Characterization of Cross-Linked Enzyme Aggregates of the Y509E Mutant of a Glycoside Hydrolase Family 52 β-xylosidase from G. stearothermophilus
Abstract
1. Introduction
2. Results and Discussion
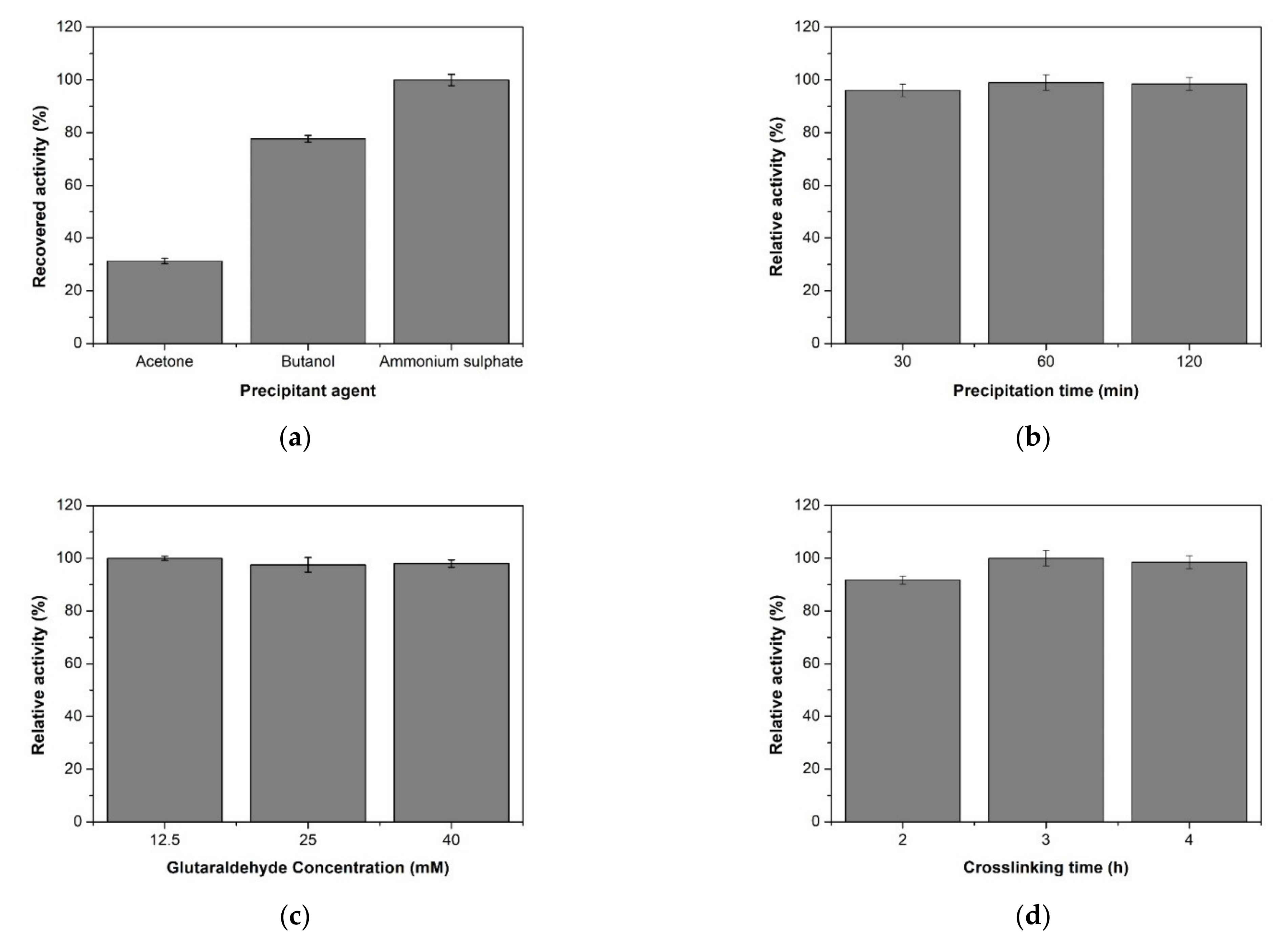
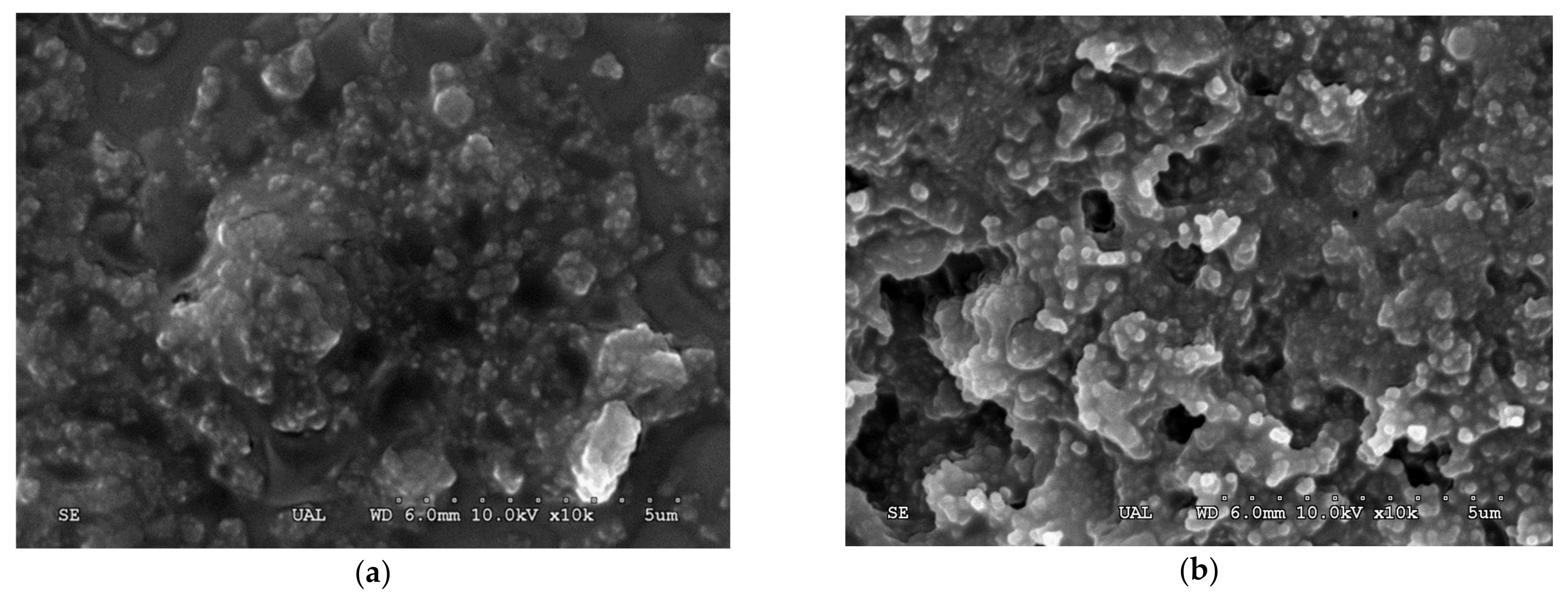
2.1. Optimization of XynB2Y509E CLEAs Preparation
2.1.1. Optimization of Precipitant Type and Precipitation Time
2.1.2. Effect of Concentration and pH in Cross-Linking Reaction
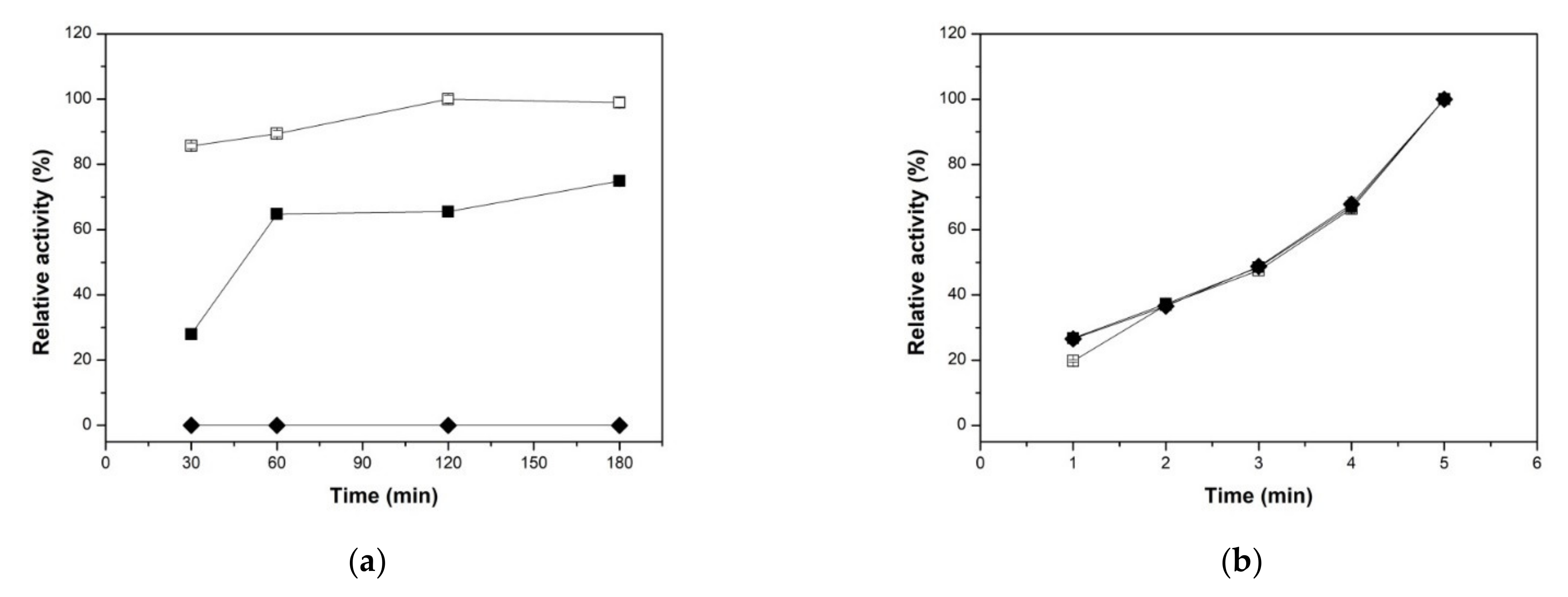
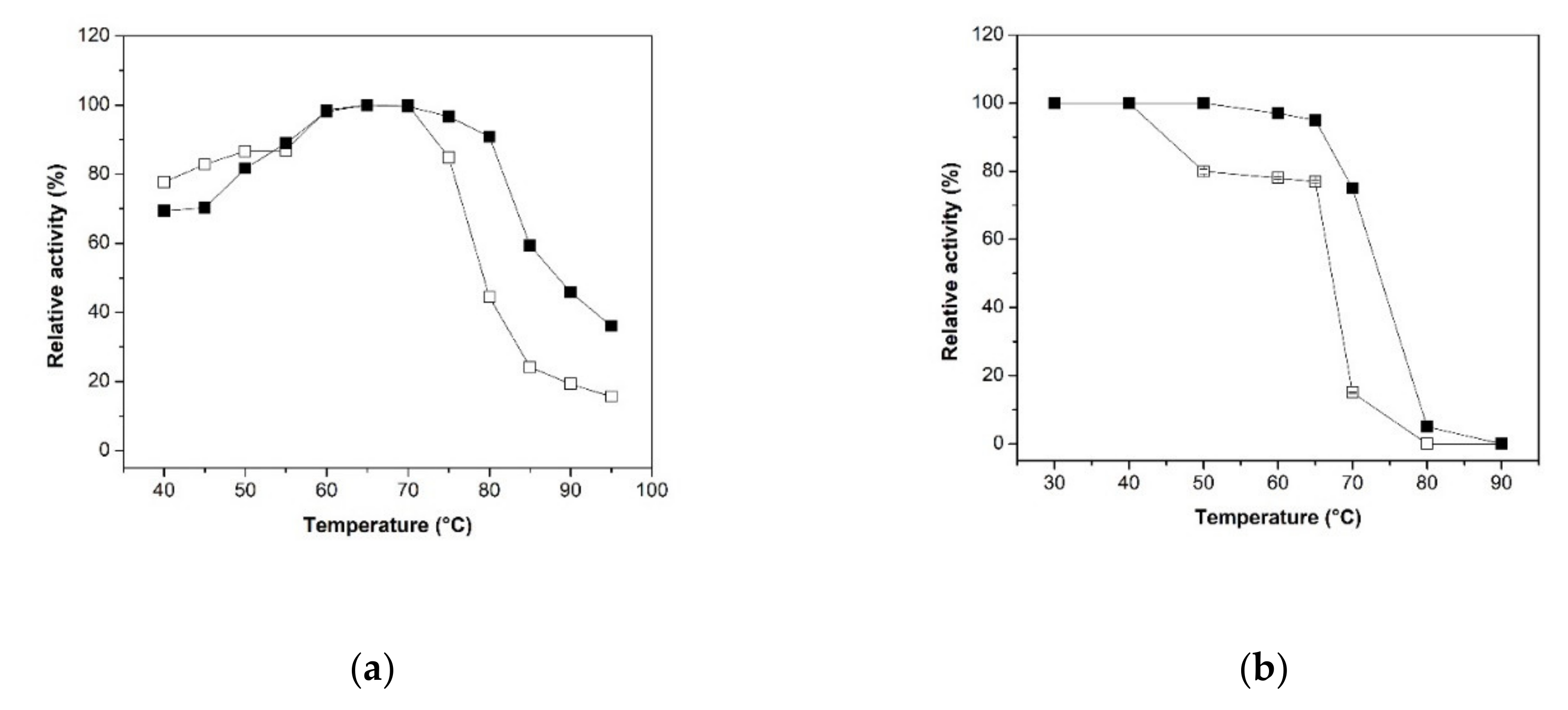
2.2. Biochemical Characterization of Free XynB2Y509E and XynB2Y509E-CLEAs
2.2.1. Effect of Reaction Times on both Xylanolytic Activities
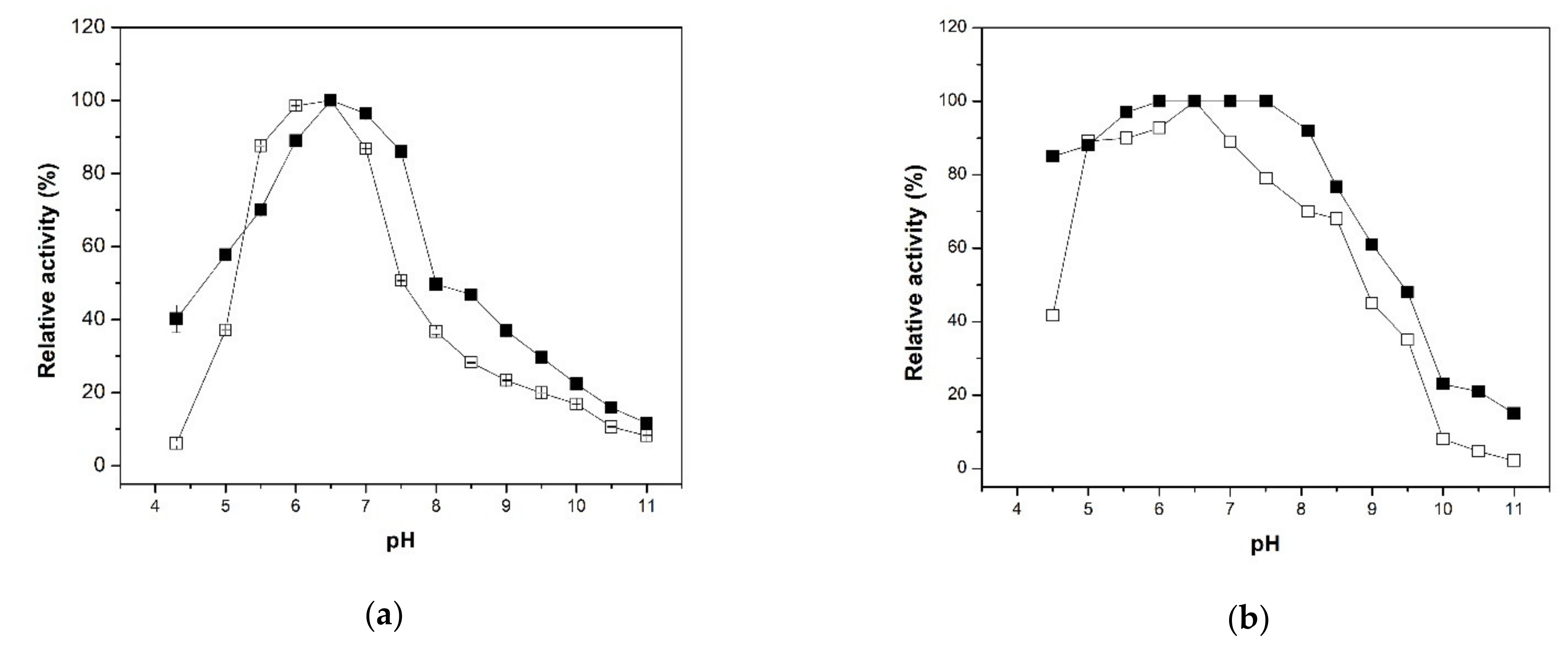
2.2.2. Effect of the Temperature and pH on Activity and Stability
2.2.3. Kinetic Parameters for the Free XynB2Y509E and XynB2Y509E-CLEAs
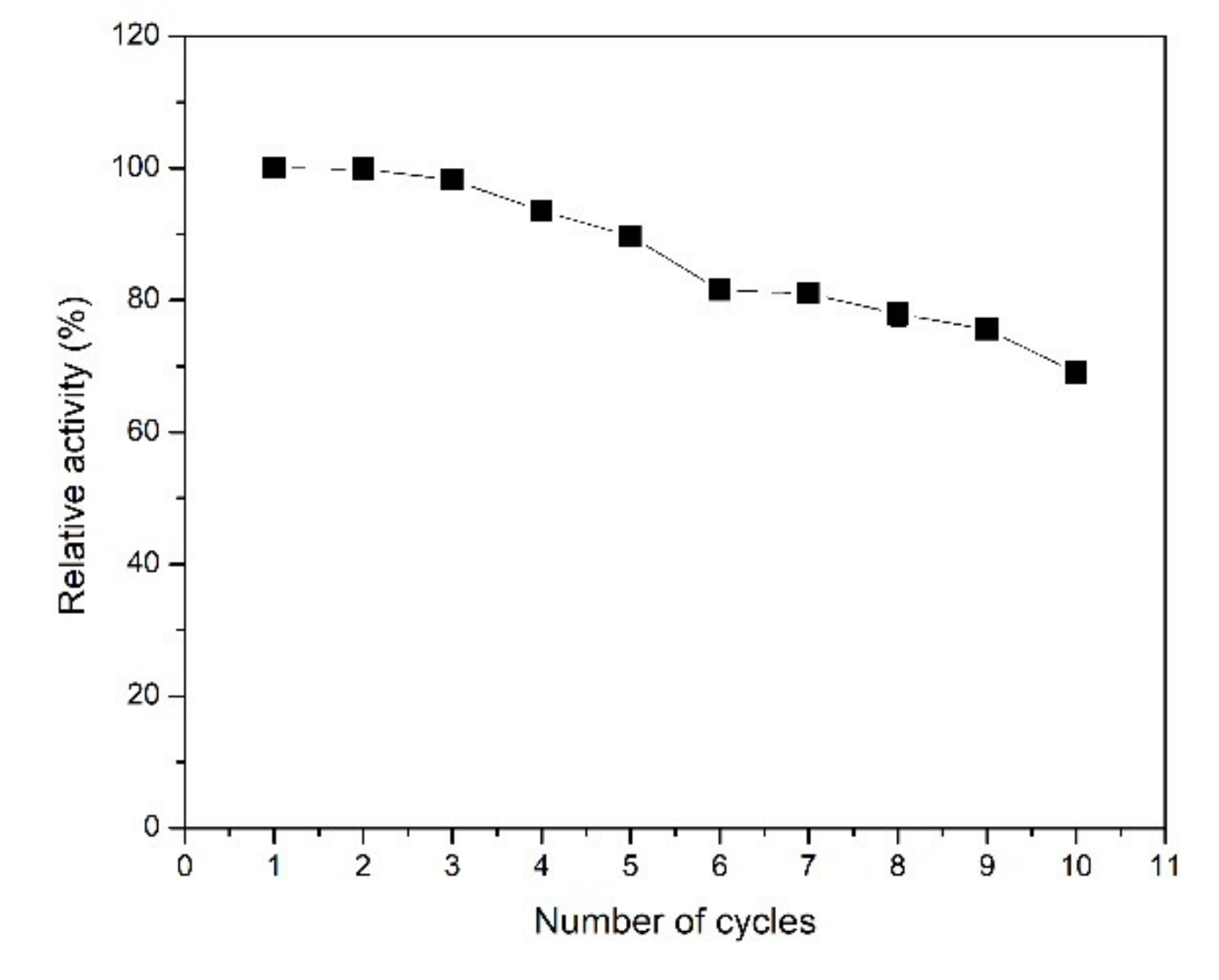
2.2.4. Reusability
3. Materials and Methods
3.1. Materials
3.2. Mutagenesis of the xynB2 Gene
- 5-GCGCGCAACAATTTAGAGTTGACAGGAAAAT-3′
- 5-ATTTTCCTGTCAACTCTAAATTGTTGCGCGC-3′
3.3. Overexpression and Partial Purification of XynB2Y509E
3.4. Enzymatic Assays
3.5. Preparation of XynB2Y509E-CLEAs
3.6. Optimization of XynB2Y509E-CLEAs Preparation
3.7. Scanning Electron Microscopy of XynB2Y509E-CLEAs
3.8. Biochemical Characterization of XynB2Y509E-CLEAs
3.8.1. Optimal Temperature and Thermal Stability of Free and XynB2Y509E-CLEAs
3.8.2. Effect of pH on Activity
3.8.3. Kinetic Parameters of Free and XynB2Y509E-CLEAs
3.8.4. Recyclability of XynB2Y509E-CLEAs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, M.Q.; Wang, S.S.; Li, L.N.; Gao, J.; Zhang, Y.W. Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 2018, 8, 460. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA® s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kiyota, Y.; Miyazaki, M. Techniques for preparation of cross-linked enzyme aggregates and their applications in bioconversions. Catalysts 2018, 8, 174. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, combi-CLEAs and ‘smart’ magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.D.; Li, N.; Zong, M.H. Cross-linked Enzyme Aggregates of β-glucosidase from Prunus domestica seeds. Biotechnol. Lett. 2012, 34, 1673–1678. [Google Scholar] [CrossRef]
- Li, L.; Li, G.; Cao, L.C.; Ren, G.H.; Kong, W.; Wang, S.D.; Guo, G.S.; Liu, Y.H. Characterization of the cross-linked enzyme aggregates of a novel β-galactosidase, a potential catalyst for the synthesis of galacto-oligosaccharides. J. Agric. Food Chem. 2015, 63, 894–901. [Google Scholar] [CrossRef]
- Hero, J.S.; Romero, C.M.; Pisa, J.H.; Perotti, N.I.; Olivaro, C.; Martinez, M.A. Designing cross-linked xylanase aggregates for bioconversion of agroindustrial waste biomass towards potential production of nutraceuticals. Int. J. Biol. Macromol. 2018, 111, 229–236. [Google Scholar] [CrossRef]
- Verma, R.; Kumar, A.; Kumar, S. Synthesis and characterization of cross-linked enzyme aggregates (CLEAs) of thermostable xylanase from Geobacillus thermodenitrificans X1. Process. Biochem. 2019, 80, 72–79. [Google Scholar] [CrossRef]
- Periyasamy, K.; Santhalembi, L.; Mortha, G.; Aurousseau, M.; Subramanian, S. Carrier-free co-immobilization of xylanase, cellulase and β-1,3-glucanase as combined cross-linked enzyme aggregates (combi-CLEAs) for one-pot saccharification of sugarcane bagasse. RSC Adv. 2016, 6, 32849–32857. [Google Scholar] [CrossRef]
- Xu, M.Q.; Li, F.L.; Yu, W.Q.; Li, R.F.; Zhang, Y.W. Combined cross-linked enzyme aggregates of glycerol dehydrogenase and NADH oxidase for high efficiency in situ NAD+ regeneration. Int J. Biol Macromol. 2020, 144, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Bravman, T.; Zolotnitsky, G.; Shulami, S.; Belakhov, V.; Solomon, D.; Baasov, T.; Shoham, G.; Shoham, Y. Stereochemistry of family 52 glycosyl hydrolases: A β-xylosidase from Bacillus stearothermophilus T-6 is a retaining enzyme. FEBS Lett. 2001, 495, 39–43. [Google Scholar] [CrossRef]
- Bravman, T.; Zolotnitsky, G.; Belakhov, V.; Shoham, G.; Henrissat, B.; Baasov, T.; Shoham, Y. Detailed kinetic analysis of a family 52 glycoside hydrolase: A β-xylosidase from Geobacillus stearothermophilus. Biochemistry 2003, 42, 10528–10536. [Google Scholar] [CrossRef] [PubMed]
- Bravman, T.; Belakhov, V.; Solomon, D.; Shoham, G.; Henrissat, B.; Baasov, T.; Shoham, Y. Identification of the catalytic residues in family 52 glycoside hydrolase, a β-xylosidas from Geobacillus stearothermophilus T-6. J. Biol. Chem. 2003, 278, 26742–26749. [Google Scholar] [CrossRef]
- Contreras, L.M.; Gómez, J.; Prieto, J.; Clemente-Jiménez, J.M.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F.; Blanco, F.; Neira, J.L. The family 52 β-xylosidase from Geobacillus stearothermophilus is a dimer: Structural and biophysical characterization of a glycoside hydrolase. Biochim. Biophys. Acta. 2008, 1784, 1924–1934. [Google Scholar] [CrossRef]
- Kurz, L.; García, V.; Wilkesman, J.; Contreras, L.M. Enzymatic characterization of the recombinant beta-xylosidase XynB2. J. Medical Biol. Sci. 2014, 1, 14–19. [Google Scholar]
- Ben-David, A.; Bravman, T.; Balazs, Y.S.; Czjzek, M.; Schomburg, D.; Shoham, G.; Shoham, Y. Glycosynthase activity of Geobacillus stearothermophilus GH52 b-xylosidase: Efficient synthesis of xylooligosaccharides from alpha-D-xylopyranosyl fluoride through a conjugated reaction. Chembiochem 2007, 8, 2145–2151. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Zhang, S.; Liu, Z. GH52 xylosidase from Geobacillus stearothermophilus: Characterization and introduction of xylanase activity by site-directed mutagenesis of Tyr509. J. Ind. Microbiol. Biotechnol. 2014, 41, 65–74. [Google Scholar] [CrossRef]
- Zhen, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catal. Sci. Technol. 2013, 3, 1937–1941. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Pletschke, B.I. Magnetic cross-linked enzyme aggregates (CLEAs): A novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enzyme Microb. Technol. 2014, 61–62, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Strategic optimization of xylanase–mannanase combi-CLEAs for synergistic and efficient hydrolysis of complex lignocellulosic substrates. J. Mol. Catal. B Enzym. 2015, 115, 140–150. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; Van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, M.; Tran, J.L.; Chu, K.H. Effective one-step saccharification of lignocellulosic biomass using magnetite-biocatalysts containing saccharifying enzymes. Sci. Total Environ. 2019, 647, 806–813. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Chen, H.; Wang, X.; Yang, Y.Y.; Ching, C.B. Cross-linked enzyme aggregates (CLEAs) with controlled particles: Application to Candida rugosa lipase. J. Mol. Catal. B Enzym. 2006, 43, 124–127. [Google Scholar] [CrossRef]
- Rojas, M.J.; Amaral-Fonseca, M.; Zanin, G.M.; Fernandez-Lafuente, R.; Giordano, R.L.C.; Tardioli, P.W. Preparation of Crosslinked Enzyme Aggregates of a Thermostable Cyclodextrin Glucosyltransferase from Thermoanaerobacter sp. Critical Effect of the Crosslinking Agent. Catalysts 2019, 9, 120. [Google Scholar] [CrossRef]
- Bibi, Z.; Qader, S.A.U.; Aman, A. Calcium alginate matrix increases the stability and recycling capability of immobilized endo-β-1,4-xylanase from Geobacillus stearothermophilus KIBGE-IB29. Extremophiles 2015, 19, 819–827. [Google Scholar] [CrossRef]
- Hormigo, D.; García-Hidalgo, J.; Acebal, C.; de la Mata, I.; Arroyo, M. Preparation and characterization of cross-linked enzyme aggregates (CLEAs) of recombinant poly-3-hydroxybutyrate depolymerase from Streptomyces exfoliatus. Bioresour. Technol. 2012, 115, 177–182. [Google Scholar] [CrossRef]
- Vršanská, M.; Voběrková, S.; Jiménez Jiménez, A.M.; Strmiska, V.; Adam, V. Preparation and optimisation of cross-linked enzyme aggregates using native isolate white rot fungi Trametes versicolor and Fomes fomentarius for the decolourisation of synthetic dyes. Int. J. Environ. Res. Public Health. 2018, 15, 23. [Google Scholar] [CrossRef]
- Xu, D.Y.; Yang, Y.; Yang, Z. Activity and stability of cross-linked tyrosinase aggregates in aqueous and nonaqueous media. J. Biotechnol. 2011, 152, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Zhang, S.; Sun, L.M. Cross-linked enzyme aggregates of phenylalanine ammonia lyase: Novel biocatalysts for synthesis of L-phenylalanine. Appl. Biochem. Biotechnol. 2012, 167, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef] [PubMed]
- Montoro-García, S.; Gil-Ortiz, F.; Navarro-Fernández, J.; Rubio, V.; García-Carmona, F.; Sánchez-Ferrer, Á. Improved cross-linked enzyme aggregates for the production of desacetyl β-lactam antibiotics intermediates. Bioresour. Technol. 2010, 101, 331–336. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, L.; Gao, G.; Jia, F.; Cao, S. Resolution of N-(2-ethyl-6-methylphenyl) alanine via cross-linked aggregates of Pseudomonas sp. lipase. J. Mol. Catal. B Enzym. 2008, 54, 7–12. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Enzyme | Km (mM) | Vmax (µmol/min) |
|---|---|---|
| Free XynB2Y509E | 0.9 ± 0.1 | 1.6 ± 0.2 |
| XynB2Y509E-CLEAs | 1.2 ± 0.2 | 1.18 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, G.; Contreras, L.M.; Aguirre, C.; Wilkesman, J.; Clemente-Jiménez, J.M.; Rodríguez-Vico, F.; Las Heras-Vázquez, F.J. Characterization of Cross-Linked Enzyme Aggregates of the Y509E Mutant of a Glycoside Hydrolase Family 52 β-xylosidase from G. stearothermophilus. Molecules 2021, 26, 451. https://doi.org/10.3390/molecules26020451
Romero G, Contreras LM, Aguirre C, Wilkesman J, Clemente-Jiménez JM, Rodríguez-Vico F, Las Heras-Vázquez FJ. Characterization of Cross-Linked Enzyme Aggregates of the Y509E Mutant of a Glycoside Hydrolase Family 52 β-xylosidase from G. stearothermophilus. Molecules. 2021; 26(2):451. https://doi.org/10.3390/molecules26020451
Chicago/Turabian StyleRomero, Gabriela, Lellys M. Contreras, Carolina Aguirre, Jeff Wilkesman, Josefa María Clemente-Jiménez, Felipe Rodríguez-Vico, and Francisco Javier Las Heras-Vázquez. 2021. "Characterization of Cross-Linked Enzyme Aggregates of the Y509E Mutant of a Glycoside Hydrolase Family 52 β-xylosidase from G. stearothermophilus" Molecules 26, no. 2: 451. https://doi.org/10.3390/molecules26020451
APA StyleRomero, G., Contreras, L. M., Aguirre, C., Wilkesman, J., Clemente-Jiménez, J. M., Rodríguez-Vico, F., & Las Heras-Vázquez, F. J. (2021). Characterization of Cross-Linked Enzyme Aggregates of the Y509E Mutant of a Glycoside Hydrolase Family 52 β-xylosidase from G. stearothermophilus. Molecules, 26(2), 451. https://doi.org/10.3390/molecules26020451








