroanalysis 2002, 14, 297–302. [Google Scholar] [CrossRef]
- Michelitsch, A.; Rittmannsberger, A.; Hüfner, A.; Rückert, U.; Likussar, W. Determination of isopropylmethylphenols in black seed oil by differential pulse voltammetry. Phytochem. Anal. 2004, 15, 320–324. [Google Scholar] [CrossRef]
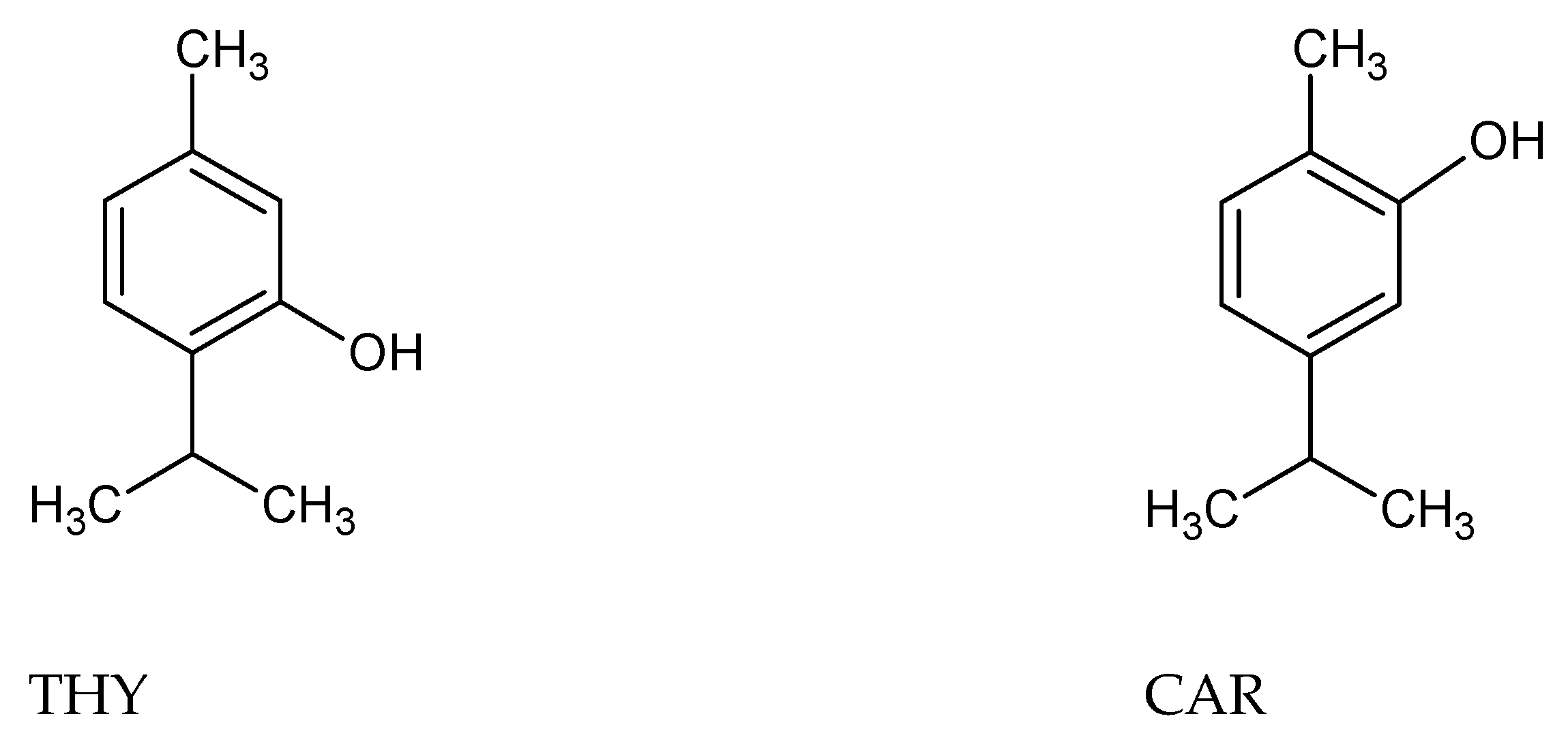


| 1) Method | Analyte | LR/µg mL−1 | LOD/µg mL−1 | Reference |
|---|---|---|---|---|
| GC-MS | THY CAR | 2 × 10−3–0.4 2 × 10−3–0.4 | 0.89 × 10−3 0.57 × 10−3 | [11] |
| GC-FID | THY | 0.1–100 | 0.05 | [13] |
| RP-LC-FLD | THY CAR | 8 × 10−3–0.2 8 × 10−3–0.2 | 1.70 × 10−3 1.56 × 10−3 | [15] |
| RP-HPLC-UV | THY CAR | 1–80 1–80 | 0.6 0.8 | [16] |
| RP-HPLC-UV | THY | 15–90 | 2.8 | [17] |
| RP-HPLC-UV | THY CAR | 15–75 1.5–7.5 | 0.17 × 10−3 0.161 × 10−3 | [18] |
| RP-HPLC-UV | THY | 4–180 | 0.4 | [19] |
| UV | THY | 0.25–10 | 0.063 | [24] |
| SWV (BDDE) | THY | 0.6–15 | 0.59 | [26] |
| DPV (BDDE) | CAR | 0.29–15.23 | 0.02 | [27] |
| DPV (BDDE) | THY CAR | 0.34–9.52 0.29–11.56 | 0.02 0.02 | [28] |
| SWV (GCE) | THY CAR | 2) nd | 0.24 0.21 | [29] |
| CV (GCE) | THY | 9.01–300.4 | 1.50 | [30] |
| LSV (GCE) | THY CAR | 12.77–195.29 11.87–180.26 | 11.87 12.92 | [31] |
| DPV (CeO2/GN/GCE) | THY | 0.015–2.7 | 7.5 × 10−3 | [32] |
| DPV (CeO2-Brij® 35/GCE) | THY | 0.10–91.03 | 0.03 | [33] |
| LSV (MWCNT-COOH-SDS/GCE) | THY | 0.075–22.53 | 0.021 | [34] |
| DPV (MWNTs/GCE) | CAR | 0.015–22.53 | 0.01 | [35] |
| DPV (Nafion/MWCNT/GCE) | THY | 0.075–0.750 | 7.5 × 10−3 | [36] |
| DPV (Ag@C@Ag/GCE) | THY | 0.015–1.50 | 3.2 × 10−3 | [37] |
| DPV (SWCNT-SPE) | THY+CAR | nd | 0.6 | [38] |
| DPV (La2O3/Co3O4/SPE) | CAR | 1.50–120.2 | 0.15 | [39] |
| DPV (MnY/CPE) | THY | 0.075–4.21 | 0.036 | [40] |
| DPV (PAM@G-MIP) | THY | 0.075–40.07 | 6.5 × 10−3 | [41] |
| Sample | DPV | GC | t-Test 4) (2.31) | F-Test 4) (6.39) | ||
|---|---|---|---|---|---|---|
| 1) Amount Found/ mg g−1 | 2)RSD/% (n = 5) | 1) Amount Found/ mg g−1 | 2)RSD/% (n = 5) | |||
| Control (rosemary matrix) | 26.88 ± 0.11 3) (101.0%) | 0.3 | 26.11 ± 0.41 3) (98.4%) | 1.6 | 1.69 | 20.2 |
| Thyme | 6.91 ± 0.31 | 3.6 | 7.21 ± 0.30 | 3.3 | 0.86 | 1.09 |
| Oregano | 13.21 ± 0.04 | 0.3 | 13.33 ± 0.48 | 2.9 | 0.31 | 125.1 |
| Savory | 8.48 ± 0.15 | 1.4 | 8.16 ± 0.34 | 3.4 | 1.06 | 5.29 |
| Herbes de Provence | 2.15 ± 0.02 | 0.6 | 2.07 ± 0.04 | 1.7 | 2.12 | 7.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, M.; Michalkiewicz, S.; Skorupa, A.; Slefarska, D. Voltammetric Determination of Isopropylmethylphenols in Herbal Spices. Molecules 2021, 26, 6095. https://doi.org/10.3390/molecules26206095
Jakubczyk M, Michalkiewicz S, Skorupa A, Slefarska D. Voltammetric Determination of Isopropylmethylphenols in Herbal Spices. Molecules. 2021; 26(20):6095. https://doi.org/10.3390/molecules26206095
Chicago/Turabian StyleJakubczyk, Magdalena, Slawomir Michalkiewicz, Agata Skorupa, and Daria Slefarska. 2021. "Voltammetric Determination of Isopropylmethylphenols in Herbal Spices" Molecules 26, no. 20: 6095. https://doi.org/10.3390/molecules26206095
APA StyleJakubczyk, M., Michalkiewicz, S., Skorupa, A., & Slefarska, D. (2021). Voltammetric Determination of Isopropylmethylphenols in Herbal Spices. Molecules, 26(20), 6095. https://doi.org/10.3390/molecules26206095






